Abstract
Candida. albicans is the most common cause of fungal infections in humans, and disseminated candidiasis has become one of the leading causes of hospital-acquired blood stream infections with a high mortality rate. However, little is known about the host–pathogen interactions and the mechanisms of antifungal immunity. Here, we report that Nedd4 is essential for signaling through Dectin-1 and Dectin-2/3. We showed that mice lacking Nedd4 globally or only in the myeloid compartment are highly susceptible to systemic C. albicans infection, which correlates with heightened organ fungal burden, defective inflammatory response, impaired leukocyte recruitment to the kidneys, and defective reactive oxygen species (ROS) expression by granulocytes. At the molecular level, Nedd4−/− macrophages displayed impaired activation of TAK-1 and NF-κB, but normal activation of Syk and PKC-δ upon C. albicans yeast and hyphal infections. These data suggest that Nedd4 regulates signaling events downstream of PKC-δ but upstream of or at TAK-1.
Introduction
Superficial infections of the skin and nails are the most common benign fungal diseases in humans and affect ~25% (or ~1.9 billion) of the general population worldwide (1). Invasive fungal infections are less common but are of greater concern because they are associated with unacceptably high mortality rates exceeding 50% (2, 3). The common encountered invasive fungal infections are caused by Candida Species (4). The most common and dangerous species that cause invasive candidiasis are C. albicans, C. glabrata, C. tropicalis, C. parapsilosis, C. krusei (5, 6) and most recently identified C. auris (7–9). Candida spp. do not cause diseases in immunocompetent individuals. However, people with immunocompromised immunity, such as Human immunodeficiency virus (HIV) patients, or those undergoing chemotherapy or organ transplantation, are of high risk of invasive fungal infections (2, 10). Prolonged hospitalization, venous catheter, denture wearers, neonatal intensive care, liver transplantation and major gut surgery are major predisposing factors for invasive Candidemia (2, 4, 11, 12). The increase in resistance to available drugs, toxicity, undesirable drug interactions, restrictions in routes of administration (internal verses external), and bioavailability among others heighten the urgency to enhance research into host defence against fungal pathogens (10, 13–15). Moreover, the Center for Disease Control and Prevention has classified C. auris as an emerging healthcare threat due to its resistance to most available drugs coupled with difficulty in identifying the organism using standard laboratory procedures (7, 8, 16).
During fungal infection, various pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and most importantly C-type lectin receptors (CLRs) in innate immune cells, sense pathogen-associated molecular patterns (PAMPs) from fungi (17–20). The CLRs dectin-1 (encoded by Clec7a in mice) and dectin-2 (encoded by Clec4n in mice) recognize C. albicans yeast cells and hyphae by binding to surface β-glucans and α-mannans on the two fungal forms respectively (21–24). These interactions induce the activation of Syk which leads to subsequent activation of protein-kinase C-δ (PKC-δ). Signalling through PKC-δ facilitates the formation of the CARD9-BCL10-MALT1 complex which subsequently activates NF-κB, thus eliciting the production of various inflammatory cytokines (25–27). Although it is well-established that NF-κB is crucial for CLR-mediated anti-fungal innate immune responses, how NF-κB activation is regulated during fungal infections is still not fully elucidated.
Nedd4 (neuronal precursor cell-expressed developmentally down-regulated 4) is a HECT (homologous E6-AP carboxyl terminus)-type E3 ubiquitin ligase which directs the ubiquitination of an array of signalling proteins (28–31). Nedd4 consists of a C-terminal HECT domain and N-terminal C2 domain and three (mouse) or four (human) WW domains. The C2 domain regulates cellular localization, the WW domains provide substrate recognition typically by binding to the PY motif (L/PPxY), and the HECT domain confers E3 ligase activity and has a ubiquitin binding surface that enables progressivity of ubiquitination (32–34). Previously, we and others have shown that Nedd4 positively regulates T cell activation and proliferation (35, 36). Although Nedd4 was reported to be involved in anti-intracellular bacteria clearance by promoting autophagy (37), in vivo biological relevance of this observation remains to be elucidated because mice deficient for Nedd4 were not used in this study. Furthermore, whether Nedd4 regulates anti-fungal innate immunity is completely unknown.
Here we report that macrophages and neutrophils lacking Nedd4 produce significantly lower amounts of pro-inflammatory cytokines including TNF-α and IL-6, and display impaired ROS expression and fungal killing activity upon infection with C. albicans yeasts and hyphae. In support of a crucial role of Nedd4 in anti-fungal innate immune responses, mice lacking Nedd4 or lacking Nedd4 specifically in myeloid cells succumb to systemic C. albicans infection. Mechanistically, Nedd4 appears to be required for CLR-mediated activation of the NF-κB signalling pathway downstream of PKC-δ but upstream of or at TAK-1.
Materials and Methods
Mice
The Nedd4 conditional knockout mice (cKO) were generated at Taconic Biosciences (Cranbury, NJ, USA) by targeting C57BL/6 embryonic stem (ES) cells followed by blastocyst microinjection for subsequent chimera generation. The targeting vector was constructed for conditional deletion of exons 2 and 3 of the mouse Nedd4 gene in a plasmid containing two LoxP sequences upstream and downstream of the target region (Fig. 1A). The long homologous arm was isolated from intron 1 and the short homologous arm was isolated from intron 3. Deletion of exons 2 and 3 led to the removal of 239 bp coding sequence, and generated a premature stop codon in the Nedd4 mRNA, thus resulting in the loss of functional Nedd4 protein. Moreover, the Nedd4 mRNA with such a deletion was degraded due to non-sense-mediated mRNA decay. Conditional KO Nedd4 (Nedd4f/f) mice were crossed with Rosa26-Cre-ERT2 mice [B6.129-Gt(ROSA)26Sortm1(cre/ERT2) in which a sequence encoding a fusion of Cre recombinase and the estrogen receptor (ERT2) was recombined into the ubiquitously expressed Rosa26 locus] to generate Nedd4f/fRosa26-Cre-ERT2 mice (called ’Nedd4/ROSACreER mice’ here) in which the Cre recombinase becomes active upon stimulation with Tamoxifen leading to systemic deletion of Nedd4 (Figure 1b). Nedd4f/f mice were also crossed to LysMcre mice [(B6N.129P2(B6)-Lyz2tm1(cre)), in which a nuclear-localized Cre recombinase is inserted into the first coding ATG of the lysozyme 2 gene (Lyz2)] (38) to generate Nedd4f/f/LYSMcre mice (referred to as Nedd4ΔM) which lack Nedd4 in myeloid specific cells (Fig. 1D). The mice were used at 8–12 weeks of age, and both male and female mice were used in this study. All animal experimentation involving systemic C. albicans infection was approved by the Institutional Animal Care and Use Committees (IACUCs) of the Ohio State University and the University of Iowa.
