Summary
Background
Alcohol use is causally linked to multiple cancers. We present global, regional, and national estimates of alcohol-attributable cancer burden in 2020 to inform alcohol policy and cancer control across different settings globally.
Methods
In this population-based study, population attributable fractions (PAFs) calculated using a theoretical minimum-risk exposure of lifetime abstention and 2010 alcohol consumption estimates from the Global Information System on Alcohol and Health (assuming a 10-year latency period between alcohol consumption and cancer diagnosis), combined with corresponding relative risk estimates from systematic literature reviews as part of the WCRF Continuous Update Project, were applied to cancer incidence data from GLOBOCAN 2020 to estimate new cancer cases attributable to alcohol. We also calculated the contribution of moderate (<20 g per day), risky (20–60 g per day), and heavy (>60 g per day) drinking to the total alcohol-attributable cancer burden, as well as the contribution by 10 g per day increment (up to a maximum of 150 g). 95% uncertainty intervals (UIs) were estimated using a Monte Carlo-like approach.
Findings
Globally, an estimated 741 300 (95% UI 558 500–951 200), or 4·1% (3·1–5·3), of all new cases of cancer in 2020 were attributable to alcohol consumption. Males accounted for 568 700 (76·7%; 95% UI 422 500–731 100) of total alcohol-attributable cancer cases, and cancers of the oesophagus (189 700 cases [110 900–274 600]), liver (154 700 cases [43 700–281 500]), and breast (98 300 cases [68 200–130 500]) contributed the most cases. PAFs were lowest in northern Africa (0·3% [95% UI 0·1–3·3]) and western Asia (0·7% [0·5–1·2]), and highest in eastern Asia (5·7% [3·6–7·9]) and central and eastern Europe (5·6% [4·6–6·6]). The largest burden of alcohol-attributable cancers was represented by heavy drinking (346 400 [46·7%; 95% UI 227 900–489 400] cases) and risky drinking (291 800 [39·4%; 227 700–333 100] cases), whereas moderate drinking contributed 103 100 (13·9%; 82 600–207 200) cases, and drinking up to 10 g per day contributed 41 300 (35 400–145 800) cases.
Interpretation
Our findings highlight the need for effective policy and interventions to increase awareness of cancer risks associated with alcohol use and decrease overall alcohol consumption to prevent the burden of alcohol-attributable cancers.
Funding
None.
Introduction
Alcohol use is associated with a vast range of injuries and diseases, including cancer, and is a leading risk factor for the global burden of disease.1, 2 The consumption of alcoholic beverages is causally linked to cancers of the upper aerodigestive tract (oral cavity, pharynx, larynx, and oesophagus) and cancers of the colon, rectum, liver, and female breast.3 Together, these cancers contributed 6·3 million cases and 3·3 million deaths globally in 2020 (data from the GLOBOCAN 2020 database).
Previous estimates of the contribution of alcohol to the burden of cancer have been published,2, 4, 5 but patterns of alcohol consumption continue to change over time across world regions.6 Alcohol consumption per capita has decreased in many European countries, especially those in eastern Europe, whereas alcohol use is on the rise in Asian countries, such as China, India, and Vietnam, and in many countries in sub-Saharan Africa.6 With these changes in alcohol consumption and more recent cancer incidence data, new estimates of the alcohol-attributable burden of cancer are warranted. We updated previous global estimates by using cancer incidence for 2020, recent relative risk estimates from the scientific literature, and alcohol consumption figures from multiple sources to calculate alcohol-attributable cancer burden. We also quantified the contribution of moderate, risky, and heavy drinking to the total burden of alcohol-attributable cancers. The overall and sex-specific world-level, regional-level, and country-level results from our study can be used to inform alcohol policy and cancer control across different settings globally.
Research in context.
Evidence before this study
Alcohol use is causally linked to numerous diseases and injuries, including several different cancer types. Using the search terms “alcohol” AND “cancer” AND “attributable”, we searched PubMed up to Sept 21, 2020, and the Institute for Health Metrics and Evaluation's research articles database up to Oct 16, 2020, for national and global studies quantifying the burden of cancer attributable to alcohol consumption. We also searched for studies in PubMed citing the latest Global Burden of Disease Study reports, and manually searched the World Cancer Research Fund Continuous Update Project reports and systematic reviews for results from meta-analyses on alcohol and cancer risk. No language restrictions were applied in any of these searches. Three major studies that had previously quantified the global alcohol-attributable burden of cancer were retrieved, but these did not use the most up-to-date estimates of cancer incidence, cancer risk, or population alcohol use.
Added value of this study
This study provides updated estimates of the global burden of cancer attributable to alcohol consumption stratified by sex, cancer site, and country, using estimates of cancer incidence from the International Agency for Research on Cancer's GLOBOCAN 2020 database and data on alcohol consumption patterns across the world from the Global Information System on Alcohol and Health. We also quantified the contribution of different levels of alcohol intake to the alcohol-attributable burden of cancer. Alcohol consumption causes a substantial burden of cancer globally, and there are large disparities between populations. Heavier drinking patterns contributed most to the global burden of alcohol-attributable cancers, but we estimate that light to moderate drinking of the equivalent of around one or two alcoholic drinks per day was accountable for more than 100 000 cases of cancer in 2020.
Implications of all the available evidence
Whereas drinking trends in European regions show an encouraging decrease in recent years, significant increases in alcohol use are predicted in several other regions, including Africa and Asia. Effective interventions to increase awareness of the causal link between alcohol and cancer among the general population might spur decreases in alcohol use and should be explored further. Tried-and-tested taxation policies have resulted in decreased population alcohol consumption in central and eastern Europe and could be implemented in other world regions that do not yet have effective alcohol policies. WHO's best buys provide the basis for cost-effective policies to reduce alcohol use among the general population and avoid the burden of cancer attributable to alcohol consumption.
