Abstract
Purpose of Review
Global malaria elimination has little chance of success without an effective vaccine. The first malaria vaccine, RTS,S/AS01e, demonstrated moderate efficacy against clinical malaria in phase III trials and is undergoing large-scale effectiveness trials in Africa. Importantly, the vaccine did not perform equally well between phase III study sites. Though reasons for the moderate efficacy and this variation are unclear, various mechanisms have been suggested. This review summarizes the recent literature on such mechanisms, with a focus on those involving landscape ecology, parasite antigenic variation, and human host genetic differences.
Recent Findings
Transmission intensity may have a role pre- and post-vaccination in modulating immune responses to the vaccine. Furthermore, malaria incidence may “rebound” in vaccinated populations living in high transmission intensity settings. There is growing evidence that both genetic variation in the parasite circumsporozoite protein and variation of human host genetic factors affect RTS,S vaccine efficacy. These genetic factors may be interacting in complex ways to produce variation in the natural and vaccine-induced immune responses that protect against malaria.
Summary
Due to the modest efficacy of RTS,S/AS01e, the combinations of factors (ecological, parasite, human host) impacting its effectiveness must be clearly understood, as this information will be critical for implementation policy and future vaccine designs.
Keywords: Plasmodium falciparum; Malaria; RTS,S vaccine; Ecology; Genetics; Immunology
Introduction
Optimism abounded in 2010 as great strides were being made to control malaria. Over the prior decade, global malaria-related deaths had declined by 20.7% and global malaria cases had declined by 7.8% in just the previous 5 years [1]. A total of 23 countries in the World Health Organization (WHO) African Region (42 in other WHO Regions) had agreed to provide insecticide-treated bed nets (ITNs) to all people at risk for malaria compared to only 2% coverage of sub-Saharan Africans in 2000. Similarly, access to indoor residual spraying (IRS) in sub-Saharan Africa had increased from 13 million people in 2005 to 75 million in 2009 [1, 2]. Supporting these interventions was a significant expansion in funding for malaria control: from US$ 200 million in 2004 to US$ 1.5 billion in 2009 [1]. This concerted effort and marked improvement triggered high-profile discussions envisioning the possibility of global malaria elimination [3–5]. However, this trajectory of improved control and elimination from endemic regions has not continued. From 2015 to 2018, estimated yearly malaria cases increased by 7 million (3.3%) even while funding for malaria elimination remained relatively constant: US$ 2.9 billion in 2015 to US$ 2.8 billion in 2018 [6]. In the 2010 World Malaria Report, the WHO anticipated this plateau in control and predicted that interruption of transmission in areas of moderate to high transmission intensity would likely require new tools [1]. Now that this plateau has been reached, evidence continues to mount that our current tools—chemotherapy, ITNs, and IRS—will likely fail to achieve the goal of malaria elimination, especially in high incidence countries [7–9].
An effective vaccine against malaria would greatly aid control and elimination efforts. However, the challenge of developing a malaria vaccine should not be underestimated. In endemic regions, repeated infection of individuals usually results in the acquisition of partial immunity to clinical malaria but does not completely prevent infection. Furthermore, this partial response appears to quickly wane without continued exposure [10]. Consequently, the goal in developing a malaria vaccine is to generate an immune response that is more effective and longer lasting than is the natural response in the typical individual. Generating a vaccine response beyond the natural response is notoriously difficult: researchers have struggled for decades to address similar problems in HIV and tuberculosis [11, 12]. It is also particularly difficult to understand and leverage the components of partial immunity as they cannot be easily separated from the ineffective responses that arise over time simply due to repeated exposure.
At present, there is only one approved vaccine for malaria: RTS,S/AS01e [13]. It is a monovalent, pre-erythrocytic vaccine targeting key portions of the Plasmodium falciparum circumsporozoite protein (CSP), which is the major surface protein densely coating the sporozoite that is injected by the infecting mosquito bite. The vaccine aims to block liver infection thereby preventing any blood stage parasites and disease. In and of itself, CSP is a complex protein in terms of both its structure and antigenic variation. Its biology is multifaceted with key roles in sporozoite development within the mosquito gut, migration to the mosquito salivary glands, travel from the skin to the liver through the human bloodstream, and then finally traversal of hepatocytes and eventual hepatocyte invasion where it is still expressed within the infected hepatocytes [14].
The vaccine does not incorporate the complete CSP sequence. It lacks the conserved N-terminal region but includes 19 NANP amino acid repeats (R in RTS,S) along with the C-terminal region representing a thrombospondin-like domain with T cell epitopes (T in RTS,S). The T cell epitopes are thought to elicit increased antibody responses which were lower when only the repeat was included. These portions of CSP are fused to the hepatitis B surface antigen (S in RTS,S). The RTS construct and a free hepatitis B surface antigen S (RTS,S) can self-assemble into a larger viral-like particle with increased immunogenicity. The vaccine also includes a highly optimized and potent adjuvant (AS01e), which consists of two immunostimulants (3-O-desacyl-4′-monophosphoryl lipid A and QS-21) that synergistically enhance humoral and cellular immune responses [15]. Overall, RTS,S has been optimized to generate high levels of anti-NANP antibodies capable of complement fixation [16, 17•]. Anti-CSP antibody titers are positively correlated with protection and, though no threshold for protection was found, an antibody titer of 121 EU/mL (95% CI: 98–153) was estimated to prevent 50% of infections [18•]. In one study, the fourth dose resulted in increased IgG1, IgG3, and IgG4 levels against all vaccine antigens after 1 month, but it did not increase IgG2 or IgM levels [19]. The complement response was most strongly correlated with IgG1 and, to a lesser extent, IgG3. IgG subclasses waned quickly over the first 18 months after vaccination, while complement fixation waned within the first 6 months [17•].
The approved RTS,S/AS01e vaccine is administered in three monthly doses around the age of 6 months, with a fourth dose recommended 18 months later [20]. This was based on the best (albeit moderate) efficacy observed in phase III clinical trials of 36.3% over the full 4 years. That a vaccine with this level of efficacy still won approval from the European Medicines Agency [21], and WHO [22] attests to the large burden of morbidity and mortality due to falciparum malaria. Currently, the vaccine is undergoing WHO-sponsored pilot implementation in Ghana, Malawi, and Kenya, which began in 2019 [20, 23]. The pilot implementation expects to vaccinate 120,000 children per year in each country and will further examine effectiveness and safety when integrated into routine vaccination schedules, although this has been disrupted by the COVID-19 pandemic. Importantly, in phase III trials, the vaccine had differential levels of efficacy in different populations; efficacy ranged from 22% in Manhiça, Mozambique, to 74.6% in Kilifi, Kenya, with efficacies below 50% in 9 of the 11 sites [20]. Furthermore, the efficacy of RTS,S wanes over time. Modeling of anti-circumsporozoite antibody dynamics and the natural acquisition of protective immunity over time indicated the half-life of the short-lived and long-lived antibody responses to be 45 days (95% CI: 42 to 48) and 591 days (95% CI: 557 to 632), respectively [18•]. Unfortunately, this long-lived response represents only 12% of the immune response after initial vaccination and 30% of the response after vaccination with the fourth dose.
