Abstract
Dexmedetomidine has been increasingly introduced into the perioperative care of surgical patients. Because a subset of anesthetics/sedatives are immunomodulatory, it is critical to understand the role of dexmedetomidine in our host immune functions. Here we reviewed the role of dexmedetomidine in different immune cells. We also reviewed published clinical articles that described the role of dexmedetomidine in organ injury, cancer surgery, and infection. In animal studies, dexmedetomidine attenuated organ injury. In clinical studies, dexmedetomidine was associated with an improvement in outcomes in cardiac surgery and transplant surgery. However, there is a paucity in research examining how dexmedetomidine is associated with these outcomes. Further studies are needed to understand its clinical application from immunological standpoints.
Keywords: Dexmedetomidine, leukocyte, outcome
Introduction
Dexmedetomidine is a highly selective α2 adrenergic agonist (α2: α1 selectivity of 1,600:1) with an imidazole ring (Ma, et al. 2004). It is a dextro-enantiomer of medetomidine. Since its approval for sedation use in intensive care units in 1999, dexmedetomidine has been increasingly used in the perioperative setting. Its pharmacological action comes from its interaction with both α2 adrenergic receptors and imidazoline receptors. The α2 receptors are G-protein coupled receptors that take norepinephrine and epinephrine as ligands. The rank order of their affinity to the α2 receptors is epinephrine >= norepinephrine (Tank and Lee Wong 2015). Their binding to the ligands results in coupling with pertussis toxin-sensitive Gi/G0 proteins to attenuate adenylyl cyclase, suppress voltage-gated Ca2+ channels and activate inwardly rectifying K+ channel for cellular hyperpolarization (Limbird 1988). The α2 receptors are expressed on the central and peripheral nervous systems as well as other cells and tissues such as leukocytes and endothelial cells. Classically the α2 adrenergic receptors were identified as presynaptic autoreceptors on the sympathetic nerve terminals, inhibiting norepinephrine release (Starke 2001). However, they are also expressed post-synaptically. Imidazoline receptors consist of three subtypes: I1R is expressed in the brain nucleus and on the endothelial cells to regulate blood pressure. I2R is expressed on the adrenal gland, fat and muscle tissues to regulate glucose use in the tissue. I3R is expressed on the pancreatic cells to regulate insulin secretion (Bousquet, et al. 2020). Dexmedetomidine’s major effects include hypnotic/analgesic effect as well as cardiovascular effects. Sedation is induced by stimulating the α2 adrenergic receptor in the locus coeruleus (LC) in the pons (Khan, et al. 1999). In the central nervous system, neuromodulators are released by neurons to alter the cellular properties of target neurons and the efficacy of their synaptic transmission (Sara 2009). The main neuromodulators include serotonin, acetylcholine, dopamine and norepinephrine. The LC clusters noradrenergic neurons. The LC, composed of only 1,500 neurons in the rat, sends projections to most brain regions including the brainstem, the cerebellum, the diencephalon and the paleo- and neocortex (Jones, et al. 1977). Analgesic effect is caused by the stimulation on the α2 adrenergic receptor in the supra-spinal and spinal sites (Fig. 1). Cardiovascular effects are derived from its direct effect on the vascular α2 receptor as well as from its indirect effect on the sympathetic nerves governing the heart.
Figure 1. The effect of dexmedetomidine on HP A axis and SAM axis.

HPA, hypothalamus-pituitary-adrenal; SAM, sympathetic-adreno-medullar ;CRH, corticotrophin-releasing hormone ;ACTH, adrenocorticotrophic hormone
The immunomodulatory properties of sedatives and anesthetics have been increasingly recognized (Carbo, et al. 2013; Koutsogiannaki, et al. 2019; Koutsogiannaki, et al. 2017; Mitsui, et al. 2020; Okuno, et al. 2019; Yuki, et al. 2013; Yuki, et al. 2012; Yuki, et al. 2020). Without exception, the immunomodulatory effect of dexmedetomidine has been investigated. Here we will review the role of dexmedetomidine in perioperative immune functions.
Perioperative stress responses
Perioperative stresses lead to metabolic, endocrinological and immunological changes in surgical patients. A surgical insult triggers a central response via afferent nerves to activate both the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) axis. The HPA axis activates the pituitary gland followed by the adrenal gland, the latter of which produces cortisol. Immune responses have been shown to be modulated by the peripheral nervous system, notably by the autonomic nervous system. The autonomic nervous system can be classified into the sympathetic nervous system, the parasympathetic nervous system, and the enteric nervous system. Among them, a sympathetic nervous system represents a major pathway involved in the crosstalk between neurons and immune system (Scanzano and Cosentino 2015). The SAM axis activates the secretion of catecholamines systemically by the adrenal gland and locally by the sympathetic nerves. The sympathetic nerves emerge from the thoracolumbar spinal cord and produce catecholamines. In contrast, the extent to which the parasympathetic neurons regulate immunity remains unresolved. The sympathetic nervous systems innervate a number of lymphoid organs and mucosa (Godinho-Silva, et al. 2019). The intensity of surgical stress depends on the severity and duration of tissue injury and may be reflected by the degree of the secretion of pituitary hormones and the activation of the sympathetic nervous system (Wang, et al. 2015).
