Abstract
This study was focused on elucidating inhibition of antibiotic efflux mechanism of cadmium adapted (CdA) Salmonella Typhi Ty2 cells. Herein, upregulated expression of efflux genes (acrB, tolC) and their regulators (soxS, marA) was observed in CdA Ty2 cells by qRT-PCR. The pathogen further elevated the expression of these genes even in the presence of three efflux pump inhibitors (EPIs), i.e., Phe-Arg-β-naphthylamide, 1-(1-naphthyl-methyl)piperazine, and 5-hydroxy-2-methyl-1,4-naphthoquinone, perhaps by sensing the pressure of the latter in addition to cadmium stress. Interaction of different EPIs with efflux pumps of CdA Ty2 cells was confirmed using ethidium bromide (EtBr) accumulation and efflux assay. All the EPIs could cause retention of EtBr which was indicated by increased fluorescence units. Considering this potential of EPIs, retention of antibiotics was evaluated in CdA Ty2 cells wherein EPIs were used in combination with selected antibiotics (instead of EtBr). A decrease in the effective concentration of antibiotics was observed. This was further validated using the clinical isolates. The data revealed the efficiency of EPIs as they could inhibit the efflux potential of even the overexpressed efflux pumps. Thus, combination of EPI(s)-antibiotics may be exploited in future as one of the strategies for combating metal induced antibiotic resistance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00492-5.
Keywords: Cadmium adaptation, Gene expression, Efflux pump inhibitors, Ethidium bromide assay, Synergy, Toxicity
Introduction
The global emergence of multidrug-resistant pathogens is a growing threat to antibiotic therapy. Under diverse physiological conditions, switching off and on of various adaptive responses has intensified the number of antibiotic resistant bacteria in the recent years. The causes of increasing resistance are multifactorial including overuse/misuse of antibiotics; poor infection control in health care settings leading to heathcare transmission; poor sanitation and hygiene; suboptimal rapid diagnosis, preventive medicine, and dosing; and mass drug administration along with travel and exposure to heavy metals in the environment [1]. Exposed to heavy metals in the natural environment, Salmonella enterica serovar Typhi (the causative agent of typhoid fever) which enters the host via feco-oral route may become resistant to the advocated antibiotics inside the host as per the co-selection theory. In view of this, recently, we have reported that increased cadmium accumulation in Salmonella Typhi Ty2 standard strain [2] and clinical isolates [3] lead to increased antibiotic resistance in these strains. Exposure to stressed conditions may prepare the organism to cause mutations of the target protein, enzymatic inactivation of the antibiotic, or inhibition of accumulation of antibiotics by over expression of efflux pumps within the cell thus contributing to emergence of multidrug resistance [4]. Unlike antibiotics, metals are not subjected to degradation and can subsequently represent an enduring selection pressure. Thus, there are concerns regarding the potential of metal contamination in maintaining a pool of antibiotic resistance genes in both environmental as well as clinical settings.
In this context, the active efflux of similar/dissimilar compounds accredits the multidrug-resistant phenotype (MDR) to the bacteria. Active efflux of both antibiotics as well as heavy metals by multidrug efflux transporter MdrL in Listeria monocytogenes has been documented [5]. Activation of MexGHI–OpmD efflux pump harbored by Pseudomonas aeruginosa resulted in increased resistance towards vanadium, ticarcillin, and clavulanic acid [6]. In Burkholderia cepacia, DsbA–DsbB system has been reported as multi-drug resistance system and metal-efflux system, which imparts resistance to multitude of antibiotics as wells as metals [7]. Hernandez et al. [8] had earlier screened Escherichia hermannii and Enterobacter cloacae from soil contaminated by refinery oil and suggested that addition of vanadate lead to induction of MDR phenotype which was promoted by membrane bound efflux system. Co-regulation of zinc (along with cadmium and cobalt) and imipenem (belonging to class carbapenem of antibiotics) resistance in Pseudomonas aeruginosa through a two component sensor protein CzcS has also been reported [9]. In Escherichia coli and Salmonella enterica serovar Typhimurium, the acquisition of MDR phenotype has been linked to the overexpression of a RND type tripartite pump AcrAB-TolC and its transcriptional regulators such as MarA and SoxS, which when induced imparts high level of intrinsic as well as adaptive resistance to numerous antibiotics [10]. However, role of this pump in metal-antibiotic co-selection has not been explored yet.
In an attempt to curb these notorious systems, diverse therapeutic options which can reverse the efflux pump activity have been explored [11]. Pharmacological inhibition of active efflux pumps using chemical, biological, and rationally designed efflux pump inhibitors (EPIs) has become an attractive goal for reversing antibiotic resistance and simultaneously improving therapeutic options [12]. In continuation to our previous finding, the present study was carried with an aim to explore the involvement of efflux mechanism in this pathogen and to find possible inhibitor(s) (chemical as well as biological) in order to combat this clinically significant menace. To the best of our knowledge, this is the first report elucidating the mechanism involved in cadmium-induced antibiotic resistance coupled with identifying its potential inhibitor, in order to disarm MDR phenotype in CdA Salmonellae.
Material and methods
Bacterial strain
S. enterica serovar Typhi Ty2, reference strain (DBL-8, David Bruce Laboratory, East Everleigh, Marlborough Wiltshire) which has been maintained in our laboratory [13] and originally procured from Central Research Institute, Kasauli, was used in the present study. The clinical isolates of serovar Typhi used for validation of efficacy of NMP-antibiotic combination in the present study were procured from Government Medical College and Hospital (GMCH), Chandigarh. Sensitivity patterns of the standard strain as well as clinical isolates have already been reported earlier by us [3].