Figure 1: Construction of the targeting vector and generation of the Floxed nedd4 allele in mice:
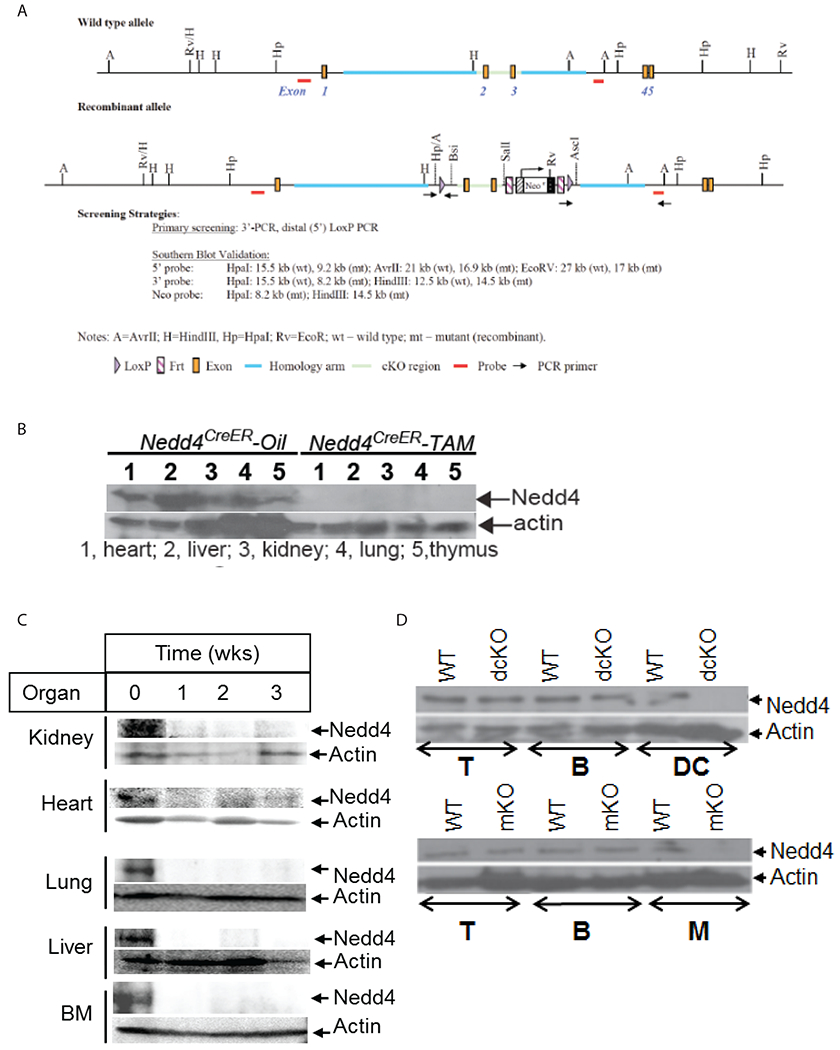
(a) schematic structure of the mouse nedd4 wild type allele and the targeting vector. The top line represent the genomic DNA structure containing exons 1-5 of the mouse wild type nedd4 allele and the lower line represents the recombinant allele. The targeting vector was constructed for conditional deletion of exons 2 and 3 in the plasmid containing both LoxP and Frt cassettes; (b) Western blots of tissue homogenates from various organs of Nedd4f/fROSACreER mice pretreated with corn oil or tamoxifen and harvested 3 days later; (c) Western blots of tissue homogenates from various organs from Nedd4f/fROSACreER mice pretreated with oil (0) or tamoxifen which were harvested 1, 2, or 3 weeks after the last injection; (d) Western blots of lysate from immune cell subsets T (T cells), B (B cells), DC (DC cells) and M (macrophages) from Nedd4f/f (WT), CD11cCre.Nedd4f/f (dcKO), and LysMCre.Nedd4f/f (mKO) mice. Data are representative of 2 independent experiments.
Reagents
Antibodies against SYK (#12358), PKC-δ (#9616) and NF-κB (#3034) were purchased from Cell Signaling Technology Inc. (Danvers, MA). Anti-TAK-1 (#166562) was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Nedd4 (#21698-AP) was purchased from Proteintech Group Inc. (Rosemont IL). The following reagents were purchased from BioLegend (San Diego, CA): PE-Cy7 conjugated anti–mouse CD45.2 antibody (#109830), PE conjugated anti–mouse CD11b (#101208), APC Cy7 conjugated anti-mouse F4/80 (#123118), PerCP conjugated anti–mouse Ly6C (#128012), Fitc conjugated anti–mouse Ly6G (#127606), and ELISA kits for mouse IL-6 (#431304) and TNF-α (#430904). Anti-phospho-SYK (Y525/526; #2711), anti-phospho-PKC-δ (Tyr311, #2055), anti-phospho-TAK-1 (Ser412 #9339), anti-phospho-p44/42 MAPK (Thr202/Tyr204, #9101), anti-phospho-SAPK/JNK (Thr183/Tyr185 #9251) anti-phospho-p38 MAPK (Thr180/Tyr182 #4631) anti-phospho-NF-κB p65 (S536; #3031), and anti-phospho-IκBα (Ser32/36) (5A5; #9246) were purchased from Cell Signaling Technology, Inc. Mouse neutrophil isolation kit (#130097658) was purchased from Miltenyi Biotec (San Diego, CA). Collagenase type IV (02195110) was purchased from MP Biomedicals (Santa Ana, CA). Concanavalin A conjugated Alexa Flour 488 and CellRox Deep Red (C10422) were purchased from ThermoFisher Scientific (Waltham, MA). TLR1-9 ligands were purchased from Novus Biologicals USA (Centennial, CO). Zymosan (#Z4250), Curdlan (#C7821) and α-mannan (#M3640) were purchased from Sigma Aldrich (St Louis, MO). Validation of the antibodies used is provided on the manufacturers’ websites.
Generation of tamoxifen inducible knockout mice
20 mg/mL tamoxifen solution was prepared by dissolving tamoxifen in corn oil and rocking it overnight at room temperature. A dose of 100 μg tamoxifen/g (100 mg/kg) body weight was injected for 4 consecutive days at 100 μl per mouse. A placebo of 100 μl corn oil per mouse was injected into control mice. Mice were transferred into a new cage 72 hrs after the last injection and were used in various experiments. All experiments were concluded within 21 days post tamoxifen injection during which time the tamoxifen induced nedd4 deletion was still observed (Fig. 1C, Supplemental Fig. 1).
Generation of bone marrow-derived macrophages (BMDMs) and isolation of mouse BM neutrophils (BMNs)
Bone marrow (BM) cells were harvested from the femurs and tibias of mice. Bones were harvested at age 8 weeks or older for myeloid specific nedd4 KO (Nedd4ΔM) mice and less than 3 weeks after tamoxifen injection for the tamoxifen inducible KO (Nedd4f/fROSACreER-Tam) mice. Cells were cultured in Iscove’s Modified Dulbecco’s medium (I3390) (Sigma-Aldrich) containing 10% FBS and 30% conditioned medium from L929 cells expressing macrophage colony stimulating factor (M-CSF). Cells were cultured for one week, non-adherent cells were removed, and adherent cells were >90% F4/80+CD11b+, as determined by flow cytometric analysis confirming the generation of BMDMs. To isolate BM neutrophils, total BM cells were recovered from femurs and tibias by flushing with RPMI medium (Sigma-Aldrich) using a 25-gauge needle, erythrocytes were lysed with red blood cell (RBC) lysis buffer (eBioscience; Dan Diego, CA) and BM neutrophils were isolated using a neutrophil isolation kit (Milteny Biotec). Neutrophil purity (>98%) was confirmed by flow cytometry.
In vitro infection of macrophages and neutrophils with C. albicans yeast and hyphal forms
A single colony of C. albicans strain SC5314 (WT) was grown overnight at 30°C in yeast extract, peptone and dextrose (YPD) medium. The cap1 yeast-only mutant, described previously (39), was obtained from Dr. Paula R. Sundstrom (Dartmouth University) was also grown similarly to the WT but at 37°C. The yeast cells were counted and the appropriate number that constituted the required MOI were washed twice with PBS. The washed yeast were resuspended in RPMI 1640 with 10% FBS and grown for 3 h at 37 °C to convert the WT yeast into hyphae. The hyphae (from the WT) and yeast (from the mutant) were washed again in PBS, and used for live stimulations. For analysis of cytokine production, 106 BMDMs or neutrophils were cultured overnight in a 12-well U-bottom plate. Cells were then infected with live C. albicans cap1 mutant or hyphae at a MOI of 1 for the times indicated; cytokine levels in the supernatant were measured by ELISA.
