Abstract
Affective prosody, or the changes in rate, rhythm, pitch, and loudness that convey emotion, has long been implicated as a function of the right hemisphere (RH), yet there is a dearth of literature identifying the specific neural regions associated with its processing. The current systematic review aimed to evaluate the evidence on affective prosody localization in the RH. One hundred and ninety articles from 1970 to February 2020 investigating affective prosody comprehension and production in patients with focal brain damage were identified via database searches. Eleven articles met inclusion criteria, passed quality reviews, and were analyzed for affective prosody localization. Acute, subacute, and chronic lesions demonstrated similar profile characteristics. Localized right antero-superior (i.e., dorsal stream) regions contributed to affective prosody production impairments, whereas damage to more postero-lateral (i.e., ventral stream) regions resulted in affective prosody comprehension deficits. This review provides support that distinct RH regions are vital for affective prosody comprehension and production, aligning with literature reporting RH activation for affective prosody processing in healthy adults as well. The impact of study design on resulting interpretations is discussed.
Keywords: prosody, emotion, communication, stroke, brain mapping
1. Introduction
Functional imaging studies have revealed large networks of bilateral frontal, temporal, and parietal regions activated during speech production and comprehension (e.g., Indefrey & Levelt, 2004; Price 2012). Yet, in most people, right hemisphere (RH) lesions do not impair the ability to produce or understand words and sentences as frequently as lesions to their left hemisphere homologues. Given this observation, why is the RH consistently activated during spoken communication?
One important communicative role of RH networks appears to be recognition and expression of affective prosody – changes in pitch, rate, rhythm, and loudness that convey emotion. Comprehension of a spoken message relies not only on the linguistic content but also on paralinguistic features, including prosody. The saying, it’s not what you say but how you say it, rings true in this discussion, especially when considering messages with orthogonal prosodic and linguistic content, such as with sarcasm. Incorrect judgment of affective prosody can result in communication breakdown between interlocutors and may negatively impact social integration (e.g., Struchen et al., 2011) and interpersonal relationships. Spouses of adults with prosodic impairments (termed aprosodia) report these deficits as significant issues (Hillis & Tippett, 2014) and can result in increased perception of caregiver burden and depression (Martinez et al., 2018). Disrupted emotional and prosodic cues are also related to poorer marital satisfaction in adults post-stroke and their spouses (Blonder et al., 2012). Thus, adults with these paralinguistic difficulties, including aprosodia, may be at an increased risk of social isolation, which is associated with poorer health outcomes after stroke (e.g., Reddy Venna & McCullough, 2015), and may negatively impact quality of life for themselves and the ones closest to them.
While a fairly extensive literature exists on the hemispheric lateralization of affective prosody (e.g., Blake et al., 2020), relatively few studies have aimed to identify specific neural structures, regions, or networks critical for processing prosody. Here we will not review the literature on lateralization (but see Blake et al., 2020) but rather those studies that have evaluated the specific RH sites of lesions associated with impaired affective prosody. The current review represents one of four research questions explored in an extensive literature search and synthesis by the Right Hemisphere Damage Writing Group of the Academy of Neurologic Communication Disorders and Sciences. The questions tackle complex questions of aprosodia: (1) characteristics of prosodic impairment related to RH damage (Stockbridge et al., in press), (2) differentiation in prosodic impairments in RH damage compared to adults with left hemisphere (LH) damage (Ukaegbe et al., in preparation), (3) co-occurrence of aprosodia with other behavioral impairments (Sheppard et al., under review), and (4) RH contributions to affective prosody processing based on impaired performance following acquired injury, which is the focus of the current review.
Functional imaging studies of healthy adults support distinct RH networks for affective prosody comprehension (ventral stream) and production (dorsal stream; Schirmer & Kotz, 2006), grossly mirroring predominantly left-lateralized ventral and dorsal streams underlying propositional language comprehension and production, respectively (e.g., Fridriksson et al., 2018; Hickok & Poeppel, 2007). A relatively recent meta-analysis on prosody recognition in healthy adults (linguistic and affective; Belyk & Brown, 2014) reported activation in right posterior and middle temporal gyri for affective prosody uniquely from linguistic prosody. Other RH structures implicated in affective prosody perception included frontal (inferior frontal and middle gyri, supplementary motor area), temporal (superior and middle temporal gyri, parahippocampal gyrus, subcallosal gyrus), and parietal (supramarginal gyrus) lobes as well as the cerebellum.
Evidence from functional imaging should be complemented by lesion studies to determine if the role of a region or network is essential to the function under investigation. That is, damage to the region should cause impaired functioning if the region is essential for the function. Therefore, if a RH region is important for affective prosody processing, then focal damage to that region, such as due to stroke, should result in impaired performance.1 Patient populations with focal lesions (e.g., stroke, tumor) are ideal for studying lesion-symptom relationships since damage is relatively focal and can be localized more readily compared to injuries (e.g., traumatic brain injury, subarachnoid hemorrhage) or diseases (e.g., dementia) associated with more diffuse brain damage or deterioration.
The seminal report by Ross (1981) identified subtypes of aprosodia based on discrete profiles of behavioral symptoms and observed distinct lesion patterns associated with each behavioral profile, findings that closely mirrored the classical aphasia syndromes (e.g., Geschwind, 1970, 1971; Graves, 1997). This early work, among others, laid the foundation for continued inquiry into neural correlates of affective aprosodia. A review by Borod (1993) investigated emotional expression in facial, prosodic, and lexical channels and concluded that expressive aprosodia did not appear to be systematically related to any given lesion location. Most studies included in that review involved patients with RH damage due to stroke, but the decision to include two studies with RH damage due to disease or unknown causes may have impacted the ability to draw specific lesion-symptom conclusions. Additionally, the included articles also varied widely in localization specificity, ranging from broad (e.g., middle cerebral artery (MCA)) to more specific (e.g., basal ganglia) structures, which also likely limited conclusions. To date, there is no review or meta-analysis available that synthesizes the available literature on RH lesions using rigorous inclusion criteria that allows a valid examination of localization of lesion-symptom relationships in affective aprosodia (receptive and expressive).
This systematic review aimed to determine if distinct lesion loci are observed for affective prosody recognition versus production impairments in patients with RH stroke. Recent support for the dual stream model in propositional language organization along with the aim to include a large pool of eligible articles in the review motivated study design to investigate ventral and dorsal prosody processing streams rather than aprosodia subtypes or syndromes. Therefore, the current review focused on affective prosody recognition and production and the neural substrates implicated in their function.
2. Methods
2.1. Search strategy
Twenty-one electronic databases (see Figure 1) were searched to identify articles from 1970 to February 1, 2020 by entering keywords and subject headings. Inclusion and exclusion of articles for review was determined based on population and publication-type criteria outlined in Table 1. To summarize, articles were included if they reported some link between RH lesions and affective prosody performance, enrolled adults (18+ years old) with focal RH damage, presented data from participants with RH damage separately from other clinical and control groups (if included), and were published in English or French in a peer-reviewed journal. Articles were excluded if lesions were too broadly characterized (e.g., rMCA stroke), they consisted of case studies each with different lesion sites, affective prosody performance was not able to be linked back to lesion location, only animal models were reported, data from participants with RH damage were not presented separately from other participant groups, participants had other progressive etiologies or co-occurring psychological disorders, the main topic was not prosody, or they did not present original data (i.e., reviews, meta-analyses).
Figure 1.
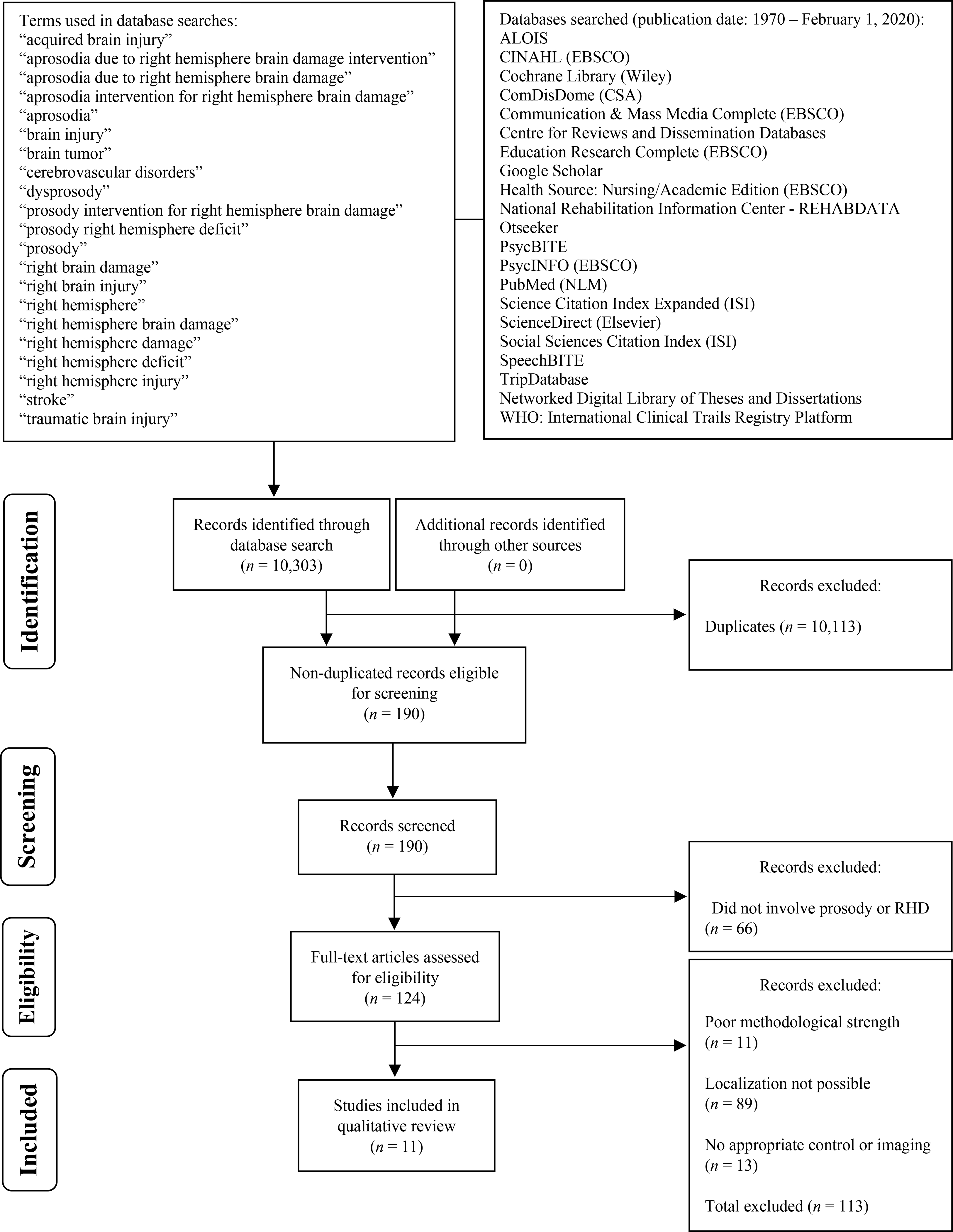
PRISMA* Flow Chart of Article Selection for Systematic Review
*Moher, D., Liberati, A., Tetzlaff, J., Altman, D.G., & The PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097.
