Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in a spectrum of clinical presentations. Evidence from Africa indicates that significantly less COVID-19 patients suffer from serious symptoms than in the industrialized world. We and others previously postulated a partial explanation for this phenomenon, being a different, more activated immune system due to parasite infections. Here, we aimed to test this hypothesis by investigating a potential correlation of co-infection with parasites with COVID-19 severity in an endemic area in Africa.
Methods: Ethiopian COVID-19 patients were enrolled and screened for intestinal parasites, between July 2020 and March 2021. The primary outcome was the proportion of patients with severe COVID-19. Ordinal logistic regression models were used to estimate the association between parasite infection, and COVID-19 severity. Models were adjusted for sex, age, residence, education level, occupation, body mass index, and comorbidities.
Findings: 751 SARS-CoV-2 infected patients were enrolled, of whom 284 (37.8%) had intestinal parasitic infection. Only 27/255 (10.6%) severe COVID-19 patients were co-infected with intestinal parasites, while 257/496 (51.8%) non-severe COVID-19 patients were parasite positive (p<0.0001). Patients co-infected with parasites had lower odds of developing severe COVID-19, with an adjusted odds ratio (aOR) of 0.23 (95% CI 0.17–0.30; p<0.0001) for all parasites, aOR 0.37 ([95% CI 0.26–0.51]; p<0.0001) for protozoa, and aOR 0.26 ([95% CI 0.19–0.35]; p<0.0001) for helminths. When stratified by species, co-infection with Entamoeba spp., Hymenolopis nana, Schistosoma mansoni, and Trichuris trichiura implied lower probability of developing severe COVID-19. There were 11 deaths (1.5%), and all were among patients without parasites (p = 0.009).
Interpretation: Parasite co-infection is associated with a reduced risk of severe COVID-19 in African patients. Parasite-driven immunomodulatory responses may mute hyper-inflammation associated with severe COVID-19.
Funding: European and Developing Countries Clinical Trials Partnership (EDCTP) – European Union, and Joep Lange Institute (JLI), The Netherlands.
Trial registration: Clinicaltrials.gov: NCT04473365
Keywords: COVID-19, Severity, parasite, africa, co-infection
Research in context.
Evidence before this study
We searched PubMed, medRxvi, and bioRxiv for [“parasite” OR “helminth” OR “Protozoa”] AND [“COVID-19″ OR “SARS-CoV-2″] until April 12, 2021, with no language restriction. Ecological studies reported an inverse correlation between incidence of COVID-19 and parasites, like with soil-transmitted helminths, schistosomiasis, or malaria. Some reports proposed that helminth co-infection may modulate, or mute COVID-19 severity in endemic regions. To the best of our knowledge, there are no reports published to date actually demonstrating a clear association between parasite co-infection and COVID-19 severity.
Added value of this study
In this unique cohort study consisting of 751 Ethiopian patients, we determined the association between co-infection with parasites and COVID-19 severity. We identified 284 (37.8%) COVID-19 patients to be co-infected with one or more parasites. We demonstrate that the proportion of COVID-19 patients with parasitic infection decreased with increasing categories of disease severity. When stratified by species-specific parasite co-infection status, we observed same trends. Using a multivariable logistic regression analysis, and after adjusting for sex, age, residence, and the presence of comorbidities, we observed that co-infection with any parasite, including protozoa, or helminths, was associated with lower odds of developing severe COVID-19. In addition, patients without parasite co-infection appeared vulnerable to worse in-hospital outcomes, including admission to the intensive-care unit, requirement for supplemental oxygen, or mechanical ventilation, and death.
Implications of all the available evidence
Co-infection with parasites is associated with reduced development of severe COVID-19 in this Africa setting. Our findings confirm the hypothesis that co-infection with parasites may mute hyper-inflammation associated with severe COVID-19. Further research is needed to unravel underlying immunology and consequences for SARS-CoV-2 vaccination in Africa. Implications might involve COVID-19 vaccine efficacy in Africa.
Alt-text: Unlabelled box
1. Introduction
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) results in a spectrum of clinical presentations. Whereas most people with COVID-19 develop asymptomatic or mild illness, at-risk patients can develop severe pneumonia and hypoxemia disease requiring hospitalization [1], [2], [3]. In severe cases, COVID-19 can be complicated by acute respiratory distress syndrome (ARDS), sepsis, multi-organ failure, including acute kidney injury, and cardiac injury [2], [3], [4], [5]. Risk factors for a severe disease course and mortality due to COVID-19 include older age, immune compromise, and underlying co-morbidities, particularly non-communicable diseases (NCDs), such as hypertension, cardiovascular disease and diabetes [2], [3], [4], [5].
Low and medium-income countries (LMICs) differ significantly in disease prevalence and conditions from high-income countries (HICs). Infectious diseases have a markedly higher prevalence in LMICs, including so-called neglected infectious diseases (NIDs). Amongst NIDs, parasitic infections affect more than 2 billion people throughout the world, with disproportionately high prevalence rates in resource-poor settings [6,7]. Multicellular and highly complex parasites such as Ascaris, hook worm, Trichuris, Enterobius, and Schistosoma, as well as unicellular organisms including Entamoeba, Giardia, Toxoplasma, Cyclospora and Cryptosporidia are among the major organisms that contribute to the global intestinal parasitic disease burden [6,7].
Chronic and/or persistent parasitic infections are common in LIMCs, and such chronic infections, possibly in part through direct modulation of the host's immune responses, were shown to alter clinical outcomes to other infections [8,9]. Pre-existing parasitic infections may also modify the host's immune response to infection with SARS-CoV-2, with postulated beneficial and detrimental effects [10], [11], [12], [13]. To the best of our knowledge, no studies to date have assessed the possible association between parasitic co-infection and COVID-19 disease severity. We and other previously hypothesized parasite infections to skew the immune system towards TH2 responses, thus precluding TH1 hyper immune activation that is characteristic of COVID-19 severity [11], [12], [13]. The objective of this study was to test this hypothesis by comparing the parasitic infections of COVID-19 patients stratified for clinical outcomes.
2. Methods
2.1. Study design and participants
This study is part of Profile-CoV project (Clinicaltrials.gov: NCT04473365), a prospective observational cohort study being undertaken in two sites in Ethiopia, with the aim of profiling of immunological response to SARS-CoV-2 in the context of persistent immune activation in Sub-Saharan Africa. This study included individuals who were recruited for Profile-CoV and subsequently screened them for intestinal parasitic infections.
