Abstract
The intestinal microbiota is a new promising avenue in cancer immunotherapy, but mechanisms remain elusive. He et al. demonstrate that butyrate, a bacterial metabolite, enhances the CD8+ T cell response and improve chemotherapy efficacy through ID2-dependent IL-12 signaling.
The intestinal microbiota is a new promising avenue in cancer immunotherapy, but mechanisms remain elusive. He et al. demonstrate that butyrate, a bacterial metabolite, enhances the CD8+ T cell response and improve chemotherapy efficacy through ID2-dependent IL-12 signaling.
Main text
The intestinal microbiota is now accepted as a potent modulator of immune responses, especially in the context of immune checkpoint inhibitor treatment for cancer. Antibiotics negatively impacts the clinical outcome of cancer patients during therapy with anti-PD-1/PD-L1 antibodies. In a study published in Cell Metabolism, He et al.1 show the role of butyrate in CD8+ T cells immunity and cancer immunotherapy.
Compared with anti-PD-1 immunotherapy responder patients, non-responders have a reduced abundance of Faecalibacterium prausnitzii or Akkermansia muciniphila, two bacteria producing short-chain fatty acids (SCFAs).2,3,4 Beyond taxonomy, microbial metabolism is indeed a key aspect of their functionality. Specific metabolites, including SCFAs such as butyrate, influence cancer development and systemic immune responses.5 Produced by bacterial fermentation of dietary fibers, SCFAs are highly abundant in the colon and are associated with the clinical response to anti-PD-1 therapy in cancer patients.6 In particular, butyrate is known to directly regulate the activity, proliferation, and apoptosis of numerous immune cells.7
However, deciphering cellular and molecular mechanisms by which bacteria modulate therapeutics efficacy remains a challenge. Two recent uncontrolled human clinical trials tested whether fecal microbiota transplantation (FMT) can affect how metastatic melanoma patients respond to anti-PD-1 immunotherapy. They showed clinical benefit in a subset of treated patients, associated with increased CD8+ T cell activation and infiltration in both the gut lamina propria and the tumor microenvironment.8,9 CD8+ T cells are considered the most important actors of anticancer immunity.10 Moreover, these patients showed increased abundance of taxa previously shown to be positively associated with response to anti-PD-1, including F. prausnitzii and A. muciniphila.9 Interestingly, microbiota-derived SCFAs, particularly butyrate, boost CD8+ T cell effector functions by modifying their cellular metabolism, favoring OXPHOS and mitochondrial respiration.7 Nevertheless, it was not known whether microbiota could directly impact the antitumor function of CD8+ T cells, or indirectly regulate their cytotoxic response through myeloid or Th1/17 cells.
To globally evaluate the anticancer effects of microbiota metabolites, He et al. provided strerile water-soluble intestinal fraction to antibiotic cocktail (ABX)-treated mice, which were then inoculated with Mc38 colon cancer cells and treated by oxaliplatin chemotherapy. ABX treatment reduced the efficacy of chemotherapy, but supplementation with gut microbial metabolites restored the therapeutic response. The significant tumor regression in mice treated by microbiota metabolites was associated with a dramatic accumulation of CD8+ T cells in the TME and an enhanced IFN-γ production. Depletion of CD8+ T cells, but not of CD4+ T cells, abolished the antitumor effect of gut microbial metabolites. Interestingly, metabolites had a direct effect on IFN- γ production by CD8+ T cells. Metabolomics analyses on colonic contents showed that the majority of metabolites that were decreased in ABX-treated mice were restored by metabolite supplementation. Among the 63 metabolites screened for their effects on CD8+ T cells in vitro, the SCFA butyrate most strongly promoted IFN- γ production. Butyrate alone recapitulated the effects of microbiota metabolites in ABX-treated mice, confirming its role in the microbiota-dependent anticancer effects. Interestingly, butyrate only had an antitumor effect when combined with chemotherapy and was not sufficient per se to control the tumor growth. RNA-seq on butyrate-treated and untreated CD8+ T cells suggested that butyrate could promote not only T cell activation but also prevent their exhaustion. Notably, the antagonist transcriptional regulators ID2 and E2A were regulated by butyrate. They are involved in the differentiation of many immune cells, but their role in tumor-infiltrating lymphocytes remains unclear. The authors found that ID2 expression was much higher in tumor-infiltrating CD8+ T cells compared to naive, activated, or memory CD8+ T cells. ID2 was indeed crucial in the antitumor effect of butyrate as demonstrated by the loss of the protective effect in mice lacking ID2 expression in CD8+ T cells. Butyrate impacts CD8+ T cell function, at least partly, via its HDAC inhibitory activity. RNA-seq analysis confirmed that ID2 upregulates activation genes in CD8+ T cells, including ifng. Moreover, IL-12 receptor, which is a target of ID2 and a critical actor for antitumor immunity, was involved in the mechanisms by which butyrate promoted antitumor CD8+ T cells.
