Abstract
Introduction
Alzheimer’s disease (AD), and idiopathic Parkinson’s disease (IPD) are the neurodegenerative diseases of the central nervous system (CNS). Cognitive impairment is on the forefront in AD. However, IPD is a movement disorder. Inflammation was suggested to have an effect in the pathophysiology of these two diseases. Neutrophil–lymphocyte ratio (NLR) was shown to be a possible marker showing the peripheral inflammation. We aimed to investigate the NLR of patiens with the diagnosis of AD, and IPD, and individuals with no neurodegenerative disease.
Materials and methods
A total of 100 patients with the diagnosis of IPD, and 94 with diagnosis of AD, and 61 healthy controls were included into the study. All the demographic, clinical, and laboratory data were retrospectively obtained from the hospital automated database system.
Results
The NLR in the IPD group was found statistically significantly higher compared with the control group and the AD group (p < 0.001, p = 0.04, respectively). The age-adjusted values were statistically analyzed because of age difference. No statistically significant difference was detected between AD and control groups in terms of NLR (p = 0.6). The age-adjusted NLR value in the Parkinson’s group was found significantly higher compared to the control group (p = 0.02) and Alzheimer’s group (p = 0.03).
Discussion
Chronic inflammation has an important role in the emergence and progression of the chronic neurodegenerative diseases of the CNS. Our results show that the inflammation in the peripheral blood in IPD was more significant compared with the inflammation in AD.
Keywords: Alzheimer’s disease, Parkinson’s disease, Neutrophil–lymphocyte ratio, Peripheral inflammation
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia in the elderly population. AD is a progressive and neurodegenerative disease (ND) of the central nervous system (CNS) characterized by impairment in the cognitive functions, being unable to perform the daily life activities and behavioral impairments. The pathophysiology of AD was tried to be explained with the amyloid hypothesis for many years [1]. According to this hypothesis, AD pathology is emerged with the accumulation of A-beta (Aβ) 42 on amyloid plaques as a result of the splitting of amyloid precursor protein with secretases. One other important pathologic event occurring in this neurodegenerative process is the hyperphosphorylation of Tau which is a microtubule-associated protein [2]. Tau hyperphosphorylation not only disrupts intracellular processes, but also causes the accumulation of neurofibrillary tangles [3]. Kinney et al. suggested in their study that inflammation was also a main component in AD pathogenesis [4]. Although inflammation was previously suggested to be only a reaction against the neuron loss, inflammation has recently been considered as the main factor in AD pathogenesis. Although the immunity reaction actually has a neuroprotective effect in the initiating of the neuropathological process [5, 6], microglia cannot clean the plaques against the permanence of the amyloid plaque. This process is the reason of the higher cytokine secretion. The cytokine secretion results with the migration of peripheral macrophages to the brain, and thus the inflammatory process is started [7]. The unknowns emerging in the inflammatory process of AD pathogenesis in the patients led the researchers to monitor the neuroinflammation developing in brain [8, 9].
Idiopathic Parkinson’s disease (IPD) is the second most common neurodegenerative movement disorder in the elderly population presenting with tremor, rigidity, bradykinesia, and postural instability. IPD is a chronic progressive ND emerging with the loss of the dopaminergic neurons of the substantia nigra (SN) pars compacta in the midbrain, and generalized accumulation of the α-synuclein aggregates [10]. Oxidative stress (OS), proteolytic stress, and inflammation are blamed for the IPD pathogenesis [11]. However, IPD is not a simple movement disorder originating from the loss of dopaminergic neurons only in SN, SN was found to be protected, and motor symptoms were found not to have developed in the early stages of the disease. This condition was accepted as an indicator that the neurodegenerative process in IPD initiated in the peripheral autonomic nervous system. Therefore, some researchers evaluate the IPD as a multisystem involved autonomic motor disease [12].
Inflammation is known to start with the increase of the serum acute phase reactants, and the tissue damage is accompanied to this process. The tissue damage results with the acute, subacute, and chronic inflammation in accordance with the cause of the inflammation, the defense mechanism against the factor, and the repaired tissue damage. The acute inflammation starts rapidly, and the symptoms period is expressed with days. However, chronic inflammation may last months, and years. Acute inflammation starts with the migration of neutrophil, and macrophages to the inflammation region through cytokines, and chemokines [13]. Macrophages, lymphocytes, and plasma cell infiltration are responsible for chronic inflammation. Acute inflammation arises as a result of the migration of neutrophils and macrophages to the inflammation site through cytokines and chemokines [14]. An increased neutrophil count is often associated with inflammation occurrence, progression, and severity, whereas a decreased lymphocyte count, as part of the immune regulatory barrier, is associated with the body’s stress response. Therefore, the neutrophil–lymphocyte ratio (NLR), as a combined inflammatory biomarker, integrates information from the two leukocyte subtypes. In particular, it avoids the disadvantage of an absolute value of a single leukocyte subtype, which may be affected by infection or dehydration, and has higher clinical significance than the other independent inflammatory biomarkers [15]. So, NLR is a marker that indicates the peripheral inflammation. It is calculated by dividing the neutrophil count by the number of the lymphocytes in the complete blood count. The obtained ratio combines the information derived from two different pathways: the neutrophils responsible for the inflammation in progress, and the lymphocytes representing regulatory pathway [16]. The association of NLR with systemic inflammation and chronic diseases has been demonstrated in many conducted studies and was suggested to be used as a biomarker such as the acute phase reactants. Fauzia et al. detected higher NLR in chronic diseases of hypertension, and diabetes mellitus in their study [17]. Also, NLR is used to predict outcomes in patients with coronary artery disease, and cancer [16]. In addition, complete blood count is a commonly used, easily accessible, and low-cost laboratory method that NLR calculation can easily be performed with the parameters obtained.
