Abstract
One of the most transformative developments in neurogastroenterology is the realization that many functions normally attributed to enteric neurons involve interactions with enteric glial cells: a large population of peripheral neuroglia associated with enteric neurons throughout the gastrointestinal tract. The notion that glial cells function solely as passive support cells has been refuted by compelling evidence that demonstrates that enteric glia are important homeostatic cells of the intestine. Active signalling mechanisms between enteric glia and neurons modulate gastrointestinal reflexes and, in certain circumstances, function to drive neuroinflammatory processes that lead to long-term dysfunction. Bidirectional communication between enteric glia and immune cells contributes to gastrointestinal immune homeostasis, and crosstalk between enteric glia and cancer stem cells regulates tumorigenesis. These neuromodulatory and immunomodulatory roles place enteric glia in a unique position to regulate diverse gastrointestinal disease processes. In this Review, we discuss current concepts regarding enteric glial development, heterogeneity and functional roles in gastrointestinal pathophysiology and pathophysiology, with a focus on interactions with neurons and immune cells. We also present a working model to differentiate glial states based on normal function and disease-induced dysfunctions.
The enteric nervous system (ENS) is an integrative neural network that provides local control over essential gastrointestinal functions through ‘brain-like’ neural circuitry composed of neurons and glia. The relative importance of intrinsic and extrinsic neurons in gastrointestinal reflexes and the characteristics of individual enteric neuron subtypes have been well described1,2. How enteric neurons organize into functional circuits3, how these circuits develop4 and how intercellular interactions with non-neuronal cells, such as immune cells5–8 and glia9, shape the output of enteric neurocircuits is only beginning to be understood. In particular, new data showing that bidirectional communication between enteric neurons and enteric glia influences the output of enteric neurocircuits is beginning to transform views of how normal enteric circuits function and of the processes that lead to dysfunction in common functional gastrointestinal disorders such as irritable bowel syndrome (IBS)9–15.
Enteric glia are a large population of peripheral neuroglia that are associated with the cell bodies and processes of enteric neurons throughout the digestive tract. Neuroglia, in general, fulfil homeostatic functions in the nervous system and this view is consistent with the known functions of enteric glia in the ENS. The latest advances in enteric glial biology have begun to define what these specific functions are and the mechanisms involved. The data emerging from these studies show that enteric glia are one of the most dynamic signalling components of the ENS and have revamped the concept of enteric glia as passive cells. Fast, bidirectional communication between enteric glia and neurons regulates enteric reflexes10,14,16,17 and the communication between extrinsic and intrinsic neurons12,18,19 that innervate the digestive tract. Likewise, enteric glia contribute to diverse disease processes, including neuroinflammation12,13, cancer20,21 and infection22, in which glia acquire pro-inflammatory or pro-tumorigenic phenotypes. In these cases, both losses and gains of glial functions contribute to altered ENS homeostasis and gastrointestinal pathophysiology.
Although much progress has been made, the field of enteric glial biology is still young and answering some of the many remaining unknowns offers an opportunity for scientific growth and meaningful advances. Little is known regarding glia in digestive organs other than the colon or ileum and glial heterogeneity within even a single region is complex. The intent of this Review is to summarize some of the currently known functions of enteric glia and highlight areas in which additional work is needed. New findings that refine the concepts of glial development, the roles of enteric glia in physiological reflexes, and that expand the known roles of glia in disease will be presented. Although current data show the vast potential of glia to be involved in diverse functions, many questions remain regarding the necessity of glia, their specific roles in neural circuits and differences in the roles of glia throughout the digestive tract. Major progress in these areas should be expected as understanding enteric glia has the potential to unlock central mechanisms that regulate gastrointestinal function and disease.
Enteric glia are heterogenous neuroglia
The term ‘enteric glia’ collectively refers to all neuroglia associated with the ENS and substantial heterogeneity exists within this broad population of cells. Glial cells are phenotypically plastic and adapt their functions in response to cues in the microenvironment to maintain local homeostasis. Presumably, the different functional roles and regional specializations of different intestinal organs promote subpopulations of glia with unique characteristics to develop, yet little is known about regional or local glial heterogeneity and how glial specializations contribute to the various digestive functions. The current framework characterizing the major subpopulations of enteric glia segregates cells based on morphology and anatomical location within the intestinal wall23,24 (TABLE 1). This classification system is useful as a means of developing a common language to discuss major populations of enteric glia with differing anatomical locations but does not capture functional heterogeneity between intestinal regions or within the major subgroups themselves. Current evidence suggests ongoing dynamic regulation of gene and protein expression within subtypes of enteric glia as well as potential differences in responsiveness to intercellular mediators within and between subtypes24,25. Single-cell transcriptional profiling data show that glial diversity differs between regions of the digestive tract and suggest that glial diversity is greater in humans than in mice26,27. These studies also show that glial gene expression is affected by circadian rhythm and age. In addition, the extent to which ‘enteric’ glia derive from differing progenitor sources, such as Schwann cell precursors28,29, and whether the current definition of enteric glia encompasses intestinal Schwann cells associated with extrinsic nerves innervating the intestine are unanswered questions30,31. Glial heterogeneity adds considerable complexity to the generalized view of enteric glia and understanding this area in more depth will be an important aspect of future work. Here, the discussion is limited to the current knowledge base suggesting local, regional and functional heterogeneity between enteric glial subtypes.
Table 1 |.
Glial subtypes
| Type | Enteric glial subtype | Defining features | Known or proposed functions | |
|---|---|---|---|---|
| Intraganglionic | Myenteric | Type IMP | Small somata and extend very short, irregularly branched processes that surround neurons; associated with neuron cell bodies in the myenteric ganglia (‘protoplasmic-like’) | Modulate myenteric neuron activity10,11,14,17–19; regulate oxidative stress99,100; provide trophic support92–98,178; regulate neuroinflammation12,13,113,133,157; gliogenesis57, neurogenesis58,102,103,119 and replenishment of mucosal glia34 |
| Submucosal | Type ISMP | Associated with neuron cell bodies within submucosal ganglia | Modulate secretomotor neuron activity16 | |
| Extraganglionic | Interganglionic | Type II | Subtype that resides in interganglionic fibre tracts (‘fibrous’) with long processes that branch infrequently and run parallel to nerve fibres connecting enteric myenteric ganglia | Signal propagation in the glial network70; neuromodulation? |
| Mucosal | Type IIImucosa | Extend a small number of long, fine unbranching processes; some follow nerve fibres and some terminate at the mucosal epithelium | Influence epithelial cell maturation36,46; potentially modulate immune responses19,37; probable modulation of neuroendocrine signaling21,35 | |
| Enteric plexus | Type IIIMP/SMP | Subtype located in the extraganglionic regions at the level of myenteric and submucosal plexuses that run along neuronal fibres and/or wrap around small blood vessels | Unknown | |
| Intramuscular | Type IV | Extend a small number of long, fine unbranching processes that follow nerve fibres in the muscularis | Unknown | |
MP, myenteric plexus; SMP, submucosal plexus.
Local heterogeneity refers to differences among glia within a given region of the gastrointestinal tract. This aspect is currently the most well defined of glial heterogeneity and major subpopulations of glia are defined based on their anatomical location throughout the intestinal wall and their localization either within or outside of enteric ganglia (TABLE 1)23,24. Based on anatomical criteria, at least six main types of enteric glia are present in the intestine. These types include glia associated with neuron cell bodies in the myenteric and submucosal plexuses (Type IMP and Type ISMP, respectively), glia within nerve fibre bundles connecting myenteric ganglia (Type II), extraganglionic glia associated with nerve fibres at the level of the myenteric and submucosal plexuses (Type-IIIMP/SMP) or the intestinal mucosa (Type IIImucosa), and glia associated with nerve fibres in the smooth muscle layers (Type IV) (FIG. 1). Current in vivo data suggest that all enteric glia within a given region of the intestine are derived from a common pool of progenitor cells within the myenteric plexus and are developmentally linked4. Nonetheless, Schwann cells present in the intestine could complicate this interpretation. In zebrafish, Schwann cell precursors that migrate from the spinal cord into the intestine give rise to postnatal enteric neurons32. Similarly, Schwann cells associated with extrinsic nerves are also capable of forming new enteric neurons and glia in mouse models of Hirschsprung disease following stimulation with glial cell-derived neurotrophic factor (GDNF)29. Understanding the extent to which Schwann cells contribute to glial diversity under normal conditions will be an important question for future work. However, distinguishing between enteric glia and Schwann cells within a similar microenvironment could prove difficult given that enteric glia are similar to Schwann cells at the transcriptional level33 and that the two cell types are driven towards a common phenotype when exposed to similar factors in cell culture28. Such local cues, rather than specified developmental programmes, seem to be the main driver of glial heterogeneity within the intestine and environmental cues are responsible for producing certain glial subtypes such as mucosal glia. Lineage tracing shows that this population derives from enteric glial cells with progenitor potential in the submucosal and myenteric plexuses and subsequently migrates to the mucosa in a process that requires cues from the microbiome34. The nature of the cues that drive this differentiation is not known. Mucosal glia are also diverse and subpopulations have specialized interactions with nerves, immune cells, enterocytes and/or enterochromaffin cells35–38. A particularly interesting subpopulation of mucosal glia is associated with the neuropod synaptic specializations of enterochromaffin cells35. These cells are ideally situated to modulate gut–brain communication and could have a major influence over intestinal sensory functions39. However, the functional significance of these cells is not currently understood and whether glia associated with the neuropods of enterochromaffin cells are actually enteric glia or Schwann cells that migrate to the intestine along extrinsic nerves is unknown30,31.
Fig. 1 |. Main populations of enteric glia and their known physiological functions.
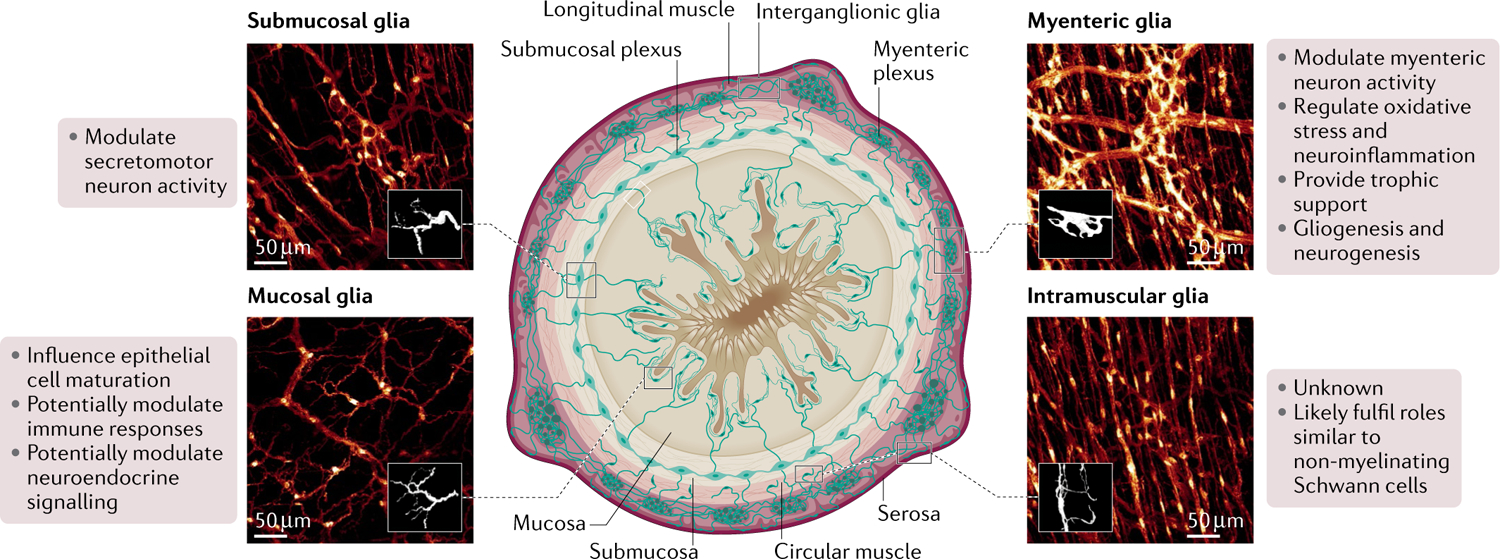
Local subpopulations of glia are defined based on their morphology, anatomical location throughout the intestinal wall, and localization either within or outside of enteric ganglia. Based on these criteria, at least six main types of enteric glia are identified in any given region of the intestine: intraganglionic glia, including glia associated with neuronal cell bodies in the myenteric and submucosal plexuses (myenteric glia or Type-IMP and submucosal glia or Type-ISMP, on the top right and left, respectively); interganglionic glia, located within nerve fibre bundles connecting myenteric ganglia (Type-II); extraganglionic glia, including glia associated with nerve fibres at the myenteric and submucosal plexus but outside the ganglia (Type-IIIMP/SMP) and in the intestinal mucosa (mucosal glia or Type-IIImucosa, bottom left); and glia associated with nerve fibres in the circular and longitudinal muscle layers (intramuscular glia or Type-IV, bottom right). Known functions of each subtype are listed beside each representative image.