2.2. Procedures
Individuals presenting to the Kuyha (Mekelle University College of Health Sciences, Mekelle), and Eka Generalized Hospital (Addis Ababa) who qualified for testing were screened for SARS-CoV-2 infection with a nasopharyngeal swab, and real-time polymerase chain reaction (RT-PCR). Following the declaration by the WHO that COVID-19 became pandemic, the Ethiopian Ministry of Health implemented a mass screening of all travelers, people who had come in contact with COVID-19 patients, those from high risk settings (e.g. health-care workers), as well as those with symptoms suggestive of COVID-19. All patients with confirmed SARS-CoV-2 infection were admitted to dedicated COVID-19 Isolation and Treatment Centers. Patients were admitted irrespective of clinical severity status. Whereas 515 patients were included from Kuyha Hospital in Mekelle between July and October 2020, the 236 cases from Eka Generalized Hospital in Addis Ababa were enrolled between February and March 2021. Admitted patients, if symptomatic, received supportive therapy according to their clinical need. Patients with severe disease received high-flow oxygen via nasal cannula or intubation as well as dexamethasone. Whereas patients admitted to the intensive care unit (ICU) were followed every day, or as needed, those with asymptomatic or mild/moderate clinical presentation were quarantined, and followed every 3–5 days, or as needed up until discharge.
Sociodemographic, clinical, and laboratory data were collected using standardized Case Record Forms (CRFs) adapted from the International Severe Acute Respiratory and Emerging Infection Consortium's (ISARIC) CRFs for emerging severe acute respiratory infections [14]. Patient's clinical status was stratified following the WHO criteria as asymptomatic, mild/moderate, severe (with dyspnea, respiratory rate ≥ 30 breaths per minute, O2 saturation ≤ 93%, lung infiltrates ≥ 50% of the lung fields within 24–48 h), and critical (with respiratory failure, septic shock, and/or multiple organ failure) [15]. All data were then entered into electronic medical records.
SARS-CoV-2 infection was confirmed by RT-PCR on samples obtained from nasopharyngeal swabs, according to manufacturer's instructions (TIB Molbiol, Berlin, Germany). Fresh stool sample specimens were obtained for examination for parasites and ova. Analysis included direct microscopic examination and modified Ritchie concentration method [16]. In addition, the intensity of infection was determined using Kato-Katz method and was calculated and reported as individuals’ eggs per gram of feces (EPG), as described previously [16], and recommended by the WHO [17]. All patients who were positive for intestinal parasites received parasite-specific therapy. None of these patients received ivermectin.
The study protocol was reviewed and approved by the Health Research Ethics Review Committee of Mekelle University College of Health Sciences (No.: ERC 1769/2020), the Ethiopian Public Health Institute (No.: EPHI 6.13/814), and Eka Kotebe General Hospital (No.: EK/150/5/32). Written informed consent was obtained by all participants, or their guardians, for participation in the study.
2.3. Outcomes
The primary outcome for this study was the proportion of severe COVID-19 among SARS-CoV-2 positive patients with and without a parasitic co-infection. Asymptomatic and mild/moderate COVID-19 cases were classified as non-severe cases and severe and critical COVID-19 cases were classified as severe. Secondary outcomes included requirement for supplemental oxygen, and/or mechanical ventilation, admission to ICU, and death.
2.4. Statistical analysis
We hypothesized that co-infection with intestinal parasite is associated with reduced risk of severe COVID-19 [13]. Exposure was intestinal parasite co-infection. The primary outcome of the study was the proportion of severe disease among SARS-CoV-2 PCR positive patients. A feasibility study showed that about 20% of COVID-19 patients with parasite co-infection developed severe COVID-19. We expected this to increase to about 50% among those without parasite co-infection. A minimum sample size of 223 patients with parasite, and 446 without parasite co-infection (at 1:2 ratio) was required to ensure 80% power using a two-sided test with a significance level of α=0.05. Assuming a dropout rate of 10%, a minimum total sample size of 738 was estimated for the cohort.
Baseline characteristics for continuous variables were expressed as the median with interquartile range (IQR), and for categorical variables as proportions. Whereas categorical variables were compared using χ² test or Fisher's exact test, continuous variables were compared by Mann-Whitney U, or Kruskal-Wallis tests, as appropriate. Normality of distribution of variables was ascertained by running Wilk test of normality, before analysis by Mann-Whitney U, or Kruskal-Wallis tests. The association between COVID-19 severity and parasitic co-infection was determined by ordinal logistic regression analysis. Independent variables, including age, sex, residence, comorbidity, parasite infection (overall and dis-aggregated by parasite type into protozoa, helminths, and species level), were included in the initial univariate analysis. We included sex, age, education level, occupation, pregnancy, body mass index and comorbidities as confounding factors as they have been associated with poor clinical outcomes among patients with COVID-19 [2], [3], [4], [5]. In addition, we adjusted for urban vs. rural residence as this has been shown to influence differential immune responses [18]. A multivariate regression analyses [adjusted odds ratio (aOR)] were calculated by including all variables that were p<0.05 by univariate analysis. P values <0.05 were considered statistically significant. Data was analyzed using STATA (Statistical package v. 14.0, StataCorp, Texas, USA). Reporting of this study was undertaken according to the STROBE statement (Supplemental Table 4).
2.5. Role of funding source
Funders had no role in the study design, study participant selection, and recruitment, data collection, analysis, data interpretation, decision to publish, or preparation of the manuscript.
3. Results
3.1. Study population characteristics
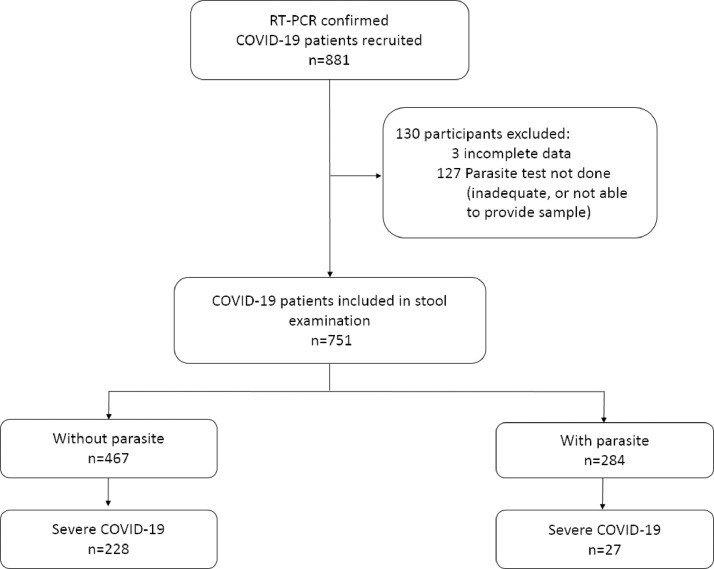
A total of 881 COVID-19 cases were recruited and 130 were excluded, either due to incomplete data or inadequate stool samples, or patient not able to provide sample (Fig. 1). Baseline socio-demographic data of the study participants is summarized in Table 1. The majority of the study population was male (63.9%). The median age of the cohort was 37 (IQR 28–50; range 3–92) years, with 53.0% being in the age range of 24 to 44 years. Those older than 60 years and above comprised only 16.1%. Patients were hospitalized for a median of 12 days (range: 3 to 45 days). Notably, 36.2% of the cohort was asymptomatic, and 29.8% had mild/moderate symptoms at the time of diagnosis; the remaining 29.0% and 4.9% had severe, and critical disease (requiring admission to the ICU), respectively (Supplemental Table 1).