Considering the role of inflammation in the pathogenesis of AD and IPD, we hypothesized that routine blood parameters in these two patient groups could have diagnostic, and predictive value and may guide the treatment. The aim of the present study was to investigate the NLR for detecting the peripheral inflammation in these two NDs of the CNS.
Material and method
A total of 100 patients with idiopathic Parkinson’s disease (pwPD) and 94 patients with Alzheimer’s disease (pwAD) who were followed up in the Neurology outpatient clinic of Tekirdag Namık Kemal University Training and Research Hospital (by the authors AU, and BA) were included in the study. All participants were investigated for the exclusion criteria for systemic or neurologic diseases. Inflammatory, autoimmune or neoplastic disease, diabetes mellitus, hypothyroidism, infection, liver or kidney failure, dyslipidemia, myocardial infarction, and history of surgery in the last 3 months were accepted as the exclusion criteria. Sixty-one healthy individuals above 60 years examined in the Internal Medicine outpatient clinic (by the author SPK) who had no disease that might affect the complete blood count parameters were included in the study as the control group.
The study protocol was approved by the Ethics Board of Tekirdag Namik Kemal University with the reference no 2020.221.09.08.
All the demographic, neuroimaging, clinical, and laboratory data were retrospectively obtained from the hospital automated database system.
The DSM-IV criteria were used to the diagnosis of AD [18]. After the cognitive evaluation conducted in accordance with these criteria, the patients who had the brain magnetic resonance images were included in the study. The demographic features, how many years they had dementia symptoms, and age of onset were identified. The disease stage was evaluated using the clinical dementia rating (CDR) score. CDR scores were evaluated in accordance with the memory, orientation, judgement, and problem solving, home and hobbies, community affairs, and personal care areas. Each area was scored as “0” (none), “0.5” (questionable), “1” (mild), “2” (moderate), and “3” (severe). A global CDR score was found based on the standard CDR protocol with the addition of scores of each of the six areas [19].
The United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria were used for the diagnosis of IPD [20]. Individuals who were diagnosed in accordance with the criteria were included in the IPD group. The demographic features, disease duration, age of disease onset, and Hoehn and Yahr (H&Y) stage of the patients were identified. The H&Y stages of the patients were identified in accordance with their latest history and neurological examination in course of the admission to the outpatient clinic. H&Y “1” was accepted as the mild stage in which only unilateral symptoms were detected, and H&Y “5” was accepted as the most severe stage in which the patients were wheelchair dependent or confined to bed [21].
The latest complete blood count data of the patients in the follow-up were investigated. White blood count (WBC), neutrophil, lymphocyte, monocyte, platelet (PLT) counts, and NLR were recorded. The obtained data were statistically investigated between the pwPD, pwAD, and the control groups.
Statistical analysis
The PASW Statistics 22 for Windows statistical software package was used for data transfer, and analysis in the present study. The mean, standard deviation, percentage, and minimum–maximum expressions were used to explain the variables. The normality distribution of the variables was evaluated using the Kolmogorov–Smirnov test, and then the independent quantitative data were analyzed. Student’s T and Pearson’s Correlation test were used to compare the variables showing a normal distribution, and the Mann–Whitney U and Spearman correlation test were used to compare the variables with non-normal distribution. Analysis of covariance (ANCOVA) was used for age-adjusted analysis to compare the groups. ROC curve analysis was used to determine the cut-off value for predicting the disease. The p-values below 0.05 were regarded as statistically significant.
Results
NLR in patients with AD
Fifty-four (57.4%) of 94 pwAD were women, and 40 (42.6%) were men. The mean age of the group was 74.2 ± 9.6 (49–92) years. The mean age of onset was 71.7 ± 9.8 years (50–92), the mean disease duration was 2.7 ± 2.4 (1–12) years, and the mean of CDR was 1.5 ± 0.8 (0.5–3).
Thirty (49.2%) of 61 healthy controls were women, and 31 (50.8%) were men, and the mean age was detected as 65.7 ± 4.6 years. The mean age of the pwAD was statistically significantly higher compared with the mean age of the control group (p < 0.001).
No statistically significant difference was detected in the comparison of the mean NLR values of the pwAD and the control group (p = 0.09). There was no difference between the pwAD, and the control groups in terms of neutrophil (p = 0.1), and lymphocyte (p = 0.3) counts. In addition, no significant difference was detected in the mean WBC, monocyte, and PLT values in AD patients compared with the values in the control group (p = 0.6, p = 0.5, and p = 0.7, respectively).