ROS assay, phagocytosis of C. albicans and fungal killing assay
For the ROS production assay, 2 × 106 BMDMs or BMNs from Nedd4f/f and Nedd4ΔM or Nedd4f/fROSAcreER-Tam mice were washed with PBS twice and replated in PBS containing 10uM luminol and 10ug/mL of horseradish peroxidase. The cells were incubated at 37 °C for 30 min and were then infected with C. albicans at an MOI = 5:1. The relative amount of ROS generated by BMDMs or BMNs was detected at regular intervals over 75 min by measuring Chemiluminescence. Relative light units (RLU) were plotted as a function of time to evaluate the chemiluminescence (CL) rate. To measure ROS expression in monocytes, macrophages and neutrophils in spleens and kidneys, Nedd4f/f and Nedd4ΔM or Nedd4f/fROSACreER-oil and Nedd4f/fROSACreER-Tam mice were infected with C. albicans by tail vein injection at a dose of 2.5 × 105 c.f.u. 48 h later, mice were sacrificed, and leukocytes from spleens and kidneys were infected with C. albicans for 30 min, and stained with CellRox and cell surface markers to determine ROS expression in monocytes (kidney: CD45.2+CD11b+Ly6C+Ly6G−; spleen: CD11b+Ly6C+Ly6G−), macrophages (kidney: CD45.2+CD11b+F4/80+Ly6C−CD11c−; spleen: CD11b+F4/80+Ly6C−Ly6G−), and neutrophils (kidney: CD45.2+CD11b+ Ly6C−Ly6G+; spleen: CD11b+Ly6G+Ly6C−). For phagocytosis of C. albicans, yeast were labelled with Concanavalin A conjugated Alexa Fluor 488 (Invitrogen) in 100 mM HEPES buffer (pH 7.5) (diluted to 1:500) and then co-cultured with BMDMs from WT or Nedd4ΔM for 45 min at 37 °C in a 6-well plate. The plate was washed twice with cold PBS to remove non phagocytosed fungi. The BMDMs where scrapped/detached and stained in a 96-well plate and the rate of phagocytosis was determined by flow cytometry (40). For the in vitro fungal killing assay, Nedd4f/f or Nedd4ΔM BMDMs and BMNs were incubated with C. albicans at an MOI of 1:500 for 24 h. After co-culture, a 100-μl suspension of a 1: 102, 103, or 104 dilution was spread on YPD plates. The plates were incubated at 37 °C for 24 h, fungal killing was determined by counting the Candida colonies, with and without the indicated cells (41)
Western blotting
To assess the phosphorylation of SYK, PKC-δ, TAK1, NF-κB ERK, JNK or P38, BMDMs from WT and Nedd4ΔM were infected with C. albicans yeast cells or hyphae (MOI = 1) at the indicated times. The cells were lysed on ice with 0.5% RIPA buffer for immunoblotting with antibodies against phospho- SYK, PKC-δ, TAK1, NF-κB ERK, JNK or P38 at 1:1000 dilution.
Detection of serum and supernatant cytokines by ELISA
For detection of TNF-α and IL-6 in BMDM or BMN culture supernatants, 105 BMDMs/BMNs from Nedd4f/f or Nedd4ΔM mice were infected with live C. albicans cap1 mutant cells or hyphae at MOI=1 for the times indicated, and cytokine production in the supernatant was measured by ELISA. Nedd4f/f and Nedd4ΔM BMDMs were also infected with A. fumigatus conidia (MOI = 1) for the times indicated, and TNF-α and IL-6 levels in the supernatant were measured by ELISA. For detection of serum IL-6 and TNF-α, Nedd4f/f/ROSAcreER-oil or Nedd4f/f/ROSAcreER-Tam mice were infected with C. albicans (2.5 × 105), sera were collected at different time points and subjected for ELISA analysis.
Induction of systemic C. albicans dissemination
For survival analysis, Nedd4f/f and Nedd4ΔM or Nedd4f/fROSACreER-oil and Nedd4f/fROSACreER-Tam mice were infected with C. albicans at 2.5-5 × 105 c.f.u. through the tail vein and monitored daily for survival. After infection, mice were weighed and monitored daily. Mice were euthanized if they lost >20% of their body weight measured at the commencement of the experiment. For purposes of serum cytokine, fungal burden, in vivo ROS, histology or immune cell recruitment, the kidneys and other organs were harvested 2 days after infection. The left kidneys were photographed and homogenized for enumeration of fungal burden. The right kidneys were fixed for histological analysis. The fungal burden in the kidneys, spleens, livers, and lungs was determined by c.f.u. in the organ homogenates. Mice were allocated to experimental groups based upon their genotypes and randomized within their sex- and age-matched groups.
Data analysis and statistical analysis
Differences in concentrations of cytokines and fungal burden were analysed using the Student’s t-test. Survival data were analysed using the Kaplan–Meier log-rank test. Differences were considered significant at P < 0.05. No animals were excluded from the analysis. Mice were allocated to experimental groups based on their genotypes and were randomized within their sex- and age-matched groups.
Results
Nedd4 regulates CLRs but not TLRs signaling
Studies on Nedd4 at the cellular and biochemical levels have been hindered by the embryonic lethality of conventional Nedd4−/− mice (42, 43). To overcome this caveat, we generated mice bearing LoxP-flanked alleles encoding Nedd4 (Nedd4f/f; Fig. 1A). Nedd4f/f mice were crossed with Rosa26-Cre-ERT2 mice [in which a sequence encoding a fusion of Cre recombinase and the estrogen receptor (ER) was recombined into the ubiquitously expressed Rosa26 locus] (44) to generate Nedd4f/fRosa26-Cre-ERT2 mice (herein referred to as ‘Nedd4f/fROSACreER mice’) in which the Cre recombinase becomes active upon stimulation with Tamoxifen leading to systemic deletion of Nedd4 (herein referred to as Nedd4f/fROSACreER-Tam). Nedd4f/fROSACreER mice injected with corn oil (herein referred to as Nedd4f/fROSACreER-oil) served as control. Figures 1B and 1C shows the efficacy of Nedd4 gene deletion whether proximal to cessation of Tamoxifen treatment (Fig. 1B) or 1, 2 and 3 weeks after treatment (Fig. 1C, Supplemental Fig. 1). We also crossed mice bearing Nedd4f/f with mice bearing Cre recombinase on the LysM gene (LysMCre) to generate Nedd4f/fLysMCre (Nedd4ΔM) mice in which Nedd4 is deleted in myeloid cells (Fig 1D). To determine whether Nedd4 regulates innate immune responses, we stimulated BMDMs from Nedd4f/f and Nedd4ΔM mice with ligands for TLRs 1-9 (45), Zymosan (ligand for dectin-1 and TLR 2) (46), Curdlan (ligand for dectin-1) (47), or α-mannan (ligand for dectin-2 and −3) (23). We found that whereas production of TNFα and IL-6 was comparable between control Nedd4f/f and Nedd4ΔM BMDMs upon TLR 1-9 ligands stimulation, TNF-α and IL-6 production was substantially lower in Nedd4ΔM BMDMs than with control Nedd4f/f BMDMs upon stimulation with zymosan, curdlan, and α-mannan (Fig. 2A). These data suggest that Nedd4 positively regulates signalling through dectin-1 and dectin-2/3, but not TLRs.