Catecholamines and cortisol play an important role in redistributing leukocytes. Epinephrine and norepinephrine induce a redistribution of neutrophils, monocytes and T cells from the marginated pool such as spleen, lungs, lymph nodes, and bone marrows into the bloodstream and temporarily increase blood leukocyte counts (Dhabhar, et al. 2012). Then, cortisol induces the movement of monocytes and T cells out of the blood stream to the surgical site or back to their origins. In contrast, neutrophil count continues to increase as a result of emergency granulopoiesis. This is in line with our general finding in the perioperative period that neutrophil number increases and NK cell and T cell number decreases postoperatively (Bartal, et al. 2010; Gaudilliere, et al. 2014). Catecholamines and cortisol also serve to modulate cytokine profiles. Proinflammatory responses are usually dominant at the surgical site first because cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-8 are primarily secreted from resident tissue macrophages. Subsequently these cytokines activate and recruit neutrophils and monocytes to inflammatory sites by interacting with their cytokine receptors (Andersson and Tracey 2011; Stoecklein, et al. 2012). Glucocorticoid receptors are expressed in neutrophils, monocytes, macrophages, T cells and B cells and cortisol shifts them to the cells with anti-inflammatory phenotype (Elenkov 2004). Catecholamine receptors are found in monocytes, macrophages, natural killer (NK) cells, B cells and T cells, and their stimulation induces anti-inflammatory responses (Glaser and Kiecolt-Glaser 2005). Anti-inflammatory responses are induced most potently by epinephrine, followed by norepinephrine, and least by cortisol (Elenkov, et al. 2008). Anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β induce regulatory T cells, a subset of Cluster of differentiation (CD) 4+ T cells with suppressive activity, from a pool of CD4+ T cells (Brenu, et al. 2013), and these regulatory T cells also bias CD4+ T cells toward Th2 cells, which are anti-inflammatory (Marik and Flemmer 2012).
The effect of dexmedetomidine on proinflammatory and anti-inflammatory cytokine production and leukocyte number in surgical patients
A meta-analysis of patient studies by Wang et al. demonstrated that intraoperative use of dexmedetomidine infusion was associated with 1) lower plasma epinephrine, norepinephrine and cortisol levels, 2) the increased number of NK cells, B cells and CD4 cells, and the decreased number of CD8 cells, and 3) increased ratios of CD4+: CD8+ and Th1: Th2 cells, and 4) decreased TNF-α, IL-6 and increased IL-10 levels (Wang, et al. 2019).
The reduction of epinephrine and norepinephrine by dexmedetomidine is intuitive given the action of dexmedetomidine on the SAM axis as described above (Fig. 1). Regarding the HPA axis, it is known that the α2 adrenergic receptors are expressed on the pineal gland (Munoz-Hoyos, et al. 2000). This will likely lead to reduced adrenocorticotropin (ACTH) and cortisol levels under dexmedetomidine (Fig. 1).
The aforementioned NK, B, T cell number change under dexmedetomidine can be explained by the fact that the effect of catecholamine and cortisol on their mobilization was attenuated because of the less catecholamine and cortisol levels. How about neutrophil number under dexmedetomidine? So far perioperative changes of neutrophil counts under dexmedetomidine has not been reported yet. Because neutrophil number in the perioperative period is largely influenced by emergency granulopoiesis, we need to know about if dexmedetomidine affects this process.
How can we explain cytokine profiles under dexmedetomidine? Although we may expect that cytokines should be proinflammatory rather than anti-inflammatory under dexmedetomidine because anti-inflammatory prone catecholamine and cortisol levels are attenuated, dexmedetomidine administration was associated with lower TNF-α, IL-6 and increased IL-10 levels in surgical patients. This may suggest that dexmedetomidine directly affects cytokine producing cells such as monocytes/macrophages. In the followings, we will review the effect of dexmedetomidine on individual leukocytes.
The expression of α2-adrenergic receptors on immune cells
The α2 receptors consist of three subtypes; α2A, α2B and α2C. The α2A subtype is expressed widely throughout both the nervous system and peripheral tissues. The α2B subtype is expressed primarily in the periphery, with the highest amounts in the kidney. The α2c subtype is expressed primarily in the central nervous system, although small amounts are present in the kidney (Link, et al. 1996). The corresponding gene names for α2A, α2B and α2C are adra2a, adra2b and adra2c, respectively. As their mRNA expression patterns are shown (Fig. 2), they are expressed on the majority of leukocyte subtypes.
Figure 2. mRNA expression profiles of the α2 adrenergic receptor subtypes on leukocyte.
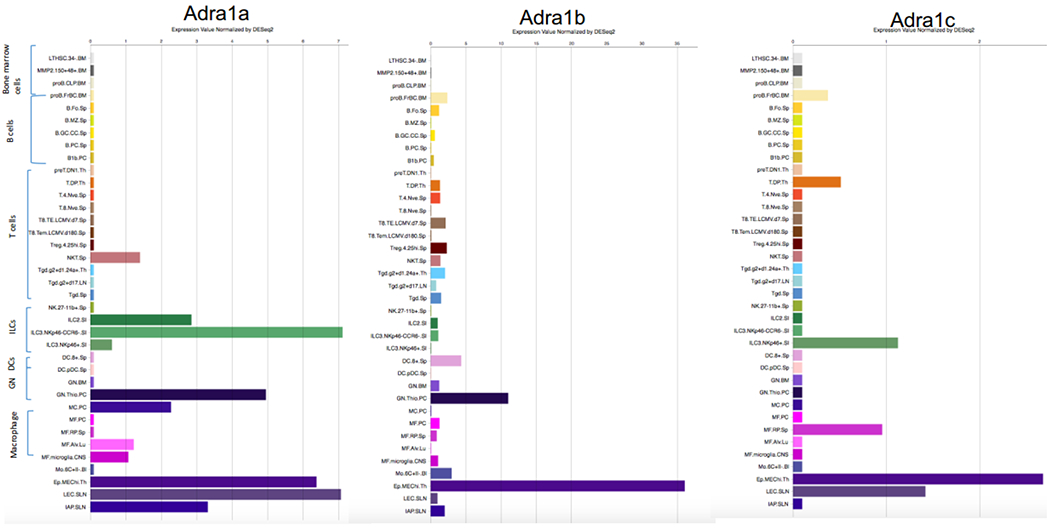
ILC, innate lymphoid cell ;DC, dendritic cell ;GN, granulocyte
Imidazoline receptors are primarily expressed on non-leukocytes. Although their association with spingosine 1-phosphate (SIP) receptors has been suggested, the role of imidazoline receptors in immunological responses is still not well determined yet (Feher, et al. 2017). Therefore, we will describe the role of dexmedetomidine in immunological responses primarily from the α2 adrenergic receptors in the followings.
