SUMMARY
Objective:
Neuroimaging studies of suicidal behavior have so far been conducted in small samples, prone to biases and false-positive associations, yielding inconsistent results. The ENIGMA-MDD working group aims to address the issues of poor replicability and comparability by coordinating harmonized analyses across neuroimaging studies of major depressive disorder and related phenotypes, including suicidal behavior.
Methods:
Here, we pool data from eighteen international cohorts with neuroimaging and clinical measurements in 18,925 participants (12,477 healthy controls and 6,448 people with depression, of whom 694 had attempted suicide). We compare regional cortical thickness and surface area, and measures of subcortical, lateral ventricular and intracranial volumes between suicide attempters, clinical controls (non-attempters with depression) and healthy controls.
Results:
We identified 25 regions of interest with statistically significant (FDR<0.05) differences between groups. Post-hoc examinations identified neuroimaging markers associated with suicide attempt including smaller volumes of the left and right thalamus and the right pallidum, and lower surface area of the left inferior parietal lobe.
Conclusions:
This study addresses the lack of replicability and consistency in several previously published neuroimaging studies of suicide attempt, and further demonstrates the need for well-powered samples and collaborative efforts. Our results highlight the potential involvement of the thalamus, a structure viewed historically as a passive gateway in the brain, and the pallidum, a region linked to reward response and positive affect. Future functional and connectivity studies of suicidal behaviours may focus on understanding how these regions relate to the neurobiological mechanisms of suicide attempt risk.
INTRODUCTION
Suicide is a leading cause of death worldwide and is a considerable health concern in both developed and developing countries (1). While region and country-specific estimates vary, the global average prevalence for suicide is estimated to be about 10.6 deaths per 100,000 (2). Suicide attempts outnumber actual suicides by twenty to thirty-fold,(3,4) which further increases the economic and social burden of suicidal behavior (5).
Suicidal behavior is more common in people living with mental illness (6–8). For a long time, suicidal behaviours were conceptualized as a symptom inherent to certain conditions, in particular major depressive disorder (MDD). It is increasingly clear that suicidal behaviour is complex (9). On the whole, a better understanding of suicidality, in terms of its underlying mechanisms, could help identify individuals at increased risk of engaging in suicidal behaviors and inform better interventions (10).
Non-invasive neuroimaging technologies, such as magnetic resonance imaging (MRI), allow brain structure and function to be studied in vivo (11,12). The analysis of brain morphometry and neuroanatomical differences between individuals with mental illness and healthy controls has already proven useful in a range of conditions such as MDD (13), bipolar disorder (14) and schizophrenia (15). Similar approaches have been used to study suicidal behaviours, albeit in small samples. Briefly, several studies have reported lower grey matter volume and cortical thickness in the frontal, prefrontal, orbitofrontal, dorsolateral and temporal lobes and white matter hyperintensities associated with suicidal behaviours (16–29).
Nonetheless, small samples and heterogeneous analysis methods have led to a lack of replicability and inconsistent results (11,30). The ENIGMA-MDD working group aims to address issues of poor replicability and comparability in neuroimaging studies by coordinating harmonized analyses of MDD and related phenotypes, including suicidal behavior. In the most recent meta-analysis of subcortical brain volumes conducted by our working group, we did not detect any significant morphological differences associated with suicidal behaviour independently of depression diagnosis (31). Identifying the neural substrates of suicide attempt is key to understanding the aetiology of suicide. This in turn might lead to novel therapeutic strategies based on behavioural neuroscience and brain stimulation (32).
Previous studies have pinpointed some of these associations, for example, alterations in the ventral and dorsal prefrontal cortex, the insula and regions involved in temporal, striatal and posterior circuits (11,30). However, studies have been performed on samples of small size and with several sources of potential bias, resulting in inconsistent findings across publications (11). Notably, subcortical associations did not replicate in our previous meta-analyses (31). Thus, we conclude that prior literature might not be sufficiently robust to support the role of specific brain structures in suicide attempt. For that reason, we decided to conduct a comprehensive exploratory investigation of neuroimaging correlates for suicide attempt in the largest participant sample to date. We perform a pooled mega-analysis of subcortical volumes and regional cortical surface area and thickness, using linear mixed model regressions in a sample of 18,925 subjects from eighteen cohorts from around the world. We aim to shed light on the neural circuits that underlie suicidal behavior by comparing brain morphometry between MDD cases with a history of suicide attempt versus those without, as well as versus healthy controls.
METHODS
Samples
We analyzed pooled data (mega-analysis) across seventeen ENIGMA-MDD working group cohorts with clinical and neuroimaging data available for participants fulfilling MDD criteria (33) (N=2,533) and healthy controls (N=4,066), and participants from the UK Biobank (N=12,326). We defined three groups: suicide attempters (SA), clinical controls (CC), that is, participants with depression and no history of suicide attempt, and healthy controls (HC). Descriptive statistics for each sample are listed in Table 1 and Supplementary Table S1. Each cohort assessed depression status and history of a suicide attempt based on available clinical information. In the UK Biobank, lifetime depression status (N=3,633) and lifetime suicide attempt (N=322) were ascertained using the Composite International Diagnostic Interview (CIDI). Participants with no history of depression or suicide attempt (N=8,411) were defined as healthy controls. A psychiatric diagnostic interview was used to diagnose participants across the ENIGMA-MDD groups. Information on the instruments used to determine suicide attempt and exclusion criteria per site are available in Supplementary Tables S2 and S3 respectively. The combined sample comprised 12,477 healthy controls and 6,448 participants with a lifetime depression diagnosis. Within the depression group, 694 participants reported at least one suicide attempt. All sites obtained approval from their local institutional ethics committees and review boards to participate in this study, and all participants provided informed consent at their local recruitment institution.
