Abstract
In this study, we increased β-glucan production from brewer’s yeast, Saccharomyces carlsbergensis RU01, by using tannic acid. High-pressure freezing and transmission electron microscopy (HPF-TEM) revealed that the yeast cell wall obtained from yeast malt (YM) medium supplemented with 0.1% w/v tannic acid was thicker than that of yeast cultured in YM medium alone. The production of β-glucan from S. carlsbergensis RU01 was optimized in 3% w/v molasses and 0.1% w/v diammonium sulfate (MDS) medium supplemented with 0.1% w/v tannic acid. The results showed that MDS medium supplemented with 0.1% w/v tannic acid significantly increased the dry cell weight (DCW), and the β-glucan production was 0.28±0.01% w/v and 11.99±0.04% w/w. Tannic acid enhanced the β-glucan content by up to 42.23%. β-Glucan production in the stirred tank reactor (STR) was 1.4-fold higher than that in the shake flask (SF) culture. Analysis of the β-glucan composition by Fourier transform infrared (FTIR) spectroscopy showed that the β-glucan of S. carlsbergensis RU01 cultured in MDS medium supplemented with 0.1% w/v tannic acid had a higher proportion of polysaccharide than that of the control. In addition, β-glucans from brewer’s yeast can be used as prebiotic and functional foods for human health and in animal feed.
Keywords: Cultivation mode, β-Glucan, Molasses diammonium sulfate (MDS) medium, Saccharomyces carlsbergensis RU01, Stirred tank reactor (STR), Tannic acid (TA)
Introduction
β-Glucans are polysaccharide molecules composed of glucose units and can be found in bacteria, algae, yeasts, mushrooms, molds, and higher plants. Their structure depends on the source of the β-1,3 linkage, and each such molecule possesses many novel properties and can improve human and animal health and the immune system. β-1,3-Glucans are classified as biological response modifiers. The molecular mass, shape, structure, and source of β-glucans that provide the most significant therapeutic benefit are highly diverse [1]. According to a previous report, the most bioactive β-glucans contain 1,6-linked sidechains branching off from the more extended β-1,3-glucan backbone and are referred to as β-1,3/1,6-glucans [2]. Many reports have suggested the production of β-glucans from different sources, such as fungi, bacteria, algae, oats, and barley [3–8], which show different linkage types, molecular weights, and degrees of branching [9, 10]. One of the sources of β-glucan is the yeast Saccharomyces cerevisiae cell wall, which is composed of approximately 55–65% β-glucan [11]. Kim and Yun [12] and Liu et al. [13] studied the technique of β-glucan production and isolation from S. cerevisiae. They achieved high productivity and purity with clean and mild treatment of β-glucan from the yeast cell wall. A method has been developed to produce a high level of yeast glucan from agricultural waste such as molasses and corn steep liquor (CSL) with fed-batch culture fermentation [14].
Tannins are water-soluble polyphenols found in plant species used in the brewing industry, e.g., grape, malts, and hops, while mashing the boiling wort [15]. Tannins are known to inhibit yeast growth and metabolism in the fermentation process [16]. For this reason, many reports have studied the interaction between wine tannins and yeast cells [17, 18]. It was suggested that yeast could fix tannins located in the cell wall. When tannins interact with the yeast cell wall, proteins and polysaccharides in the cell wall are precipitated [19]. According to the interaction, colloidal aggregates can form, and these aggregates have a limited size and remain stable. The result is the prevention of glycosyl moieties forming multiple bridges between tannins and their protein parts. Subsequently, hydrophilic and negatively charged aggregates are formed. This process stops yeast cell growth [19, 20]. In addition, it has been suggested that under tannin stress conditions, yeast can protect and maintain internal homeostasis using β-glucan accumulation in the cell wall. According to microscopic observations, the yeast cells appeared thicker [17]. Conventionally, brewer’s yeast strains are divided into two categories, namely, top-fermenting (ale) and bottom-fermenting (lager) yeasts. Strains of S. cerevisiae are commonly used to produce ales in the temperature range of 16–25 °C. On the other hand, Saccharomyces carlsbergensis strains are industrially used to produce lagers in the temperature range from 8 to 15 °C [21]. The yeast S. carlsbergensis is commonly used for beer production. The waste production from this beer fermentation process is high. S. carlsbergensis, however, is rich in protein, vitamin B, chitin, and β-glucans, which possess several physiological functions. Interestingly, breweries can generate additional revenue by isolating β-glucan from spent beer yeast as a high-value product. Tian et al. [22] used alkali treatment at high pressure to isolate β-glucan from spent beer yeast. An extraction rate of 78.38% with 78.11% β-D-glucan content was achieved under optimal conditions. Homogenization of cell walls was found to increase the yield of β-glucan [23, 24]. Alkaline treatment was used to isolate β-glucan from the cell walls of spent brewer’s yeast; β-glucan with minimal structural changes could be obtained by combination of sonication and spray-drying, and the formed particles exhibited an insignificant amount of agglomerate formation [24]. However, these properties of β-glucans were shown to be affected by differences in isolation and drying procedures. They found that lyophilized preparations exhibited the highest oil-binding capacity and lowest swelling, and air-dried preparations showed enhanced swelling. It has also been shown that β-glucans obtained from yeast homogenized cell walls exhibit relatively high apparent viscosity, emulsion-stabilizing capacity, and water-holding capacity than commercial β-glucans from baker’s yeast [23]. A benefit of β-glucans is also its ability to act as a potent stimulator of the immune system against infection by viruses, bacteria, and fungi, which leads to cancer and stress-related immune suppression [25–27].
