Abstract
Vaccines and monoclonal antibodies (mAb) offer promising strategies to treat substance use disorders (SUD) and prevent overdose. Despite vaccines and mAb against SUD demonstrating proof of efficacy, selectivity, and safety in animal models, it is unknown whether the mechanism of action of these immunotherapeutics relies exclusively on formation of antibody:drug complexes, or also involves antibody-mediated effector functions. Hence, this study tested whether the efficacy of active and passive immunization against drugs of abuse requires phagocytosis, intact Fc portion of the anti-drug antibody, Fcγ receptors (FcγR), or the neonatal Fc receptor (FcRn). The efficacy of a lead vaccine against oxycodone was not diminished in mice after depletion of macrophages or granulocytes. Anti-oxycodone F(ab’)2 fragments resulted in lower serum level of F(ab’)2 compared to intact mAb, and F(ab’)2 were not as effective as the parent mAb in reducing distribution of oxycodone to the brain. Efficacy of vaccine and mAb against oxycodone was preserved in either FcγIII or FcγI-IV ablated (−/−) mice, suggesting FcγR are not required for antibody efficacy. Finally, both active and passive immunization against oxycodone in FcRn−/− mice yielded reduced efficacy compared to wild-type control mice. These data identified a role for FcRn, but not for phagocytosis or Fc-dependent effector functions, in mediating efficacy of vaccines and mAb against SUD. This study supports rational design of vaccines and mAb engineered for maximal neutralization activity and optimal FcRn binding.
INTRODUCTION
Substance abuse disorders (SUD) and drug-related overdoses are a worldwide public health threat, and have a dramatic economic impact due to crime and lost productivity (1). In the United States, opioid use disorders (OUD) and fatal overdoses related to opioids have reached epidemic proportions (2). Since the 1990s and early 2000s, OUD has spiked partly due to over-prescription of opioid analgesics such as oxycodone and hydrocodone, which often act as gateway drugs to heroin intravenous use (3). More recently, widespread access to illicit fentanyl and its analogues has exacerbated the incidence of accidental overdoses from exposure to street mixtures containing opioids or psychostimulants laced with fentanyl (4). Finally, during the COVID-19 pandemic caused by the SARS-CoV-2 novel coronavirus, the incidence of opioid fatal and non-fatal overdoses increased in the United States (5). Available treatments, consisting of pharmacological agonists and antagonists of the μ-opioid receptor, have not been sufficient to prevent the occurrence of OUD and fatal overdoses, highlighting the need for new medical interventions in this area.
Therapeutic or prophylactic use of vaccines and monoclonal antibodies (mAb) may offer a complementary strategy against SUD, OUD and related overdoses. Vaccines and mAb have demonstrated efficacy, selectivity, and safety in animal models involving drug-induced antinociception, locomotor activity, respiratory depression, bradycardia, lethality, and opioid self-administration in preclinical models including mice, rats, and non-human primates (6–10). Vaccine-induced polyclonal antibodies and mAb act by selectively binding the target drug, forming antibody:drug complexes that retain drugs in serum or extracellular fluids, thus preventing opioids from crossing the blood-brain barrier and reducing opioid-induced pharmacological effects (reviewed in (11, 12)). Vaccine-induced antibodies targeting small molecules such as nicotine have also been shown to reduce drug distribution to tissues including muscle, spleen, liver, heart, kidney, and adipose compartment (13). Additionally, the antibody-opioid complex is not active at the μ-opioid receptor in vitro (10), which limits concerns for secondary effects at peripheral receptors. To date, it is not known whether the mechanism of action of these immunotherapeutics relies exclusively on antibody:drug complexes reducing the concentration of unbound (free) drug in circulation, or whether active processes including antibody-mediated effector functions are involved. While the role of antibody effector functions is well established for antibody-mediated pathogen clearance (14, 15), their contribution to vaccine efficacy against drugs of abuse or other small molecules is less clear. A recent study found that IgG specific for small molecule neuromuscular-blocking agents used in anaesthesia triggered anaphylaxis in human patients, correlated with markers of FcγR and neutrophil activation (16). These data highlight the importance of studying effector functions associated with beneficial or detrimental effects on vaccine or mAb efficacy. As vaccines and mAb against SUD move into the clinical space, such studies will provide additional evidence of safety.
Antibodies consist of an antigen binding fragment [F(ab)], which is sufficient for antigen binding and neutralization, and the fragment crystallizable (Fc) constant region, which is required for functions including phagocytosis, cytotoxic killing, and complement activation, enabled by binding to Fc gamma receptor (FcγR) and complement (17, 18). Additionally, Fc interaction with neonatal Fc receptor (FcRn) acts to increase Ab half-life through a cellular recycling mechanism, in which the endocytosed Ab is rescued from degradation and returned to circulation (19, 20). To evaluate the contribution of antibody-dependent Fc receptor mechanisms for anti-opioid vaccines, this study tested whether the efficacy of active and passive immunization against oxycodone requires only neutralization, or also incorporates other downstream mechanisms involving Fc-mediated interactions.
Anti-drug conjugate vaccines stimulate T cell-dependent B cell activation and germinal center (GC) formation to generate target-specific antibodies (11). While several cellular and molecular targets are involved in this process, our laboratory has found that vaccine efficacy against opioids in mice is impacted by interleukin-4 (IL-4) signalling (21, 22). Specifically, blockade of IL-4 increased vaccine efficacy against oxycodone and fentanyl, which correlated with increased levels of IgG1, IgG2a and IgG3 subclasses compared to control. Immunization of IL-4 receptor−/− and STAT6−/− mice showed an increase in IgG2a and IgG3 levels alone, without an associated change in IgG1 levels, but did not increase vaccine efficacy against oxycodone. Increased efficacy associated with higher levels of IgG1, IgG2a and IgG3 subclasses has also been reported for vaccine formulations against nicotine (23) and cocaine (24). While activation of IgG subclasses may be related to simply more mature class switch events in B cells, these preliminary studies also suggested the possibility that differential binding of IgG subclasses to Fc receptors may be important for efficacy.
Understanding whether neutralization only, or instead Fc-mediated clearance, is the main underlying mechanism of action of antibodies against drugs of abuse will inform development of more effective vaccine formulations and mAb engineering. If Fc-mediated effector functions are involved, efforts shall be focused on characterization of the role of IgG isotypes and Fc glycosylation, two major factors that affect Fc receptor binding. If FcRn-mediated antibody recycling is involved, efforts shall be focused on engineering mAb to display Fc sequences to optimize FcRn binding and recycling (25, 26), which would increase or maintain functional antibody levels in vivo. Hence, this study compared the efficacy of anti-oxycodone F(ab’)2 fragments to the parent mAb, and whether the efficacy of vaccines and mAb against opioids would be reduced by pharmacological inhibition of phagocytes or by genetic ablation of FcγIII, FcγI-IV, or FcRn in mice. These data identified a role for FcRn, but not for phagocytosis or Fc-dependent effector functions, in mediating efficacy of vaccines and mAb against SUD or OUD. These data suggest that opioid:antibody complexes are not likely to trigger FcγR-related side effects in immunized subjects, minimizing concerns for immune-related adverse events following anti-opioid active or passive immunization. Additionally, this study supports rational design of vaccines and mAb engineered for maximal neutralization activity and optimal FcRn binding.
MATERIALS AND METHODS
Animals.
All studies were approved by the Hennepin Healthcare Research Institute (HHRI) and the University of Minnesota Animal Care and Use Committees. Male BALB/c mice were purchased from Envigo (Indianapolis, IN) and male C57BL/6, FcγRIII−/−, and FcRn−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Female and male FcγRI-IV−/− mice (27, 28) were a gift from Dr. Sjef Verbeek (Leiden University Medical Center, Leiden, NL). Experiments were conducted on 6–10 week old mice, with the exception of experiments using FcγRI-IV−/− mice aged 15–20 weeks obtained from an in-house breeding program. Mice were group-housed in conventional environment with a 14/10 hour light/dark cycle (light cycle 6 am to 8 pm) and fed ad libitum.
Vaccine and mAb formulations.
