Abstract
A real time polymerase chain reaction (real-time PCR) assay was developed to detect and quantify the chicken infectious anemia virus (CIAV). The two sets of primers specific to VP1 region of CIAV were designed and their sensitivity and efficacy were studied. Both the primers designed in this study were highly sensitive and were able to detect upto 0.01 fg/μl or 82 × 102 copy number of plasmid DNA. The efficiency of the real time PCR was 100.9%. The results have also shown that the present qPCR assay is 100 times more sensitive than regular qualitative PCR. Both primer sets were validated using 28 field poultry samples and showed good results. The optimized real-time quantitative PCR will be useful in quick detection of field outbreaks, sub-clinical infection in poultry flocks, virus pathogenesis studies and for detecting vaccine contamination.
Keywords: Chicken infectious anemia, Real-time PCR, Diagnosis, Poultry
Chicken Infectious Anemia (CIA) is an economically important disease of poultry industry worldwide causing severe anemia, hemorrhages, lymphoid atrophy, stunted growth and increased mortality and severe immunosuppression [3, 7, 11, 15, 16]. The clinical disease is mainly observed in young chickens of 3–4 weeks, which is usually transmitted vertically or horizontally. Spread by vaccine contamination is also observed as one of the sources of infections in recent times [9]. Major economic loss by CIA is due to secondary bacterial infections, poor immune response to other vaccines due to immune-suppression and mortality. The causative agent, Chicken Anaemia Virus (CAV) is one of the smallest, non-enveloped DNA viruses belonging the genus Gyrovirus under Circoviridae family. The genome consists of 2.3 kbp circular single-stranded negative sense DNA, coding for three viral proteins (VP1, VP2 and VP3) from single major transcript of 2 bp size coded by three overlapping reading frames. VP1 gene codes for major capsid protein that induce neutralizing antibodies [8].
Early detection of CAV in poultry flocks is necessitated to avoid the spread, subsequent vaccine failures, secondary bacterial infections of young flocks and mortality. Virus isolation in MDCC-MSB1 cell line is gold-standard technique for identification; however, it is tedious and time-consuming. Molecular detecting techniques have been developed including conventional PCR, Dot-blot hybridization and nested PCR [5]. Very few studies attempted for developing quantitative real time PCR for CAV [4, 10]. Hence, development of quick and sensitive assay for quantitation of CAV will help in checking the extraneous contamination of CAV in chicken embryo passaged vaccines, sub-clinical CAV infection in poultry flocks and also in virus pathogenesis studies. In the present study, we describe the development and application of VP1 gene specific real time PCR assay for quick and sensitive detection and quantification for CIAV.
The gene sequences of CAU65414 strain was used for designing the primers. The primers were designed with NCBI Primer blast program (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Conserved region of VP1 gene was targeted and primers were designed to have approximately 50% GC content with no hetero or homo-dimer formation and not more than 3 wobbles. The designed primers were also blast analyzed against GenBank database to check the suitability for detection of field isolates. Primer sequences designed are given in Table 1. Primer sets were synthesized from IDT technologies (USA). Plasmid DNA (TOPO cloning vector) having the VP1 region insert (990 bp region of VP1 gene) was used to optimize the qPCR assay (Invitrogen, Carlsbad, CA). Plasmid DNA was purified from the positive recombinant clone by using standard procedure with plasmid purification kit (Qiagen, Netherlands). Positive DNA samples of Marek’s disease virus (MDV), Avian Leukosis (ALV) and Reticuloendotheliosis virus (REV) maintained at Avian Health Lab, ICAR-DPR were used. The primer sets were checked for amplification in conventional PCR with following conditions. Initial denaturation at 94 °C for 3 min, followed by 35 cycles of 94 °C for 1 min, 57 °C for 1 min and 72 °C for 2 min, with final extension at 72 °C for 5 min. The PCR amplification products were analyzed by 1.5% agarose gel electrophoresis. The reaction mixture (25 µl) consisted of 2.5 µl 10× buffer, 1.25U of Dream Taq Polymerase with MgCl2, 0.5 µl dNTPs (10 mM each), 2 µl of plasmid DNA (500 ng/µl), 0.5 µl of each forward and reverse primer (20 pmol) and nuclease free water.
Table 1.