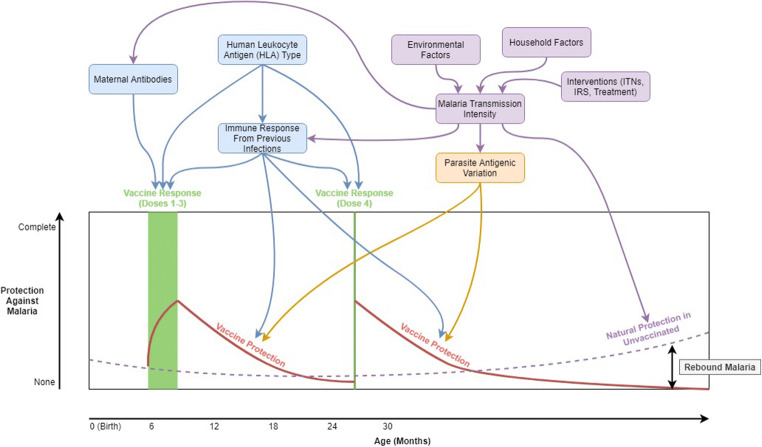
The exact mechanisms behind the observed efficacy and its variation between sites and individuals are still poorly understood. Dissecting these mechanisms and determining factors impacting them are daunting tasks due to the complexity and heterogeneity of malaria epidemiology, the multifaceted biology and variant nature of the CSP, and the convolution of natural and vaccine-induced immune responses. Recent work provides growing evidence that environmental, parasite, and human variation all play a role in determining RTS,S/AS01e vaccine efficacy in various settings. Figure 1 displays theoretical mechanisms through which these factors might impact vaccine efficacy over time. Some mechanisms impact the quality of the host immune responses to the vaccination (maternal antibodies, antibodies from previous infections, HLA type), while others determine the protection the vaccine affords when a vaccinated individual is challenged with malaria exposure during follow-up (antibodies from previous infections, parasite antigenic variation). One mechanism even operates through the immune response of those who are unvaccinated. Rebound malaria occurs when the protection of the vaccine falls below the level of naturally acquired immunity to clinical malaria in the unvaccinated population (determined by transmission intensity), essentially increasing the relative likelihood of infection and severe disease. This paper expands upon these mechanisms using recent literature, summarizing the current knowledge and evidence for the use of RTS,S/AS01e in different contexts.
Fig. 1.
Impacts of ecology, parasite antigenic variation, and human genetics on RTS,S/AS01e malaria vaccine efficacy
Ecology
In vaccine trials, an ecological perspective is seldom employed. However, previous analyses have shown that ecology can play a key role in determining the impact of vaccine interventions. For example, analysis of a cholera vaccine in Bangladesh found spatial heterogeneity in vaccine coverage, leading to varying levels of herd protection in the population and thus differing neighborhood vaccine efficacies [24, 25]. Environmental and demographic characteristics were correlated with vaccine efficacy, after controlling for neighborhood vaccine coverage [24, 25]. The ecological vaccine approach altered the scientific consensus on the efficacy of the oral cholera vaccine and was key in gaining support for the implementation of the vaccine [26, 27].
Falciparum malaria is inextricably linked to the environment due to the behavior of the Anopheles mosquito [28, 29]. The ideal larval habitat for these mosquitoes is clean water with vegetation which is most common in rural areas [30, 31]. Thus, those residing in certain ecologies experience higher rates of malaria than in others. Importantly, studies of phase II and III data have suggested potential differences in vaccine efficacy by transmission intensity, most suggesting efficacy to be higher in lower transmission intensity settings [32–37]. It is important to note that efficacy is a ratio measure and, thus, a vaccine with homogenous efficacy will have a greater absolute impact (in terms of cases averted) in high transmission settings. Even though the vaccine seemed to have a lower efficacy in high transmission settings during the trials, most analyses found that the impact of the vaccine over the full follow-up period (~4 years) was overall protective in high transmission areas and that more cases were prevented than in low transmission areas.
There are several mechanisms through which malaria transmission intensity might impact the efficacy of the vaccine. One mechanism is through antibodies present pre-vaccination. Antibodies can be acquired through maternal transmission or from early-life infections, both of which will occur more often in higher transmission intensity settings. In one study, higher malaria exposure was associated with a poorer induction of functional antibodies, meaning that the vaccine might be less effective in these populations [17•]. In infants aged 6–12 weeks in the phase III trial, higher baseline anti-CSP antibodies were associated with lower antibodies post-vaccination, suggesting that maternal antibodies may dampen the response to the vaccine [18•].
Additionally, natural infections post-vaccination may alter the vaccine response through multiple mechanisms. For instance, previous exposure to malaria may result in innate training altering subsequent responses [38•]. Also, natural infection post-vaccination was found to induce antibodies to other falciparum proteins which are not vaccine-induced but are correlated with protection [39•]. This suggests that such antigens should be considered in subsequent multivalent vaccines and that increased exposure to malaria might increase the efficacy of the vaccine. This mechanism is also impacted by landscape ecology, as ecology will determine (through transmission intensity) the frequency of malaria infections post-vaccination. In line with malaria exposure affecting response, the timing and dosage of the vaccine itself may affect response: initial controlled human challenge studies suggest that vaccination with smaller (fractional) doses and larger time intervals may improve efficacy [40–42] and such modified schedules are currently undergoing clinical trials in malaria-endemic regions.
Another potential mechanism through which transmission intensity might impact vaccine efficacy is the phenomenon of “rebound malaria” [43]. This mechanism is unique because it acts through the immune response of those unvaccinated. In high transmission intensity areas, vaccinated individuals are initially afforded partial protection against malaria, while unvaccinated individuals remain susceptible. As vaccinated individuals gradually lose protection because vaccine-induced immunity wanes, unvaccinated individuals are repeatedly exposed to malaria and develop partial immunity through natural infection [44]. Thus, after a certain period, vaccinated individuals are more susceptible to infection than unvaccinated individuals—identified through estimates of negative efficacy [36, 43]. The fourth dose of the vaccine, administered around 18 months after the third dose, provides a temporary boost to immunity, but this effect wanes as well, only delaying the rebound effect [20]. In low transmission intensity areas, the vaccine response still wanes, but the build-up of natural immunity in the unvaccinated group is minor. Thus, when estimating the efficacy of the RTS,S/AS01e vaccine, this effect will be dependent on the background transmission intensity of the study site and the length of follow-up considered. For example, a 7-year extended phase II trial in Kenya and Tanzania found that vaccine efficacy waned quicker in high transmission intensity areas, to the point of a negative efficacy point estimate beginning around the third year of follow-up [34••]. The authors further examined within-site variation by estimating, through active surveillance, malaria prevalence within a 1-km buffer of each participant. They found that individuals with high background prevalence estimates experienced a rebound in malaria infections while those with low prevalence estimates did not [34••, 45]. An analysis of three-phase III trial sites in Burkina Faso and Kenya found similar results. In the last 3 of 7 years, negative efficacy was observed for both the three- and four-dose groups in the highest incidence site and for only the three-dose group in the second-highest incidence site, while efficacy remained positive in the lowest incidence site [35••].