Figure 2: Nedd4 regulates CLRs but not TLRs signaling:
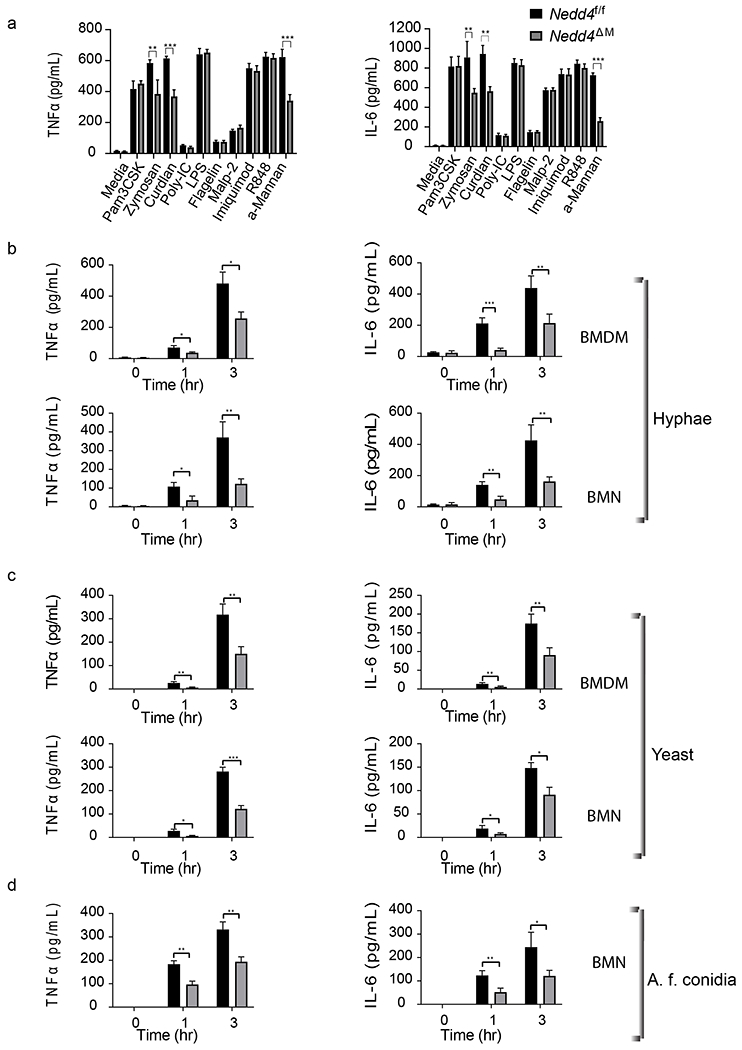
(a) Control Nedd4f/f and Nedd4ΔM BMDMs were stimulated with TLR ligands 1 to 9, α-mannan, zymosan, and curdlan for 24 h; TNFα (left) and IL-6 (right) levels in the supernatant were detected using ELISA. Data are pooled from 3 independent experiments with three replicates of each sample per experiment. Error bars are mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired two-tailed Student’s t test. (b-c) BMDMs (top) or BMNs (bottom) from control Nedd4f/f and Nedd4ΔM were infected with C. albicans hyphae (b) or yeast (c) at MOI of 1:1 for 0, 1 or 3 hrs; (d) control Nedd4f/f and Nedd4ΔM BMDM were infected with Conidia from A. fumigatus. TNF-α and IL-6 content in supernatant was measured using ELISA. For Fig 2B – 2D, data are pooled from 3 independent experiments with three replicates of each sample per experiment. Error bars are mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001; unpaired two-tailed Student’s t test.
We and others have shown that C. albicans yeast generate signals through dectin-1 by recognition of β-glucan on the yeast cell, whereas the hyphal form signals through dectin-2 and −3 by recognition of α-mannans on the hyphal cell wall (21–23). To further confirm this observation and to determine whether Nedd4 is important in antifungal immunity, we infected BMDMs and BMNs of control Nedd4f/f and Nedd4ΔM mice with a C. albicans hyphae or yeast-only mutant (cap1; hereafter referred to as yeast), in which the adenylate-cyclase-associated protein-1 (Cap1) gene is disrupted, causing the failure of yeast–hypha transition due to the lack of cAMP (39). BMDMs and BMNs lacking Nedd4 displayed a significant reduction in the production of TNFα and IL-6 compared with BMDMs or BMNs sufficient for Nedd4 upon infection with C.albicans hyphae (Fig. 2B) or yeast (Fig. 2C). To confirm whether Nedd4 plays a similar role in response to other fungal species, we infected Nedd4f/f and Nedd4ΔM BMDMs with Aspergillus fumigatus Conidia and then determined TNFα and IL-6 levels in the supernatant. We observed that BMDMs lacking Nedd4 produced significantly lesser amounts of TNFα and IL-6 compared to control Nedd4f/f BMDMs (Fig. 2D).
BMDMs and BMNs lacking Nedd4 display reduced ROS activity and defective killing of C. albicans
Oxidative stress is one of the initial mechanisms that phagocytes utilize in killing pathogens and release of ROS is an important function of oxidative stress (48–50). C. albicans infection has been shown to trigger ROS production by macrophages and neutrophils (24, 51, 52). To determine the role of Nedd4 in regulation of ROS production, we measured ROS production by co-culturing hyphae with BMDMs/BMNs from control Nedd4f/f and Nedd4ΔM mice. We found that Nedd4ΔM BMDMs and BMNs produced less ROS than Nedd4f/f controls (Fig. 3A). Consistent with this observation, BMDMs and BMNs lacking Nedd4 displayed impaired fungal killing abilities (Fig. 3B). However, phagocytosis of C. albicans, an important process use to internalize pathogens by phagocytic cells (53), was not decreased by BMDMs and BMNs lacking Nedd4, as compared to that by BMDMs and BMNs sufficient for Nedd4 (Nedd4f/f; Fig. 3C).
Figure 3: Nedd4 deficiency impairs ROS production and fungal killing in BMDMs and BMNs:
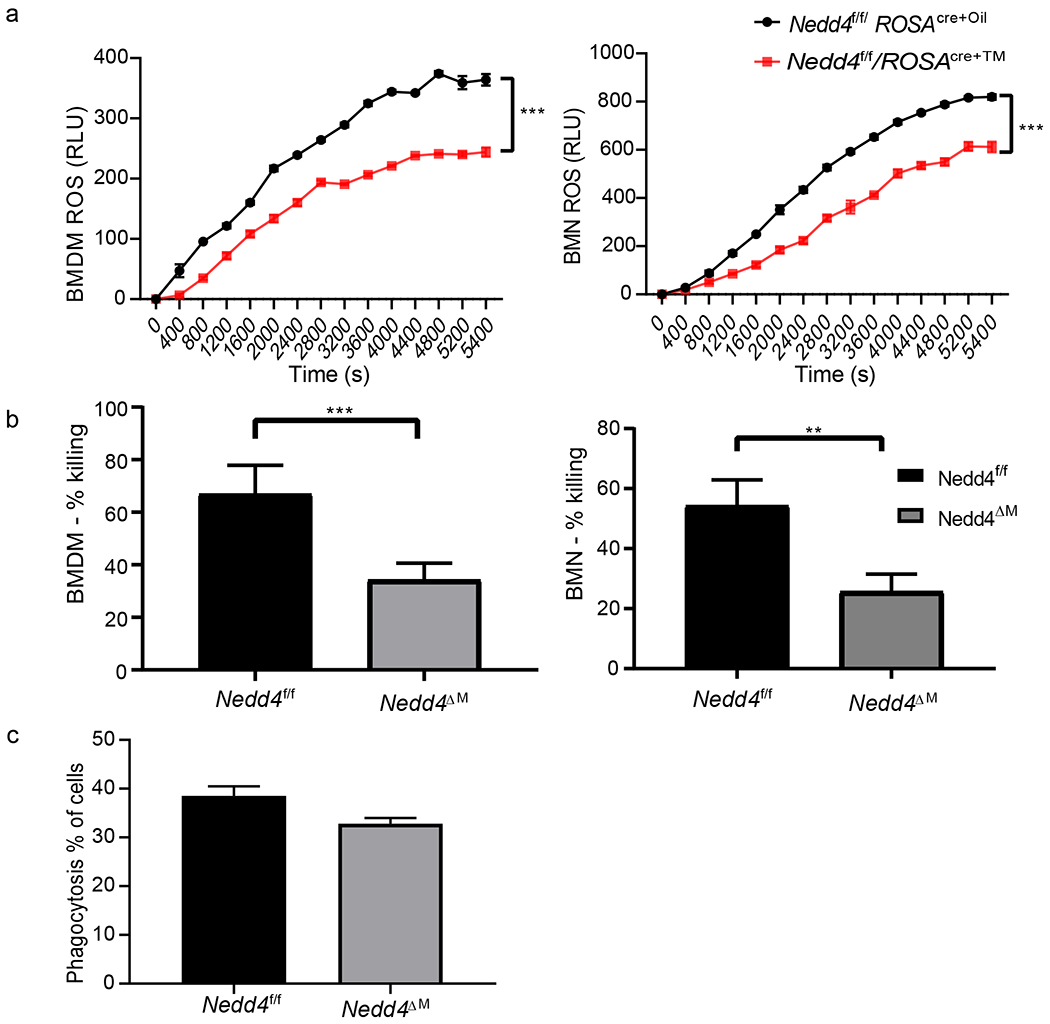
(a) ROS production by control Nedd4f/fROSACreER-Oil or Nedd4f/fROSACreER-Tam BMDM (left) and BMNs (right) was determined by co-culture with C. albicans yeast at MOI = 5:1 and monitored in real time over the indicated time period using chemiluminescence assay. Values are expressed as relative light units (RLU) and represent mean values of 4 replicates in each sample. Data were analyzed using student’s t-test; (b) control Nedd4f/f or Nedd4ΔM BMDM were co-cultured with C. albicans yeast (cap1 mutant) at an MOI of 40 :1 for 24 hrs (left), control Nedd4f/f or Nedd4ΔM BMN were co-cultured with C. albicans yeast (cap1 mutant) at an MOI of 5 :1 for 2 hrs (right). The fungal/BMDM or fungal/BMN homogenates were plated on YPD media overnight and fungal killing determined. Data are pooled from 3 independent experiments, with each group having 4 samples per experiment. Error bars are mean ± s.d. **P < 0.01, ***P < 0.001 by Student’s t test; (c) Control Nedd4f/f or Nedd4ΔM BMDMs were co-cultured with ConA Alexa Fluor 488–labeled C. albicans. After 45 min of co-culture, control Nedd4f/f or Nedd4ΔM BMDMs were analyzed for C. albicans uptake by flow cytometry. Data are pooled from 2 independent experiments, with each group having 4 samples per experiment. Error bars are mean ± s.d.