Fig. 1.
Flow diagram of study participants.
Table 1.
Clinical features among COVID-19 patients without, or with parasite co-infection.
| Characteristic | All patients n = 751 | Without parasite n = 467 | With parasite n = 284 | p-Value |
|---|---|---|---|---|
| Socio–demographic features: | ||||
| Gender | ||||
| Male | 480 (63.9%) | 307 (65.7%) | 173 (60.9%) | 0.011 |
| Female (not pregnant) | 257 (34.2%) | 147 (31.5%) | 110 (38.7%) | |
| Female (pregnant) | 14 (1.9%) | 13 (2.8%) | 1 (0.4%) | |
| Age in years | 37 (28–50) | 40 (30–57) | 32 (25–42) | 0.064 |
| Age group [years] | ||||
| < 24 | 92 (12.3%) | 42 (9.0%) | 50 (17.6%) | 0.0001 |
| 24 – 44 | 398 (53.0%) | 224 (48.0%) | 174 (61.3%) | |
| 45 – 59 | 140 (18.6%) | 99 (21.2%) | 41 (14.4%) | |
| ≥ 60 | 121 (16.1%) | 102 (21.8%) | 19 (6.7%) | |
| Residence | ||||
| Rural | 141(18.8%) | 68 (14.6%) | 73 (25.7%) | 0.0001 |
| Urban | 593 (79.0%) | 393 (84.2%) | 200 (70.4%) | |
| Undetermined | 17 (2.3%) | 6 (1.3%) | 11 (3.9%) | |
| Education level | ||||
| ≤ Primary | 215 (28.6%) | 124 (26.5%) | 91 (32.0%) | 0.210 |
| Secondary | 203 (27.0%) | 126 (27.0%) | 77 (27.1%) | |
| College/university | 333 (44.3%) | 217 (46.5%) | 116 (40.9%) | |
| Occupation | ||||
| Unemployed | 250 (33.3%) | 149 (31.9%) | 101 (35.6%) | 0.587 |
| Employed | 421 (56.0%) | 267 (57.2%) | 154 (54.2%) | |
| Health care workers | 80 (10.7%) | 51 (10.9%) | 29 (10.2%) | |
| Clinical symptoms and signs: | ||||
| Fever | 248 (33.0%) | 200 (42.8%) | 48 (16.9%) | <0.0001 |
| Dyspnea | 199 (26.5%) | 176 (37.7%) | 23 (8.1%) | <0.0001 |
| Cough (any type) | 339 (45.1%) | 259 (55.5%) | 80 (28.2%) | <0.0001 |
| Non–productive cough | 148 (19.7%) | 94 (20.1%) | 54 (19.0%) | 0.0001 |
| Productive cough | 191 (25.4%) | 165 (35.3%) | 26 (9.2%) | |
| Hemoptysis | 21 (2.8%) | 19 (4.1%) | 2 (0.7%) | 0.007 |
| Chest pain | 47 (6.3%) | 39 (8.4%) | 8 (2.8%) | 0.002 |
| Sore throat | 70 (9.3%) | 54 (11.6%) | 16 (5.6%) | 0.007 |
| Head ache | 223 (29.7%) | 181 (38.8%) | 42 (14.8%) | <0.0001 |
| Nasal congestion | 42 (5.6%) | 23 (4.9%) | 19 (6.7%) | 0.307 |
| Loss of smell and/or taste | 59 (7.9%) | 36 (7.7%) | 23 (8.1%) | 0.847 |
| Nausea/vomiting | 76 (10.1%) | 67 (14.4%) | 9 (3.2%) | <0.0001 |
| Abdominal pain | 44 (5.9%) | 35 (7.5%) | 9 (3.2%) | 0.014 |
| Diarrhea | 39 (5.2%) | 32 (6.9%) | 7 (2.5%) | 0.009 |
| Myalgia | 112 (14.9%) | 98 (21.0%) | 14 (4.9%) | <0.0001 |
| Body mass index | ||||
| 18.5–24.9 | 668 (88.9%) | 399 (85.4%) | 269 (94.7%) | <0.0001 |
| <18.5 | 4 (0.5%) | 3 (0.6%) | 1 (0.4%) | |
| 25.0–29.9 | 56 (7.5%) | 42 (9.0) | 14 (4.9%) | |
| ≥30.0 | 23 (3.1%) | 23 (4.9%) | 0 (0.0%) | |
| Temperature > 37.3 °C | 50 (6.7%) | 41 (8.8%) | 9 (3.2%) | 0.003 |
| Temperature | 36.0 (36.0–36.9) | 36.2 (36.0–37.0) | 36.0 (36–36.7) | <0.00001 |
| Systolic blood pressure (mmHg) | 120 (110–130) | 120 (110–130) | 115 (110–125) | 0.0009 |
| Diastolic blood pressure (mmHg) | 75 (68–80) | 75 (68–80) | 75 (68–80) | 0.8395 |
| Respiratory rate (breaths/minute) | 22 (20–25) | 23 (21–28) | 22 (19–23) | <0.00001 |
| Heart rate (beats/minute) | 85 (76–94) | 86 (78–96) | 83 (76–90) | <0.00001 |
| Laboratory data* | ||||
| Lymphocyte, x109/L | 1.2 (0.8–1.6) | 1.2 (0.8–1.6) | 1.3 (0.9–1.7) | 0.7008 |
| Hematocrit,% | 44.0 (40.1–46.9) | 44.2 (40.6–47.4) | 42.4 (38.2–45.7) | 0.0296 |
| Platelet count, x109 /L | 217 (163–294) | 215 (159–288) | 247(204–334) | 0.0488 |
| Alanine aminotransferase concentration, U/L | 40 (25–67) | 40 (25–67) | 48 (36–55) | 0.6841 |
| Creatinine concentration, mg/dL | 0.87 (0.68–1.04) | 0.88 (0.70–1.04) | 0.62 (0.48–1.47) | 0.0135 |
| Comorbidities | ||||
| Comorbidity (at least 1) | 216 (28.8%) | 178 (38.1%) | 38 (13.4%) | <0.0001 |
| Non–communicable disease (NCDs) comorbidities | 179 (23.8%) | 150 (32.1%) | 29 (10.2%) | <0.0001 |
| Diabetes | 97 (12.9%) | 84 (18.0%) | 13 (4.6%) | <0.0001 |
| Hypertension | 87 (11.6%) | 74 (15.9%) | 4.6 (4.5%) | <0.0001 |
| Cardio vascular diseases | 19 (2.5%) | 18 (3.9%) | 1 (0.4%) | 0.003 |
| Chronic obstructive lung diseases and asthma | 23 (3.1%) | 19 (4.1%) | 4 (1.4%) | 0.040 |
| Chronic liver disease | 8 (1.1%) | 8 (1.7%) | 0 (0.0%) | 0.027 |
| Chronic kidney disease | 11 (1.5%) | 9 (1.9%) | 2 (0.7%) | 0.176 |
| Surgery cases | 15 (2.0%) | 12 (2.6%) | 3 (1.1%) | 0.151 |
| Communicable disease comorbidities | ||||
| HIV | 17 (2.3%) | 14 (0.3.0%) | 3 (1.1%) | 0.083 |
| Tuberculosis | 1 (0.1%) | 1 (0.2%) | 0 (0.0%) | 0.435 |
| Outcomes | ||||