Molecular and functional data suggest added complexity within and between the major glial subtypes defined earlier. For example, immunohistochemical and single-cell transcriptomics data from mice and humans show variability in the extent to which enteric glia express major glial markers such as glial fibrillary acidic protein (GFAP), S100β, proteolipid protein 1 (PLP1) and Sox10 (REFs24–26,33). GFAP is dynamic and its observed expression varies depending on glial state12, subtype24, genetic targeting strategy10,40 and antibody used and therefore results should be interpreted with caution41. Differing transgene insertion sites cause variability in genomic models that use GFAP as a glial-specific driver10,40 and enteric glia express multiple GFAP isoforms42–44. Thus, the ability of antibodies to identify one or more GFAP isoform will affect the observed extent of GFAP in immunolabelling experiments41,44. For example, GFAP is reportedly absent in intramuscular glia and in subsets of mucosal and submucosal glia in mice based on immunolabeling data, but it is unclear whether this is a true absence or if these data reflect variable antibody efficacy33. Single-cell transcriptional sequencing data show that all subtypes of myenteric glia within the mouse ileum express GFAP, albeit at differing levels25. Bulk RNA sequencing data of myenteric glia from the mouse colon also indicate comparable levels of GFAP, PLP1 and S100β transcription under steady-state conditions33. However, these data should be interpreted with caution given that they reflect population levels of RNA and RNA sequencing is a proxy for protein expression. It is unclear how well these data reflect variability within individual cells or functional protein expression.
Regional heterogeneity refers to differences among populations of enteric glia present in various locations of the gastrointestinal tract; however, the vast majority of work has focused on enteric glia located in the colon or distal ileum. The main subsets already discussed are prominent in these regions but fewer are present in the oesophagus and stomach since these regions lack a well-defined submucosal plexus2. Glial cells in the oesophagus and stomach could derive from a different pool of progenitor cells than glial cells in the intestine, as distinct populations of enteric precursors differently colonize the oesophagus and stomach than the intestine in mice30. Thus, both developmental programmes and functional specializations driven by the local environment could contribute to glial heterogeneity between the intestine and organs of the upper digestive tract. Distinct glial populations with unique transcriptional profiles have been described in the adult ENS of mice and humans25,26. The number of glial subsets varies according to species and location along the gastrointestinal tract and could relate to the complexity of motor circuits (FIG. 2). This area is still underdeveloped and would benefit from future work.
Fig. 2 |. Transcriptionally distinct glial subsets in the adult enteric nervous system.
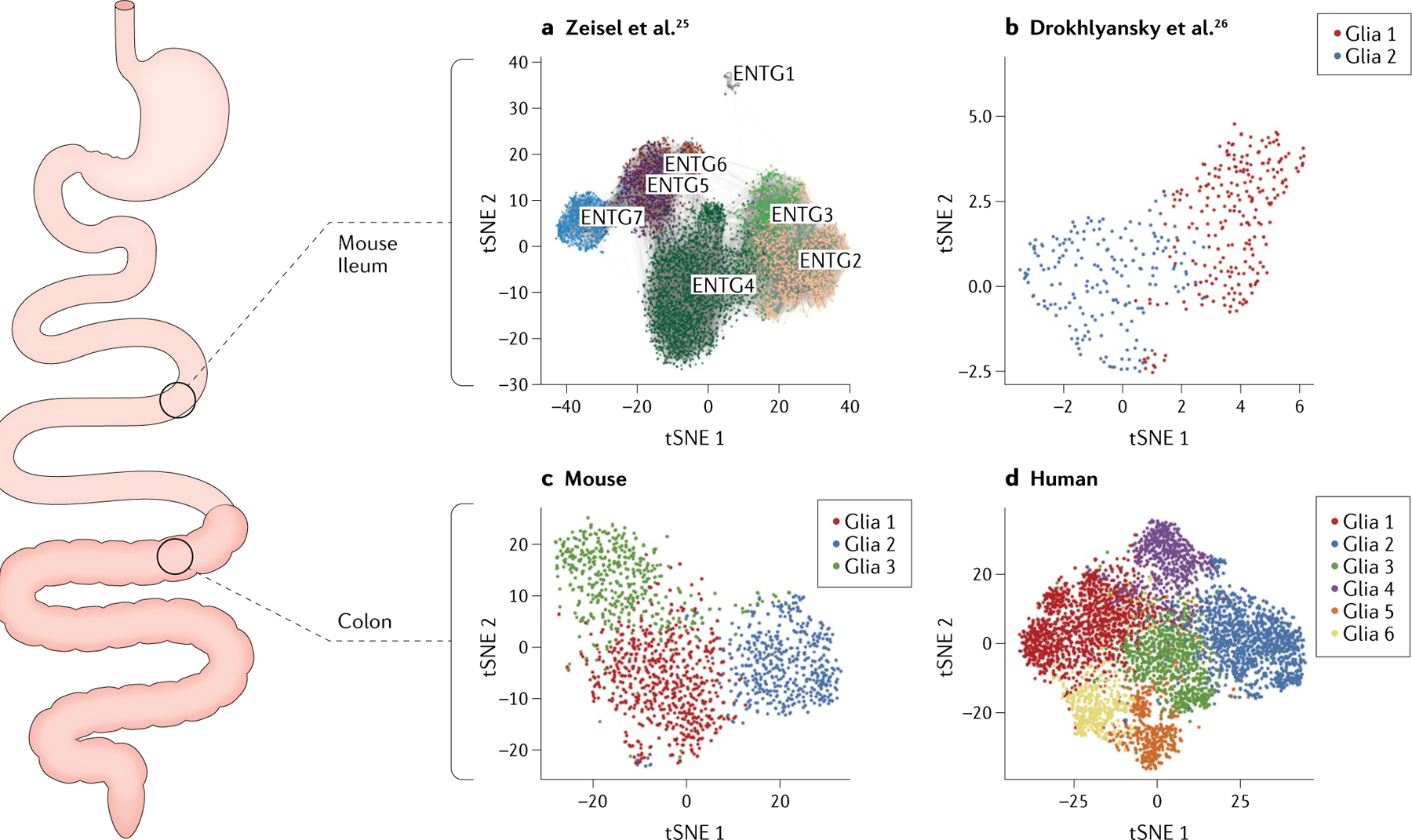
Distinct subsets of enteric glia display unique transcriptional profiles in the enteric nervous system. Data from single-cell sequencing experiments indicate the presence of distinct glial populations that vary according to location along the gastrointestinal tract and species. The mouse ileum exhibits seven glial populations according to data from Zeisel et al.25 (part a) and two glial subpopulations according to data from Drokhlyansky et al.26 (part b). The mouse colon displays three glial subsets according to data from Drokhlyansky et al.26 (part c) and the human colon displays six glial subsets (part d; also data from Drokhlyansky et al.). tSNE, t-distributed stochastic neighbour embedding. Part a was adapted from REF.25, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). Parts b–d were adapted with permission from REF.26, Elsevier.
Local and regional functional heterogeneity among enteric glia remains poorly defined. Glia within the myenteric and submucosal plexuses support and modulate neuronal signalling in mice11,14,16,45, whereas glia within the mucosa appear to influence enterocyte development36,46,47 and immune responses in cell culture19,37,48. To what extent these roles are conserved between regions is unclear. Differences among protein expression, responsiveness to various intercellular mediators and transcriptional profiles have also been observed between myenteric glia in the ileum and colon of mice24,25. Functional specializations and differential distributions of enteric glial subtypes might operate in tandem with specified neuronal networks to regulate specific functions in different regions and segments of the digestive tract49. However, whether these differences represent true functionally defined subsets of glia or a snapshot of ongoing glial plasticity within a more homogeneous population is unknown.
Glial development and maintenance
The ENS of vertebrates derives mostly from a rostral portion of the neural crest, referred to as vagal. The vagal crest itself is composed of two subpopulations of precursors for the ENS: cells adjacent to somites 1 and 2 that produce Schwann cell precursors associated with the vagus nerve and contribute more to the autonomic ganglia in the oesophagus and stomach than the intestine, and cells adjacent to somites 3–7, the cervical region of the trunk crest, which contribute to form sympathetic ganglia and colonize most of the digestive tract30. Furthermore, enteric precursors also derive from the sacral neuronal crest, which contributes 20% of the neurons in the descending colon and rectum30. Enteric glia and enteric neurons are derived from enteric precursor cells that express the transcription factor Sox10 and undergo extensive proliferation as they colonize the bowel in a rostral to caudal progression50,51. Associated changes in the expression of transcription factors promote differentiation into neurons and glia. For example, Sox10 is maintained in glial precursors and in the majority of mature enteric glia but is progressively downregulated in neural crest precursor cells committed to a neural fate52,53. Subpopulations of cells within the migrating neural crest precursor cells include bipotential progenitors that can form both neurons and glia, fate-restricted gliogenic precursors with variable proliferative potential, and fate-restricted neurogenic precursors with limited proliferative capacity4. Together, these cells have the potential to give rise to multiple neuron subtypes and to all types of enteric glia in the intestine. However, Schwann cell precursors that invade the intestines with extrinsic nerves make a substantial contribution to neurogenesis later in development and produce many of the intrinsic sensory neurons that express the calcium-binding protein calbindin in mice31. Schwann cell precursors also contribute to post-embryonic neurogenesis in zebrafish32 and to post-embryonic neurogenesis and gliogenesis in themouse ENS under certain circumstances29. Furthermore, given the different strategies engaged by ENS progenitors to migrate in the small intestine and the colon54, the development and organization of neuron–glia networks could be driven differently in distinct gastrointestinal tract regions.
The development of the ENS is a dynamic process that continues postnatally and is influenced by the gut microbiota and the immune system34,55,56. Likewise, myenteric glia develop during the initial colonization of the myenteric plexus around E12.5 in mice and subsequently give rise to submucosal and mucosal glia4. Enteric glia are not observed in the lamina propria at birth in mice and the colonization of the mucosa begins during the suckling period and does not reach adult levels until after weaning34. This population is continually replenished by enteric glia with progenitor potential within the myenteric plexus in a process that requires the gut microbiota. By contrast, myenteric glia are relatively stable and the rate of gliogenesis in the myenteric plexus is low under steady state conditions. Data from BrdU labelling studies suggest that only 2–3% of myenteric glia are either proliferating or newly born during a 12-week period in adult mice57. Whether the myenteric cells that incorporated BrdU over this period reflect enteric glia with progenitor potential that support glial turnover in the mucosa or local replenishment of glia in the myenteric plexus is unclear. The rates of glial turnover in the myenteric plexus, submucosal plexus and mucosa are currently uncharacterized and could be complicated by the fact that standard markers (such as BrdU and EdU) do not always capture cells that transdifferentiate or that migrate to the ENS along extrinsic nerves29. However, gliogenesis increases in response to injury, inflammation, exercise and other challenges under normal conditions and the maintenance of glia is supported by progenitor cells that are presumably the same subset of enteric glia with progenitor potential that form mucosal glia and exhibit neurogenic potential58.
Role in gastrointestinal physiology
The fundamental function of neuroglia, a classification that encompasses enteric glia, is homeostasis of the nervous system59. In this regard, enteric glia can be defined as homeostatic cells of the ENS. Functional data support this definition and highlight multiple mechanisms whereby enteric glia set the tone of neurotransmission in the ENS and regulate the intestinal reflexes and processes underlying neuroinflammation in the intestine10,14,60–62. Multiple independent lines of evidence support a major role for enteric glia in gastrointestinal reflexive activities10,14,63,64, but the contributions of glia to other functions, such as mucosal barrier regulation, remain debatable11. Here, discussion is focused on the current understanding of the role of enteric glia in the regulation of molecular, cellular and organ-level homeostasis in the intestine.
Regulation of enteric neural reflexes.