An oxycodone-based hapten containing a tetraglycine linker (OXY) was conjugated to either native keyhole limpet hemocyanin (KLH) or subunit KLH (sKLH) using carbodiimide chemistry (29, 30). The OXY-KLH and OXY-sKLH conjugates were purified by filtration (Amicon, MilliporeSigma, Burlington, MA) and characterized as described (29). Vaccine formulations included 75 μg of OXY-KLH or OXY-sKLH adsorbed on aluminum adjuvant (Alhydrogel-85 2%, InvivoGen, San Diego, CA) in sterile physiological saline prior to injection. An anti-oxycodone mAb (clone HY1–3G8) was isolated from mice immunized with OXY-sKLH as described (31). F(ab’)2 fragments were generated from the parent mAb by enzymatic digestion with agarose-immobilized ficin (Thermo Fisher, Waltham, MA) with 4 mM cysteine, and purified with Protein A agarose to remove Fc fragment and undigested IgG. The mAb and F(ab’)2 were prepared in sterile physiological saline.
Active and passive immunizations.
Mice were immunized intramuscularly on days 0, 14 and 28 (unless otherwise specified) with either unconjugated carrier protein (KLH or sKLH) or vaccine (OXY-KLH or OXY-sKLH) in a total injection volume of 60 μL via two injections into contralateral caudal thigh muscles. As detailed in specific experiments, mice were immunized with either OXY-KLH or OXY-sKLH concurrently with a neutralizing anti-IL-4 mAb antibody (clone 11B11, BioXCell, West Lebanon, NH) on days −2 and +1 of the vaccination series (0.5 mg/200 μL i.p.) as described previously (21, 22). In passive immunization studies, anti-oxycodone mAb or F(ab’)2 were delivered intraperitoneally (i.p.) 24 hours prior to the oxycodone challenge.
Macrophage poisoning and granulocyte depletion.
Immunized mice were administered intravenously 1 mg of lipid vesicles encapsulating clodronate (Liposoma BV, Amsterdam, Netherlands) 12 hours prior to oxycodone challenge. Mice were administered i.p. with 0.5 mg of anti-Gr-1 antibody (clone RB6-BC5, a gift from Dr. Thomas Griffith, University of Minnesota Center for Immunology) 12 and 2 hours prior to the oxycodone challenge. Both reagents were administered in sterile PBS in a total volume of 200 μL. To verify successful treatment of macrophage and granulocyte populations, spleen and lymph nodes were collected at the conclusion of the experiment and analyzed by flow cytometry (Figure S1).
Antibody analysis.
Serum oxycodone-specific polyclonal IgG antibody titers and subclasses were analyzed by indirect ELISA as previously described (8). Briefly, 96-well plates were coated with 5 ng/well of either OXY-OVA conjugates or unconjugated OVA control in carbonate buffer at pH 9.6 and blocked with 1% gelatin. Serum was added at an initial dilution of 1:200. Primary antibodies were incubated with goat anti-mouse IgG conjugated to horseradish peroxidase (Jackson ImmunoResearch), and visualized with OPD substrate (Sigma). Serum titers of oxycodone-specific mAb or F(ab’)2 were analyzed with indirect ELISA using a secondary antibody specific to mouse IgG1 (Alpha Diagnostics Int’l Cat# 40126) or F(ab’)2 (Millipore Sigma Cat# SAB3700993–2MG).
Vaccine, mAb and F(ab’)2 efficacy against oxycodone in vivo.
Vaccine, mAb, and F(ab’)2 efficacy was tested against oxycodone-induced antinociception on a 54°C hot plate, a test of centrally-mediated analgesia commonly used to screen for vaccine potency in blocking opioid pharmacological effects. Behavioral tests were performed during the light cycle, between 10 am and 2 pm. Latency to respond was measured as time to first hind-paw flick or lift, observed by an experimenter blinded to treatment condition, and was measured at baseline and 15 or 30 min post-oxycodone administration. Maximum possible effect (MPE) was calculated as (post-drug latency-baseline)/(60-baseline)x100 = %MPE. After oxycodone challenge, mice were euthanized to collect serum and brain for measurement of oxycodone concentrations by either gas chromatography–mass spectrometry (8) or liquid chromatography-mass spectrometry as described (32). Briefly, brains were weighed and homogenized in four parts 0.1M phosphate buffer, pH 6.0, and serum and brain homogenate samples were extracted with Bond Elut Plexa PCX 3mL extraction cartridges (Agilent, Santa Clara, CA), and analyzed by LC-MS/MS with Zorbax Eclipse Plus C18 column (Agilent) and G6470A TQ (Agilent).
Statistical Analyses.
Data are expressed as means ± standard error of the mean (SEM). All experiments were performed once with n ≥ 3 per group. Between-group comparisons were determined by one-way ANOVA paired with a Tukey’s multiple comparison post hoc test. Any p values lower than 0.05 were accepted as significant. All analyses were made using Prism 8.3.0 (GraphPad, La Jolla, CA).
RESULTS
Monocytes, macrophages, and granulocytes are not required for vaccine efficacy against oxycodone.
Because it is unknown whether IgG-induced phagocytosis is involved in the mechanism of action of anti-drug polyclonal antibodies or mAb, we tested whether a major cell subset that performs this antibody-dependent effector function contributes to vaccine efficacy against oxycodone in mice. Because macrophages and granulocytes constitute the majority of circulating phagocytic cells (33), these cell populations were depleted by treatment with liposome-embedded clodronate or anti-granulocyte receptor-1 (Gr-1) mAb respectively. Balb/c mice were immunized with either KLH or OXY-KLH, and clodronate or anti-Gr-1 treatment was administered within 12 hours prior to the oxycodone challenge. To verify successful vaccination, oxycodone-specific serum IgG antibody titers were analyzed 24 hours prior to the drug challenge (Figure 1A). Absence of macrophages (F4/80+) and granulocytes (Gr-1+) was confirmed by flow cytometry in spleen and lymph nodes (Figure S1). After drug challenge, serum oxycodone concentration was increased in immunized groups compared to the control group immunized with unconjugated KLH (Figure 1B). Vaccination with OXY-KLH in untreated, clodronate-treated, and anti-Gr-1-treated mice reduced the brain-to-serum ratio of oxycodone concentrations compared to KLH controls (Figure 1C), and no statistically significant differences were found between vaccinated groups after clodronate or anti-Gr-1 treatment. These data suggested that macrophages or Gr-1+ cells are not necessary for vaccine efficacy against acute oxycodone challenge.
Figure 1. Role of granulocytes and macrophages in efficacy of vaccines against opioids.