Methods
Study design and data sources
In this population-based study, we used the most recent International Agency for Research on Cancer (IARC) monograph on personal habits to select cancer types with sufficient evidence of a causal relationship with the consumption of alcoholic beverages (appendix p 6).3 Country-specific estimates of incident cancer cases were extracted from the GLOBOCAN 2020 database for lip and oral cavity cancer, pharyngeal cancer, oesophageal cancer, colon cancer, rectal cancer, liver cancer, laryngeal cancer, breast cancer (female only), and all cancers combined, excluding non-melanoma skin cancer (defined using International Classification of Diseases, tenth revision; appendix p 2). Due to the specific causality with hepatocellular carcinoma and oesophageal squamous cell carcinoma, estimates of these cancers were obtained from two studies that have estimated the distributions of the histological subtypes of liver and oesophageal cancer using cancer registry data7 (hepatocellular carcinoma estimates: Rumgay H, unpublished). Hepatocellular carcinoma and oesophageal squamous cell carcinoma were defined according to International Classification of Diseases for Oncology (third edition; appendix p 2). We included cancers of the stomach and pancreas in sensitivity analysis due to evidence suggesting a causal association with alcohol consumption in World Cancer Research Fund (WCRF) classifications, but an absence of sufficient evidence in the IARC monograph classification (appendix p 6).3, 8 In our aim to quantify the burden of avoidable cancers, we did not include the potential reduction in kidney cancer incidence despite probable evidence of a protective effect from alcohol intake of up to 30 g per day.8 Details on the cancer site selection and incidence estimates are shown in the appendix (p 2).
Relative risk estimates for current drinking were obtained from the systematic literature reviews done as part of the WCRF Continuous Update Project (appendix pp 2–3, 7).8 Former drinking, defined as lifetime alcohol use but not in the past 12 months, was included in sensitivity analysis using sex-specific relative risks from multiple sources, as detailed in the appendix (pp 3, 7–8).
Assuming a 10-year latency period between exposure and cancer diagnosis (appendix p 2), alcohol consumption estimates for 2010 were obtained from the Global Information System on Alcohol and Health as adult per capita alcohol consumption in litres of alcohol per year by country disaggregated by age (15–19, 20–24, 25–34, 35–49, 50–64, and 65 years and older) and sex.9 We then converted the alcohol consumption estimates to grams of alcohol per day. To minimise the effect of bias in reporting of alcohol use, the per capita alcohol consumption data (ie, population-level alcohol exposure data) were derived from multiple sources: recorded, unrecorded, and tourist per capita alcohol consumption. Details on the sources of the alcohol consumption data and the methods to estimate the distribution of population alcohol use are summarised in the appendix (pp 3–4).
Statistical analysis
We calculated the effect of alcohol consumption on the incidence of cancer worldwide in 2020 using a Levin-based population attributable fraction (PAF) method10 adapted from Shield and colleagues5 and based on a theoretical minimum-risk exposure of lifetime abstention from alcohol consumption (appendix pp 2–5). We calculated PAFs for each age, sex, country, and cancer site by combining the age-specific, sex-specific, and country-specific prevalence of current drinking (PCD) with the cancer relative risks of current drinking (RRCD) using the following formula:
Amount of alcohol consumed for current drinking (x) was modelled with an upper integration limit of 150 g per day. We modelled the contribution of different levels of alcohol consumption by splitting alcohol prevalence into three categories: moderate drinking (<20 g per day, the equivalent of up to two alcoholic drinks per day), risky drinking (20–60 g per day, the equivalent of between two and six alcoholic drinks per day), and heavy drinking (>60 g per day, the equivalent of more than six alcoholic drinks per day). We also stratified alcohol consumption by 10 g per day increments from less than 10 g per day to 140–150 g per day. Details on the estimation of PAF by drinking category and former drinking are included in the appendix (pp 4–5).
Using the age-specific PAFs for each country, sex, and cancer site, we derived the number of cancer cases attributable to alcohol consumption for each country, sex, and cancer site (appendix p 5). Alcohol-attributable age-standardised incidence rates per 100 000 people were calculated using the age-specific, sex-specific, and country-specific number of alcohol-attributable cases. Countries were categorised into 17 world regions based on UN definitions. Alcohol PAFs for ten countries with missing alcohol prevalence data were imputed using the average age-specific, sex-specific, and cancer-specific PAFs from each world region in which they were located. World region totals were subsequently recalculated including the imputed estimates of alcohol-attributable cases. We also grouped countries into the Human Development Index categories using the UN Development Programme human development data for 2019. More details on the country groupings are described in the appendix (p 5).
Estimates of uncertainty were modelled using a Monte Carlo-like approach where 1000 estimates of the drinking status, mean, and SD of the alcohol consumption estimates and relative risks were randomly simulated based on their respective uncertainty distributions (appendix p 5). The 2·5th and 97·5th percentiles were taken from the 1000 modelled PAF estimates to construct the 95% uncertainty intervals (UIs). All analyses were carried out using R (version 3.6.1).
Role of the funding source
There was no funding source for this study.