Neutrophils
Neutrophils are the first-line defense immune cells against tissue injury and invading pathogens via rapid mobilization, engulfment, intracellular killing, release of antimicrobial factors and neutrophil extracellular traps. In mRNA level, human neutrophils express β3 > β2 > α1A > α1B ~ α2A ~ β1 = α2C (Scanzano, et al. 2015). Among the α2 subtypes, neutrophils express α2A on protein level (Herrera-Garcia, et al. 2014).
The effect of dexmedetomidine on neutrophils was previously studied by co-incubating neutrophils with dexmedetomidine in vitro. The study by Nishina et al. did not find any effect of dexmedetomidine at clinically relevant concentrations on neutrophil phagocytosis, chemotaxis or superoxide production (Nishina, et al. 1999). Similarly, the study by Chen et al. did not find any effect of dexmedetomidine at clinically relevant concentrations on neutrophil respiratory burst (Chen, et al. 2016). Although circulating epinephrine and norepinephrine are primarily released from the adrenal gland and the sympathetic ganglia, they have been identified within human neutrophils as well (Cosentino, et al. 1999). This may indicate that neutrophils also synthesize and release catecholamines. The contribution of neutrophil derived catecholamine in neutrophil effector functions is likely very minimal, however, even if adrenergic receptors are involved in catecholamine production.
Despite the very minimal effect of dexmedetomidine in vitro, in the murine acute inflammation model using the air pouch, α2 adrenergic agonists xylazine and UK14304 increased the resistance of neutrophil extravasation from the endothelial cells (Herrera-Garcia, et al. 2014). This was explained by the attenuation of intercellular adhesion molecule-1 (ICAM-1) expression on the endothelial cells by these α2 agonists, rather than their effects on neutrophils. Inada et al. tested the effect of dexmedetomidine in the air pouch model (Inada, et al. 2017). They found neutrophil recruitment was significantly reduced by dexmedetomidine administration at least in part due to the reduction of neutrophil chemoattractants CXCL1 and CXCL2 production. The authors speculated that CXCL1 and CXCL2 would be produced in the lining cells such as mast cells in this model. Although these murine experiments supported the in vivo effect of dexmedetomidine on neutrophil functions, these phenotypes are likely caused indirectly, which is in line with the aforementioned in vitro studies. So far, the effect of dexmedetomidine on neutrophils in vivo was primarily studied in the context of neutrophil recruitment.
Norepinephrine seems to be inhibitory for neutrophil activation. Norepinephrine was shown to suppress neutrophil chemotaxis, activation and phagocytosis (Nicholls, et al. 2018). Because dexmedetomidine administration is associated with a reduction in norepinephrine level, it is possible that dexmedetomidine administration may attenuate the reduction of neutrophil phagocytosis, which would occur in the perioperative setting. In the experimental polymicrobial abdominal sepsis model induced by cecal ligation and puncture (CLP) surgery, dexmedetomidine administration was associated with a better survival associated with decreased proinflammatory cytokine levels than non-exposure arm (Chen, et al. 2015; Wu, et al. 2020). However, bacterial loads or neutrophil effector functions were not compared in these infection models. Thus, it is important to determine in the future whether or not neutrophil effector functions against microbes are better in vivo under dexmedetomidine.
Monocytes/ Macrophages
Monocytes and macrophages are key components of innate immune cells. They can phagocytize and present antigens. Monocytes and macrophages are also major cytokine producing cells. The α2 adrenergic receptors are expressed on monocytes and macrophages (Fig. 2) (Piazza, et al. 2016).
Perioperative dexmedetomidine has been shown to be associated with less TNF-α, IL-6 and IL-8 levels immediately and one day after surgery, and with higher IL-10 level one day after surgery in a meta-analysis by Li et al (Li, et al. 2015) (Fig. 3). In line, dexmedetomidine attenuated the production of TNF-α, IL-6 and IL-8 in lipopolysaccharide (LPS) stimulated whole blood in vitro (Kawasaki, et al. 2013). Similarly norepinephrine and clonidine also attenuated the production of TNF-α, IL-6 and IL-8 in LPS stimulated whole blood in vitro (Maes, et al. 2000). Then, can the reduced TNF-α, IL-6 and IL-8 levels be explained by the direct effect of dexmedetomidine on monocytes/ macrophages?
Figure 3. The effect of dexmedetomdine on local and systemic responses.