Chemicals
Phe-Arg-β-naphthylamide (PAβN), 1-(1-naphthyl-methyl)-piperazine (NMP), and plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone from Plumbago indica), ampicillin, ciprofloxacin, chloramphenicol, ceftizoxime, and TRIZOL reagent were procured from Sigma-Aldrich (St Louis, MO, USA). Cadmium chloride (CdCl2) salt was procured from Sisco Research Laboratories Pvt. Ltd. (Mumbai). cDNA reaction kit and 2X iQ SYBR Green Supermix were purchased from Bio-Rad Laboratories (India) Pvt. Ltd. PAβN was dissolved in sterile Milli-Q water and NMP, plumbagin were dissolved in DMSO. For agents dissolved in DMSO, the final concentration of 1% DMSO was used. Since antibiotics are effective at much lower concentrations as compared to other agents, the antibiotics were stored as standard stocks of 1 g/L, whereas other three agents (EPIs) were stored as standard stocks of 10 g/L at −20°C.
qRT-PCR of soxS, marA, acrB, and tolC genes
Cadmium-adapted (CdA) serovar Typhi cells were obtained by carrying out sequential propagation (ten passages) in nutrient broth supplemented with cadmium chloride at its sub-MIC (0.5 mM) under laboratory conditions, as described by us earlier [3]. Differential expression of soxS, marA, acrB, and tolC genes encoding the efflux pumps was determined in CdA Ty2 and unadapted cells (CdunA) using real-time PCR studies. Primers were designed using NCBI primer designing tool (Table S1.) and were procured from Eurofins Genomics Pvt Ltd. The PCR protocol used on Applied Biosystem step one real-time PCR system started with the first step of initial denaturation and enzyme activation at 95°C for 3 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing and extension at 54 °C for 30 s. Melt curve analysis was performed by heating the samples from 55 to 95 °C with an increment of 0.5 °C and fluorescence was recorded. Under these experimental conditions, GAPDH was used as reference gene.
Selection of inhibitors
Three efflux pump inhibitors (EPIs), namely Phe-Arg-β- naphthylamide (PAβN), 1-(1-naphthyl-methyl)-ppiperazine (NMP), and plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone from Plumbago indica), were selected upon literature survey [11, 14–16] keeping in view the above mentioned genes for the efflux pumps.
Determination of minimum inhibitory concentrations of the agents
Minimum inhibitory concentrations (MICs) of EPIs, antibiotics, and EPI(s)-antibiotic(s) combination were determined against reference Ty2 strain before and after cadmium adaptation by broth dilution techniques (CLSI, 2012), as described by us earlier [3]. To evaluate efficacy of EPI(s)-antibiotic(s) combinations, a two-fold serial dilution of antibiotics and EPIs were made in Mueller Hinton broth in 96-well microtiter plates, making different combinations of antibiotics plus EPI(s) [12]. Each well was then supplemented with 5×105 CFU/ml CdunA and CdA Ty2 cells and then the plate was incubated at 37 °C. Wells not containing any antibacterial agent were used as positive growth controls. The fractional inhibitory concentration (FIC) index of various antibiotics in combination with EPIs was also calculated for CdA and CdunA cells. The FIC was calculated by dividing the MIC of the tested agent in combination with the MICs of the tested agent obtained when agent used alone.
The FIC index was interpreted as indicating a synergistic effect when it was ≤0.5, as additive or indifferent when it was >0.5 and 2.0, and as antagonistic when it was >2.0 [2]. A twofold or greater reduction in MIC values of various antibiotics after addition of EPIs was considered significant. Thus, the minimum effective concentration of the inhibitor (minimal concentration of EPI that produced the maximal reduction in MIC of antibiotics) was found to be 1/4th of the MIC of inhibitors that was also confirmed by carrying out time kill studies (Data S1.). Therefore, in the subsequent experiments, EPIs were used at 1/4th of their MICs.
Assessment of membrane destabilizing effect of EPIs
Membrane-destabilizing effect of EPIs, if any, was evaluated using NPN permeabilization [17]. Briefly, 100 μl of 107 CFU/ml CdA Ty2 cells were pre-treated with EPIs (at their different MICs) for 2 h at 37 °C. After treatment, cells were recovered and re-suspended in PBS. A total of 200 μl EPI(s) treated CdA Ty2 cells and 10mM 1-N-phenylnapthylamine (NPN) were pipette out in a 96-well plate. Fluorescence intensity was recorded in Genetix microtiter plate reader at 350 nm excitation and 420 nm emission wavelengths. Untreated cells were considered as control.
Assessment of relative gene expression in the CdA cells in the presence of EPIs
The impact of the EPIs at 1/4th MIC on the relative expression of soxS, marA, acrB, and tolC in the CdA cells was assessed using the same qRT-PCR conditions as mentioned above.
Toxicity assays of EPIs
Hemolysis assay
Assay was performed using the standard protocol of Ghosh et al. [18]. Heparinized human blood was washed thrice with PBS by centrifugation at 900g 4 °C for 5 min. Fifty microliters of 2×108 RBC/ml were added to the fresh Eppendorf tubes already containing 50 μl of EPIs at their 1/4th MIC. The tubes containing reaction mixture were kept at room temperature for 2 h and after centrifugation at 900g for 5 min, the activity was expressed in terms of hemoglobin leakage in the suspension which was monitored by measuring absorbance at 541 nm. PBS-treated cells were taken as a positive control and percentage of lysis was calculated for each sample after treating cells with distilled water.
Cytotoxicity assay
The toxicity of EPIs was assessed in human pancreatic carcinoma MIA PaCa-2 cell line. Briefly, 105confluent cells/ml were seeded in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal calf serum (FCS) in 6-well microtiter plate and incubated for 24 h at 37 °C in a humidified incubator with 5% CO2. These cells were then exposed to the EPIs at their 1/4th MIC and were examined after 24 h incubation. Untreated cells served as control. Cells were analyzed using light microscopy for morphological alterations [19].
Elucidation of activity of efflux pumps using fluorometric analysis of ethidium bromide efflux
Ethidium bromide (EtBr) is a known substrate for efflux pumps in family Enterobacteriaceae and is widely recognized as the best candidate for monitoring the efflux pump activity [20, 21], as well as for elucidating the potential of efflux pump inhibitors (EPIs) in Gram-negative bacteria [22, 23]. Therefore, in the present study, in an attempt to analyze the effect of different EPIs (at their 1/4 MIC), we monitored the kinetics of EtBr accumulation and efflux in CdA Ty2 cells by fluorimetric method. The concentration of EtBr, EPIs, and glucose used were 1 mg/l (MIC of EtBr-10 mg/l), 1/4th MICs, and 0.4%, respectively. Before determining the efflux activity, it is necessary to optimize the conditions of EtBr accumulation; thus, the assay was performed in two steps in the presence of glucose which has the tendency to stimulate the efflux of EtBr by energizing the efflux pumps:
Accumulation assay
CdA Ty2 cells were grown in 20 ml nutrient broth to an OD600 nm of 0.6. The culture was centrifuged at 13000rpm for 3 min. The supernatant was discarded; the pellet obtained was once washed and resuspended in PBS. Again, the OD600 nm was readjusted to 0.3 with PBS. To the one set of 1 ml bacterial suspension, glucose, EtBr, and EPI(s) were added. Other sets, (i) no EPI(s) + no glucose and (ii) no EPI(s) + glucose, served as controls. Fluorescence was recorded at excitation and emission wavelengths of 530 nm and 585 nm for 35 min using a multimode microplate reader (BioTek). The assay was repeated thrice with reproducible results.