The bidirectional communication between enteric neurons and glia is a mechanism that regulates intestinal reflexes. Enteric glia surround enteric neurons and monitor multiple forms of neural activity through neurotransmitter receptor signalling65. The potential for enteric neuron-to-glia communication was originally described in 1972 by Gabella, who observed presynaptic specializations in nerve processes contacting enteric glia in the myenteric plexus of the guinea pig ileum66. Gabella noted that these “emi-junctions” are “so frequent that it seems unlikely that any glial cell is totally devoid of them”. The functional importance of neuron–glia junctions remained in question for nearly 40 years until Ca2+ imaging studies demonstrated functional responses in enteric glia evoked by exogenous neurotransmitters and neurotransmitters released from endogenous neurons in vitro9,64,67–70. It is now clear that multiple neurotransmitter systems evoke glial activity67,71,72 encoded by intracellular Ca2+ responses and that glial Ca2+ responses, in turn, evoke the release of gliotransmitters such as ATP and GABA through membrane channels composed of connexin 43 (Cx43, also known as GJA1)13,60 and/or through the reversal of neurotransmitter transporters73 (FIG. 3). Glial Ca2+ responses are evoked in the myenteric plexus by neuronal activity during the active, contractile phase of colonic migrating motor complexes (CMMCs) that is dominated by activity in excitatory cholinergic neurons located in the myenteric plexus64. Myenteric glia are ‘innervated’ by cholinergic neurons, and neurotransmitters released by this class of neurons, such as acetylcholine and ATP, evoke Ca2+ responses in enteric glia in Ca2+ imaging studies of whole-mount preparations of myenteric plexus in mice9,14,66. By contrast, glia are relatively silent during the intervening quiescent periods of tonic inhibition that are driven by activity within myenteric inhibitory motor neurons that produce nitric oxide64. These data support the concept that myenteric glia in the colon are preferentially activated by excitatory neurotransmission and are either not responsive to or are under tonic inhibition by inhibitory neurons74. Little is known regarding enteric neuron–glia signalling in the submucosal plexus, although purinergic neuron-to-glia signalling is also prominent16.
Fig. 3 |. Mechanisms of bidirectional communication between enteric neurons and glia in enteric circuits.
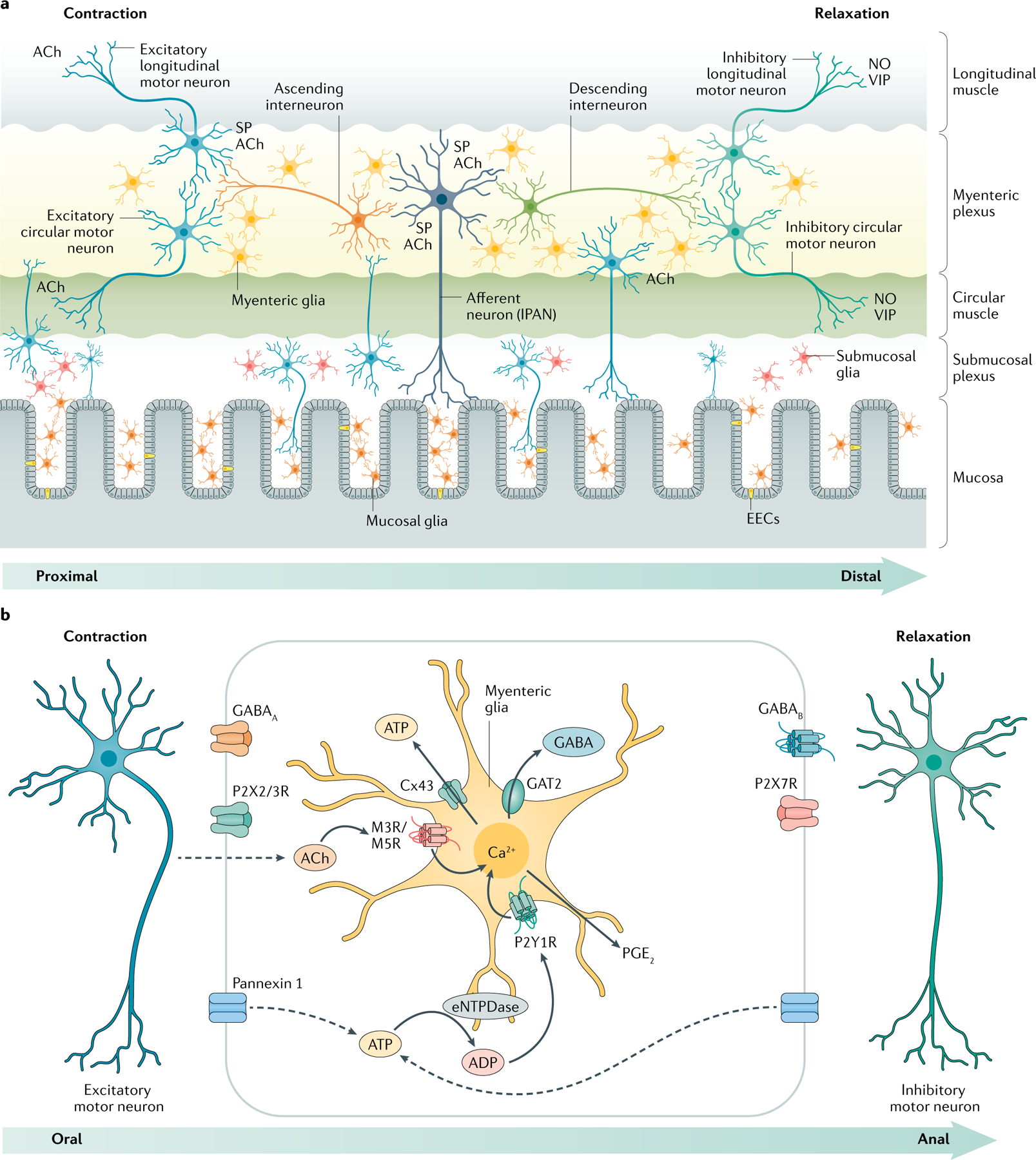
a | Enteric glia surround neurons in the myenteric and submucosal plexuses and are associated with nerve fibres in the mucosa. b | Glial Ca2+ responses are evoked in the myenteric plexus by neurotransmitters such as acetylcholine (ACh) and ATP, which, in turn, induce the release of gliotransmitters such as ATP and GABA through membrane channels composed of connexin 43 (Cx43) and/or through the reversal of neurotransmitter transporters (such as GABA transporter 2 or GAT2). These gliotransmitters act on receptors expressed by excitatory and inhibitory neurons to exert reciprocal effects on enteric neural circuits that control the intestinal motility. EEC, enteroendocrine cells; eNTPDase, ectonucleoside triphosphate diphosphohydrolase 2; NO, nitric oxide; SP, substance P; VIP, vasoactive intestinal peptide.
Several studies have attempted to test the functional significance of enteric glia in gastrointestinal reflexes by using glial metabolic poisons or glial ablation animal models. However, these approaches have proven inconsistent and produce widely variable effects in terms of gastrointestinal motility17,45,75, secretions, barrier function45,76,77 and survival17,45,76–78. For example, animals exposed to the glial metabolic poison fluorocitrate exhibit impaired small intestinal transit and ileal neurogenic contractions but do not exhibit marked changes in colonic motor function17,75. By contrast, depleting enteric glia with genetic targeting strategies does not overtly affect small intestinal transit but does impair colonic motility45 and, in some models, is lethal76,77. Although these studies do support a role for glia in enteric reflexes, methodological complications limit a clear understanding of how it relates to active glial signalling. Neither technique specifically addresses glial signalling and the presence of confounding factors that do not directly involve glial signalling, such as reactive gliosis produced by fluorocitrate79, variable efficacy of glial depletion45, and adverse effects on epithelial and immune cells45,76,77, limit the insight into the role of glial signalling in a physiological system. Variable efficacy in glial depletion45,76 is particularly important when considering the potential effects on gastrointestinal motility given the extensive redundancy in the ENS. For example, neurons are integral to gastrointestinal motility but the ability to generate rhythmic propagating neurogenic colonic motor complexes is maintained in mice that have lost at least half of their enteric neurons80. Thus, the partial depletion of myenteric glia might not produce a robust effect on motility. Notably, current studies do not address the possibility that glial ablation models preferentially affect certain glial subtypes, which could add considerable complexity to the interpretation of these data as subtype-specific effects might produce effects that are more or less pronounced on certain functions, whereas a non-selective depletion of all glia could mask important contributions of relevant subpopulations. The effects of glial ablation on enteric neuron function and neurocircuitry are also uncharacterized. Similar issues have been partly responsible for fuelling debate surrounding the role of astrocytes in neural signalling in the brain and have made it clear that more selective methods are required to disentangle the roles of glia in neural signalling81,82,.
Manipulating specific glial signalling mechanisms produces a clear effect on intestinal reflexes without the complications of altered cellular survival or phenotype. For example, reducing glial intercellular signalling by ablating glial Gja1 slows intestinal motility, impairs CMMCs, impairs secretomotor reflexes and dampens neuromuscular functions in the mouse colon11,13,60. These observations suggest that glial signalling mediated by Cx43 exerts a modulatory effect that is required to maintain the normal strength of intestinal reflexes. Complementary studies using designer receptors activated by designer drugs (DREADDs) in mice to selectively activate Ca2+ responses in enteric glia extend this observation and suggest that glial cells have the potential to modulate but also to activate intestinal reflexes12,14. In these in vitro and in vivo experiments with mice, the chemogenetic receptor hM3Dq was expressed under the control of the GFAP promoter to model the activation of enteric glia by neurotransmitters acting through Gq-coupled receptors such as acetylcholine and purines10,14. The data show that this mechanism of glial activation is powerful and drives neurogenic contractions in the ileum and colon as effectively as stimulating neurons or smooth muscle10,11. In addition, augmenting glial activation during CMMCs potentiates CMMC strength, propagation speed and frequency10,14. These effects are absent in the presence of tetrodotoxin, indicating that the pro-contractile effects of glial activation are mediated by intercellular signalling with neurons. The same is true for secretomotor reflexes, where activating enteric glia via DREADDs indirectly drives secretomotor responses through neural signalling and possibly through direct signalling with epithelial cells11. Although the DREADD system relies on the expression of a foreign receptor to activate glia, it does replicate endogenous glial M3 acetylcholine receptor mechanisms14 and shows that triggering these glial mechanisms affects the enteric neural circuitry that underlies motility and secretomotor reflexes in these models.
How enteric glia exert their effects on enteric neural reflexes is mostly uncharacterized, but it is possible that subsets of enteric glia are specialized to detect and modulate specific neural pathways. Astrocytes, a related type of neuroglia in the central nervous system, are circuit-specific cells and function to tune neurotransmission on a synapse-by-synapse basis83–85. Enteric glia express the specific molecular machinery to detect excitatory neurotransmitters71, are recruited by excitatory neurons during reflexive activity, and release excitatory gliotransmitters, such as ATP and GABA13,73, that activate P2X2/3 and GABAA receptors expressed by excitatory neurons in ascending motility pathways86–88. No corresponding positive interactions with inhibitory neurons or effects on inhibitory neurotransmission have been observed. By contrast, glia secrete mediators, such as prostaglandin E2 (PGE2)89 and GABA73, that suppress inhibitory pathways through EP2 and GABAB receptors, respectively, expressed by nitrergic neurons25. These data support the concept that enteric neuron–glia signalling is circuit specific, but this hypothesis remains to be directly tested. Enteric glia are also recruited by some forms of activity in extrinsic sympathetic18, parasympathetic19 and sensory neurons12 innervating the intestine. Additional work is needed to understand how enteric glia integrate synaptic information and modify enteric neural networks in response to these various forms of activation.
Supportive roles.
Enteric glia are often compared to astrocytes in terms of their homeostatic roles that support neural networks90. Supportive roles fulfilled by astrocytes are diverse and well characterized91, but the evidence supporting the notion that enteric glia share similar functions is largely circumstantial or absent (Supplementary Table 1). Enteric glia do support enteric neurotransmission through astrocyte-like mechanisms that include supplying neurotransmitter precursors92,93, regulating neurotransmitter availability94–98, regulating oxidative stress99,100, supplying neurotrophins46,101 and, potentially, supporting neurogenesis102,103. Glia also extend these supportive roles to non-neuronal cells, such as intestinal epithelial cells, where glial factors influence the maturation and differentiation of the intestinal epithelium36,104–106. Furthermore, it is unknown whether enteric glia have a major role in other well-characterized astrocytic homeostatic functions such as ion homeostasis, regulation of pH, chemosensing, regulation of energy balance, sleep, control of blood–brain barrier and lymphatic systems, regulation of local blood flow, or glycogen synthesis and storage. The necessity of glial support in enteric networks is also unclear as depleting enteric glia in Plp1CreER;Rosa26DTA mice does not seem to cause overt changes in neuron survival; however, this aspect has not been rigorously tested45,107.