This study aimed to investigate the effect of tannic acid on cell morphology, dry cell weight (DCW), and β-glucan production in S. carlsbergensis RU01. The cultivation mode of β-glucan production in molasses and diammonium sulfate (MDS) medium supplemented with tannic acid was studied.
Materials and Methods
Yeast Strain
S. carlsbergensis strain RU01 was isolated from beer beverage waste. For long-term maintenance, the strain was stored at −20 °C in yeast malt (YM; Himedia) medium supplemented with 0.1% w/v tannic acid (Sigma) and 20% w/v glycerol (Sigma). Before the experiment, the strain was propagated twice in YM medium supplemented with 0.1% w/v tannic acid.
Transmission Electron Microscopy (TEM) of the Yeast Cell Wall
S. carlsbergensis RU01 was available as a cryofixed (by the high-pressure freezing (HPF) method) unit that exhibits reliable physical performance in yeast cells [28]. The cells of S. carlsbergensis strain RU01 were grown in YM supplemented with 0.1% w/v tannic acid and in YM as a control at 30 °C for 48 h. Some of the cultures were starved in a refrigerator at 4 °C, and some were suspended in 20% v/v glycerol for at least 4 h. S. carlsbergensis RU01 was cryofixed by HPF using an HPM 010 (BAL-TECAG, Liechtenstein) at −193 °C and 210 MPa. The cells were observed after cryocutting at −175 to −185 °C. Yeast cell sections with a thickness of 100 nm were prepared. The samples were imaged with a Philips CM10 transmission electron microscope. The structures of yeast in near-native state were studied.
Yeast Cultivation Mode
Shake Flask (SF) Culture
S. carlsbergensis RU01 was cultured in 3% w/v molasses and 0.1% w/v diammonium sulfate (MDS) medium supplemented with 150 mL of 0.1% w/v tannic acid in a 250-mL Erlenmeyer flask at 30 °C, pH 5.0, and 200 rpm for 36 h. The growth rate was measured at 600 nm. The dry weight was determined. Then, 2 mL of culture was diluted to 50 mL, filtered through a predried filter (0.45 μm pore size), and washed twice with 50 mL of normal saline solution. Cells on the filters were dried at 100 °C in an oven until constant weight and were subsequently cooled at room temperature in a desiccator for 2 h before weighing. The DCW was the difference between the filter weights with or without yeast.
Bioreactor-Scale Culture
Scale-up of the culture was performed in a 7.5-L bioreactor (BIOFLO310, New Brunswick, USA) with a 5-L working volume. S. carlsbergensis RU01 was cultured in MDS medium supplemented with 0.1% w/v tannic acid at 30 °C and pH 5.0 for 48 h. The medium was inoculated with an initial concentration of approximately 6.0×107 CFU/mL (250 mL). Three agitation rates (100, 200, and 400 rpm) and two aeration rates (0.5 and 1.0 vvm) were used.
β-Glucan Extraction and Determination
Alkaline Extraction
The cell wall of S. carlsbergensis RU01 was extracted by a modified alkaline extraction method [29]. The cell wall was extracted with 6% w/v NaOH at 90 °C for 2 h and centrifuged at 6000 rpm and 4 °C for 10 min; the supernatant was discarded. The sediment was washed three times in distilled water, and the supernatant was removed by centrifugation at 6000 rpm and 4 °C for 10 min. Subsequently, the pH was adjusted to 6.0–7.0 with 1% v/v HCl. The isolated material was designated β-glucan because it contained this polysaccharide as the main component, mixed with a small number of other polysaccharides.