Table 1.
Article inclusion/exclusion criteria for review
| Study Status | Criterion |
|---|---|
| Included in review (study met all criteria for inclusion) | 1. Study reported RH lesion localization and some link between lesion and performance (quantitative or qualitative comparison) 2. Study participants were 18+ years of age 3. Study participants had acquired focal cortical and/or subcortical RH regions (e.g., stroke, tumor, surgery (e.g., AVM repair)* 4. Study provided quantitative information on participants with RH damage separately if multiple patient groups included (e.g., RH, LH, cerebellar, etc.) 5. Study publication date between 01/01/1970–02/01/2020 (includes studies in press) 6. Study written/published in English or French 7. Full study published in a peer-reviewed journal 8. Study included original data addressing emotional prosody processing |
| Excluded from review (study met at least one criterion for exclusion) | 1. Study compared participants with RH damage and participants without brain damage only** 2. RH localization was too broadly characterized (e.g., rMCA stroke, frontoparietotemporal lesion) 3. Affective prosody performance was not able to be linked back to lesion location (e.g., reported lesion location at the group-level only) 4. Article was a case study that reported multiple lesion sites 5. Publication was an abstract only 6. Study did not present original data (e.g., review, meta-analysis) 7. Reported animal models only 8. Participants with RH damage were not quantitatively identified separate from other clinical groups 9. Study participants enrolled had progressive etiologies or psychological disorders 10. Emotional prosody was not the focus of the study 11. Study published in a non-peer-reviewed journal 12. Study not published in English or French |
Note. RH = Right hemisphere; LH = Left hemisphere; AVM = Arteriorvenous malformation; rMCA = Right middle cerebral artery
This inclusion criteria was later refined to include articles including participants with focal damage due to ischemic stroke only
Relevant to reviews assessing aprosodia in participants with RH vs. LH only
The overarching search strategy was prosody AND right hemisphere damage. Several combinations of search terms were used to capture a large group of studies to evaluate with our inclusion/exclusion criteria. Table 2 provides an example search strategy, and Supplementary Table 1 (S1) contains the list of databases and searches completed for each database. These search terms were identified by members of the Academy of Neurogenic Communication Disorders and Sciences RH Brain Damage Writing Group (RHDWG), which is part of the Evidence-Based Clinical Research Committee. Consensus on the databases selected to search was obtained given descriptions of their subject matter. Each search algorithm (i.e., pairing search terms) was entered into the 21 databases (see Figure 1).
Table 2.
Database Search Strategy Example
| Search term combinations for PubMed (NLM) |
|---|
| “prosody” OR “aprosodia” AND “right hemisphere damage” OR “right hemisphere deficit” OR “right brain injury” OR “acquired brain injury” OR “traumatic brain injury” OR “brain tumor” OR “cerebrovascular disorders” OR “stroke” |
2.2. Article screening process
Articles were evaluated multiple times to determine inclusion/exclusion in the current review. First, articles were screened by title and abstract by research assistants and verified by two writing group members to identify articles that met inclusion criteria or identify articles that could eliminated based on exclusion criteria. Eligible articles were then entered into a spreadsheet along with article citations and relevant participant demographic information (i.e., number of participants (including a breakdown of participants with RH damage vs. LH damage vs. controls) and participants’ age range). Articles were also cross-referenced with the database in which they were found as well as for full-text availability across open-access to closed-access (subscription-based) platforms. Electronic versions of the articles were uploaded into an online repository accessible to all members participating in the screening and subsequent rating process. Next, members of the RHDWG checked the spreadsheet for duplicate articles as well as articles that did not meet all the inclusion criteria. Any flagged articles were then removed. The remaining articles were assigned to pairs of RHDWG members for extraction of article methods, outcomes, and results for further quality and strength evaluation.
2.3. Quality and methodological review
Seven reviewers independently evaluated the methodological strength and quality of included articles using a rubric modified from Downs and Black (1998; see Supplementary Table 2 (S2)). Ratings assigned to each of the 17 categories included 0 (weak quality/strength), 1 (moderate quality/strength), or 2 (strong quality/strength), with total quality/strength scores ranging from 0–34. Each article was rated by two reviewers, and the final rating was the average of the two reviewers’ total scores. For any article in which the two ratings differed by more than two points, a third reviewer provided an independent rating, and the final rating was the average of the two most similar ratings. Articles identified as being of low quality (i.e., total score < 8) were excluded from the review.
3. Results
3.1. Article selection and review
The database searches yielded 10,303 articles. After removal of duplicates, only 190 articles remained. Sixty-six articles were removed because they did not include prosody or participants with RH damage (n = 124). An additional 11 articles were excluded due to poor methodological strength, leaving 113 articles eligible for screening for RH localization of affective prosody processing. Eighty-nine articles were excluded due to inability to determine localization (e.g., localization information through medical records only, lesion descriptions such as frontopartietotemporal; n = 24). Thirteen articles were removed for inadequate imaging or control information provided, resulting in the inclusion of 11 articles. During the methodological quality and strength rating process, it was decided the review would be restricted to patients who sustained strokes (infarct or hemorrhage) only because other lesion types are associated with more diffuse brain damage (e.g., trauma, neurodegenerative disease) or substantial reorganization of structure-function relationships prior to surgery (e.g., tumor, refractory epilepsy; n = 2). All quality and strength ratings of included articles were within two points of each other. Most ratings (80%) were identical across raters, and 18% of ratings were within one point. Only four ratings (out of 187 total ratings, 2%) differed by two points.
3.2. Time post-stroke and brain imaging considerations
Results are divided into studies of patients with acute stroke (<1 week) versus those with subacute (1 week – 3 months) or chronic (>3 months) because different approaches to brain imaging and mapping are needed depending on the time post-onset at which patients are studied. Studies that included participants with LH damage were noted where appropriate, but as the search was restricted to studies of prosody and RH damage, the results were interpreted for only RH localization.
Acute lesion-deficit association studies use either follow-up head CT or diffusion-weighted imaging (DWI; revealing the infarct within minutes of onset) with or without perfusion-weighted imaging (PWI; revealing hypoperfused, dysfunctional tissue surrounding the infarct). Since patients are studied before substantial reorganization or recovery, all patients with acute lesions can be included to identify an association between the deficit (present or absent, severity) and the brain region (lesion present or absent, severity). The brain region may be parsed into voxels, grey and white matter parcels, lobes and structures, or other regions of interest (ROI). This approach is less valid at later recovery stages because lesion-symptom relationships change as patients recover (i.e., a change in deficit without a change in presence or absence of lesion in each ROI).
Many early subacute and chronic lesion-symptom association studies used head computed tomography (CT) scans to identify lesion sites and followed one of two approaches. One approach was to identify patients who had impaired affective prosody at some point after stroke and then to identify the lesions associated with those deficits, which comprised articles in our review. The second approach was to identify patients who had RH lesions in the past and then test them for affective prosody deficits. Using this second, arguably flawed, approach to study lesion-symptom correlates, it would be difficult to know if included patients without emotional prosody impairments had impairments earlier after stroke but then recovered prior to their participation in the study. This appraoch would also fail to indicate the lesion(s) (originally) necessary for emotional prosody function. While this type of study may be useful for identifying lesions associated with poorer prosody recovery, it does not help to identify the networks typically engaged during affective prosody processing.
For example, one study included mostly patients with very small subcortical RH strokes with normal cerebral blood flow on Positron Emission Tomography (PET; Bradvik et al., 1991). The finding of no difference in affective prosody production between patients (as a group) and healthy controls provides no information about the role of cortical regions in prosody, as most had no cortical lesions. What can be concluded is that these patients with right subcortical lesions either had no prosodic production deficits or their deficits recovered (patients were tested “a considerable time” after stroke). This study did show that the total volume of dysfunctional tissue (infarct on CT or hypoperfusion on PET) correlated with the degree of emotional prosody recognition impairment, suggesting that the few patients with cortical (larger) lesions had deficits. Therefore, studies following this second approach (Bradvik et al., 1991; Karow, Marquardt, & Marshall, 2001; Ross & Monnot, 2011; Ross, Thompson, & Yenkosky, 1997; Wertz et al., 1998) were excluded from the current review as part of the rating of methodological quality and strength.