Primer sequences designed for qPCR assay
| Primers | Sequence (5′ to 3′) | Length (bp) | Amplicon size (bp) | Annealing region Accession No. CAU65414 |
|---|---|---|---|---|
| CAV1 | F: ACTATCCGCTTCCAAGGAGTCATC | 24 | 155 | VP1 gene: 1036 to 1190 |
| R: GTTAGGTTCATTGACGCTAGCAGG | 24 | |||
| CAV2 | F: GCGGACGGGTCTAAATCACA | 20 | 217 | VP1 gene 1234 to 1450 |
| R: CAATGTGTCGGAACAGGTGC | 20 |
The qPCR assay was performed in Insta Q96™ Real-time PCR system (Himedia, India). The optimal reaction condition was established by using matrix method. Amplification reaction (20 µl) was carried out with 20 µl of TB Green Premix Ex Taq II (Takara), 2 µl of template plasmid DNA and 0.8 µl each of forward and reverse primers (20 pmol), 0.4 µl of ROX reference dye and 6 µl of nuclease free water. Initial denaturation was done at 95 °C for 30 s, followed by amplification profile with 40 cycles of 95 °C for 5 sec, 60 °C for 30 s and 72 °C for 1 min. The amplification was followed by melting stage at 60 °C. For determination of sensitivity, serial 10-fold dilution of the plasmid DNA (upto 10−12) with initial concentration of 1 µg/ µl was done and each dilution was used as template for real time PCR. The reactions were carried out in duplicates and separately carried out for both the primer sets. The sensitivity of the primer sets were first checked in conventional PCR and followed by real time PCR. The highest dilution of plasmid DNA that gives visible band in conventional PCR and the cut off Ct (Ct = 39) value in real time PCR were taken as the detection limit for both assays. The Ct values were plotted against logarithm values of serially dilution plasmid DNA to generate standard curve. Slope and equation were calculated for both primer sets. Number of DNA copies was calculated with the formula = Amount (ng) × 6.022 × 1023/ length (bp) × 1 × 109 x 660. The efficiency of qPCR was calculated using the following formula. Efficiency % = (E − 1) x 100, wherein E = 10−1/slope of standard curve) The efficiency percentage of qPCR for both primers was calculated.
BLAST analysis with GenBank database showed the primer sets specifically detects the CIAV VP1 gene with no non-specific aligning. The primer sets amplified the amplicons of 155 bp and 217 bp VP1 regions specifically in conventional PCR. The visible amplicon was detected upto 10−6 of plasmid DNA in conventional PCR. Hence, the highest limit of detection by the primer sets was calculated as 1 pg/µl by conventional PCR. In real time PCR, the maximum detection Ct value (39) was observed for 10−9 dilution of plasmid DNA. Hence, the detection limit was calculated as 1 fg/ µl by real time PCR. The qPCR assay was able to detect 1000 times more sensitive than conventional PCR. The copy number of detection limit was calculated as 1.82 × 104and 82 × 102 copies for conventional PCR and qPCR respectively. Mean Tm value for the primer sets were 84.14 °C and 86.31 °C respectively. Both the primer sets did not amplify the DNA from MD, ALV and REV DNA samples, showing the high specificity for the primer sets. The standard curve analysis the linear equations y = − 3.3345x + 29.30 and y = − 3.2986x + 28.88 were obtained for the primer sets, respectively. Slope value of − 3.3345 and − 3.2986 was obtained for both sets of primers from standard curve. E value was calculated as 2.009 and the efficiency % for the qPCR assay was 100.9%. The Ct values gradually increased with increased dilution of DNA. An increase of approximately 3.32 Ct value was observed for every ten-fold dilution of plasmid DNA. This indicates almost 100% efficiency of qPCR assay. For validation with field samples, poultry flocks of 4 lines maintained at DPR, Hyderabad were used. Serum samples from four colored parent lines (n = 28 each, total = 112) were collected and subjected to iELISA for CAV specific antibodies with commercial IDEXX kit. Seroconversion of 85–90% were observed in the serum samples indicating the exposure to CAV in these flocks. Blood samples from birds showing symptoms like dullness, paleness and ruffled feather from these flocks were collected (n = 28 from all four lines), DNA extracted by standard procedure and subjected to real time PCR by using the optimized primers following the above-mentioned protocol. Out of 28 samples 6 samples showed the presence of virus DNA indicated by the cut-off Ct values, indicating the presence of circulating virus in the flock.