Analyses of β-Glucan
According to the manufacturer’s instructions, the 1,3/1,6-β-glucans were determined in quadruplicate using an assay kit [30]. The β-glucan content was determined by subtracting the α-glucan content from the total glucan content. The total glucan/α-glucan levels and the D-glucose in the oligosaccharide, sucrose, and free-D-glucose contents were measured in both steps. The enzymatic assay test for detecting 1,3/1,6-β-glucans in yeast is a complete method for quantitative determination of specific linked β-glucans in yeast. All glucans were split into their glucose monomers and measured spectrophotometrically.
Fourier Transform Infrared (FTIR) Spectroscopic Analysis
Powdered glucan was analyzed by FTIR spectroscopy (Bruker Invenio S instrument, UK). FTIR spectra were obtained in absorption mode at room temperature. Spectra were recorded from 400 to 4000 cm−1. Spectra were preprocessed with OPUS-TOUCH software. The curve fitting used to quantify the ratio of polysaccharides, proteins, and lipids was based on a least-square method using Gaussian bands.
Statistical Analysis
All experiments were performed in triplicate, and analysis of variance (ANOVA) with a confidence interval of 95% (p<0.05) was reported. The significance of the results was validated using SPSS (version 18.0).
Results and Discussion
Cell Wall Remodeling Accompanies Cell Volume Changes During Tannic Acid Stress
In a preliminary study, it was found that MDS medium interfered with the HPF technique. In this report, YM medium was used instead of MDS medium to study the cell wall structure. Moreover, YM medium is consistently applied in large-scale fermentation at the initial stages since it comprises all the essential elements and biosynthetic building blocks necessary for yeast cell propagation [31]. S. carlsbergensis RU01 cells were cultured in YM medium with or without 0.1% w/v tannic acid supplementation at 30 °C for 48 h. Subsequently, the cells were fixed with HPF. HPF-TEM showed that the cell wall of S. carlsbergensis RU01 contains two outer layers of mannoprotein and an inner β-glucan-chitin layer. During stress, the yeast cells were cultured in tannic acid, leading to significant changes in the cell wall. If one assumes that the yeast cell wall does not change in YM medium (Fig. 1a), it is the tannic acid in the supplemented medium that changes the cell wall composition. In other words, the β-glucan-chitin layer is likely to become thicker, while the mannoprotein layers become thinner (Fig. 1b). The structured side of the yeast is in a near-native state. This analysis revealed dramatic changes in cell wall architecture immediately following supplementation with or without tannic acid. Mongkontanawat et al. [32] observed that the mannoprotein content decreased, while the β-glucan content increased, in the S. cerevisiae cell wall. Bzducha-Wróbel et al. [33] found that the β-glucan content increased in the Candida utilis cell wall. Moreover, Osumi [34] examined septum formation during cell division after cryofixation by HPF. The α-1,3 and β-1,3 glucans were found in the invaginating nascent septum, and highly branched β-1,6-glucan was later observed on the second septum. However, Mekoue et al. [20] suggested that mannan and β-glucan in yeast cell walls were increased to protect the cells from tannins.