The age-adjusted values were statistically analyzed because a statistically significant age difference was detected between the control and pwAD groups. No statistically significant difference was detected between the groups for age-adjusted NLR (p = 0.6), neutrophil (p = 0.2), and lymphocyte (p = 0.4) counts. All of the values and analysis results are presented in Table 1.
Table 1.
The comparison of the mean values of WBC, neutrophil count, lymphocyte count, monocyte count, PLT count, and NLR in AD, IPD, and control groups
| AD | IPD | Control | p-value | |||
|---|---|---|---|---|---|---|
| AD vs C | IPD vs C | AD vs IPD | ||||
| Patient number | 94 | 100 | 61 | |||
| WBC count mm3 (mean ± SD) |
6.6 ± 1.7 (2.9–10.9) |
6.7 ± 1.6 (3.4–11.2) |
6.5 ± 1.4 (3.9–10.3) |
0.6 | 0.4 | 0.7 |
| Neutrophil count mm3 (mean ± SD) |
3.9 ± 1.3 (1.5–7.1) |
4.1 ± 1.3 (1.8–8.4) |
3.7 ± 1.0 (1.8–6.3) |
0.1 | 0.06 | 0.4 |
| Lymphocyte count mm3 (mean ± SD) |
1.9 ± 0.6 (0.6–3.8) |
1.8 ± 0.5 (0.4–3.8) |
2.1 ± 0.6 (0.9–4.2) |
0.3 | 0.007* | 0.1 |
| Monocyte count mm3 (mean ± SD) |
0.4 ± 0.1 (0.1–1.2) |
0.4 ± 0.1 (0.1–1.2) |
0.5 ± 0.2 (0.2–1.9) |
0.5 | 0.9 | 0.6 |
| Platelet count mm3 (mean ± SD) |
249.7 ± 73.0 (127–564) |
245.7 ± 78.1 (83–545) |
243.8 ± 55.5 (142–428) |
0.7 | 0.9 | 0.7 |
| NLR |
2.2 ± 1.2 (0.7–8.1) |
2.5 ± 1.7 (0.7–17.1) |
1.9 ± 0.7 (0.9–4.7) |
0.09 | < 0.001* | 0.04* |
*The p-values below 0.05 were regarded as statistically significant
We evaluated the association between the age of disease onset, disease duration, and the disease stage with peripheral white blood cell counts in the correlation analysis. A positive correlation was detected between age and NLR, and a negative correlation with lymphocyte count. The age of onset and NLR showed a positive correlation (r = 0.2, p = 0.03). A negative correlation was observed between the age of onset and lymphocyte (r = − 0.3, p < 0.001), and PLT (r = − 0.2, p = 0.03). No association was detected between the disease duration and stage with the parameters (p > 0.05). The lymphocyte and PLT counts were getting lower, and NLR getting higher with the increase of age in pwAD.
NLR in patients with IPD
Fifty-one (51%) of 100 pwPD were women, and 49 (49%) were men. The mean age was 68.2 ± 9.3 (39–87) years. The mean age of onset was 62.5 ± 10.1 (36–81), the mean disease duration was 5.6 ± 4.0 (1–24) years, and the mean H&Y was 1.9 ± 0.8 (1–4). The mean age of the pwPD was statistically significantly higher compared with the mean age of the control group (p = 0.01).
In the analysis, the mean NLR value of pwPD was found statistically higher compared with the mean value of the control group (p < 0.001). The mean lymphocyte count of the patient group was found statistically significantly lower compared with the control group (p = 0.007). No significant difference was detected in the WBC, neutrophil, monocyte, and PLT values (p = 0.4, p = 0.06, p = 0.9, and p = 0.9, respectively) between the patient and control groups (Table 1).
The age-adjusted NLR value in the Parkinson’s group (p = 0.02) and age-adjusted neutrophil value were statistically significantly higher in the Parkinson’s group (p = 0.02) compared to control. We found age-adjusted lymphocyte count statistically lower in the pwPD (p = 0.02) (Table 2).
Table 2.
