Abstract
Purpose
Some women undergoing stimulated cycles have elevated serum progesterone (P) on the day of ovulation trigger, but its effect on embryo quality is unclear. We analyze embryo quality among patients with high and low serum P undergoing preimplantation genetic testing for aneuploidy (PGT-A).
Methods
This retrospective study included 1597 patients divided into two groups by serum P values: < 1.5 ng/mL or ≥ 1.5 ng/mL. A gonadotrophin-releasing hormone (GnRH) antagonist protocol was established for each patient. Serum P levels were measured on the day of triggering. Propensity score matching and Poisson regression were done. Age, body mass index, and ovarian sensitivity index were also compared.
Results
Elevated serum P was not significantly associated with euploid embryo rate or other embryo-quality variables evaluated in our study. Age was the only variable associated with euploidy rate (per MII oocyte, P < 0.001; per biopsied embryo, P = 0.008), embryo biopsy rate (P < 0.001), absolute number of euploid embryos (P = 0.008), and top-quality embryo rate (P = 0.008). Categorical variables decreased in value for every year of increased age in patients with high serum P.
Conclusions
Elevated serum P did not affect the number of euploid and good-quality embryos for transfer in GnRH antagonist intracytoplasmic sperm injection (ICSI) cycles. Contrary to the clear influence of premature P elevation on endometrial receptivity based on literature, our results may help to tip the balance towards the absence of a negative effect of P elevation on embryo competence. More studies are needed to fully understand the effect of P elevation on reproductive outcomes.
Keywords: Progesterone, Euploidy, Blastocyst, Embryo quality, PGT-A
Introduction
Progesterone (P) elevation in the late follicular phase occurs in about 2 to 35% of patients who undergo stimulated cycles [1–3]. The effect that this premature elevation may have on clinical outcomes has been studied in recent decades. In 1991, Schoolcraft et al. [4] described that high serum P values on the day of human chorionic gonadotrophin (hCG) administration negatively affects clinical pregnancy. Since then, many studies have attempted to explain this relationship [3], resulting in many hypotheses regarding the increased P level. Most plausibly, P may increase due to high follicle-stimulating hormone (FSH) concentrations and an excess number of follicles after stimulation [5].
Women undergoing a fresh autologous cycle who present high P values on the day of triggering have decreased pregnancy rates compared to women who undergo a freeze-all embryos policy [6]. This is likely due to asynchrony between the embryo and the endometrium, as a result of changes during endometrial maturation [7]. Indeed, significant endometrial changes at the gene expression level have been associated with premature P elevation in the late follicular phase, which may displace the endometrial receptivity window [8, 9].
Serum P elevation at the end of the follicular phase may also negatively affect embryo viability. However, available evidence on the impact of circulating P levels on oocyte and embryo quality following stimulation is inconclusive. Several clinical evidences support a negative impact on embryo quality when P is above 1.5 ng/mL on the day of hCG administration [10–12], while other reports claim to see no effect [13–17]. Some studies [13, 18] that did not observe an association between elevated P and embryo quality described a threshold value lower than 1 ng/mL, value that seems to have been chosen arbitrarily. Meanwhile, the ones observing an association with poorer clinical outcomes use higher reference values [1].
However, there is still no consensus on the cut-off value of P. In a meta-analysis of more than 60,000 cycles, proposed values among the different papers to act as a threshold P on the day of hCG varied from 0.5 to 3.0 ng/mL [3], and the methodology used for the measurement of P varied as well. P is measured mainly in blood; however, the intrafollicular measurement is presented as a more appropriate representation on the intrinsic follicular properties. Shufaro et al. [19] discussed the origin of elevated blood P and proposed a new index to measure it based on the average P secretion per follicle instead of total blood P. These considerations warrant new studies.
To determine whether there is an association between serum P values and embryo chromosomal constitution, we analyzed whether serum P values determined on the day of ovulation trigger affect embryo quality. We assessed euploidy rate and other clinical outcomes such as biopsy rate, top-quality embryo rate, absolute number of euploid embryos, and live birth rate in patients undergoing preimplantation genetic testing for aneuploidy (PGT-A). Advancing the understanding of the ovarian response and the gonadotrophin dose in stimulation protocols will help elucidate the complex mechanisms that contribute to elevated P in the late follicular phase and its influence on pregnancy outcomes.