Efflux inhibition assay
Based on the results obtained from the accumulation studies, EtBr was accumulated in the CdA Ty2 cells in the presence of NMP (EPI that caused highest level of EtBr accumulation), by incubating tubes containing the reaction mixture for 45 min in order to get maximum EtBr retention as no glucose was added. Briefly, after accumulation of EtBr, CdA Ty2 cells were recovered by centrifugation at 13000 rpm for 3 min and all the NMP was removed. The O.D600 nm of the bacterial suspension was readjusted to 0.3 and 100 μl cell suspension was transferred to a 96-well (black) plate and different EPIs were added. Later, glucose was added, which stimulates the efflux of EtBr by energizing the efflux pumps [23]. Replicates that did not receive an EPI served as a control for the assessment of EtBr efflux. The fluorescence was measured at excitation and emission wavelengths of 530 nm and 585 nm, respectively, at 1-min intervals for 45 min and the relative fluorescence was calculated. The experiment was performed thrice and in triplicates.
Assessment of interaction of NMP with CdA cells using field emission scanning electron microscopy
For FE-SEM studies, 106 CFU/ml log phase CdA Ty2 cells were incubated with 100, 200, and 400 mg/l NMP for 2 h at 37 °C. After incubation, pellets were collected by centrifugation at 1957g for 5 min and washed five times with 0.5 mM phosphate buffered saline (PBS), pH 7.2. Subsequently, the bacterial pellets were chemically fixed with 500 μl of 2% glutaraldehyde (v/v) for 1 h at 4°C. After washing the pellet twice with PBS, it was dehydrated with graded ethanol bath (10, 30, 50, 70, 90, and 100% for 5–10 min each). Thereafter, the suspension was transferred onto a glass cover slip and then subjected to air drying. Cover slips containing samples were mounted on aluminum stubs, coated with gold palladium at a thickness of 200 Å and examined for any change in morphology [2].
Validation of NMP antibiotic combination
Freshly procured clinical serovar Typhi strains were adapted to cadmium by sequentially propagating in cadmium supplemented media. Validation of selected combination was done by determining MIC against CdA clinical isolates of serovar Typhi, as described above. The FIC index of various antibiotics in combination with EPIs was also calculated for CdA isolates.
Statistical analysis
Data were expressed as mean ± standard deviation of three independent experiments. Statistical data analysis was done using SPSS 16.2 for Windows (SPSS Inc., Chicago, IL) and GraphPad Prism 5 software by evaluating significance of data using Student’s two sample t test and one-way analysis of variance (ANOVA). During data analysis, p-values of 0.001, 0.01, 0.05, or less (p<0.001, p<0.01, p˂0.05) were considered significant.
Results
Quantification of the relative gene expression of soxS, marA, acrB, and tolC
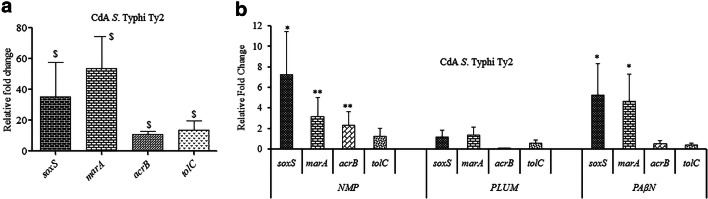
The qRT-PCR studies revealed significant (p<0.001) upregulation in the expression of soxS, marA, acrB, and tolC corresponding to 35.4, 53.9, 10.8, and 13.7-fold, respectively, in CdA cells as compared to CdunA Ty2 cells (Fig. 1a).
Fig. 1.
Quantification of the relative mRNA expression levels of different genes in CdA Ty2 cells (a) in comparison to CdunA Ty2 cells ($p<0.001) (b) upon treatment with different EPIs (**p<0.001 and *p<0.05 as compared to untreated CdA Ty2 cells). CdunA cells were taken as the control with basal level expression indicated as 1
Determination of MICs of EPIs
For both CdA and CdunA Ty2 cells, MICs of PAβN and Plumbagin were evaluated to be more than 200 mg/l whereas for it was observed to 400mg/l for NMP. Such high concentrations of these agents indicate that the agents do not have any direct antimicrobial activity.
Efficacy of antibiotics in the presence of EPIs
All the EPIs potentiated the activity of antibiotics against CdA Ty2 cells (Table 1). In case of CdA Ty2 cells, upon treatment with NMP (1/4th MIC), maximum reduction ~16-fold in MIC of ampicillin and chloramphenicol was observed, respectively. With PAβN, for ciprofloxacin, chloramphenicol, and ceftizoxime ~8, 16 and eight-fold decrease in the MIC was observed. With PAβN (1/4th MIC), maximum 16-fold MIC reduction of chloramphenicol was observed, while with plumbagin (1/4th MIC) 32-fold MIC reduction of chloramphenicol was recorded. Potential of EPIs to reduce the effective concentration of antibiotics indicate that they may hinder with the efflux of the antibiotics.
Table 1.