What limited evidence is available does suggest that enteric glia maintain homeostasis in the microenvironment surrounding enteric neurons. Glia regulate neurotransmitter availability through their expression of transporters such as the GABA transporter 2 (GAT2)73,95 and by degrading neuroactive compounds in the extracellular space via cell-surface enzymes, including nucleoside triphosphate diphosphohydrolase 2 (NTPDase2)96–98. Enteric glia are immunoreactive for neurotransmitter precursors and synthesis mechanisms (including L-arginine92,108 and glutamine synthetase93), which could indicate that glia support nitrergic and glutamatergic neurotransmission similarly to astrocytes90, but this hypothesis has not been directly tested. Glutamine synthetase is also responsible for the detoxification of ammonia; enteric glia sense ammonia and modify neuromuscular transmission through GABAergic signalling with neurons in mice73. Type‐III enteric glia in the extraganglionic region might also be involved in supplying nutrients to enteric neurons and/or shedding metabolic waste as they extend their processes to wrap around small blood vessels; however, neither role has been tested24. Enteric glia do express potassium channels109–111 and voltage-gated potassium currents have been recorded in cultured enteric glial cells109,110, although it is unknown whether enteric glia play a major role in potassium homeostasis in tissue. Enteric glia might also contribute to neurogenesis and neuronal network maturation given their ability to produce trophic compounds such as pro-epidermal growth factor46, nerve growth factor101,112 and vascular endothelial growth factor113 in vitro as well as IL-1β113–115 (which exhibits neurotrophic effects at certain concentrations113) and S100β116 in vivo. However, the bulk of this evidence is based on cell culture studies and the relevance of glial trophic support in vivo is untested.
Data from targeted enteric glial ablation models are conflicting regarding the necessity of glial support in enteric networks. Glial ablation models that rely on glial-targeted CD8+ and CD4+ T cells in mice77,117, transgenic mice expressing herpes simplex virus thymidine kinase from the mouse GFAP promoter76 (GFAPHSV-TK), and the gliotoxin 6-aminonicotinamide78,118 result in severe intestinal issues such as fulminant jejuno-ileitis, enterocolitis and death. By contrast, depleting glia in mice that express diphtheria toxin under the control of Cre recombinase directed to glia using the Plp1 promoter (PLPCreER;Rosa26DTA) does not cause overt changes beyond an increase in colonic migrating motor complex frequency in female mice45. The reason for this discrepancy between model organisms is not fully understood but could involve differing penetrance in model systems, differential effects on glial subtypes, and bystander and/or compensatory effects in the surrounding cells45. However, it is worthwhile to note that the specific attributes of neuron signalling and health have not been assessed in any of these models. Data from the PLPCreER;Rosa26DTA mouse model show that the depletion of glia does not substantially alter enteric neuron survival under steady-state conditions, which argues against a requirement for glia in the maintenance of enteric neuron survival and ongoing neurogenesis45,107. This conclusion is strengthened by prior data that failed to observe any major level of neurogenesis in the adult ENS in steady-state conditions in mice58. By contrast, gliogenesis is consistently detected under steady-state conditions in the adult mouse gut and its rate increases after certain types of ENS injury, including inflammation, irradiation, benzalkonium chloride treatment and partial gut stenosis57,58.
It is possible that the neurogenic potential of glia is only enacted during challenges that drive the system away from homeostasis and data showing that enteric glia contribute to the formation of new neurons during colitis102 in response to chemical injury58 and in the presence of oestrogen receptor-β agonists in mouse models of enteric neuronal damage119 are in line with this conclusion. Interestingly, the depletion of glial cells in the PLPCreER;Rosa26DTA mouse model does not substantially alter the susceptibility to dextran sodium sulfate-induced colitis45, whereas genetic mouse models targeting specific glial mechanisms involved in inflammatory responses (such as the glial enzyme NTPDase2) do exhibit increased susceptibility to dextran sodium sulfate-induced colitis97. These differences suggest that future work assessing the specific glial maintenance functions might require subtle perturbations that only affect the mechanism of interest to avoid confounding factors in depletion models.
Enteric neurons could also directly fulfil some of the supportive roles attributed to glia in other systems. For example, glia regulate oxidative stress by contributing to glutathione synthesis but both enteric neurons and glia express components of the glutathione biosynthesis machinery and it is likely that neurons are able to fulfil this function in the absence of glia99. Other non-neuronal cells also support enteric neuron survival, such as intestinal smooth muscle, an important source of GDNF, which promotes neurogenesis and survival in the ENS120. Similar questions have arisen regarding the role of glia in the support of intestinal epithelial barrier function. Glia are the only neuron-type cell body at the level of the intestinal mucosa and are considered potent modulators of intestinal epithelial barrier function104. Enteric glia extend processes to the mucosal crypts and to the tips of the villi, ensheathing the neuronal processes involved in the control of mucosal secretions121. In addition, glia release barrierenhancing factors in vitro, including 15-hydroxyeicosatetraenoic acid122, S-nitrosoglutathione47 and GDNF123, and levels of these factors are decreased in inflammatory bowel disease (IBD). Data from the GFAPHSV-TK mouse model show that ablating enteric glia produces a profound loss of intestinal barrier function76,77. However, complications with toxicity to non-glial cells and bystander effects in intestinal epithelial cells in this model confound a clear interpretation of the results45. Experiments using the PLPCreER;Rosa26DTA mouse model did not demonstrate toxicity in neighbouring cells nor that intestinal barrier function was affected45. Similarly, intestinal barrier function is unaffected in transgenic mouse models that have perturbed glial signaling11 and in germ-free mice that lack mucosal glia34,124. Mice can tolerate a large loss of enteric glia, which makes the understanding of the contribution of enteric glia to intestinal epithelial barrier integrity and functions difficult to extrapolate. Glia clearly have the capacity to influence intestinal epithelial cells in culture systems36,104,125; however, whether this aspect is essential under homeostatic conditions in vivo remains contested.
Role in gastrointestinal pathophysiology
Neuropathology can be broadly defined as a failure of the nervous system to maintain local homeostasis. In this sense, it is essential to consider glia in any study of neuropathology given their contributions to maintaining local homeostasis in concert with other resident cells126,127. This aspect is particularly relevant in disorders of gut–brain interactions (previously known as functional gastrointestinal and motility disorders), in which abnormal motility and pain are considered disturbances of neurogastroenterology128, and in age-related digestive disorders in which altered ENS homeostasis contributes to a decline in gastrointestinal neuromuscular function60,129–131. Enteric glia influence disease processes in intestinal disorders that are seemingly diverse in nature through mechanisms that regulate neuroplasticity and immune responses12,27,113. These mechanisms are also relevant for inflammatory disorders and new observations suggest that they also contribute to colon carcinogenesis20, enteric neuroinflammation132 and age-related gut motility disorders60,129.
Activated, reactive and dysfunctional glia.
The interchangeable usage of terminology such as ‘activated’, ‘reactive’ and ‘gliopathy’ to describe different states of enteric glia has led to considerable ambiguity within the existing literature. These terms are used extensively despite a lack of clear defining criteria. Here, we provide a set of guidelines that summarize the defining characteristics of activated enteric glia, reactive enteric glia and enteric gliopathy (TABLE 2).
Table 2 |.
Criteria for and examples of activated glia, reactive glia or gliopathy
| Glial phenotype | Criteria | Examples from experimental evidence |
|---|---|---|
| Activated enteric glia | Response to physiological stimuli; normal signalling mechanisms whereby enteric glia monitor the activity of cells in the surrounding tissue; primarily enacts glial mechanisms that exert beneficial, homeostatic effects | Enteric glia exhibit activity encoded by intracellular Ca2+ signalling in response to neurotransmitters released by intrinsic (enteric)16,60,64,66,68,70,71,179 and extrinsic12,18 neurons; enteric glial activation encoded by intracellular Ca2+ responses modulates enteric excitatory motor10,14,17,60 and secretomotor11 neurocircuits; enteric glia modulate local immune response in vitro and in vivo37,48,151,157; enteric glia influence the maturation and differentiation of the intestinal epithelium in vitro46,104–106,180 |
| Reactive enteric glia | Response to pathophysiological perturbation of any severity; involves altered molecular composition, structure and/or function; changes undergone are progressive and depend on the type and severity of injury, glial subtype and specific molecular signals received; changes have the potential to alter glial activities through both gain and loss of functions that can be either beneficial or detrimental to the surrounding neural and non-neuronal cells | Enteric glia respond to intestinal inflammation12,13,62,116,148,155 and infection in vitro and in vivo22,148,170,172; enteric glia undergo changes in GFAP expression, morphology, cytokine release and gene expression profile during intestinal inflammation in vitro and in vivo 12,113,133; enteric glia contribute to neuron death during acute intestinal inflammation13,147; enteric glia contribute to vagal anti-inflammatory effects on resident intestinal immune cells following intestinal injury19,157; enteric glia influence visceral sensitivity through interactions with muscularis macrophages48 |
| Enteric gliopathy | Dysfunctional or maladaptive response and/or survival of glial cells; might be primary and result from genetic or acquired dysfunction emanating from glia, or secondary whereby glial dysfunction results from effects in the surrounding tissue; exerts detrimental effects that contribute to disease | Glial support of epithelial barrier homeostasis is impaired in Crohn’s disease in vitro122,181; enteric glial networks are impaired and display dysfunctional responses in patients with Crohn’s disease77,90 |
Activation describes a normal physiological process whereby enteric glia detect extracellular signals. Enteric glia express diverse receptors and are constantly monitoring their extracellular environment. In this sense, enteric glia are nearly always activated and only rarely, if ever, at rest. Glial activation triggers physiological intracellular signal transduction cascades such as those mediated by Ca2+ and cAMP10,14,133. The downstream effects of glial activation are generally considered beneficial and function to modulate intestinal reflexes and/or maintain homeostasis14,17,19,44.
‘Reactive’ describes the status of enteric glia that are responding to a pathophysiological perturbation of any severity. Reactive enteric gliosis is a highly complex continuum of dynamic states that is context dependent and disease specific. Reactive gliosis is often considered a deleterious response in which glia exhibit an increase in pro-inflammatory functions at the expense of homeostatic functions such as during acute colitis in mice12. However, this assumption is misleading as reactive glia also exert beneficial effects and it could be argued that reactive gliosis is an attempt to maintain homeostasis. Criteria that define reactive enteric gliosis are currently lacking and experimental assessment relies on assessing changes in morphology and/or the expression of markers such as GFAP and S100β134–136. Whether or not changes in morphology or GFAP expression occur is not a definitive indication of a reactive glial phenotype as not all insults will produce detectable changes in morphology or GFAP expression and glial heterogeneity will affect how glial subtypes respond. More definitive guidelines for defining reactive astrogliosis have been proposed and are relevant when defining reactive enteric gliosis137,138. In general, reactive enteric gliosis encompasses four main features and reactive enteric glia can display one or more of the these changes depending on the severity and type of insult: (1) enteric glia can be considered reactive if they alter their molecular composition, structure and/or function in response to a pathophysiological perturbation of any severity; (2) the degree to which these changes occur varies with the nature and severity of the insult, with progressive alterations in molecular expression, progressive cellular hypertrophy and, in severe cases, proliferation; (3) the changes undergone by reactive enteric glia depend upon the type and severity of the insult, time following insult, the type of enteric glia, location within the intestine and the specific molecular signals received; and (4) the changes undergone during reactive gliosis have the potential to alter enteric glial activities through both the gain and loss of functions that can be either beneficial or detrimental to the surrounding neural and non-neuronal cells.
Enteric gliopathy refers to a dysfunctional or maladaptive response and/or survival of enteric glia. Gliopathies can result from genetic or acquired abnormalities that cause dysfunction specifically in glia (primary) or from effects in the surrounding tissue that produce glial dysfunction (secondary). An important difference between reactive gliosis and gliopathies is that gliopathies are considered maladaptive and are therefore always detrimental. Reactive gliosis is also considered a transient process, whereas a gliopathy is permanent. However, whether chronic reactive gliosis can produce a gliopathy is not understood.
Enteric glia in neuroplasticity.
Neuronal plasticity involving alterations to the sensitivity and/or survival of intrinsic and extrinsic neurons innervating the intestine produces disturbed intestinal motility and visceral pain in both functional and organic gastrointestinal disorders139–142. Key features of enteric neuroplasticity include the facilitation of presynaptic transmission, increased excitability of sensory neurons and interneurons, defects in purinergic neuromuscular transmission, the degeneration of inhibitory neurons and the development of reactive gliosis13,14,139,143–147. As noted, reactive gliosis can include deleterious effects that contribute to neurodegeneration and changes in neuromuscular function driven by acute inflammation12,13,147, infection148 or metabolic challenges78. In these examples, enteric glia are reacting to a potentially harmful environment to defend the nervous system from damage. Alternatively, reactive enteric gliosis can be triggered by intense activity in sensory neurons and enteric neurons in the absence of other inflammatory stimuli12,13. In this context, neurogenic inflammation, or inflammation arising from the nervous system itself, drives reactive gliosis through axon reflexes that can initiate a self-perpetuating process that results in permanent changes in neural circuitry. In either case, the development of reactive gliosis contributes to neuroinflammation and, subsequently, to neuronal plasticity and functional abnormalities (FIG. 4a).