Results
Globally, an estimated 741 300 (95% UI 558 500–951 200; PAF 4·1% [3·1–5·3]) of all new cases of cancer in 2020 were attributable to alcohol consumption. In males, there were 568 700 (76·7%; 95% UI 422 500–731 100; PAF 6·1% [4·6–7·9]) alcohol-attributable cancer cases, and in females there were 172 600 (23·3%; 135 900–220 100; 2·0% [1·6–2·5]) alcohol-attributable cancer cases (table). The global age-standardised incidence rate was 8·4 (95% UI 6·2–10·9) alcohol-attributable cancer cases per 100 000 people: 13·4 (10·0–17·4) cases per 100 000 males and 3·7 (2·7–5·0) cancer cases per 100 000 females. The cancers with the highest PAFs were cancers of the oesophagus (31·6% [95% UI 18·4–45·7]), pharynx (22·0% [9·0–37·8]), and lip and oral cavity (20·2% [12·1–32·3]), with considerable differences by sex; for example, 39·2% (22·7–55·6) of oesophageal cancers in males were attributable to alcohol, compared with 14·3% (9·0–23·5) in females. The cancer sites that contributed the most attributable cases were cancers of the oesophagus (189 700 cases [95% UI 110 900–274 600]), liver (154 700 cases [43 700–281 500]), and breast (98 300 cases [68 200–130 500]; table). For distribution of cancer sites according to world region, see the appendix (p 35).
Table.
Global number of alcohol-attributable cancer cases, population attributable fraction, and age-standardised incidence rate of alcohol-attributable cases in 2020, by cancer site and sex
|
Males |
Females |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Alcohol-attributable cases | Population attributable fraction | Age-standardised incidence rate per 100 000 males | Alcohol-attributable cases | Population attributable fraction | Age-standardised incidence rate per 100 000 females | Alcohol-attributable cases | Population attributable fraction | Age-standardised incidence rate per 100 000 people | |
| Lip and oral cavity cancer (C00–C06) | 66 700 (40 000–105 300) | 25·9% (15·6–40·9) | 1·6 (0·9–2·5) | 8200 (4600–14 300) | 7·3% (4·1–12·7) | 0·2 (0·1–0·3) | 74 900 (44 600–119 600) | 20·2% (12·1–32·3) | 0·9 (0·5–1·4) |
| Pharyngeal cancer (C09–C10, C12–C13) | 37 000 (15 200–63 400) | 25·3% (10·4–43·4) | 1·8 (0·7–3·1) | 2500 (940–4400) | 7·4% (2·8–13·4) | 0·1 (0·0–0·2) | 39 400 (16 100–67 800) | 22·0% (9·0–37·8) | 0·5 (0·4–1·6) |
| Oesophageal cancer (C15)* | 163 100 (94 200–231 000) | 39·2% (22·7–55·6) | 3·9 (2·2–5·5) | 26 600 (16 700–43 700) | 14·3% (9·0–23·5) | 0·6 (0·4–0·9) | 189 700 (110 900–274 600) | 31·6% (18·4–45·7) | 2·1 (1·3–3·1) |
| Colon cancer (C18) | 76 900 (57 700–95 400) | 13·0% (9·7–16·1) | 1·8 (1·3–2·2) | 14 600 (10 600–19 100) | 2·7% (1·9–3·5) | 0·3 (0·2–0·4) | 91 500 (68 300–114 500) | 8·1% (6·0–10·1) | 1·0 (0·7–1·2) |
| Rectal cancer (C19–C20) | 57 300 (42 700–71 800) | 13·0% (9·7–16·3) | 1·4 (1·0–1·7) | 7800 (5800–10 300) | 2·7% (2·0–3·6) | 0·2 (0·1–0·2) | 65 100 (48 500–82 000) | 9·0% (6·7–11·3) | 0·7 (0·5–0·9) |
| Liver cancer (C22)† | 141 300 (39 600–255 000) | 22·7% (6·4–40·9) | 3·3 (0·9–6·0) | 13 400 (4100–26 400) | 5·0% (1·5–9·8) | 0·3 (0·1–0·5) | 154 700 (43 700–281 500) | 17·3% (4·9–31·6) | 1·7 (0·5–3·2) |
| Laryngeal cancer (C32) | 26 400 (15 100–41 600) | 16·6% (9·5–26·1) | 0·6 (0·4–1·0) | 1200 (620–1700) | 4·7% (2·5–7·0) | 0·0 (0·0–0·0) | 27 600 (15 700–43 300) | 15·0% (8·6–23·6) | 0·3 (0·2–0·5) |
| Breast cancer (C50) | .. | .. | .. | 98 300 (68 200–130 500) | 4·4% (3·0–5·8) | 2·2 (1·3–3·2) | 98 300 (68 200–130 500) | 4·4% (3·0–5·8) | 1·1 (0·7–1·6) |
| All sites excluding non-melanoma skin cancer (C00–C97 excluding C44) | 568 700 (422 500–731 100) | 6·1% (4·6–7·9) | 13·4 (10·0–17·4) | 172 600 (135 900–220 100) | 2·0% (1·6–2·5) | 3·7 (2·7–5·0) | 741 300 (558 500–951 200) | 4·1% (3·1–5·3) | 8·4 (6·2–10·9) |
Data in parentheses are 95% uncertainty intervals. Cancer types were defined according to International Classification of Diseases (tenth revision; ICD-10) and International Classification of Diseases for Oncology (third edition; ICD-O-3).
Alcohol-attributable cases of oesophageal cancer calculated as estimates of squamous cell carcinoma (ICD-10 code C15; ICD-O-3 codes 8050–8078, 8083–8084); the population attributable fraction is of all oesophageal cancer cases (ICD-10 code C15).
Alcohol-attributable cases of liver cancer calculated as estimates of hepatocellular carcinoma (ICD-10 code C22; ICD-O-3 code, 8170–8175); the population attributable fraction is of all liver cancer cases (ICD-10 code C22).