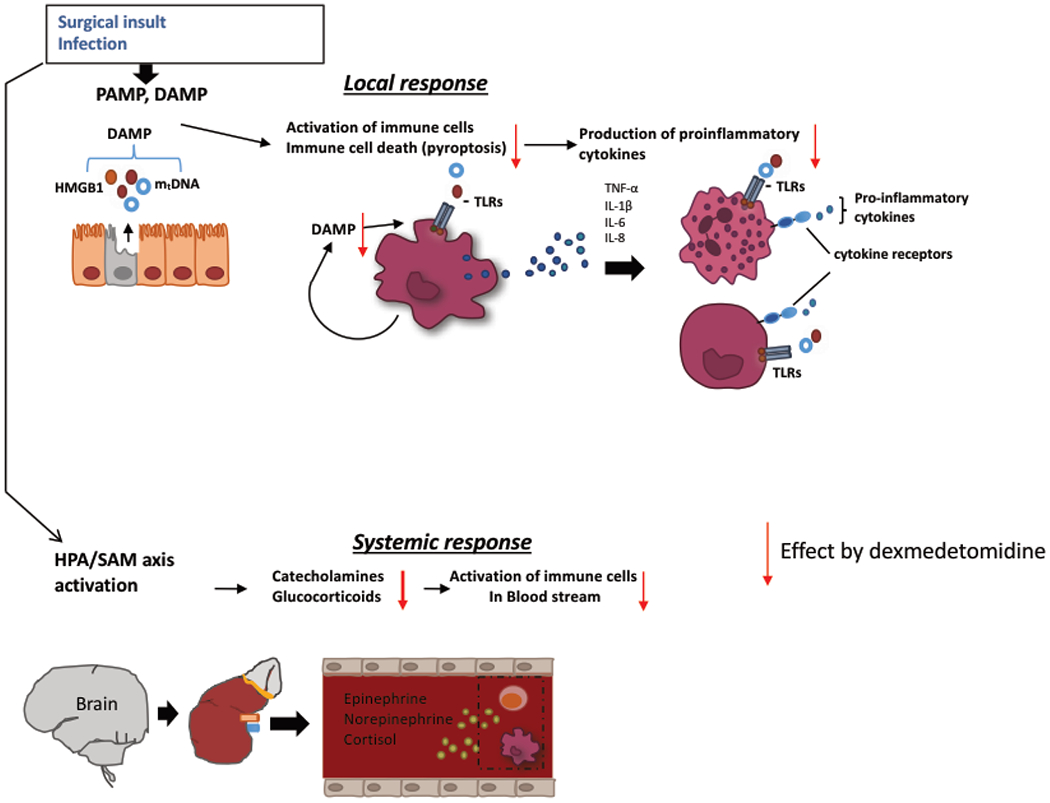
DAMP, damage-associated molecular pattern ; PAMP, pathogen-associated molecular pattern; HMGB1, high mobility group box 1; mtDNA, mitochondorial DNA ; TLR, toll-like receptor
In monocytes and macrophages, TNF-α, IL-6 and IL-8 are produced via the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling pathway. The study by Li et al. showed that dexmedetomidine (1 μM) attenuated NFκB-p65 phosphorylation, and TNF-α and IL-1β production from LPS-stimulated murine BV-2 microglial cells and RAW264.7 macrophage cells (Li, et al. 2020). The study by Sun et al. also showed that dexmedetomidine attenuated proinflammatory cytokine production in BV-2 cells (Sun, et al. 2018). The recently study by Zhou et al. also showed that dexmedetomidine treatment attenuated TNF-a and IL-6 production and enhanced IL-10 secretion from bone marrow-derived macrophages (BMDMs) stimulated by LPS (Zhou, et al. 2020). In contrast, Piazza et al. tested the effect of dexmedetomidine on proinflammatory cytokines in human primary alveolar macrophages and found that dexmedetomidine did not affect the level of proinflammatory cytokines (Piazza, et al. 2016). Thus, the effect of dexmedetomidine on proinflammatory cytokine production may be dependent on the type of cells. Further clarification is required to determine whether or not dexmedetomidine directly affects the production of proinflammatory cytokines by macrophages and monocytes. Because IL-10 suppresses TNF-α, IL-6 and IL-8 via the signaling through IL-10 receptor (de Waal Malefyt, et al. 1991; van der Poll, et al. 1994; Wang, et al. 1994), the cytokine profile on postoperative day one may be explained merely by elevated IL-10. Because IL-10 can be produced by a number of immune cells which use different promotor regions depending on the type of cells, it is also important to determine how IL-10 production was increased under dexmedetomidine in vitro and in vivo.
Dexmedetomidine affects other monocyte and macrophage functions? In the study by Zhou et al., dexmedetomidine promoted BMDMs M2 activation as evidenced by increased Arg1 and Mrc1 gene induction and decreased iNOS gene induction (Zhou, et al. 2020). Dexmedetomidine attenuated the production of reactive oxygen species (ROS) in LPS stimulated U937 monocyte cells in vitro (Chai, et al. 2020). In addition, dexmedetomidine attenuated the adhesion of U937 cells to the endothelial cells by reducing the expression of adhesion molecules lymphocyte function-associated antigen-1 (LFA-1) and very late activation antigen-4 (VLA-4) in vitro. Both functional alternation under dexmedetomidine was considered to be due to the reduction of connexin Cx43. The interaction between the α2 adrenergic receptor and Cx43 has not been determined yet. The effect of dexmedetomidine on in vivo monocyte function was examined about phagocytosis. Dexmedetomidine attenuated monocyte phagocytosis in LPS stimulated mice (Wu, et al. 2015). Other monocyte functions need to be examined in vivo.
NK cells
NK cells are a phenotypically distinct population of lymphocytes (CD56+/CD3−) that eradicate tumor cells or microbes using constitutively expressed lytic machinery independent of prior immunization (Vivier, et al. 2008). NK cells also express the α2 receptors (Fig. 2) (Jetschmann, et al. 1997; Xiao, et al. 2010).
Gratz et al. examined NK cell function perioperatively. NK cell cytotoxicity was attenuated following surgery using K562 tumor cells as target cells. However, dexmedetomidine administration attenuated the reduction of NK cell cytotoxicity in patients receiving general anesthesia (Gratz 1999). However, dexmedetomidine did not affect NK cell cytotoxicity against K562 cells in vitro (Tazawa, et al. 2017). NK cell function is subjected to the modulation by the central nervous system (CNS). Cortisol produced by the HPA axis attenuates NK cell cytotoxicity (Capellino, et al. 2020). Because cortisol level increases under surgical stress, a reduction in NK cell function in the perioperative period can be explained at least in part by the cortisol production. Because dexmedetomidine infusion attenuates cortisol secretion in patients and in vitro experiment did not affect NK cell cytotoxicity, the preservation of NK cell cytotoxicity by dexmedetomidine in patients is largely explained by its CNS effect.