Though the fourth dose of RTS,S/AS01e delays the rebound of malaria infections, it is not clear that it will reliably do so. Since the fourth dose is meant to be given at a time when children do not have a scheduled interaction with the healthcare system, there is concern that many children will not receive it [46]. Malaria has a higher incidence in rural areas, where children will also likely have to travel further to receive the fourth dose of the vaccine, which may decrease the chance that they receive it. Future analyses should investigate the spatial distribution of the receipt of the fourth dose during implementation, relative to the distribution of malaria incidence. High transmission intensity areas could be targeted with education campaigns, stressing the importance of returning on time for a fourth dose, or providing monetary compensation for doing so. Additionally, pairing vaccination with other interventions, like ITNs and IRS, could help cost-effectively reduce the rebound effect [43, 47].
The three mechanisms that connect ecological factors to vaccine efficacy are all mediated through transmission intensity. Further research is needed to identify the ecological variables that might modify the impact of RTS,S through these mechanisms. In Lilongwe, Malawi, proximity to wetlands, identified using satellite imagery, was found to be related to RTS,S/AS01e efficacy, possibly because there was rebound malaria in higher transmission intensity areas [37]. The environmental context of households must be analyzed in conjunction with household ecological variables such as ITN and IRS use as well as household socioeconomic context. Each participant’s household roof type (metal versus grass), which is a socioeconomic indicator, was measured in the Malawi phase III trial site but was not found to be related to vaccine efficacy [37]. However, a recent analysis from Kintampo, Ghana, found that vaccine efficacy was higher in high-SES households, partially determined by household construction materials [48]. For these ecological variables and for others, a careful analysis that considers all three potential mechanisms of ecological modification is needed. Drivers of transmission intensity in Lilongwe, a peri-urban setting, or Kintampo, a more rural setting, might differ from drivers of transmission intensity elsewhere, so future analyses in other settings should identify locally important variables to transmission intensity and determine whether they impact the efficacy of the vaccine.
It is unclear whether temporally or seasonally varying variables modify vaccine efficacy. Within the pre- and post-vaccination exposure mechanisms, the season of vaccination and follow-up could play a role. Considering seasonal transmission within the rebound malaria mechanism, it may be that the magnitude of the rebound effect is impacted by the degree of seasonality of malaria in a particular setting. However, it is less clear whether seasonality would cause vaccine efficacy to change over time within one study area. One analysis found that RTS,S/AS01e efficacy in Malawi did not vary by the amount of recent rainfall, which is a seasonally varying environmental driver of malaria in that setting [49]. This absence of effect could be consistent with the rebound malaria theory, as the process of acquiring natural immunity takes time and the impact of the period of increased malaria incidence on efficacy would likely occur in the span of months and years, rather than weeks. However, it presents an issue for the theory that exposure to malaria post-infection should increase vaccine efficacy. An interesting follow-up to this analysis would be to investigate the amount of rainfall in the period between birth and vaccination and its impact on vaccine efficacy.
It is also unclear the degree to which herd protection will play a role in the impact of RTS,S. A small proportion of the population will be vaccinated since only children under 5 (representing ~15% of the population in sub-Saharan Africa) are being vaccinated during implementation and other age groups account for the majority of malaria infections [50]. Since efficacy wanes quickly, these older groups will not be protected by a vaccine received at 6 months of age. Additionally, vaccination may only protect against clinical disease rather than infection, implying that even with 100% vaccine coverage, transmission may remain high due to asymptomatic infections.
Parasite Antigenic Variation
P. falciparum is a complex eukaryotic organism with a 22 megabase genome containing over 5000 proteins [51]. The genome is also notable for a high AT content and abundant repetitive seqeunce between genes as well as marked numbers of tandem amino acid repeats within proteins. Over 10% of the genome represents duplicated highly polymorphic gene families (e.g., var, rifin, stevor). It is a recent human pathogen having jumped from gorillas into the human population approximately 10,000 years ago [52]. The jump resulted in a severe population bottleneck essentially erasing the vast majority of standing neutral sequence variation. Thus, currently observed highly variant regions in P. falciparum likely exist because of strong selection due to adaptive advantages since the bottleneck rather than just by chance.
In this light, both the variation in the NANP repeat and the marked amino acid polymorphism in the C-terminal TH2R and TH3R are likely of functional consequence. Supporting this, the TH2R and TH3R are remarkable in that virtually all observed DNA variation is nonsynonymous mutations leading to amino acid substitutions. Most strains differ from each other by 5–10 amino acids [53–56]. This suggests strong balancing frequency-dependent selection, which is often a signature of immune evasion. This is of particular concern for RTS,S efficacy as the vaccine only represents one strain, and given the number of differences, this may result in poor response to non-vaccine-like strains. Indeed, based on a sieve analysis, RTS,S efficacy appears to be dependent on the infecting strain’s TH2R-TH3R amino acid haplotype [55••]. In this analysis of initial clinical malaria episodes across all 11 sites of the phase III trial, the vaccine was 50.3% (95% CI: 34.6 to 62.3) effective in protecting against vaccine matching TH2R-TH3R haplotype over the course of 1 year while it was 33.4% (95% CI: 29.3 to 37.2) effective against non-vaccine haplotype strains. Breaking this down separately to TH2R and TH3R matching yielded similar results [55••]. Importantly, efficacy declined as the number of TH2R-TH3R amino acid differences from 3D7 increased and declined particularly with 4 or more amino acid differences [55••]. A secondary analysis better accounting for multi-strain infections found an overall higher efficacy of 60% vs 44% for 3D7 matching and nonmatching strains [57]. On the other hand, analysis of the NANP region was reassuring as there was no detectable difference in efficacy related to the number of repeats which varied from 34 to 42 [55••].
How this C-terminal variation plays a role in differential efficacy is still unknown. The traditional view is that the vast majority of vaccine efficacy is attributed to the high levels of anti-NANP antibodies that are already present when a patient is faced with an infective mosquito bite and invading sporozoites. In this scenario, the role of the C-terminal region is inducing prior CD4 helper T cell support to drive more potent and longer-lived anti-NANP antibodies [58, 59]. Given that antibody levels at the time of invasion are fait accompli and directed to the NANP repeat, there is no reason that there should be differences in efficacy due to TH2R-TH3R relatedness.
This suggests that TH2R-TH3R amino acid variation is affecting a separate response apart from the development of preformed anti-NANP antibodies. One possible mechanism is that TH2R-TH3R strain specific cytotoxic T cells (C8+) are engendered by the vaccine and contribute to efficacy by killing infected hepatocytes. Cytotoxic CD8 T cells are known to be key effectors in mouse models of attenuated sporozoite and in human whole sporozoite responses [60]. RTS,S is not formulated to drive a cytotoxic response, and measures of CD8 have been absent or low and thought to be relatively inconsequential [61].
Another more likely mechanism may be that the boosts from TH2R-TH3R-specific CD4+ T cells are orchestrating a more efficacious response indirectly through cytokine production apart from antibody production. Increased frequency of RTS,S-induced IFN-gamma-secreting CD4 T cells has been correlated with protection [62–64]. A broad panel of cytokine responses confirms the central role for IFN-gamma as well as IL-15, whereas IL-5 and RANTES were associated with malaria susceptibility [65]. Combined with the high antibody levels, these associated specific T cell and cytokine responses may be mainly providing protection by activating and driving antibody-dependent killing mechanisms of neutrophils, macrophages and NK cells. Recent functional studies of controlled human challenges of RTS,S find that the functional role is important [66–68]. In fact, a comprehensive systems immunomics approach found that the key factors that are most correlated with protection in human challenge trials were the functional ability of the antibodies. The study measured close to 100 different factors, and lasso analysis revealed two key features that best accounted for protection: anti-NANP antibody–dependent cellular phagocytosis and anti-NANP binding to Fc Gamma receptor 3A that is key to NK antibody–dependent killing [67••]. Importantly, there was no appreciable predictive contribution of the overall levels of the anti-NANP antibodies which has been the best correlate until now [67••].