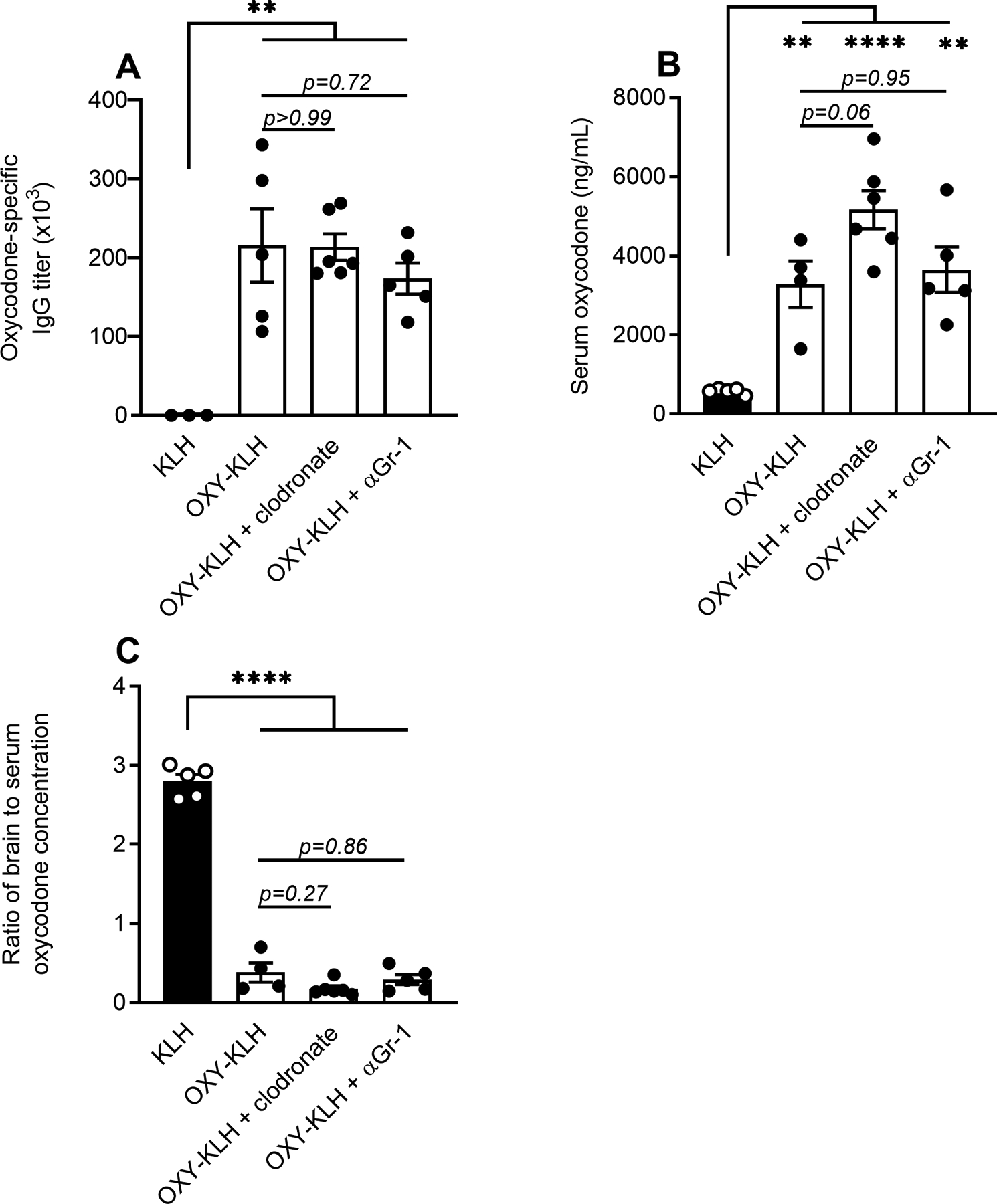
Balb/c mice were immunized with either OXY-KLH or KLH on days 0, 14, and 28. Oxycodone-specific serum IgG antibody titers were measured on day 34. On day 35, mice vaccinated with OXY-KLH were treated in vivo with either liposome-embedded clodronate to pharmacologically poison macrophages, or αGr-1 to deplete granulocytes. After 24 hours, mice were challenged s.c. with 5.0 mg/kg oxycodone. A) Oxycodone-specific serum IgG titers assessed by treatment group prior to depletion. Oxycodone concentration in B) serum and C) as a ratio of brain to serum oxycodone concentration at 30 minutes after drug challenge. Data are mean ± SEM. Sample size: n=3–6 mice per treatment group. Statistical symbols: **, ***, **** p≤ 0.01, 0.001, 0.0001.
F(ab’)2 fragments are not as effective as intact mAb against acute opioid challenge.
Next, we tested whether Fc-mediated effector functions are involved in the efficacy of vaccines against oxycodone. To this end, the functional Fc fragment of oxycodone-specific mAb was enzymatically cleaved to produce oxycodone-specific F(ab’)2. Compared to mAb, F(ab’)2 retain capacity for antigen binding and neutralization by formation of opioid:antibody complexes, but not Fc receptor binding. Mice were passively immunized with either anti-oxycodone mAb or the molar equivalent dose of the oxycodone-specific F(ab’)2. While no observable differences in the hot plate test of antinociception were detected (Figure S2A), after a 2.25 mg/kg oxycodone challenge, serum oxycodone concentrations were significantly increased in both mAb- and F(ab’)2-immunized mice compared to control (Figure 2A). However, mAb was more effective than F(ab’)2 in retaining serum oxycodone (Figure 2A), which was associated with a greater reduction in brain-to-serum ratio in mAb-treated mice (Figure 2B). To further characterize the reduced efficacy of F(ab’)2 compared to intact mAb, a dose-response study tested whether higher doses of F(ab’)2 fragments could recapitulate the effect of mAb. In this experiment, mice were passively immunized with 40 mg/kg anti-oxycodone mAb, F(ab’)2 at dose equivalent to 1–100 mg/kg mAb, or PBS control. Oxycodone-specific mAb and F(ab’)2 titers (Figure 2C) were assessed by ELISA at 2 hours prior to the drug challenge. While increasing doses of F(ab’)2 yielded higher titers, serum levels did not equal those of the mAb. When mice were challenged with 2.0 mg/kg oxycodone, higher F(ab’)2 dosing resulted in a trend toward higher retention of oxycodone in the serum compartment, but serum oxycodone concentrations did not match those of mAb-treated mice (Figure 2D). However, mice receiving a dose of 40 mg/kg or higher of F(ab’)2 fragments displayed significantly reduced brain-to-serum ratios compared to control (Figure 2E). These data demonstrate that higher doses of F(ab’)2 could be potentially deployed to protect from oxycodone, though the intact mAb demonstrated higher efficacy at lower doses. The serum titer of F(ab’)2 and IgG before challenge showed a strong negative correlation with brain oxycodone concentration (Figure 2F), supporting that doses of F(ab’)2 sufficient to match serum IgG concentrations could reach similar efficacy to intact IgG. Discrepancies in mAb or F(ab’)2 efficacy against oxycodone may be explained by altered tissue distribution of F(ab’)2 or opioid:F(ab’)2 complexes, and a longer serum half-life for mAb compared to F(ab’)2 (34, 35), suggesting that mAb-based immunotherapeutic interventions against SUD or OUD should retain an intact Fc for optimal duration of action.
Figure 2. Role of Fc in IgG-mediated reduction of opioid distribution to the brain.