3.3. Receptive aprosodia
In all the studies reviewed, affective prosody recognition was assessed by matching auditory stimuli spoken with various emotions to the corresponding emotion label. Most studies used linguistic stimuli that were recordings of neutral content sentences while some presented sentences with content that was either congruent or incongruent with the prosody emotion (e.g., “all the puppies were dead” spoken in a happy or sad tone; Starkstein et al., 1994). A few studies also used vowels or sequences of monosyllables (e.g., babababa; Ross & Monnot, 2008).
3.3.1. Acute lesions
Starkstein and colleagues (1994) found that nearly half of their patients with RH or LH lesions assessed within the first week of stroke demonstrated emotional prosody recognition impairments. Those with deficits were more likely to have right temporoparietal cortex and basal ganglia lesions than patients without deficits. Sixteen patients with severe emotional prosody comprehension deficits had lesions including the right temporal cortex. Similarly, another study found that 16 out of 23 patients assessed within five days of RH stroke had impaired affective prosody recognition. Using multivariable regression, this deficit was associated with right posterior superior temporal cortical infarct and age, but not total lesion volume or damage to other regions (Sheppard et al., 2020). The amygdala was a significant predictor only for recognition of fearful prosody. Right superior temporal cortex was also implicated in impaired affective prosody recognition in a case series by Wright and colleagues (2018). Two patients with acute stroke and receptive aprosodia (attributed to impaired extraction of prosodic features from speech) had anterior or superior temporal and insular lesions. One participant also had amygdala damage.
3.3.2. Subacute and chronic lesions
Ross (1981) observed that patients with sensory aprosodia (impaired affective prosody recognition and imitation but spared production in spontaneous speech) or transcortical sensory aprosodia (impaired affective prosody recognition but spared repetition and spontaneous production) had temporal and/or parietal lesions. Specifically, sensory aprosodia was observed in a patient with posterior superior temporal and inferior parietal lobe infarction, encompassing the right homologue of Wernicke’s area. Temporal damage associated with transcortical sensory aprosodia included middle and inferior regions anteriorly and narrowing to the middle temporal lobe posteriorly. Another study with similar results included 73 patients evaluated seven to ten days and again about 20 days post-stroke (Darby, 1993). Four patients with sensory aprosodia were identified; three had lesions that included the inferior division of the right middle cerebral artery (MCA) territory focusing on RH homologue of Wernicke’s area, and the fourth had damage to the left inferior division of the MCA territory. Lesion size varied considerably across participants, including inferior and middle parietal lobe, posterior frontal lobe, anterior occipital lobe, and more extensive temporal lobe damage. No patients with superior division (frontoparietal) or subcortical lacunar infarcts demonstrated sensory aprosodia. Two of the patients with right temporal strokes continued to have sensory aprosodia at about 20 days post-stroke.
Additional studies have reported right temporoparietal lesions and receptive affective aprosodia (Baum & Pell, 1997; Cancelliere & Kertesz, 1990; Gorelick & Ross, 1987; Hughes, Chan, & Su, 1983; Ross & Monnot, 2008). Spared right temporal and/or parietal cortex resulted in functional or unimpaired recognition of affective prosody (e.g., Gorelick & Ross, 1987; Hughes, Chan, & Su, 1983). Even though the focus of Baum and Pell’s (1997) study was prosody production, they found that half of the participants with right temporal and/or parietal lesions had impaired affective prosody recognition. None of the other patients with confirmed lesions in left parietal or temporoparietal cortex had impaired affective prosody recognition. Among studies of subacute and chronic stroke, other RH regions associated with receptive aprosodia include the inferior frontal lobe (Cancelliere & Kertesz, 1990), insula (Cancelliere & Kertesz, 1990), thalamus (Ross & Monnot, 2008), and basal ganglia (Cancelliere & Kertesz, 1990; Gorelick & Ross, 1987; Ross & Monnot, 2008).
3.3.3. Summary of receptive aprosodia
Studies in RH acute, subacute, and chronic stroke strongly converge on temporoparietal damage and impaired affective prosody recognition. The basal ganglia as well as inferior frontal infarcts were less often but still frequently identified in adults with receptive aprosodia. Other subcortical structures that were reported even less in association with receptive aprosodia symptoms include the thalamus, internal capsule, insula, and amygdala. Effect sizes on affective prosody recognition tasks were reported or calculable for four articles (Cancelliere & Kertesz, 1990; Ross & Monnot, 2008; Sheppard et al., 2020; Starkstein et al., 1994; see Table 5). Interpretation of effect sizes using Cohen’s d (1988) revealed large effects for patients with RH lesions compared to LH lesions performing poorly on affective prosody recognition in semantically congruent and incongruent sentences (see Table 4). However, when comparing patients with RH or LH lesion or patients with right temporoparietal or basal ganglia lesions compared to lesions outside this region, effect sizes were trivial (Starkstein et al., 1994). Also in acute stroke, Sheppard and colleagues (2020) observed large effects from regression models predicting recognition accuracy and small to moderate effects when assessing receptive aprosodia symptoms in patients with right posterior superior temporal gyrus damage compared to those without damage to this RH region. During affective prosody recognition and discrimination tasks for subacute and chronic stroke, Ross and Monnot (2008) observed a large effect for patients with LH lesions and impaired recognition improving their recognition performance with simpler linguistic stimuli (word vs. monosyllabic vs. asyllabic). Participants with RH lesions did not show this effect, demonstrating support for RH involvement in the recognition of prosody over and above linguistic context.
Table 5.
Articles reporting relationships between RH lesions and receptive/expressive aprosodia symptoms
Note. Baum and Pell (1997) describe a patienťs lesion as temporoparietal-occipital, so likely the lesion is in the anterior aspect, but this was not stated specifically
RH = right hemisphere, pSTG = posterior superior temporal gyrus, SMG = supramarginal gyrus
Table 4.
Effect sizes (reported and calculated) across affective prosody tasks in reviewed articles
| Article | Task | Effect size for lesion analyses |
|---|---|---|
| ACUTE STROKE | ||
| Wright et al. (2018) | - | na |
| Starkstein et al. (1994)* | Recognition in sentences (emotion expressed via prosody and semantics) | Congruent sentences: d = −0.72; Incongruent sentences: d = −0.62 (RHD < LHD); Likelihood of RHD > LHD for aprosodia presence: φ = 0.01; Likelihood of right temporoparietal or basal ganglia lesion causing aprosodia: φ = 0.03 |
| Patel et al. (2018) | - | na |
| Sheppard et al. (2020)* | Recognition in sentences | Multivariable regression (reported): All emotions: Adjusted R2 = 0.52; pSTG Β = −0.903; Fear: Adjusted R2 = 0.77; Participants without and with pSTG lesions (t-tests; calculated): Happy: d = 0.38; Sad: d = 0.30; Angry: d = 0.25; Bored: d = 0.18 |
| SUBACUTE/CHRONIC STROKE | ||
| Ross (1981) | - | na |
| Gorelick & Ross (1987) | - | na |
| Hughes et al. (1983) | - | na |
| Baum & Pell (1997) | - | na |
| Ross & Monnot (2008) | Discrimination & Recognition (asyllabic, monosyllabic, and sentence) | Partial η2 = 0.15 (group (impaired RHD vs. impaired LHD vs. healthy controls)*task (discrimination, asyllabic recognition, monosyllabic recognition, sentence recognition)) |
| Repetition (asyllabic, monosyllabic, and sentence) | Partial η2 = 0.23 (group (impaired RHD vs. impaired LHD vs. healthy controls)*task (word, monosyllabic, asyllabic)) | |
| Conversational discourse | Adjusted R2 = 0.62 (RHD vs. LHD) | |
| Darby (1993) | - | na |
| Cancelliere & Kertesz (1990)* | Recognition in sentences | Deep central: d = −2.25; Posterior: d = −2.75; Central cortical: d = −4.58; Frontal: d = −4.42 |
| Sentence repetition (affective prosody | Deep central: d = −1.30; Posterior: d = −1.66; Central cortical: d = −2.10; Frontal: d = −2.13 | |
| Sentence repetition (neutral prosody) | Deep central: d = −1.16; Posterior: d = −1.03; Central cortical: d = −1.35; Frontal: d = −1.04 | |
Note.
= Denotes calculation of population effect size (patient group mean - control group mean)/control group standard deviation)or phi (√X2/N) if not reported in original article; Control groups used for calculation of effect size comprised healthy adults except for Starkstein et al. (1994) when the control group comprised participants with LHD for performance on congruent vs. incongruent sentences; pSTG = Posterior superior temporal gyrus; RHD = Right hemisphere damage; LHD = Left hemisphere damage
3.4. Expressive aprosodia
3.4.1. Acute lesions
Only two identified studies associated acute stroke lesions with impaired affective prosody production. Patel et al. (2018) conducted magnetic resonance imaging (MRI) and narrative speech acoustic analyses within 48 hours of onset in 41 patients with RH ischemic stroke. They measured 25 acoustic features and found that coefficient of variation in fundamental frequency (CoVF0) and spectral flatness were most strongly correlated with listener judgement of affective prosody. Using multivariable logistic regression, impairment – defined as the lowest quartile – of these features was predicted by sex, age, lesion volume, and percent damage to nine RH grey and white matter regions (expressive affective prosody: inferior frontal gyrus pars opercularis, supramarginal gyrus, angular gyrus, inferior frontal occipital fasciculus (IFOF), superior frontal occipital fasciculus, superior longitudinal fasciculus (SLF), uncinate fasciculus (UF); receptive affective prosody: superior temporal gyrus, sagittal stratum). Independent predictors of CoVF0 included age and percent damage to right supramarginal gyrus and SLF. Independent predictors of impaired spectral flatness were sex, lesion volume, and damage to right inferior frontal gyrus pars opercularis, IFOF, and UF. Percent damage to ventral stream receptive prosody regions was not associated with either acoustic feature.