Nedd4 is required for the protection against systemic C. albicans infection
To assess whether Nedd4 positively regulates anti-fungal immunity in vivo, we infected Nedd4f/f/ROSACreER-Oil and Nedd4f/f/ROSACreER-Tam, or Nedd4f/f and Nedd4ΔM mice, with a lethal dose (5 x 105 CFU) of C. albicans through the tail vein to monitor survival and with a sub-lethal dose (2.5 x 105 CFU) to measure serum cytokines and fungal burden. We found that mice lacking Nedd4 were highly susceptible to lethal systemic infection with C. albicans. Whereas all the mice lacking Nedd4 (systemically or myeloid-cell specifically) died by day 8, more than 50% of WT mice survived beyond day 10 (Fig. 4A). This correlated with dampened levels of TNF-α and IL-6 in the sera of Nedd4 deficient mice (Fig. 4B), as well as higher fungal burden in the kidney, lung, spleen and liver (Fig. 4C), compared with those of WT mice. Kidney histology also revealed massive inflammatory cell damage and more hyphae dwelling in the cells of Nedd4f/f/ROSACreER-Tam mice compare with WT mice upon PAS and H&E staining (Fig. 4D).
Figure 4: Nedd4 deficient mice have reduced survival and higher fungal burden during C. albicans infection:
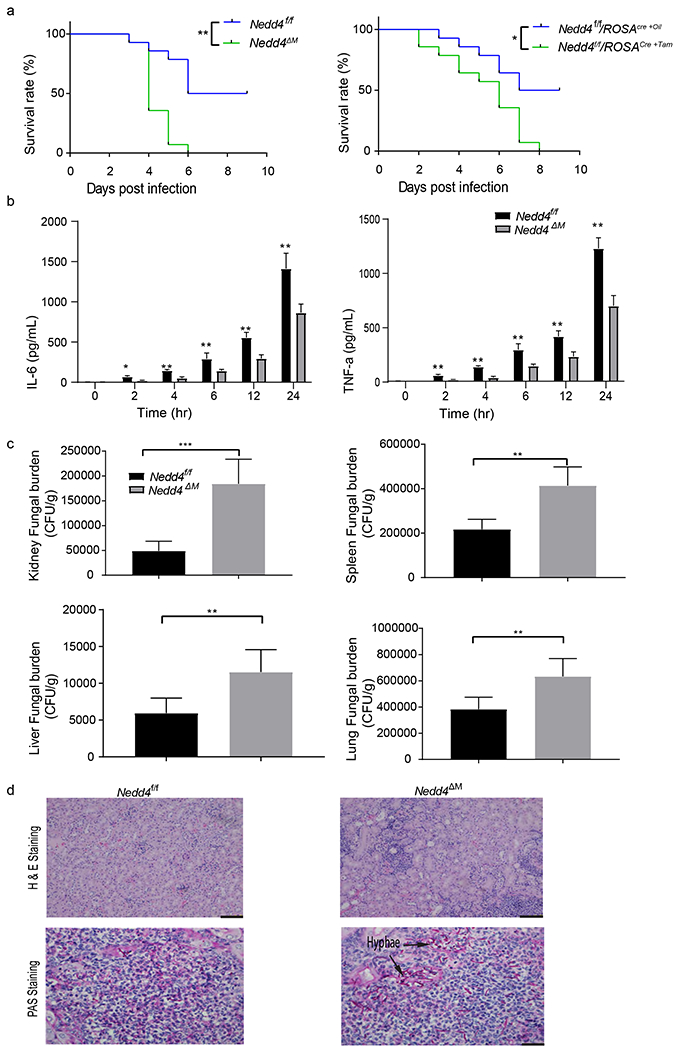
(a) Mice lacking Nedd4 in myeloid cells (left) or systemically (right) and control Nedd4f/f were injected with 5 x 105 CFU of C. albicans through the tail vein and monitored for survival; Kaplan-Meier survival curve was plotted to determine survival rate. Data are are pooled from three independent experiments with 5 mice per group per experiment. P< 0.05 by Log-rank test. (b-c) Control Nedd4f/f and Nedd4ΔM mice were injected with 2.5 x 105 CFU of C. albicans through the tail vein for 48 hrs, (b) serum TNFα and IL-6 production at the stated times were determined by ELISA. Data are are pooled from three independent experiments with 5 mice per group per experiment. Error bars are mean ± s.d. **P < 0.01 ***P < 0.001; by unpaired two-tailed Student’s t-test. (c) Another set of mice were sacrificed 48 hrs post infection and the kidney, spleen, liver and lung homogenates were plated on YPD media for 24 hrs then colonies were counted and fungal burden determined. Data are are pooled from three independent experiments with 5 mice per group per experiment. Error bars are mean ± s.d. **P < 0.01 ***P < 0.001; by unpaired two-tailed Student’s t-test (d) Kidney histopathology analysis by H&E staining (top) and PAS staining (bottom) to visualize fungal burden (hyphae); (n = 5 mice per group). Images are representative of two independent experiments (biological replicates). Scale bars, 100 μm.
During Candidemia, C. albicans enter the blood stream and subsequently localize in the viscera (54) with the kidney been the most preferred site. Protective effector activity requires the recruitment of phagocytes and other immune cells to sites of infection. We further assessed recruitment of immune cells to the kidneys, spleens, livers and lungs. We observed that more immune cells including macrophages and neutrophils were recruited to the kidneys of control Nedd4f/f than Nedd4ΔM mice (Fig. 5A). To confirm the effector activities of these cells ex vivo, we monitored ROS expression in monocytes, macrophages and neutrophils from control Nedd4f/f and Nedd4ΔM spleens and kidneys by Cell-Rox dye using flow cytometry. We observed that, similar to in vitro C. albicans infection of BMDMs and BMNs (Fig. 3B), macrophages and neutrophils from kidneys and spleens of mice lacking Nedd4 produced less ROS than controls (Nedd4f/f; Fig. 5B). While kidney monocytes lacking Nedd4 were defective in ROS compared with monocytes sufficient for Nedd4, there was no difference in ROS production of monocytes from control Nedd4f/f and Nedd4ΔM spleen (Fig. 5B).