Finally, antibodies directed at TH2R-TH3R could play a role in protection. The crystal structure of the C-terminal domain revealed an unexpected fold (or pocket) suggestive of functional activity [69]. While the function of the pocket is unknown, it may be related to hepatocyte invasion. This pocket is surrounded by the variation in the TH2R and TH3R and further analysis demonstrated that the majority of changes were charge changes potentially more consistent with protein-protein interactions, such as an antibody or receptor [53]. While this is an intriguing hypothesis, there is minimal supporting evidence as functional antibodies from RTS,S- or sporozoite-vaccinated individuals have not been recovered in appreciable amounts, nor have these antibodies demonstrated significant neutralization [70]. It is interesting that there appears to be a relatively greater antibody response to the C-terminal region in natural infections [71].
The TH2R-TH3R sequence included in RTS,S was based simply on the standard lab strain (3D7) for which a subclone had been qualified for human trials (NF54). Interestingly, although 3D7 originates from Africa, it is by no means highly representative [72]. Site-specific studies in Malawi and Zambia both found that only 5% of samples had the same TH2R-TH3R haplotype as 3D7 [54, 56]. In Zambia, the 3D7-matched haplotype was the 5th most common, and the vast majority of mismatched haplotypes differed by 5 and 8 different amino acids. A more extensive examination of 2635 CSP sequences derived from publicly available whole genomes (Pf3k Project), and representing nine East and West African countries, found the 3D7 haplotype represented 5.3% of sequences [56]. For the phase III trial, the prevalence of 3D7-matching strain was 6.7% in unvaccinated controls with only 3 of the sites (Agogo, Ghana; Kintampo, Ghana; and Lambarene, Gabon) having a prevalence above 10% [55••]. This low frequency within the parasite population has obvious ramifications. First, it suggests that including or switching to more prevalent strains could significantly increase efficacy. It also suggests that the vaccine may not be rapidly susceptible to vaccine escape since it in essence is already in place given the vast majority of strains already differ significantly from the vaccine strain. However, this does not alleviate concern for the potential further escape long term due to new mutations in TH2R-TH3R or repeat. While groups are monitoring for vaccine escape, it is unclear even with the pilot implementation vaccinating numerous children whether this will result in significant selective pressure given they represent only a small proportion of the overall population harboring parasites [50].
Human Genetics and Immunology
Human genetic variation is another source of variability affecting immune responses. In general, vaccine responses have been shown to be highly heritable [73] and association studies have identified variants across not only immune-related genes but also pathways related to pathogen biology and entry [74, 75]. The most common association across vaccines is with human leukocyte antigen (HLA) genes on chromosome 6 [76, 77]. This is not surprising given HLA genes are central to the immune response. The proteins in the HLA complex are highly polymorphic, and the polymorphism lies in the grove of the HLA proteins where short peptide fragments are presented to T cells allowing for the recognition of self and non-self (pathogen) [78]. Key HLA genes central to adaptive responses are class I proteins that are present on every cell and aid in internal antigen presentation for the destruction of infected cells by CD8 T cells predominantly, and class II proteins that present extracellular antigens to promote CD4 T cell activation and B cell proliferation. The polymorphism in HLA genes is the result of balancing selection called overdominance whereby selection acts towards having two different alleles at every gene to better allow recognition and response to a broad array of pathogens or strain variation responses. In terms of vaccines, variations in class I HLA-A and HLA-B and class II HLA-DRB1 have been most often associated with differential responses [74].
In natural infections, HLA variation in class II genes has been shown to affect antibody responses to P. falciparum proteins, including other liver stage antigens [79–86]. In natural infections, there is evidence that variant epitopes of CSP may be selected by HLA-restricted CTL responses and that responses to such variants may be mutually antagonistic as has been reported in a study conducted in Gambia [81]. This conforms with previous associations of class I and class II alleles with protection from severe malaria [87]. Recent computational binding studies of class II HLA-DRB1 and CSP variation found relatively weak and restricted responses consistent with selection for TH2-TH3 variants that particularly escape binding to common HLA-DRB1 alleles in Africa [88•]. To date, the only HLA association study with RTS,S efficacy has been a combined analysis of 222 subjects from ten phase II control human infection trials to the vaccine strain [89•]. Specifically, the analysis was limited to 37 different serotypes relating to two MHC class I genes (HLA-A, HLA-B) and one MHC class II gene (HLA-DRB1). Three (HLA-A*01, HLA-B*08, and HLA DRB1*15/16) of the 37 broader serogroups assessed had statistically significant protective effects, while three others (HLA-A*03, HLA-B*53, and HLA DRB1*07) were associated with decreased efficacy [89•]. The study was relatively underpowered and likely at least a few of the associations were false positives. While class II associations could be expected, it is interesting that associations of class I variation were found as well and potentially suggestive of variation in C8 T cell responses. If the differential response is due to protective alleles better presenting peptides derived from TH2R-TH3R, then future work could aim to determine additional parasite TH2R-TH3R haplotypes that could be incorporated into a vaccine in order to engender better protective response across individuals.
To date, there have not been any genome-wide association studies of efficacy nor studies in the African context where the vaccine is planned to be most broadly delivered. Such large-scale studies in Africa are certainly warranted to more fully understand the impact of variation on the vaccine response. In future studies, it will be important to try to disentangle what may be part of the hepatitis B surface antigen upon which RTS,S is built. The genetics of the response to hepatitis B have been extensively studied [74]. Associations have been identified across the genome (ITGAL, FOXP1, variants in class II DRB1, DQB1, and DPQ1) [74]. Such studies should also incorporate ecologic and parasite variables, which ultimately need to be analyzed in concert to truly disentangle their impact on efficacy.
Conclusion
Global malaria elimination has little chance of success without an effective vaccine. The first malaria vaccine, RTS,S/AS01e, is now undergoing implementation in three African countries. The vaccine demonstrated moderate efficacy against clinical malaria in phase III trials; however, the vaccine did not perform equally well in different populations, with efficacy ranging between 22 and 74.6% between sites. This variability may be due to environmental, parasite, or host factors. Therefore, understanding combinations of these factors that modulate vaccine efficacy is critical for guiding vaccine use. Research must uncover these mechanisms and evaluate their relative importance in order for vaccine implementation to fully contribute towards the goal of malaria elimination. Furthermore, any malaria vaccine will not be used in isolation, but rather as part of an integrated program leveraging other control measures. Due to the modest efficacy of RTS,S/AS01e, it will be crucial to understand how to best integrate vaccination into specific control programs, as this information will be critical for policy decisions concerning vaccine implementation.
Footnotes
This article is part of the Topical Collection on Infectious Disease Epidemiology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Michael Emch, Email: emch@unc.edu.