Fig. 4 |. Intercellular glial signalling mechanisms that contribute to neuroplasticity during gastrointestinal inflammation.
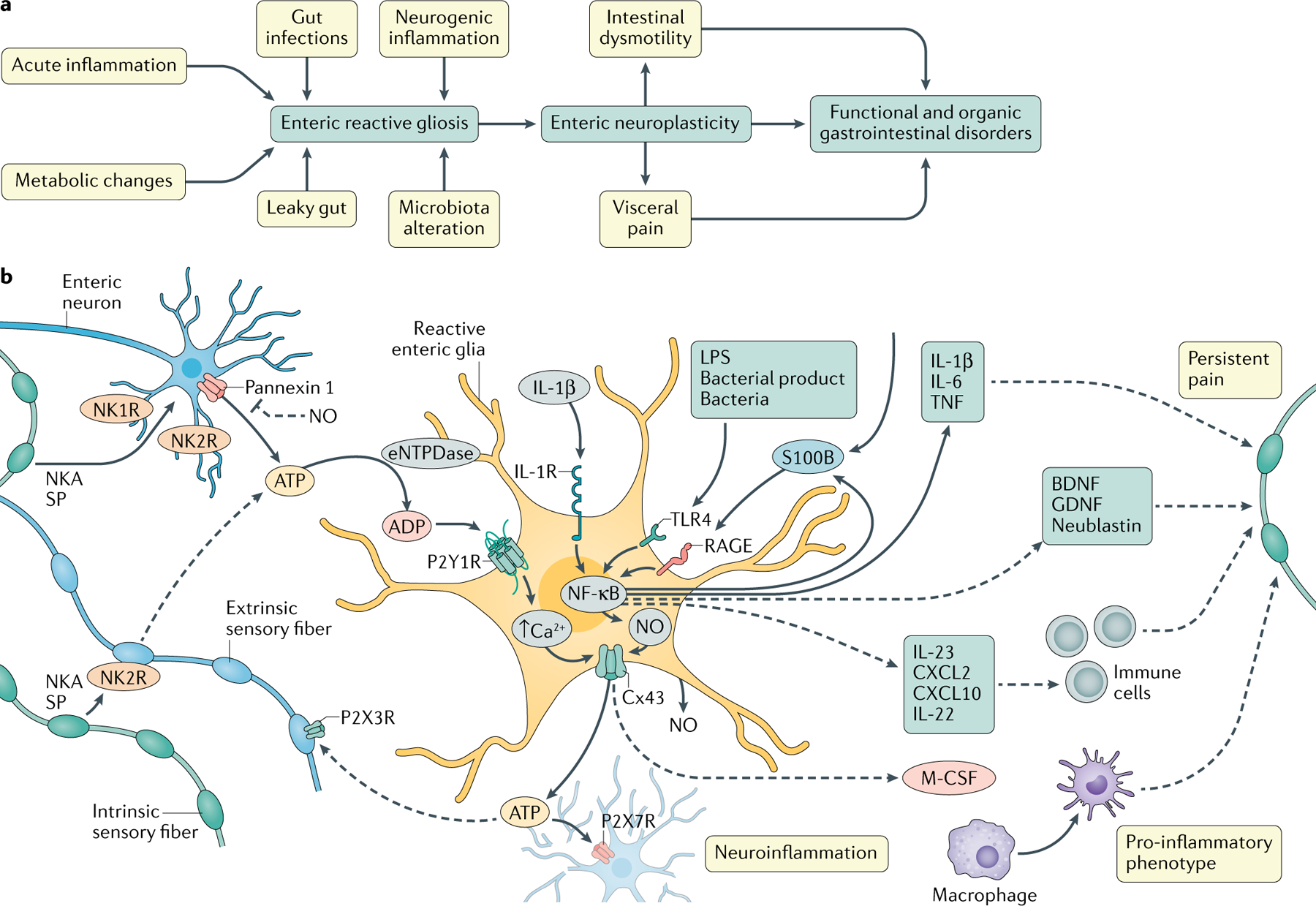
a | Reactive enteric gliosis is a protective response to potentially harmful stimuli. Reactive enteric glia contribute to functional abnormalities that underlie both functional and organic gastrointestinal disorders by promoting neuronal plasticity. b | Enteric glial signalling mechanisms active during acute inflammatory responses. Enteric reactive gliosis may be driven by adenosine triphosphate (ATP) release via pannexin 1 channels from enteric neurons intensively stimulated by neuronal mediators, such as neurokinin A (NKA), substance P (SP) or ATP. A self-perpetuating process derives from the activation of P2Y1Rs in the surrounding glial cells and their release of ATP through connexin 43 (Cx43) hemichannels. ATP released by glia drives P2X7R-mediated neuroinflammation and nociceptive neuron activation, likely via P2X3Rs. Glial activation also mediates the Cx43-dependent release of macrophage colony-stimulating factor (M-CSF) and other mediators that potentially regulate the activation of muscularis macrophages and visceral sensitivity in intestinal inflammation. Pro-inflammatory signals, such as S100β, IL-1β and bacterial products, increase the release of nitric oxide (NO) and other inflammatory signals via NF-κB that directly or indirectly affect the normal glia–neuron purinergic signalling and/or neuronal sensitivity through actions on local immune responses. eNTPDase, ectonucleoside triphosphate diphosphohydrolase 2; GDNF, glial cell-derived neurotrophic factor; LPS, lipopolysaccharide.
Enteric glia alter the survival and function of neurons through active signalling mechanisms and through more passive processes that regulate the secretion and availability of neuroactive compounds. Active glial signalling during neurogenic inflammation has a strong purinergic component that is required for the development of reactive gliosis, neuron death and long-term changes in neuromuscular function12,13,147. This process is initiated by ATP released from neurons through pannexin 1 channels and is subsequently perpetuated and amplified by the activation of the surrounding glial cells and their release of ATP through Cx43 hemichannels13. The ATP released by glia in this context acts on neuronal P2X7 receptors to promote neuronal death and neuroinflammatory processes13,147 (FIG. 4b). Interestingly, similar mechanisms of glial recruitment by neurotransmitters and gliotransmitter release are involved in normal enteric reflexes but do not cause neuroinflammation14. One reason for this finding is that pro-inflammatory mediators, such as nitric oxide generated by glia during acute inflammation, interact with normal signalling mechanisms to enhance ATP release13. Normal neuron–glia signalling does not have a prominent nitric oxide component as the glial increase in inducible nitric oxide synthase expression only occurs during inflammation, thereby differentiating between physiological and pathological signalling. Additionally, physiological enteric neuron-to-glia signalling involves cholinergic signalling and the activation of glial cholinergic receptors does not cause neuroinflammation14. This finding implies that receptor-specific information is encoded in glial Ca2+ responses because glial purinergic and cholinergic receptors both signal through intracellular pathways that utilize Ca2+. Furthermore, enteric glia do not seem to propagate Ca2+ responses throughout the glial network under normal conditions and glial activation is spatially restricted within so-called neuron–glia units in which only glial cells that surround a given neuron are activated in vitro9. How glia encode or interpret this information to differentiate between pathological and physiological signals is not yet understood.
Transcriptional profiling of mouse and human reactive enteric glia suggests that glial cytokine secretion, neurotrophin secretion and the regulation of neuromodulator availability contribute to neuroplasticity during inflammation12,113,133 (FIG. 4b). Reactive enteric glia increase their production of pro-inflammatory and anti-inflammatory cytokines and chemokines but the specific effects of glial-derived cytokines and/or chemokines are not well defined. Certain glial proinflammatory proteins, such as IL-1β and IL-6, enhance neuronal excitability by modifying glial and neuronal signaling149,150, while others, such as CXCL2, CXCL10, IL-22 and IL-23, might indirectly affect neuronal sensitivity through actions on local immune responses12,133,151. For example, an increase in the production of IL-1β by enteric glia is considered an important mechanism in the development of postoperative ileus by modulating ENS activity152 and glial macrophage colony-stimulating factor (M-CSF) production influences visceral sensitivity following intestinal inflammation through Cx43-dependent signalling with muscularis macrophages48. Glial neurotrophins might also contribute to neuronal sensitization and links between enteric glia, nerve growth factor112 and substances produced in response to brain-derived neurotrophic factor153 have been proposed. In addition, comparing transcriptomics datasets to assess the potential ‘interactome’ between enteric glia and colon-projecting dorsal root ganglion neurons supports the concept that neurotrophins could be potential molecular substrates of interactions (published as pre-print154). However, these interactions have not yet been directly tested. More conclusive data show that S100β protein release via glial RAGE–NF-κB signalling pathways is associated with the onset and maintenance of nitric oxide-dependent inflammation in the human gut (rectal biopsy samples from patients with ulcerative colitis155) and that the S100β–RAGE interaction promotes the release of pro-inflammatory cytokines, including IL-1β, TNF and nitric oxide, as well as the recruitment of immune cells such as macrophages and mast cells to the intestinal mucosa in mice and humans. Mouse and human transcriptomics datasets indicate that inflammation also induces major changes in glial molecular mechanisms that regulate neuroactive compounds such as ATP, histamine and serotonin, but the effect of these changes on neuronal signalling is mostly undefined12,97,133.
Enteric glial–immune interactions.
The intestine is the largest immune organ and glial cells are ideally positioned to bridge interactions between the immune and nervous systems156. Yet, little is known about bi-directional interactions between enteric glia and immune cells, their physiological relevance, or the specific mechanisms involved. Glial transcriptional responses to experimental colitis in mice and lipopolysaccharide in vitro exhibit an increased expression of genes associated with immune responses12,113,148. Many of these genes reflect the modulatory effects on innate immune responses, which is supported by data in mice showing that glia secrete neurotrophins that fine-tune innate IL-22 production from group 3 innate lymphoid cells37. Likewise, data show that enteric glia promote muscularis macrophages to adopt a pro-inflammatory phenotype through mechanisms that involve Cx43 and M-CSF release during chronic colitis in mice48. Enteric glia also modulate adaptive immune responses and have immunosuppressive effects on T cells in vitro151. The mechanisms whereby enteric glia influence T cells are not known, but human and mouse enteric glia do express major histocompatibility complex class II in response to pathogenic microorganisms in vitro148 and glial antigen presentation contributes to interactions with T cells in a mouse transgenic model of Crohn’s disease77. Enteric glia also modulate local immune responses and gut barrier integrity after injury by transmitting vagal anti-inflammatory signals to resident immune cells157. In addition to the modulatory effects, enteric glia might also initiate immune responses. Support for this concept comes from in vitro work showing that the exposure to pathogenic enteroinvasive Escherichia coli bacteria elicits protective responses in enteric glia that include the upregulation of immunomodulatory mechanisms148. The relative importance of glial-driven immune responses or modulation in vivo is still unclear and will be an important area for future research.
Enteric glia in cancer.
Glia have a central role in tumorigenesis in the nervous system and current data suggest that the same is true in the intestine, where enteric glia contribute to multiple aspects of tumorigenesis. For example, human duodenal gastrinomas associated with mutations in the MEN1 gene are composed of cells expressing markers of enteric glia21. Under these circumstances, glial gastrin expression is induced in a feed-forward signalling loop that involves the activation of cholecystokinin B receptors and results in the loss of menin protein and the de-repression of gastrin messenger RNA in mice21. Other mechanisms of tumorigenesis identified by in vitro studies show that bi-directional signalling between enteric glia and colon cancer stem cells drives tumorigenesis through mechanisms that involve IL-1 release from tumour epithelial cells and PGE2 release from enteric glia20. In cultured biopsy samples, the increased expression of glial S100β also parallels tumour progression in the human colon and could contribute to the carcinogenic microenvironment by sequestering the pro‐apoptotic factor wild-type p53 and upregulating pro‐inflammatory and proangiogenic mediators158. Interestingly, enteric glia only acquire a pro-tumorigenic phenotype when exposed to tumour epithelial cell-derived IL-1 in vitro20. This finding suggests that glia might not initiate tumorigenesis but, once activated by a tumour, can stimulate further tumorigenesis. Tumour cells also utilize the ENS as a substrate for tumour invasion and, according to coculture studies, neurons are the predominant adhesion partner of tumour cells; the role of glia in this process is unclear159.
Enteric glia in extraintestinal diseases.