Figure 5: Nedd4 enhances effector functions of innate immune cells:
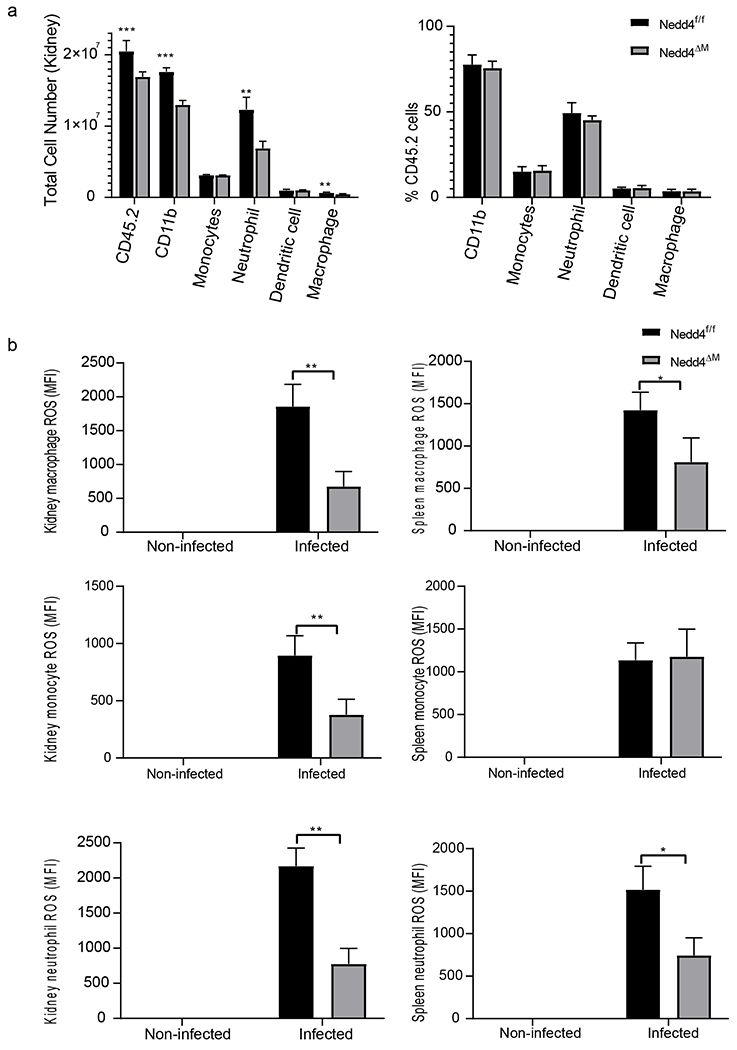
Control Nedd4f/f and Nedd4 deficient mice were injected with 2.5 x 105 CFU of C. albicans through the tail vein for 48 hrs. (a) Immune cell recruitment to the kidney was measure by flow cytometry; (b) ROS production by myeloid cells in the kidney and spleen measured using flow cytometry. For Fig 5A and 5B, data are pooled from three independent experiments. For each experiment there were 5 mice per group, each with three replicate samples. Error bars are mean ± s.d. *P < 0.05, unpaired two-tailed Student’s t test.
Nedd4 is required for NF-κB activation downstream of PKC-δ but upstream of or at TAK-1
During fungal infection, CLRs binding to their ligands leads to downstream signalling, which induces the activation of Syk which subsequently activates PKC-δ. Signalling through PKC-δ facilitates the formation of the CARD9-BCL10-MALT1 complex which subsequently activates NF-κB. Having shown that Nedd4 positively regulates the dectin-1- and dectin-2/3-mediated immune responses (Fig. 2A), we wanted to determine the potential molecular mechanism(s) of Nedd4 in anti-fungal innate immune responses. To this end, we differentiated BMDM from Nedd4f/f/ROSACreER-Oil and Nedd4f/f/ROSACreER-Tam mice, then infected them with C. albicans yeasts or hyphae for 5, 15, 30 or 60 minutes. We then determined the activation of Syk, PKC-δ, TAK-1, NF-κB, ERK, JNK and p38 MAPK. Although Syk and PKC-δ phosphorylation was comparable between the controls and Nedd4f/f/ROSACreER-Tam, phosphorylation of TAK-1 and NF-κB was markedly reduced in Nedd4 deficient BMDMs upon infection with C. albicans yeasts and hyphae (Fig. 6). These results suggest Nedd4 regulates the CLR signalling downstream of PKC-δ but at or upstream of TAK-1.
Figure 6: Nedd4 is required for TAK1 and NF-κB but not Syk and PKC-δ activation:
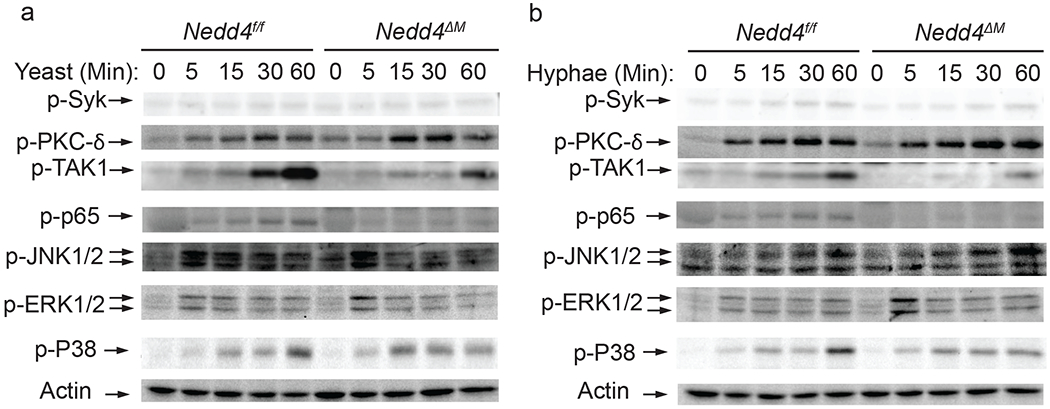
(a-b) Immunoblot analysis of the indicated proteins from the cell lysate of control Nedd4f/f and Nedd4ΔM BMDMs after infection with hyphae (a) and yeast (b) from C. albicans. Images are representative of three independent experiments (biological replicates).
Discussion
Recognition of the surface β-glucans and α-mannans on C. albicans yeasts and hyphae, respectively, by dectin-1 and dectin-2/3 expressed on neutrophils and macrophages results in release of inflammatory cytokines, which is critical for anti-fungal immunity (23–25, 55, 56). However, how the CLR pathway is regulated during fungal infection is still not fully understood. Here we showed Nedd4 regulates the CLR signalling pathway during C. albicans infections. Whilst we did not observe any difference in the production of TNF-α and IL-6 by control and Nedd4ΔM BMDMs upon stimulation with TLR 1-9 ligands (Fig. 2A), BMDMs lacking Nedd4 produced significantly less amounts of TNF-α and IL-6 when stimulated with zymosan, which triggers dectin-1 and TLR-2 (46), curdlan, which triggers dectin-1 only (47), and α-mannan, which triggers both dectin-2 and dectin-3 (23) compared to their WT controls. These data collectively indicate that Nedd4 positively regulates signalling derived from CLRs, but not TLRs during fungal infection. In support of this notion, Nedd4ΔM BMDMs secreted less TNF-α and IL-6 than control BMDMs upon infection with yeast and hyphae of C. albicans. Furthermore, mice lacking Nedd4 systemically or in myeloid cells succumbed to systemic C. albicans infection, with lower levels of serum TNF-α and IL-6 and higher fungal burdens in kidneys, spleens, livers, and lungs. Therefore, our data collectively demonstrate that Nedd4 expression in myeloid cells is essential for anti-fungal innate immune responses mediated by the dectin family of CLRs.
ROS have been shown to play a significant role in the fungal killing (49, 57) and the ability of phagocytes to produce ROS enhances their fungal killing effector functions (53, 58–60). Our results established that BMDMs and BMNs lacking Nedd4 exhibited reduced capability to produce ROS during fungal infection (Fig. 3). Although the precise mechanism for this observation is currently not defined, defective signalling events mediated by Nedd4 upon C. albicans infection may be responsible for impaired ROS activity. It has been shown that ROS production is impaired in Card9−/− macrophages (61), and that neutrophils from patients with a CARD9 mutation display defective fungal killing (62). Therefore, it is highly possible that Nedd4 may regulate CARD9-mediated signalling pathways.
E3 ubiquitin ligases have been commonly shown to negatively regulate signalling pathways and when specific E3 ubiquitin ligases are eliminated or inactivated, these pathways often exhibit enhanced signalling. In regards to innate responses after Candida challenge, our group and others have previously shown the E3 ubiquitin ligase Cbl-b to dampen signals generated through the dectin-1 and dectin-2 CLRs. When Cbl-b is eliminated or inactivated, innate immune responses are greatly enhanced and mice are now protected from lethal C. albicans infection (24, 63, 64). This is in contrast to the results presented herein, where elimination of the E3 ubiquitin ligase Nedd4 has the opposite effect with innate responses mediated through CLRs diminished, and mice lacking Nedd4 either globally or within the myeloid compartment now much more sensitive to C. albicans challenge. This strongly implies that Nedd4 supports or enhances signalling through CLRs as opposed to the negative regulation enforced by Cbl-b. This is consistent with previous studies demonstrating Nedd4 to enhance T cell activation and proliferation (35, 36). The reason for these opposing effects is not fully understood, but may reflect K48 versus K63 linked ubiquitination (65). Although K48 polyubiquitinated substrates are targets for degradation, K63-linked ubiquitination has been associated with proteasome-independent roles within the cell, a number of which support key cellular functions (65). Of interest, K63-linked ubiquitination has been shown to support downstream NF-κB activation in several systems (66) and suggests Nedd4 may play a similar role in dectin receptor signalling pathways.