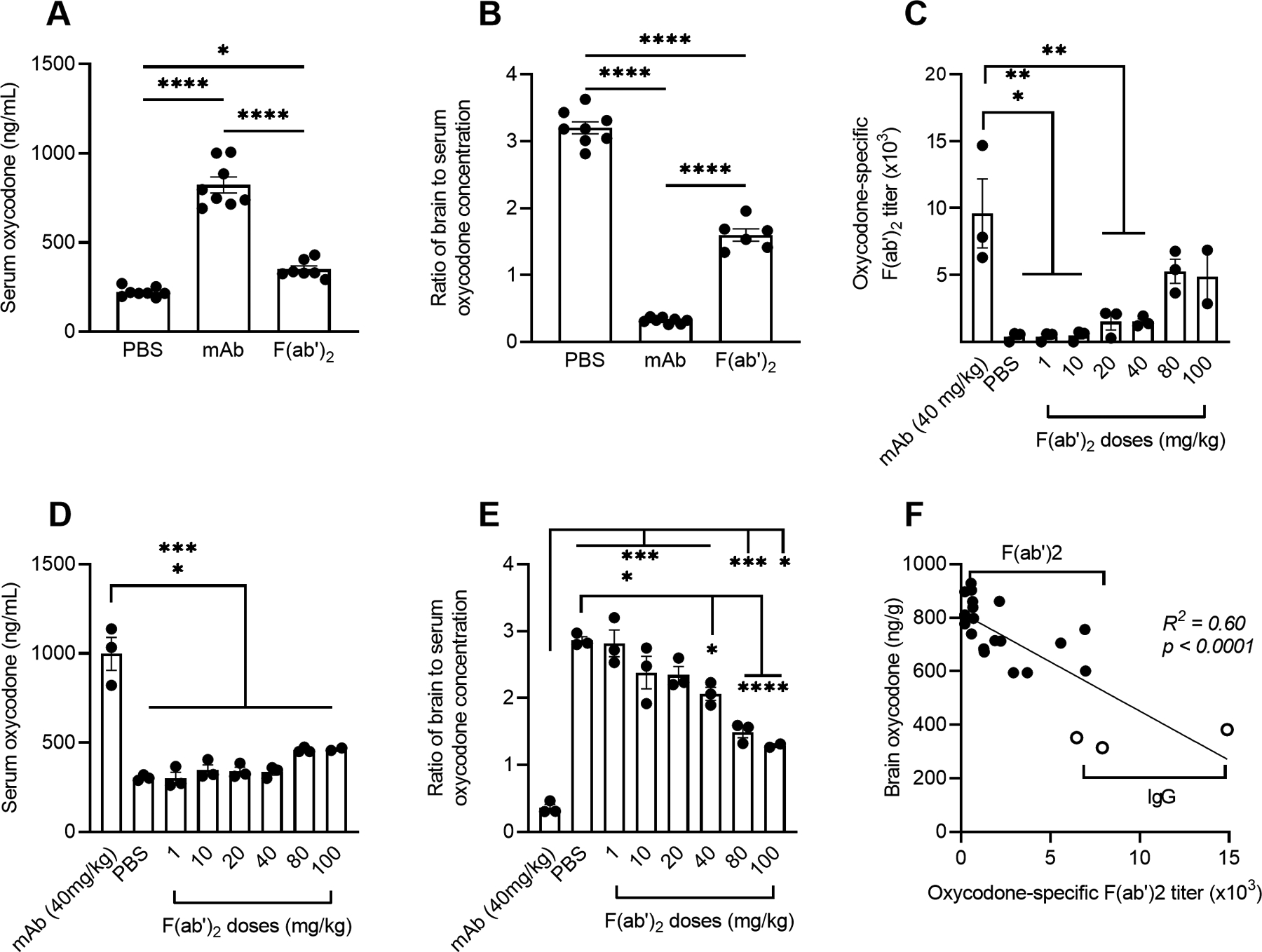
Balb/c mice were passively immunized with oxycodone-specific mAb (40 mg/kg), its derived F(ab’)2 fragment, or PBS as control. At 24 hours after treatment, mice were challenged s.c. with oxycodone (2.25 mg/kg) (A-B) or (2.0 mg/kg) (C-E). Shown: A) oxycodone concentration in serum and B) as a ratio of brain to serum oxycodone concentration. Mice were passively immunized i.p. with oxycodone-specific mAb (40 mg/kg) or F(ab’)2 fragments (1–100 mg/kg IgG dose equivalent). C) total IgG oxycodone-specific antibody or antibody fragment titers were assessed 22 hours post-passive immunization to drug challenge in immunized mice. Oxycodone concentration in D) serum and E) as a ratio of brain to serum oxycodone concentration 30 min after drug challenge. F) Correlation between serum titer of IgG and F(ab’)2 shown in panel C and brain oxycodone concentration by simple linear regression. Data are mean ± SEM. Sample size: n≥3 each group. Statistical symbols: *, **, ***, **** p≤ 0.05, 0.01, 0.001, 0.0001.
The Fcγ receptor III is not required for generation of effective anti-oxycodone polyclonal antibody responses.
Activation of FcγRIII can mediate inflammatory processes including phagocytosis, cytokine production, and antibody-dependent cellular cytotoxicity (36, 37). Hence, we tested the role of FcγRIII by immunizing C57BL/6 wild-type and FcγRIII−/− mice with OXY-KLH in the presence of a neutralizing mAb against IL-4 (αIL-4), which has been shown to enhance total IgG, IgG2 and IgG3 titers, and increase vaccine efficacy against opioids (21). Seven days after completion of the third immunization, mice were challenged with 2.5 mg/kg oxycodone and assessed for oxycodone-induced antinociception in the hot plate test. In this first challenge, there was no distinguishable difference between OXY-KLH immunized mice and KLH control immunized mice (Figure S2B). Therefore, mice were vaccinated a fourth time 14 days after the last immunization, and presented with a second oxycodone challenge consisting of two sequential 2.5 mg/kg doses at 15 minutes interval, for a cumulative dose of 5.0 mg/kg (Figure 3). Oxycodone-specific total IgG titers and IgG subclass titers were assessed 24 hours prior to drug challenge (Figure 3A). There were no significant differences between immunized groups in vaccine-induced titers, and immunized mice in both wild-type and FcγRIII−/− groups demonstrated reduced antinociception compared to KLH controls, with a trend toward a greater reduction in FcγRIII−/− mice (Figure 3B). In both wild-type and FcγRIII−/− mice immunized with OXY-KLH, serum oxycodone levels were significantly higher compared to control (Figure 3C), and brain-to-serum ratios of oxycodone concentration were reduced in groups immunized with the active vaccine (Figure 3D). These data show that FcγRIII is not necessary for the induction of anti-oxycodone polyclonal antibodies, nor critical for efficacy in blocking oxycodone distribution to the brain during acute drug challenges.
Figure 3. Role of FcγRIII in efficacy of vaccines against opioids.
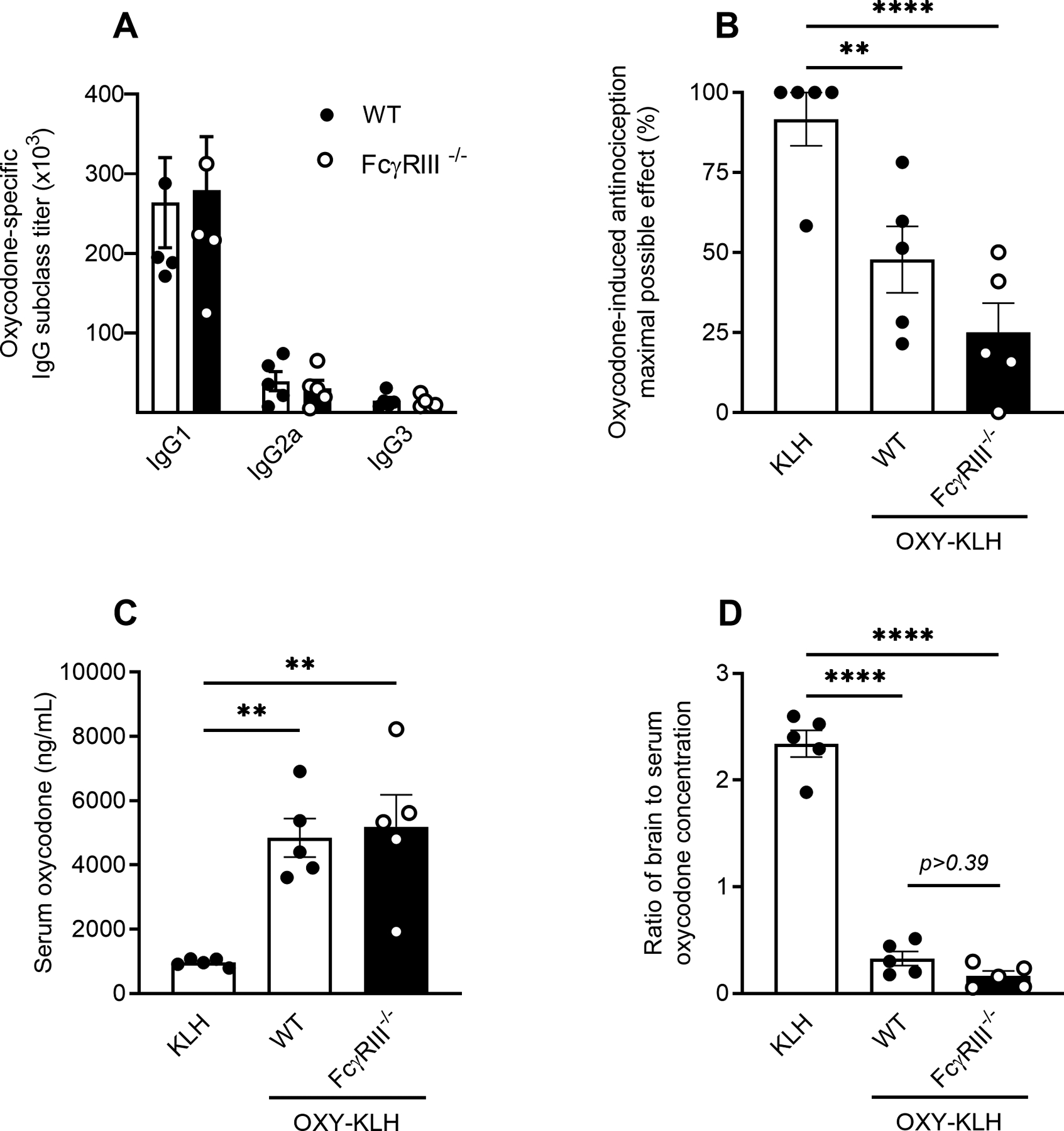
C57BL/6 wild-type or FcγRIII−/− mice were immunized with either OXY-KLH (+ αIL-4 on days −2 and +1) or KLH on days 0, 14, 28, and 42. Oxycodone-specific serum IgG antibodies were analyzed on day 48. On day 49, mice were challenged s.c. with 5.0 mg/kg oxycodone to test for vaccine efficacy in reducing oxycodone-induced antinociception measured on a hot plate. A) Oxycodone-specific serum total IgG and subclass antibody titers, and B) oxycodone-induced antinociception expressed as a percent of maximum possible effect (MPE). Oxycodone concentration in C) serum and D) as a ratio of brain to serum oxycodone concentration. Data are mean ± SEM. Sample size: n=5 mice in each treatment group. Statistical symbols: **, **** p≤ 0.01, 0.0001.