CIAV is one of the emerging and economically important pathogen worldwide poultry Industry including India [5, 13]. Seroprevalence and clinical disease was observed in Indian poultry causing severe economic loss [1]. CIAV is considered as ubiquitous and mainly cause sub-clinical infection. It also enhances the mortality rate as co-infecting pathogen in respiratory disease complex [6]. CIAV mainly cause vaccination failures due to immunosuppression, sub-optimal flock performance and predisposing to other respiratory diseases [2, 17]. Prevalence rate of 73% was reported by PCR detection from clinical samples from different states of India [19]. Genetic analyses of CIA virus strains circulating in Indian poultry also showed it is closely related with strains of major poultry production countries. In recent years, it has been reported more frequently that CIAV is transmitted through contaminated live vaccine [9, 14]. Use of CIAV contaminated SPF chicken embryos is one of the important reasons for vaccine contamination.
Virus load in contaminated vaccines and/or sub-clinical infections are generally at very low level, scope of detection by conventional techniques such as virus isolation, identification and conventional PCR is limited. Virus isolation in cell culture or in embryonated eggs is considered the gold standard procedure for CIAV detection and has been used routinely, but it is tedious, expensive and time-consuming [20]. Hence, a high throughput, quick and sensitive method is need of the time. The methods such as ELISA, immuoperoxidase and immunohistochemistry etc., require extensive sample processing procedure. Sensitivity, specificity and cost are the limiting factors for these diagnostic methods. On the other hand, PCR based techniques are sensitive, fast and reliable method. Conventional PCR techniques have been earlier used [18, 12]. However, real time PCR assays are quicker and more sensitive than conventional PCR. In the present study, we developed and optimized the real time PCR assay for the quantitative detection of CAV. The developed qPCR assay showed 100 times higher sensitivity than conventional PCR and the detection limit was 0. 01 fg/ µl. The efficiency of the PCR was also calculated as 100.5%. Conventional PCR for CIAV have been earlier reported for molecular diagnosis. Very few reports used real time PCR for quantification of CIAV [4]. Recently, digital droplet PCR also have been reported [10], however, this assay needs elaborate laboratory setup and instruments. The developed assay will be useful for the quick screening of extraneous contamination of live vaccines, to detect sub-clinical infections and also in quantification of virus load in cell culture assays (Fig. 1).
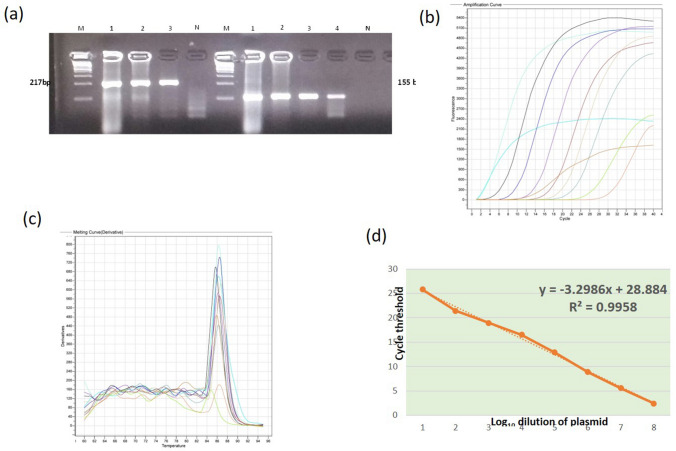
Fig.1.
a Amplification of VP1 gene regions by primer sets in conventional PCR. M: 100 bp ladder, lane 1–4 positive control showing amplification of 217 bp and 155 bp products with primer sets, N: No template control b Amplification of serially diluted plasmid DNA in real time PCR assay (c) Melting curve for primer set 2 in real time PCR assay d Standard curve for Primer set 1. R2: the coefficient of determination
Compliance with ethical standards
Conflict of interest
The authors declared that they have no conflict of interest.