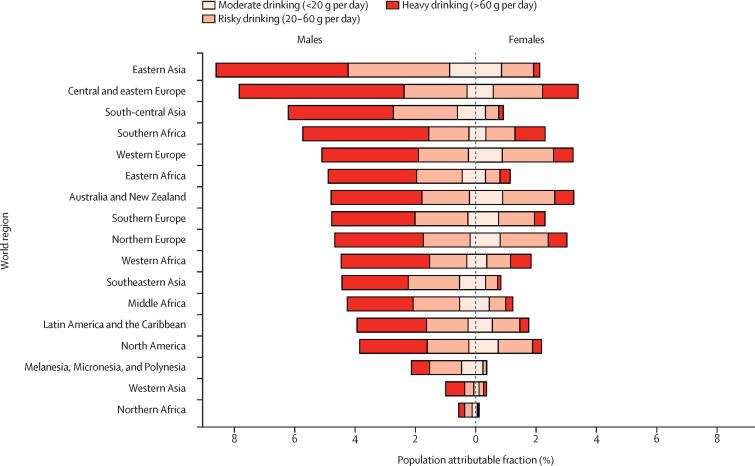
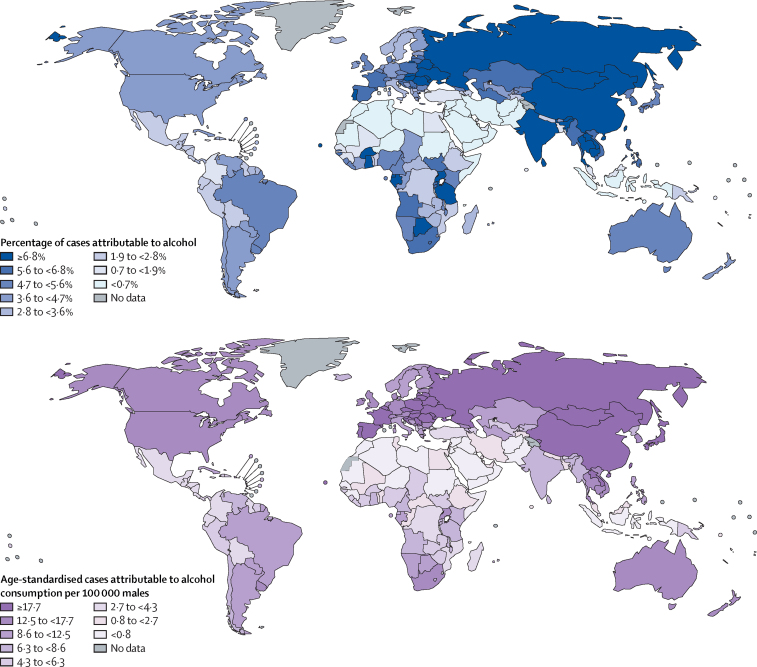
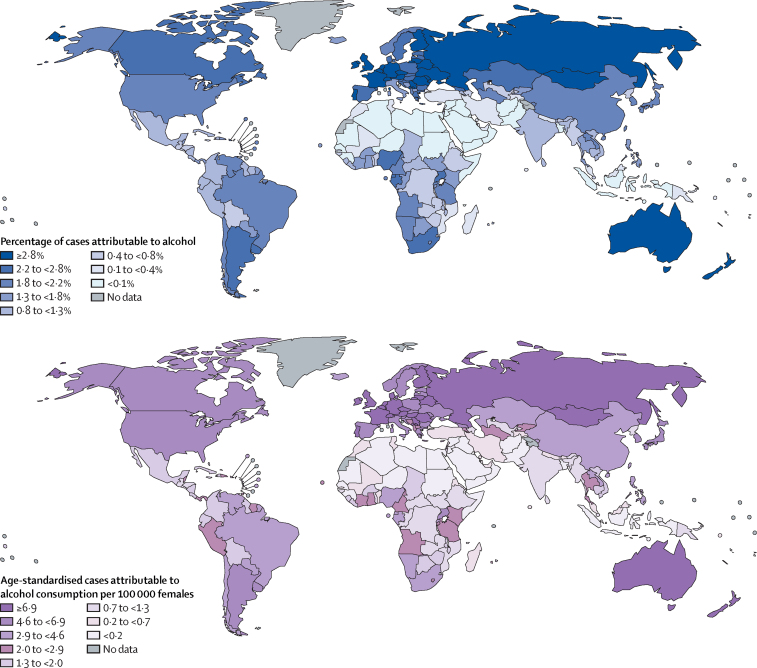
The highest PAFs of all new cases of cancer were observed in Mongolia, China, Moldova, and Romania (appendix p 33), which are reflected at the regional level where eastern Asia and central and eastern Europe had the highest PAFs (5·7% [95% UI 3·6–7·9] and 5·6% [4·6–6·6], respectively; appendix pp 9–10); we found the lowest PAFs in northern African (0·3% [0·1–3·3]) and western Asian (0·7% [0·5–1·2]) countries, including Kuwait, Libya, and Saudi Arabia (appendix pp 13, 18, 33). The national and regional patterns in males were similar to the average for both sexes combined, with the largest PAFs found in eastern Asia and central and eastern Europe (Figure 1, Figure 2; appendix pp 9–10). Among females, the largest PAFs were found in central and eastern Europe—driven by the highest national PAFs in females in Belarus, Moldova, Romania, and Russia—as well as in Australia and New Zealand and western Europe (Figure 1, Figure 3; appendix pp 9–10, 19). For all country-specific estimates, see the appendix (pp 11–25).
Figure 1.
Population attributable fractions, by alcohol consumption category, sex, and world region
Figure 2.