Minimum inhibitory concentration (MIC) values and fractional inhibitory concentration indexes (FICI) (mg/l) of different antibiotics in the presence of EPIs against CdA cells
| CdA Ty2 cells | |||||||
|---|---|---|---|---|---|---|---|
| Antibiotics | MIC (mg/l) | MIC of antibiotics in the presence of fixed concentrations of EPI | |||||
|
PAβN (50 mg/l) |
FICI (PAβN + antibiotics) |
NMP (100 mg/l) |
FICI (NMP + antibiotics) |
Plumbagin (50 mg/l) |
FICI (Plum + antibiotics) |
||
| AMP | 16 | 2 (8)* | 0.375 | 1 (16) | 0.3125 | 2 (8) | 0.375 |
| CIPRO | 1 | 0.125 (8) | 0.375 | 0.125 (8) | 0.375 | 0.25 (4) | 0.5 |
| CHLOR | 8 | 0.5 (16) | 0.3125 | 0.5 (16) | 0.3125 | 0.25 (32) | 0.28 |
| CEFTI | 0.0625 | 0.0156 (4) | 0.5 | 0.0078 (8) | 0.375 | 0.0156 (4) | 0.5 |
*(...) value within the round brackets indicates fold reduction in the MIC value
Effect of EPIs on the membrane stabilization
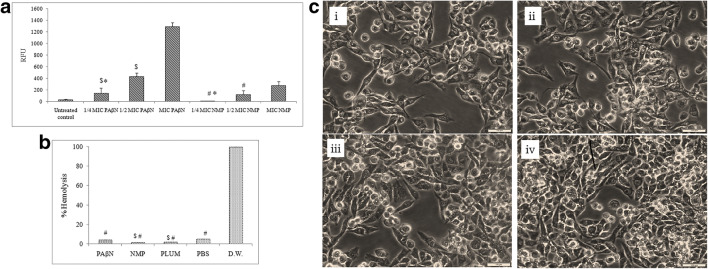
Though at higher concentration of NMP and PAβN, membrane destabilizing effect was observed which was indicated by increase in the fluorescence of NPN, but at 1/4th of MICs, no significant change (p>0.001) with respect to control was observed (Fig. 2a). During the assay, the data generated was insignificant for plumbagin, as decrease in the fluorescence intensity (values in negative) as compared to the control was observed with increase in the concentration of plumbagin. This can be attributed to the intrinsic fluorescence properties of plumbagin that led to the spectral overlap of plumbagin and NPN, thus, quenching the fluorescence signal.
Fig. 2.
a Effect of different concentrations of EPIs (NMP and PAβN) on the outer membrane permeability of the CdA Salmonella Typhi Ty2 cells evaluated using NPN assay. Values are expressed as mean ± standard deviation of three individual values ($p< 0.001 versus MIC of PAβN, #p< 0.01 versus MIC of NMP, *p>0.01versus untreated control). b Percentage hemolysis of RBC after treatment with EPIs. Values are expressed as mean ± standard deviation of three individual values ($p< 0.01 versus PBS and #p< 0.001 versus D.W). c In vitro cytotoxicity assay using human MIA PaCa-2 cell lines: cell line (i) control, (ii) NMP (100mg/l), (iii) PAβN (50mg/l), and (iv) plumbagin (50mg/l)
Expression of genes encoding efflux pump in the presence of EPIs
The effect of EPIs on the expression of selected genes was explored quantitatively using qRT-PCR. These agents at 1/4th MICs upregulated the expression of tolC and acrB genes which were shown to govern (Fig. 1b) the efflux activity in CdA cells. Also, the upregulation in the expression of transcriptional regulators marA and soxS was evident in EPI-exposed CdA Ty2 cells. Though the fold increase was there in the presence of EPIs, it was significantly (p<0.001 and p<0.05) upregulated when NMP was used as inhibitor. It may be inferred from these observations that these agents interact with the efflux pumps.
Thus, the above mentioned parameters showed that (i) based upon high MIC values obtained, EPIs are not direct acting; (ii) NPN studies showed that at 1/4th MIC, EPIs does not cause membrane destabilization; (iii) gene expression analysis shows that EPIs at 1/4th MIC are specific in their action in binding with the efflux pumps.
Non-toxicity of EPIs
Upon interaction with the normal red blood cells, the observations showed that EPIs (at their 1/4th MIC) (p<0.01) were devoid of any hemolytic activity (Fig. 2b). Moreover, cytotoxicity assay using human MIA PaCa-2 cell lines showed no toxicity of EPIs (Fig. 2c) indicating absence of any cell damaging properties at their 1/4th MIC. The results of the SEM analysis, hemolysis, and cytotoxicity assay support the fact that EPIs at selected concentration fulfills the criteria of being solely an efflux inhibitor, as no direct antimicrobial activity was observed.
EtBr accumulation and efflux inhibition assay
Ethidium bromide accumulation and efflux real-time fluorometric assay was conducted for demonstration of strength with which different EPIs inhibit the efflux activity of CdA cells.
Accumulation assay
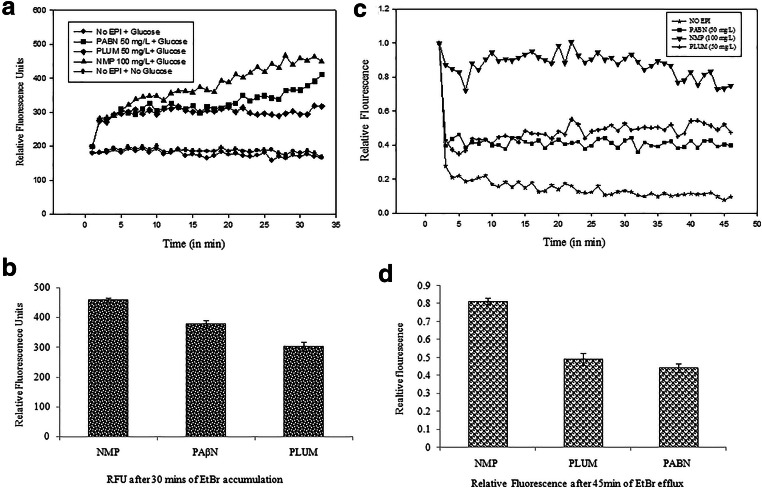
In this assay, glucose was added in the reaction mixture right in the beginning in order to see the effect of EPIs and to select the best inhibitor causing maximum accumulation, indicated by increase in the fluorescence. The level of EtBr accumulation was lowest in the control groups where no EPI treatment was given to the cells. However, out of the three EPIs, addition of NMP at 100 mg/l resulted in maximum ethidium bromide accumulation (p< 0.001), followed by PAβN (50 mg/l) (p<0.001), whereas plumbagin (50 mg/l)-treated cells showed the lowest level of accumulation (p<0.001). The RFU calculated for NMP, PAβN, and plumbagin were 459, 365, and 294, respectively, after 30 min of EtBr accumulation (Fig. 3a and b).