Table 1.
Demographics and clinical measures across studied groups
| HC | CC | SA | |
|---|---|---|---|
| Total N (%) | 15,269 (71) | 5,557 (26) | 694 (3) |
| Females N (%) | 7,637 (50) | 3,642 (66) | 466 (67) |
| Males N (%) | 7,632 (50) | 1,915 (34) | 228 (33) |
| Age mean (sd) | 57.6 (14.8) | 53.2 (15.4) | 49.2 (16.3) |
| BDI mean (sd)¥Ω | 3.6 (4.0) | 18 (10.9) | 23 (11.8) |
| HDRS mean (sd)¥Ω | 1.3 (2.0) | 11.0 (6.9) | 13.8 (6.9) |
| Depression age of onset¥ | NA | 29.8 (14.3) | 23.3 (14.1) |
| Antidepressant use %¥ | 0.1% | 36% | 21% |
| Depression recurrence¥ % | NA | 21% | 36% |
HC- Healthy controls; CC- Clinical controls; SA - Suicide attempters.
BDI- Beck Depression Inventory; HDRS- Hamilton Depression Rating Scale
Data available only for a subset of the sample.
Sum-score excluding the suicidal behaviours item.
Image processing and analysis
T1-weighted MRI structural brain scans were acquired and analyzed locally at each site using the validated and automated segmentation software FreeSurfer (34) (available at http://surfer.nmr.mgh.harvard.edu/). Image acquisition parameters and software versions and descriptions are detailed in Supplementary Table S2. The segmentation of cortical and subcortical phenotypes was visually inspected for accuracy following standardized protocols designed to facilitate harmonized image analysis across multiple sites (http://enigma.ini.usc.edu/protocols/imaging-protocols/). Within each cohort, measures were visually verified for accuracy and excluded if they were not properly segmented. Within-cohort outliers (defined as measurement greater than three standard deviations away from the mean) were excluded from the analysis. We examined five global brain measures including: intracranial volume (ICV), total surface area of the left and right hemispheres and mean cortical thickness of the left and right hemispheres, 16 subcortical brain volume measures, and cortical surface area and thickness measures for 68 brain regions of interest (ROI) as defined by the Desikan-Killiany atlas (35).
Ascertainment of suicide attempt history
In this study, a suicide attempt was defined as any self-harm act with the intent to die. In this study, we focused on lifetime suicide attempt, as opposed to other suicidal behaviours, to reduce potential heterogeneity arising from different suicide risk assessment instruments used across cohorts. Attempt severity was not assessed due to a lack of information in individual studies. A description of instruments used to measure suicide attempt in each site is available in Supplementary Table S2. Cohorts also provided (where available) information on i) whether participants have used antidepressants, ii) depression severity, coded either as the Hamilton Depression Rating Scale (HDRS) score excluding the suicide item, or the number of DSM-IV MDD criteria endorsed (ranging from 0 to 9), iii) age of depression onset and iv) whether depression was recurrent or a single episode.
Statistical analyses
Linear mixed-effects models
Statistical analyses were performed in R v3.6.1 using the statistical package nlme. Linear mixed-effects models were used to account for site variation (with a random intercept for scan site) while correcting for desired covariates as fixed effects. We modeled each regional measure as an outcome while using an indicator variable per group of interest: healthy controls (HC), MDD patients with no suicide attempt history (clinical controls; CC), and MDD patients with attempt history (SA). All models were adjusted for age and sex, while surface area and volumetric analyses also adjusted for ICV (except when ICV was the measure of interest). Main effects of groups (i.e., differences between groups of HC, CC, and SA) were identified by performing a type II analysis of variance (F-test) over the fitted linear mixed-effects model described above. We conducted follow-up (post-hoc) analyses to assess whether the effects were driven by suicide attempt. ROI were compared between suicide attempters and clinical controls; between suicide attempters and healthy controls; and between clinical controls and healthy controls. Finally, we conducted several sensitivity analyses. First, to assess the effects of severity, recurrence, and age of onset of depression, and history of antidepressant use on the observed associations, we repeated the post-hoc analyses of the four regions showing evidence of association with suicide attempt, including additional covariates one at a time. Then, to evaluate the contribution of the largest cohort in the analysis to the observed results on the four ROI mentioned above, we conducted the analyses excluding the UK Biobank cohort.
Statistical significance definition
We corrected for multiple comparisons using a false discovery rate (FDR) procedure (36) for each set of morphometry measures separately. The significance threshold to define ROI for post-hoc analyses was set at FDR p-value <0·05. For the post hoc tests of the ROI identified above, we used a matrix spectral decomposition to identify the number of effective variables (37,38) coupled with Bonferroni correction to keep the type I error rate at 5%. In this manuscript, a significant result survived post-hoc multiple testing corrections (p<Bonferroni corrected threshold), whereas a nominally significant result was only significant before correction (p<0.05).
RESULTS
Suicide attempt prevalence and sample demographics
Details on the sample of each cohort included in this analysis are summarized in Table 1 and Supplementary Table S1. Notably, not all cohorts had cases of suicide attempt, but still contributed data to the healthy or clinical control groups. The pooled mean age was 56.22 years old, with a standard deviation of 15.17 years. Differences in age and sex composition across cohorts were detected (Supplementary Table S1) and used as covariates for all the analyses. The total sample size comprised 18,925 subjects, of which 3.67% (N=694) had at least one past suicide attempt. Furthermore, 30.04% (N=5,574) of the total sample was diagnosed with depression but did not report a previous suicide attempt. Methodological differences (e.g., scanner used or different parameters for the scan) between participating cohorts are listed in Supplementary Table S2.