Materials and methods
Study population
This study was retrospective and non-interventional, involving a single-center cohort of patients who underwent PGT-A at our fertility center between January 2016 and December 2018. A total of 1597 patients were enrolled in the study (see Table 2). No other inclusion/exclusion criteria were applied. Patient age ranged from 25 to 46 years old. The study was approved by the Ethical Committee of Clinical Research at IVI Valencia (1503-VLC-017-AM).
Table 2.
Baseline characteristics of the study population before and after propensity score matching
| Parameter Mean ± SD |
Unmatched | Matched | ||
|---|---|---|---|---|
| P < 1.5 ng/mL (n = 1465) |
P ≥ 1.5 ng/mL (n = 132) |
P < 1.5 ng/mL (n = 36) |
P ≥ 1.5 ng/mL (n = 36) |
|
| Age (years) | 38 ± 3 | 37 ± 4 | 37 ± 4 | 37 ± 4 |
| BMI (kg/m2) | 23.3 ± 4 | 22.6 ± 3.4 | 22 ± 2.6 | 22 ± 3.6 |
| Progesterone (ng/mL) | 0.6 ± 0.4 | 2.1 ± 0.7 | 0.7 ± 0.4 | 2 ± 0.7 |
| Ovarian sensitivity index | 4.6 ± 5.9 | 4.7 ± 4.6 | 4.7 ± 4.5 | 4.7 ± 4.6 |
| Estradiol (pg/mL) | 2176 ± 1616 | 3956 ± 2205 | 2759 ± 1369 | 2971 ± 2199 |
| Cause of infertility (%) | ||||
| Advanced age | 67.3 | 52.3 | 52.5 | 50 |
| Implantation failure | 10.4 | 19.7 | - | – |
| Male factor | 7.9 | 7.6 | 22.2 | 30.6 |
| Recurrent miscarriage | 5.7 | 9.1 | 3.1 | - |
| Genetic | 8.4 | 9.8 | 22.2 | 19.4 |
| Others | 0.3 | 1.5 | - | – |
| MII oocytes retrieved | 9 ± 6 | 16 ± 9 | 14 ± 8 | 15 ± 9 |
| Fertilized oocytes | 6 ± 5 | 11 ± 7 | 11 ± 7 | 11 ± 7 |
| Fertilization rate (%) | 66.7 | 68.8 | 78.6 | 73.3 |
| Oocyte origin (%) | ||||
| Fresh | 98 | 100 | 100 | 100 |
| Mixed | 2 | – | – | – |
| Biopsied embryos | 3 ± 3 | 5 ± 4 | 6 ± 5 | 6 ± 4 |
| Normal (%) | 41.4 | 45.3 | 44.1 | 43.8 |
| Abnormal (%) | 58.6 | 54.7 | 55.9 | 56.2 |
SD, standard deviation; BMI, body mass index
Ovarian stimulation
Ovarian stimulation was performed with antagonists following the respective protocol explained in the European Society of Human Reproduction and Embryology guidelines on ovarian stimulation for in vitro fertilization and intracytoplasmic sperm injection (ICSI) [20].
Hormone measurement
Serum P was determined on the day of triggering by electrochemiluminescence immunoassay (COBAS® e411 analyzer, Roche Diagnostics GmbH, Germany) with an analytical sensitivity of 0.03 ng/mL. Intra- and inter-assay coefficients of variation for the P determinations were 1.2–11.8% and 3.6–23.1%, respectively, for P values between 0.045 and 54.5 ng/mL. Hormones administered were FSH, luteinizing hormone (LH), and human menopausal gonadotrophin (hMG). All hormones were measured in international units (IU).
Fertilization assessment
MII oocytes were inseminated by ICSI following routine protocol established in the clinical practice. Fertilization was checked 16-20 hours post-insemination and considered successful when the presence of two pronuclear structures and two polar bodies were observed.