Fig. 3.
a Effect of EPIs on the accumulation EtBr (1mg/l) in CdA Ty2 cells ($p< 0.001 versus no EPIs). b Relative fluorescence units for different EPIs after EtBr accumulation. c Efflux of ethidium bromide by CdA Ty2 cells. Concentrations of PAβN, plumbagin, and NMP are at 1/4th their MICs ($p< 0.001 versus no EPIs, #p< 0.001 versus plumbagin, %p< 0.001 versus PAβN). d Relative fluoresence for different EPIs after EtBr efflux
Efflux inhibition assay
In this assay, first, the accumulation of EtBr was done in the presence of the selected inhibitor (in absence of glucose) considering its appreciable binding with the efflux pumps resulting in the retention of EtBr. Then, the inhibitor was removed from the supernatant by centrifugation. It was followed by the addition of all the EPIs separately along with glucose to evaluate whether there is an efflux of the EtBr, which is indicated by reduction in the fluorescence. Under the selected conditions, only the control cells without EPIs extruded the EtBr, resulting in significant decrease in fluorescence over the assay period, thereby indicating that EtBr efflux from the bacterial cells. Compared to this, in the presence of each EPIs, fluorescence was not significantly reduced (p<0.001) reflecting a strong interference in the efflux of ethidium bromide by EPI(s). Thus, stability in the relative fluorescence of NMP throughout the period of 45 min even in the presence of glucose was observed as compared to other inhibitors, suggesting that NMP was most effective in inhibiting the efflux of ethidium bromide out of the CdA cells, followed by plumbagin, which proved to be better than PAβN (Fig. 3c and d).
Effect of NMP on the morphology of CdA cells
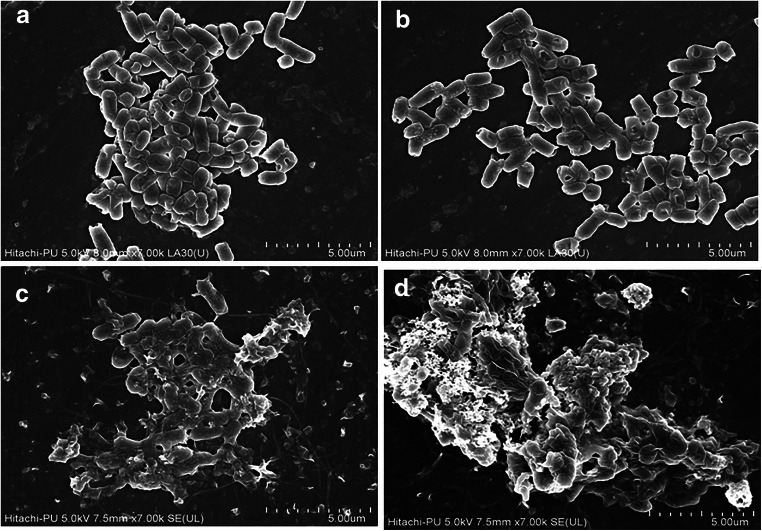
FE-SEM analysis of untreated cells of CdA Ty2 cells (Fig. 4) showed intact surface suggesting no alterations. Similarly, CdA cells treated with NMP (at 1/4th MIC) showed no alteration in the cellular morphology, as compared to control cells. However, at 1/2 MIC and MIC, a dose-dependent damaging effect was observed. Thus, NMP at its 1/4th MIC exhibited no direct antimicrobial activity (DAA).
Fig. 4.
Field emission scanning electron micrographs of CdA Ty2 cells upon treatment with various concentrations of NMP (a control, b ¼ MIC, c 1/2 MIC, and d MIC)
Validation of NMP-antibiotic combination
Out of ten clinical strains (as per disk diffusion and microbroth dilution analysis, seven CdunA clinical strains were found to be sensitive and three CdunA strains were intermediately sensitive to selected antibiotics namely cefotaxime, co-trimoxazole, chloramphenicol, nalidixic acid, norfloxacin, oxytetracycline, ceftazidime, ceftriaxone, ciprofloxacin, ceftizoxime, ampicillin) (data of microbroth dilution testing provided in Table 2). After adaptation, maximum of ~8, 16, 16, and four-fold increase in the MIC of ampicillin, ciprofloxacin, chloramphenicol, and ceftizoxime, respectively, was observed in clinical CdA Typhi strains. In the CdA clinical isolates, the combination of NMP-ampicillin, NMP-ciprofloxacin, NMP-chloramphenicol showed synergistic effect against all the isolates, whereas NMP and ceftizoxime showed either synergistic or additive effect for different clinical isolates (Table 2). MIC values obtained after using the antibiotics-NMP combination against CdA cells were close to the MIC values observed against CdunA cells. NMP-ampicillin, NMP-ciprofloxacin, and NMP-chloramphenicol combinations acted synergistically against CdA cells. Although NMP-ceftizoxime acted synergistically against most of the CdA strains, its additive effect was also observed against two CdA strains.
Table 2.
Minimum inhibitory concentration (MIC) values of different antibiotics against cadmium unadapted and cadmium adapted clinical S. enterica serovar Typhi isolates. Fractional inhibitory concentration indexes (FICI) of different antibiotics in the presence of NMP (1/4th MIC) against CdA clinical S. enterica serovar Typhi isolates
| Sr. No. |
CdunA cells | CdA cells + NMP (100mg/l) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/l) | MIC (mg/l) | FICI | MIC (mg/l) | FICI | MIC (mg/l) | FICI | MIC (mg/l) | FICI | |||||||||
| Isolates | AMP | CIPRO | CHLOR | CEFTI | AMP | AMP+NMP | CIPRO | CIPRO+NMP | CHLOR | CHLOR +NMP | CEFTI | CEFTI+ NMP | |||||
| 1. | 9485/18 | 2 | 0.25 | 2 | 0.03125 | 4 (2) | 0.5 | 0.375 | 0.5 (2) | 0.125 | 0.5 | 4 (2) | 0.5 | 0.375 | 0.0625 (2) | 0.0156 | 0.5 |
| 2. | 9170/18 | 2 | 0.25 | 2 | 0.03125 | 16 (8) | 2 | 0.375 | 1 (4) | 0.125 | 0.375 | 8 (4) | 1 | 0.375 | 0.125 (4) | 0.0625 | 0.75 |
| 3. | 9907/18 | 2 | 1 | 2 | 0.0156 | 8 (4) | 1 | 0.375 | 2 (2) | 0.5 | 0.5 | 4 (2) | 05 | 0.375 | 0.0625 (4) | 0.03125 | 0.75 |
| 4. | 9331/18 | 1 | 0.25 | 2 | 0.0625 | 4 (4) | 1 | 0.5 | 2 (8) | 0.125 | 0.3125 | 8 (4) | 1 | 0.375 | 0.0625 | 0.0156 | 0.375 |
| 5. | 10,817/18 | 2 | 0.25 | 1 | 0.0625 | 8 (4) | 2 | 0.5 | 2 (8) | 0.5 | 0.5 | 8 (8) | 2 | 0.5 | 0.125 (2) | 0.03125 | 0.5 |
| 6. | 10,346/18 | 4 | 0.125 | 0.5 | 0.0625 | 16 (4) | 2 | 0.375 | 0.5 (4) | 0.125 | 0.5 | 8 (16) | 2 | 0.5 | 0.25 (4) | 0.0625 | 0.5 |
| 7. | 10,335/18 | 2 | 0.5 | 4 | 0.03125 | 8 (4) | 1 | 0.375 | 2 (4) | 0.125 | 0.3125 | 16 (4) | 4 | 0.5 | 0.0625 (2) | 0.0156 | 0.5 |
| 8. | 657/19 | 1 | 0.125 | 2 | 0.03125 | 4 (4) | 0.5 | 0.375 | 2 (16) | 0.5 | 0.5 | 8 (4) | 2 | 0.5 | 0.125 (4) | 0.0156 | 0.375 |
| 9. | 668/18 | 1 | 1 | 1 | 0.0156 | 8 (8) | 2 | 0.5 | 4 (4) | 0.5 | 0.375 | 8 (8) | 0.5 | 0.3125 | 0.0625 (4) | 0.0156 | 0.5 |