Subcortical volumetric measures
The thalamus (right and left), right pallidum, and total ICV exhibited a statistically significant group effect (i.e., any difference between healthy controls, clinical controls, or suicide attempters) after correcting for multiple comparisons. The left pallidum and the right nucleus accumbens showed a nominally significant difference but did not survive correction for multiple comparisons (Supplementary Table S4). Depressed attempters exhibited smaller volumes in the left and right thalamus and right pallidum compared to both clinical (Cohen’s d=−0.13, −0.14 and −0.12, respectively) and healthy controls (Figure 1). Depressed attempters also exhibited smaller ICV compared to healthy controls (Cohen’s d =−0.13). This association did not reach significance, when comparing attempters and clinical controls, after accounting for multiple testing. None of the regions with a significant group effect showed a significant difference when comparing clinical to healthy controls (Supplementary Table S5).
Figure 1. Group differences in subcortical volumes.

Effect sizes are shown for regions that displayed a statistically significant difference in subcortical volumes between the groups: attempters compared to clinical controls (left panel) and attempters compared to healthy controls (right panel). No difference between clinical and healthy controls reached statistical significance after correction for multiple comparisons. Significant results are the bilateral thalamus and right pallidum.
Cortical surface area
Eight of the 68 cortical regions under analysis displayed a significant group effect (Supplementary Table S6). These regions included the left and right pericalcarine, left and right cuneus, left inferior parietal, left rostral-middle frontal, right lingual, and right fusiform gyri. Depressed attempters exhibited, on average, a smaller surface area of the left cuneus, left inferior parietal, left rostral middle frontal and right pericalcarine regions, compared to healthy controls. Furthermore, clinical controls exhibited smaller surface areas in the right pericalcarine and right fusiform gyri compared to healthy controls and clinical controls (Supplementary Table S7). The left and right cuneus, left inferior parietal, right pericalcarine, and right lingual also exhibited nominally significant differences between attempters and clinical controls (p<0.05, uncorrected). After correcting for post-hoc multiple testing, only the left inferior parietal surface area was significantly different between attempters and clinical controls (Cohen’s d =−0.12; Figure 2).
Figure 2. Group differences in cortical surface area.

Effect sizes are shown for regions that displayed a (top) nominally significant (p<0.05) or (bottom) a statistically significant (p<0.00333 threshold after multiple test correction) post-hoc difference between attempters and healthy controls (left panel), attempters and clinical controls (middle panel) and clinical controls compared to healthy controls (right panel). All of the colored regions showed a statistically significant group effect (FDR<0.05).
Cortical thickness
Widespread cortical thickness differences between the three groups were observed (Supplementary Table S8). Five of these regions showed a significant difference when comparing attempters to healthy controls, and three out of those five (left fusiform, left insula and left rostral middle frontal) showed nominally significant lower cortical thickness in depressed attempters compared to clinical controls. Only the left rostral middle frontal region displayed a statistically significant difference between attempters and healthy controls (Supplementary Table S9). The left fusiform, and the left insula also showed a nominally significant difference between clinical and healthy controls. Conversely, the left rostral middle frontal did not show a significant difference between clinical and healthy controls. All regions with a nominally significant difference between attempters and clinical controls were in the left hemisphere. After adjusting for multiple testing in the post-hoc tests, none of the cortical thickness differences between attempters and clinical controls reached statistical significance (Figure 3 bottom left).
Figure 3. Group differences in cortical thickness.
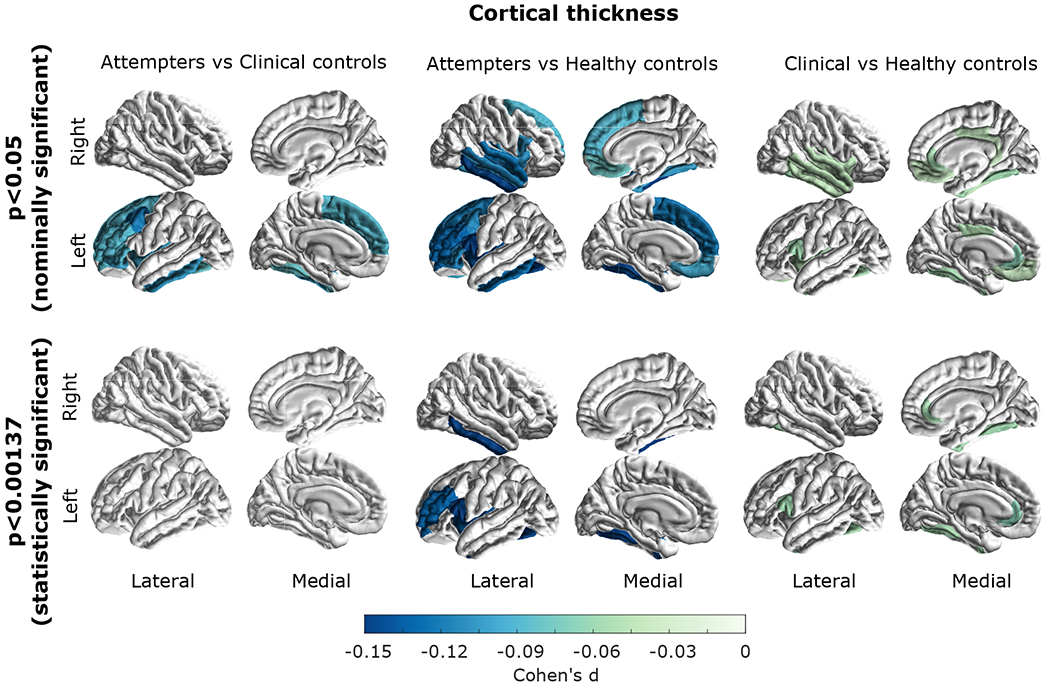
Effect sizes are shown for regions that displayed a (top) nominally significant (p<0.05) or (bottom) a statistically significant (p<0.001373 threshold after multiple test correction) post-hoc difference between attempters and healthy controls (left panel), attempters and clinical controls (middle panel) and clinical controls compared to healthy controls (right panel). All of the colored regions showed a statistically significant group effect (FDR<0.05).