Jeffrey A. Bailey, Email: jeffrey_bailey@brown.edu
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Organization WH, Others (2012) World malaria report 2010. 2010. Geneva: World Health Organization 238:
- 2.Cibulskis RE, Alonso P, Aponte J, Aregawi M, Barrette A, Bergeron L, Fergus CA, Knox T, Lynch M, Patouillard E, Schwarte S, Stewart S, Williams R. Malaria: global progress 2000 - 2015 and future challenges. Infect Dis Poverty. 2016;5:61. doi: 10.1186/s40249-016-0151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feachem RGA, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, Sabot O, Rodriguez MH, Abeyasinghe RR, Ghebreyesus TA, Snow RW. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–1578. doi: 10.1016/S0140-6736(10)61270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feachem RGA, Phillips AA, Targett GA, Snow RW. Call to action: priorities for malaria elimination. Lancet. 2010;376:1517–1521. doi: 10.1016/S0140-6736(10)61500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das P, Horton R. Malaria elimination: worthy, challenging, and just possible. Lancet. 2010;376:1515–1517. doi: 10.1016/S0140-6736(10)61551-6. [DOI] [PubMed] [Google Scholar]
- 6.WHO. World malaria report. 2020:2019.
- 7.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, Gimnig JE, Fish D, Killeen GF. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith DL, McKenzie FE, Snow RW, Hay SI. Revisiting the basic reproductive number for malaria and its implications for malaria control. PLoS Biol. 2007;5:e42. doi: 10.1371/journal.pbio.0050042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rabinovich RN, Drakeley C, Djimde AA, et al. malERA: an updated research agenda for malaria elimination and eradication. PLoS Med. 2017;14:e1002456. doi: 10.1371/journal.pmed.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moormann AM, Nixon CE, Forconi CS. Immune effector mechanisms in malaria: an update focusing on human immunity. Parasite Immunol. 2019;41:e12628. doi: 10.1111/pim.12628. [DOI] [PubMed] [Google Scholar]
- 11.Haynes BF, Burton DR. Developing an HIV vaccine. Science. 2017;355:1129–1130. doi: 10.1126/science.aan0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McShane H. Insights and challenges in tuberculosis vaccine development. Lancet Respir Med. 2019;7:810–819. doi: 10.1016/S2213-2600(19)30274-7. [DOI] [PubMed] [Google Scholar]
- 13.Duffy PE, Patrick Gorres J. Malaria vaccines since 2000: progress, priorities, products. NPJ Vaccines. 2020;5:48. doi: 10.1038/s41541-020-0196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coppi A, Natarajan R, Pradel G, Bennett BL, James ER, Roggero MA, Corradin G, Persson C, Tewari R, Sinnis P. The malaria circumsporozoite protein has two functional domains, each with distinct roles as sporozoites journey from mosquito to mammalian host. J Exp Med. 2011;208:341–356. doi: 10.1084/jem.20101488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Didierlaurent AM, Laupèze B, Di Pasquale A, Hergli N, Collignon C, Garçon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16:55–63. doi: 10.1080/14760584.2016.1213632. [DOI] [PubMed] [Google Scholar]
- 16.Moorthy VS, Ballou WR. Immunological mechanisms underlying protection mediated by RTS,S: a review of the available data. Malar J. 2009;8:312. doi: 10.1186/1475-2875-8-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtovic L, Agius PA, Feng G, Drew DR, Ubillos I, Sacarlal J, Aponte JJ, Fowkes FJI, Dobaño C, Beeson JG. Induction and decay of functional complement-fixing antibodies by the RTS,S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med. 2019;17:45. doi: 10.1186/s12916-019-1277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis. 2015;15:1450–1458. doi: 10.1016/S1473-3099(15)00239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez L, Vidal M, Jairoce C, et al. Antibody responses to the RTS, S/AS01 E vaccine and Plasmodium falciparum antigens after a booster dose within the phase 3 trial in Mozambique. NPJ Vaccines. 2020;5:1–16. doi: 10.1038/s41541-020-0192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RTS,S Clinical Trials Partnership Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015;386:31–45. doi: 10.1016/S0140-6736(15)60721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First malaria vaccine receives positive scientific opinion from EMA. https://www.ema.europa.eu/en/documents/press-release/first-malaria-vaccine-receives-positive-scientific-opinion-ema_en.pdf
- 22.de la mondiale SO, Organization WH, Others Malaria vaccine: WHO position paper--January 2016. Weekly Epidemiological Record= Relevé épidémiologique hebdomadaire. 2016;91:33–52. [PubMed] [Google Scholar]
- 23.van den Berg M, Ogutu B, Sewankambo NK, Biller-Andorno N, Tanner M. RTS,S malaria vaccine pilot studies: addressing the human realities in large-scale clinical trials. Trials. 2019;20:316. doi: 10.1186/s13063-019-3391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali M, Emch M, von Seidlein L, Yunus M, Sack DA, Rao M, Holmgren J, Clemens JD. Herd immunity conferred by killed oral cholera vaccines in Bangladesh: a reanalysis. Lancet. 2005;366:44–49. doi: 10.1016/S0140-6736(05)66550-6. [DOI] [PubMed] [Google Scholar]
- 25.Emch M, Ali M, Acosta C, Yunus M, Sack DA, Clemens JD. Efficacy calculation in randomized trials: global or local measures? Health Place. 2007;13:238–248. doi: 10.1016/j.healthplace.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Health Organization W Cholera vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85:117–128. [PubMed] [Google Scholar]
- 27.Farmer P, Almazor CP, Bahnsen ET, Barry D, Bazile J, Bloom BR, Bose N, Brewer T, Calderwood SB, Clemens JD, Cravioto A, Eustache E, Jérôme G, Gupta N, Harris JB, Hiatt HH, Holstein C, Hotez PJ, Ivers LC, Kerry VB, Koenig SP, LaRocque RC, Léandre F, Lambert W, Lyon E, Mekalanos JJ, Mukherjee JS, Oswald C, Pape JW, Gretchko Prosper A, Rabinovich R, Raymonville M, Réjouit JR, Ronan LJ, Rosenberg ML, Ryan ET, Sachs JD, Sack DA, Surena C, Suri AA, Ternier R, Waldor MK, Walton D, Weigel JL. Meeting cholera’s challenge to Haiti and the world: a joint statement on cholera prevention and care. PLoS Negl Trop Dis. 2011;5:e1145. doi: 10.1371/journal.pntd.0001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janko MM, Irish SR, Reich BJ, Peterson M, Doctor SM, Mwandagalirwa MK, Likwela JL, Tshefu AK, Meshnick SR, Emch ME. The links between agriculture, Anopheles mosquitoes, and malaria risk in children younger than 5 years in the Democratic Republic of the Congo: a population-based, cross-sectional, spatial study. Lancet Planet Health. 2018;2:e74–e82. doi: 10.1016/S2542-5196(18)30009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Midega JT, Smith DL, Olotu A, Mwangangi JM, Nzovu JG, Wambua J, Nyangweso G, Mbogo CM, Christophides GK, Marsh K, Bejon P. Wind direction and proximity to larval sites determines malaria risk in Kilifi District in Kenya. Nat Commun. 2012;3:674. doi: 10.1038/ncomms1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G. Spatial distribution and habitat characterization of anopheline mosquito larvae in Western Kenya. Am J Trop Med Hyg. 1999;61:1010–1016. doi: 10.4269/ajtmh.1999.61.1010. [DOI] [PubMed] [Google Scholar]