In a second study of acute patients (Wright et al., 2018), CoVF0 was unimpaired in two out of three patients, both with lesions involving temporal cortex and insula. The third patient had a fronto-insular lesion and reduced CoVF0 in sentence reading. CoVF0 improved when given cues indicating prosodic features of the target emotion (e.g., Sad: slow rate, low pitch, low volume).
3.4.2. Subacute and chronic lesions
Ross (1981) reported that three patients with motor aprosodia (impaired affective prosody production in spontaneous speech and repetition without impaired comprehension) had frontal lesions including the inferior frontal lobe and anterior inferior parietal lobe. One individual with transcortical motor aprosodia (intact repetition and comprehension but poor spontaneous use of affective prosody) demonstrated a subcortical lesion comprising the head of the caudate nucleus, putamen, and anterior limb of the internal capsule. Cancelliere and Kertesz (1990) also observed overlap in the inferior frontal lobe, insula, inferior parietal lobe, anterior temporal lobe, basal ganglia, and corona radiata among participants with motor or transcortical motor aprosodia. Another study in subacute stroke revealed that patients with motor aprosodia had lesions predominantly in the right frontal operculum (posterior inferior frontal gyrus) with extension to anterior inferior parietal and anterior temporal cortices (Gorelick & Ross, 1987). In these patients, posterior and superior mid-temporal cortex was spared. In contrast, patients with normal affective prosody production had spared right frontal opercula. Hughes, Chan, and Su (1983) observed that of their 12 patients with RH stroke, the two patients with motor aprosodia had frontal lesions, and those with spared emotional prosody production did not have frontal lesions. One patient’s lesion encompassed almost the entire posterior and middle frontal lobe with extension into the anterior parietal lobe. The other patient had a relatively focused area of infarct in the inferior frontal lobe.
In these early subacute and chronic studies, prosody production was subjectively evaluated. However, subsequent studies have used objective acoustic analyses, such as measuring pitch range and CoVF0, to assess for expressive aprosodia. Baum and Pell (1997) had seven participants with RH lesions produce sentences with varying linguistic and affective prosody and compared values of duration, fundamental frequency, and relative amplitude. There were no differences on affective prosody tasks for measures of sentence duration or fundamental frequency across participants with LH damage, RH damage, or healthy adult controls. However, at the participant level, two adults with temporal lesions had duration values outside the range of typical values for healthy controls during repetition, and one of these individuals produced fundamental frequency values outside the typical range for terminal sentence segments during repetition. There was a main effect of group for relative amplitude during sentence reading wherein participants with RHD did not vary the amplitude relative to the target emotion when sentences were void of semantic information. Baum and Pell (1997) concluded that evidence did not support previous claims of the RH uniquely engaged during affective prosody production. Rather, their findings suggest the RH is engaged during “global” prosodic control, which, when impaired, may contribute to the perception of flat affect among patients with RH lesions. Of note, no participants in the study had frontal lesions from what was disclosed in the article.
Additional support for the link between anterior lesions and emotional prosody production came from a study of 18 patients with LH and 21 with RH stroke compared to 43 age-matched controls. Impaired affective prosody production measured by CoVF0 was associated with right frontal damage, including the frontal operculum and anterior insula (Ross & Monnot, 2008). Though not statistically significant, all participants with deep lesions comprising the posterior caudate and globus pallidus demonstrated impaired spontaneous affective prosody use.
3.4.3. Summary of expressive aprosodia
To summarize, expressive aprosodia following RH stroke is most commonly associated with frontal lesions, primarily the inferior frontal lobe. Other important regions, including the insula, anterior (inferior) parietal lobe, and anterior temporal lobe have also been reported, but less consistently, as resulting in expressive aprosodia. Three studies also reported involvement of the basal ganglia, and two identify white matter structures, including the corona radiata, internal capsule, SLF, IFOF, and UF that, when damaged, were associated with impaired expressive affective prosody production. The IFOF and UF, though both ventrally-situated, may subserve aspects of affective prosody expression and recognition like their LH homologues (e.g., Basilakos et al., 2014; Bonilha et al., 2019; Harvey et al., 2013; Ivanova et al., 2016; Mirman et al., 2015), but future work is needed to better understand the contribution of these important white matter pathways. Only two studies (Cancelliere & Kertesz, 1990; Ross & Monnot, 2008) reported or allowed for calculations of effect sizes, and both studies reported large effects of performance on affective prosody production tasks when comparing RH to LH lesions and unimpaired controls as well as for varying RH lesion loci (deep central, cortical central, anterior/frontal, & posterior; Table 4).
3.5. Comparison of lesion loci and aprosodia type
The articles included in the current review span fifty years of investigation into aprosodia following RH stroke and represent varying quantitative and qualitative analytical approaches. More qualitative reports of lesion loci laid the foundation for future, more quantitative analyses of lesion-symptom relationships. However, this heterogeneous presentation of study findings renders cross-study comparisons, namely statistical in nature, a challenge. Much of the data were published in the 1980s and 1990s, with the median year of publication being 1995 and only three of the 11 articles published within the last five years. Fewer than half of the studies employed MRI to evaluate lesion loci, making formal analyses of lesion-symptom mapping unrealistic with this dataset. Additionally, we did not have access to the raw (lesion) data in all the articles, and without access to this lesion data specifically, recreation of lesion overlay or subtraction maps to compare cross-study lesion-symptom correlates of affective aprosodia was not feasible. Only one study (Patel et al., 2018) reported lesion volume information for their RH participants (37.2±67.0 cc).
As a method to compare lesion loci and observance of expressive and/or receptive aprosodia, we calculated the odds (relative risk) of observing symptoms of receptive or expressive aprosodia by the total observations of aprosodia in broad RH regions. For instance, to calculate the odds that receptive aprosodia was observed more often than expressive aprosodia following RH frontal lobe damage, we calculated [the proportion of articles reporting cases of receptive aprosodia compared to the total cases of aprosodia following frontal lobe damage] divided by [the proportion of articles reporting cases of expressive aprosodia compared to the total cases of aprosodia following frontal lobe damage], i.e., [(3/12)/(9/12) = 0.33]. To calculate the odds of expressive aprosodia occurring over receptive aprosodia for the right frontal lobe, the proportions were flipped [(9/12)/(3/12) = 3]. Due to the small number of articles in the review and relatively large number of RH lesion loci, aprosodia-type likelihood was calculated for lobes (frontal, temporal, parietal, occipital, and insula separately), subcortical nuclei (basal ganglia, thalamus, amygdala), and white matter pathways (IFOF, SLF, UF, internal capsule, corona radiata) and presented in Table 5.
Lesions to the frontal lobe, insula, and white matter pathways appeared to be more often associated with expressive aprosodia (expressive vs. receptive; Frontal lobe: 3 vs. 0.33, Insula: 1.50 vs. 0.67, White matter pathways: 5 vs. 0.20), providing support for more frontal lesions resulting in expressive aprosodia symptoms. Damage to white matter pathways appear to be more often implicated in cases of expressive aprosodia, but few studies investigated white matter contributions to aprosodia symptoms and thus limit conclusions, which will be discussed in further detail in the following section. Posterior RH regions, including the temporal and occipital lobes, as well as subcortical nuclei were more often observed with symptoms of receptive aprosodia (receptive vs. expressive; Temporal lobe: 4 vs. 0.25, Occipital lobe: 2 vs. 0.50, Subcortical nuclei: 2.33 vs. 0.43). There appeared to be a slightly higher proportion of articles reporting parietal damage and receptive aprosodia compared to expressive aprosodia (1.14 vs. 0.88), but these ratios were much closer and similar in value compared to other RH regions. We acknowledge that continued work is vital to validate these claims and that these findings should be interpreted cautiously. Though these proportions were not able to be statistically compared, they provide an estimate of the likelihood that lesions occurring in varying RH lesions will contribute to expressive and/or receptive aprosodia symptoms.
4. Discussion
This systematic review synthesized the literature from the past five decades on lesion localization of affective prosody in the RH following stroke. Our findings suggest that distinct RH regions are recruited for comprehension versus production of affective prosody, resulting in receptive and expressive aprosodia, respectively, upon infarct or hemorrhage. Namely, damage to frontal regions, particularly the (inferior) frontal lobe and insula, was more consistently observed with expressive over receptive aprosodia. Receptive aprosodia was more often associated with posterior damage to the temporal lobe and subcortical nuclei, including the basal ganglia, thalamus, and amygdala, compared to reported cases of expressive aprosodia following stroke to these RH regions. The anterior/posterior distinction for expression/comprehension of affective prosody was first suggested by Ross (1981) approximately 50 years ago, and evidence from a variety of studies utilizing valid imaging tools in acute, subacute, and chronic lesion studies support that claim. As will be discussed in further detail in coming paragraphs, the likelihood of a specific lesion-symptom relationship (e.g., inferior frontal lobe damage = expressive aprosodia) is not absolute and dichotomous, highlighting the need for further inquiry into aprosodia and RH localization to understand the intricacies of the neural network(s) engaged.