Embryo quality assessment
Embryo development post-fertilization was evaluated until the day of biopsy (day 5) under laboratory conditions (37 °C; 6% CO2; 5% O2). Evaluation incorporated the morphologic criteria established by the Association for the Study of Reproductive Biology [21]. Blastocysts were evaluated according to the following morphological parameters: (i) day of vitrification (5 vs. 6); (ii) the degree of blastocyst expansion based on cavitation, full expansion, or hatching out of the zona; (iii) inner cell mass quality (defined as A, B, C, or D); and (iv) trophectoderm quality (defined as A, B, C, or D).
Embryo biopsy
Each embryo was hatched on day 3 by laser technology (OC-TAX Navilase). Then, embryos were cultured in a fresh medium until day 5 or 6 of development. Embryos that developed beyond the full blastocyst stage were selected for biopsy, taken on day 5 of embryo development. Biopsies were performed via the pulling or flicking techniques described by de los Santos et al. [22]. Around five cells from the trophectoderm were collected to perform PGT-A by standard protocols.
Statistical analyses
Continuous and categorical variables are presented as mean ± standard deviation (SD) and percentages (%), respectively, based on sample distribution. To avoid bias, patients were divided into two groups according to serum P values on the day of ovulation trigger. In the absence of a clear consensus among authors on the specific threshold for P, the selected cut-off was 1.5 ng/mL. It was chosen based on our clinical practice, exposed in a previous report of poor clinical outcomes above this value [1]. P was categorized as either low (P < 1.5 ng/mL) or high (P ≥ 1.5 ng/mL). Other variables such as age, body mass index (BMI), ovarian sensitivity index (OSI), estradiol (E2), number of MII oocytes, and embryo ploidy were also considered in the model. The main outcome variable was the euploidy rate in relation to both the number of retrieved MII oocytes and the number of biopsied embryos. Other categorical parameters such as biopsy rate, defined as the number of embryo biopsies per number of retrieved MII oocytes; top-quality embryo rate, defined as the number of A + B quality embryos per biopsied embryos; the absolute number of euploid embryos; and the live birth rate, defined as the delivery of at least one live newborn (> 24 weeks of gestation) per cycle, were all measured. Three percent of participants repeated cycles.
Based on the considerable difference in sample size between groups of high and low P values, it was decided to add to the statistical analysis another approach based on the propensity score matching. The type of matching done was 1:1 without replacement. A nearest-neighbor matching algorithm was used to obtain the propensity score of each patient according to their baseline characteristics. Based on this value, patients with high and low serum P who shared a similar propensity score were matched. A t test confirmed no significant differences between both matched P groups for each variable included in the study. After propensity score matching, the association between serum P and outcome variables in both matched and unmatched patients were assessed by Poisson regression. OSI was calculated as the number of MII oocytes retrieved divided by the total dose of gonadotrophins administered (in this case FSH, LH and hMG; see Eq. 1). OSI values were multiplied by 1000 to present graphs > 1 and were log-transformed due to skewed distribution. During data analysis, a 95% confidence interval (CI) was calculated and P value ≤ 0.05 was considered statistically significant.
| 1 |
Results
Study population
The study included 1597 patients, of whom 1465 (91.7%) had serum P below 1.5 ng/mL and 132 (8.3%) had serum P above or equal to 1.5 ng/mL. Each baseline characteristic was calculated before and after the matching algorithm (Table 2). Age was the only variable with the same mean in the two matched groups (37±4 years), being advanced age the principal indicator of infertility for both groups (52.5% vs 50%). OSI had similar means of 4.6 (± 4.5) and 4.7 (± 4.6), for low and high P, respectively. P had averages of 0.7 ng/mL (± 0.4) for low P and 2 ng/mL (± 0.7) for high P. The oocyte source was fresh in each patient. The whole number of retrieved MII oocytes per patient was 14 ± 8 vs 15 ± 9 (low vs high), with subsequent fertilization rates of 78.6% vs 73.3% (low vs high). These data reflect the general type of ovarian response shown in our population. After PGT-A, the percentages of euploid embryos per biopsy were 44.1% in patients with low P (< 1.5), while in patients with high P (≥ 1.5), euploid embryos per biopsy were 43.8%.