T cells
Like other leukocytes, T cells also express the α2 adrenergic receptors (Fig. 2)(Heng, et al. 2008). The subtype of T helper cells consists of precursor helper T (Th0), T helper 1 (Th1), T helper 2 (Th2), T helper 17 (Th17) and regulatory T (Treg) cells. Th1 cells produce IFN-γ and favor cell-mediated immune responses (Kurosawa and Kato 2008). Th2 cells produce IL-4 and/or IL-10 and favor humoral immunity by antibody production. Th17 cells are differentiated in an IL-23 dependent manner and produces IL-17 (Webster and Galley 2009). Treg cells are important for the induction and maintenance of peripheral tolerance, therefore preventing excessive immune responses and autoimmunity (Romano, et al. 2019). Th1/Th2 and Th17/Treg ratios can be indicated by IFN-γ/IL-4 and IL-17/IL-10 ratios, respectively. Dexmedetomidine administration was associated with IFN-γ/IL-4 and IL-17/IL-10 ratios, which indicated a shift toward Th1 and Th17 (Lee, et al. 2018). The study by Wang et al. showed that dexmedetomdine arm has higher Th1 cells and Treg cells (Wang, et al. 2021). Th17 population was not examined in the study by Wang et al.
In addition, dexmedetomidine was associated with the expression of programmed cell death-1 (PD-1) and its ligand (PD-L1) on T cells (Wang, et al. 2021). Because stress responses can lead to immunosuppressive T cell phenotype with PD-1 expression (Qiao, et al. 2018), this can be likely explained by the attenuation of stress responses by dexmedetomidine. Dexmedetomidine did not attenuate IL-2 production from T cells or proliferation in vitro (Yuki, et al. 2011). However, the experiment using splenocytes explanted from mice receiving dexmedetomidine showed that dexmedetomidine attenuated T cell proliferation (Wu, et al. 2015). It is unclear if in vivo exposure of dexmedetomidine altered T cell phenotype. Overall the effect of dexmedetomidine on T cell function remains to be studied further. The effect of dexmedetomidine on B cells was limitedly studies, and we will not discuss here.
Dexmedetomidine and immunological outcomes
Overall dexmedetomidine administration attenuates perioperative stress responses, favoring anti-inflammatory state. Whether or not dexmedetomidine administration affects clinical immunological outcomes is an important topic. A large number of studies have been done in preclinical models but here we primarily focus on clinical studies.
Cancer outcome
The association between the choice of anesthetic regimens/ drugs and cancer outcomes is one of the most studied areas (Hiller, et al. 2018). For example, a number of studies compared the outcome of patients undergoing cancer resection surgeries either under volatile anesthetic-based general anesthesia or intravenous anesthetic-based general anesthesia (Yap, et al. 2019). The negative effect of perioperative stress on cancer outcome was also shown (Armaiz-Pena, et al. 2013; Ben-Eliyahu 2003). Dexmedetomidine can attenuate stress response and may be an advantageous drug in cancer resection surgery from this aspect. Perioperative impaired NK cell function is also associated with cancer recurrence (Freeman and Buggy 2018), but dexemedetomidine seems to preserve it (Gratz 1999). Dexmedetomidine promotes M2 macrophage polarization. M2 macrophages can facilitate angiogenesis and tumor growth (Jayasingam, et al. 2019).
Levon et al. tested the effect of dexmedetomidine in tumor growth in the rodent tumor model. In contrast to the prediction, the size of breast, lung and colon cancer was significantly larger in mice receiving dexmedetomidine (Bruzzone, et al. 2008; Lavon, et al. 2018; Szpunar, et al. 2013). In these studies, dexmedetomidine administration induced tumor growth and metastasis by directly activating the α2 adrenergic receptors on tumor cells or by activating the α2 adrenergic receptors on host stromal cells. Other explanation is by promotion of M2 macrophages.
In consistent with the animal study, the study of 1,404 patients by Cata et al. showed that intraoperative dexmedetomidine administration was associated with a decrease in the overall survival of propensity-matched patients after non-small cell lung cancer surgery (Cata, et al. 2017). However, more studies are needed to know about its clinical impact in cancer surgery.
Organ injury
A subset of invasive surgeries such as cardiac surgery and transplant surgery are known to be associated with high incidence of morbidities and mortalities. Ischemia-reperfusion involving these surgeries is one of major causes for morbidities and mortalities. Damage-associated molecular pattern (DAMP) molecules such as high mobility group box 1 (HMGB1) are released from injured cells. DAMPs activate cells via various pattern recognition receptors such as Toll-like receptors (TLRs) and the receptor for advanced glycation end products (RAGE) on various cells, which can lead them into cell death including pyroptosis, apoptosis and necrosis.
Pyroptosis is a caspase-1 dependent programmed cell death, and dexmedetomidine was shown to demonstrate cyto-protective property by attenuating pyroptosis (Ji, et al. 2019) (Fig. 3).
The study of a total of 1,134 patients undergoing coronary artery bypass (CABG) surgeries and CABG plus valvular surgeries by Li et al. showed that perioperative dexmedetomidine use was associated with a decrease in postoperative mortality up to one year and decreased incidence of postoperative complications (Ji, et al. 2013). Another study of 724 patients undergoing CABG surgeries by the same group also showed that perioperative dexmedetomidine use was associated with better in-hospital, 30-day and 1-year survival rates (Ji, et al. 2014). The similar result was shown by Cheng et al (Cheng, et al. 2016). In infant cardiac surgery, neurological complications post cardiopulmonary bypass as well as perioperative sedation are one of serious comorbidities (Whiting, et al. 2015). Dexmedetomidine has shown to be neuroprotective in animal models (Rajakumaraswamy, et al. 2006; Sifringer, et al. 2015), and the role of dexmedetomidine in infant cardiac surgery has been under evaluation currently (Zuppa, et al. 2019).