Enteric glia are gaining substantial interest due to their role as an early ‘sentinel’ of neuroplastic changes that develop during various extraintestinal pathologies. Enteric glia exhibit a reactive phenotype during the earliest stages of extraintestinal diseases, such as Parkinson disease160 and obesity161, and enteric glia are the first site of entry to the nervous system in certain intestinal infections that spread to the brain such as prion disease and JC virus infection22,162. Glial GFAP expression and phosphorylation is elevated in colonic biopsy samples from patients with Parkinson disease and is associated with increased permeability and mucosal inflammation163,164. This local glial reaction might be driven by extracellular α‐synuclein or other neurotropic agents that breach the intestinal epithelial barrier and trigger the initial α‐synuclein aggregation in axon terminals in the enteric plexuses according to the Braak hypothesis165,166. Glial transition to a reactive phenotype might, in turn, exacerbate local inflammatory responses and synaptic dysfunction and facilitate the ascension of Parkinson disease pathology to the CNS167. Active parts played by enteric glia in the neuroinvasion process of prion disease provide support for this hypothesis. In mice, enteric glia act as a reservoir and replication site for misfolded prion protein before its replication in the brain and enteric glia exhibit the same alterations observed later in central astrocytes, including altered morphology and co-localization of misfolded prion protein in GFAP+ cells168. The observation that JC virus preferentially infects myenteric glia in patients with chronic idiopathic intestinal pseudo-obstruction reinforces the concept that glia act as an initial site of entry for intestinal pathogens that disrupt neuromuscular function22. Thus, a growing body of evidence suggests that glia contribute to widespread nervous system disorders by facilitating the spread of pathogens that cross an impaired intestinal barrier. Whether glial changes are causative or reactionary to neuroplastic or barrier abnormalities remains to be clarified. Traumatic brain injury does increase colonic glial Sox10 and GFAP expression along with mucosal permeability in mice169, suggesting descending modulatory mechanisms. By contrast, intracolonic administration of HIV1 Tat protein in rats triggers ascending dysfunction reflected by increases in Cx43 (GJA1) and inducible nitric oxide synthase expression in S100β+ cells in the submucosal plexus followed by similar increases in the spinal cord and frontal cortex and, ultimately, cognitive performance impairment170. These observations suggest that glia contribute to bi-directional communication between the periphery and the brain during pathophysiological states, although the mechanisms regulating glial crosstalk remain unknown.
Translational implications
The neuromodulatory and immunomodulatory properties of enteric glia are increasingly attractive as therapeutic targets for diverse gastrointestinal diseases (TABLE 3), including diseases with active inflammatory components such as IBD (to modulate immune responses), diseases that involve neuroplasticity such as IBS or IBD in remission (to tune the sensitivity of neurons), and diseases with unclear aetiology such as postoperative ileus or chronic intestinal pseudo-obstruction (to improve motor function). Given the evolving role of enteric glia in tumorigenesis, the targeting of glial mechanisms could be explored as a potential cancer treatment. Drugs or therapies that are specifically designed to affect glial mechanisms do not exist. However, glial mechanisms are affected by many of the recently approved or new emerging therapies for functional bowel disorders171. For example, at least some of the beneficial effects of drugs for IBS, such as the neurokinin 2 receptor antagonist ibodutant, muscarinic M3 receptor antagonists and the glutamine synthetase product glutamine, might involve effects on glia based on in vitro and in vivo data that show glial expression and the function of these mechanisms12,14,73,171. Similarly, the beneficial actions of P2X7 receptor antagonists on visceral pain in individuals with IBD170 suggest that purinergic neuron–glia signalling in the context of acute inflammation contributes to visceral pain and experiments in animal colitis models support this mechanism12. The same might be true for pannexin 1 antagonists such as spironolactone, which is currently used as a treatment for hypertension but has not yet been explored for its effects in gastrointestinal disorders. The reduction of reactive gliosis in animal models of intestinal inflammation improves the survival of enteric neurons and protects against long-term changes in intestinal motor functions12,62,172,173. Based on these experimental data, drugs that reduce reactive gliosis, such as the PPARα agonist palmitoylethanolamide62,172, cannabidiol135, pentamidine173, the RAGE inhibitors174,175, the IL-1 receptor antagonist anakinra152,176 or ibodutant177, could be beneficial in the treatment of gastrointestinal diseases with inflammatory or neuroinflammatory processes. Data from animal models show that glia have the capacity to control enteric motor circuits and that targeting the conserved mechanisms in humans could be a powerful therapeutic approach for motility disorders.
Table 3 |.
Targeting glia for translational benefit
| Glial mechanism | Potential benefit | Available drugs |
|---|---|---|
| Neuron–glia communication in acute inflammation | Reduce neuroinflammation, neuroprotection and visceral hypersensitivity (pain and dysmotility) | P2X7R antagonists: AZD9056, CE-224,535, GSK1482160 (REFs147,177,182,183) Pannexin 1 antagonists: spironolactone178 Neurokinin 2 receptor antagonists: ibodutant177 |
| Pathways triggering gliosis | Inflammation, inflammatory postoperative ileus, acute inflammation, inflammation, bacterial infections or in gastrointestinal diseases involving bacterial translocation, and neuroinflammation | Anakinra: IL-1R antagonist152 Proliferator-activated receptor-α agonist: palmitoylethanolamide62 Toll-like receptor, receptor for advanced glycation endproducts or nitric oxide synthase antagonists: L-NMMA, PF-04494700 (REFs13,61,148,174,175,184) Peroxisome proliferator-activated receptor-γ agonist: cannabidiol135 S100β inhibitor: pentamidine148,173,184,185 |
| Gliotransmission | Motility disorders such as postoperative ileus, chronic intestinal pseudo-obstruction or chronic constipation | None |
Conclusions
Enteric glia are one of the most dynamic cell types in the gastrointestinal tract and fulfil diverse roles in neuronal support, mucosal integrity, neuroprotection, neurogenesis, neuroimmune interactions and synaptic transmission. Although the diversity of glial functions is only beginning to be unveiled, those currently known demonstrate incredible plasticity, wide heterogeneity and place enteric glia in a crucial regulatory role in all major gut activities. Increasingly sophisticated genetic and imaging technologies continue to facilitate the understanding of specific functions of enteric glia and have led to major progress in terms of understanding the functional relevance of glial Ca2+ response in motor circuits. However, the complexity of the signalling between enteric glia, neurons and non-neuronal cells is still poorly defined and the influence of these interactions on gut health and disease is unclear. The specificity of glial signalling in the digestive tract, molecular signatures and functional characteristics of glial subtypes, and how enteric glia contribute to gastrointestinal dysfunction and disorders of the gut–brain axis remain open questions (BOx 1). Answering these questions represents a stimulus for growth in neurogastroenterology and an opportunity to identify new therapeutic strategies for gastrointestinal and extraintestinal disorders.
Box 1 |. Outlook and open questions.
Outlook
Bi-directional enteric neuron–glia communication is a fundamental mechanism that regulates enteric nervous system functions.
Enteric glia display phenotypic plasticity and glial heterogeneity is present within regions, between regions and during pathophysiological states.
Enteric glia communicate with immune cells and influence immune homeostasis.
Enteric glia have complex interactions with nerves, immune cells, microbiota, enterocytes and neuroendocrine cells at the mucosal interface.
Enteric glia encode and decode physiological and pathological information in intracellular Ca2+ transients.
Enteric glia have a key role in gastrointestinal tumorigenesis.
Enteric glia regulate and are influenced by inflammation; changes in gut functions during inflammation affect local gastrointestinal functions and gut–brain signalling.
Open questions
Are glial signalling mechanisms specific to individual neuron types and pathways? If so, how do glia encode this information and use it to influence neurons and other cell types that coordinate gastrointestinal functions?
How extensive is glial heterogeneity in the digestive system? What are the combinatorial codes that define different glial subtypes by distinct protein expression, responsiveness to various intercellular mediators and transcriptional profiles?
What is the role of enteric glia in gut–brain communication? Are specific glial subtypes devoted to intrinsic and extrinsic neurons and what is their role in gut–brain signalling?
To what extent do glia contribute to neurogenesis and gliogenesis under normal and pathophysiological conditions? Is a specific progenitor subtype responsible? What are the mechanisms that promote neurogenesis versus gliogenesis?
How do glia regulate immune homeostasis and how is this altered by disease?
How do specific reactive glial phenotypes contribute to disease?
Example experimental approaches for open questions
Functional imaging of enteric neuron–glia networks.
Glial and neuronal directed cellular actuators; for example, designer receptors exclusively activated by designer drugs and indicators (such as genetically encoded Ca2+ indicators).
Slide-seq, single-cell RNA sequencing and RNAscope to study distinct glial subtypes and assess molecular changes under diverse conditions.
In vivo Ca2+ imaging of gut-projecting neurons, combined with glial designer receptors exclusively activated by designer drugs to study how enteric glia influence those neurons.
Single-cell lineage tracing studies combined with pseudotime transcriptional analyses of molecular changes associated with progenitor-to-neuron and progenitor-to-glia differentiation.
Models that perturb glial-specific immune-modifying signalling pathways and test the outcomes on immune homeostasis in health and disease.
Single-cell transcriptional analysis of reactive glia throughout disease progression and resolution.
Supplementary Material
Key points.
Enteric glia are a heterogeneous population of peripheral neuroglia that regulate homeostasis in the enteric nervous system.
Bidirectional communication between enteric glia and neurons modulates intestinal reflexes.
Enteric glia are central players in neuroinflammation and contribute to neuroplasticity through interactions with neurons and immune cells.
Enteric glia regulate disease processes involved in tumorigenesis and extragastrointestinal diseases.
Therapies targeting glial mechanisms, such as gliotransmitter release or signalling pathways that promote gliosis, could substantially advance the treatment of common gastrointestinal diseases.
Acknowledgements
B.D.G. receives support from grants R01DK103723 and R01DK120862 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The content is solely the responsibility of the Authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks W. Boesmans, M. Hao, K. Sharkey and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41575-021-00423-7.
References
- 1.Furness JB The enteric nervous system: normal functions and enteric neuropbbathies. Neurogastroenterol. Motil 20, 32–38 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Furness JB The Enteric Nervous System (John Wiley & Sons, 2008). [Google Scholar]
- 3.Fung C & Vanden Berghe P Functional circuits and signal processing in the enteric nervous system. Cell Mol. Life Sci 77, 4505–4522 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasrado R et al. Lineage-dependent spatial and functional organization of the mammalian enteric nervous system. Science 356, 722–726 (2017).This paper describes how the developing enteric nervous system is organized into overlapping clonally related units that exhibit synchronous activity in response to network stimulation.
- 5.Jarret A et al. Enteric nervous system-derived IL-18 orchestrates mucosal barrier immunity. Cell 180, 50–63.e12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klose CSN et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 549, 282–286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardoso V et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 17, 755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller PA et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boesmans W et al. Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 67, 1167–1178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClain JL, Fried DE & Gulbransen BD Agonistevoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell. Mol. Gastroenterol. Hepatol 1, 631–645 (2015).This study uses a chemogenetic mouse model to activate Ca2+ signalling in enteric glia; the results showed that glial activity enhances and/or activates myenteric motor neurocircuits.
- 11.Grubišić V & Gulbransen BD Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J. Physiol 595, 3409–3424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvalle NM et al. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell. Mol. Gastroenterol. Hepatol 6, 321–344 (2018).This study shows that extrinsic sensory neurons promote neuroinflammation through effects on enteric glia and also provides a database of transcriptional changes in reactive enteric glia during experimental colitis.
- 13.Brown IAM, McClain JL, Watson RE, Patel BA & Gulbransen BD Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol 2, 77–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delvalle NM, Fried DE, Rivera-Lopez G, Gaudette L & Gulbransen BD Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol 315, G473–G483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vergnolle N & Cirillo C Neurons and glia in the enteric nervous system and epithelial barrier function. Physiology 33, 269–280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fung C et al. VPAC receptor subtypes tune purinergic neuron-to-glia communication in the murine submucosal plexus. Front. Cell. Neurosci 11, 118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasser Y et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol 291, G912–G927 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Gulbransen BD, Bains JS & Sharkey KA Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J. Neurosci 30, 6801–6809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costantini TW et al. Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am. J. Physiol. Gastrointest. Liver Physiol 299, G1308–G1318 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valès S et al. Tumor cells hijack enteric glia to activate colon cancer stem cells and stimulate tumorigenesis. EBioMedicine 49, 172–188 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundaresan S et al. Gastrin induces nuclear export and proteasome degradation of menin in enteric glial cells. Gastroenterology 153, 1555–1567.e15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selgrad M et al. JC virus infects the enteric glia of patients with chronic idiopathic intestinal pseudo-obstruction. Gut 58, 25–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanani M & Reichenbach A Morphology of horseradish peroxidase (HRP)-injected glial cells in the myenteric plexus of the guinea-pig. Cell Tissue Res 278, 153–160 (1994). [DOI] [PubMed] [Google Scholar]
- 24.Boesmans W, Lasrado R, Vanden Berghe P & Pachnis V Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63, 229–241 (2015).This study uses immunohistochemistry and Ca2+ imaging to provide evidence of heterogeneity among enteric glia in the myenteric plexus of the mouse colon.
- 25.Zeisel A et al. Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018).This study demonstrates functional and phenotypic heterogeneity across distinct enteric glia subtypes.