Propensity score matching
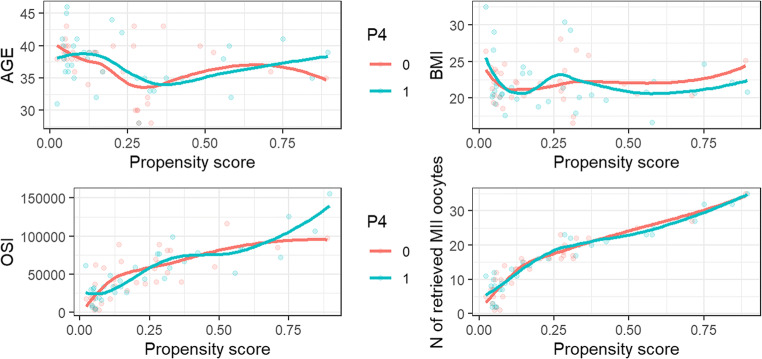
Patients from both groups with similar propensity scores were chosen for propensity score matching. This resulted in 36 pairs of one patient with serum P value less than 1.5 ng/mL and one patient with a serum P value greater than 1.5 ng/mL. Among these 72 observations, two matched subgroups of patients with similar baseline characteristics were created to study individually the effect of serum P. Figure 1 shows the behavior of the variables included in the study according to the propensity score estimated and differentiated by the two groups. These graphs show similar trends for each variable in both groups. A t test (Table 1) indicates no influence on any of the variables for both matched groups.
Fig. 1.
Estimated propensity score of each clinical variable in both groups after matching. Group 0 (pink line) corresponds to patients with serum P below 1.5 ng/mL and group 1 (blue line) represents patients with serum P above 1.5 ng/mL
Table 1.
Results of t test analysis in the matched groups
| Variable | P < 1.5 ng/mL | P ≥ 1.5 ng/mL | 95% CI | P value |
|---|---|---|---|---|
| Age | 37.03 | 37.42 | − 2.28 to 1.51 | NS |
| Body mass index | 21.99 | 22.04 | − 1.51 to 1.42 | NS |
| Ovarian sensitivity index | 4.66 | 4.7 | − 4.29 to 4,05 | NS |
| MII oocytes retrieved | 13.89 | 15.19 | − 5.22 to 2.61 | NS |
CI, confidence interval; NS, non-significant (P value > 0.05); ovarian sensitivity index is represented logarithmically
Serum P and euploidy rate in matched patients
Patients with elevated serum P values on the day of ovulation trigger did not have significantly different euploidy rates (20.3% vs 17.5%) per MII oocyte (P = 0.908) or (44% vs 43.9%) per biopsied embryo (P = 0.338). After analysis (Table 3), the only statistically significant variable with an impact on euploidy rate per MII oocyte (P <0.001) and per biopsied embryo (P = 0.008) was patient age. For every year that patient age increased, the euploidy rate per MII oocyte and per biopsied embryo decreased by 8% and 5%, respectively. BMI (P = 0.798 per MII oocyte; P = 0.918 per biopsied embryo) and OSI (P = 0.473 per MII oocyte; P = 0.247 per biopsied embryo) did not significantly impact euploidy rate.
Table 3.