In the study of 780 patients undergoing kidney transplantation by Chen et al., dexmedetomidine administration was associated with a decrease in delayed graft failure, risk of acute rejection, infection and overall complications in the early post-transplantation phase (Chen, et al. 2020). Furthermore, in a meta-analysis of 20 studies by Biccard et al., dexmedetomidine use was associated with a trend towards improved all-cause mortality, non-fatal myocardial infarction and myocardial ischemia (Biccard, et al. 2008). The results from these clinical studies are in line with the cyto-protective property of dexmedetomidine.
Infection
Although dexmedetomidine administration favored CLP sepsis as described above, it is unclear if the outcome result could be explained by the attenuation of proinflammatory cytokine production by dexmedetomidine or due to other reasons. In LPS model, dexmedetomidine attenuated neurological damage (Sun, et al. 2019). Pathogen-associated molecular pattern (PAMP) molecules such as LPS activate inflammatory cells and can induce cell death. Dying cells can release DAMPs. Sun et al. showed that dexmedetomidine not only decreased cell death (pyroptosis) by LPS but also decreased the release of DAMPs (Fig. 3), suggesting that it could potentially serve to attenuate organ injury in the setting of infection.
In the clinical study of sepsis, dexmedetomidine administration was not significantly associated with any improvement of mortality or ventilator-free days (Kawazoe, et al. 2017). Although the data regarding surgical infections has been shown by other sedatives/ anesthetics (Shibamura-Fujiogi, et al. 2020), the effect of dexmedetomidine on surgical site infections remains to be determined.
Summary
Dexmedetomidine attenuates surgical stress responses and poses anti-inflammatory shift in our immunological responses. In animal models, dexmedetomidine showed cyto-protective property to attenuate organ damage. However, it increased tumor size. In clinical studies, dexmedetomidine administration was associated with an improvement of outcomes in cardiac surgery and transplant surgery. However, it was associated with a decrease in survival in tumor surgery, indicating that its clinical benefit may be depending on the clinical context. However, there is a paucity in research examining how dexmedetomidine is associated with these outcomes. Further studies are needed to understand its clinical application from immunological standpoints.
Highlights.
Dexmedetomidine is a highly specific α2 adrenergic agonist with increasing use in the perioperative medicine.
A number of immune cells express the α2 adrenergic receptors.
Preclinical and clinical studies demonstrated both benefit and disadvantage of dexmedetomidine use depending on clinical context.
Acknowledgments
Finance:
This work is in part supported by NIH GM118277/ GM127600 (K.Y.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
None
Competing interests:
Author declared no conflict of interest.
References
- Andersson U, and Tracey KJ 2011. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29:139–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaiz-Pena GN, et al. 2013. Neuroendocrine influences on cancer progression. Brain Behav Immun 30 Suppl:S19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartal I, et al. 2010. Immune perturbations in patients along the perioperative period: alterations in cell surface markers and leukocyte subtypes before and after surgery. Brain Behav Immun 24(3):376–86. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S 2003. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun 17 Suppl 1:S27–36. [DOI] [PubMed] [Google Scholar]
- Biccard BM, Goga S, and de Beurs J 2008. Dexmedetomidine and cardiac protection for non-cardiac surgery: a meta-analysis of randomised controlled trials. Anaesthesia 63(1):4–14. [DOI] [PubMed] [Google Scholar]
- Bousquet P, et al. 2020. Imidazoline Receptor System: The Past, the Present, and the Future. Pharmacol Rev 72(1):50–79. [DOI] [PubMed] [Google Scholar]
- Brenu EW, et al. 2013. Heat shock proteins and regulatory T cells. Autoimmune Dis 2013:813256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone A, et al. 2008. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol 155(4):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellino S, Claus M, and Watzl C 2020. Regulation of natural killer cell activity by glucocorticoids, serotonin, dopamine, and epinephrine. Cell Mol Immunol 17(7):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbo C, et al. 2013. Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model. J Anesth 27(2):261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cata JP, et al. 2017. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol 33(3):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, et al. 2020. Dexmedetomidine Attenuates LPS-Induced Monocyte-Endothelial Adherence via Inhibiting Cx43/PKC-alpha/NOX2/ROS Signaling Pathway in Monocytes. Oxid Med Cell Longev 2020:2930463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. 2020. Perioperative Dexmedetomidine Improves Outcomes of Kidney Transplant. Clin Transl Sci 13(6):1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, et al. 2016. In vitro effect of dexmedetomidine on the respiratory burst of neutrophils. Genet Mol Res 15(2). [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. 2015. Dexmedetomidine Ameliorate CLP-Induced Rat Intestinal Injury via Inhibition of Inflammation. Mediators Inflamm 2015:918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, et al. 2016. The Effect of Dexmedetomidine on Outcomes of Cardiac Surgery in Elderly Patients. J Cardiothorac Vasc Anesth 30(6):1502–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M, et al. 1999. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci 64(11):975–81. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R, et al. 1991. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174(5):1209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, et al. 2012. Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology 37(9):1345–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ 2004. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 1024:138–46. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, et al. 2008. Low- versus high-baseline epinephrine output shapes opposite innate cytokine profiles: presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans? J Immunol 181(3):1737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher A, et al. 2017. Analysing the effect of I1 imidazoline receptor ligands on DSS-induced acute colitis in mice. Inflammopharmacology 25(1):107–118. [DOI] [PubMed] [Google Scholar]