| Admission to ICU | 56 (7.5%) | 44 (9.4%) | 12 (4.2%) | 0.009 |
| Supplemental oxygen | 243 (32.4%) | 216 (46.3%) | 27 (9.5%) | <0.0001 |
| Invasive mechanical ventilation | 53 (7.1%) | 46 (9.9%) | 7 (2.5%) | <0.0001 |
| Death | 11 (1.5%) | 11 (2.4%) | 0 (0.0%) | 0.009 |
| Severe COVID–19 | 255 (34.0%) | 228 (48.8%) | 27 (9.5%) | <0.0001 |
Data are expressed as n (%) or median (IQR). p values are from χ² test, or Fisher's Exact test (for categorical variables), and Mann-Whitney U, or Kruskal-Wallis tests (for continuous variables), as appropriate.
*Data missing for 490, 487, 492, 622, and 540 patients for lymphocyte, hematocrit, platelet, alanine aminotransferase, and creatinine concentrations, respectively.
Patients with severe, or critical disease at presentation were older and had more symptoms, including fever, dyspnoea, cough, hemoptysis, chest pain, sore throat, head ache, nausea/vomiting, abdominal pain, diarrhea, and myalgia, when compared to those presenting with asymptomatic, or mild/moderate clinical status (Supplemental Table 1). Nasal congestion, and loss of smell/taste was reported less frequently in severe, or critical cases. Co-morbid conditions were significantly higher among severe, and critical COVID-19 cases when compared to non-severe cases (Supplemental Table 1). In addition, COVID-19 patients with NCDs were older, the majority (45.3%) being ≥ 60 years old (p = 0.0001), and more symptomatic (Supplemental Table 2). Severe/critical clinical manifestation was more frequent in COVID-19 patients with NCDs compared to those without NCDs [(p<0.0001), Supplemental Table 2].
3.2. Prevalence of intestinal parasites
Of the total 751 individuals enrolled in the study, 284 (37.8%) harbored one or more intestinal parasites (Table 2). Of the patients included in the study, protozoa and helminth infections comprised 20.2% and 24.5%, respectively. The most common protozoa infections were Entamoeba spp. (16.8%) and Giardia (3.6%). For helminths, the most common infections were H. nana (15.1%), S. mansoni (4.5%), and A. lumbricoides (3.6%). The proportion of patients harboring multiple parasites (>1) was 8.7% (Table 2). There was no significant difference in sex and age distribution between those with parasites and those without (Table 1). However, those without parasite co-infection appeared more symptomatic for COVID-19. In addition, the proportion of comorbid conditions, in particular NCDs, was significantly higher in COVID-19 patients without parasitic co-infection (Table 1).
Table 2.
Prevalence of parasitic infections in the different COVID–19 severity category.
| Parasite co–infection | All patients (n = 751) | COVID-19 severity category |
p-Value | |||
|---|---|---|---|---|---|---|
| Asymptomatic (n = 272) | Mild/moderate (n = 224) | Severe (n = 218) | Critical (n = 37) | |||
| Any parasite | 284 (37.8%) | 150 (55.2%) | 107 (47.8%) | 22 (10.1%) | 5 (13.5%) | <0.0001 |
| Protozoa – all | 152 (20.2%) | 77 (28.3%) | 59 (26.3%) | 12 (56.5%) | 4 (10.8%) | <0.0001 |
| Entamoeba spp. cyst | 109 (14.5%) | 63 (23.2%) | 38 (17.0%) | 6 (2.8%) | 2 (5.4%) | <0.0001 |
| Entamoeba histolytica trophozoite | 17 (2.3%) | 5 (1.8%) | 10 (4.5%) | 2 (0.9%) | 0 (0.0%) | 0.087 |
| Giardia lamblia cyst | 12 (1.6%) | 4 (1.5%) | 4 (1.5%) | 2 (0.9%) | 2 (5.4%) | 0.227 |
| Giardia lamblia trophozoite | 15 (2.0%) | 6 (2.2%) | 7 (3.1%) | 2 (0.9%) | 0 (0.0%) | 0.393 |
| Helminth – all | 184 (24.5%) | 103 (37.9%) | 68 (30.4%) | 12 (5.5%) | 1 (2.7%) | <0.0001 |
| Hymenolopis nana | 113 (15.1%) | 59 (21.7%) | 47 (21.0%) | 7 (3.2%) | 0 (0.0%) | <0.0001 |
| Schistosoma mansoni | 34 (4.5%) | 22 (8.1%) | 10 (4.5%) | 2 (0.9%) | 0 (0.0) | 0.001 |
| Ascaris lumbricoides | 27 (3.6%) | 13 (4.8%) | 10 (4.5) | 3 (1.4%) | 1 (2.7%) | 0.142 |
| Trichuris trichiura | 11 (1.5%) | 8 (2.9%) | 3 (1.3%) | 0 (0.0%) | 0 (0.0%) | 0.050 |
| Hook worm | 12 (1.6%) | 8 (2.9%) | 3 (1.3%) | 1 (0.5%) | 0 (0.0%) | 0.169 |
| Taenia spp. | 3 (0.4%) | 3 (1.1%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0.239 |
| Soil–transmitted helminths only | 48 (6.4%) | 26 (9.6%) | 17 (7.6%) | 4 (1.8%) | 1 (2.7%) | 0.002 |
| Poly–parasitism – any | 65 (8.7%) | 37 (13.6%) | 25 (11.2%) | 3 (1.4%) | 0 (0.0%) | <0.0001 |
| Protozoa plus helminth | 50 (6.7%) | 30 (11.0%) | 18 (8.1%) | 2 (0.9%) | 0 (0.0%) | <0.0001 |
| Helminth plus helminth | 19 (2.5%) | 11 (4.0%) | 7 (3.1%) | 1 (0.5%) | 0 (0.0%) | 0.046 |
Data are expressed as n (%). P values are from χ² test, or Fisher's Exact test, as appropriate.