Relationship between P and the different outcome variables in matched patients (N = 72)
| Euploidy rate per MII | |||
|---|---|---|---|
| Variable | Estimate | 95% CI | P value |
| Progesterone ≥ 1.5 ng/mL | 1.02 | 0.76–1.35 | NS |
| Age | 0.92 | 0.88–0.95 | < 0.001* |
| Body mass index | 1.01 | 0.96–1.05 | NS |
| Ovarian sensitivity index | 0.81 | 0.46–1.46 | NS |
| Euploidy rate per biopsied embryo | |||
| Progesterone ≥ 1.5 ng/mL | 1.15 | 0.86–1.55 | NS |
| Age | 0.95 | 0.92–0.99 | 0.008* |
| Body mass index | 1.00 | 0.96–1.05 | NS |
| Ovarian sensitivity index | 0.55 | 0.21–1.55 | NS |
| MII oocytes retrieved | 1.02 | 0.98–1.06 | NS |
| Biopsy rate per mii | |||
| Progesterone ≥ 1.5 ng/mL | 0.91 | 0.75–1.11 | NS |
| Age | 0.96 | 0.93–0.98 | < 0.001* |
| Body mass index | 1.00 | 0.97–1.03 | NS |
| Ovarian sensitivity index | 0.84 | 0.58–1.24 | NS |
| Top-quality embryo rate | |||
| Progesterone ≥ 1.5 ng/mL | 1.15 | 0.86–1.55 | NS |
| Age | 0.95 | 0.92–0.99 | 0.008* |
| Body mass index | 1.00 | 0.96–1.05 | NS |
| Ovarian sensitivity index | 0.55 | 0.21–1.55 | NS |
| MII oocytes retrieved | 1.02 | 0.98–1.06 | NS |
| Absolute number of euploid embryos | |||
| Progesterone ≥ 1.5 ng/mL | 0.99 | 0.75–1.32 | NS |
| Age | 0.90 | 0.87–0.94 | 0.008* |
| Body mass index | 1.00 | 0.95–1.04 | NS |
| Ovarian sensitivity index | 0.68 | 0.28–1.75 | NS |
| MII oocytes retrieved | 1.08 | 1.05–1.12 | < 0.001* |
| Live birth rate | |||
| Progesterone ≥ 1.5 ng/mL | 2.00 | 0.74–5.53 | NS |
| Age | 0.95 | 0.83–1.08 | NS |
| Body mass index | 1.10 | 0.93–1.31 | NS |
| Ovarian sensitivity index | 0.56 | 0.04–9.51 | NS |
| MII oocytes retrieved | 1.07 | 0.95–1.22 | NS |
Estimate represents the estimated coefficient resulting from Poisson regression. Values < 1.0 imply a reduction in each outcome variable due to an increase in the main variable. Values > 1.0 imply an improvement in each outcome variable due to an increase in the main variable
CI, confidence interval; NS, non-significant (P value > 0.05); ovarian sensitivity index is represented logarithmically; * indicates statistical significance
Serum P and outcome variables in matched patients
Propensity score matching for the clinical variables related to embryo quality are presented in Table 3. Patients with serum P ≥ 1.5 ng/mL on the day of ovulation trigger did not have significantly different biopsy rate (P = 0.357), top-quality embryo rate (P = 0.338), absolute number of euploid embryos (P = 0.958), or live birth rate (P = 0.34) compared to patients with serum P < 1.5 ng/mL. With the exception of live birth rate, all other categorical variables had a significant correlation with age in the subgroup of matched patients. For every year increase in patient age, the biopsy rate decreased by 4% (P < 0.01), the top-quality embryo rate decreased by 5% (P = 0.008), and the absolute number of euploid embryos decreased by 10% (P = 0.008). Neither BMI nor OSI statistically affected any of the measured embryo-quality variables (P > 0.05).
Serum P and outcome variables in unmatched patients
Poisson regression was applied to the unmatched patients to determine the impact of elevated serum P in our outcome variables (Table 4). Patients from the unmatched group with high P on the day of ovulation trigger did not have significantly different euploidy rates (P = 0.3 per MII oocyte; P = 0.64 per biopsied embryo), biopsy rate (P = 0.32), top-quality embryo rate (P = 0.84), or live birth rate (P = 0.17). However, serum P on the day of triggering was correlated with the absolute number of euploid embryos. Patients with high serum P levels had 27% more absolute number of euploid embryos than those with low P levels (P <0.001). Advanced age was significantly associated with poor outcome variables (P < 0.001). On the other hand, OSI had no association with euploidy rate, either per MII oocyte (P = 0.08) or per biopsied embryo (P = 0.21), top-quality embryo rate (P = 0.08), or live birth rate (P = 0.68), but it was correlated with biopsy rate and the absolute number of euploid embryos (P < 0.001). For every factor of 10 increment of OSI, the biopsy rate decreased by 13% and the absolute number of euploid embryos increased by 258%. BMI had no effect on outcome variables (P > 0.05).
Table 4.