The comparison of the age-adjusted values of WBC, neutrophil count, lymphocyte count, monocyte count, PLT count, and NLR in AD, IPD, and control group
| AD | Control | p-value | IPD | Control | p-value | AD | IPD | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| WBC count mm3 (mean ± SD) | 6.7 ± 0.1 | 6.4 ± 0.2 | 0.3 | 6.7 ± 0.1 | 6.5 ± 0.2 | 0.3 | 6.7 ± 0.1 | 6.6 ± 0.1 | 0.9 |
| Neutrophil count mm3 (mean ± SD) | 3.9 ± 0.1 | 3.7 ± 0.1 | 0.2 | 4.1 ± 0.1 | 3.7 ± 0.1 | 0.027* | 3.9 ± 0.1 | 4.1 ± 0.1 | 0.3 |
| Lymphocyte count mm3 (mean ± SD) | 2.0 ± 0.06 | 1.9 ± 0.08 | 0.4 | 1.8 ± 0.05 | 2.07 ± 0.07 | 0.02* | 2.1 ± 0.06 | 1.7 ± 0.06 | 0.007* |
| Monocyte count mm3 (mean ± SD) | 0.4 ± 0.02 | 0.5 ± 0.02 | 0.3 | 0.4 ± 0.02 | 0.5 ± 0.02 | 0.4 | 0.484 ± 0.01 | 0.489 ± 0.01 | 0.8 |
| Platelet count mm3 (mean ± SD) | 253.1 ± 7.2 | 238.5 ± 9.1 | 0.2 | 246.4 ± 7.0 | 242.7 ± 9.0 | 0.7 | 253.2 ± 7.9 | 242.3 ± 7.6 | 0.3 |
| NLR | 2.1 ± 0.1 | 2.0 ± 0.1 | 0.6 | 2.4 ± 0.1 | 1.9 ± 0.1 | 0.023* | 2.1 ± 0.1 | 2.6 ± 0.1 | 0.03* |
*The p-values below 0.05 were regarded as statistically significant
A positive correlation was detected between the age and NLR (r = 0.2, p = 0.01), and a negative correlation was detected with the lymphocyte counts (r = − 0.2, p = 0.003) in the pwPD. No association was detected between the age of onset and all the parameters. The disease duration showed negative correlation with the WBC (r = − 0.2, p = 0.005), neutrophil (r = − 0.1, p = 0.04), and monocyte (r = − 0.2, p = 0.02) counts. The disease stage, and the WBC (r = − 0.3, p = 0.001), neutrophil (r = − 0.2, p = 0.01), lymphocyte (r = − 0.2, p = 0.02), and monocyte (r = − 0.3, p < 0.001) counts showed negative correlation.
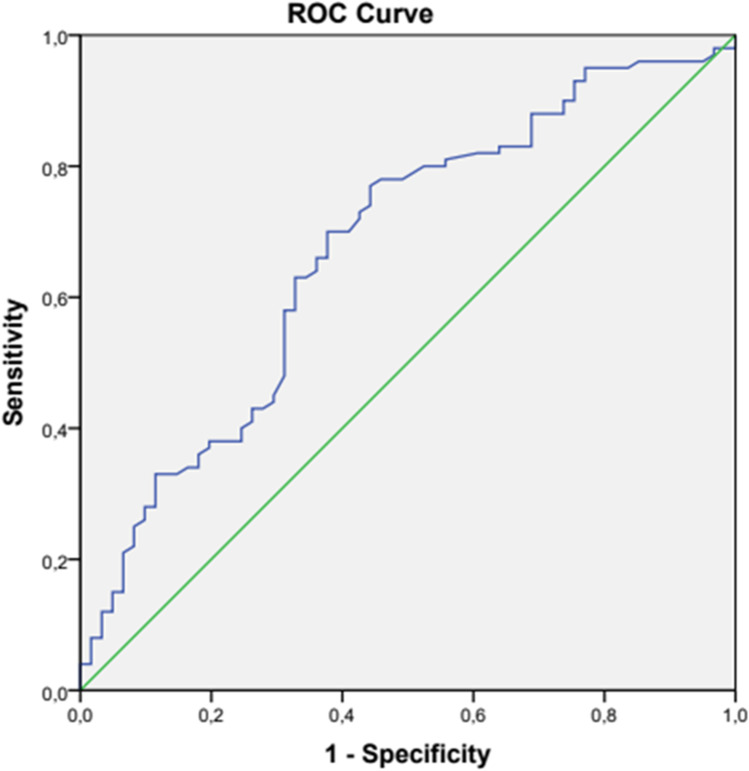
In the ROC analysis, the NLR cut-off value in pwPD was found as 1.86 with 70% sensitivity, and 62.3% specificity (p < 0.001) (Fig. 1, Table 3).
Fig. 1.
ROC curve analysis of NLR to predict IPD
Table 3.
Cut-off value of NLR to predict IPD
| AUC (95%) | Cut-off | p | Sensitivity | Specificity (%) | |
|---|---|---|---|---|---|
| NLR | 0.67 (0.58–0.75) | 1.86 | < 0.001 | 70 | 62.3 |
Comparison of NLR in the patients with AD and IPD
There was a statistical difference for age between the Alzheimer’s and Parkinson’s groups (p < 0.001). The evaluation of the means of NLR revealed that the mean of the pwPD was significantly higher compared with the pwAD (p = 0.04). No statistical difference was detected between the mean values of WBC, neutrophil, lymphocyte, monocyte, and PLT (p = 0.7, p = 0.4, p = 0.1, p = 0.6, p = 0.7, respectively) between the two groups (Table 1).
The age-adjusted NLR value in the Parkinson’s groups was found significantly higher (p = 0.03). The age-adjusted lymphocyte value in the Parkinson’s groups was significantly lower (p = 0.007). No statistical difference was detected for WBC (p = 0.9), neutrophil (p = 0.3), monocyte (p = 0.8), and PLT (p = 0.3) between the two groups (Table 2).
Discussion
The studies reporting the important role of chronic inflammation in the emergence and progression of the disease in chronic NDs of the CNS are currently receiving attention in the literature. Researchers discussed that NLR which is regarded as a marker demonstrating the peripheral inflammation in chronic diseases might provide data in the disease course, and treatment response. Inflammation was investigated in details in a group of NDs [22–27]. After conducting a detailed literature research, we suggested that the comparison of the NLR ratios of two NDs might be used as a guide. Therefore, in this study, we compared the NLR values in cases diagnosed with IPD, and AD with the healthy control group. We found that in IPD, the NLR ratio was higher compared with the level in both AD and healthy control groups, and NLR in IPD might show the peripheral inflammation.