Population attributable fraction and age-standardised incidence rate of alcohol-attributable cancer cases in males in 2020, by country
Figure 3.
Population attributable fraction and age-standardised incidence rate of alcohol-attributable cancer cases in females in 2020, by country
The global and regional patterns of age-standardised incidence rates differed slightly to those of the PAFs: at the national level, many central and eastern European countries, including Moldova, Slovakia, and Romania, had the highest age-standardised incidence rates in males, which is reflected in the region having the highest age-standardised incidence rate (23·1 [95% UI 19·0–26·6] per 100 000 males; figure 2; appendix pp 9, 19, 34); the next highest regional age-standardised incidence rates were found in eastern Asia (21·5 [13·4–29·6] per 100 000 males), followed by western Europe (17·3 [13·8–20·4] per 100 000 males) and Australia and New Zealand (17·0 [12·7–20·7] per 100 000 males; appendix pp 9–10, 34). Among females, the highest national-level age-standardised incidence rates were in northern and western European countries, including Belgium, France, and Ireland (figure 3; appendix pp 20–21), but at the regional level, the age-standardised incidence rate was highest in females in Australia and New Zealand (10·2 [95% UI 6·3–15·2] per 100 000 females), followed by western Europe (9·4 [6·2–12·8] per 100 000 females) and northern Europe (9·1 [5·9–12·8] per 100 000 females; appendix pp 9–10, 34). In every world region, the age-standardised incidence rate was higher in males than in females; the smallest relative differences between males and females were found in Australia and New Zealand, northern Europe, and western Europe (male-to-female ratios 1·7, 1·7, and 1·8, respectively), whereas the largest relative differences were observed in south-central and southeastern Asia, where males had a 5·6–6·8 times higher rate than females.
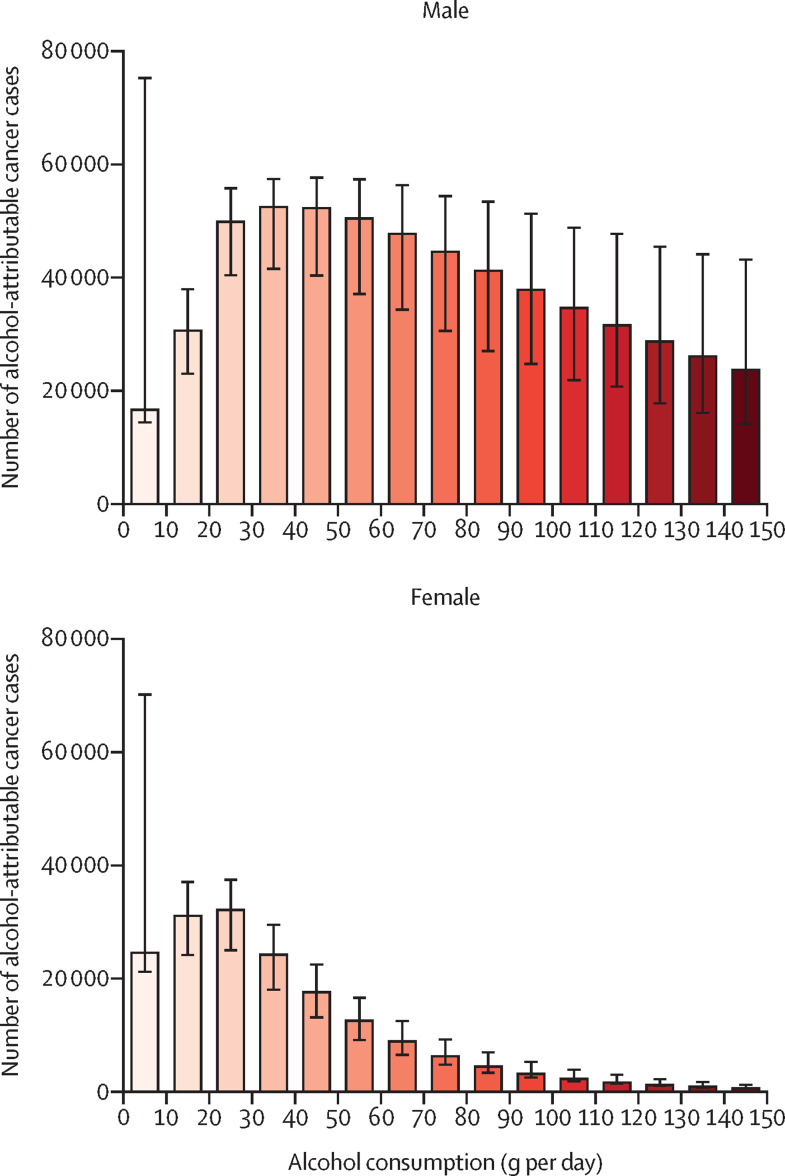
Of 741 300 cases, when separated into drinking categories, moderate drinking (<20 g per day) contributed 103 100 (13·9%; 95% UI 82 600–207 200) cases of alcohol-attributable cancer, risky drinking (20–60 g per day) contributed 291 800 (39·4%; 227 700–333 100) cases, and heavy drinking (>60 g per day) contributed 346 400 cases (46·7%; 227 900–489 400; appendix p 29). The proportion of cases of cancer attributable to heavy drinking in males was highest in the following regions: southern Africa (2100 [72·9%] of 2800) and central and eastern Europe (34 800 [69·7%] of 49 900); in females, it was highest in the following regions: southern Africa (590 [43·4%] of 1400) and western Africa (990 [37·2%] of 2700; figure 1; appendix pp 26–27). For regional estimates by consumption category, see the appendix (pp 26–29). After further stratifying alcohol consumption into 10 g per day increments, drinking up to 10 g per day contributed 41 300 (95% UI 35 400–145 800) alcohol-attributable cancer cases: 16 700 (14 300–75 400) cases were found in males and 24 600 (21 100–70 400) in females (16 700 [2·9%] of 568 700 and 24 600 [14·3%] of 172 600 alcohol-attributable cases among males and females, respectively), although the highest frequencies of alcohol-attributable cancers were in males drinking from 30 to less than 40 g per day and 40 to less than 50 g per day and in females drinking from 10 to less than 20 g per day and 20 to less than 30 g per day (figure 4; appendix p 30).