Relationship between P and the different outcome variables in unmatched patients (N = 1597)
| Euploidy rate per MII | |||
|---|---|---|---|
| Variable | Estimate | 95% CI | P value |
| Progesterone ≥ 1.5 ng/mL | 1.07 | 0.93–1.21 | NS |
| Age | 0.91 | 0.90–0.93 | < 0.001* |
| Body mass index | 0.99 | 0.98–1.00 | NS |
| Ovarian sensitivity index | 0.91 | 0.80–1.02 | NS |
| Euploidy rate per biopsied embryo | |||
| Progesterone ≥ 1.5 ng/mL | 1.03 | 0.89–1.19 | NS |
| Age | 0.94 | 0.92–0.95 | < 0.001* |
| Body mass index | 1.00 | 0.98–1.01 | NS |
| Ovarian sensitivity index | 1.16 | 0.92–1.45 | NS |
| MII oocytes retrieved | 1.00 | 0.99–1.01 | NS |
| Biopsy rate per MII | |||
| Progesterone ≥ 1.5 ng/mL | 1.05 | 0.96–1.14 | NS |
| Age | 0.98 | 0.97–0.98 | < 0.001* |
| Body mass index | 0.99 | 0.99–1.01 | NS |
| Ovarian sensitivity index | 0.87 | 0.80–0.94 | < 0.001* |
| Top-quality embryo rate | |||
| Progesterone ≥ 1.5 ng/mL | 1.02 | 0.83–1.25 | NS |
| Age | 0.92 | 0.90–0.94 | < 0.001* |
| Body mass index | 1.00 | 0.98–1.02 | NS |
| Ovarian sensitivity index | 1.34 | 0.96–1.84 | NS |
| MII oocytes retrieved | 1.06 | 0.98–1.06 | < 0.001* |
| Absolute number of euploid embryos | |||
| Progesterone ≥ 1.5 ng/mL | 1.27 | 1.12–1.45 | < 0.001* |
| Age | 0.92 | 0.90–0.93 | < 0.001* |
| Body mass index | 1.00 | 0.99–1.01 | NS |
| Ovarian sensitivity index | 3.58 | 3.13–4.08 | < 0.001* |
| Live birth rate | |||
| Progesterone ≥ 1.5 ng/mL | 1.08 | 0.65–1.75 | NS |
| Age | 0.92 | 0.88–0.96 | < 0.001* |
| Body mass index | 1.01 | 0.97–1.04 | NS |
| Ovarian sensitivity index | 1.94 | 0.98–3.87 | NS |
| MII oocytes retrieved | 1.02 | 0.98–1.06 | NS |
Estimate represents the estimated coefficient resulting from Poisson regression. Values < 1.0 imply a reduction in each outcome variable due to an increase in the main variable. Values > 1.0 imply an improvement in each outcome variable due to an increase in the main variable
CI, confidence interval; NS, non-significant (P value > 0.05); ovarian sensitivity index is represented logarithmically; * indicates statistical significance
Discussion
Premature rise of serum P in the late follicular phase occurs in a variable proportion of patients who undergo ovarian stimulation for assisted reproduction cycles. The detrimental effect of this elevation on pregnancy outcomes has been debated for decades; although evidence indicated that increased P in this phase of the cycle affects the endometrium in terms of endometrial receptivity, it is unclear whether oocyte and embryo quality are affected. Most studies that focus on this impact have different P cut-off values that vary from 0.5 to 3.0 ng/mL, different types of ovarian responders in their study population, or different stimulation protocols, with variables results [3]. This inconsistency in the design of the studies, added to the results reported in recent publications [10–12], leads us to question the effect of high serum P on embryo and oocyte quality.
In this retrospective study, we evaluated the association between premature serum P rise on the day of triggering and aneuploidy embryo rate. To that end, we collected data from 1597 patients undergoing PGT-A during 2016 to 2018. Results indicated that the high serum P value does not show a significant correlation either with euploid embryos or with other variables related to embryo quality after applying propensity score matching. We also assessed the association between the number of embryos that reached the blastocyst stage per MII oocyte defined as biopsy rate and high serum P in the late follicular phase. Against all odds, we found no difference in biopsy rates between low and high serum P groups. Live birth rate was also not impacted by elevated P. Further, neither OSI nor BMI presented any effect on euploidy rate, biopsy rate, absolute number of euploid embryos, top-quality embryo rate, or live birth rate in matched groups. Age, however, significantly impacted euploidy rate, biopsy rate, absolute number of euploid embryos, and top-quality embryo rate in patients with high serum P (≥ 1.5 ng/ml). These results confirm that both embryo and oocyte quality are compromised by maternal age, even when patients present high serum P in the late follicular phase.