The proinflammatory cytokines in AD were shown in both CNS and in the periphere, and the presence of a strong immune response in all processes of the disease was emphasized. The impairments emerging in the regulation of Aβ peptide are accepted to cause the appearence of this immune response [28–30]. The comparison of the peripheral leukocytes with the healthy control showed that they increased in AD, and passed to the CNS through the blood–brain barrier (BBB), and were accumulated in the neuronal tissue [31]. However, it has also been discussed that neuroinflammation occurs only in the late and advanced stages of AD. In particular, it was thought that glial cell activation accompanied but did not significantly contribute to amyloid pathology. CNS microglia, which are responsible for protecting and reshaping synapses, have been shown to be closely related to the brain tissue changes in AD. In these tissues, it was observed that macrophages derived from microglia and monocytes infiltrating from peripheral blood surrounded the Aβ plaques, and when the morphology of parenchymal microglia was examined, they showed changes in response to inflammation. In vivo studies have shown that soluble Aβ oligomers and Aβ fibrils binded to receptors expressed by microglia and it caused the release of inflammatory factors such as interleukin-1 (IL-1), IL-6, granulocyte–macrophage colony stimulating factor (GM-CSF), IL-12, IL-23, and tumor necrosis factor (TNF) in the brain and CSF of the pwAD [32https://doi.org/10.1038/nrn3880]. In this context, although neuroinflammation is not typically associated with the onset of AD, it plays an essential role in increasing the severity of the disease by exacerbating Aβ and Tau pathologies [33]. In addition, some studies reported that the increased mitochondrial OS in the lymphocytes of the pwAD also caused the progression of the disease [34]. As a mechanism, it has been suggested that neuroinflammation in AD triggers mitochondrial stress in neurons by increasing proinflammatory cytokine concentration in the microenvironment [35]. Another factor known to be associated with the prodromal phase of AD is OS [36]. Cell membranes being rich in polyunsaturated fatty acids and low antioxidant capacity make neurons more sensitive to OS [37]. OS also seems to have an important role in the severity and spread of AD. Indeed, in AD patients, Aβ has been shown to improve OS and may be a source of oxygen and nitrogen radicals [36]. Oxygen radicals can diffuse towards the membrane and oxidize proteins and nucleic acids, and in particular, nucleic acid oxidation can cause fatal damage to the cell [38]. An elevated OS state also disrupts the balance between pro- and anti-apoptotic processes, leading to apoptosis and subsequent neurodegeneration [35].
In studies related to NLR, which is a marker of peripheral inflammation, it was found that NLR was higher [39, 40] in pwAD compared to the control group, but showed a weak correlation with neocortical amyloid deposition [41]. We found no difference in the NLR ratio in the comparison of AD with the healthy controls in our study and the NLR ratio increased with the increase of age, and age of disease onset. This means that the statistics be corrected for age or that the groups are homogeneous in terms of age is important for real results.
Anti-inflammatory drugs have shown promising therapeutic effects on microglia and inflammation. However, the BBB severely inhibits drug delivery to microglial cells in the CNS. It has been reported that nanoparticles (NPs) that emerged with the developing technology could be useful tools for anti-inflammatory drugs throughout the BBB to inhibit the excessive activation of microglia and neuroinflammation. Therefore, NPs with proper biocompatibility have the potential to be developed as an effective carrier to help drugs cross the BBB or as a therapeutic agent for the treatment of neuroinflammation-mediated NDs [42]. According to the results of our study, it should be considered that the use of peripheral pathways in the struggle against inflammation may be insufficient in pwAD due to the peripheral inflammation being background compared to CSN inflammation. In future studies, it can be thought that the combination of treatments with NPs will give better outcomes.
While neuroinflammation is prominent in advanced AD stages [32], we could not find a study investigating the relationship between stage and NLR, but there were studies evaluating NLR in patients with mild cognitive impairment (MCI) and AD. Analyses of these studies showed that there was no significant difference in NLR values between these two groups [41, 43]. In our study, no relationship was found between disease duration and stage and peripheral inflammation. Due to the short disease duration and early clinical stage in our AD population, the evolution of pathology in patients with advanced stages and the effect of peripheral inflammation-suppressing treatments are subjects waiting to be supported by new studies.
In fact, in all NDs, inflammatory processes help flush out toxins and unwanted pathogens, while promoting cytotoxicity and neurodegeneration [44]. IPD is one of these NDs. At the end of the twentieth century, cytokines and complement proteins, which are components of the immune system, were found to be high in the serum, brain, and CSF of pwPD [45]. Also, an association between inflammation and IPD was shown with the post mortem, in vivo [46], clinical and animal modelling [47] studies. The increase of cytokine in the peripheral blood also has a significant role in the progression of the IPD [48]. Another part of IPD pathology is free oxygen radicals, which are heavily generated during the enzymatic breakdown of dopamine and mitochondrial degradation [49, 50].