- 26.Drokhlyansky E et al. The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622.e23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bon-Frauches AC & Boesmans W The enteric nervous system: the hub in a star network. Nat. Rev. Gastroenterol. Hepatol 17, 717–718 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Dulac C & Le Douarin NM Phenotypic plasticity of Schwann cells and enteric glial cells in response to the microenvironment. Proc. Natl Acad. Sci. USA 88, 6358–6362 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soret R et al. Glial cell derived neurotrophic factor induces enteric neurogenesis and improves colon structure and function in mouse models of Hirschsprung disease. Gastroenterology 159, 1824–1838 (2020).Data in this study suggest that Schwann cells associated with extrinsic nerves are capable of forming new enteric neurons and glia under certain circumstances.
- 30.Espinosa-Medina I et al. Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proc. Natl Acad. Sci. USA 114, 11980–11985 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uesaka T, Nagashimada M & Enomoto H Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J. Neurosci 35, 9879–9888 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Nachef WN & Bronner ME De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types. Development 147, dev186619 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao M et al. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia 63, 2040–2057 (2015).This study provides the first transcriptional analysis of enteric glia and compares transcriptional profiles to other populations of glia.
- 34.Kabouridis PS et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 85, 289–295 (2015).Results from this study show that enteric glia at the level of the lamina propria are continuously renewed by cells that originate in the submucosal and myenteric plexuses.
- 35.Bohórquez DV et al. An enteroendocrine cell – enteric glia connection revealed by 3D electron microscopy. PLoS ONE 9, e89881 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neunlist M et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-beta1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol 292, G231–G241 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Ibiza S et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 535, 440–443 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YA et al. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterol. Motil 25, e324–e338 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Kaelberer MM et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin W et al. Interferon-gamma induced medulloblastoma in the developing cerebellum. J. Neurosci 24, 10074–10083 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jessen KR, Thorpe R & Mirsky R Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. J. Neurocytol 13, 187–200 (1984). [DOI] [PubMed] [Google Scholar]
- 42.Park YM, Chun H, Shin JI & Lee CJ Astrocyte specificity and coverage of hGFAP-CreERT2 [Tg(GFAP-Cre/ERT2)13Kdmc] mouse line in various brain regions. Exp. Neurobiol 27, 508–525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Z et al. The appropriate marker for astrocytes: comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. BioMed. Res. Int 2019, 9605265 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jessen KR & Mirsky R Glial fibrillary acidic polypeptides in peripheral glia. Molecular weight, heterogeneity and distribution. J. Neuroimmunol 8, 377–393 (1985). [DOI] [PubMed] [Google Scholar]
- 45.Rao M et al. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153, 1068–1081.e7 (2017).This study highlights complications with glial ablation models that draw the necessity of glial support into question.
- 46.Van Landeghem L et al. Enteric glia promote intestinal mucosal healing via activation of focal adhesion kinase and release of proEGF. Am. J. Physiol. Gastrointest. Liver Physiol 300, G976–G987 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savidge TC et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132, 1344–1358 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Grubišić V et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep 32, 108100 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z et al. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. eLife 8, 899 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avetisyan M, Schill EM & Heuckeroth RO Building a second brain in the bowel. J. Clin. Invest 125, 899–907 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiyama C et al. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat. Neurosci 15, 1211–1218 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Young HM, Bergner AJ & Müller T Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J. Comp. Neurol 456, 1–11 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Hao MM, Capoccia E, Cirillo C, Boesmans W & Vanden Berghe P Arundic acid prevents developmental upregulation of S100B expression and inhibits enteric glial development. Front. Cell. Neurosci 11, 42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burns AJ & Le Douarin NM Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat. Rec 262, 16–28 (2001). [DOI] [PubMed] [Google Scholar]
- 55.Kabouridis PS & Pachnis V Emerging roles of gut microbiota and the immune system in the development of the enteric nervous system. J. Clin. Invest 125, 956–964 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hao MM et al. Enteric nervous system assembly: functional integration within the developing gut. Dev. Biol 417, 168–181 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Joseph NM et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Invest 121, 3398–3411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laranjeira C et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Invest 121, 3412–3424 (2011).Joseph et al. and Laranjeira et al. provide complementary evidence that suggests enteric glia are able to form neurons and glia in culture, but only seem to give rise to neurons in vivo in response to intestinal injury.
- 59.Verkhratsky A, Ho MS, Zorec R & Parpura V The concept of neuroglia. Adv. Exp. Med. Biol 1175, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McClain JL et al. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507.e1 (2014).This study shows that connexin 43 hemichannels are an important molecular mediator of glia intercellular communication and that perturbing this mechanism impairs intestinal motility in mice.
- 61.MacEachern SJ, Patel BA, McKay DM & Sharkey KA Nitric oxide regulation of colonic epithelial ion transport: a novel role for enteric glia in the myenteric plexus. J. Physiol 589, 3333–3348 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esposito G et al. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 63, 1300–1312 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Grubišić V & Parpura V Two modes of enteric gliotransmission differentially affect gut physiology. Glia 65, 699–711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ & Smith TK Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol 590, 335–350 (2012).This study provides important evidence showing that glial activity is recruited during physiological patterns of neural activity that generate the colonic migrating motor complex.
- 65.Grubišić V & Gulbransen BD Enteric glia: the most alimentary of all glia. J. Physiol 595, 557–570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gabella G Fine structure of the myenteric plexus in the guinea-pig ileum. J. Anat 111, 69–97 (1972). [PMC free article] [PubMed] [Google Scholar]
- 67.Kimball BC & Mulholland MW Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J. Neurochem 66, 604–612 (1996).This study provides early evidence that neuro- transmitters evoke activity in enteric glia and defined the intracellular signal transduction mechanisms involved.
- 68.Gomes P et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol. Motil 21, 870–e62 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Boesmans W, Hao MM & Vanden Berghe P Optical tools to investigate cellular activity in the intestinal wall. J. Neurogastroenterol. Motil 21, 337–351 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulbransen BD & Sharkey KA Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136, 1349–1358 (2009).This study shows that enteric glia sense neuro- transmitters released by enteric neurons and display activity encoded by intracellular Ca2+ responses.
- 71.Boesmans W et al. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol. Motil 25, e151–e160 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Garrido R, Segura B, Zhang W & Mulholland M Presence of functionally active protease-activated receptors 1 and 2 in myenteric glia. J. Neurochem 83, 556–564 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Fried DE, Watson RE, Robson SC & Gulbransen BD Ammonia modifies enteric neuromuscular transmission through glial γ-aminobutyric acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol 313, G570–G580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith TK & Koh SD A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am. J. Physiol. Gastrointest. Liver Physiol 312, G1–G14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Antonioli L et al. Colonic dysmotility associated with high-fat diet-induced obesity: role of enteric glia. FASEB J 34, 5512–5524 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Bush TG et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998). [DOI] [PubMed] [Google Scholar]
- 77.Cornet A et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn’s disease? Proc. Natl Acad. Sci. USA 98, 13306–13311 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aikawa H & Suzuki K Enteric gliopathy in niacin-deficiency induced by CNS glio-toxin. Brain Res 334, 354–356 (1985). [DOI] [PubMed] [Google Scholar]
- 79.McClain JL & Gulbransen BD The acute inhibition of enteric glial metabolism with fluoroacetate alters calcium signaling, hemichannel function, and the expression of key proteins. J. Neurophysiol 117, 365–375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ro S, Hwang SJ, Muto M, Jewett WK & Spencer NJ Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am. J. Physiol. Gastrointest. Liver Physiol 290, G710–G718 (2006). [DOI] [PubMed] [Google Scholar]
- 81.Savtchouk I & Volterra A Gliotransmission: beyond black-and-white. J. Neurosci 38, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiacco TA & McCarthy KD Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J. Neurosci 38, 3–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martín R, Bajo-Grañeras R, Moratalla R, Perea G & Araque A Glial cell signaling. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Chai H et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu X et al. Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99, 1170–1187.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Auteri M, Zizzo MG & Serio R GABA and GABA receptors in the gastrointestinal tract: from motility to inflammation. Pharmacol. Res 93, 11–21 (2015). [DOI] [PubMed] [Google Scholar]
- 87.Grider JR & Makhlouf GM Enteric GABA: mode of action and role in the regulation of the peristaltic reflex. Am. J. Physiol 262, G690–G694 (1992). [DOI] [PubMed] [Google Scholar]
- 88.King BF Purinergic signalling in the enteric nervous system (An overview of current perspectives). Auton. Neurosci 191, 141–147 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Murakami M, Ohta T, Otsuguro K-I & Ito S Involvement of prostaglandin E2 derived from enteric glial cells in the action of bradykinin in cultured rat myenteric neurons. Neuroscience 145, 642–653 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Cabarrocas J, Savidge TC & Liblau RS Role of enteric glial cells in inflammatory bowel disease. Glia 41, 81–93 (2003). [DOI] [PubMed] [Google Scholar]
- 91.Verkhratsky A & Nedergaard M Physiology of astroglia. Physiol. Rev 98, 239–389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aoki E, Semba R & Kashiwamata S Evidence for the presence of L-arginine in the glial components of the peripheral nervous system. Brain Res 559, 159–162 (1991). [DOI] [PubMed] [Google Scholar]
- 93.Jessen KR & Mirsky R Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J. Neurosci 3, 2206–2218 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ruhl A, Hoppe S, Frey I, Daniel H & Schemann M Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J. Comp. Neurol 490, 1–11 (2005). [DOI] [PubMed] [Google Scholar]
- 95.Fletcher EL, Clark MJ & Furness JB Neuronal and glial localization of GABA transporter immunoreactivity in the myenteric plexus. Cell Tissue Res 308, 339–346 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Lavoie EG et al. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am. J. Physiol. Gastrointest. Liver Physiol 300, G608–G620 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Grubišić V et al. NTPDase1 and −2 are expressed by distinct cellular compartments in the mouse colon and differentially impact colonic physiology and function after DSS colitis. Am. J. Physiol. Gastrointest. Liver Physiol 317, G314–G332 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braun N et al. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 45, 124–132 (2004). [DOI] [PubMed] [Google Scholar]
- 99.Brown IAM & Gulbransen BD The antioxidant glutathione protects against enteric neuron death in situ, but its depletion is protective during colitis. Am. J. Physiol. Gastrointest. Liver Physiol 314, G39–G52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdo HH et al. Enteric glial cells protect neurons from oxidative stress in part via reduced glutathione. FASEB J 24, 1082–1094 (2010). [DOI] [PubMed] [Google Scholar]
- 101.von Boyen GBT et al. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J. Neuroendocrinol 18, 820–825 (2006). [DOI] [PubMed] [Google Scholar]
- 102.Belkind-Gerson J et al. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep 7, 2525 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Le Berre-Scoul C et al. A novel enteric neuron-glia coculture system reveals the role of glia in neuronal development. J. Physiol 595, 583–598 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neunlist M et al. The digestive neuronal-glialepithelial unit: a new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol 10, 90–100 (2013). [DOI] [PubMed] [Google Scholar]
- 105.Soret R et al. Characterization of human, mouse, and rat cultures of enteric glial cells and their effect on intestinal epithelial cells. Neurogastroenterol. Motil 25, e755–e764 (2013). [DOI] [PubMed] [Google Scholar]
- 106.Bach-Ngohou K et al. Enteric glia modulate epithelial cell proliferation and differentiation through 15-deoxy-12,14-prostaglandin J2. J. Physiol 588, 2533–2544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matheis F et al. Adrenergic signaling in muscularis macrophages limits infection-induced neuronal loss. Cell 180, 64–78.e16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nagahama M, Semba R, Tsuzuki M & Aoki E L-arginine immunoreactive enteric glial cells in the enteric nervous system of rat ileum. Biol. Signals Recept 10, 336–340 (2001). [DOI] [PubMed] [Google Scholar]
- 109.Bannerman PG, Mirsky R & Jessen KR Analysis of enteric neurons, glia and their interactions using explant cultures of the myenteric plexus. Dev. Neurosci 9, 201–227 (1987). [DOI] [PubMed] [Google Scholar]
- 110.Broussard DL, Bannerman PG, Tang CM, Hardy M & Pleasure D Electrophysiologic and molecular properties of cultured enteric glia. J. Neurosci. Res 34, 24–31 (1993). [DOI] [PubMed] [Google Scholar]
- 111.Hanani M et al. Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am. J. Physiol. Gastrointest. Liver Physiol 278, G644–G651 (2000).This study characterizes the electrophysiological properties of enteric glia.
- 112.Long X et al. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol. Motil 30, e13227 (2018). [DOI] [PubMed] [Google Scholar]
- 113.Rosenbaum C et al. Activation of myenteric glia during acute inflammation in vitro and in vivo. PLoS ONE 11, e0151335 (2016).This study highlights genomic changes that reflect a reactive phenotype of myenteric glia in vivo driven by exposure to lipopolysaccharide.