3.3. Association of parasitic co-infection with COVID-19 severity
The proportion of severe, or critical COVID-19 was significantly higher in patients without parasitic co-infection (Table 1). The proportion of severe COVID-19 in patients without parasites (196/467 [42.0%, CI 37.56–46.52]) was significantly higher than in those with parasites (22/284 [7.8%, CI 5.14–11.51]); p<0.0001. Likewise, the proportion of critical COVID-19 in patients without any parasite infection (32/467 [6.9%, CI: 4.88–9.54]) was significantly higher than in those co-infected with parasites (5/284 [1.8, CI 0.73–4.18]; p<0.0001). The proportion of severe COVID-19 was higher in the in the non-protozoa group (206/599 [34.4%, CI 30.68–38.30]) when compared to the protozoa group (12/152 [7.9%, CI 4.51–13.47]; p<0.0001), and in the helminth negative group (206/567 [36.3%, CI 32.46–40.39]) compared to the helminth co-infected group (12/184 [6.5%, CI 3.72–11.19]; p = 0.0001). Furthermore, the proportion of critical COVID-19 cases was higher in the in the non-protozoa group (33/599 [5.5%, CI 3.94–7.66]) when compared to the protozoa group (4/152 [2.6%, CI 0.98–6.87]; p<0.0001), and in the helminth negative group (36/567 [6.4%, CI 4.61–8.69]) compared to the helminth co-infected group (1/184 [0.5%, CI 0.08–3.82]; p = 0.0001). We did not observe any correlation between helminth egg-load and COVID-19 severity.
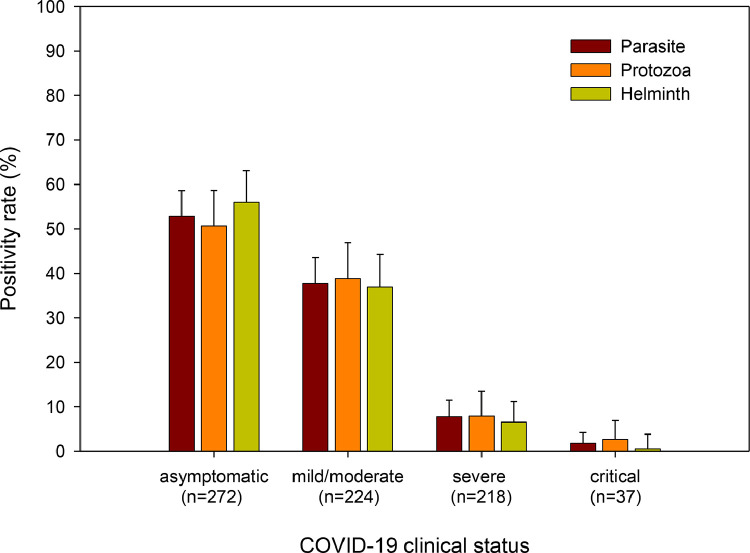
Thus, parasitic infections were inversely correlated with COVID-19 severity. A higher proportion of patients without parasitic co-infection were admitted to the ICU, required supplemental oxygen, and/or mechanical ventilation, or died compared to those with parasitic co-infection (Table 1). The proportion of COVID-19 patients with any parasite infection, or protozoa, or helminth infection decreased with increasing categories of disease severity (Table 2; Fig. 2). When stratified by species-specific parasite co-infection status, we observed similar trends with significant decrease in COVID-19 severity for those co-infected with poly-parasites, soil-transmitted helminths, Entamoeba spp. cyst, H. nana, S. mansoni, and T. trichiura compared to those without (Supplemental Fig. 1).
Fig. 2.
Relationship of parasite co-infection with categories of COVID-19 severity. Proportion of patients with different categories of COVID-19 severity co-infected with any parasite, protozoa, or helminths. Error bars indicate 95% CI. p<0.0001 (for parasite, and protozoa), and p = 0.001 (for helminths) by Kruskal-Wallis rank test.