- 31.Depinay J-MO, Mbogo CM, Killeen G, Knols B, Beier J, Carlson J, Dushoff J, Billingsley P, Mwambi H, Githure J, Toure AM, Ellis McKenzie F. A simulation model of African Anopheles ecology and population dynamics for the analysis of malaria transmission. Malar J. 2004;3:29. doi: 10.1186/1475-2875-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bejon P, White MT, Olotu A, Bojang K, Lusingu JPA, Salim N, Otsyula NN, Agnandji ST, Asante KP, Owusu-Agyei S, Abdulla S, Ghani AC. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13:319–327. doi: 10.1016/S1473-3099(13)70005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olotu A, Fegan G, Wambua J, Nyangweso G, Awuondo KO, Leach A, Lievens M, Leboulleux D, Njuguna P, Peshu N, Marsh K, Bejon P. Four-year efficacy of RTS, S/AS01E and its interaction with malaria exposure. N Engl J Med. 2013;368:1111–1120. doi: 10.1056/NEJMoa1207564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, Bejon P. Seven-year efficacy of RTS,S/AS01 malaria vaccine among young African children. N Engl J Med. 2016;374:2519–2529. doi: 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinto H, Otieno W, Gesase S, Sorgho H, Otieno L, Liheluka E, Valéa I, Sing'oei V, Malabeja A, Valia D, Wangwe A, Gvozdenovic E, Guerra Mendoza Y, Jongert E, Lievens M, Roman F, Schuerman L, Lusingu J. Long-term incidence of severe malaria following RTS,S/AS01 vaccination in children and infants in Africa: an open-label 3-year extension study of a phase 3 randomised controlled trial. Lancet Infect Dis. 2019;19:821–832. doi: 10.1016/S1473-3099(19)30300-7. [DOI] [PubMed] [Google Scholar]
- 36.Penny MA, Verity R, Bever CA, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387:367–375. doi: 10.1016/S0140-6736(15)00725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell GJ, Loop MS, Mvalo T, Juliano JJ, Mofolo I, Kamthunzi P, Tegha G, Lievens M, Bailey J, Emch M, Hoffman I. Environmental modifiers of RTS,S/AS01 malaria vaccine efficacy in Lilongwe, Malawi. BMC Public Health. 2020;20:910. doi: 10.1186/s12889-020-09039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrum JE, Crabtree JN, Dobbs KR, et al. Cutting edge: Plasmodium falciparum Induces trained innate immunity. J Immunol. 2018;200:1243–1248. doi: 10.4049/jimmunol.1701010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobaño C, Ubillos I, Jairoce C, et al. RTS,S/AS01E immunization increases antibody responses to vaccine-unrelated Plasmodium falciparum antigens associated with protection against clinical malaria in African children: a case-control study. BMC Med. 2019;17:157. doi: 10.1186/s12916-019-1378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pallikkuth S, Chaudhury S, Lu P, Pan L, Jongert E, Wille-Reece U, et al. A delayed fractionated dose RTS,S AS01 vaccine regimen mediates protection via improved T follicular helper and B cell responses. Elife. 2020. 10.7554/eLife.51889. [DOI] [PMC free article] [PubMed]
- 41.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Kathcart AK, Hauns KD, Komisar JL, Qabar AN, Davidson SA, Dutta S, Griffith ME, Magee CD, Wojnarski M, Livezey JR, Kress AT, Waterman PE, Jongert E, Wille-Reece U, Volkmuth W, Emerling D, Robinson WH, Lievens M, Morelle D, Lee CK, Yassin-Rajkumar B, Weltzin R, Cohen J, Paris RM, Waters NC, Birkett AJ, Kaslow DC, Ballou WR, Ockenhouse CF, Vekemans J. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214:762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 42.Moon JE, Ockenhouse C, Regules JA, Vekemans J, Lee C, Chuang I, Traskine M, Jongert E, Ivinson K, Morelle D, Komisar JL, Lievens M, Sedegah M, Garver LS, Sikaffy AK, Waters NC, Ballou WR, Ofori-Anyinam O, RTS,S Malaria Vaccine Working Group. Cicatelli SB, Duncan EH, Mills KT, Lee CE, Epstein JE, Cowden JJ, Spring MD, Hamer MJ, Copeland NK, Ngauy V, Tosh DM, Curley JM, Bennett JW, Riddle M, Waterman PE, Koren MA, Hutter JN, Bergmann-Leitner E, Kooken J, Angov E, Peterson K, Leprince A, Murray L, Cicatelli SB, Duncan EH, Mills KT, Lee CE, Epstein JE, Cowden JJ, Spring MD, Hamer MJ, Copeland NK, Ngauy V, Tosh DM, Curley JM, Bennett JW, Riddle M, Waterman PE, Koren MA, Hutter JN, Bergmann-Leitner E, Kooken J, Angov E, Peterson K, Leprince A, Murray L. A phase IIa controlled human malaria infection and immunogenicity study of RTS,S/AS01E and RTS,S/AS01B delayed fractional dose regimens in malaria-naive adults. J Infect Dis. 2020;222:1681–1691. doi: 10.1093/infdis/jiaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dicko A, Greenwood B. Malaria vaccination and rebound malaria. Lancet Infect Dis. 2019;19:790–791. doi: 10.1016/S1473-3099(19)30282-8. [DOI] [PubMed] [Google Scholar]
- 44.Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 45.Olotu A, Fegan G, Wambua J, Nyangweso G, Ogada E, Drakeley C, Marsh K, Bejon P. Estimating individual exposure to malaria using local prevalence of malaria infection in the field. PLoS One. 2012;7:e32929. doi: 10.1371/journal.pone.0032929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulland A. Malaria vaccine difficult to roll out because four doses are needed, WHO says. BMJ. 2015;351:h5706. doi: 10.1136/bmj.h5706. [DOI] [PubMed] [Google Scholar]
- 47.Bell GJ, Loop M, Topazian HM, et al. Case reduction and cost-effectiveness of the RTS,S/AS01 malaria vaccine alongside bed nets in Lilongwe, Malawi. Vaccine. 2020. 10.1016/j.vaccine.2020.04.031. [DOI] [PMC free article] [PubMed]
- 48.Gyaase S, Asante KP, Adeniji E, Boahen O, Cairns M, Owusu-Agyei S. Potential effect modification of RTS,S/AS01 malaria vaccine efficacy by household socio-economic status. BMC Public Health. 2021;21:240. doi: 10.1186/s12889-021-10294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han L, Hudgens MG, Emch ME, Juliano JJ, Keeler C, Martinson F, et al. RTS,S/AS01 malaria vaccine efficacy is not modified by seasonal precipitation: results from a phase 3 randomized controlled trial in Malawi. Sci Rep. 2017. 10.1038/s41598-017-07533-w. [DOI] [PMC free article] [PubMed]