Some studies investigating peripheral inflammation showed that the increased CRP and fibrinogen levels were detected in IPD [51], and some studies showed that the use of NSAID decreased the risk of the development of sporadic IPD [52]. There are also strongly accepted studies supporting that the gastrointestinal inflammation emerging in IPD caused the increase of the proinflammatory cytokine levels [53]. In this case, the peripheral inflammatory reactions emerging associated with the gastroitestinal system in IPD pathophysiology are highly accepted.
The studies conducted about the NLR ratio in IPD were started in 2015. Akil et al. reported that the NLR levels of 51 pwPD were higher than the levels in the healthy control group [10]. The studies demonstrating that the NLR ratio was higher in IPD [10, 54], however, there are some studies suggesting the opposite [55]. In addition, researchers in the studies reported a positive association between the NLR and disease severity in pwPD [16, 56]. The mean disease duration of the patient group in our study was 5.6 years, and the mean disease severity in accordance with the H&Y staging score was 2. We found no association between the disease stage and duration with the peripheral inflammation. The decrease of peripheral blood cell with the increase of disease duration and severity suggested the increase of CNS inflammation, and migration of cells to the brain in the IPD pathogenesis. Altogether evaluation of these results, we suggest that peripheral inflammation in IPD was higher compared with the AD, and therefore it will be more appropriate to use the treatment on peripheral and CNS inflammation.
In this context, when we look at the studies on the suppression of inflammation and OS, the antioxidant, anti-inflammatory, and anti-apoptotic activity of polyphenol quercetin, a flavonoid that exhibits the ability to repair impaired mitochondrial activity, can be suggested for the theraphy of NDs [57, 58]. In addition, AD, which has a role in the pathogenesis of oxidative damage, mitochondrial dysfunction, and neuroinflammation, can also modulate and suppress neuroinflammation of the brain with various approaches, where various phytochemicals such as curcumin, resveratrol, propolis, polyunsaturated fatty acids, and ginsenosides can modulate and suppress the neuroinflammation of the brain, as in IPD. It has been observed that it reduced infiltration through the BBB, and leads to neuroprotection by directly penetrating the brain parenchyma [59]. In treatment studies aimed at reducing OS, it has been revealed that glabridin reduces serum cytokine levels such as IL-1β and TNF-α and improves OS markers [60]. The antioxidant effect of silyamarin, which is known to be hepatoprotective [61], may be on the agenda in the treatment of NDs in the future. However, the clinical use of the mentioned molecules is still pending and future studies on these molecules are needed. As a result of our study, while the importance of passing BBB in AD for the effectiveness of these treatments has emerged, it can be thought that peripheral application may be sufficient in IPD.
Limitations
We evaluated the NLR, and white blood cell counts as a peripheral inflammatory measure in two NDs in our study. We included the healthy controls aged over 60 years with no history of chronic disease, and used no drugs; however, the mean age of the individuals meeting these criteria was lower. Therefore in analysis, we preferred to use age-adjusted values. The studies using the age, and sex-matched control groups, and long time longitudinal studies and all peripheral inflammatory indicators even their association with cytokines and OS will enable us to obtain more concrete evidence.
Conclusion
In IPD, compared to healthy controls, changes in peripheral blood cells, increase in NLR, decrease in WBC, neutrophil, and monocyte counts were detected with the increase of disease stage. NLR value was not indicative for the AD group and was found to be unrelated to disease stage. This result shows that inflammation in peripheral blood is more significant in IPD than in AD. For the evaluation of these two NDs, the significant value of peripheral inflammation in IPD may also be associated with the detection of non-motor symptoms. However, more questions have arisen that need to be answered in the future research. Basic research on the pathological progression of AD and IPD is also needed. At the same time, according to the results of our study, it should be kept in mind that treatment studies for inflammation, mitochondrial damage, and suppression of OS may differ in IPD, where peripheral inflammation is more prominent, and in AD, where peripheral inflammation is not significant.
Author contribution
Conceptualization: Sonat Pınar Kara, Bengu Altunan, Aysun Unal; methodology: Sonat Pınar Kara, Bengu Altunan, Aysun Unal; formal analysis and investigation: Bengu Altunan; writing — review and editing: Sonat Pınar Kara, Bengu Altunan, Aysun Unal; resources: Sonat Pınar Kara, Bengu Altunan, Aysun Unal; supervision: Aysun Unal.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethical approval
None.
Conflict of interest
The authors declare no competing interests.
Informed consent
The study was approved by the Ethics Board of Tekirdag Namik Kemal University and informed consent was obtained from all participants included in the study.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sonat Pınar Kara, Email: sonatmert2004@yahoo.com.
Bengü Altunan, Email: bertanaltunan@gmail.com.
Aysun Unal, Email: aysuneu@yahoo.com.