As to signal transduction through the dectin receptors, binding by β-glucans and α-mannans is known to activate Syk. Activated Syk then phosphorylates PKC-δ, which phosphorylates CARD9. This facilitates complex formation with BCL10 and MALT1, thus eliciting NF-κB activation (27). Intriguingly, although BMDMs lacking Nedd4 display defective activation of NF-κB and TAK-1, the activation of Syk and PKC-δ is not altered upon infection with C. albicans yeasts and hyphae (Fig. 6). These data strongly indicate that Nedd4 lies downstream of PKC-δ but upstream of or at TAK-1. The target(s) of Nedd4 in dectin-mediated signalling pathways is currently under investigation.
In summary, we found that Nedd4 expression in myeloid cells is required for inflammatory responses and fungal killing. Nedd4 is essential for the activation of NF-κB in myeloid cells mediated by the dectin family of CLRs downstream of PKC-δ but upstream of or at TAK-1.
Supplementary Material
Key points.
Nedd4 regulates CLR but not TLR signalling during fungal infection
Macrophages and neutrophils lacking Nedd4 have impaired effector functions
CLR-mediated activation of NF-κB signalling pathway is enhanced by Nedd4
Acknowledgments
Acknowledgments
We thank Dr. Amy Lovett-Racke for critical review of the manuscript. We are also grateful to Dr Dao-Fu Dai for his help interpreting the histology data in Fig. 4D.
Support
This work was supported by National Institute of Allergy and Infectious Diseases/National Institutes of Health Awards R01 AI123253, R01 AI 121196 and R01 AI 090901 to J.Z. P.N-G was supported by an Administrative Supplement awarded to R01AI123253. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.Z. also receives support as a University of Iowa Health Care Distinguished Scholar.
Abbreviations used in this article
- BCL10
B-cell lymphoma/leukemia 10
- BMDM
Bone marrow-derived macrophage
- BMN
Bone marrow neutrophil
- CARD9
Caspase recruitment domain-containing protein 9
- Cbl-b
Casitas B lymphoma-b
- CLR
C-type lectin receptor
- HECT
Homologous to E6-AP C-terminus
- Nedd4
Neural precursor cell expressed developmentally down-regulated 4
- PAMP
Pathogen-associated molecular patterns
- PKC-δ
Protein kinase C-δ
- PRR
Pattern recognition receptor
- RLU
Relative light unit
- ROS
Reactive oxygen species
- SYK
Spleen tyrosine kinase
- TAK-1
Transforming growth factor-beta-activating kinase-1
- YPD
Yeast Extract-Peptone-Dextrose
Footnotes
Disclosures
T.J.W. is a cofounder of ImmunoNanoMed, a start-up with business interests in the development of nanoparticle-based vaccines against infectious diseases.
References
- 1.Havlickova B, Czaika VA, and Friedrich M. 2008. Epidemiological trends in skin mycoses worldwide. Mycoses 51 Suppl 4: 2–15. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, and White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4: 165rv113. [DOI] [PubMed] [Google Scholar]
- 3.Lionakis MS, and Levitz SM. 2018. Host Control of Fungal Infections: Lessons from Basic Studies and Human Cohorts. Annu Rev Immunol 36: 157–191. [DOI] [PubMed] [Google Scholar]
- 4.Bitar D, Lortholary O, Le Strat Y, Nicolau J, Coignard B, Tattevin P, Che D, and Dromer F. 2014. Population-based analysis of invasive fungal infections, France, 2001-2010. Emerg Infect Dis 20: 1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antinori S, Milazzo L, Sollima S, Galli M, and Corbellino M. 2016. Candidemia and invasive candidiasis in adults: A narrative review. Eur J Intern Med 34: 21–28. [DOI] [PubMed] [Google Scholar]
- 6.McCarty TP, and Pappas PG. 2016. Invasive Candidiasis. Infect Dis Clin North Am 30: 103–124. [DOI] [PubMed] [Google Scholar]
- 7.Spivak ES, and Hanson KE. 2018. Candida auris: an Emerging Fungal Pathogen. J Clin Microbiol 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saris K, Meis JF, and Voss A. 2018. Candida auris. Curr Opin Infect Dis 31: 334–340. [DOI] [PubMed] [Google Scholar]
- 9.Sears D, and Schwartz BS. 2017. Candida auris: An emerging multidrug-resistant pathogen. Int J Infect Dis 63: 95–98. [DOI] [PubMed] [Google Scholar]
- 10.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, and Rex JH. 2010. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9: 719–727. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, Rangel-Frausto MS, Rinaldi MG, Saiman L, Wiblin RT, Wenzel RP, and National G Epidemiology of Mycoses Survey Study. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clin Infect Dis 33: 177–186. [DOI] [PubMed] [Google Scholar]
- 12.Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F, and French Mycosis Study G. 2014. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010). Intensive Care Med 40: 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning DW, and Hope WW. 2010. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol 18: 195–204. [DOI] [PubMed] [Google Scholar]
- 14.Jackson A, and Hosseinipour MC. 2010. Management of cryptococcal meningitis in sub-saharan Africa. Curr HIV/AIDS Rep 7: 134–142. [DOI] [PubMed] [Google Scholar]
- 15.Dewsnup DH, Galgiani JN, Graybill JR, Diaz M, Rendon A, Cloud GA, and Stevens DA. 1996. Is it ever safe to stop azole therapy for Coccidioides immitis meningitis? Ann Intern Med 124: 305–310. [DOI] [PubMed] [Google Scholar]
- 16.Bidaud AL, Chowdhary A, and Dannaoui E. 2018. Candida auris: An emerging drug resistant yeast - A mini-review. J Mycol Med 28: 568–573. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TA, Pang KC, and Masters SL. 2017. Intercellular communication for innate immunity. Mol Immunol 86: 16–22. [DOI] [PubMed] [Google Scholar]
- 18.Faure-Dupuy S, Vegna S, Aillot L, Dimier L, Esser K, Broxtermann M, Bonnin M, Bendriss-Vermare N, Rivoire M, Passot G, Lesurtel M, Mabrut JY, Ducerf C, Salvetti A, Protzer U, Zoulim F, Durantel D, and Lucifora J. 2018. Characterization of Pattern Recognition Receptor Expression and Functionality in Liver Primary Cells and Derived Cell Lines. J Innate Immun 10: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haider M, Dambuza IM, Asamaphan P, Stappers M, Reid D, Yamasaki S, Brown GD, Gow NAR, and Erwig LP. 2019. The pattern recognition receptors dectin-2, mincle, and FcRgamma impact the dynamics of phagocytosis of Candida, Saccharomyces, Malassezia, and Mucor species. PLoS One 14: e0220867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schorey JS, and Lawrence C. 2008. The pattern recognition receptor Dectin-1: from fungi to mycobacteria. Curr Drug Targets 9: 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, and Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, Komatsu R, Miura N, Adachi Y, Ohno N, Shibuya K, Yamamoto N, Kawakami K, Yamasaki S, Saito T, Akira S, and Iwakura Y. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681–691. [DOI] [PubMed] [Google Scholar]
- 23.Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, and Lin X. 2013. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity 39: 324–334. [DOI] [PubMed] [Google Scholar]
- 24.Xiao Y, Tang J, Guo H, Zhao Y, Tang R, Ouyang S, Zeng Q, Rappleye CA, Rajaram MV, Schlesinger LS, Tao L, Brown GD, Langdon WY, Li BT, and Zhang J. 2016. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat Med 22: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardison SE, and Brown GD. 2012. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol 13: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drummond RA, and Brown GD. 2013. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog 9: e1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J, Lin G, Langdon WY, Tao L, and Zhang J. 2018. Regulation of C-Type Lectin Receptor-Mediated Antifungal Immunity. Front Immunol 9: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Li B, Rezaeian AH, Xu X, Chou PC, Jin G, Han F, Pan BS, Wang CY, Long J, Zhang A, Huang CY, Tsai FJ, Tsai CH, Logothetis C, and Lin HK. 2017. H3 ubiquitination by NEDD4 regulates H3 acetylation and tumorigenesis. Nat Commun 8: 14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S, Park S, Lee H, Han S, Song JM, Han D, and Suh YH. 2019. Nedd4 E3 ligase and beta-arrestins regulate ubiquitination, trafficking, and stability of the mGlu7 receptor. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, and Jiang X. 2007. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell 128: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang X, Chen J, Cao W, Yang L, Chen Q, He J, Yi Q, Huang H, Zhang E, and Cai Z. 2019. The many substrates and functions of NEDD4-1. Cell Death Dis 10: 904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An H, Krist DT, and Statsyuk AV. 2014. Crosstalk between kinases and Nedd4 family ubiquitin ligases. Mol Biosyst 10: 1643–1657. [DOI] [PubMed] [Google Scholar]