FcγRI-IV are not necessary for generation of polyclonal antibodies nor for vaccine efficacy against oxycodone.
Fcγ receptors I, II, III, and IV play a role in cell signaling activation and inhibition, binding to IgG subclasses with varying affinities (17, 36, 37). To investigate the role of these receptors in reducing oxycodone distribution to the brain, we immunized male and female wild-type and FcγRI-IV−/− mice (27, 28) with sKLH control or OXY-sKLH. Oxycodone-specific IgG titers and subclasses were assessed within 24 hours prior to the drug challenge. There was no difference in total oxycodone-specific IgG antibodies or in IgG subclasses in FcγRI-IV−/− mice compared to wild-type mice immunized with OXY-sKLH (Figure 4A), except for a small but statistically significant reduction in IgG3 titers in female FcγRI-IV−/− mice compared to female wild-type (Figure S3B). Thirty minutes after a 5.0 mg/kg oxycodone challenge, serum oxycodone levels were increased in both male and female OXY-KLH immunized mice, and there were no significant differences in serum oxycodone concentrations between wild-type or FcγRI-IV−/− mice (Figure 4B). Similarly, both male and female mice receiving OXY-KLH demonstrated a significant reduction in oxycodone brain-to-serum ratio compared to KLH control mice (Figure 4C). Additionally, no statistically significant differences were seen between immunized wild-type and FcγRI-IV−/− mice with male and female mice analyzed separately (Figure S3E–F). To distinguish between the effects of FcγRs on development of antibody over time and acute effects during drug challenge, an independent passive immunization study was conducted with anti-oxycodone mAb in wild-type and male FcγRI-IV−/− mice. Similar to active immunization, treatment with mAb resulted in similar serum oxycodone levels (Figure 4D) and brain-to-serum ratios of oxycodone concentration (Figure 4E) in wild-type and FcγRI-IV−/− mice thirty minutes after 5.0 mg/kg oxycodone challenge. These studies support that the FcγRI-IV are not necessary to mediate vaccine or mAb efficacy against the target drug.
Figure 4. Role of FcγRI-IV in efficacy of vaccines against opioids.
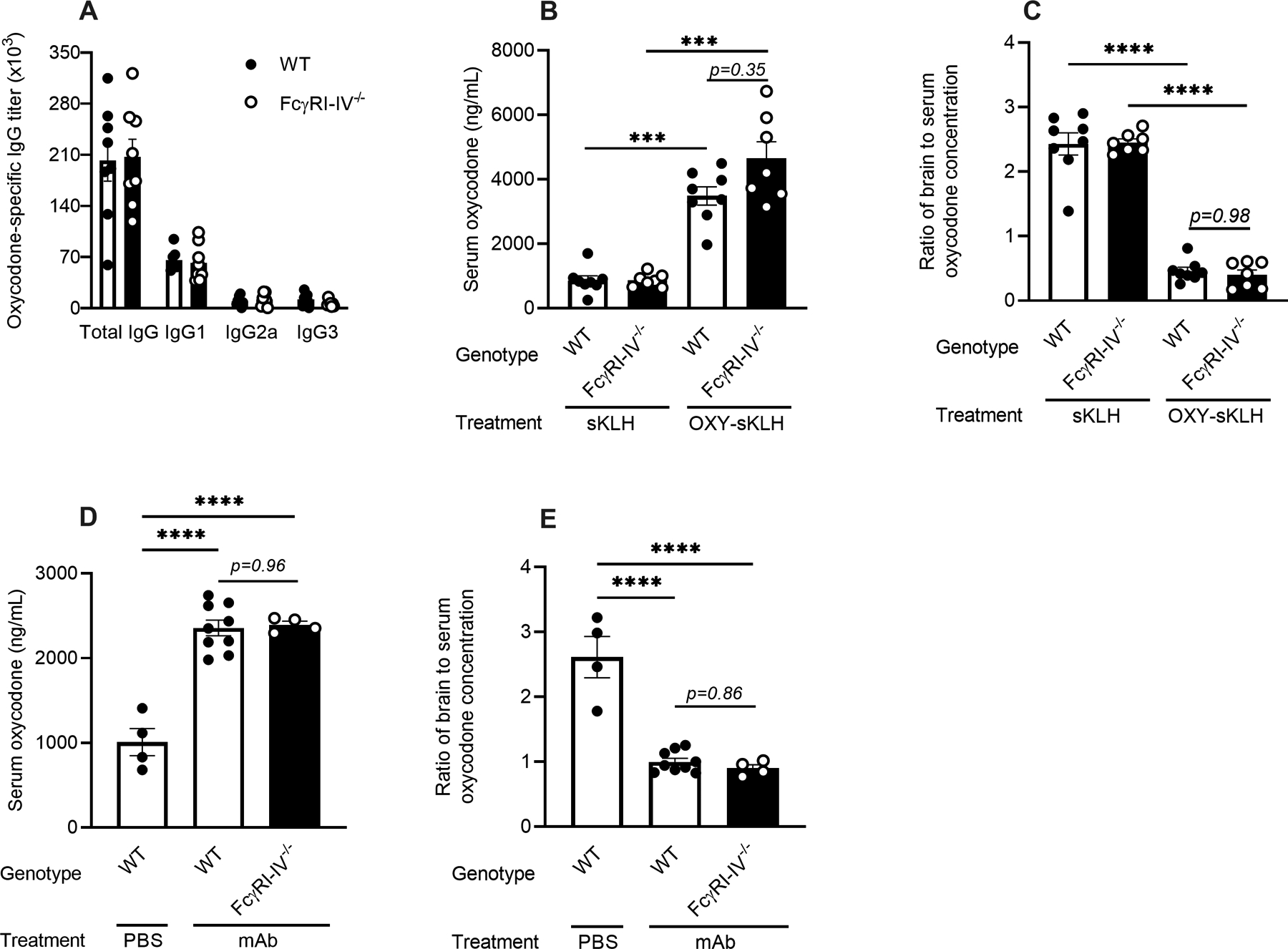
C57BL/6 wild-type and FcγRI-IV−/− male and female mice were actively immunized with either OXY-KLH or unconjugated KLH on days 0, 14, and 28, or passively immunized with an anti-oxycodone mAb (40 mg/kg, i.p.) at 24 hours prior to an oxycodone challenge. Oxycodone-specific serum IgG and subclasses titers were assessed prior to drug challenge (A). At 30 minutes after a s.c. challenge with 5.0 mg/kg oxycodone, drug concentration was assessed in serum (B) and brain, as a ratio of brain to serum oxycodone concentration (C). Serum (D) and brain-to-serum ratio (E) oxycodone concentration in passively immunized male mice. Data are mean ± SEM. Sample size: n=4–5/treatment group. Statistical symbols: ***, **** p≤ 0.001, 0.0001.
FcRn is necessary for generation of anti-drug polyclonal antibodies as well as efficacy of vaccine and mAb against oxycodone.