References
- 1.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avila J, Lucas JJ, Perez M, et al. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361–384. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- 3.Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 4.Kinney JW, Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/S0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 6.Simard AR, Rivest S. Neuroprotective properties of the innate immune system and bone marrow stem cells in Alzheimer’s disease. Mol Psychiatry. 2006;11:327–335. doi: 10.1038/sj.mp.4001809. [DOI] [PubMed] [Google Scholar]
- 7.Stalder AK, Ermini F, Bondolfi L, et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J Neurosci. 2005;25:11125–11132. doi: 10.1523/JNEUROSCI.2545-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradburn S, Murgatroyd C, Ray N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: a meta-analysis. Ageing Res Rev. 2019;50:1–8. doi: 10.1016/j.arr.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Sayed A, Bahbah EI, Kamel S, et al. The neutrophil-to-lymphocyte ratio in Alzheimer’s disease: current understanding and potential applications. J Neuroimmunol. 2020;349:577398. doi: 10.1016/j.jneuroim.2020.577398. [DOI] [PubMed] [Google Scholar]
- 10.Akıl E, Bulut A, Kaplan İ, et al. The increase of carcinoembryonic antigen (CEA), high-sensitivity C-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol Sci. 2015;36:423–428. doi: 10.1007/s10072-014-1976-1. [DOI] [PubMed] [Google Scholar]
- 11.Alexander GE. Biology of Parkinson’s disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci. 2004;6:259–280. doi: 10.31887/DCNS.2004.6.3/galexander. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djaldetti R, Lev N, Melamed E. Lesions outside the CNS in Parkinson’s disease. Mov Disord. 2009;24:793–800. doi: 10.1002/mds.22172. [DOI] [PubMed] [Google Scholar]
- 13.Hannoodee S, Nasuruddin DN (2020) Acute ınflammatory response. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing [PubMed]
- 14.Pahwa R, Goyal A, Bansal P, Jialal I (2020) Chronic inflammation. In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing [PubMed]
- 15.Petrone AB, Eisenman RD, Steele KN, et al. Temporal dynamics of peripheral neutrophil and lymphocytes following acute ischemic stroke. Neurol Sci. 2019;40:1877–1885. doi: 10.1007/s10072-019-03919-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uçar CA, Çokal BG, Artık HAÜ, et al. Comparison of neutrophil-lymphocyte ratio (NLR) in Parkinson’s disease subtypes. Neurol Sci. 2017;38:287–293. doi: 10.1007/s10072-016-2758-8. [DOI] [PubMed] [Google Scholar]
- 17.İmtiaz F, Shafique K, Mirza SS, et al. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. IntArchMed. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoehn MM, Yahr MD. Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/WNL.17.5.427. [DOI] [PubMed] [Google Scholar]
- 22.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio-Perez JM, Morillas-Ruiz JM. A review: inflammatory process in Alzheimer’s disease, role of cytokines. Sci World J. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solito E, Sastre M. Microglia function in Alzheimer’s disease. Front Pharmacol. 2012;3:14. doi: 10.3389/fphar.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappellano G, Carecchio M, Fleetwood T, et al. Immunity and inflammation in neurodegenerative diseases. Am J Neurodegener Dis. 2013;2:89–107. [PMC free article] [PubMed] [Google Scholar]
- 26.Enciu AM, Popescu BO. Is there a causal link between inflammation and dementia? Biomed Res Int. 2013;2013:316495. doi: 10.1155/2013/316495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch MA. The impact of neuroimmune changes on development of amyloid pathology; relevance to Alzheimer’s disease. Immunology. 2014;141:292–301. doi: 10.1111/imm.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferretti MT, Cuello AC. Does a pro-inflammatory process precede Alzheimer’s disease and mild cognitive impairment? Curr Alzheimer Res. 2011;8:164–174. doi: 10.2174/156720511795255982. [DOI] [PubMed] [Google Scholar]
- 29.Strang F, Scheichl A, Chen YC, et al. Amyloid plaques dissociate pentameric to monomeric C-reactive protein: a novel pathomechanism driving cortical inflammation in Alzheimer’s disease? Brain Pathol. 2012;22:337–346. doi: 10.1111/j.1750-3639.2011.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillot-Sestier MV, Town T. Innate immunity in Alzheimer’s disease: a complex affair. CNS Neurol Disord Drug Targets. 2013;12:593–607. doi: 10.2174/1871527311312050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heppner F, Ransohoff R, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 33.Zotova E, Nicoll JA, Kalaria R, Holmes C, et al. Inflammation in Alzheimer’s disease: relevance to pathogenesis and therapy. Alzheimers Res Ther. 2010;2:1. doi: 10.1186/alzrt24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultana R, Baglioni M, Cecchetti R, et al. Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radic Biol Med. 2013;65:595–606. doi: 10.1016/j.freeradbiomed.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calabrò M, Rinaldi C, Santoro G, et al. The biological pathways of Alzheimer disease: a review. AIMS Neurosci. 2020;8:86–132. doi: 10.3934/Neuroscience.2021005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llanos-Gonzalez E, Henares-Chavarino AA, Pedrero-Prieto CM, et al. Interplay between mitochondrial oxidative disorders and proteostasis in Alzheimer’s disease. Front Neurosci. 2019;13:1444. doi: 10.3389/fnins.2019.01444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller VM, Lawrence DA, Mondal TK, et al. Reduced glutathione is highly expressed in white matter and neurons in the unperturbed mouse brain—implications for oxidative stress associated with neurodegeneration. Brain Res. 2009;1276:22–30. doi: 10.1016/j.brainres.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuyumcu ME, Yesil Y, Oztürk ZA, et al. The evaluation of neutrophil-lymphocyte ratio in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;34:69–74. doi: 10.1159/000341583. [DOI] [PubMed] [Google Scholar]