Sensitivity analyses
Finally, we performed sensitivity analyses to assess the effect of additional covariates on the associations discovered. Namely, we tested whether our results were robust to adjustment for previous antidepressant use, depression severity, age of onset, and recurrence. These analyses had lower statistical power as data on these variables was available in fewer cohorts. Participants with a history of suicide attempt continued to show a smaller volume of the right thalamus (p<0.05) even after adjusting for history antidepressant use, depression severity, age of onset and recurrence (Supplementary Tables S10–S13). No other region remained significant after adjusting for any of these variables. A final sensitivity analysis was performed by excluding the UK-Biobank cohort. While all of the effects were on the same direction, only the association between suicide attempt and a lower right thalamus volume remained statisitcally significant (p=0.004; Supplementary table S14).
DISCUSSION
The present study addressed the lack of statistical power, which may have resulted in low replicability and consistency in prior neuroimaging studies of suicide attempt. Our analyses revealed four regions associated explicitly with suicide attempt, above and beyond the effect of MDD, supporting the hypothesis of suicidality-associated neural differences (30). The relatively few regions identified, and the overall small effect sizes should be taken as a warning for future studies attempting to perform these types of analyses in small samples. However, we have also identified a need for reducing heterogeneity across cohorts, for example by including information on suicide attempt lethality, this is increasingly challenging as sample size increases.
We observed statistically significant volume reductions of the left and right thalamus, right pallidum, and a smaller surface area in the left inferior parietal cortex in depressed participants with a history of suicide attempt compared to both clinical and healthy controls. Prior studies have suggested that these regions are associated with suicidal behaviors;(17,21,39–41) however, the lack of evidence for associations in better-powered studies (31) and the lack of consensus in the field (11,13) made it challenging to reach definitive conclusions. A recent overview of a brain model for suicidal behaviour identified at least four functional diathetic elements to suicide. These include: subjective distress, impaired decision making, learning or memory deficits and social distortion as part of a cognitive impairment (42). The regions identified here are involved in decision making behaviours such as impulsivity and planning, as well as attention and concept of self (see below). All of these factors are related to the four risk increasing components discussed above.
The pallidum has been linked to reward response, social activity mediation, and positive affect (43,44). Furthermore, a recent structural MRI study has linked the pallidum to suicidal ideation severity and impulsivity in a small sample of Korean MDD patients (45). Thus alterations in the pallidum may reflect changes in affect and impulsivity known to be associated with suicidal behaviours (46,47). The thalamus, historically viewed as a passive gateway linking different brain regions, has been recently proposed to operate as an integrative hub of the brain (48), relaying signals between regions such as the basal-ganglia and the cortex, but also frontal-subcortical connections which have been linked to suicide attempt through fractional anisotropy (42). A growing body of evidence suggests the different nuclei within the thalamus are involved in high-order cognition (49). The thalamus serves as a region where the integration of cortico-striatal-thalamic-cortical takes place. These circuits modulate several behaviours including emotional drive and planning (50). Our results showing abnormalities in part of the basal-ganglia (pallidum) and the thalamus would be consistent with implicating these circuits with suicide attempt. Furthermore, thalamic abnormalities and lesions have been linked to disorders such as addiction (51), bipolar disorder,(52) and schizophrenia (53,54). Therefore, an impaired function in the thalamus, and its related circuits with the basal-ganglia and cortex, might mediate suicide attempt through impaired affect, empathy, and processing of stimuli-response relationships.
As previously reviewed (28), the parietal region is part of the executive control network, which exerts control over thought, emotion and behavior. The inferior parietal lobe is further part of the posterior parietal cortex (55), known for its role in attention networks (56) and processing of visual information. The inferior parietal lobe has been linked to schizophrenia through structural and functional differences including executive function and “concept of self” functions (57). It has further been linked to suicide attempt within bipolar disorder (58). Connectivity and functional studies are necessary to complement our results, however studying suicidality using fMRI carries important challenges such as the immediate need to reduce suicide risk and establishing a valid (i.e. relevant) timeframe before or after a suicide attempt.
Conversely, no cortical thickness differences survived post-hoc multiple testing correction. Some regions differed between attempters and healthy controls, but the lack of differences between clinical controls and suicide attempters suggests that these associations may be driven by depression status, rather than suicide attempt. The fact that subcortical volume and surface area associations had a stronger association with suicide attempt than with cortical thickness could be of interest in light of genetic analyses that identified substantial differences in the genetic etiology of cortical morphometry phenotypes (59–61). For example, surface area measurements were reported to have a higher heritability, and be more influenced by early developmental genetic influences compared to cortical thickness,(60) suggesting that biomarkers associated with surface area are likely established earlier in life. In contrast, cortical thickness phenotypes may be more variable and more susceptible to adverse environmental effects later in life, such as substance use or having a psychiatric condition.