| 10. | 614/19 | 2 | 1 | 2 | 0.125 | 8 (4) | 2 | 0.5 | 2 (2) | 0.125 | 0.3125 | 4 (2) | 1 | 0.5 | 0.25 (2) | 0.0625 | 0.5 |
Discussion
Active efflux of antimicrobial compounds is one of the mechanisms which renders the pathogens multidrug-resistant (MDR). The widely distributed efflux pumps belonging to resistance-nodulation-division (RND) superfamily have broad specificity with a generalized role in protecting the cell from toxic compounds including antibiotics, biocides, organic solvents, and metals which are encountered in the environment and thus contribute to virulence within host [24–26]. Enhanced expression of two genes acrB and tolC (encoding AcrAB-TolC, a RND type tripartite pump in Salmonella [27, 28] along with other two genes marA and soxS which encode the transcriptional regulators MarA and SoxS [29] of this pump) was observed in CdA Salmonellae. In Salmonella and E. coli, soxS and marA have been reported to be under the control of soxRS and marRAB regulon which is activated under oxidative stress conditions [10]. Furthermore, these transcriptional activators have been found to activate acrAB transcription by binding to its promoter and contribute to increased antibiotic resistance in Gram-negative bacteria [30]. Thus, induction of overexpression of soxS and marA observed in the present study might have elevated the expression of AcrAB-TolC efflux pump leading to appearance of MDR phenotype in CdA Salmonellae which is in agreement with the study done by Alekshun, Levy [31].
An inverse relationship in the AcrAB expression and growth rate of E. coli has been reported by Eaves et al. [10] which strengthened our previous report wherein marked delay in various phases of growth were observed in the CdA cells [3]. Moreover, increased intracellular survival of CdA Salmonellae and enhanced biofilm forming potential observed in our earlier study might have also been attributed to the enhanced expression of acrB and marA as reported previously by other workers also for E. coli [32].
After observing an increased expression of efflux pumps in CdA Salmonellae, attempts were made to identify possible inhibitors which can disarm these efflux pumps. Keeping this in mind, EPI potential of PAβN, NMP, and Plumbagin was explored. The effect of these EPIs along with the antibiotics was also evaluated against CdA Ty2 and Cdun Ty2 strain. Previously, we reported that CdA serovar Typhi Ty2 became resistant to antibiotics as per its MICs, as compared to MICs observed before its adaptation (in CdunA cells) [3]. Therefore, in continuation to this, when all the EPIs at their sub-MICs were used along with ampicillin, ciprofloxacin, chloramphenicol, and ceftizoxime, the MICs of the agents in combination were significantly decreased to values close to the ones required for eliminating the CdunA Ty2 cells (Table S2). Thus, selected concentrations of the combination of EPI and antibiotics were found to be reasonably effective, as blocking of efflux pumps by the EPI might have helped in retention of the antibiotics inside the CdA cells. On the whole, the combination acted powerfully that it could eliminate the bacterial population consisting of a consortium of sensitive (CdunA) as well resistant (CdA) Salmonellae.
Increased efficacy of antibiotics in the presence of EPIs was ascertained by time kill assay using EPIs at their sub-MICs along with antibiotics (supplementary Data S1.). Results revealed that NMP had the best potential in eliminating the resistant CdA Ty2 cells. Although both NMP and PAβN have been reported as EPIs of RND efflux pumps, PAβN acted intermediately. This observation was supported by the study carried out by Schumacher et al. [33] wherein it was demonstrated that the activity of PAβN is different from that of NMP, suggesting different modes of action of both EPIs. Though the effect of plumbagin was faster, it was found to be least effective as it might not have sustained in the medium for a prolonged period of 24 h which can be ascribed to its volatile nature as well as its conversion to less toxic methylated form by the bacterial cells [34–36]. Furthermore, the role of EPIs to act as membrane destabilizing agents at a particular concentration has already been reported by Misra et al. (2015). In view of this, when evaluated using NPN assay, no such membrane destabilization effect was observed at 1/4th MIC of EPIs.
When quantitatively determined, further enhanced expression of acrB, tolC, marA, and soxS in EPI-exposed CdA Ty2 cells was observed, thereby indicating the interaction of these EPIs (at their 1/4th MIC) with the bacterial efflux pumps, which was maximal in case of NMP. A strong binding of the NMP to the efflux pump might have caused a profound effect on the gene expression of AcrAB-TolC and its transcriptional regulators in the CdA Ty2 cells. Within the cell, NMP has been reported to interfere with the normal process of pumping, by moving from the proximal to the distal pocket of AcrAB-TolC, thus straddling the G-loop [37]. This binding would have resulted in intracellular stress in response to which the cells again initiate transcription of the efflux genes in an attempt to get rid of the dual pressure conditions of cadmium as well as inhibitors. Our results are in agreement with the observations of Anes et al. [38] who reported that NMP upregulates the expression of a cascade of genes involved in activity of AcrAB-TolC efflux pump in Klebsiella pneumoniae. All these responses in the presence of different EPIs show the impeccable efforts put in by the bacterium in order to maintain cellular homeostasis during EPI stress.