Fig. 1.
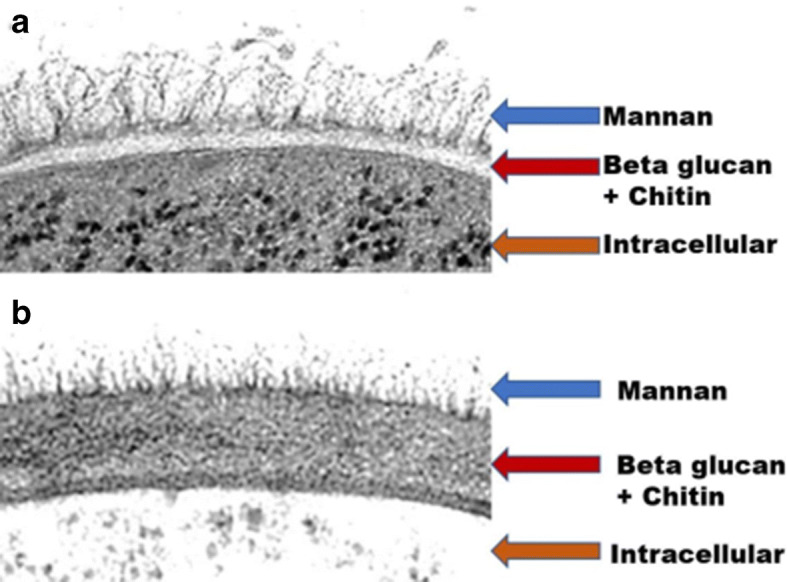
For transmission electron microscopy (TEM) of the Saccharomyces carlsbergensis RU01 cell wall, the cells were fixed with high-pressure freezing (HPF) after cultivation in YM medium without (a) or with (b) 0.1% w/v tannic acid supplementation
Effect of Tannic Acid on β-Glucan Production and DCW
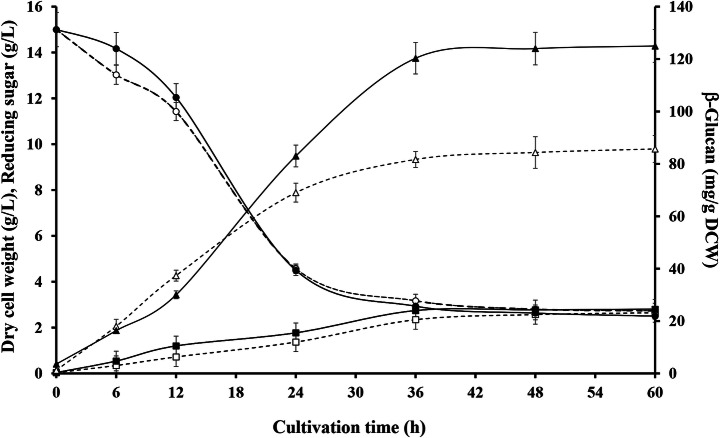
The culture time for β-glucan production from S. carlsbergensis RU01 in MDS medium with or without tannic acid supplementation was investigated. The β-glucan content, DCW, and reducing sugar content were determined as shown in Fig. 2. As the reducing sugar content decreased, a β-glucan content of 11.99% w/w was obtained in the medium supplemented with 0.1% w/v tannic acid, which was higher than that in the medium without tannic acid (8.43% w/w) after 36 h of cultivation. MDS medium supplemented with tannic acid yielded the highest DCW of S. carlsbergensis RU01 at 0.28% w/v (Table 1). Yeast cells can protect themselves and maintain internal homeostasis under stress by synthesizing thicker cell walls which increases carbohydrate levels. The cells may elicit a response to tannins by increasing the synthesis of β-glucan, which builds a thicker cell wall [35]. This is consistent with the results of Chotigavin et al. [36], who found that tannic acid at low concentrations enhanced the growth of the yeast S. carlsbergensis. Kim et al. [14] found that molasses and corn steep liquor (CSL) increased the cell mass of S. cerevisiae JUL3. In addition, Mongkontanawat et al. [37] indicated that YM medium supplemented with additive chemical stress factors, such as EDTA and SDS, increased β-glucan production in S. cerevisiae by approximately 7–40%. Phenolic compounds, which are chemical stress factors present in malva nut juice wastewater, were also reported to enhance β-glucan production in S. cerevisiae [38]. This indicated that tannins interfered with the outer structure of the yeast cell wall. Tannins had effects on the mannoprotein layers, which caused them to aggregate. Therefore, the structure of the cell wall could be changed by increasing β-glucan production.
Fig. 2.
Culture time for production of β-glucan in Saccharomyces carlsbergensis RU01 grown in 3% w/v molasses and 0.1% w/v diammonium sulfate (MDS) medium. The slash lines and unfilled symbols indicate MDS medium, and dark lines and solid symbols indicate MDS medium supplemented with 0.1% w/v tannic acid. Symbols: rectangles, dry cell weight (DCW); circles, reducing sugar; and triangles, β-glucan
Table 1.