- Freeman J, and Buggy DJ 2018. Modelling the effects of perioperative interventions on cancer outcome: lessons from dexmedetomidine. Br J Anaesth 120(1):15–17. [DOI] [PubMed] [Google Scholar]
- Gaudilliere B, et al. 2014. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med 6(255):255ra131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, and Kiecolt-Glaser JK 2005. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5(3):243–51. [DOI] [PubMed] [Google Scholar]
- Godinho-Silva C, Cardoso F, and Veiga-Fernandes H 2019. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu Rev Immunol 37:19–46. [DOI] [PubMed] [Google Scholar]
- Gratz I, Larijani G, Gouldberg ME, Cantillo J, Deal E, Afshar M, Zafeiridis A, Plett A, Gardner E, Murasko D 1999. Dexmedetomidine maintain natural killer activity during surgery under general anesthesia. Anesth Analg 88:339S. [Google Scholar]
- Heng TS, Painter MW, and Consortium Immunological Genome Project 2008. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9(10):1091–4. [DOI] [PubMed] [Google Scholar]
- Herrera-Garcia AM, et al. 2014. Prevention of neutrophil extravasation by alpha2-adrenoceptor-mediated endothelial stabilization. J Immunol 193(6):3023–35. [DOI] [PubMed] [Google Scholar]
- Hiller JG, et al. 2018. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol 15(4):205–218. [DOI] [PubMed] [Google Scholar]
- Inada T, et al. 2017. Mitigation of inflammation using the intravenous anesthetic dexmedetomidine in the mouse air pouch model. Immunopharmacol Immunotoxicol 39(4):225–232. [DOI] [PubMed] [Google Scholar]
- Jayasingam SD, et al. 2019. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol 9:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetschmann JU, et al. 1997. Expression and in-vivo modulation of alpha- and beta-adrenoceptors on human natural killer (CD16+) cells. J Neuroimmunol 74(1-2):159–64. [DOI] [PubMed] [Google Scholar]
- Ji F, et al. 2013. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 127(15):1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji F, et al. 2014. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth 28(2):267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, et al. 2019. Dexmedetomidine protects against high mobility group box 1-induced cellular injury by inhibiting pyroptosis. Cell Biol Int 43(6):651–657. [DOI] [PubMed] [Google Scholar]
- Jones BE, et al. 1977. Ascending projections of the locus coeruleus in the rat. I. Axonal transport in central noradrenaline neurons. Brain Res 127(1):1–21. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, et al. 2013. Dexmedetomidine suppresses proinflammatory mediator production in human whole blood in vitro. J Trauma Acute Care Surg 74(5):1370–5. [DOI] [PubMed] [Google Scholar]
- Kawazoe Y, et al. 2017. Effect of Dexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial. JAMA 317(13):1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZP, Ferguson CN, and Jones RM 1999. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 54(2):146–65. [DOI] [PubMed] [Google Scholar]
- Koutsogiannaki S, et al. 2019. Volatile Anesthetic Attenuates Phagocyte Function and Worsens Bacterial Loads in Wounds. J Surg Res 233:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsogiannaki S, et al. 2017. From the Cover: Prolonged Exposure to Volatile Anesthetic Isoflurane Worsens the Outcome of Polymicrobial Abdominal Sepsis. Toxicol Sci 156(2):402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa S, and Kato M 2008. Anesthetics, immune cells, and immune responses. J Anesth 22(3):263–77. [DOI] [PubMed] [Google Scholar]
- Lavon H, et al. 2018. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth 120(1):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, et al. 2018. Immunomodulatory effects of intraoperative dexmedetomidine on T helper 1, T helper 2, T helper 17 and regulatory T cells cytokine levels and their balance: a prospective, randomised, double-blind, dose-response clinical study. BMC Anesthesiol 18(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, et al. 2015. Anti-inflammatory Effects of Perioperative Dexmedetomidine Administered as an Adjunct to General Anesthesia: A Meta-analysis. Sci Rep 5:12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, et al. 2020. Dexmedetomidine Exerts an Anti-inflammatory Effect via alpha2 Adrenoceptors to Prevent Lipopolysaccharide-induced Cognitive Decline in Mice. Anesthesiology 133(2):393–407. [DOI] [PubMed] [Google Scholar]
- Limbird LE 1988. Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. FASEB J 2(11):2686–95. [DOI] [PubMed] [Google Scholar]
- Link RE, et al. 1996. Cardiovascular regulation in mice lacking alpha2-adrenergic receptor subtypes b and c. Science 273(5276):803–5. [DOI] [PubMed] [Google Scholar]
- Ma D, Rajakumaraswamy N, and Maze M 2004. alpha2-Adrenoceptor agonists: shedding light on neuroprotection? Br Med Bull 71:77–92. [DOI] [PubMed] [Google Scholar]
- Maes M, et al. 2000. The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res 96(3):245–53. [DOI] [PubMed] [Google Scholar]
- Marik PE, and Flemmer M 2012. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg 73(4):801–8. [DOI] [PubMed] [Google Scholar]
- Mitsui Y, et al. 2020. Volatile Anesthetic Sevoflurane Attenuates Toll-Like Receptor 1/2 Activation. Anesth Analg 131(2):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Hoyos A, et al. 2000. Effect of clonidine on plasma ACTH, cortisol and melatonin in children. J Pineal Res 29(1):48–53. [DOI] [PubMed] [Google Scholar]
- Nicholls AJ, et al. 2018. Activation of the sympathetic nervous system modulates neutrophil function. J Leukoc Biol 103(2):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina K, et al. 1999. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesth Analg 88(2):452–8. [DOI] [PubMed] [Google Scholar]