In addition, this review further validates Ross’s (1981) initial observations and subsequent findings (e.g., Ross & Monnot, 2008) of localization and aprosodia type. With the current focus in aphasia research on dorsal and ventral language processing streams (e.g., Fridriksson et al., 2018) and less on aphasia subtype classification (e.g., Broca’s aphasia), along with the interesting observations of similar language function organization across cerebral hemispheres, the current review also focused on ventral and dorsal streams in terms of receptive and expressive aprosodia RH localization. This review was specific to RH localization of aprosodia as part of an effort by the RHDWG to better understand and disseminate information on aprosodia and the RH.
4.1. Role of the dorsal stream
As shown, frontal RH regions were more often associated with expressive compared to receptive aprosodia. This observation converges with similar findings in LH homologues: frontal regions, particularly the inferior frontal lobe, are critical for speech-language production (e.g., Fridriksson et al., 2018; Hickok & Poeppel, 2007; Indefrey & Levelt, 2004). According to some models of receptive aprosodia (Grandjean, 2020; Sammler, 2015; Schirmer & Kotz, 2006), this region is also critical for (affective) prosody recognition processes, which aligns with cases of receptive aprosodia following damage to the inferior right frontal lobe. Thus, findings suggest that the connections to frontal lobe structures are also important to consider. White matter pathways, including the SLF, IFOF, UF, internal capsule, and corona radiata were more likely to be associated with expressive over receptive aprosodia. White matter pathway involvement was reported by only three articles, with Patel and colleagues (2018) reporting SLF, IFOF, and UF involvement in impaired affective prosody use in discourse. Sammler and colleagues (2015) used multi-fiber probabilistic tractography to estimate white matter pathways connecting cortical RH regions activated during linguistic prosody recognition. They observed a ventral connection between posterior superior temporal sulcus and inferior frontal gyrus via the middle longitudinal fasciculus as well as a dorsal connection between the two regions via the arcuate fasciculus and SLF. Thus, with connections from both dorsal and ventral white matter pathways, it is not surprising that the inferior frontal lobe (in this example) is implicated in both expressive and receptive aprosodia when damaged.
It may be the case that this (inferior) frontal region is critical for a process that underlies or is necessary for both expression and recognition of affective prosody. Wright and colleagues (2018) proposed a cognitive architecture that includes a stage of matching acoustic-prosodic features to the semantic representation of the emotion, similar to the lemma stage of language processing or the orthographic lexicon. This stage, access to Abstract Representations of Acoustic Characteristics that Convey Emotion (ARACCE) is vital and shared for both encoding and decoding affective prosody. Schirmer and Kotz (2006) outline in their model that the right inferior frontal gyrus is critical for evaluative judgment of affective prosody. Therefore, it follows from previous LH research that frontal regions are critical for linguistic production processes, but these regions may also serve important, multifunctional roles shared with linguistic comprehension/recognition.
Findings for the right parietal lobe and aprosodia type were interesting as neither aprosodia type appeared to occur more frequently over the other. Articles reporting cases were nearly split in half – eight articles reported cases of parietal damage and subsequent receptive aprosodia while seven articles reported cases of parietal damage and expressive aprosodia. Of note, all cases in articles reporting parietal stroke and expressive aprosodia reported anterior involvement apart from Patel and colleagues (2018) who observed that damage to the supramarginal gyrus was a significant independent predictor of CoVF0. Straddling the posterior termination point of the Sylvian fissure, the supramarginal gyrus plays a key sensorimotor role integrating information from posterior and anterior brain regions (e.g., Hickok & Poeppel, 2007). Thus, the parietal lobe serves as shared ground for language production and comprehension processes with more anterior portions recruited for expressive affective prosody and posterior portions playing an integrative role with both expression and recognition of affective prosody.
4.2. Role of the ventral stream
Temporal regions were frequently associated with receptive over expressive aprosodia cases. Within the temporal lobe, the (posterior) superior temporal gyrus was the most frequently reported region, and it may help with processing acoustic-prosodic features from speech (Schirmer & Kotz, 2006; Wright et al., 2018). Subcortically, the basal ganglia were most often implicated in affective prosody compared to the thalamus and amygdala. Articles reporting cases of basal ganglia lesion associations did not show a prominence for receptive or expressive aprosodia (4 vs. 3 articles). The basal ganglia are posited to play a role in elements of both expression and recognition of affective prosody. The basal ganglia are a multifunctional set of nuclei responsible for many tasks, among which are emotion and speech-language perception (e.g., Grandjean, 2020; Lim, Fiez, & Holt, 2014; Paulmann, Ott, & Kotz, 2011) and production (e.g., see Grandjean, 2020 and Silveri, 2021 for reviews). How the basal ganglia differentially impact affective prosody performance remains to be clarified with future investigations, including more descriptive speech-language profiles (e.g., dysarthria) of individuals using comparable, quantitative measures, to help understand the relationship of impaired speech, language, and cognitive contributions to aprosodia symptoms (see Sheppard et al., under review). Additionally, many patients with basal ganglia lesions have hypoperfusion beyond the infarct, resulting in cortical dysfunction and later extension of the infarct (Hillis et al., 2002). Thus, an infarct within the basal ganglia could have led to different patterns of cortical hypoperfusion, not captured on imaging or classified in most of the reviewed articles, contributing to varying behavioral observations among patients with this stroke pattern.
As mentioned previously, some studies evaluated lesion sites associated with more specific underlying cognitive processes (Sheppard et al., in press; Wright et al., 2018) or vascular syndromes akin to the classic aphasia syndromes (e.g., Ross, 1981). However, limited data available would not support review at this time.
4.3. Longitudinal stroke and aprosodia recovery
The importance of time post-stroke in determination of affective prosody lesion-symptom relationships is demonstrated by the few longitudinal studies available. Darby (1993) first assessed emotional prosody recognition in patients 7–10 days post-stroke and again about 20 days post-stroke. He found that about half of patients who initially demonstrated deficits later recovered. Clearly, some lesions associated with deficits acutely would no longer be associated with deficits chronically. Similarly, Sheppard and colleagues (2020) studied patients within 5 days of stroke and conducted follow-up testing in a subset of patients at least 6 months post-stroke. They found that 40% of patients with impaired prosody acutely were no longer impaired chronically. As their lesions did not change, lesion-deficit correlations would be different at the acute versus chronic stage in these participants.
4.5. Findings from healthy adults
Review findings also converge with data from functional imaging of healthy adults. Many studies with healthy adults have reported right-lateralized frontotemporal activation during emotional prosody processing (e.g., George et al., 1996; Mitchell et al., 2003; Wiethoff et al., 2008; Wildgruber et al., 2005). Other studies have reported bilateral activation (e.g., Beaucousin et al., 2007; Buchanan et al., 2000; Ethofer et al., 2006; Ethofer et al., 2012; Grandjean et al., 2005; Kotz et al., 2003; Mitchell & Ross, 2008; Seydell-Greenwald et al., 2020; Thönnessen et al., 2010), which has been proposed to reflect several stages of processing. For example, a recent study of emotional prosody recognition by Seydell-Greenwald et al. (2020) compared content-neutral sentences spoken with emotional prosody to those with neutral prosody. For emotional sentences, they found frontotemporal activation in the RH along with bilateral activation in pars orbitalis, amygdala, and anterior insula. They concluded that emotional prosody processing is initially lateralized to the RH, but once acoustic features associated with specific emotions are identified in right temporal cortex, the information is processed bilaterally in inferior frontal cortex for evaluation and integration with semantic content.
In addition to functional neuroimaging, repetitive transcranial magnetic stimulation (rTMS), a temporary neurodisruptive technique that mimics cortical lesions, has also been used to investigate emotional prosody processing in healthy populations. Alba-Ferrera et al. (2012) investigated emotional prosody recognition in a group of healthy adults using rTMS targeting left vs. right superior temporal gyrus (STG). They found that emotional prosody recognition was disrupted following right but not left STG stimulation and concluded that right temporal regions were crucial for prosodic decoding while left temporal regions were not. In another rTMS study, van Rijn et al. (2005) observed significantly more disrupted emotional prosody detection than emotional semantic processing for fear and sadness following stimulation of the right fronto-parietal operculum, which they inferred as playing a role in withdrawal emotional processing.
5. Current review limitations and future research considerations
The main limitation of this review is data quality in available studies. Most affective prosody studies have been small (<100 patients) and provide qualitative descriptions of lesions or lesion overlap in patients with a specified deficit. Only a few studies reported statistical associations between lesions and deficits. As mentioned in Section 3.1, the type of brain imaging (CT, MRI) may limit interpretation of affective prosody lesion-symptom relationships. Even though CT and MRI are reported to have equal sensitivity in acute hemorrhage identification, DWI can detect acute ischemia with superior sensitivity (e.g., Chalela et al., 2007; Kertesz et al., 1987; Merino & Warach, 2010; Vilela & Rowley, 2017) and better characterizes acute lesions compared to head CT (e.g., see Merino & Warach, 2010; Vilela & Rowley, 2017). Impaired blood flow to nonlesioned tissue may also confound reported lesion data. Hypoperfusion of spared brain regions can result in (sub)acute (e.g., Hillis et al., 2001a,b; Hills et al., 2002) and even chronic (e.g., Richardson et al., 2011) deficits. Thus, impaired affective prosody performance may be due to presence and severity of not only the infarct but also the hypoperfused tissue, which may or may not reperfuse and improve function. Only one reviewed article (Wright et al., 2018) reported perfusion information, and one (Ross & Monnot, 2008) provided rationale for including participants beyond the time frame for reversible acute pathophysiologic effects (e.g., diaschisis, edema). Future acute lesion-symptom mapping studies would benefit from inclusion of perfusion data to help determine contributions of hypoperfusion, reperfusion, and infarct characteristics on affective prosody performance and on longitudinal stroke communication recovery mechanisms.