Dry cell weight, β-glucan content, and FTIR range ratios of the polysaccharide, protein, and lipid content of S. carlsbergensis RU01 cultured in MDS medium (control) with or without 0.1% w/v tannic acid (TA) supplementation at 36 h
| Medium | DCW (%w/v) | β-Glucan content (%w/w) | Band area (%) | Range ratio (1:2:3) | ||
|---|---|---|---|---|---|---|
| Range 1 925–1190 cm−1 (polysaccharide) | Range 2 1500–1700 cm−1 (protein) | Range 3 2800–3000 cm−1 (lipid) | ||||
| MDS | 0.25±0.01a | 8.43±0.21a | 11.16 | 10.57 | 6.42 | 1.7:1.6:1.0 |
| MDS + TA | 0.28±0.01b | 11.99±0.04b | 12.65 | 9.66 | 6.67 | 1.9:1.5:1.0 |
Data are shown as the mean±SD derived from three replicates. Means within a column followed by a different letter are significantly different (p≤ 0.05)
Scale-up of β-Glucan Production in Batch Fermentation
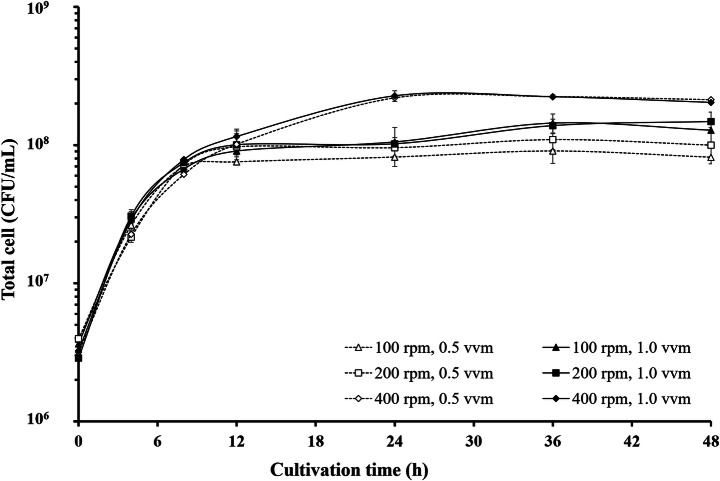
At aeration rates of 0.5 and 1.0 vvm, similar amounts of cells were obtained at the same agitation rate; the aeration rate did not affect cell growth, while agitation at 100, 200, and 400 rpm affected the total yeast cell count, as shown in Fig. 3. The cell count was proportional to the agitation rate, and 400 rpm yielded the highest amount of yeast cells. Therefore, the optimum agitation rate for cell growth was 400 rpm with aeration rates of 0.5 and 1.0 vvm. The results in Table 2 show that the β-glucan levels at 36 h were 14.61 and 17.08% w/w, whereas at 48 h, the β-glucan levels were 16.19 and 14.95% w/w. Based on the results, agitation at 400 rpm with aeration at 1.0 vvm afforded the highest β-glucan content at 36 h. It was suggested that the oxygen concentration influenced the β-glucan content. According to Baez and Shiloach [39], oxygen content affects bacteria and yeast growth by accelerating cell division, but it affects antioxidants and causes deterioration of the repair pathway itself. The cells may have thinner cell walls and decreased amounts of β-glucan.
Fig. 3.
Number of S. carlsbergensis RU01 cells in a 5-L stirrer tank reactor under various agitation and aeration conditions
Table 2.
β-Glucan content of S. carlsbergensis RU01 cultured in a 5-L stirred tank reactor in MDS medium supplemented with 0.1% w/v tannic acid at 36 and 48 h
| Agitation/aeration rate | β-Glucan content (%w/w) | |||
|---|---|---|---|---|
| 36 h | 48 h | |||
| 0.5 vvm | 1.0 vvm | 0.5 vvm | 1.0 vvm | |
| 100 rpm | 4.37±0.52 | 5.44±0.45 | 4.80±0.48 | 5.90±0.37 |
| 200 rpm | 7.87±0.32 | 10.10±0.60 | 9.67±0.81 | 12.41±0.61 |
| 400 rpm | 14.61±0.42 | 17.08±0.55 | 16.19±0.61 | 14.95±0.43 |
Data are shown as the mean±SD derived from three replicates
The S. carlsbergensis RU01 DCW, β-glucan content, and fold change in β-glucan yield are shown in Table 3. The relation between growth and fermentation rates was first established. After 36 h of cultivation in a shake flask (SF), approximately 119.89 mg/g DCW was obtained. Batch fermentation contributed to an increase in β-glucan production of 172.26 mg/g DCW. Thus, the efficacy of batch fermentation in β-glucan production was higher than that of batch culture. In addition, the β-glucan yield coefficient (Yp/s) for the cultivation mode of S. carlsbergensis RU01 was defined as the amount of β-glucan produced per unit of reducing sugar consumed at 36 h. The results showed that batch fermentation and SF cultivation consumed 0.059 and 0.028 g/g reducing sugars, respectively.
Table 3.