The substantial overlap between depression severity and suicide attempt (e.g., our Table 1 shows that participants with a past suicide attempt also have higher HDRS and Beck Depression Inventory (BDI) sum scores even after removing the suicide item) makes it hard to differentiate whether an identified statistical effect is driven by depression severity or suicide attempt status. This is also supported by the fact that several cortical associations also displayed an effect when comparing clinical to healthy controls, and that those results did not remain significant after sensitivity analyses that controlled for proxies of depression severity. Overall, effect sizes were smaller when comparing clinical to healthy controls than those when comparing suicide attempters to either control group. That might be due to the previously discussed collinearity between suicidal behaviours and depression severity. Many of the most severely depressed cases will be among the depressed suicidal group, thus reducing the strength of any signals associated with depression severity.
In our previous study, we investigated intracranial and subcortical volumes for their association with suicidal behaviour, ideation, plan and attempt in a smaller sample (N=3,097). Notably, we did ot detect any significant associations and a post hoc power estimation analysis suggested that a sample size of > 2290 cases (suicide attempters) would be required just for a subcortical volume study. In the present study, the total the sample size and the number of contributing cohorts more than doubled. Furthermore, we focused on suicide attempt only to reduce heterogeneity; and expanded the number and type of brain morphology phenotypes under analysis (including cortical brain measures). Lastly, we used a different methodology based on pooling individual-level data and using linear mixed models, as compared to a classic meta-analysis as in our previous study. By using a mega-analytical approach instead of a meta-analytical approach, we could include more samples with a relatively small number of suicide attempt cases. Accordingly, we implemented a linear mixed-model framework, which allowed us to adjust for the differences across sites by modeling imaging-site as a random effect without significantly compromising statistical power.
This study examined cohorts from the ENIGMA-MDD working group and a subset of participants fulfilling MDD diagnosis criteria from the UK Biobank. Heterogeneity across cohorts is a potential confounder of any large scale collaboration. For example, the mechanisms underlying suicide have been shown to vary with age (62). Furthermore, different groups might have used different MRI scanners, acquisition parameters, studied treatment naive participants or assessed suicide attempt using different instruments. To account for this, we used linear mixed models which adjust for the effects of different sites, and relevant covariates such as age (and depression age of onset or antidepressant usage as sensitivity analyses), while preserving statistical power. All individuals with a history of suicide attempt also had an MDD diagnosis. Thus, we cannot conclude whether the associations observed in this study would also be correlated with suicide attempt history in other mental illnesses. While the direction of effects were highly consistent in the sensitivity analyses, only the right thalamus was associated with suicide attempt above and beyond potential covariates such as depression severity, recurrence, and history of antidepressant use. Furthermore, the right thalamus remained associated even after excluding the UK Biobank cohort, suggesting it is a generalisable and robust association. The observation of other ROI no longer showing an association with suicide attempt after correcting for these additional clinical variables was expected for several reasons. First, suicidality is highly collinear with depression severity, including recurrence of depressive episodes. Second, the lack of information on these variables in some of the contributing cohorts greatly reduced the sample size for the sensitivity analyses, resulting in reduced statistical power to detect the same small effect sizes observed for these brain measures across the whole sample.
It is important to acknowledge that a range of suicide risk assessment, MRI instruments and acquisition parameters were used across cohorts. We have tackled this in two ways: i) Cohorts ascertained lifetime suicide attempt using standard instruments with help from a mental health expert and ii) We used a statistical framework that corrects for heterogeneity and confounders arising from systematic cohort differences such as MRI scanner used.
While we identify alterations associated with suicide attempt, the direction of causality is less clear. It is plausible that these alterations are a consequence of suicidal thoughts causing a brain rewiring. Suicide attempts may also affect brain morphometry. Longitudinal analyses are perhaps the best approach to help us understand whether these alterations are a cause or consequence of suicide attempt. Other limitations include the lack of detailed information on suicide attempt method and lethality, as well as detailed information for sensitivity analyses such as antidepressant type and prescription regime used. Furthermore, there is a known non-disclosure effect driven by suicide attempt being considered a taboo across several cultures. This would cause some actual cases to be reported as controls. Thus, our power to detect different neural mechanisms would be hindered by both the non-disclosure effect and a lack of detailed clinical information. Finally, this study was performed within participants with depression, and care should be taken when generalising to other disorders.
This international collaboration has yielded valuable insights into the neurobiology of suicide. The number of associations discovered and their small effect sizes indicates that morphological differences between attempters and non-attempters will likely not be useful for clinical diagnosis or risk stratification. Even so, if several neural circuits underlie suicidal behaviours with relatively small effect sizes, the aggregation of them might still be useful for risk stratification. Heterogeneity, which can be increased by several factors including lack of information on the lethality of suicide attempt, also contributes to reduced power and might explain the small effect sizes. Thus, lowering heterogeneity, increasing sample size, examining more detailed suicide attempt related phenotypes such as number of attempts, lethality and age of first attempt and increasing the resolution of neuroimaging studies (e.g., to the vertex level) as well as integrating neuroimaging measurements with other modalities (e.g., functional imaging data), will help advance the field.
In summary, our results suggest that suicide attempt is associated with volumetric reductions within the thalamus, right pallidum and surface area reductions in the left interior parietal lobe, over and above the effects of depression alone. Our findings suggest that several regions are associated with suicide attempt, albeit with relatively small effect sizes. This study addressed the lack of replicability and consistency in several previously published neuroimaging studies of suicide attempt and further demonstrated the need for well-powered samples and collaborative efforts to avoid reaching biased or misleading conclusions.