The FcRn is widely known for its role in recycling antibodies and contributing to antibody half-life. To test for the role of FcRn in mediating vaccine efficacy against opioids, we immunized wild-type and FcRn−/− mice with OXY-KLH. FcRn−/− mice developed oxycodone-specific antibodies at significantly lower levels than wild-type mice (Figure 5A). OXY-KLH-immunized FcRn−/− mice displayed reduced antinociception compared to KLH-control immunized FcRn−/− mice upon a 5.0 mg/kg oxycodone challenge (Figure S2C). Only wild-type immunized mice demonstrated significantly increased serum oxycodone levels (Figure 5B) compared to FcRn−/− mice or KLH controls. However, both wild-type and FcRn−/− immunized mice demonstrated a reduced brain-to-serum ratio in oxycodone concentration compared to their respective KLH controls (Figure 5C), but the reduction was significantly larger in wild-type immunized mice compared to FcRn−/− mice. As a control study to account for the reduced anti-oxycodone antibody production in FcRn−/− mice, wild-type and FcRn−/− mice were passively immunized with anti-oxycodone mAb 24 hours prior to the drug challenge. Passively immunized FcRn−/− mice showed a significant increase in serum oxycodone levels compared to control treated mice (Figure 5E) and a significant reduction in brain-to-serum ratio of oxycodone concentration compared to control treated mice (Figure 5F), but the reduction in brain-to-serum ratio was greater in immunized wild-type mice. Passively immunized FcRn−/− mice also showed lower serum retention of mAb compared to wild-type mice (Figure 5D), indicating that reduced vaccine performance in FcRn−/− mice is likely a result of loss of FcRn recycling function to maintain serum antibody levels. Because high levels of anti-drug polyclonal or monoclonal antibodies are required for efficacy against the target drug, these data suggest that FcRn plays a critical role in maintaining efficacious levels of serum antibody upon active vaccination, and is necessary to achieve optimal pharmacokinetic performance of both vaccines and mAb.
Figure 5. Role of FcRn in efficacy of vaccines against opioids.
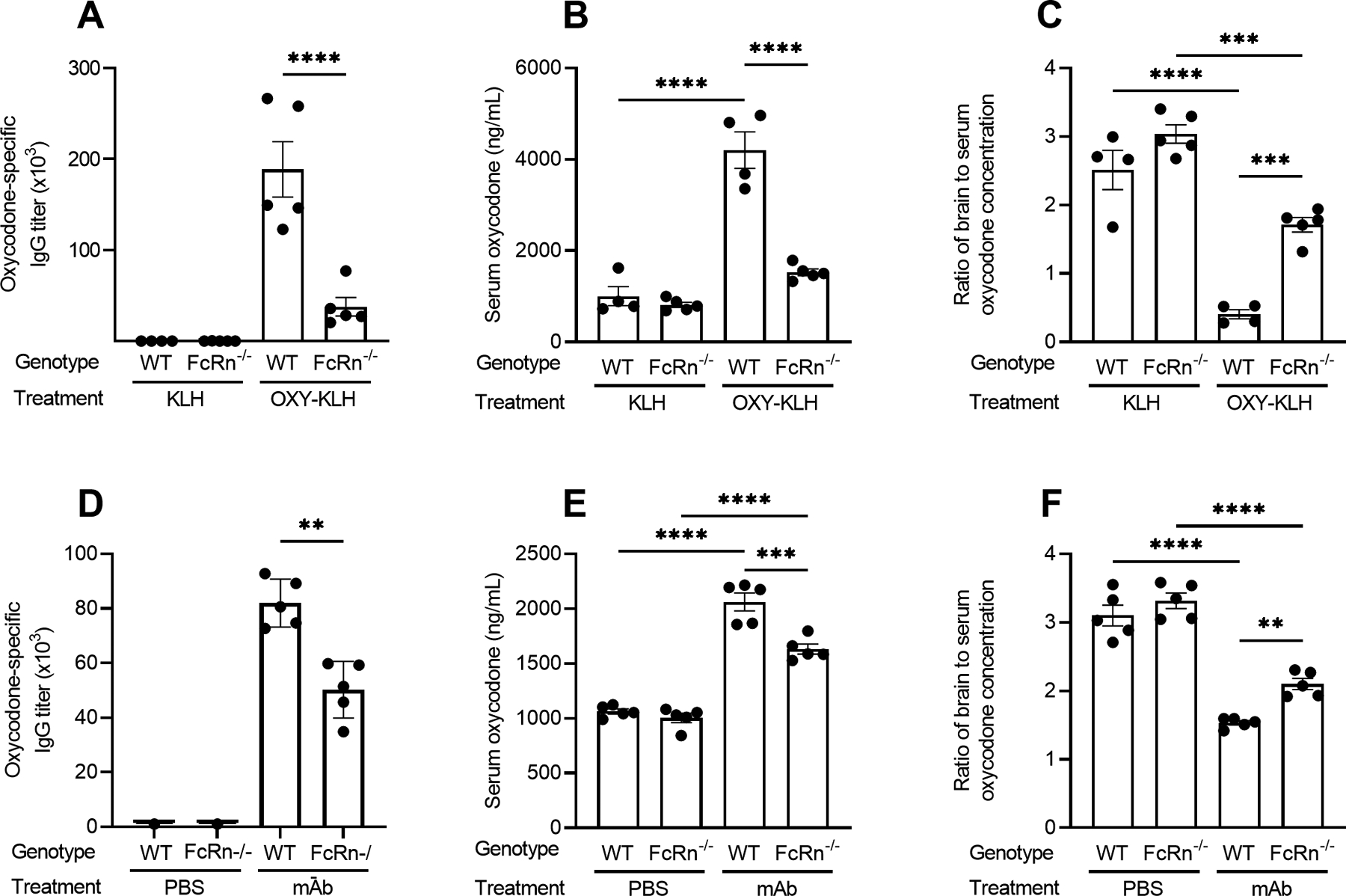
C57BL/6 wild-type and FcRn−/− mice were immunized with either OXY-KLH or unconjugated KLH on days 0, 14, and 28. Oxycodone-specific serum IgG titers were assessed prior to drug challenge (A). On day 35 mice were challenged s.c. with 5.0 mg/kg oxycodone. At 30 min post-drug challenge, oxycodone concentration was evaluated in B) serum and C) as a ratio of brain to serum oxycodone concentration. C57BL/6 wild-type or FcRn−/− mice were passively immunized with an anti-oxycodone mAb (40 mg/kg, i.p.) at 24 hours prior to a 5.0 mg/kg oxycodone challenge. Serum mAb was assessed 2 hours prior to drug challenge (D), and at 30 min post-drug challenge, oxycodone concentration was evaluated in E) serum and F) as a ratio of brain to serum oxycodone concentration. Data are mean ± SEM. Sample size: n=5/treatment group. Statistical symbols: *, **, ***, **** p≤ 0.05, 0.01, 0.001, 0.0001.
DISCUSSION
Vaccines have been one of the most effective medical innovations against infectious disease, and their introduction resulted in significant prolongation of human lives (38). Beyond infectious diseases, vaccines have been tested against a number of non-communicable chronic diseases such as Alzheimer’s, Parkinson, dementia, and SUD. As an alternative or complementary treatment option to pharmacotherapy for SUD involving agonists and antagonists of the opioid receptor, vaccines and mAb have been proposed as a viable strategy to treat OUD and prevent overdose (12). While the cellular and molecular mechanisms underlying vaccine- or mAb-induced protection against infectious diseases is an active area of investigation, less is known about the immunological mechanism of vaccines and mAb against drugs of abuse, or against other small molecules such as nerve agents or other toxic chemicals. Understanding the cellular and molecular processes associated with vaccine or mAb efficacy will speed and optimize translation of safe, long-lasting, and cost-effective therapeutic or prophylactic interventions.
Because binding of small molecule drug targets to drug-specific antibodies are thought to be unlikely to cause antibody aggregation or to induce conformational changes in the Fc region to promote Fc receptor engagement, it is expected that antibody-mediated neutralization of opioids does not depend on FcγR. However, no previous studies have directly tested these hypotheses. While previous reports found that IgG subclasses correlate with vaccine efficacy in mice, it is not clear whether the increased vaccine efficacy associated with IgG2a and IgG3 responses is due to their ability to trigger effector functions such as phagocytosis, or is simply a reflection of a more mature B cell response or higher B cell numbers involving extensive class switch and recombination events which would lead to a more effective polyclonal antibody response. Specifically, our lab showed a primarily IgG1 polyclonal anti-oxycodone antibody response to OXY-KLH vaccines in Balb/c mice, and that IL-4 depleted or IL-4−/− mice had increased levels of antibody, but also a skewing toward IgG2a that resulted in enhanced vaccine efficacy (21). However, it was unclear in these studies whether the presence of increased IgG2a increased efficacy due to subclass-dependent effector functions, or because of increased concentration of antibody overall.