References
- 1.Bhatt P, Shukla SK, Mahendran M, Dhama K, Chawak MM, Kataria JM. Prevalence of chicken infectious anaemia virus (CIAV) in commercial poultry flocks of northern India: a serological survey. Transbound Emerg Dis. 2011;58:458–60. doi: 10.1111/j.1865-1682.2011.01215.x. [DOI] [PubMed] [Google Scholar]
- 2.De Boer GF, Van Roozelaar DJ, Moormann RJ, Jeurissen SH, Wijngaard JC, Hilbink F, Koch G. Interaction between chicken anaemia virus and live Newcastle disease vaccine. Avian Pathol. 1994;23:263–275. doi: 10.1080/03079459408418994. [DOI] [PubMed] [Google Scholar]
- 3.Bülow V, Schat KA. Chicken infectious anemia. In: Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. Diseases of poultry. 10. Ames: Iowa State University Press; 1977. pp. 739–56. [Google Scholar]
- 4.Chansiripornchai N, et al. Application of real-time polymerase chain reaction for quantitative detection of chicken infectious anemia virus. Thai J Vet Med. 2012;12:533–6. [Google Scholar]
- 5.Dhama K, Mahendran M, Somvanshi R, Chawak MM. Chicken infectious anemia virus: an immunosuppressive pathogen of poultry – a review. Indian J Vet Pathol. 2008;32:158–67. [Google Scholar]
- 6.Gowthaman V, Singh SD, Dhama, Kuldeep R, Barathidasan S, Palani NK, Mahajan, Ramakrishnan M. Molecular characterization of chicken infectious anemia virus isolated from commercial poultry with respiratory disease complex in India. Adv Anim Vet Sci. 2014;2:171–6. doi: 10.14737/journal.aavs/2014/2.3.171.176. [DOI] [Google Scholar]
- 7.Hagood LT, Kelly TF, Wright JC, Hoerr FJ. Evaluation of chicken infectious anemia virus and associated risk factors with disease and production losses in broilers. Avian Dis. 2000;44(4):803–8. doi: 10.2307/1593052. [DOI] [PubMed] [Google Scholar]
- 8.Koch G, van Roozelaar DJ, Verschueren CA, van der Eb AJ, Noteborn MH. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine. 1995;13(8):763–70. doi: 10.1016/0264-410X(94)00034-K. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Hu Y, Cui S, et al. Molecular characterization of chicken infectious anemia virus from contaminated live-virus vaccines. Poult Sci. 2017;96(5):1045–51. doi: 10.3382/ps/pew406. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Zhang Y, Meng F, et al. (2019) A new strategy for the detection of chicken infectious anemia virus contamination in attenuated live vaccine by droplet digital PCR. Biomed Res Int. 2750472. [DOI] [PMC free article] [PubMed]
- 11.McNulty MS. Chicken anaemia agent: a review. Avian Pathol. 1991;20(2):187–203. doi: 10.1080/03079459108418756. [DOI] [PubMed] [Google Scholar]
- 12.van Santen VL, Toro H. Rapid selection in chickens of subpopulations within ArkDPI-derived infectious bronchitis virus vaccines. Avian Pathol. 2008;37:293306. doi: 10.1080/03079450802043783. [DOI] [PubMed] [Google Scholar]
- 13.Schat KA. Chicken infectious anaemia. Curr Top Microbiol Immunol. 2009;331:151–183. doi: 10.1007/978-3-540-70972-5_10. [DOI] [PubMed] [Google Scholar]
- 14.Su Q, Li Y, Meng F, Cui Z, Chang S, Zhao P. Newcastle disease virus-attenuated vaccine co-contaminated with fowl adenovirus and chicken infectious anemia virus results in inclusion body hepatitis-hydropericardium syndrome in poultry. Vet Microbiol. 2018;218:52–9. doi: 10.1016/j.vetmic.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Todd D. Circoviruses: immunosuppressive threats to avian species: a review. Avian Pathol. 2000;29:373. doi: 10.1080/030794500750047126. [DOI] [PubMed] [Google Scholar]
- 16.Todd D. Avian circovirus diseases: lessons for the study of PMWS. Vet Microbiol. 2004;98:169. doi: 10.1016/j.vetmic.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Toro H, Gonzalez C, Cerda L, Hess M, Reyes E, Geissea C. Chicken anemia virus and fowl adenoviruses: association to induce the inclusion body hepatitis/ hydropericardium syndrome. Avian Dis. 2000;44:51–8. doi: 10.2307/1592507. [DOI] [PubMed] [Google Scholar]
- 18.Toro H, van Santen VL, Li L, Lockaby SB, van Santen E, Hoerr FJ. Epidemiological and experimental evidence for immunodeficiency affecting avian infectious bronchitis. Avian Pathol. 2006;35(6):455–64. doi: 10.1080/03079450601028811. [DOI] [PubMed] [Google Scholar]
- 19.Wani MY, Dhama K, Barathidasan R, Gowthaman V, Tiwari R, Bhatt P, Kataria JM. Molecular detection and epidemiology of chicken infectious anaemia virus in India. South Asian J Exp Biol. 2013;3(4):145–151. [Google Scholar]
- 20.Yuasa N, Taniguchi T, Yoshida I. Isolation and some characteristics of an agent inducing anemia in chickens. Avian Dis. 1979;23:366385. doi: 10.2307/1589567. [DOI] [Google Scholar]