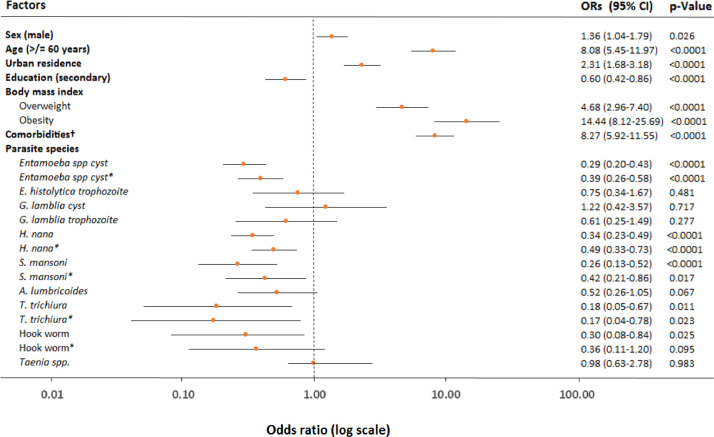
Ordinal logistic regression analysis was used in order to ascertain the association between parasite infection and COVID-19 severity. In univariate analysis, male sex, age ≥ 60 years, urban residence, primary education level, being overweight, or obese, and comorbidities, in particular, NCDs including diabetes, hypertension, cardio-vascular diseases, and chronic liver disease were all associated with increased odds of severe COVID-19 (Table 3). However, occupation, pregnancy, chronic obstructive lung disease, including asthma, chronic kidney disease, surgery, and HIV-1 infection appeared not associated with severe COVID-19. In contrast, having any parasitic, protozoal, or helminth infection was associated with lower odds of developing severe COVID-19. In multivariate analysis, age ≥ 60 years, urban residence, obesity, comorbidity, in particular NCDs including hypertension, and chronic liver disease were associated with increased odds of severe COVID-19 (Table 3). After adjustment for sex, age, residence, obesity, and the presence of comorbidities, COVID-19 patients with any parasitic co-infection (aOR 0.35 [95% CI 0.26–0.48]; p<0.0001), protozoal co-infection (aOR 0.51 [95% CI 0.36–0.73]; p<0.0001), as well as helminth co-infection (aOR 0.37 [95% CI 0.27–0.52]; p<0.0001) had a lower odds of developing severe COVID-19 when compared to patients without a parasitic co-infection (Table 3). We also noted that harboring multiple parasites was strongly associated with less severe COVID-19 (aOR 0.43 [95% CI 0.26–0.71]; p<0.0001; Table 3). Further analysis stratified by parasite species level revealed that patients co-infected with Entamoeba cyst spp (aOR 0.39 [95% CI 0.26–0.58]; p<0.0001), or Hymenolopis nana (aOR 0.49 [95% CI 0.33–0.73]; p<0.0001), or Schistosoma mansoni (aOR 0.42 [95% CI 0.21–0.86; p = 0.017)], or T. trichiura (aOR 0.17 [95% CI 0.04–0.78); p = 0.023) co-infection tended to have lower odds of developing severe COVID-19 (Fig. 3). Covid-19 patients co-infected with Hook worm also tended to exhibit decreased risk of severe disease, but this was not statistically significant (Fig. 3). Smaller sample sizes precluded observations for the remaining parasite species. We could not determine association between death (n = 11), and (lack-of) co-infection with parasites because of the small number of death outcomes. Notably, after adjusting for sex, age, residence, pregnancy, education, occupation, and body mass index we found that the odds of having a NCD was significantly lower in COVID-19 patients with parasitic co-infection (aOR 0.35 [95% CI 0.21–0.60], p<0.0001), or helminth infection (aOR 0.21 [95% CI 0.10–0.43]; p<0.0001; Supplemental Table 3).
Table 3.
Factors associated with severe COVID–19.
| Characteristic | Univariable model |
Multivariable model |
||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Gender | ||||
| Male | 1 | .. | 1 | .. |
| Female (not pregnant) | 0.70 (0.53–0.92) | 0.011 | 0.86 (0.64–1.15) | 0.313 |
| Female (pregnant) | 1.72 (0.69–4.28) | 0.242 | 2.09 (0.79–5.59) | 0.139 |
| Age (≥60 years vs. <60) | 8.08 (5.45–11.97) | <0.0001 | 3.41 (2.13–5.46) | <0.0001 |
| Rural vs. urban residence | 0.38 (0.28–0.516) | <0.0001 | 0.52 (0.38–0.72) | <0.0001 |
| Education level | ||||
| ≤ Primary | 1 | .. | 1 | .. |
| Secondary | 0.60 (0.42–0.86) | 0.006 | 1.02 (0.68–1.54) | 0.911 |
| College/university | 0.91 (0.66–1.25) | 0.569 | .. | .. |
| Occupation | ||||
| Unemployed | 1 | .. | 1 | .. |
| Employed | 1.01 (0.76–1.35) | 0.939 | .. | .. |
| Health care workers | 1.26 (0.81–1.94) | 0.304 | .. | .. |
| Body mass index | ||||
| 18.5–24.9 | 1 | .. | 1 | .. |
| <18.5 | 0.52 (0.04–6.34) | 0.610 | .. | .. |
| 25.0–29.9 | 4.68 (2.96 –7.40) | <0.0001 | 1.34 (0.61–2.94) | 0.460 |
| ≥30.0 | 14.44 (8.12–25.69) | <0.0001 | 2.68 (1.22–5.87) | 0.014 |
| Comorbidity (at least 1)* | 8.27 (5.92–11.55) | <0.0001 | 5.43 (3.50–48.42) | <0.0001 |
| Non–communicable disease (NCDs) comorbidities* | 10.58 (7.36–15.20) | <0.0001 | 7.74 (4.64–12.90) | <0.0001 |
| Diabetes | 6.41 (4.22–9.76) | <0.0001 | 1.61 (0.78–3.32) | 0.198 |
| Hypertension | 9.63 (6.10–15.18) | <0.0001 | 2.87 (1.63–5.907) | <0.0001 |
| Cardio vascular diseases | 5.27 (2.37–11.74) | <0.0001 | 1.25 (0.504–3.12) | 0.633 |
| Chronic obstructive lung diseases | 1.90 (0.90–4.04) | 0.093 | .. | .. |
| Chronic kidney disease | 2.13 (0.72–6.74) | 0.174 | .. | .. |
| Chronic liver disease | 3.57 (1.0.8–11.78) | 0.037 | 4.67 (1.30–16.82) | 0.018 |
| Surgery cases | 0.64 (0.25–1.66) | 0.362 | .. | .. |
| Communicable disease comorbidities | ||||
| HIV | 2.05 (0.83–5.07) | 0.121 | .. | |
| Tuberculosis | 1.82e+09 (..–..) | 0.998 | .. | .. |
| Parasite co–infection** | ||||
| Any parasite (at least 1) | 0.23 (0.17–0.30) | <0.0001 | 0.35 (0.26–0.48) | <0.0001 |
| Protozoa | 0.37 (0.26–0.51) | <0.0001 | 0.51 (0.36–0.73) | <0.0001 |
| Helminth | 0.26 (0.19–0.35) | <0.0001 | 0.37 (0.27–0.52) | <0.0001 |
| Poly–parasitism – any | 0.31 (0.19–0.51) | <0.0001 | 0.43 (0.26–0.71) | 0.001 |
| Protozoa plus helminth | 0.29 (0.17–0.50) | <0.0001 | 0.36 (0.20–0.65) | 0.001 |
| Helminth plus helminth | 0.33 (0.14–0.79) | 0.013 | 0.52 (0.21–1.25) | 0.143 |
| Soil–transmitted helminths only | 0.39 (0.23–0.68) | 0.001 | 0.43 (0.24–0.77) | 0.004 |
OR=odds ratio. *Adjusted for gender, age, residence, education level, body mass index; **Adjusted for gender, age, residence, education level, body mass index, and comorbidities.
Fig. 3.
ORs for the association between species-specific parasite co-infection and COVID-19 severity. All models and those stratified by species-specific parasitic co-infection were analysed by univariable ordinal logistic regression analysis. *Multivariable models adjusted for sex, age, residence, education, body mass index, and comorbidities† (including diabetes, hypertension, cardio-vascular diseases, and chronic liver diseases). ORs=odds ratios. Lines are 95% CIs.