Dry cell weight and yield and fold change of β-glucan in S. carlsbergensis RU01 cultured in a shake flask or 5-L stirred tank reactor in MDS medium supplemented with 0.1% w/v tannic acid for 36 h
| Cultivation mode | DCW (g/L) |
β-Glucan content (mg/g DCW) | (Yp/s) (g/g reducing sugar consumed) |
Fold change of β-glucan |
|---|---|---|---|---|
| Shake flask | 2.81±0.11a | 119.89±0.38a | 0.028 | 1.0 |
| Stirred tank reactor | 4.07±0.16b | 172.26±9.05b | 0.059 | 1.4 |
The data are shown as the mean±SD derived from three replicates. Means within a column followed by a different letter are significantly different (p≤0.05)
Composition of β-Glucan from S. carlsbergensis RU01 by FTIR Analysis
The FTIR spectrum was used to study three main regions, corresponding to polysaccharides (925–1190 cm−1), proteins (1500–1700 cm−1), and lipids (2800–3000 cm−1), in yeast cells [31, 40]. Curve fitting and band area determination have been previously employed to analyze yeast cell walls [41, 42]. FTIR analysis of the extracted β-glucan from S. carlsbergensis RU01 was performed. The polysaccharide, protein, and lipid levels of the glucan were calculated from the band area (%). The alkaline extract of S. carlsbergensis RU01 cell walls was designated β-glucan because it contained this polysaccharide as the main component, mixed with proteins and lipids (Table 1). The extracted β-glucan of S. carlsbergensis RU01, which was cultured in MDS medium supplemented with 0.1% w/v tannic acid, showed a higher ratio (1.9:1.5:1.0) and %polysaccharide than the control. The results showed that the polysaccharide content was higher than that in the control, while the protein content was slightly lower than that in the control. It was suggested that tannic acid enhanced the β-glucan content and decreased the protein content in the cell wall of S. carlsbergensis RU01.
Conclusion
β-Glucan production in S. carlsbergensis RU01 was enhanced by tannic acid. The cell wall of S. carlsbergensis RU01 became thick, and β-glucan production increased significantly. The β-glucan content was enhanced 42.23% by tannic acid and 1.4-fold upon changing the mode of cultivation. The extracted β-glucans were mainly polysaccharides, mixed with proteins and lipids. In addition, β-glucans from brewer’s yeast can be used as a prebiotic and functional foods for human health and in animal feed.
Acknowledgements
We would like to thank Assist. Prof. Varaporn Veraplakorn, Department of Biotechnology, Faculty of Science, Ramkhamhaeng University, Thailand.
Funding
This work was supported by the National Research Council of Thailand under research number 2560A11802129.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stone, B. A., & Clarke, A. E. (1992). Chemistry and biology of (1→3)-β-glucans. La Trobe University Press.
- 2.Akramiene D, Kondrotas A, Didziapetriene J, Kevelaitis E. Effects of beta-glucans on the immune system. Medicina (Kaunas, Lithuania) 2007;8:597–606. doi: 10.3390/medicina43080076. [DOI] [PubMed] [Google Scholar]
- 3.Yan JK, Wang WQ, Wu JY. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. Journal of Functional Foods. 2014;6:33–47. doi: 10.1016/j.jff.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhanja SK, Rout D, Patra P, Sen IK, Nandan CK, Islam SS. Water-insoluble glucans from the edible fungus Ramaria botrytis. Bioactive Carbohydrates and Dietary Fibre. 2014;3(2):52–58. doi: 10.1016/j.bcdf.2014.01.004. [DOI] [Google Scholar]
- 5.Stack HM, Kearney N, Stanton C, Fitzgerald GF, Ross RP. Association of beta-glucan endogenous production with increased stress tolerance of intestinal Lactobacilli. Applied and Environmental Microbiology. 2010;76(2):500–507. doi: 10.1128/AEM.01524-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vetvicka V, Dvorak B, Vetvickova J, Richter J, Krizan J, Sima P, Yvin JC. Orally administered marine (1→3)-beta-D-glucan phycarine stimulates both humoral and cellular immunity. International Journal of Biological Macromolecules. 2007;40(4):291–298. doi: 10.1016/j.ijbiomac.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Yoo HU, Ko MJ, Chung MS. Hydrolysis of beta-glucan in oat flour during subcritical-water extraction. Food Chemistry. 2020;308:125670. doi: 10.1016/j.foodchem.2019.125670. [DOI] [PubMed] [Google Scholar]
- 8.Du B, Bian ZX, Xu BJ. Skin health promotion effects of natural beta-glucan derived from cereals and microorganisms: A review. Phytotherapy Research. 2014;28(2):159–166. doi: 10.1002/ptr.4963. [DOI] [PubMed] [Google Scholar]
- 9.Petravić-Tominac V, Zechner-Krpan V, Grba S, Srečec S, Panjkota-Krbavčić I, Vidović L. Biological effects of yeast β-glucans. Agriculturae Conspectus Scientificus. 2010;75(4):149–158. [Google Scholar]
- 10.Li, X., & Cheung, P. C. K. (2019). Application of natural β-glucans as biocompatible functional nanomaterials. Food Science and Human Wellness.