Here, we explored the role of antibody effector functions in mediating vaccine efficacy against oxycodone. We found that phagocytic macrophages and granulocytes are dispensable for mediating vaccine efficacy. Depletion of macrophages and granulocytes at the time of acute drug challenge did not affect vaccine efficacy, demonstrating that phagocytosis is not necessary for mediating vaccine efficacy. Use of oxycodone-specific F(ab’)2 showed that antibodies lacking the Fc portion were still able to reduce oxycodone distribution to the brain in a dose-dependent manner, providing initial evidence that the Fc portion is not required for neutralization. However, F(ab’)2 was not as effective as mAb, where even a dose equivalent to 100 mg/kg mAb was not sufficient to match the efficacy of 40 mg/kg intact mAb. There are likely several factors contributing to this effect. First, the serum distribution of F(ab’)2 to serum was low compared to mAb 24 hours after passive immunization, which may reflect reduced serum half-life typical of F(ab’)2 and other Fab isoforms lacking Fc region (34, 35). Also, the reduced molecular weight of F(ab’)2 may render it more able to permeate the blood-brain barrier, or increase its distribution to other tissues.
While one factor complicating analysis was the somewhat variable antinociceptive response of mouse strains to doses of oxycodone used in these studies, concerns were mitigated by data reporting the efficacy of vaccines and mAb in altering the distribution of oxycodone to the serum and brain compartments. Balb/c mice used for macrophage and granulocyte experiments (Figure 1) and F(ab’)2 experiments (Figure 2) exhibited moderate but individually variable antinociception after low dose (2.0–2.5 mg/kg) oxycodone challenge (Figure S2A). However, C57BL/6 wild-type and FcγRIII−/− mice displayed only mild antinociception in response to 2.5 mg/kg oxycodone challenge (Figure S2B). Hence, for later experiments with C57BL/6 mice, doses of 5.0 mg/kg oxycodone were used for acute challenges, in order to achieve a relevant pharmacological effect of the drug. Notably, FcRn−/− mice immunized with control vaccine showed higher antinociception in response to oxycodone compared to wild-type control mice (Figure S2C). Because of individual variability and strain-dependent differences, particularly when genetic deletion of immune receptors that may have unknown physiological impact is involved, the direct measurement of drug sequestration by brain to serum oxycodone ratio provided a more reliable measure of performance of these vaccines.
Ablation of FcγRIII alone, and then FcγRI-IV, did not affect efficacy of an OXY-KLH vaccine. Collectively, this indicates that neutralization is the predominant mechanism for vaccine and mAb efficacy. Analysis of oxycodone-specific antibody titers from actively immunized mice showed that loss of these receptors did not impact overall level of vaccine-elicited antibodies. Because genetic deletion of FcγR may cause alterations in immune response to vaccine that could produce differences in antibody affinity or stability not captured by antibody titers, additional studies were conducted using passive immunization with our lead oxycodone-specific mAb. As with active immunization, passive immunization of FcγRI-IV−/− mice showed similar efficacy as in wild-type mice, further supporting that these receptors are not necessary for efficacy.
Importantly, a significant role in vaccine efficacy was identified for FcRn, as deletion of FcRn resulted in substantial reduction both of vaccine-induced antibody and of vaccine efficacy during oxycodone challenge. Passive immunization of these mice with oxycodone-specific mAb also demonstrated reduced mAb retention in serum and performance of mAb against oxycodone in the absence of FcRn, indicating that this receptor is needed for optimal in vivo half-life and correlates with increased efficacy. Future studies will investigate antibody engineering at the Fc portion to achieve optimal engagement of FcRn, and improve the pharmacokinetic/pharmacodynamic profile of mAb against opioids or other drugs of abuse (26, 39). Furthermore, clinical studies of vaccines against OUD or SUD should take in account that individual variability may originate from genetic polymorphisms related to the FcRn pathway.
While these results suggest that Fc receptors other than FcRn do not contribute to efficacy in mice, differences between mouse and human IgG and Fc receptors (reviewed in (40)) must be considered as vaccines and mAb for SUD advance into clinical trials, both to optimize efficacy and to predict occurrence of side effects. Another limitation of this study is that experiments focused on the role of IgG, while the role of other Ig classes was not investigated. For instance, it would be of interest to test the efficacy of IgA mAb and subsequently IgA Fc receptors against drugs of abuse. Past studies have shown that vaccine-induced IgA in the bronchoalveolar space may have the ability to retain nicotine upon cigarette smoke inhalation (41). Because some drugs are at high concentrations at the mucosal interface and IgA can be translocated there at higher concentrations, a future area of exploration could focus on development of vaccines and mAb based on IgA against inhaled drugs of abuse and other toxic inhalants.
In conclusion, this study provides the basis for focusing development of immunotherapeutic formulations providing high affinity neutralizing polyclonal antibodies or mAb, while less emphasis should be focused on assessment of the antibody isotype or its FcγRI-IV-binding capabilities. These data support the notion that efforts should be devoted to development of mAb variants engineered for high neutralization capacity, increased FcRn binding, and extended serum half-life.
Supplementary Material
Key Points:
Phagocytosis and FcγRI-IV are not required for efficacy of oxycodone vaccine.
Loss of FcRn in mice reduces serum antibody levels and oxycodone vaccine efficacy.
Acknowledgments.
The authors thank Dr. Sjef Verbeek (Leiden University Medical Center, Leiden, NL) for the generous gift of FcγRI-IV−/− mice; the University of Minnesota Flow Cytometry Resource core facilities for technical and research support; and Dr. Thomas Griffith at the University of Minnesota Center for Immunology for providing antibody reagents for depletion of Gr-1+ immune cells.
Funding acknowledgement:
This work was supported by R01DA041730 (MP), T32DA037183-05 (AHK), and T32DA007097 (CB).
Footnotes
Disclosure of Conflicts of Interest. Pravetoni is the inventor of “Cytokine Signaling Immunomodulators and Methods”, and Baehr and Pravetoni are the inventors of “Anti-opioid compounds and methods of making and using same”. The other authors declare no conflict of interest.