Patients with LH stroke were not always included in investigations of prosody localization. While many studies indicate a RH lateralization of affective (but not linguistic) prosody (e.g., Blonder et al., 1991; Cancelliere & Kertesz, 1990; Pell, 2006), others fail to support this lateralization (e.g., Blake et al., 2020). Studies employing functional imaging (e.g., Buchanan et al., 2000) and event-related potentials (e.g., Pihan et al., 2000) also indicate a RH lateralization of emotional prosody. More experimental designs including patients with LH stroke and patients with RH stroke are needed to validate previous findings of RH affective prosody specialization.
Stimuli-related factors may also limit review findings. Early investigations tended to rely on subjective tasks and measures (e.g., ratings) or live productions of emotional prosody sentences with more recent work utilizing acoustic analyses and pre-recorded stimuli. However, only some stimuli have been validated. Decontextualized tasks may pose another challenge by taxing attenuated cognitive resources (e.g., Tompkins, 2012) and obscuring true processing abilities. Patients with RH stroke benefit from contextual supports that aim to reduce cognitive demands (e.g., Blake et al., 2015; Tompkins, 1991; Tompkins & Flowers, 1987; Zezinka & Tompkins, 2015), and more implicit tasks may better approximate everyday emotional prosody processing demands. Tompkins and Flowers (1987) found that adults with RH stroke improved in emotional prosody recognition following a congruent mood-priming paragraph similarly to adults with LH stroke and adults without brain damage. Likewise, Zezinka and Tompkins (2015) observed increased negative affect word use in discourse samples of patients with RH stroke following completion of a negatively-biasing task. More work is needed to understand how affective prosody task demands (i.e., cognitive-linguistic) impact neural processing patterns and behavioral performance in this population.
Because of the possible influence of these task demands, the type of task completed by participants may have influenced performance. Table 6 outlines the list of the aprosodia assessments completed by participants across studies and the proposed cognitive-linguistic skills required for successful processing. It may be the case that assessment tasks grouped together to investigate RH lesion-symptom relationships for expressive or receptive aprosodia were more heterogeneous rather than homogenous in nature, impacting the neural substrates recruited and ultimately the observations and conclusions drawn from study findings. To clarify, assessments of receptive aprosodia included discrimination (participants judge if a pair of emotional stimuli are the same or different) and recognition (participants judged the emotion of a sentence). Though both are assessing input prosody processing, these tasks recruit different degrees of cognitive-linguistic skills or processes. For instance, recognition of emotion from a sentence would require access to the unique profile of acoustic characteristics that denote the specific emotion (Wright et al., 2018), and access to the semantic representation of the target emotion, whereas discrimination does not require such higher-level processes. This need to integrate sensory and conceptual information during emotion recognition in sentences likely taxes cognitive processes, such as working memory capacity, more so than auditory discrimination tasks. Thus, when comparing performance across studies employing varying recognition (or production) tasks, neural regions necessary for processing may not be as obvious or congruent. Future work is vital to continue to understand the contributions of various networks underlying and supporting affective prosody and its processes.
Table 6.
Aprosodia behavioral assessment methods of reviewed studies
| Affective prosody task(s) | Proposed relevant processes for emotional prosody task completion | Study/studies |
|---|---|---|
| Discrimination | Acoustic/prosodic feature recognition, auditory-verbal working memory, selective attention | Hughes et al. (1983); Ross & Monnot (2008) |
| Recognition in sentences (no linguistic content) | Acoustic/prosodic feature recognition, ARACCE access (sound-tomeaning mapping), emotion semantic knowledge, auditory-verbal working memory, selective attention | Baum & Pell (1997); Ross & Monnot (2008) |
| Recognition in sentences (semantically-neutral, congruent, and incongruent; ±multiple choice) | Acoustic/prosodic feature recognition, ARACCE access (sound-tomeaning mapping), emotion semantic knowledge, auditory-verbal working memory (varying demands), selective attention (varying demands), inhibition (semantically-incongruent sentences) | Baum & Pell (1997); Darby (1993); Cancelliere & Kertesz (1990); Gorelick & Ross (1987); Hughes et al., 1983); Ross (1981); Ross & Monnot (2008); Sheppard et al. (2020); Starkstein et al. (1994); Wright et al. (2018) |
| A/Monosyllabic repetition | Acoustic-prosodic feature recognition, motor planning/programming, motor execution, auditory-verbal working memory, sustained/selective attention (lesser motor-speech demands compared to sentence repetition) | Ross & Monnot (2008) |
| Sentence repetition (sentence presented with affective prosody) | Acoustic-prosodic feature recognition, motor planning/programming, motor execution, auditory-verbal working memory, sustained/selective attention | Cancelliere & Kertesz (1990); Darby (1993); Gorelick & Ross (1987); Hughes et al. (1983); Ross (1981); Ross & Monnot (2008) |
| Sentence repetition (sentence presented with neutral prosody) | Acoustic-prosodic feature recognition, motor planning/programming, motor execution, auditory-verbal working memory, sustained/selective attention; emotion semantic knowledge, ARACCE access (meaning-tosound mapping) | Cancelliere & Kertesz (1990); Hughes et al. (1983) |
| Sentence reading | Orthographic decoding, emotion semantic knowledge, ARACCE access (meaning-to-sound mapping), motor planning/programming, motor execution | Wright et al. (2018) |
| Picture description | Visuospatial processing, inferencing, narrative discourse planning & production, emotion semantic knowledge, ARACCE access (meaningto-sound mapping), motor planning/programming, motor execution | Patel et al. (2018) |
| Conversational discourse | Emotion semantic knowledge, ARACCE access (meaning-to-sound mapping), motor planning/programming, motor execution, pragmatics, language comprehension, narrative discourse planning & production, memory (type – declarative, episodic – depends on conversation topic) | Gorelick & Ross (1987); Ross (1981); Ross & Monnot (2008) |
Note. ARACCE = Abstract Representation of Acoustic Characteristics that Convey Emotion; It is unclear from Darby (1993) for the affective prosody expression task if participants were presented with the sentence orthographically or auditorily
Finally, future work would benefit from inclusion of more comprehensive patient communication profiles. Of the articles reviewed, only a subset reported on concomitant motor speech/facial muscle weakness (6/11), cognitive-linguistic skills (excluding emotional facial/gestural recognition; 8/11), and hearing acuity (4/11). Reported post-stroke communication abilities also varied from scores on specific assessments to (inconsistent) comments of impairment presence or absence. It is yet unclear how often affective prosody deficits may be attributed to breakdowns of task-specific processes (e.g., matching prosodic features to specific emotions; Wright et al., 2018) domain-general cognitive processes (e.g., immediate and working memory, attention, suppression (see Tompkins, 2012)), and/or sensory processes (e.g., acoustic feature extraction). Broader assessment of patients’ communication abilities is recommended to further develop the taxonomy of RH communication disorders (e.g., Blake et al., 2002; Tompkins, 2012). Sheppard and colleagues (under review) assessed prevalence of aprosodia and other co-occurring deficits in adults with RH lesions and observed that hemispatial neglect co-occurred with amusia and linguistic but not affective prosody deficits. Receptive affective aprosodia co-occurred with deficits in interpersonal interactions and less often with impairments of emotional semantic access and emotional facial expression recognition. As with the current review, the authors highlight the significant gap in the literature regarding co-occurrence of aprosodia with other deficits.
6. Conclusion
Despite the limitations and future work needed to understand affective prosody localization in the RH, the reviewed studies indicate an essential role of right frontal (dorsal stream) regions in affective prosody expression, right temporal and subcortical (ventral stream) regions in affective prosody recognition, and right parietal regions for shared processes, mirroring similar network organization to that of propositional language in the LH.
Supplementary Material
Table 3.