Figure 4.
Global number of alcohol-attributable cancer cases, by 10 g per day increase in alcohol consumption and sex
Error bars indicate 95% uncertainty intervals.
In a sensitivity analysis, when stomach and pancreatic cancers were included, the total alcohol-attributable cases reached 808 700 (95% UI 616 300–1 034 800; PAF 4·5% [3·4–5·8]; age-standardised incidence rate 9·1 [6·8–11·8]) from 50 000 (13 200–95 900; PAF 10·1% [2·7–19·4], age-standardised incidence rate 0·5 [0·1–1·0]) pancreatic cancer cases and 17 400 (810–36 900; PAF 1·6% [0·1–3·4], age-standardised incidence rate 0·2 [0·0–0·4]) stomach cancer cases (data not shown). Furthermore, sensitivity analysis, in which former drinking was included, added an additional 135 000 (95% UI 102 200–171 900) cases, which increased the total number of cases to 925 900 (705 900–1 187 500; 713 200 [543 600–910 400] in males and 212 700 [162 400–277 100] in females), the world total PAF to 5·2% (3·9–6·6; males 7·7% [5·9–9·8], females 2·4% [1·9–3·2]), and the age-standardised incidence rate to 10·3 (7·7–13·4) cases per 100 000 people (16·7 [12·7–21·4] per 100 000 males, 4·5 [3·2–6·1] per 100 000 females; data not shown).
Discussion
Globally, about 741 000, or 4·1%, of all new cases of cancer in 2020 were attributable to alcohol consumption. About three-quarters of alcohol-attributable cancer cases were in males, and the cancer sites contributing the most attributable cases were oesophageal, liver, and breast (in females). PAFs were lowest in northern Africa and western Asia in both sexes, and highest among males in eastern Asia and central and eastern Europe, and among females in central and eastern Europe, Australia and New Zealand, and western Europe. Risky and heavy drinking contributed most to the burden of alcohol-attributable cancers; however, moderate drinking still contributed one in seven alcohol-attributable cases and more than 100 000 cancer cases worldwide.
Our estimated global PAF was lower than the previous global estimates of 5·5% of cancer cases in 2012,4 4·8% of cancer deaths in 2016,5 and 4·9% of cancer deaths in 2019.2 This difference could be due to genuine decreases in consumption of alcohol in several world regions, such as in southern Europe and central and eastern Europe, as Shield and colleagues reported a 5·5% decrease in the global alcohol-attributable age-standardised rate of death from cancer between 2000 and 2016.5 Furthermore, there were differences in the numbers of included cancer sites; in all previous studies,2, 4, 5 total liver cancer was used, whereas in the current study we used hepatocellular carcinoma-specific incidence, which represents 80% of total primary liver cancers (Rumgay H, unpublished). Praud and colleagues also included pancreatic and gallbladder cancers in their main analysis, accounting for about 40 000 alcohol-attributable cases.4 Shield and colleagues incorporated former drinking into their main analysis,5 and inclusion of former drinking in our sensitivity analysis resulted in a more similar PAF. One of the major differences, however, is that the previous studies estimated cancer mortality attributable to alcohol, whereas our study covered cancer incidence only. The previous studies assumed that the increased risk from drinking alcohol was the same for both cancer incidence and mortality and that the latency period between alcohol exposure and cancer mortality did not change. For breast cancer and colorectal cancer, which have much lower mortality rates than incidence in many populations, the burden of alcohol-attributable deaths was much lower than alcohol-attributable cases in the study by Praud and colleagues.4 Despite differences in the relative risks used and source of alcohol consumption data, our country-specific estimates were consistent with those of previous national studies, including those done in Chile, the UK, and the USA.11, 12, 13 Due to the consistent methodology and data sources used in our study, we consider our results to provide the most comparable estimates between countries and world regions.
There are several biological pathways by which the consumption of alcohol, as ethanol, can lead to cancer development, including DNA, protein, and lipid alterations or damage by acetaldehyde, the carcinogenic metabolite of ethanol;14 oxidative stress;15 and alterations to the regulation of hormones such as oestrogens and androgens.16 Ethanol might also promote cancer development indirectly by acting as a solvent for other carcinogenic agents such as chemicals in tobacco.17 Evidence shows that humans who carry the aldehyde dehydrogenase-2*2 (ALDH2*2) variant allele of ALDH2—the main enzyme that metabolises acetaldehyde—have a substantially increased risk for development of cancers of the upper aerodigestive tract.18 It is estimated that between 28% and 45% of eastern Asian populations are carriers of the ALDH2*2 polymorphism;18 therefore, a proportion of the alcohol-associated cancers in eastern Asian populations in our study could be due to the increased risk from this genetic variant. However, it is thought that some self-selection takes place whereby people who are slow metabolisers of acetaldehyde experience a flushing reaction that might be unpleasant for the individual and they might prefer to avoid drinking alcohol;18 this hypothesis is in contrast with the observed increase in alcohol use in a number of eastern Asian populations.6
Consistent with patterns of alcohol per capita consumption, PAFs were lowest in countries such as Saudi Arabia and Kuwait, where religious-based policies have ensured that population alcohol consumption remains low and lifetime abstention rates remain high.6 On the other end of the spectrum, alcohol consumption in central and eastern Europe has historically outranked that of other world regions, but has decreased in recent years,6 whereas increases in alcohol consumption, linked with countries' economic development, are projected in Asian countries such as China and India. With regard to the effect of social and economic development, increases in alcohol consumption in women have been reported as women have taken on a larger share of paid employment.6 This finding is clearly reflected in countries highly indexed in development, where we saw the highest burden of alcohol-attributable cancers in women and the most similar male-to-female ratios of alcohol-attributable cancer rates; in these regions, breast cancer was the main driver of the high alcohol-attributable cancer incidence rates among women. Global changes in alcohol drinking patterns by region and sex alongside demographic changes and a growing cancer burden might mark an increase in alcohol-attributable cases in several world regions,19 such as eastern and south-central Asia, which should be countered by comprehensive national cancer control plans that cover cancer prevention.