- 33.Ingham RJ, Gish G, and Pawson T. 2004. The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23: 1972–1984. [DOI] [PubMed] [Google Scholar]
- 34.Yang B, and Kumar S. 2010. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ 17: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Qiao G, Ying H, Li Z, Zhao Y, Liang Y, Yang L, Lipkowitz S, Penninger JM, Langdon WY, and Zhang J. 2012. E3 ubiquitin ligase Cbl-b regulates Pten via Nedd4 in T cells independently of its ubiquitin ligase activity. Cell Rep 1: 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao G, Li Z, Molinero L, Alegre ML, Ying H, Sun Z, Penninger JM, and Zhang J. 2008. T-cell receptor-induced NF-kappaB activation is negatively regulated by E3 ubiquitin ligase Cbl-b. Mol Cell Biol 28: 2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pei G, Buijze H, Liu H, Moura-Alves P, Goosmann C, Brinkmann V, Kawabe H, Dorhoi A, and Kaufmann SHE. 2017. The E3 ubiquitin ligase NEDD4 enhances killing of membrane-perturbing intracellular bacteria by promoting autophagy. Autophagy 13: 2041–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, and Akira S. 1999. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10: 39–49. [DOI] [PubMed] [Google Scholar]
- 39.Bahn YS, and Sundstrom P. 2001. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J Bacteriol 183: 3211–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swamydas M, Luo Y, Dorf ME, and Lionakis MS. 2015. Isolation of Mouse Neutrophils. Curr Protoc Immunol 110: 3 20 21–23 20 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirnsberger G, Zwolanek F, Stadlmann J, Tortola L, Liu SW, Perlot T, Jarvinen P, Durnberger G, Kozieradzki I, Sarao R, De Martino A, Boztug K, Mechtler K, Kuchler K, Klein C, Elling U, and Penninger JM. 2014. Jagunal homolog 1 is a critical regulator of neutrophil function in fungal host defense. Nat Genet 46: 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawabe H, Neeb A, Dimova K, Young SM Jr., Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang DE, Umikawa M, Kariya K, Goebbels S, Nave KA, Rosenmund C, Jahn O, Rhee J, and Brose N. 2010. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron 65: 358–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Oppenheim RW, Sugiura Y, and Lin W. 2009. Abnormal development of the neuromuscular junction in Nedd4-deficient mice. Dev Biol 330: 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, and Jacks T. 2007. Restoration of p53 function leads to tumour regression in vivo. Nature 445: 661–665. [DOI] [PubMed] [Google Scholar]
- 45.Takeda K, and Akira S. 2005. Toll-like receptors in innate immunity. Int Immunol 17: 1–14. [DOI] [PubMed] [Google Scholar]
- 46.Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S, Unutmaz D, and Pulendran B. 2006. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 116: 916–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu CC, Zhao GQ, Lin J, Hu LT, Xu Q, Peng XD, Wang X, and Qiu S. 2015. Dectin-1 agonist curdlan modulates innate immunity to Aspergillus fumigatus in human corneal epithelial cells. Int J Ophthalmol 8: 690–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown GD 2011. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol 29: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arana DM, Alonso-Monge R, Du C, Calderone R, and Pla J. 2007. Differential susceptibility of mitogen-activated protein kinase pathway mutants to oxidative-mediated killing by phagocytes in the fungal pathogen Candida albicans. Cell Microbiol 9: 1647–1659. [DOI] [PubMed] [Google Scholar]
- 50.Komalapriya C, Kaloriti D, Tillmann AT, Yin Z, Herrero-de-Dios C, Jacobsen MD, Belmonte RC, Cameron G, Haynes K, Grebogi C, de Moura AP, Gow NA, Thiel M, Quinn J, Brown AJ, and Romano MC. 2015. Integrative Model of Oxidative Stress Adaptation in the Fungal Pathogen Candida albicans. PLoS One 10: e0137750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown AJ, Haynes K, and Quinn J. 2009. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr Opin Microbiol 12: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogdan C, Rollinghoff M, and Diefenbach A. 2000. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol 12: 64–76. [DOI] [PubMed] [Google Scholar]
- 53.Nicola AM, Casadevall A, and Goldman DL. 2008. Fungal killing by mammalian phagocytic cells. Curr Opin Microbiol 11: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kullberg BJ, and Arendrup MC. 2015. Invasive Candidiasis. N Engl J Med 373: 1445–1456. [DOI] [PubMed] [Google Scholar]
- 55.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, and Brown GD. 2007. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roesner LM, Ernst M, Chen W, Begemann G, Kienlin P, Raulf MK, Lepenies B, and Werfel T. 2019. Human thioredoxin, a damage-associated molecular pattern and Malassezia-crossreactive autoallergen, modulates immune responses via the C-type lectin receptors Dectin-1 and Dectin-2. Sci Rep 9: 11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dupre-Crochet S, Erard M, and Nubetae O. 2013. ROS production in phagocytes: why, when, and where? J Leukoc Biol 94: 657–670. [DOI] [PubMed] [Google Scholar]
- 58.Ding Y, Li Z, Li Y, Lu C, Wang H, Shen Y, and Du L. 2016. HSAF-induced antifungal effects in Candida albicans through ROS-mediated apoptosis. RSC Adv 6: 30895–30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, Suzuki K, Maeda N, and Koyama H. 2006. Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol 55: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y, Chang Q, Cheng L, Xiong S, Jia X, Lin X, and Zhao X. 2018. C-Type Lectin Receptor CD23 Is Required for Host Defense against Candida albicans and Aspergillus fumigatus Infection. J Immunol 201: 2427–2440. [DOI] [PubMed] [Google Scholar]
- 61.Wu W, Hsu YM, Bi L, Songyang Z, and Lin X. 2009. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat Immunol 10: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 62.Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, van Hamme JL, van Leeuwen EM, Roos D, Scalais E, de Beaufort C, Janssen H, van den Berg TK, and Kuijpers TW. 2013. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121: 2385–2392. [DOI] [PubMed] [Google Scholar]
- 63.Wirnsberger G, Zwolanek F, Asaoka T, Kozieradzki I, Tortola L, Wimmer RA, Kavirayani A, Fresser F, Baier G, Langdon WY, Ikeda F, Kuchler K, and Penninger JM. 2016. Inhibition of CBLB protects from lethal Candida albicans sepsis. Nat Med 22: 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu LL, Luo TM, Xu X, Guo YH, Zhao XQ, Wang TT, Tang B, Jiang YY, Xu JF, Lin X, and Jia XM. 2016. E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor-mediated antifungal innate immunity. J Exp Med 213: 1555–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim KL, and Lim GG. 2011. K63-linked ubiquitination and neurodegeneration. Neurobiol Dis 43: 9–16. [DOI] [PubMed] [Google Scholar]
- 66.Chen ZJ, and Fuchs SY. 2004. Ubiquitin-dependent activation of NF-kappaB: K63-linked ubiquitin chains: a link to cancer? Cancer Biol Ther 3: 286–288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