- 11.Klis FM, Mol P, Hellingwerf K, Brul S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiology Reviews. 2002;26(3):239–256. doi: 10.1111/j.1574-6976.2002.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim KS, Yun HS. Production of soluble β-glucan from the cell wall of Saccharomyces cerevisiae. Enzyme and Microbial Technology. 2006;39(3):496–500. doi: 10.1016/j.enzmictec.2005.12.020. [DOI] [Google Scholar]
- 13.Liu X, Wang Q, Cui S, Liu H. A new isolation method of β-D-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocolloids. 2008;22(2):239–247. doi: 10.1016/j.foodhyd.2006.11.008. [DOI] [Google Scholar]
- 14.Kim YH, Kang S, Lee J, Chang HL, Yun CW, Paik HD, Kang CW, Kim S. High cell density fermentation of Saccharomyces cerevisiae JUL3 in fed-batch culture for the production of β-glucan. Journal of Industrial and Engineering Chemistry. 2007;13:153–158. [Google Scholar]
- 15.Fumi MD, Galli R, Lambri M, Donadini G, De Faveri DM. Effect of full-scale brewing process on polyphenols in Italian all-malt and maize adjust lager beer. Journal of Food Composition and Analysis. 2011;24(4–5):568–573. doi: 10.1016/j.jfca.2010.12.006. [DOI] [Google Scholar]
- 16.Wauters T, Iserentant D, Verachtert H. Sensitivity of Saccharomyces cerevisiae to tannic acid is due to iron deprivation. Canadian Journal of Microbiology. 2001;47(4):290–293. doi: 10.1139/w01-006. [DOI] [PubMed] [Google Scholar]
- 17.Wauters T, Verachtert H, Iserentant D. Isolation of mutants of Saccharomyces cerevisiae with a changed cell wall composition by screening on resistance to tannic acid. Food Technology and Biotechnology. 1999;34(4):271–275. [Google Scholar]
- 18.Kanpiengjai A, Chui-Chai N, Chaikaew S, Khanongnuch C. Distribution of tannin-'tolerant yeasts isolated from Miang, a traditional fermented tea leaf (Camellia sinensis var. assamica) in northern Thailand. International Journal of Food Microbiology. 2016;238:121–131. doi: 10.1016/j.ijfoodmicro.2016.08.044. [DOI] [PubMed] [Google Scholar]
- 19.Mekoue NJ, Vernhet A, Siechzkowski N, Brillouet JM. Interactions of condensed tannins with Saccharomyces cerevisiae yeast cells and cell walls: Tannin location by microscopy. Journal of Agricultural and Food Chemistry. 2015;63:39–45. doi: 10.1021/jf505339q. [DOI] [PubMed] [Google Scholar]
- 20.Mekoue NJ, Pocet-Legrand C, Sieczkowski N, Vernhet A. Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and β-glucan. Food Chemistry. 2016;210:671–682. doi: 10.1016/j.foodchem.2016.04.050. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira IMPLVO, Pinho O, Vieira E, Tavarela JG. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends in Food Science and Technology. 2010;21(2):77–84. doi: 10.1016/j.tifs.2009.10.008. [DOI] [Google Scholar]
- 22.Tian X, Yang P, Jiang W. Effect of alkali treatment combined with high pressure on extraction efficiency of β-D-glucan from spent brewer’s yeast. Waste and Biomass Valorization. 2019;10(5):1131–1140. doi: 10.1007/s12649-017-0130-8. [DOI] [Google Scholar]
- 23.Thammakiti S, Suphantharika M, Phaesuwan T, Verduyn C. Preparation of spent brewer’s yeast β-glucans for potential applications in the food industry. International Journal of Food Science & Technology. 2004;39(1):21–29. doi: 10.1111/j.1365-2621.2004.00742.x. [DOI] [Google Scholar]
- 24.Zechner-Krpan V, Petravić-Tominac V, Galović P, Galović V, Filipović-Grčić J, Srečec S. Application of different drying methods on β-glucan isolated from spent brewer’s yeast using alkaline procedure. Agriculturae Conspectus Scientificus. 2010;75:45–50. [Google Scholar]
- 25.Barsanti L, Passarelli V, Evangelista V, Frassanito AM, Gualtieri P. Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Natural Product Reports. 2011;3:457–466. doi: 10.1039/c0np00018c. [DOI] [PubMed] [Google Scholar]
- 26.Ensley HE, Tobias B, Pretus HA, McNamee RB, Jones EL, Browder IW, Williams DL. NMR spectral analysis of a water-insoluble (1→3)-beta-D-glucan isolated from Saccharomyces cerevisiae. Carbohydrate Research. 1994;258:307–311. doi: 10.1016/0008-6215(94)84098-9. [DOI] [PubMed] [Google Scholar]
- 27.Yan J, Allendorf DJ, Brandley B. Yeast whole glucan particle (WGP) beta-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opinion on Biological Therapy. 2005;5(5):691–702. doi: 10.1517/14712598.5.5.691. [DOI] [PubMed] [Google Scholar]
- 28.Müller-Reichert T, Srayko M, Hyman AA, O’Toole E, McDonald K. Correlative light and electron microscopy of early Caenorhabditis elegans embryos in mitosis. Methods in Cell Biology. 2007;79:101–119. doi: 10.1016/S0091-679X(06)79004-5. [DOI] [PubMed] [Google Scholar]
- 29.Manners DJ, Masson AJ, Patterson JC. The structure of a β-(1-3)-D-glucan from yeast cell walls. Biochemical Journal. 1973;135(1):31–36. doi: 10.1042/bj1350031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Megazyme. (2011). Enzymatic yeast beta glucan. Megazyme International.