There is low awareness of the link between alcohol and cancer risk among the general public, but adding cancer warnings to alcohol labels, similar to those used on tobacco products, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,20 which could then confer increased public support for alcohol policies.21 WHO developed its list of so-called best buys for tackling non-communicable diseases, and for alcohol these involve policies to increase taxation, limit purchasing availability, and reduce marketing of alcohol brands to the public;22 yet their effective implementation relies on enforcement and regulation—processes that are not always available in low-income or middle-income settings. In such settings, there is also a scarcity of research into effective alcohol policies: for example, in the sub-Saharan African regions where heavy drinking had the largest contribution to alcohol-attributable cases, only 16 of 46 countries have national or subnational alcohol strategies.23 A good understanding of the local context is essential for successful policy implementation and is paramount in reducing the alcohol-attributable burden of cancer.
We believe that the main results of our study are conservative estimates; we only included cancer sites with sufficient evidence of a causal link according to the most recent IARC monograph.3 We also only considered current drinking in our main analysis, with inclusion of former drinking in the sensitivity analysis. Other strengths of our study include the use of meta-analyses of risk estimates from cohort studies of the highest quality, and the specific model used to estimate alcohol prevalence, which corrects for under-reporting of alcohol consumption in survey data using population data on per capita consumption of alcohol, as described by Shield and colleagues.5 However, this method of adjustment does not account for differential degrees of under-reporting by age and sex, and changes in alcohol consumption before and after 2010, and it does not address survey biases that lead to an under estimation of the prevalence of former drinkers. Furthermore, variations in the quality of data on per capita consumption of alcohol and surveys exist between countries. In particular, data from countries with a high volume of unrecorded alcohol consumption and data from countries that do not have high-quality nationally representative surveys are more susceptible to biases.
Another limitation of our study is that we did not consider the synergistic effect between alcohol and tobacco, which is reported as a true interaction for most upper aerodigestive tract cancers. It is possible that some alcohol-attributable cases in our study could have been caused by tobacco due to residual confounding in the current and former drinking relative risks used. Similarly, a proportion of alcohol-attributable liver cancers could be the result of synergism with hepatitis B or hepatitis C virus infection or aflatoxin exposure. This could have been the case in Mongolia, where hepatitis B and hepatitis C viruses were estimated to have caused around 44% and 46% of liver cancer cases, respectively, in 2012.24 Another cofactor that we did not consider was obesity, despite some evidence of an interaction between alcohol and obesity on liver disease and risk of hepatocellular carcinoma.25 Along with alcohol, the cofactors discussed are associated with social inequalities both between and within countries, and the determinants of these inequalities should be explored further to understand the observed disparities. In addition, by not using population-specific relative risks, we might have underestimated the alcohol-attributable cancer burden in populations in which a higher risk is observed, such as in people with a history of cancer, and in eastern Asian populations carrying the ALDH2*2 allele. Furthermore, high-quality prospective aetiological studies in low-income and middle-income settings are scarce, so differences in risk are still largely unknown. High-quality estimates of risk in different populations and ethnicities would further define the true global picture of the alcohol-attributable burden of cancer.
It is also important to consider the impact of the global COVID-19 pandemic when estimating health outcomes for the year 2020.26 The cancer incidence estimates for 2020 used in our study do not account for changes in the reporting of cancer due to disruptions caused by health system closures and the concerns of individuals, among other reasons. One study in the Netherlands reported a 27% decrease in cancer diagnoses in the early phase of the pandemic response, with some evidence of this returning to pre-pandemic rates.27 The COVID-19 pandemic could have also affected individuals' total consumption of alcohol, as shown by a reported increase in the proportion of the UK population binge drinking or drinking four or more times a week observed during national lockdowns in the UK.28 However, any changes in drinking patterns among individuals are not yet evident for current cancer rates, but could be reflected in the next decades.
In summary, we found that alcohol use causes a substantial burden of cancer, a burden that could potentially be avoided through cost-effective policy and interventions to increase awareness of the risk of alcohol and decrease overall alcohol consumption. General population strategies, such as WHO's best buys, include a reduction of availability, an increase in price via taxation, and a ban on marketing, and are most effective for an outcome such as alcohol-attributable cancer, where even lower levels of drinking can increase the risk of cancer.22 With increases in alcohol consumption predicted until at least 2030 in several world regions, action must be taken to reduce the avoidable burden of cancer attributable to alcohol.