Participant Demographics Summary of Articles Assessing Emotional Prosody Lesion Localization in Right Hemisphere Stroke
| Study | N | Participants | Age (range) | Education (years, range) | Time post stroke (range) | Imaging modality |
|---|---|---|---|---|---|---|
| ACUTE STROKE | ||||||
| Wright et al. (2018) | 4 | RHD (3), FTD | 56–61 | 1 RHD: 16+ | RHD: 1/3 <48 hours, 2/3 nr; FTD: dx 1 year prior to testing | MRI |
| Starkstein et al.(1994) | 76 | RHD & LHD (59), NBD | LHD & RHD: No
aprosodia: M = 52.3; Mild aprosodia: M = 59.9; Severe aprosodia: M = 60.9 (range nr); NBD: 33–62 |
LHD & RHD: No
aprosodia: M = 11.3; Mild aprosodia: M = 8.6; Severe aprosodia: M = 10.0 (range nr); NBD: nr |
RHD & LHD: No aprosodia: M = 6.1 days; Mild aprosodia: M = 5.9 days; Severe aprosodia: M = 7.2 days (range nr) |
CT |
| Patel et al. (2018) | 41 | RHD | M = 62.7 (range nr) | M = 14.4 (range nr) | <48 hours | MRI |
| Sheppard et al. (2020) | 33 | RHD (23), NBD | RHD M = 55.09; NBD M = 53.33 (range nr) | nr | <5 days; 9/23 RHD retested at 6+ months | MRI |
| SUBACUTE/CHRONIC STROKE | ||||||
| Ross (1981) | 10 | RHD | 34–76 | nr | < 24hrs-5 months | CT |
| Gorelick & Ross (1987) | 14 | RHD | 36–82 | nr | 0.5–2 months | CT |
| Hughes et al. (1983) | 19 | RHD (12), NBD | 32–70 | 0–16 | 0.4–27 months | CT |
| Baum & Pell (1997) | 21 | LHD (4), RHD (7), NBD | 43–85 | nr | 9–76 months | nr |
| Ross & Monnot (2008) | 82 | RHD (21), LHD (18), NBD | 43–78 | 8–17 | 3–8 weeks | MRI |
| Darby (1993) | 84 | RHD (26), LHD (16), ns lacunar infarcts (31), NBD |
57–86 (aprosodia subset) | nr | Time 1: 2–3 days; Time 2: 14107 days (aprosodia subset) | CT |
| Cancelliere & Kertesz (1990) | 66 | RHD (28), LHD (18) | RHD & LHD: M = 62.5; NBD: M = 62.4 (range nr) | nr | M = 25.6 days (range nr) | CT |
Note. LHD = Left Hemisphere Brain Damage; RHD = Right Hemisphere Brain Damage; NBDs = Controls without Brain Damage; FTD = Frontotemporal Dementia; M = Mean; nr = Not Reported; dx = Diagnosis; ns = Not specified
Affective prosody refers to tone of voice or inflection changes that convey emotion
Affective prosody processing is often impaired after right hemisphere (RH) stroke
Systematic review of affective prosody localization following RH stroke is lacking
Affective prosody production/recognition engages right dorsal/ventral brain regions
Considerations for future affective prosody localization research are discussed
Acknowledgements
Additional members of the ANCDS RH Brain Damage Writing Group (Laura Murray, PhD, Kristine Lundgren, PhD, Lynsey M. Keator, MA, Melissa Stockbridge, PhD, Jerry Hoepner, PhD, Hiram Brownell, PhD, Joseph Duffy, PhD, and Yves Joanette, PhD) contributed substantially to the conceptualization of the study as well as to identifying and rating articles.
Funding and Declaration of Competing Interest
This work was partially funded by the National Institutes of Health (NIDCD; R01 DC015466). We gratefully acknowledge this support. The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Zezinka Durfee and Dr. Sheppard report that they received salary support from NIH through R01 DC015466; Dr. Hillis reports that she received salary support from NIH through R01 DC015466 and monetary support from the American Heart Association as Deputy Editor of Stroke and from Elsevier as Associated Editor of Practice Update: Neurology. Dr. Blake has no financial disclosures.
Footnotes
How performance is impacted, or what aspect of affective prosody processing (e.g., recognition of prosodic features, mapping prosodic features to emotional representations, cognitive evaluation of emotional prosody) is impaired following damage to a specific neural region or structure is another question requiring further investigation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alba-Ferrara L, Ellison A, & Mitchell RLC (2012). Decoding emotional prosody: Resolving differences in functional neuroanatomy from fMRI and lesion studies using TMS. Brain Stimulation, 5(3), 347–353. [DOI] [PubMed] [Google Scholar]
- Basilakos A, Fillmore PT, Rorden C, Guo D, Bonilha L, & Fridriksson J (2014). Regional white matter damage predicts speech fluency in chronic aphasia. Frontiers in Human Neuroscience, 8, Article 845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum SR & Pell MD (1997). Production of affective and linguistic prosody by brain-damaged patients. Aphasiology, 11(2), 177–198. [Google Scholar]
- Beaucousin V, Lacheret A, Turbelin MR, Morel M, Mazoyer B, & Tzourio-Mazoyer N (2007). FMRI study of emotional speech comprehension. Cerebral Cortex, 17(2), 339–352. [DOI] [PubMed] [Google Scholar]
- Belyk M & Brown S (2014). Perception of affective and linguistic prosody: An ALE-meta-analysis of neuroimaging studies. Social Cognitive and Affective Neuroscience, 9(9), 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake ML, Duffy JR, Myers PS, & Tompkins CA (2002). Prevalence and patterns of right hemisphere cognitive/communicative deficits: Retrospective data from an inpatient rehabilitation unit. Aphasiology, 16(4–6), 537–547. [Google Scholar]
- Blake ML, Lundgren K, Keator L, Murray L, Sheppard SM, & Stockbridge M (2020). A systematic review of the lateralization of aprosodia. Accepted for presentation at the 50th Annual Clinical Aphasiology Conference; Hawaii. [Google Scholar]
- Blake ML, Tompkins CA, Scharp VL, Meigh KM, & Wambaugh J (2015). Contextual Constraint Treatment for coarse coding deficit in adults with right hemisphere brain damage: Generalization to narrative discourse comprehension. Neuropsychological Rehabilitation, 25(1), 15–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder LX, Bowers D, & Heilman KM (1991). The role of the right hemisphere in emotional communication. Brain, 114, 1115–1127. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Hillis AE, Wilmskoetter J, Hickok G, Basilakos A, Munsell B, Rorden C, & Fridriksson J (2019). Neural structures supporting spontaneous and assisted (entrained) speech fluency. Brain, 142, 3951–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borod JC (1993). Cerebral mechanisms underlying facial, prosodic, and lexical emotional expression: A review of neuropsychological studies and methodological issues. Neuropsychology, 7(4), 445–463. [Google Scholar]
- Bradvik B, Dravins C, Holtas S, Rosen I, Ryding E, & Ingvar DH (1991). Disturbances of speech prosody following right hemisphere infarcts. Acta Neurologica Scandinavica, 84, 114–126. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lutz K, Mirzazade S, Specht K, Shah NJ, Zilles K, & Jäncke L (2000). Recognition of emotional prosody and verbal components of spoken language: An fMRI study. Cognitive Brain Research, 9(3), 227–238. [DOI] [PubMed] [Google Scholar]
- Cancelliere AEB & Kertesz A (1990). Lesion localization in acquired deficits of emotional expression and comprehension. Brain and Cognition, 13, 133–147. [DOI] [PubMed] [Google Scholar]
- Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, Hill MD, Patronas N, Latour L, & Warach S (2007). Magnetic resonance imaging and computer tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet, 369, 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum. [Google Scholar]
- Darby DG (1993). Sensory aprosodia: A clinical cue to lesions of the inferior division of the right middle cerebral artery? Neurology, 43, 567–572. [DOI] [PubMed] [Google Scholar]
- Downs SH & Black N (1998). The feasibility of creating a checklist for the assessment of methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology and Community Health, 52(6), 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethofer T, Anders S, Wiethoff S, Erb M, Herbert C, Saur R, Grodd W, & Wildgruber D (2006). Effects of prosodic emotional intensity on activation of associative auditory cortex. Neuroreport, 17(3), 249–253. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Gschwind M, Kreifelts B, Wildgruber D, & Vuilleumier P (2012). Emotional voice areas: anatomic location, functional properties, and structural connections revealed by combined fMRI/DTI. Cerebral Cortex, 22(1), 191–200. [DOI] [PubMed] [Google Scholar]
- Fridriksson J, den Ouden D-B, Hillis AE, Hickok G, Rorden C, Basilakos A, Yourganov G, & Bonilha L (2018). Anatomy of aphasia revisited. Brain, 141(4), 848–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Parekh PI, Rosinsky N, Ketter TA, Kimbrell TA, Heilman KM, Herscovitch P, & Post RM (1996). Understanding emotional prosody activates right hemisphere regions. Archives of Neurology, 53(7), 665–670. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1970). The organization of language and the brain. Science, 170(3961), 940–944. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1971). Aphasia. The New England Journal of Medicine, 28(12), 654–656. [DOI] [PubMed] [Google Scholar]
- Gorelick PB & Ross ED (1987). The aprosodias: Further functional-anatomical evidence for the organisation of affective language in the right hemisphere. Journal of Neurology, Neurosurgery and Psychiatry, 50(5), 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean D (2020). Brain networks of emotional prosody processing. Emotion Review, 13(1), 34–43. [Google Scholar]
- Grandjean D, Sander D, Pourtois G, Schwartz S, Seghier ML, Scherer KR, & Vuilleumier P (2005). The voices of wrath: Brain responses to angry prosody in meaningless speech. Nature Neuroscience, 8(2), 145–146. [DOI] [PubMed] [Google Scholar]
- Graves RE (1997). The legacy of the Wernicke-Lichtheim Model. Journal of the History of the Neurosciences, 6(1), 3–20. [DOI] [PubMed] [Google Scholar]
- Harvey DY, Wei T, Ellmore TM, Cris Hamilton A, & Schnur TT (2013). Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia, 51, 789–801. [DOI] [PubMed] [Google Scholar]
- Hickock G & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8, 393–402. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kane A, Tuffiash E, Ulatowski JA, Barker PB, Beauchamp NJ, & Wityk RJ (2001a). Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain and Language, 79, 495–510. [DOI] [PubMed] [Google Scholar]