- 31.Hahn-Hägerdal B, Karhumaa K, Larsson CU, Gorwa-Grauslund MF, Görgens JF, van Zyl WH. Role of cultivation media in the development of yeast strains for large scale industrial use. Microbial Cell Factories. 2005;4(1):31. doi: 10.1186/1475-2859-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mongkontanawat N, Sanguandeekul R, Prakitchaiwattana C, Xiao H, McLandsborough LA, Methacanon P. Effect of three additives on the cell morphology and β-glucan production in Saccharomyces cerevisiae. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2011;2:283–295. [Google Scholar]
- 33.Bzducha-Wróbel A, Pobiega K, Blazejak S, Kieliszek M. The scale-up cultivation of Candida utilis in waste potato juice water with glycerol affects biomass and β(1,3)/(1,6)-glucan characteristic and yield. Biotechnological Products and Process Engineer. 2018;102:9131–9145. doi: 10.1007/s00253-018-9357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osumi M. Visualization of yeast cells by electron microscopy. Journal of Electron Microscopy. 2012;61:343–365. doi: 10.1093/jmicro/dfs082. [DOI] [PubMed] [Google Scholar]
- 35.Bzducha-Wróbel A, Blazejak S, Molenda M, Reczek L. Biosynthesis of β(1,3)/(1,6)-glucan of cell wall of the yeast Candida utilis ATCC 9950 strains in the culture media supplemented with deproteinated potato juice water and glycerol. European Food Research and Technology. 2015;240(5):1023–1034. doi: 10.1007/s00217-014-2406-6. [DOI] [Google Scholar]
- 36.Chotigavin, N. (2019). The production of beta-glucan from Saccharomyces carlsbergensis RU01 by tannin containing medium. MS Thesis, King Mongkut’s Institute of Technology Ladkrabang, Thailand
- 37.Mongkontanawat N, Sanguandeekul R, Phakitchaiwattana C, Xiao H, McLandsborough LA, Methacanon P. Influence of additives on Saccharomyces cerevisiae β-glucan production. International Food Research Journal. 2013;20:1953–1959. [Google Scholar]
- 38.Mongkontanawat N, Wasikadilok N, Phuangborisut S, Chanawanno T, Khunphutthiraphi T. β-Glucan production of Saccharomyces cerevisiae by using malva nut juice production wastewater. International Food Research Journal. 2018;25:499–503. [Google Scholar]
- 39.Baez A, Shiloach J. Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds. Microbial Cell Factories. 2014;13(1):181. doi: 10.1186/s12934-014-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pengkumsri N, Sivamaruthi BS, Sirilun S, Peerajan S, Kesika P, Chaiyasut K, Chaiyasut C. Extraction of β-glucan from Saccharomyces cerevisiae: Comparison of different extraction methods and in vivo assessment of immunomodulatory effect in mice. Food Science and Technology. 2017;37(1):124–130. doi: 10.1590/1678-457x.10716. [DOI] [Google Scholar]
- 41.Adt I, Toubas D, Pinon JM, Manfait M, Sockalingum GD. FTIR spectroscopy as a potential tool to analyze structural modifications during morphogenesis of Candida albicans. Archives of Microbiology. 2006;85:277–285. doi: 10.1007/s00203-006-0094-8. [DOI] [PubMed] [Google Scholar]
- 42.Galichet A, Sockalingum GD, Belabi A, Manfait M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiology Letters. 2001;197(2):179–186. doi: 10.1111/j.1574-6968.2001.tb10601.x. [DOI] [PubMed] [Google Scholar]