Supplementary Material
KEY RESOURCE TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Deposited Data; Public Database | UK Biobank access under application number 25331 | https://www.ukbiobank.ac.uk/enable-your-research/apply-for-access | data fields: 26721-26921; 26923-26988; RRID: SCR_012815 | |
| Deposited Data; Public Database | ENIGMA MDDinidivudal cohort data (not publically available) | http://enigma.ini.usc.edu/ | SCR_005515 | |
| Software; Algorithm | R v 3.6.1 and packages nlme, ppcor, xlsx | https://cran.r-project.org/ | SCR_015655 | |
| Software; Algorithm | MATLAB r2019b | Mathworks | RRID:SCR_001622 | |
| Other |
ACKNOWLEDGEMENTS
The work reported here was supported in part by many public and private agencies across the world. Core funding for ENIGMA was provided by the NIH Big Data to Knowledge (BD2K) program under consortium grant U54 EB020403. AFFDIS was supported by the University Medical Center Göttingen and the German Federal Ministry of Education and Research (Bundesministerium fuer Bildung und Forschung, BMBF: 01 ZX 1507, “PreNeSt - e:Med”). The BiDirect study was supported by grants from the German Federal Ministry of Education and Research (BMBF; grants FKZ-01ER0816 and FKZ-01ER1506). ETPB study at NIH is funded by the National Institute of Mental Health Intramural Program. The FOR2107 study was supported by the German Research Foundation (DFG) grant Nos Ki588/14-1 and Ki588/14-2 to TK. The IMH Singapore cohort was supported by the National Healthcare Group Research Grant (SIG/15012) awarded to KS. The LOND study was supported in part by the Biomedical Research Centre, South London and Maudsley NHS Foundation Trust, London, UK. The Stanford study was supported in part by the National Institute of Mental Health Grant R37-MH101495 to IHG. The Melbourne study was supported by Australia’s National Health and Medical Research Council (NHMRC) Project Grants 1064643 (principal investigator, BJH) and 1024570 (principal investigator, CGD). The QTIM study was supported by the National Institute of Child Health and Human Development (R01-HD050735), and the National Health and Medical Research Council (NHMRC 486682, 1009064), Australia (principal investigator MJW). The Pharmo study in Amsterdam was supported by faculty resources of the Academic Medical Center, University of Amsterdam, and 11.32050.26 ERA-NET PRIOMEDCHILD FP 6 (EU). The UCSF study was funded in part by the National Institute of Mental Health (R01MH085734 and K01MH117442), National Center for Complementary and Integrative Health (NCCIH 1R61AT009864) and the American Foundation for Suicide Prevention (AFSP). The Edinburgh group is funded by a Wellcome Trust Strategic Award “Stratifying Resilience and Depression Longitudinally” (STRADL) (Reference 104036/Z/14/Z). The SHIP cohort is part of the Community Medicine Research Network of the University Medicine Greifswald, which is supported by the German Federal State of Mecklenburg- West Pomerania. AIC is supported by a UQ Research Training Scholarship from The University of Queensland (UQ). MER thanks the support of the NHMRC and Australian Research Council (ARC) through a Research Fellowship (APP1102821). BJH and CGD were supported by NHMRC Career Development Fellowships (1124472 and 1061757, respectively). SEM thanks funding support from NHMRC grants APP1103623, APP1158127, and APP1172917. TTY is funded in part by NCCIH 1R61AT009864. LS and NJ received support from the National Institute of Mental Health of the National Institutes of Health (USA) under Award Number R01-MH117601. This project used data from the UK-Biobank under application number 25331. This study was posted as a preprint in medRxiv to comply with open access requirements of funding institutions.
Financial disclosures
Hans J. Grabe has received travel grants and speakers honoraria from Fresenius Medical Care, Neuraxpharm, Servier, and Janssen Cilag as well as research funding from Fresenius Medical Care; Carlos A. Zarate is a full-time U.S. government employee. He is listed as a co-inventor on a patent for the use of ketamine and its metabolites in major depression and suicidal ideation. Dr. Zarate has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. Neda Jahanshad and Paul M. Thompson are MPI of a research related grant from Biogen, Inc., for work unrelated to this manuscript. Ian B. Hickie is funded by NHMRC Fellowship (2013-2017 and 2018-22).He is the Chief Scientific Advisor to, and a 5% equity shareholder in, InnoWell Pty Ltd. InnoWell was formed by the University of Sydney (45% equity) and PwC (Australia; 45% equity) to deliver the $30 M Australian Government funded Project Synergy (2017- 20. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript.