Based on these findings, butyrate could be a strong ally in cancer therapy. The authors assessed the effect of butyrate in other cancer settings. Butyrate also improved oxaliplatin efficacy in mice in the absence of ABX, as well as in a colitis-associated colorectal cancer model. Butyrate enhanced anti-PD-L1 treatment but did not improve the efficacy of non-immunogenic drugs, such as cisplatin, potentially because their therapeutic activity is less dependent on the immune reaction. Finally, butyrate level correlated with oxaliplatin efficacy in human patients with cancer and increased IFN-γ and ID2 expression in human CD8+ T cells, suggesting the human relevance of these results.
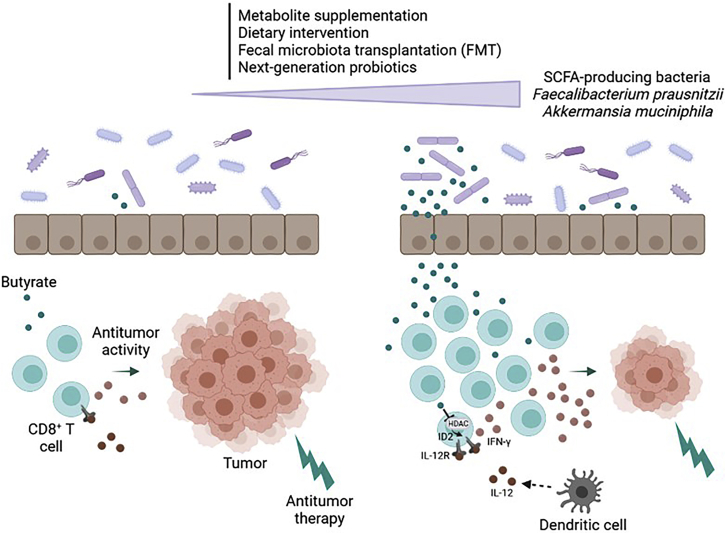
These findings prove that butyrate increases the tumor-suppressing effects of immunogenic cancer therapies. Microbial metabolites interact with the host immune system to maintain homeostasis and fight diverse disease states. An attractive strategy is to transfer certain bacteria or defined bacterial consortia that have been associated with health, the so-called live biotherapeutic products (Figure 1). Initially, the fact that anti-inflammatory bacteria, such as F. prausnitzii, were associated with positive response to cancer immunotherapy was counterintuitive. This study provides elements that could explain why SCFA-producing bacteria are crucial in the antitumor response and opens promising therapeutic perspectives for such next-generation probiotics.
Figure 1.
The gut microbiota metabolite butyrate promotes antitumor immunity of CD8+ T cells and improves chemotherapy efficacy through ID2-dependent IL-12 signaling
By inhibiting HDAC activity in CD8+ T cells, butyrate induces ID2 expression, which leads to the upregulation of IFN-g and IL-12 receptor expression. The gut microbiota can be modulated through various strategies: metabolite supplementation, dietary intervention, fecal microbiota transplantation (FMT), or the use of next-generation probiotics producing SCFA for instance, such as F. prausnitzii and A. muciniphila.
Acknowledgments
Declaration of interests
HS received unrestricted study grants from Danone, Biocodex, and Enterome; board membership, consultancy, or lecture fees from Carenity, Abbvie, Astellas, Danone, Ferring, Mayoly Spindler, MSD, Novartis, Roche, Tillots, Enterome, Maat, BiomX, Biose, Novartis, and Takeda; and a co-founder of Exeliom bioscience.
Footnotes
Present address: Service de Gastro-entérologie, Hôpital Saint-Antoine, 184 rue du Faubourg Saint-Antoine, 75571 Paris Cedex 12, France
References
- 1.He Y., Fu L., Li Y., Wang W., Gong M., Zhang J., Dong X., Huang J., Wang Q., Mackay C.R. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021;33:988–1000.e7. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Routy B., Le Chatelier E., Derosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 4.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zitvogel L., Daillère R., Roberti M.P., Routy B., Kroemer G. Anticancer effects of the microbiome and its products. Nat. Rev. Microbiol. 2017;15:465–478. doi: 10.1038/nrmicro.2017.44. [DOI] [PubMed] [Google Scholar]
- 6.Nomura M., Nagatomo R., Doi K., Shimizu J., Baba K., Saito T., Matsumoto S., Inoue K., Muto M. Association of Short-Chain Fatty Acids in the Gut Microbiome With Clinical Response to Treatment With Nivolumab or Pembrolizumab in Patients With Solid Cancer Tumors. JAMA Netw Open. 2020;3:e202895. doi: 10.1001/jamanetworkopen.2020.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaudel C., Sokol H. The Gut Microbiota at the Service of Immunometabolism. Cell Metab. 2020;32:514–523. doi: 10.1016/j.cmet.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 9.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houot R., Schultz L.M., Marabelle A., Kohrt H. T-cell-based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol. Res. 2015;3:1115–1122. doi: 10.1158/2326-6066.CIR-15-0190. [DOI] [PubMed] [Google Scholar]