From the above mentioned data, it is indicated that (i) EPIs do not have any direct antimicrobial activity, (ii) do not destabilize the outer membrane, and (iii) as they further upregulate the expression of efflux genes which indicates their interaction with the efflux pumps at 1/4th of their MIC. Therefore, to substantiate the efflux pump binding potential of the EPIs, we assessed the accumulation and efflux inhibition of ethidium bromide, which is also considered as a substrate of the efflux pumps [22]. The difference observed in efficacy of these inhibitors may be due to the structural differences in the binding pockets of these molecules which directly interact with the bacterial efflux pumps. In addition to this, FE-SEM studies using NMP at different concentrations also strengthened the fact that 1/4th MIC of NMP did not have any damaging effect on the CdA Ty2 cells. Thus, out of all the inhibitors used, NMP was singled out as the best EPIs in inhibiting the efflux potential of even the overexpressed efflux pump AcrAB-TolC in CdA Ty2 cells. The above observations were further strengthened in the CdA clinical Salmonella Typhi isolates when NMP acted synergistically with ampicillin, ciprofloxacin, and chloramphenicol thereby reducing the effective concentration(s) of all the antibiotics.
This study thus indicates the involvement of AcrAB-TolC efflux pump which was found to be directly upregulated upon cadmium exposure, thereby leading to cadmium-induced antibiotic resistance in Salmonella enterica serovar Typhi Ty2 as per the co-selection theory. Both chemical and biological inhibitors, devoid of any toxicity, at selected concentration interacted with AcrAB-TolC efflux pump (ethidium bromide assay) and upregulated the cascade of efflux related genes, signifying the extent of generation of stressful conditions in CdA cells. All the agents at the selected concentrations restored the MIC of antibiotics; however, the limitations associated with PAβN and plumbagin, and left NMP to be the most suitable EPI which rendered the efflux activity of CdA cells ineffective. The concept was validated using CdA clinical isolates, wherein the reversal in the efficacy of antibiotics when used in combination with NMP was observed. These observations suggest that NMP has a better MDR reversal affect than other the two inhibitors for cadmium adapted Salmonella enterica serovar Typhi isolates, which might be due to the inhibition of efflux potential of the AcrAB pump. Thus, the present study emphasizes on (i) the urgency of reframing the existing guidelines for management of waste disposal, especially the heavy metals, as the latter aid in maintenance of pool of antibiotic-resistant determinants in the environment and (ii) the future studies that may be designed with an aim to explore suitable cost-effective and eco-friendly agents.
Supplementary Information
(DOCX 405 kb).
Funding
This work was supported by the financial assistance provided by the Indian Council of Medical Research (ICMR), India, to Prof. Praveen Rishi (Grant No. 5/3/3/9/2013-ECD-I). The support of Department of Science and Technology (DST), India, is duly acknowledged for providing fellowship to Ujjwal Jit Kaur (IF120542) for the initial phase.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Castro-Sánchez E, Moore LS, Husson F, Holmes AH. What are the factors driving antimicrobial resistance? Perspectives from a public event in London, England. BMC Infect Dis. 2016;16:465. doi: 10.1186/s12879-016-1810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rishi P, Thakur R, Kaur UJ, Singh H, Bhasin KK. Potential of 2, 2′-dipyridyl diselane as an adjunct to antibiotics to manage cadmium-induced antibiotic resistance in Salmonella enterica serovar Typhi Ty2 strain. J Microbiol. 2017;55:737–744. doi: 10.1007/s12275-017-7040-0. [DOI] [PubMed] [Google Scholar]
- 3.Kaur UJ, Preet S, Rishi P. Augmented antibiotic resistance associated with cadmium induced alterations in Salmonella enterica serovar Typhi. Sci Rep. 2018;8:12818. doi: 10.1038/s41598-018-31143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy SK, Kumari N, Gupta S, Pahwa S, Nandanwar H, Jachak SM. 7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against Mycobacterium smegmatis mc2 155. Eur J Med Chem. 2013;66:499–507. doi: 10.1016/j.ejmech.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Mata MT, Baquero F, Perez-Diaz JC. A multidrug efflux transporter in Listeria monocytogenes. FEMS Microbiol Lett. 2000;187:185–188. doi: 10.1111/j.1574-6968.2000.tb09158.x. [DOI] [PubMed] [Google Scholar]
- 6.Aendekerk S, Ghysels B, Cornelis P, Baysse C. Characterization of a new efflux pump, MexGHI-OpmD, from Pseudomonas aeruginosa that confers resistance to vanadium. Microbiology. 2002;148:2371–2381. doi: 10.1099/00221287-148-8-2371. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi S, Abe M, Kimoto M, Furukawa S, Nakazawa T. The dsbA-dsbB disulfide bond formation system of Burkholderia cepacia is involved in the production of protease and alkaline phosphatase, motility, metal resistance, and multi-drug resistance. Microbiol Immunol. 2000;44:41–50. doi: 10.1111/j.1348-0421.2000.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 8.Hernández A, Mellado RP, Martínez JL. Metal accumulation and vanadium-induced multidrug resistance by environmental isolates of Escherichia hermannii and Enterobacter cloacae. Appl Environ Microbiol. 1998;64:4317–4320. doi: 10.1128/AEM.64.11.4317-4320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perron K, Caille O, Rossier C, Van Delden C, Dumas JL, Köhler T. CzcR-CzcS, a two-component system involved in heavy metal and carbapenem resistance in Pseudomonas aeruginosa. J Biol Chem. 2004;279:8761–8768. doi: 10.1074/jbc.M312080200. [DOI] [PubMed] [Google Scholar]