4. Discussion
This study demonstrates for the first time that co-infection with enteric parasites, both protozoa and helminths, is associated with lower odds of developing severe COVID-19 in African patients. Notably, this association was maintained even after adjusting for sex, age, residency, education level, and presence of comorbid conditions, factors that are commonly associated with COVID-19 severity [2], [3], [4], [5]. Previous reports have demonstrated that helminthic correlates with a lower risk for development of diabetes, and metabolic syndrome in humans [19], [20], [21], [22], [23], [24]. This is consistent with our finding that patients co-infected with parasites had lower proportions of NCDs. This finding suggests that the inverse correlation between parasitic infection and COVID-19 severity in our cohort can be attributed at least partly to decreased NCDs risk. In this study, we noted that NCDs, in particular hypertension, chronic liver disease, overweight, and obesity were all associated with increased risk of COVID-19 severity. Nonetheless, pregnancy, and NCDs such as chronic obstructive lung disease, chronic kidney disease, surgery, and HIV-1 infection appeared not associated with severe COVID-19, most likely due to the smaller sample size of patients with these conditions.
The pathogenesis of severe COVID-19 has been linked to the phenomenon of immune hyperactivation [25], which resembles that of chronic inflammatory condition, such as hypertension, obesity, diabetes, and inflammatory bowel diseases [26], [27], [28]. Lifestyle factors, such as high calorie diet, physical inactivity, and higher standard of living, coupled with decreased rates of helminthic infections, in HICs has been linked with the advent of chronic inflammatory conditions – compatible with the theory of hygiene hypothesis [29,30]. Indeed, several authorities proposed that lack of co-infection with parasites may lead to increased risk of COVID-19 severity in HICs [[11], [12], [13], 29,30]. It is possible, therefore, that parasites mute COVID-19 severity through their effects in modulating systemic immune response. Chronic intestinal parasitic infections are often associated with the development of T helper-2 (TH2)-skewed, alternatively activated macrophages (M2), and type 2 innate lymphoid cells. These responses are accompanied with the induction of cytokines such as IL-4, IL-5, IL-13, and enhanced eosinophilia, and IgE responses [8,9]. Parasite-driven TH-2 responses are important in controlling parasitic infections, as well as play an important role in the repair of tissue damage as a result of parasite infections [8,9]. TH2 immune responses during parasitic infections is also accompanied by the induction of a strong T cell regulatory (Treg) responses that is relevant for survival and chronic persistence of the parasite itself, that may affect responses to heterologous infection [8,9,29]. On the contrary, severe COVID-19 is associated with increased hyperinflammation characterized by increased production of pro-inflammatory cytokines [25]. Thus, persistent parasite-driven TH2, and Treg responses, in turn, may counterbalance overactive TH1 responses, which have been described in severe COVID-19 [13]. In addition, parasite-driven gut microbiome changes may modulate the host's immune response [13]. Thus, it is possible that parasitic infections may affect pathogenesis both through direct modulation of the immune system as well as through an indirect parasite-driven microbiome balance [8,9,13]. Indeed, it has been demonstrated in animal models that enteric helminths can protect against pulmonary viral infections through interaction with microbiota [31]. Paradoxically, lack of hygienic practice in parasite endemic areas of LMICs may increase the risk of transmission and infection of SARS-CoV-2 [29].
The strengths of the current study include its prospective nature. However, our study has some limitations. First, it was not possible to collect stool samples for every consecutive patient which may have resulted in a potential selection bias. Second, stool examination was determined by microscopy only. Although PCR has been shown to be superior to microscopy with increased sensitivity and specificity [32], the presence of very low intensity of infection determined by PCR, might indeed preclude the effects on immune modulation. Third, some of the parasite species-specific associations with COVID-19 severity could not be ascertained in the current study because of small sample sizes/prevalence. Fourth, it is difficult to ascertain whether the cases included in our study can be considered representative of the wider population, given the fact that there is lack of data from the country. Finally, the inclusion of a smaller proportion of critical cases (which are rare in our settings) [33], as compared to other categories within the clinical spectrum of COVID-19 in our cohort may potentially bias the results. Confounding through other treatments the patients may have received on their COVID-19 severity in our cohorts is negligible, as very few patients (n = 5) took remdesivir. In addition, none of the patients co-infected with parasites included in the cohort did receive anti-parasitic drug ivermectin, that has been shown to exhibit in-vitro activity against SARS-CoV-2 [34].
In conclusion, our study is the first to show a significant inverse correlation between the presence of intestinal parasites and COVID-19 severity, suggesting that parasite co-infection, with both protozoa and helminths, may protect against progression to severe COVID-19. This is corroborated by the observed low COVID-19 fatality rate in LMIC settings where parasitic infections are endemic [11,12]. Nonetheless, causality cannot be inferred from the current study design. Thus, more evidence corroborating this association is needed, with corroboration in other LMIC settings, and studies with larger sample sizes that permit testing of possible interplay between the parasite microbiome on COVID-19 severity [13]. Unraveling the parasite-modulated mechanisms underlying severe COVID-19 offers avenues for novel preventive and therapeutic interventions. Moreover, parasitic infections might have repercussions for efficacy of current COVID-19 vaccines administered in Africa through COVAX initiative [35], and could lead to alternative approaches, such as: first deworm, than vaccinate. Studies in this area are recommended.
5. Contributors
DW, VH, and TRW were involved in funding acquisition, and conceived and designed the study. TG, ZGA, YK, AG, GT, MA, KLE, TGH, and FKM followed the patients, collected clinical samples and data. TG, AH, GT, and SA did the laboratory analysis. HEA and AD collected data. GT, MT, and EA were involved in funding acquisition, and study design. BCU and HHDFS were involved in funding acquisition, study design, data interpretation, and draft writing and review. DW, and TRW wrote the first draft of the manuscript; both were responsible for access and data accession of the raw data. All authors contributed to data interpretation, critically reviewed the manuscript, and approved the final version for submission.
Declaration of Competing Interest
DW is European and Developing Countries Clinical Trials Partnership (EDCTP) Senior Research Fellow, and received funding for EvaLAMP project on Leishmaniasis Diagnostics; he serves as Strategic and Scientific Advisory Board of the Research Networks for Health Innovations in Sub-Saharan Africa (German Federal Ministry of Education and Research), and has received an honorarium for lectures and presentations from the Ethiopian Ministry of Science and Higher Education. VH received grants from Netherlands organization for Health Research and Development, VaillantFonds, and she serves as Gilead advisory board, and has received an honorarium from Medtalks, and Gilead. In addition, she serves as head of expertise group for Federal Medical Specialists, and is reviewer for COVID-19 grants for Netherlands organization for Health Research and Development. TRW is employee of PharmAccess Foundation, is Board Member of Mondial Diagnostics, and Advisory Board member of Healthinc, The Netherlands. All other authors have no declarations to disclose.