- 114.Murakami M, Ohta T & Ito S Lipopolysaccharides enhance the action of bradykinin in enteric neurons via secretion of interleukin-1beta from enteric glial cells. J. Neurosci. Res 87, 2095–2104 (2009). [DOI] [PubMed] [Google Scholar]
- 115.Gougeon P-Y et al. The pro-inflammatory cytokines IL-1β and TNFα are neurotrophic for enteric neurons. J. Neurosci 33, 3339–3351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cirillo C et al. S100B protein in the gut: the evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol 17, 1261–1266 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Magnusson FC et al. Direct presentation of antigen by lymph node stromal cells protects against CD8 T-cell-mediated intestinal autoimmunity. Gastroenterology 134, 1028–1037 (2008). [DOI] [PubMed] [Google Scholar]
- 118.Aikawa H & Suzuki K Lesions in the skin, intestine, and central nervous system induced by an antimetabolite of niacin. Am. J. Pathol 122, 335–342 (1986). [PMC free article] [PubMed] [Google Scholar]
- 119.D’Errico F et al. Estrogen receptor β controls proliferation of enteric glia and differentiation of neurons in the myenteric plexus after damage. Proc. Natl Acad. Sci. USA 115, 5798–5803 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zoumboulakis D, Cirella KR, Gougeon PY, Lourenssen SR & Blennerhassett MG MMP-9 processing of intestinal smooth muscle-derived GDNF is required for neurotrophic action on enteric neurons. Neuroscience 443, 8–18 (2020). [DOI] [PubMed] [Google Scholar]
- 121.Betageri KR et al. Enteric glial networks visualized using SOX10 fluorescent reporter in optically-cleared full thickness intestinal tissues. FASEB J 34 (Suppl. 1), 1–1 (2020). [Google Scholar]
- 122.Pochard C et al. Defects in 15-HETE production and control of epithelial permeability by human enteric glial cells from patients with Crohn’s disease. Gastroenterology 150, 168–180 (2016). [DOI] [PubMed] [Google Scholar]
- 123.Bauman BD et al. Enteric glial-mediated enhancement of intestinal barrier integrity is compromised by morphine. J. Surg. Res 219, 214–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lomasney KW et al. Selective influence of host microbiota on cAMP-mediated ion transport in mouse colon. Neurogastroenterol. Motil 26, 887–890 (2014). [DOI] [PubMed] [Google Scholar]
- 125.Cavin JB, Cuddihey H, MacNaughton WK & Sharkey KA Acute regulation of intestinal ion transport and permeability in response to luminal nutrients: the role of the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol 318, G254–G264 (2020). [DOI] [PubMed] [Google Scholar]
- 126.De Schepper S et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 175, 400–415.e13 (2018). [DOI] [PubMed] [Google Scholar]
- 127.Obata Y et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 578, 284–289 (2020). [DOI] [PubMed] [Google Scholar]
- 128.Drossman DA Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. G astroenterology 150, 1262–1279.e2 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Phillips RJ, Kieffer EJ & Powley TL Loss of glia and neurons in the myenteric plexus of the aged Fischer 344 rat. Anat. Embryol 209, 19–30 (2004). [DOI] [PubMed] [Google Scholar]
- 130.Camilleri M, Cowen T & Koch TR Enteric neurodegeneration in ageing. Neurogastroenterol. Motil 20, 418–429 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Saffrey MJ Aging of the mammalian gastrointestinal tract: a complex organ system. Age 36, 9603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Seguella L, Sarnelli G & Esposito G Leaky gut, dysbiosis, and enteric glia activation: the trilogy behind the intestinal origin of Parkinson’s disease. Neural Regen. Res 15, 1037–1038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liñán-Rico A et al. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype: implications for GI infection, IBD, POI, neurological, motility, and GI disorders. Inflamm. Bowel Dis 22, 1812–1834 (2016).This study provides a comprehensive analysis of human reactive enteric glia.
- 134.Baydas G, Nedzvetskii VS, Tuzcu M, Yasar A & Kirichenko SV Increase of glial fibrillary acidic protein and S-100B in hippocampus and cortex of diabetic rats: effects of vitamin E. Eur. J. Pharmacol 462, 67–71 (2003). [DOI] [PubMed] [Google Scholar]
- 135.De Filippis D et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS ONE 6, e28159 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nogueira LT et al. The involvement of mast cells in the irinotecan-induced enteric neurons loss and reactive gliosis. J. Neuroinflammation 14, 79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sofroniew MV Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32, 638–647 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sofroniew MV & Vinters HV Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Krauter EM et al. Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig. Neurogastroenterol. Motil 19, 990–1000 (2007). [DOI] [PubMed] [Google Scholar]
- 140.Hoffman JM, McKnight ND, Sharkey KA & Mawe GM The relationship between inflammation-induced neuronal excitability and disrupted motor activity in the guinea pig distal colon. Neurogastroenterol. Motil 23, 673–e279 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Roberts JA, Durnin L, Sharkey KA, Mutafova-Yambolieva VN & Mawe GM Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J. Physiol 591, 3725–3737 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Spencer NJ & Hu H Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol 17, 338–351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lomax AE, Fernández E & Sharkey KA Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol. Motil 17, 4–15 (2005). [DOI] [PubMed] [Google Scholar]
- 144.Lomax AE, O’Hara JR, Hyland NP, Mawe GM & Sharkey KA Persistent alterations to enteric neural signaling in the guinea pig colon following the resolution of colitis. Am. J. Physiol. Gastrointest. Liver Physiol 292, G482–G491 (2007). [DOI] [PubMed] [Google Scholar]
- 145.Linden DR, Sharkey KA & Mawe GM Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J. Physiol 547, 589–601 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Strong DS et al. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J. Physiol 588, 847–859 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gulbransen BD et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med 18, 600–604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Turco F et al. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut 63, 105–115 (2014). The results of this study show that pathogens and probiotics have differential effects on enteric glia. [DOI] [PubMed] [Google Scholar]
- 149.Murakami M, Ohta T & Ito S Interleukin-1β enhances the action of bradykinin in rat myenteric neurons through up-regulation of glial B1 receptor expression. Neuroscience 151, 222–231 (2008). [DOI] [PubMed] [Google Scholar]
- 150.Buckley MM, O’Halloran KD, Rae MG, Dinan TG & O’Malley D Modulation of enteric neurons by interleukin-6 and corticotropin-releasing factor contributes to visceral hypersensitivity and altered colonic motility in a rat model of irritable bowel syndrome. J. Physiol 592, 5235–5250 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kermarrec L, Durand T, Neunlist M, Naveilhan P & Neveu I Enteric glial cells have specific immunosuppressive properties. J. Neuroimmunol 295–296, 79–83 (2016). [DOI] [PubMed] [Google Scholar]
- 152.Stoffels B et al. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology 146, 176–187.e1 (2014).This study shows that pro-inflammatory mediators such as IL-1β disrupt intestinal motility through effects on enteric glia. This is one example of the detrimental effects of reactive enteric glia.
- 153.Wang P et al. BDNF contributes to IBS-like colonic hypersensitivity via activating the enteroglia-nerve unit. Sci. Rep 6, 20320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wangzhou A et al. A pharmacological interactome platform for discovery of pain mechanisms and targets. bioRxiv 10.1101/2020.04.14.041715 (2020). [DOI] [PMC free article] [PubMed]
- 155.Cirillo C et al. Increased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil 21, 1209–e112 (2009). [DOI] [PubMed] [Google Scholar]
- 156.Chow AK & Gulbransen BD Potential roles of enteric glia in bridging neuroimmune communication in the gut. Am. J. Physiol. Gastrointest. Liver Physiol 312, G145–G152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Langness S, Kojima M, Coimbra R, Eliceiri BP & Costantini TW Enteric glia cells are critical to limiting the intestinal inflammatory response after injury. Am. J. Physiol. Gastrointest. Liver Physiol 312, G274–G282 (2017).Data in this study show that enteric glia modulate local immune responses and gut barrier integrity after injury by transmitting vagal anti-inflammatory signals to resident immune cells.
- 158.Seguella L et al. Pentamidine niosomes thwart S100B effects in human colon carcinoma biopsies favouring wtp53 rescue. J. Cell. Mol. Med 24, 3053–3063 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duchalais E et al. Colorectal cancer cells adhere to and migrate along the neurons of the enteric nervous system. Cell. Mol. Gastroenterol. Hepatol 5, 31–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Clairembault T et al. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem 130, 805–815 (2014). [DOI] [PubMed] [Google Scholar]
- 161.Stenkamp-Strahm C, Patterson S, Boren J, Gericke M & Balemba O High-fat diet and age-dependent effects on enteric glial cell populations of mouse small intestine. Auton. Neurosci 177, 199–210 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seelig DM, Mason GL, Telling GC & Hoover EA Chronic wasting disease prion trafficking via the autonomic nervous system. Am. J. Pathol 179, 1319–1328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corbillé A-G et al. What a gastrointestinal biopsy can tell us about Parkinson’s disease? Neurogastroenterol. Motil 28, 966–974 (2016). [DOI] [PubMed] [Google Scholar]
- 164.Devos D et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis 50, 42–48 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Perez-Pardo P et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68, 829–843 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Chen QQ et al. Age-dependent alpha-synuclein accumulation and aggregation in the colon of a transgenic mouse model of Parkinson’s disease. Transl. Neurodegener 7, 13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dutta SK et al. Parkinson’s disease: the emerging role of gut dysbiosis, antibiotics, probiotics, and fecal microbiota transplantation. J. Neurogastroenterol. Motil 25, 363–376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Albanese V et al. Evidence for prion protein expression in enteroglial cells of the myenteric plexus of mouse intestine. Auton. Neurosci 140, 17–23 (2008). [DOI] [PubMed] [Google Scholar]
- 169.Ma EL et al. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav. Immun 66, 56–69 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Esposito G et al. HIV-1 Tat-induced diarrhea evokes an enteric glia-dependent neuroinflammatory response in the central nervous system. Sci. Rep 7, 7735 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Camilleri M et al. Pharmacological, pharmacokinetic, and pharmacogenomic aspects of functional gastrointestinal disorders. Gastroenterology 150, 1319–1331.e20 (2016). [DOI] [PubMed] [Google Scholar]
- 172.Sarnelli G et al. HIV-1 Tat-induced diarrhea is improved by the PPARalpha agonist, palmitoylethanolamide, by suppressing the activation of enteric glia. J. Neuroinflammation 15, 94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Costa DVS et al. 5-Fluorouracil induces enteric neuron death and glial activation during intestinal mucositis via a S100B-RAGE-NFκB-dependent pathway. Sci. Rep 9, 665 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Angelo MF et al. The proinflammatory RAGE/NF-κB pathway is involved in neuronal damage and reactive gliosis in a model of sleep apnea by intermittent hypoxia. PLoS ONE 9, e107901 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lue LF et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer’s disease: identification of a cellular activation mechanism. Exp. Neurol 171, 29–45 (2001). [DOI] [PubMed] [Google Scholar]
- 176.Moraes CA et al. Activated microglia-induced deficits in excitatory synapses through IL-1β: implications for cognitive impairment in sepsis. Mol. Neurobiol 52, 653–663 (2015). [DOI] [PubMed] [Google Scholar]
- 177.Tack J et al. The neurokinin-2 receptor antagonist ibodutant improves overall symptoms, abdominal pain and stool pattern in female patients in a phase II study of diarrhoea-predominant IBS. Gut 66, 1403–1413 (2017). [DOI] [PubMed] [Google Scholar]
- 178.Good ME et al. Pannexin 1 channels as an unexpected new target of the anti-hypertensive drug Spironolactone. Circ. Res 122, 606–615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fried DE & Gulbransen BD In situ Ca2+ imaging of the enteric nervous system. J. Vis. Exp 95, 52506 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Van Landeghem L et al. Regulation of intestinal epithelial cells transcriptome by enteric glial cells: impact on intestinal epithelial barrier functions. BMC Genomics 10, 507 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Coquenlorge S et al. The arachidonic acid metabolite 11β-prostaglandinF2α controls intestinal epithelial healing: deficiency in patients with Crohn’s disease. Sci. Rep 6, 25203 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eser A et al. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm. Bowel Dis 21, 2247–2253 (2015). [DOI] [PubMed] [Google Scholar]
- 183.Burnstock G, Jacobson KA & Christofi FL Purinergic drug targets for gastrointestinal disorders. Curr. Opin. Pharmacol 37, 131–141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Esposito G et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology 133, 918–925 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Asano T et al. Arundic acid (ONO-2506) ameliorates delayed ischemic brain damage by preventing astrocytic overproduction of S100B. CNS Neurol. Disord. Drug Targets 4, 127–142 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