- Okuno T, et al. 2019. Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll-like receptor 4 system. FASEB J 33(12):14528–14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza O, et al. 2016. Effect Of alpha2-Adrenergic Agonists And Antagonists On Cytokine Release From Human Lung Macrophages Cultured In Vitro. Transl Med UniSa 15:67–73. [PMC free article] [PubMed] [Google Scholar]
- Qiao G, et al. 2018. Adrenergic Signaling: A Targetable Checkpoint Limiting Development of the Antitumor Immune Response. Front Immunol 9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumaraswamy N, et al. 2006. Neuroprotective interaction produced by xenon and dexmedetomidine on in vitro and in vivo neuronal injury models. Neurosci Lett 409(2):128–33. [DOI] [PubMed] [Google Scholar]
- Romano M, et al. 2019. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front Immunol 10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ 2009. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10(3):211–23. [DOI] [PubMed] [Google Scholar]
- Scanzano A, and Cosentino M 2015. Adrenergic regulation of innate immunity: a review. Front Pharmacol 6:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzano A, et al. 2015. Adrenergic modulation of migration, CD11b and CD18 expression, ROS and interleukin-8 production by human polymorphonuclear leukocytes. Inflamm Res 64(2):127–35. [DOI] [PubMed] [Google Scholar]
- Shibamura-Fujiogi M, et al. 2020. The Role of Anesthetic Management in Surgical Site Infections After Pediatric Intestinal Surgery. J Surg Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifringer M, et al. 2015. Neuroprotective effect of dexmedetomidine on hyperoxia-induced toxicity in the neonatal rat brain. Oxid Med Cell Longev 2015:530371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K 2001. Presynaptic autoreceptors in the third decade: focus on alpha2-adrenoceptors. J Neurochem 78(4):685–93. [DOI] [PubMed] [Google Scholar]
- Stoecklein VM, Osuka A, and Lederer JA 2012. Trauma equals danger--damage control by the immune system. J Leukoc Biol 92(3):539–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YB, et al. 2019. Dexmedetomidine inhibits astrocyte pyroptosis and subsequently protects the brain in in vitro and in vivo models of sepsis. Cell Death Dis 10(3):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, et al. 2018. The effect of dexmedetomidine on inflammatory inhibition and microglial polarization in BV-2 cells. Neurol Res 40(10):838–846. [DOI] [PubMed] [Google Scholar]
- Szpunar MJ, et al. 2013. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 6(12):1262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank AW, and Lee Wong D 2015. Peripheral and central effects of circulating catecholamines. Compr Physiol 5(1):1–15. [DOI] [PubMed] [Google Scholar]
- Tazawa K, et al. 2017. The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett 266:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poll T, et al. 1994. Regulation of interleukin 10 release by tumor necrosis factor in humans and chimpanzees. J Exp Med 180(5):1985–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier E, et al. 2008. Functions of natural killer cells. Nat Immunol 9(5):503–10. [DOI] [PubMed] [Google Scholar]
- Wang K, et al. 2019. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth 123(6):777–794. [DOI] [PubMed] [Google Scholar]
- Wang P, et al. 1994. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol 153(2):811–6. [PubMed] [Google Scholar]
- Wang XW, et al. 2015. Effect of perioperative dexmedetomidine on the endocrine modulators of stress response: a meta-analysis. Clin Exp Pharmacol Physiol 42(8):828–36. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. 2021. Effect of dexmedetomidine on CD4+ T cells and programmed cell death protein-1 in postoperative analgesia: a prospective, randomized, controlled study. Minerva Anestesiol. [DOI] [PubMed] [Google Scholar]
- Webster NR, and Galley HF 2009. Immunomodulation in the critically ill. Br J Anaesth 103(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting D, Yuki K, and DiNardo JA 2015. Cardiopulmonary bypass in the pediatric population. Best Pract Res Clin Anaesthesiol 29(2):241–56. [DOI] [PubMed] [Google Scholar]
- Wu H, et al. 2020. Effects of dexmedetomidine on stress hormones in patients undergoing cardiac valve replacement: a randomized controlled trial. BMC Anesthesiol 20(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RS, et al. 2015. Different cellular responses of dexmedetomidine at infected site and peripheral blood of emdotoxemic BALB/c mice. Environ Toxicol 30(12):1416–22. [DOI] [PubMed] [Google Scholar]
- Xiao J, et al. 2010. Modulation of natural killer cell function by alpha-adrenoreceptor-coupled signalling. Neuro Endocrinol Lett 31(5):635–44. [PubMed] [Google Scholar]
- Yap A, et al. 2019. Anesthetic technique and cancer outcomes: a meta-analysis of total intravenous versus volatile anesthesia. Can J Anaesth 66(5):546–561. [DOI] [PubMed] [Google Scholar]
- Yuki K, et al. 2013. Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin alphaIIbbeta3. PLoS One 8(4):e60415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, et al. 2012. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J 26(11):4408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, et al. 2020. Mechanistic consideration of the effect of perioperative volatile anesthetics on phagocytes. Clin Immunol 222:108635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki K, Soriano SG, and Shimaoka M 2011. Sedative drug modulates T-cell and lymphocyte function-associated antigen-1 function. Anesth Analg 112(4):830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, et al. 2020. Dexmedetomidine preconditioning alleviated murine liver ischemia and reperfusion injury by promoting macrophage M2 activation via PPARgamma/STAT3 signaling. Int Immunopharmacol 82:106363. [DOI] [PubMed] [Google Scholar]
- Zuppa AF, et al. 2019. Results of a phase 1 multicentre investigation of dexmedetomidine bolus and infusion in corrective infant cardiac surgery. Br J Anaesth 123(6):839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]