- 50.Filipe JAN, Riley EM, Drakeley CJ, Sutherland CJ, Ghani AC. Determination of the processes driving the acquisition of immunity to malaria using a mathematical transmission model. PLoS Comput Biol. 2007;3:e255. doi: 10.1371/journal.pcbi.0030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DMA, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu W, Li Y, Shaw KS, Learn GH, Plenderleith LJ, Malenke JA, Sundararaman SA, Ramirez MA, Crystal PA, Smith AG, Bibollet-Ruche F, Ayouba A, Locatelli S, Esteban A, Mouacha F, Guichet E, Butel C, Ahuka-Mundeke S, Inogwabini BI, Ndjango JBN, Speede S, Sanz CM, Morgan DB, Gonder MK, Kranzusch PJ, Walsh PD, Georgiev AV, Muller MN, Piel AK, Stewart FA, Wilson ML, Pusey AE, Cui L, Wang Z, Färnert A, Sutherland CJ, Nolder D, Hart JA, Hart TB, Bertolani P, Gillis A, LeBreton M, Tafon B, Kiyang J, Djoko CF, Schneider BS, Wolfe ND, Mpoudi-Ngole E, Delaporte E, Carter R, Culleton RL, Shaw GM, Rayner JC, Peeters M, Hahn BH, Sharp PM. African origin of the malaria parasite Plasmodium vivax. Nat Commun. 2014;5:3346. doi: 10.1038/ncomms4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aragam NR, Thayer KM, Nge N, Hoffman I, Martinson F, Kamwendo D, Lin F-C, Sutherland C, Bailey JA, Juliano JJ. Diversity of T cell epitopes in Plasmodium falciparum circumsporozoite protein likely due to protein-protein interactions. PLoS One. 2013;8:e62427. doi: 10.1371/journal.pone.0062427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey JA, Mvalo T, Aragam N, Weiser M, Congdon S, Kamwendo D, Martinson F, Hoffman I, Meshnick SR, Juliano JJ. Use of massively parallel pyrosequencing to evaluate the diversity of and selection on Plasmodium falciparum csp T-cell epitopes in Lilongwe, Malawi. J Infect Dis. 2012;206:580–587. doi: 10.1093/infdis/jis329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neafsey DE, Juraska M, Bedford T, et al. Genetic diversity and protective efficacy of the RTS,S/AS01 malaria vaccine. N Engl J Med. 2015;373:2025–2037. doi: 10.1056/NEJMoa1505819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pringle JC, Carpi G, Almagro-Garcia J, Zhu SJ, Kobayashi T, Mulenga M, Bobanga T, Chaponda M, Moss WJ, Norris DE. RTS,S/AS01 malaria vaccine mismatch observed among Plasmodium falciparum isolates from southern and central Africa and globally. Sci Rep. 2018;8:6622. doi: 10.1038/s41598-018-24585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Follmann D, Huang C-Y. Sieve analysis using the number of infecting pathogens. Biometrics. 2018;74:1023–1033. doi: 10.1111/biom.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazmin D, Nakaya HI, Lee EK, Johnson MJ, van der Most R, van den Berg RA, Ballou WR, Jongert E, Wille-Reece U, Ockenhouse C, Aderem A, Zak DE, Sadoff J, Hendriks J, Wrammert J, Ahmed R, Pulendran B. Systems analysis of protective immune responses to RTS,S malaria vaccination in humans. Proc Natl Acad Sci U S A. 2017;114:2425–2430. doi: 10.1073/pnas.1621489114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurens MB. RTS,S/AS01 vaccine (MosquirixTM): an overview. Hum Vaccin Immunother. 2020;16:480–489. doi: 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abuga KM, Jones-Warner W, Hafalla JCR. Immune responses to malaria pre-erythrocytic stages: implications for vaccine development. Parasite Immunol. 2021;43(2):e12795. [DOI] [PMC free article] [PubMed]
- 61.Moris P, Jongert E, van der Most RG. Characterization of T-cell immune responses in clinical trials of the candidate RTS,S malaria vaccine. Hum Vaccin Immunother. 2018;14:17–27. doi: 10.1080/21645515.2017.1381809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ansong D, Asante KP, Vekemans J, Owusu SK, Owusu R, Brobby NAW, Dosoo D, Osei-Akoto A, Osei-Kwakye K, Asafo-Adjei E, Boahen KO, Sylverken J, Adjei G, Sambian D, Apanga S, Kayan K, Janssens MH, Lievens MJJ, Olivier AC, Jongert E, Dubois P, Savarese BM, Cohen J, Antwi S, Greenwood BM, Evans JA, Agbenyega T, Moris PJ, Owusu-Agyei S. T cell responses to the RTS, S/AS01 E and RTS, S/AS02 D malaria candidate vaccines administered according to different schedules to Ghanaian children. PLoS One. 2011;6:e18891. doi: 10.1371/journal.pone.0018891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, Schwenk R, Nielsen RA, Debebe Z, Pinelis E, Juompan L, Williams J, Dowler M, Stewart VA, Wirtz RA, Dubois MC, Lievens M, Cohen J, Ballou WR, Heppner, Jr DG, RTS,S Vaccine Evaluation Group Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS, S/AS01B and RTS, S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 64.Schwenk RJ, Richie TL. Protective immunity to pre-erythrocytic stage malaria. Trends Parasitol. 2011;27:306–314. doi: 10.1016/j.pt.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 65.Moncunill G, Mpina M, Nhabomba AJ, Aguilar R, Ayestaran A, Sanz H, Campo JJ, Jairoce C, Barrios D, Dong Y, Díez-Padrisa N, Fernandes JF, Abdulla S, Sacarlal J, Williams NA, Harezlak J, Mordmüller B, Agnandji ST, Aponte JJ, Daubenberger C, Valim C, Dobaño C. Distinct helper T cell type 1 and 2 responses associated with malaria protection and risk in RTS,S/AS01E Vaccinees. Clin Infect Dis. 2017;65:746–755. doi: 10.1093/cid/cix429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chaudhury S, Ockenhouse CF, Regules JA, Dutta S, Wallqvist A, Jongert E, Waters NC, Lemiale F, Bergmann-Leitner E. The biological function of antibodies induced by the RTS,S/AS01 malaria vaccine candidate is determined by their fine specificity. Malar J. 2016;15:301. doi: 10.1186/s12936-016-1348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Suscovich TJ, Fallon JK, Das J, et al. Mapping functional humoral correlates of protection against malaria challenge following RTS,S/AS01 vaccination. Sci Transl Med. 2020. 10.1126/scitranslmed.abb4757Systematic investigation of humoral response correlating protection with anti-NANP receptor binding to phagocytes and NK cells with no contribution of overall levels of anti-NANP antibody. [DOI] [PubMed]