Data sharing
All cancer incidence and population estimates are available to the public through the Global Cancer Observatory. All statistical code (ie, R code) and input files used to produce the results presented in this paper are available to the public on request to the corresponding author. All results from this study are available to the public through the dedicated Cancers Attributable to Alcohol tool in the Global Cancer Observatory.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The work by HR reported in this paper was undertaken during a PhD studentship at the International Agency for Research on Cancer (Lyon, France). We thank all population-based cancer registries and their staff who have contributed in sharing the cancer incidence data used to build the estimates used in this study. We also thank Melina Arnold (Cancer Surveillance Branch, International Agency for Research on Cancer) for providing the oesophageal squamous cell carcinoma estimates and Teresa Norat and Doris Chan (both at the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK) for earlier enquiries about the World Cancer Research Fund results. Where authors are identified as personnel of the International Agency for Research on Cancer and WHO, the authors alone are responsible for the views expressed in this Article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer and WHO.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
HR, IS, and KS conceived the study. HR, HC, and KS contributed to study design and analysis. HR and KS contributed to data collection and verified all underlying data. HR, IS, KS, PF, JR, IO, and BS interpreted the results. HR and IS wrote the first draft of the manuscript. All authors critically reviewed the manuscript and agreed with the decision to submit for publication. The corresponding author had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Rehm J, Gmel GE, Sr, Gmel G. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction. 2017;112:968–1001. doi: 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJL, Aravkin AY, Zheng P. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer IARC monographs on the evaluation of carcinogenic risks to humans volume 100E. Personal habits and indoor combustion. 2012. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Personal-Habits-And-Indoor-Combustions-2012 [PMC free article] [PubMed]
- 4.Praud D, Rota M, Rehm J. Cancer incidence and mortality attributable to alcohol consumption. Int J Cancer. 2016;138:1380–1387. doi: 10.1002/ijc.29890. [DOI] [PubMed] [Google Scholar]
- 5.Shield K, Manthey J, Rylett M. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. 2020;5:e51–e61. doi: 10.1016/S2468-2667(19)30231-2. [DOI] [PubMed] [Google Scholar]
- 6.Manthey J, Shield KD, Rylett M, Hasan OSM, Probst C, Rehm J. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet. 2019;393:2493–2502. doi: 10.1016/S0140-6736(18)32744-2. [DOI] [PubMed] [Google Scholar]
- 7.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 8.World Cancer Research Fund/American Institute for Cancer Research Diet, nutrition, physical activity and cancer: a global perspective. Continuous Update Project Expert Report 2018. https://www.wcrf.org/wp-content/uploads/2021/02/Summary-of-Third-Expert-Report-2018.pdf
- 9.WHO Global information system on alcohol and health. 2019. https://www.who.int/data/gho/data/themes/global-information-system-on-alcohol-and-health
- 10.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9:531–541. [PubMed] [Google Scholar]
- 11.Rezende LFM, Murata E, Giannichi B. Cancer cases and deaths attributable to lifestyle risk factors in Chile. BMC Cancer. 2020;20:693. doi: 10.1186/s12885-020-07187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown KF, Rumgay H, Dunlop C. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer. 2018;118:1130–1141. doi: 10.1038/s41416-018-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Islami F, Goding Sauer A, Miller KD. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31–54. doi: 10.3322/caac.21440. [DOI] [PubMed] [Google Scholar]
- 14.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 15.Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278–290. doi: 10.1079/pns2006496. [DOI] [PubMed] [Google Scholar]
- 16.Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- 17.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 18.Chang JS, Hsiao J-R, Chen C-H. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci. 2017;24:19. doi: 10.1186/s12929-017-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Stockwell T, Vallance K, Hobin E. The effects of alcohol warning labels on population alcohol consumption: an interrupted time series analysis of alcohol sales in Yukon, Canada. J Stud Alcohol Drugs. 2020;81:225–237. [PubMed] [Google Scholar]
- 21.Weerasinghe A, Schoueri-Mychasiw N, Vallance K. Improving knowledge that alcohol can cause cancer is associated with consumer support for alcohol policies: findings from a real-world alcohol labelling study. Int J Environ Res Public Health. 2020;17:398. doi: 10.3390/ijerph17020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Tackling NCDs: best buys and other recommended interventions for the prevention and control of non-communicable diseases. 2017. https://apps.who.int/iris/handle/10665/259232
- 23.Ferreira-Borges C, Esser MB, Dias S, Babor T, Parry CDH. Alcohol control policies in 46 African countries: opportunities for improvement. Alcohol Alcohol. 2015;50:470–476. doi: 10.1093/alcalc/agv036. [DOI] [PubMed] [Google Scholar]
- 24.Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–2477. doi: 10.1002/ijc.31280. [DOI] [PubMed] [Google Scholar]
- 25.Mahli A, Hellerbrand C. Alcohol and obesity: a dangerous association for fatty liver disease. Dig Dis. 2016;34(suppl 1):32–39. doi: 10.1159/000447279. [DOI] [PubMed] [Google Scholar]
- 26.Valencia DN. Brief review on COVID-19: the 2020 pandemic caused by SARS-CoV-2. Cureus. 2020;12 doi: 10.7759/cureus.7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinmohamed AG, Visser O, Verhoeven RHA. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedzwiedz CL, Green MJ, Benzeval M. Mental health and health behaviours before and during the initial phase of the COVID-19 lockdown: longitudinal analyses of the UK Household Longitudinal Study. J Epidemiol Community Health. 2021;75:224–231. doi: 10.1136/jech-2020-215060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All cancer incidence and population estimates are available to the public through the Global Cancer Observatory. All statistical code (ie, R code) and input files used to produce the results presented in this paper are available to the public on request to the corresponding author. All results from this study are available to the public through the dedicated Cancers Attributable to Alcohol tool in the Global Cancer Observatory.