- 40.Shad KF, Aghazadeh Y, Ahmad S, et al. Peripheral markers of Alzheimer’s disease: surveillance of white blood cells. Synapse. 2013;67:541–543. doi: 10.1002/syn.21651. [DOI] [PubMed] [Google Scholar]
- 41.Rembach A, Watt AD, Wilson WJ, et al. An increased neutrophil-lymphocyte ratio in Alzheimer’s disease is a function of age and is weakly correlated with neocortical amyloid accumulation. J Neuroimmunol. 2014;273:65–71. doi: 10.1016/j.jneuroim.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Zhu FD, Hu YJ, Yu L, et al. Nanoparticles: a hope for the treatment of ınflammation in CNS. Front Pharmacol. 2021;12:683935. doi: 10.3389/fphar.2021.683935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong X, Nao J, Shi J, et al. Predictive value of routine peripheral blood biomarkers in Alzheimer’s disease. Front Aging Neurosci. 2019;11:332. doi: 10.3389/fnagi.2019.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badanjak K, Fixemer S, Smajić S, et al. The contribution of microglia to neuroinflammation in Parkinson’s disease. Int J Mol Sci. 2021;22:4676. doi: 10.3390/ijms22094676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to ınfectious agents and pesticides and the occurrence of brain ınjuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin-Bastida A, Tilley BS, Bansal S, et al. Iron and inflammation: in vivo and post-mortem studies in Parkinson’s disease. J Neural Transm. 2021;128:15–25. doi: 10.1007/s00702-020-02271-2. [DOI] [PubMed] [Google Scholar]
- 47.Ramsey CP, Tansey MG. A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson’s disease and potential molecular targets. Exp Neurol. 2014;256:126–132. doi: 10.1016/j.expneurol.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins LM, Toulouse A, Connor TJ, et al. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology. 2012;62:2154–2168. doi: 10.1016/j.neuropharm.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 49.Herrera A, Muñoz P, Steinbusch H, et al. Are dopamine oxidation metabolites involved in the loss of dopaminergic neurons in the nigrostriatal system in Parkinson’s disease? ACS Chem Neurosci. 2017;8:702–711. doi: 10.1021/acschemneuro.7b00034. [DOI] [PubMed] [Google Scholar]
- 50.Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 51.Song IU, Kim YD, Cho HJ. Is neuroinflammation involved in the development of dementia in patients with Parkinson’s disease? Intern Med. 2013;52:1787–1792. doi: 10.2169/internalmedicine.52.0474. [DOI] [PubMed] [Google Scholar]
- 52.Singh A, Tripathi P, Singh S. Neuroinflammatory responses in Parkinson’s disease: relevance of Ibuprofen in therapeutics. Inflammopharmacology. 2021;29:5–14. doi: 10.1007/s10787-020-00764-w. [DOI] [PubMed] [Google Scholar]
- 53.Harsanyiova J, Buday T, Trancikova AK. Parkinson’s disease and the gut: future perspectives for early diagnosis. Front Neurosci. 2020;14:626. doi: 10.3389/fnins.2020.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solmaz V, Pekdaş EG, Aksoy D, et al. Serum neutrophil-lymphocyte ratios, C-reactive protein and sedimentation levels in Parkinson’s disease. Cukurova Med J. 2018;43:305–311. doi: 10.17826/cumj.341649. [DOI] [Google Scholar]
- 55.Jin H, Gu HY, Mao CJ, et al. Association of inflammatory factors and aging in Parkinson’s disease. Neurosci Lett. 2020;736:135259. doi: 10.1016/j.neulet.2020.135259. [DOI] [PubMed] [Google Scholar]
- 56.Moghaddam HS, Sherbaf FG, Zadeh MM, et al. Association between peripheral inflammation and DATSCAN data of the striatal nuclei in different motor subtypes of Parkinson disease. Front Neurol. 2018;9:234. doi: 10.3389/fneur.2018.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karuppagounder SS, Madathil SK, Pandey M, et al. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Ahn TB, Jeon BS. The role of quercetin on the survival of neuron-like PC12 cells and the expression of α-synuclein. Neural Regen Res. 2015;10:1113–1119. doi: 10.4103/1673-5374.160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Song Y, Chen Z, et al. Connection between systemic ınflammation and neuroinflammation underlies neuroprotective mechanism of several phytochemicals in neurodegenerative diseases. Oxid Med Cell Longev. 2018;2018:1972714. doi: 10.1155/2018/1972714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parlar A, Annac E, Arslan SO, et al. Pretreatment with glabridin prevents carrageenan-ınduced ınflammation: the roles for cytokines and oxidative stress production. Farmacia. 2021;69:135–141. doi: 10.31925/farmacia.2021.1.18. [DOI] [Google Scholar]
- 61.Mandegary A, Saeedi A, Eftekhari A, et al. Hepatoprotective effect of silyamarin in individuals chronically exposed to hydrogen sulfide; modulating influence of TNF-α cytokine genetic polymorphism. Daru. 2013;21:28. doi: 10.1186/2008-2231-21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.