References
- 1.World Health Organization (n.d.): Suicide Data. World Health Organization. Retrieved January 27, 2021, from https://www.who.int/en/news-room/fact-sheets/detail/suicide [Google Scholar]
- 2.The Global Health Observatory, World Health Organisation (n.d.): Suicide mortality rate. World Health Statistics Data Visualizations Dashboard. World Health Organization. Retrieved January 27, 2021, from http://apps.who.int/gho/data/node.sdg.3-4-viz-2?lang=en [Google Scholar]
- 3.Lifeline Australia (n.d.): Statistics on Suicide in Australia. Retrieved January 27, 2021, from https://www.lifeline.org.au/about-lifeline/lifeline-information/statistics-on-suicide-in-australia
- 4.Zalsman G, Hawton K, Wasserman D, van Heeringen K, Arensman E, Sarchiapone M, et al. (2016): Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry 3: 646–659. [DOI] [PubMed] [Google Scholar]
- 5.Shepard DS, Gurewich D, Lwin AK, Reed GA Jr, Silverman MM (2016): Suicide and Suicidal Attempts in the United States: Costs and Policy Implications. Suicide Life Threat Behav 46: 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesney E, Goodwin GM, Fazel S (2014): Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 13: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertolote JM, Fleischmann A (2002): Suicide and psychiatric diagnosis: a worldwide perspective. World Psychiatry 1: 181–185. [PMC free article] [PubMed] [Google Scholar]
- 8.Rihmer Z (2007): Suicide risk in mood disorders. Curr Opin Psychiatry 20. 10.1097/YCO.0b013e3280106868 [DOI] [PubMed] [Google Scholar]
- 9.Stone DM, Simon TR, Fowler KA, Kegler SR, Yuan K, Holland KM, et al. (2018): Vital Signs: Trends in State Suicide Rates - United States, 1999-2016 and Circumstances Contributing to Suicide - 27 States, 2015. MMWR Morb Mortal Wkly Rep 67: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pompili M (2018, August 11): The increase of suicide rates: the need for a paradigm shift. The Lancet, vol. 392. pp 474–475. [DOI] [PubMed] [Google Scholar]
- 11.Domínguez-Baleón C, Gutiérrez-Mondragón LF, Campos-González AI, Rentería ME (2018): Neuroimaging Studies of Suicidal Behavior and Non-suicidal Self-Injury in Psychiatric Patients: A Systematic Review. Front Psychiatry 9: 500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Structural and functional neuroimaging studies of the suicidal brain (2011): Prog Neuropsychopharmacol Biol Psychiatry 35: 796–808. [DOI] [PubMed] [Google Scholar]
- 13.Schmaal L, Veltman DJ, van Erp TGM, Samann PG, Frodl T, Jahanshad N, et al. (2016): Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 21: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. (2018): Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry 23: 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. (2018): Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry 84. 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlösser RG (2011): Structural brain alterations in patients with major depressive disorder and high risk for suicide: evidence for a distinct neurobiological entity? Neuroimage 54. 10.1016/j.neuroimage.2010.08.082 [DOI] [PubMed] [Google Scholar]
- 17.Taylor WD, Boyd B, McQuoid DR, Kudra K, Saleh A, MacFall JR (2015): Widespread white matter but focal gray matter alterations in depressed individuals with thoughts of death. Prog Neuropsychopharmacol Biol Psychiatry 62. 10.1016/j.pnpbp.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosnell SN, Velasquez KM, Molfese DL, Molfese PJ, Madan A, Fowler JC, et al. (2016): Prefrontal cortex, temporal cortex, and hippocampus volume are affected in suicidal psychiatric patients. Psychiatry research Neuroimaging 256. 10.1016/j.pscychresns.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang JP, Lee TW, Tsai SJ, Chen TJ, Yang CH, Lirng JF, Tsai CF (2010): Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. J Geriatr Psychiatry Neurol 23. 10.1177/0891988710363713 [DOI] [PubMed] [Google Scholar]
- 20.Besteher B, Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Schlösser R, et al. (2016): Pronounced prefronto-temporal cortical thinning in schizophrenia: Neuroanatomical correlate of suicidal behavior? Schizophr Res 176. 10.1016/j.schres.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 21.Giakoumatos CI, Tandon N, Shah J, Mathew IT, Brady RO, Clementz BA, et al. (2013): Are Structural Brain Abnormalities Associated With Suicidal Behavior In Patients With Psychotic Disorders? J Psychiatr Res 47: 1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlösser RG (2012): Prefrontal cortical thickness in depressed patients with high-risk for suicidal behavior. J Psychiatr Res 46. 10.1016/j.jpsychires.2012.07.013 [DOI] [PubMed] [Google Scholar]
- 23.Lijffijt M, Rourke ED, Swann AC, Zunta-Soares GB, Soares JC (2014): Illness-course modulates suicidality-related prefrontal gray matter reduction in women with bipolar disorder. Acta Psychiatr Scand 130. 10.1111/acps.12314 [DOI] [PubMed] [Google Scholar]
- 24.Rüsch N, Spoletini I, Wilke M, Martinotti G, Bria P, Trequattrini A, et al. (2008): Inferior frontal white matter volume and suicidality in schizophrenia. Psychiatry Res 164. 10.1016/j.pscychresns.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 25.Aguilar EJ, García-Martí G, Martí-Bonmatí L, Lull JJ, Moratal D, Escartí MJ, et al. (2008): Left orbitofrontal and superior temporal gyrus structural changes associated to suicidal behavior in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 32. 10.1016/j.pnpbp.2008.06.016 [DOI] [PubMed] [Google Scholar]
- 26.Soloff PH, Pruitt P, Sharma M, Radwan J, White R, Diwadkar VA (2012): Structural brain abnormalities and suicidal behavior in borderline personality disorder. J Psychiatr Res 46. 10.1016/j.jpsychires.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soloff P, White R, Diwadkar VA (2014): Impulsivity, aggression and brain structure in high and low lethality suicide attempters with borderline personality disorder. Psychiatry Res 222: 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Heeringen C, Bijttebier S, Godfrin K (2011): Suicidal brains: a review of functional and structural brain studies in association with suicidal behaviour. Neurosci Biobehav Rev 35. 10.1016/j.neubiorev.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 29.Pompili M, Innamorati M, Mann JJ, Oquendo MA, Lester D, Del Casale A, et al. (2008): Periventricular white matter hyperintensities as predictors of suicide attempts in bipolar disorders and unipolar depression. Prog Neuropsychopharmacol Biol Psychiatry 32. 10.1016/j.pnpbp.