- 10.Eaves DJ, Ricci V, Piddock LJ. Expression of acrB, acrF, acrD, marA, and soxS in Salmonella enterica serovar Typhimurium: role in multiple antibiotic resistance. Antimicrob Agents Chemother. 2004;48:1145–1150. doi: 10.1128/AAC.48.4.1145-1150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pagès JM, Masi M, Barbe J. Inhibitors of efflux pumps in Gram-negative bacteria. Trends Mol Med. 2005;11:382–389. doi: 10.1016/j.molmed.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Rishi P, Vij S, Maurya IK, Kaur UJ, Bharati S, Tewari R. Peptides as adjuvants for ampicillin and oxacillin against methicillin-resistant Staphylococcus aureus (MRSA) Microb Pathog. 2018;124:11–20. doi: 10.1016/j.micpath.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Chanana V, Majumdar S, Rishi P. Tumour necrosis factor α mediated apoptosis in murine macrophages by Salmonella enterica serovar Typhi under oxidative stress. FEMS Immunol Med Microbiol. 2006;47:278–286. doi: 10.1111/j.1574-695X.2006.00090.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohene-Agyei T, Mowla R, Rahman T, Venter H. Phytochemicals increase the antibacterial activity of antibiotics by acting on a drug efflux pump. Microbiologyopen. 2014;3:885–896. doi: 10.1002/mbo3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opperman TJ, Nguyen ST. Recent advances toward a molecular mechanism of efflux pump inhibition. Front Microbiol. 2015;6:421. doi: 10.3389/fmicb.2015.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venter H, Mowla R, Ohene-Agyei T, Ma S. RND-type drug efflux pumps from Gram-negative bacteria: molecular mechanism and inhibition. Front Microbiol. 2015;6:377. doi: 10.3389/fmicb.2015.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chusri S, Na-Phatthalung P, Siriyong T, Paosen S, Voravuthikunchai SP. Holarrhena antidysenterica as a resistance modifying agent against Acinetobacter baumannii: its effects on bacterial outer membrane permeability and efflux pumps. Microbiol Res. 2014;169:417–424. doi: 10.1016/j.micres.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh JK, Shaool D, Guillaud P, Cicéron L, Mazier D, Kustanovich I, Shai Y, Mor A. Selective cytotoxicity of dermaseptin s3 toward intraerythrocytic Plasmodium falciparum and the underlying molecular basis. J Biol Chem. 1997;272:31609–31616. doi: 10.1074/jbc.272.50.31609. [DOI] [PubMed] [Google Scholar]
- 19.Thakur R, Pathania P, Kaur N, Joshi V, Kondepudi KK, Suri RC, Rishi P. Prophylactic potential of cytolethal distending toxin B (CdtB) subunit of typhoid toxin against Typhoid fever. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-54690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jernaes MW, Steen HB. Staining of Escherichia coli for flow cytometry: influx and efflux of ethidium bromide. Cytometry. 1994;17:302–309. doi: 10.1002/cyto.990170405. [DOI] [PubMed] [Google Scholar]
- 21.Raherison S, Gonzalez P, Renaudin H, Charron A, Bebear C, Bebear CM. Increased expression of two multidrug transporter-like genes is associated with ethidium bromide and ciprofloxacin resistance in Mycoplasma hominis. Antimicrob Agents Chemother. 2005;49:421–424. doi: 10.1128/AAC.49.1.421-424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues L, Wagner D, Viveiros M, Sampaio D, Couto I, Vavra M, Kern WV, Amaral L. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J Antimicrob Chemother. 2008;61:1076–1082. doi: 10.1093/jac/dkn070. [DOI] [PubMed] [Google Scholar]
- 23.Paixão L, Rodrigues L, Couto I, Martins M, Fernandes P, De Carvalho CC, Monteiro GA, Sansonetty F, Amaral L, Viveiros M. Fluorometric determination of ethidium bromide efflux kinetics in Escherichia coli. J Biol Eng. 2009;3:18. doi: 10.1186/1754-1611-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcalde-Rico M, Hernando-Amado S, Blanco P, Martínez JL. Multidrug efflux pumps at the crossroad between antibiotic resistance and bacterial virulence. Front Microbiol. 2016;7:1483. doi: 10.3389/fmicb.2016.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco P, Hernando-Amado S, Reales-Calderon JA, Corona F, Lira F, Alcalde-Rico M, Bernardini A, Sanchez MB, Martinez JL. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms. 2016;4:14. doi: 10.3390/microorganisms4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nies DH. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 27.Baucheron S, Imberechts H, Chaslus-Dancla E, Cloeckaert A. The AcrB multidrug transporter plays a major role in high-level fluoroquinolone resistance in Salmonella enterica serovar Typhimurium phage type DT204. Microb Drug Resist. 2002;8:281–289. doi: 10.1089/10766290260469543. [DOI] [PubMed] [Google Scholar]
- 28.Whitehead RN, Overton TW, Kemp CL, Webber MA. Exposure of Salmonella enterica serovar Typhimurium to high level biocide challenge can select multidrug resistant mutants in a single step. PLoS One. 2011;6:e22833. doi: 10.1371/journal.pone.0022833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Regan E, Quinn T, Pagès JM, McCusker M, Piddock L, Fanning S. Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar enteritidis: involvement of RamA and other global regulators. Antimicrob Agents Chemother. 2009;53:1080–1087. doi: 10.1128/AAC.01005-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrari RG, Galiana A, Cremades R, Rodríguez JC, Magnani M, Tognim MCB, Oliveira TC, Royo G. Expression of the marA, soxS, acrB and ramA genes related to the AcrAB/TolC efflux pump in Salmonella enterica strains with and without quinolone resistance-determining regions gyrA gene mutations. Braz J Infect Dis. 2013;17:125–130. doi: 10.1016/j.bjid.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alekshun MN, Levy SB. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob Agents Chemother. 1997;41:2067–2075. doi: 10.1128/AAC.41.10.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soto SM. Role of efflux pumps in the antibiotic resistance of bacteria embedded in a biofilm. Virulence. 2013;4:223–229. doi: 10.4161/viru.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schumacher A, Steinke P, Bohnert JA, Akova M, Jonas D, Kern WV. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Enterobacteriaceae other than Escherichia coli. J Antimicrob Chemother. 2005;57:344–348. doi: 10.1093/jac/dki446. [DOI] [PubMed] [Google Scholar]
- 34.Lin CN, Syu WJ, Sun WSW, Chen JW, Chen TH, Don MJ, Wang SH. A role of ygfZ in the Escherichia coli response to plumbagin challenge. J Biomed Sci. 2010;17:84. doi: 10.1186/1423-0127-17-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bothiraja C, Joshi PP, Dama GY, Pawar AP. Rapid method for isolation of plumbagin, an alternative medicine from roots of Plumbago zeylanica. Eur J Integr Med. 2011;3:39–42. doi: 10.1016/j.eujim.2011.02.008. [DOI] [Google Scholar]
- 36.Sumsakul W, Plengsuriyakarn T, Na-Bangchang K. Pharmacokinetics, toxicity, and cytochrome P450 modulatory activity of plumbagin. BMC Pharmacol Toxicol. 2016;17:50. doi: 10.1186/s40360-016-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XZ, Plésiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin Microbiol Rev. 2015;28:337–418. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anes J, Sivasankaran SK, Muthappa DM, Fanning S, Srikumar S. Exposure to sub-inhibitory concentrations of the chemosensitizer 1-(1-naphthylmethyl)-piperazine creates membrane destabilisation in multi-drug resistant Klebsiella pneumoniae. Front Microbiol. 2019;10:92. doi: 10.3389/fmicb.2019.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 405 kb).