REFERENCES
- 1.UNODC. 2019. World Drug Report 2019,. UN. [Google Scholar]
- 2.Wilson N, Kariisa M, Seth P, Smith H IV, D. N 2020. Drug and Opioid-Involved Overdose Deaths — United States, 2017–2018. MMWR Morb Mortal Wkly Rep 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cicero TJ, Ellis MS, Surratt HL, and Kurtz SP. 2014. The Changing Face of Heroin Use in the United States: A Retrospective Analysis of the Past 50 Years. JAMA Psychiatry 71: 821–826. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell J, Gladden RM, Mattson CL, Hunter CT, D. N 2020. Vital Signs: Characteristics of Drug Overdose Deaths Involving Opioids and Stimulants — 24 States and the District of Columbia, January–June 2019. MMWR Morb Mortal Wkly Rep 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alter A Y. C 2020. COVID-19 Impact on US National Overdose Crisis. Overdose Detection Mapping Application Program. [Google Scholar]
- 6.Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, and Pentel PR. 2014. Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS ONE 9: e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pravetoni M, le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, and Pentel PR. 2013. Reduced Antinociception of Opioids in Rats and Mice by Vaccination with Immunogens Containing Oxycodone and Hydrocodone Haptens. Journal of Medicinal Chemistry 56: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pravetoni M, le Naour M, Harmon TM, Tucker AM, Portoghese PS, and Pentel PR. 2012. An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. The Journal of pharmacology and experimental therapeutics 341: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pravetoni M, Raleigh MD, le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, and Pentel PR. 2012. Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30: 4617–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F, Carroll FI, Comer SD, Navarro HA, Langston TL, Runyon SP, Winston S, Pravetoni M, and Pentel PR. 2017. Safety and efficacy of an oxycodone vaccine: Addressing some of the unique considerations posed by opioid abuse. PLOS ONE 12: e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pravetoni M 2016. Biologics to treat substance use disorders: Current status and new directions. Human Vaccines & Immunotherapeutics 12: 3005–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pravetoni M, and Comer SD. 2019. Development of vaccines to treat opioid use disorders and reduce incidence of overdose. Neuropharmacology 158: 107662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, and Pentel PR. 2003. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. International immunopharmacology 3: 957–970. [DOI] [PubMed] [Google Scholar]
- 14.Lu LL, Suscovich TJ, Fortune SM, and Alter G. 2018. Beyond binding: antibody effector functions in infectious diseases. Nature reviews. Immunology 18: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanderven HA, and Kent SJ. 2020. The protective potential of Fc-mediated antibody functions against influenza virus and other viral pathogens. Immunology & Cell Biology 98: 253–263. [DOI] [PubMed] [Google Scholar]
- 16.Jönsson F, de Chaisemartin L, Granger V, Gouel-Chéron A, Gillis CM, Zhu Q, Dib F, Nicaise-Roland P, Ganneau C, Hurtado-Nedelec M, Paugam-Burtz C, Necib S, Keita-Meyer H, le Dorze M, Cholley B, Langeron O, Jacob L, Plaud B, Fischler M, Sauvan C, Guinnepain M-T, Montravers P, Aubier M, Bay S, Neukirch C, Tubach F, Longrois D, Chollet-Martin S, and Bruhns P. 2019. An IgG-induced neutrophil activation pathway contributes to human drug-induced anaphylaxis. Science Translational Medicine 11: eaat1479. [DOI] [PubMed] [Google Scholar]
- 17.Bournazos S, Wang TT, Dahan R, Maamary J, and v Ravetch J. 2017. Signaling by Antibodies: Recent Progress. Annual review of immunology 35: 285–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruhns P, and Jönsson F. 2015. Mouse and human FcR effector functions. Immunological Reviews 268: 25–51. [DOI] [PubMed] [Google Scholar]
- 19.Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, and Blumberg RS. 2019. The neonatal Fc Receptor (FcRn): A misnomer? Frontiers in Immunology 10: 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roopenian DC, and Akilesh S. 2007. FcRn: The neonatal Fc receptor comes of age. Nature Reviews Immunology 7: 715–725. [DOI] [PubMed] [Google Scholar]
- 21.Laudenbach M, Baruffaldi F, Robinson C, Carter P, Seelig D, Baehr C, and Pravetoni M. 2018. Blocking interleukin-4 enhances efficacy of vaccines for treatment of opioid abuse and prevention of opioid overdose. Scientific Reports 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crouse B, Robinson C, Huseby Kelcher A, Laudenbach M, Abrahante JE, and Pravetoni M. 2020. Mechanisms of interleukin 4 mediated increase in efficacy of vaccines against opioid use disorders. npj Vaccines 5: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Hu Y, Harmon T, Pentel PR, Ehrich M, and Zhang C. 2018. Hybrid nanoparticle-based nicotine nanovaccines: Boosting the immunological efficacy by conjugation of potent carrier proteins. Nanomedicine : nanotechnology, biology, and medicine 14: 1655–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks MJ, De BP, Rosenberg JB, Davidson JT, Moreno AY, Janda KD, Wee S, Koob GF, Hackett NR, Kaminsky SM, Worgall S, Toth M, Mezey JG, and Crystal RG. 2011. Cocaine analog coupled to disrupted adenovirus: a vaccine strategy to evoke high-titer immunity against addictive drugs. Molecular therapy : the journal of the American Society of Gene Therapy 19: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghetie V, Popov S, Borvak J, Radu C, Matesoi D, Medesan C, Ober RJ, and Ward ES. 1997. Increasing the serum persistence of an IgG fragment by random mutagenesis. Nature Biotechnology 15: 637–640. [DOI] [PubMed] [Google Scholar]
- 26.Booth BJ, Ramakrishnan B, Narayan K, Wollacott AM, Babcock GJ, Shriver Z, and Viswanathan K. 2018. Extending human IgG half-life using structure-guided design. mAbs 10: 1098–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hobday PM, Auger JL, Schuneman GR, Haasken S, Verbeek JS, and Binstadt BA. 2014. Fcγ receptor III and Fcγ receptor IV on macrophages drive autoimmune valvular carditis in mice. Arthritis & rheumatology (Hoboken, N.J.) 66: 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fransen MF, Benonisson H, van Maren WW, Sow HS, Breukel C, Linssen MM, Claassens JWC, Brouwers C, van der Kaa J, Camps M, Kleinovink JW, Vonk KK, van Heiningen S, Klar N, van Beek L, van Harmelen V, Daxinger L, Nandakumar KS, Holmdahl R, Coward C, Lin Q, Hirose S, Salvatori D, van Hall T, van Kooten C, Mastroeni P, Ossendorp F, and Verbeek JS. 2018. A Restricted Role for FcγR in the Regulation of Adaptive Immunity. Journal of immunology (Baltimore, Md. : 1950) 200: 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baruffaldi F, Kelcher AH, Laudenbach M, Gradinati V, Limkar A, Roslawski M, Birnbaum A, Lees A, Hassler C, Runyon S, and Pravetoni M. 2018. Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Molecular Pharmaceutics 15: 4947–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baruffaldi F, Raleigh MD, King SJ, Roslawski MJ, Birnbaum AK, Hassler C, Carroll FI, Runyon SP, Winston S, Pentel PR, and Pravetoni M. 2019. Formulation and Characterization of Conjugate Vaccines to Reduce Opioid Use Disorders Suitable for Pharmaceutical Manufacturing and Clinical Evaluation. Molecular pharmaceutics 16: 2364–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baehr C, Huseby Kelcher A, Khaimraj A, Reed DE, Pandit SG, AuCoin D, Averick S, and Pravetoni M. 2020. Monoclonal Antibodies Counteract Opioid-Induced Behavioral and Toxic Effects in Mice and Rats. Journal of Pharmacology and Experimental Therapeutics 375: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson C, Gradinati V, Hamid F, Baehr C, Crouse B, Averick S, Kovaliov M, Harris D, Runyon S, Baruffaldi F, LeSage M, Comer S, and Pravetoni M. 2020. Therapeutic and Prophylactic Vaccines to Counteract Fentanyl Use Disorders and Toxicity. Journal of Medicinal Chemistry 63: 14647–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosales C, and Uribe-Querol E. 2017. Phagocytosis: A Fundamental Process in Immunity. BioMed research international 2017: 9042851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Covell DG, Barbet J, Holton OD, Black CDV, Weinstein JN, and Parker RJ. 1986. Pharmacokinetics of monoclonal immunoglobulin G1 F(ab’ ‘)2, and fab’ in mice. Cancer Research 46: 3969–3978. [PubMed] [Google Scholar]
- 35.Holton OD, Black CD, Parker RJ, Covell DG, Barbet J, Sieber SM, Talley MJ, and Weinstein JN. 1987. Biodistribution of monoclonal IgG1, F(ab’)2, and Fab’ in mice after intravenous injection. Comparison between anti-B cell (anti-Lyb8.2) and irrelevant (MOPC-21) antibodies. The Journal of Immunology 139. [PubMed] [Google Scholar]
- 36.Daëron M 1997. Fc Receptor Biology. Annual Review of Immunology 15: 203–234. [DOI] [PubMed] [Google Scholar]
- 37.Ravetch J. v., and Bolland S. 2001. IgG Fc Receptors. Annual Review of Immunology 19: 275–290. [DOI] [PubMed] [Google Scholar]
- 38.Rappuoli R 2020. Timeline: Vaccines. Cell 183: 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu ML, Goulet DR, Teplyakov A, and Gilliland GL. 2019. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruhns P 2012. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119: 5640–5649. [DOI] [PubMed] [Google Scholar]
- 41.Pravetoni M, Keyler DE, Raleigh MD, Harris AC, Lesage MG, Mattson CK, Pettersson S, and Pentel PR. 2011. Vaccination against nicotine alters the distribution of nicotine delivered via cigarette smoke inhalation to rats. Biochemical pharmacology 81: 1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