Acknowledgments
Funding
This research was supported by grants from the European and Developing Countries Clinical Trials Partnership (EDCTP), supported by the European Union (RIA-2020EF-2095) and Joep Lange Institute for Global Health and Development, The Netherlands.
Data sharing
The consortium welcomes request for data and material access through the Research Steering Committee. Data collected for the study, including individual anonymized participant data, a data dictionary defining each field in the set, study protocol, and consent forms will be made available. Data will be shared after approval of the application, and with a signed data access agreement.
Acknowledgments
We would like to express our gratitude to the medical, nursing, management, and support staff at Ayder General Hospital, and Eka Kotebe General Hospital for their commitment in caring the patients, and towards combatting COVID-19 in Ethiopia.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101054.
Appendix. Supplementary materials
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotez P.J., Alvarado M., Basanez M.G. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herricks J.R., Hotez P.J., Wanga V. The global burden of disease study 2013: what does it mean for the NTDs? PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005424. e0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White M.P.J., McManus C.M., Maizels R.M. Regulatory T-cells in helminth infection: induction, function and therapeutic potential. Immunology. 2020;160:248–260. doi: 10.1111/imm.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabé M., Lokmer A., Ségurel L. Gut protozoa: friends or foes of the human gut microbiota? Trends Parasitol. 2017;33:925–934. doi: 10.1016/j.pt.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury R.S., Piedrafita D., Greenhill A., Mahanty S. Will helminth co-infection modulate COVID-19 severity in endemic regions? Nat Rev Immunol. 2020;20:342. doi: 10.1038/s41577-020-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutman J.R., Lucchi N.W., Cantey P.T. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020;103:572–577. doi: 10.4269/ajtmh.20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hays R., Pierce D., Giacomin P., Loukas A., Bourke P., McDermott R. Helminth coinfection and COVID-19: an alternate hypothesis. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008628. e0008628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolday D., Tasew G., Amogne W. Interrogating the impact of intestinal parasite-microbiome on the pathogenesis of COVID-19 in Sub-Saharan Africa. Front Microbiol. 2021;12:766–772. doi: 10.3389/fmicb.2021.614522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). COVID-19 CRF. https://isaric.tghn.org/COVID-19- CRF/. (Accessed Aug 28, 2020).
- 15.(COVID-19): WHO; Geneva, Switzerland: 2019. World Health Organization. Report of the WHO-China joint mission on coronavirus disease. https://www.who.int/docs/default-source/coronaviruse/who-china-jointmission-on-covid-19-final-report.pdf. (Accessed 01 July 2020) [Google Scholar]
- 16.Mahmud M.A., Spigt M., Bezabih A.M. Efficacy of handwashing with soap and nail clipping on intestinal parasitic infections in school-aged children: a factorial cluster randomized controlled trial. PLos Med. 2015;12 doi: 10.1371/journal.pmed.1001837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:1–57. i–vi. [PubMed] [Google Scholar]
- 18.de Ruiter K., Jochems S.P., Tahapary D.L. Helminth infections derive heterogeneity in human type 2 and regulatory cells. Sci Transl Med. 2020;12:eaaw3703. doi: 10.1126/scitranslmed.aaw3703. [DOI] [PubMed] [Google Scholar]
- 19.Duan Q., Xiong L., Liao C. Population based and animal study on the effects of Schistosoma japonicum infection in the regulation of host glucose homeostasis. Acta Trop. 2018;180:33–41. doi: 10.1016/j.actatropica.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Hays R., Esterman A., Giacomin P., Loukas A., McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian Aboriginal adults. Diabetes Res Clin Pract. 2015;107:355–361. doi: 10.1016/j.diabres.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Vasunilashorn S., Crimmins E.M., Kim J.K. Blood lipids, infection, and inflammatory markers in the Tsimane of Bolivia. Am J Hum Biol. 2010;22:731–740. doi: 10.1002/ajhb.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magen E., Bychkov V., Ginovker A., Kashuba E. Chronic Opisthorchis felineus infection attenuates atherosclerosis—An autopsy study. Int J Parasitol. 2013;43:819–824. doi: 10.1016/j.ijpara.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Rajamanickam A., Munisankar S., Dolla C. Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanya R.E., Webb E.L., Zziwa C. The effect of helminth infections and their treatment on metabolic outcomes: results of a cluster-randomized trial. Clin Infect Dis. 2020;71:601–613. doi: 10.1093/cid/ciz859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19. JAMA Intern Med. 2020;180:1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 26.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F., Wang M., Wang J., Li R., Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol. 2019;9:206–217. doi: 10.3389/fcimb.2019.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw K.A., Bertha M., Hofmekler T. Dysbiosis, inflammation, and response to treatment: a longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Med. 2016;8:75–88. doi: 10.1186/s13073-016-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cepon-Robins T.J., Gildner T.E. Old friends meet a new foe: a potential role for immune-priming parasites in mitigating COVID-19 morbidity and mortality. Evol Med Public Health. 2020;2020:234–248. doi: 10.1093/emph/eoaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naidoo P., Ghazi T., Chuturgoon A.A. SARS-CoV-2 and helminth co-infections, and environmental pollution exposure: an epidemiological and immunological perspective. Environ Int. 2021;156 doi: 10.1016/j.envint.2021.106695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFarlane A.J., McSorley H.J., Davidson D.J. Enteric helminth-induced type I interferon signaling protects against pulmonary virus infection through interaction with the microbiota. J Allergy Clin Immunol. 2017;140:1068–1078. doi: 10.1016/j.jaci.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Llewellyn S., Inpankaew T., Nery S.V. Application of a multiplex quantitative PCR to assess prevalence and intensity of intestinal parasite infections in a controlled clinical trial. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraha H.E., Gessesse Z., Gebrecherkos T. Clinical features and risk factors associated with morbidity and mortality among patients with COVID-19 in northern Ethiopia. Int J Infect Dis. 2021;105:776–783. doi: 10.1016/j.ijid.2021.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolday D., Ndungu F.M., Gómez-Pérez G.P. Chronic immune activation and CD4+ T cell lymphopenia in healthy African individuals: perspectives for SARS-CoV-2 vaccine efficacy. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.693269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.