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 30.Schmaal L, van Harmelen A-L, Chatzi V, Lippard ETC, Toenders YJ, Averill LA, et al. (2020): Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies. Mol Psychiatry 25: 408–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rentería ME, Schmaal L, Hibar DP, Couvy-Duchesne B, Strike LT, Mills NT, et al. (2017): Subcortical brain structure and suicidal behaviour in major depressive disorder: a meta-analysis from the ENIGMA-MDD working group. Transl Psychiatry 7: e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Torre D, Della Torre A, Chirchiglia D, Volpentesta G, Guzzi G, Lavano A (2020): Deep brain stimulation for treatment-resistant depression: a safe and effective option. Expert Rev Neurother 20. 10.1080/14737175.2020.1749049 [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association (2013): Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- 34.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- 35.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 36.Website (n.d.): Retrieved January 27, 2021, from Benjamini Y, & Hochberg Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57 (1), 289–300. Retrieved January 27, 2021, from http://www.jstor.org/stable/2346101 [Google Scholar]
- 37.Nyholt DR (2004): A Simple Correction for Multiple Testing for Single-Nucleotide Polymorphisms in Linkage Disequilibrium with Each Other. Am J Hum Genet 74: 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Ji L (2005): Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95. 10.1038/sj.hdy.6800717 [DOI] [PubMed] [Google Scholar]
- 39.Vang FJ, Ryding E, Träskman-Bendz L, van Westen D, Lindström MB (2010): Size of basal ganglia in suicide attempters, and its association with temperament and serotonin transporter density. Psychiatry Res 183: 177–179. [DOI] [PubMed] [Google Scholar]
- 40.Jia Z, Wang Y, Huang X, Kuang W, Wu Q, Lui S, et al. (2014): Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci 39: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z, Zhang H, Jia Z, Zhong J, Huang X, Du M, et al. (2015): Magnetization transfer imaging of suicidal patients with major depressive disorder. Sci Rep 5: 9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann JJ, Rizk MM (2020): A Brain-Centric Model of Suicidal Behavior. Am J Psychiatry 177. 10.1176/appi.ajp.2020.20081224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Napier TC, Mickiewicz AL (2010): The role of the ventral pallidum in psychiatric disorders. Neuropsychopharmacology 35: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith KS, Tindell AJ, Wayne Aldridge J, Berridge KC (2009): Ventral Pallidum Roles in Reward and Motivation. Behav Brain Res 196: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim K, Shin J-H, Myung W, Fava M, Mischoulon D, Papakostas GI, et al. (2019): Deformities of the Globus Pallidus are Associated with Severity of Suicidal Ideation and Impulsivity in Patients with Major Depressive Disorder. Sci Rep 9: 7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conner KR, Meldrum S, Wieczorek WF, Duberstein PR, Welte JW (2004): The association of irritability and impulsivity with suicidal ideation among 15- to 20-year-old males. Suicide Life Threat Behav 34: 363–373. [DOI] [PubMed] [Google Scholar]
- 47.Smith AR, Witte TK, Teale NE, King SL, Bender TW, Joiner TE (2008): Revisiting impulsivity in suicide: implications for civil liability of third parties. Behav Sci Law 26: 779–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang K, Bertolero MA, Liu WB, D’Esposito M (2017): The Human Thalamus Is an Integrative Hub for Functional Brain Networks. J Neurosci 37: 5594–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolff M, Vann SD (2019): The Cognitive Thalamus as a Gateway to Mental Representations. J Neurosci 39: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haber SN, Calzavara R (2009): The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull 78. 10.1016/j.brainresbull.2008.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balleine BW, Morris RW, Leung BK (2015): Thalamocortical integration of instrumental learning and performance and their disintegration in addiction. Brain Res 1628: 104–116. [DOI] [PubMed] [Google Scholar]
- 52.Bielau H, Trübner K, Krell D, Agelink MW, –G. Bernstein H, Stauch R, et al. (2005): Volume deficits of subcortical nuclei in mood disorders. Eur Arch Psychiatry Clin Neurosci 255: 401–412. [DOI] [PubMed] [Google Scholar]
- 53.Pinault D (2011): Dysfunctional thalamus-related networks in schizophrenia. Schizophr Bull 37: 238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. (2014): Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex 24: 3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter M, Amunts K, Mohlberg H, Bludau S, Eickhoff SB, Zilles K, Caspers S (2019): Cytoarchitectonic segregation of human posterior intraparietal and adjacent parieto-occipital sulcus and its relation to visuomotor and cognitive functions. Cereb Cortex 29. 10.1093/cercor/bhy245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allan PG, Briggs RG, Conner AK, O’Neal CM, Bonney PA, Maxwell BD, et al. (2019): Parcellation-based tractographic modeling of the dorsal attention network. Brain Behav 9. 10.1002/brb3.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torrey EF (2007): Schizophrenia and the inferior parietal lobule. Schizophr Res 97. 10.1016/j.schres.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 58.Benedetti F, Radaelli D, Poletti S, Locatelli C, Falini A, Colombo C, Smeraldi E (2011): Opposite effects of suicidality and lithium on gray matter volumes in bipolar depression. J Affect Disord 135: 139–147. [DOI] [PubMed] [Google Scholar]
- 59.Elliott LT, Sharp K, Alfaro-Almagro F, Shi S, Miller KL, Douaud G, et al. (2018): Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grasby KL, Jahanshad N, Painter JN, Colodro-Conde L, Bralten J, Hibar DP, et al. (2020): The genetic architecture of the human cerebral cortex. Science 367. 10.1126/science.aay6690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hibar DP, Stein JL, Renteria ME, Arias-Vasquez A, Desrivières S, Jahanshad N, et al. (2015): Common genetic variants influence human subcortical brain structures. Nature 520: 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGirr A, Renaud J, Bureau A, Seguin M, Lesage A, Turecki G (2008): Impulsive-aggressive behaviours and completed suicide across the life cycle: a predisposition for younger age of suicide. Psychol Med 38. 10.1017/S0033291707001419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