- Hillis AE & Tippett DC (2014). Stroke recovery: Surprising influences and residual consequences. Advances in Medicine, 2014. doi: 10.1155/2014/378263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, & Metter EJ (2002). Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain, 125(Pt 5), 1094–1104. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, & Selnes OA (2001b). Hypoperfusion of Wernicke’s area predicts severity of semantic deficit in acute stroke. Annals of Neurology, 50, 561–566. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Chan JL, & Su MS (1983). Aprosodia in Chinese patients with right cerebral hemisphere lesions. Archives of Neurology, 40(12), 732–736. [DOI] [PubMed] [Google Scholar]
- Indefrey P & Levelt WJM (2004). The spatial and temporal signatures of word production components. Cognition, 92, 101–144. [DOI] [PubMed] [Google Scholar]
- Ivanova MV, Isaev DY, Dragoy OVS, Petrushevskiy AG, Fedina ON, Shklovsky VM, & Dronkers NF (2016). Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex, 85, 165–181. [DOI] [PubMed] [Google Scholar]
- Karow CM, Marquardt TP, & Marshall RC (2001). Affective processing in left and right hemisphere brain-damaged subjects with and without subcortical involvement. Aphasiology, 15(8), 715–729. [Google Scholar]
- Kertesz A, Black SE, Nicholson L, & Carr R (1987). The sensitivity and specificity of MRI in stroke. Neurology, 37, 1580–1585. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Meyer M, Alter K, Besson M, von Cramon DY, & Friederici AD (2003). On the lateralization of emotional prosody: An event-related functional MR investigation. Brain and Language, 86(3), 366–376. [DOI] [PubMed] [Google Scholar]
- Lim S-J, Fiez JA, & Holt LL (2014). How may the basal ganglia contribute to auditory categorization and speech perception? Frontiers in Neuroscience, 8. doi: 10.3389/fnins.2014.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M, Multani N, Anor CJ, Misquitta K, Tang-Wat DF, Keren R, Fox S, Lang AE, Marras C, & Tartaglia MC (2018). Emotion detection deficits and decreased empathy in patients with Alzheimer’s disease and Parkinson’s disease affect caregiver mood and burden. Frontiers in Aging and Neuroscience, 10, Article 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino JG & Warach S (2010). Imaging of acute stroke. Nature Reviews Neurology, 6, 560–571. [DOI] [PubMed] [Google Scholar]
- Mirman D Zhang Y, Branch Coslett H, & Schwartz MF (2015). The ins and outs of meaning: Behavioral and neuroanatomical dissociation of semantically-driven word retrieval and multimodal semantic recognition in aphasia. Neuropsychologia, 76, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RL, Elliott R, Barry M, Cruttenden A, & Woodruff PW (2003). The neural response to emotional prosody, as revealed by functional magnetic resonance imaging. Neuropsychologia, 41(10), 1410–1421. [DOI] [PubMed] [Google Scholar]
- Mitchell RL & Ross ED (2008). fMRI evidence for the effect of verbal complexity on lateralisation of the neural response associated with decoding prosodic emotion. Neuropsychologia, 46(12), 2880–2887. [DOI] [PubMed] [Google Scholar]
- Patel S, Oishi K, Wright A, Sutherland-Foggio H, Saxena S, Sheppard SM, & Hillis AE (2018). Right hemisphere regions critical for expression of emotion through prosody. Frontiers in Neurology, 9, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmann S, Ott DVM, & Kotz SA (2011). Emotional Speech Perception Unfolding in Time: The Role of the Basal Ganglia. PLOS ONE, 6(3), e17694. doi: 10.1371/journal.pone.0017694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell MD (2006). Cerebral mechanisms for understanding emotional prosody in speech. Brain and Language, 96(2), 221–234. [DOI] [PubMed] [Google Scholar]
- Pihan H, Altenmuller E, Hertrich I, & Ackermann H (2000). Cortical activation patterns of affective speech processing depend on concurrent demands on the subvocal rehearsal system. A DC-potential study. Brain, 123(Pt 11), 2338–2349. [DOI] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62, 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy Venna V & McCullough LD (2015). Role of social factors on cell death, cerebral plasticity and recovery after stroke. Metabolic Brain Disease, 30(2), 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JD, Baker JM, Morgan PS, Rorden C, Bonilha L, & Fridriksson J (2011). Cerebral perfusion in chronic stroke: Implications for lesion-symptom mapping and functional MRI. Behavioural Neurology, 24, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn S, Aleman A, van Diessen E, Berckmoes C, Vingerhoets G, & Kahn RS (2005). What is said or how it is said makes a difference: Role of the right fronto‐parietal operculum in emotional prosody as revealed by repetitive TMS. European Journal of Neuroscience, 21(11), 3195–3200. [DOI] [PubMed] [Google Scholar]
- Ross ED (1981). The aprosodias: Functional-anatomic organization of the affective components of language in the right hemisphere. Archives of Neurology, 38, 561–569. [DOI] [PubMed] [Google Scholar]
- Ross ED & Monnot M (2008). Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain and Language, 104, 51–74. [DOI] [PubMed] [Google Scholar]
- Ross ED & Monnot M (2011). Affective prosody: What do comprehension errors tell us about hemispheric lateralization of emotions, sex and aging effects, and the role of cognitive appraisal. Neuropsychologia, 49, 866–877. [DOI] [PubMed] [Google Scholar]
- Ross ED, Thompson RD, & Yenkosky J (1997). Lateralization of affective prosody in brain and the callosal integration of hemispheric language functions. Brain and Language, 56, 27–54. [DOI] [PubMed] [Google Scholar]
- Sammler D, Grosbras M-H, Anwander A, Bestelmeyer PEG, & Belin P (2015). Dorsal and ventral pathways for prosody. Current Biology, 25(23), 3079–3085. [DOI] [PubMed] [Google Scholar]
- Schirmer A & Kotz SA (2006). Beyond the right hemisphere: Brain mechanisms mediating vocal emotional processing. Trends in Cognition Sciences, 10(1), 24–30. [DOI] [PubMed] [Google Scholar]
- Seydell-Greenwald A, Chambers CE, Ferrara K, & Newport EL (2020). What you say versus how you say it: Comparing sentence comprehension and emotional prosody processing using fMRI. NeuroImage, 209, 116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SM, Keator LM, Breining BL, Wright AE, Saxena S, Tippett DC, & Hillis AE (2020). Right hemisphere ventral stream for emotional prosody identification: Evidence from acute stroke. Neurology, 94(10), e1013–e1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard SM, Stockbridge MD, Keator LM, Murray LL, & Blake ML (under review). The company prosodic deficits keep following right hemisphere stroke: A systematic review. Journal of the International Neuropsychological Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MC (2021). Contribution of the cerebellum and the basal ganglia to language production: Speech, word fluency, and sentence construction – Evidence from pathology, The Cerebellum, 20, 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Federoff JP, Price TR, Leiguarda R, Robinson RG (1994). Neuropsychological and neuroradiologic correlates of emotional prosody comprehension. Neurology, 44(3 Pt 1), 515–522. [DOI] [PubMed] [Google Scholar]
- Stockbridge MD, Sheppard SM, Keator LM, Murray LL, Blake LM, & Right hemisphere disorders working group, Evidence-based clinical research committee, Academy of Neurological Communication Disorders and Sciences. (accepted). Aprosodia subsequent to right hemisphere brain damage: A systematic review and meta-analysis. Journal of the International Neuropsychological Society. [Google Scholar]
- Struchen MA, Pappadis MR, Sander AM, Burrows CS, & Myszka KA (2011). Examining the contribution of social communication abilities and affective/behavioral functioning to social integration outcomes for adults with traumatic brain injury. Journal of Head Trauma Rehabilitation, 26(1), 30–42. [DOI] [PubMed] [Google Scholar]
- Thönnessen H, Boers F, Dammers J, Chen YH, Norra C, & Mathiak K (2010). Early sensory encoding of affective prosody: Neuromagnetic tomography of emotional category changes. NeuroImage, 50(1), 250–259. [DOI] [PubMed] [Google Scholar]
- Tompkins CA (1991). Automatic and effortful processing of emotional intonation after right or left hemisphere brain damage. Journal of Speech and Hearing Research, 4, 820–830. [DOI] [PubMed] [Google Scholar]
- Tompkins CA (2012). Rehabilitation for cognitive-communication disorders in right hemisphere brain damage. Archives of Physical Medicine and Rehabilitation, 93(1 Suppl), S61–69. [DOI] [PubMed] [Google Scholar]
- Tompkins CA & Flowers CR (1987). Contextual mood priming following left and right hemisphere damage. Brain and Cognition, 6, 361–376. [DOI] [PubMed] [Google Scholar]
- Ukaegbe O, Holt B, Keator L, Brownell H, & Lundgren K (in preparation). Aprosodia following right hemisphere damage: What’s right and what’s left? [DOI] [PubMed] [Google Scholar]
- Vilela P & Rowley HA (2017). Brain ischemia: CT and MRI techniques in acute ischemic stroke. European Journal of Radiology, 96, 162–172. [DOI] [PubMed] [Google Scholar]
- Wertz RT, Henschel CR, Author LL, Ashford JR, & Kirshner HS (1998). Affective prosodic disturbance subsequent to right hemisphere stroke: A clinical application. Journal of Neurolinguistics, 11(1–2), 89–102. [Google Scholar]
- Wiethoff S, Wildgruber D, Kreifelts B, Becker H, Herbert C, Grodd W, & Ethofer T (2008). Cerebral processing of emotional prosody – influence of acoustic parameters and arousal. NeuroImage, 39(2), 885–893. [DOI] [PubMed] [Google Scholar]
- Wildgruber D, Riecker A, Hertrich I, Erb M, Grodd W, Ethofer T, & Ackermann H (2005). Identification of emotional intonation evaluated by fMRI. NeuroImage, 24(4), 1233–1241. [DOI] [PubMed] [Google Scholar]
- Wright AE, Saxena S, Sheppard SM, & Hillis AE (2018). Selective impairments in components of affective prosody in neurologically impaired individuals. Brain and Cognition, 124, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezinka A & Tompkins CA (2015). Negative word production in adults with right hemisphere brain damage: Effects of implicit assessment and contextual bias. American Journal of Speech-Language Pathology, 24(4), S815–S827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