- 68.Dobaño C, Sanz H, Sorgho H, Dosoo D, Mpina M, Ubillos I, Aguilar R, Ford T, Díez-Padrisa N, Williams NA, Ayestaran A, Traore O, Nhabomba AJ, Jairoce C, Waitumbi J, Agnandji ST, Kariuki S, Abdulla S, Aponte JJ, Mordmüller B, Asante KP, Owusu-Agyei S, Tinto H, Campo JJ, Moncunill G, Gyan B, Valim C, Daubenberger C. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun. 2019;10:2174. doi: 10.1038/s41467-019-10195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doud MB, Koksal AC, Mi L-Z, Song G, Lu C, Springer TA. Unexpected fold in the circumsporozoite protein target of malaria vaccines. Proc Natl Acad Sci U S A. 2012;109:7817–7822. doi: 10.1073/pnas.1205737109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scally SW, Murugan R, Bosch A, Triller G, Costa G, Mordmüller B, Kremsner PG, Sim BKL, Hoffman SL, Levashina EA, Wardemann H, Julien JP. Rare PfCSP C-terminal antibodies induced by live sporozoite vaccination are ineffective against malaria infection. J Exp Med. 2018;215:63–75. doi: 10.1084/jem.20170869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calle JM, Nardin EH, Clavijo P, Boudin C, Stüber D, Takacs B, Nussenzweig RS, Cochrane AH. Recognition of different domains of the Plasmodium falciparum CS protein by the sera of naturally infected individuals compared with those of sporozoite-immunized volunteers. J Immunol. 1992;149:2695–2701. doi: 10.4049/jimmunol.149.8.2695. [DOI] [PubMed] [Google Scholar]
- 72.Preston MD, Campino S, Assefa SA, Echeverry DF, Ocholla H, Amambua-Ngwa A, Stewart LB, Conway DJ, Borrmann S, Michon P, Zongo I, Ouédraogo JB, Djimde AA, Doumbo OK, Nosten F, Pain A, Bousema T, Drakeley CJ, Fairhurst RM, Sutherland CJ, Roper C, Clark TG. A barcode of organellar genome polymorphisms identifies the geographic origin of Plasmodium falciparum strains. Nat Commun. 2014;5:4052. doi: 10.1038/ncomms5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newport MJ, Goetghebuer T, Weiss HA, Whittle H, Siegrist C-A, Marchant A, MRC Gambia Twin Study Group Genetic regulation of immune responses to vaccines in early life. Genes Immun. 2004;5:122–129. doi: 10.1038/sj.gene.6364051. [DOI] [PubMed] [Google Scholar]
- 74.Mentzer AJ, O’Connor D, Pollard AJ, Hill AVS. Searching for the human genetic factors standing in the way of universally effective vaccines. Philos Trans R Soc Lond Ser B Biol Sci. 2015;370:20140341. doi: 10.1098/rstb.2014.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Connor D, Png E, Khor CC, Snape MD, AVS H, van der Klis F, Hoggart C, Levin M, Hibberd ML, Pollard AJ. Common genetic variations associated with the persistence of immunity following childhood immunization. Cell Rep. 2019;27:3241–3253.e4. doi: 10.1016/j.celrep.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 76.Li Z-K, Nie J-J, Li J, Zhuang H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: a meta-analysis. Vaccine. 2013;31:4355–4361. doi: 10.1016/j.vaccine.2013.06.108. [DOI] [PubMed] [Google Scholar]
- 77.Posteraro B, Pastorino R, Di Giannantonio P, Ianuale C, Amore R, Ricciardi W, Boccia S. The link between genetic variation and variability in vaccine responses: systematic review and meta-analyses. Vaccine. 2014;32:1661–1669. doi: 10.1016/j.vaccine.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 78.Cao K, Moormann AM, Lyke KE, Masaberg C, Sumba OP, Doumbo OK, Koech D, Lancaster A, Nelson M, Meyer D, Single R, Hartzman RJ, Plowe CV, Kazura J, Mann DL, Sztein MB, Thomson G, Fernandez-Vina MA. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens. 2004;63:293–325. doi: 10.1111/j.0001-2815.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 79.Banic DM, Goldberg AC, Pratt-Riccio LR, De Oliveira-Ferreira J, Santos F, Gras-Masse H, Camus D, Kalil J, Daniel-Ribeiro CT. Human leukocyte antigen class II control of the immune response to p126-derived amino terminal peptide from Plasmodium falciparum. Am J Trop Med Hyg. 2002;66:509–515. doi: 10.4269/ajtmh.2002.66.509. [DOI] [PubMed] [Google Scholar]
- 80.Beck HP, Felger I, Barker M, Bugawan T, Genton B, Alexander N, Jazwinska E, Erlich H, Alpers M. Evidence of HLA class II association with antibody response against the malaria vaccine SPF66 in a naturally exposed population. Am J Trop Med Hyg. 1995;53:284–288. [PubMed] [Google Scholar]
- 81.Gilbert SC, Plebanski M, Gupta S, Morris J, Cox M, Aidoo M, Kwiatkowski D, Greenwood BM, Whittle HC, Hill AV. Association of malaria parasite population structure, HLA, and immunological antagonism. Science. 1998;279:1173–1177. doi: 10.1126/science.279.5354.1173. [DOI] [PubMed] [Google Scholar]
- 82.Johnson AH, Leke RGF, Mendell NR, Shon D, Suh YJ, Bomba-Nkolo D, Tchinda V, Kouontchou S, Thuita LW, van der Wel AM, Thomas A, Stowers A, Saul A, Zhou A, Taylor DW, Quakyi IA. Human leukocyte antigen class II alleles influence levels of antibodies to the Plasmodium falciparum asexual-stage apical membrane antigen 1 but not to merozoite surface antigen 2 and merozoite surface protein 1. Infect Immun. 2004;72:2762–2771. doi: 10.1128/IAI.72.5.2762-2771.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.May J, Meyer CG, Kun JF, Lell B, Luckner D, Dippmann AK, Bienzle U, Kremsner PG. HLA class II factors associated with Plasmodium falciparum merozoite surface antigen allele families. J Infect Dis. 1999;179:1042–1045. doi: 10.1086/314661. [DOI] [PubMed] [Google Scholar]
- 84.Riley EM, Olerup O, Bennett S, Rowe P, Allen SJ, Blackman MJ, Troye-Blomberg M, Holder AA, Greenwood BM. MHC and malaria: the relationship between HLA class II alleles and immune responses to Plasmodium falciprum. Int Immunol. 1992;4:1055–1063. doi: 10.1093/intimm/4.9.1055. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Q, Xue X, Xu X, Wang C, Chang W, Pan W. Influence of HLA-DRB1 alleles on antibody responses to PfCP-2.9-immunized and naturally infected individuals. J Clin Immunol. 2009;29:454–460. doi: 10.1007/s10875-009-9281-0. [DOI] [PubMed] [Google Scholar]
- 86.Migot-Nabias F, Luty AJ, Minh TN, Fajardy I, Tamouza R, Marzais F, Charron D, Danzé PM, Renaut A, Deloron P. HLA alleles in relation to specific immunity to liver stage antigen-1 from plasmodium falciparum in Gabon. Genes Immun. 2001;2:4–10. doi: 10.1038/sj.gene.6363713. [DOI] [PubMed] [Google Scholar]
- 87.Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, Bennett S, Brewster D, McMichael AJ, Greenwood BM. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 88.Khan S, Parrillo M, Gutierrez AH, Terry FE, Moise L, Martin WD, De Groot AS. Immune escape and immune camouflage may reduce the efficacy of RTS,S vaccine in Malawi. Hum Vaccin Immunother. 2020;16:214–227. doi: 10.1080/21645515.2018.1560772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nielsen CM, Vekemans J, Lievens M, Kester KE, Regules JA, Ockenhouse CF. RTS,S malaria vaccine efficacy and immunogenicity during Plasmodium falciparum challenge is associated with HLA genotype. Vaccine. 2018;36:1637–1642. doi: 10.1016/j.vaccine.2018.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]