Our findings are supported by previous works describing no alteration in oocyte/embryo quality associated with premature P elevation [13, 18, 23]. However, these decades-old publications lack a certain sustainability in their study design as they do not share a precise cut-off value. Recently, Racca et al. [17] published a retrospective analysis excluding any detrimental effect of high P (≥ 1.5 ng/mL) on reproductive outcomes of recipients in oocyte donation cycles. Other recent studies, however, draw other conclusions [10–12]. Huang et al. argued, in a retrospective study of 4236 fresh IVF cycles, a pronounced reduction in top quality embryo when P was ≥ 2 ng/mL on the day of hCG trigger. In parallel, another retrospective study that enrolled 986 GnRH antagonist IVF/ICSI cycles showed a detrimental effect in blastocyst quality with a value of P above 1.5 ng/ml. Further, studies differ in their selection criteria for embryo quality. Whereas Racca et al. and Huang et al. focused on day 3 embryo rate and morphological characteristics, Vanni et al. described detrimental impacts on blastocyst stage when P levels were high. Despite these findings, it has not been elucidated whether high serum P on the day of triggering affects embryo quality. More research is needed to determine the effect of elevated P, perhaps focusing on progesterone-responsive genes that may act as mediators in the regulation of oocyte competence as seen in previous animal models [24, 25].
Our results suggest that any patient with elevated serum P value on the day of ovulation trigger should not experience impaired embryo quality. However, the ovarian response would play a key role and could question this claim. Considering that the biological nature of the elevated P lies in the follicle, its elevation at the intrafollicular level would not affect the reproductive cycle outcomes in the same way as its elevation in the blood. After ovarian stimulation, a high responder may recruit additional follicles, leading to the production of a greater quantity of oocytes and a higher probability of pregnancy. This scenario may also contribute to high serum P without the worsening of clinical outcomes. A poor responder with elevated intrafollicular P values on the day of hCG administration may experience an abnormal follicular development resulting in poor oocyte and embryo quality [19]. These patients were not part of our study group but should be a part of further investigation.
Oocyte and embryo quality may be impacted by elevated P levels in the late follicular phase and the ovarian response. Therefore, variables such as the number of oocytes retrieved should also be considered. We used propensity score matching to analyze the effect of serum P separately and found no effect on embryo aneuploidy rate. Based on our results, no different embryo replacement strategies were indicated. However, it is advisable to establish other strategies to avoid genetic changes caused by P elevated values, which can lead to an important asynchrony between endometrium and embryo [8]. This negative impact on endometrial receptivity can be eradicated with other strategies such as cryopreservation, allowing endometrial recovery after exposure to P [7, 26]. Interestingly, in the case of cryopreservation, it has been seen that the potential benefits of freeze-all policy decline with worsening ovarian response. Although frozen cycles are more successful than fresh cycles in high responders, poor responders do not report significant advantages with this strategy [27, 28]. Cryopreservation is used daily in laboratory practice, but there are no clinical data to support the widespread use of a freeze-all strategy for all patients [29]. Individualization of controlled stimulation treatment has been proposed as a better strategy against premature P elevation. The optimal dose of gonadotrophin varies between patients depending on ovarian response, hormonal parameters, baseline characteristics, and other factors [30].
The current study presents some limitations. This was a retrospective study where conclusions were extracted based on an observational analysis of the data. A prospective controlled trial might better extrapolate the results to the population. In addition, the average age of our study matched patients was 37 years. This was an advantage since most of the patients who undergo a reproductive cycle have an advanced age but unfortunately does not provide information about the effect of premature P elevation on young patients with poor ovarian response. Only 8.3% of our patients presented serum P values above 1.5 ng/mL, which may limit the power of our study. Exposures such as smoking and alcohol use were not included in the database.
In conclusion, premature P rise on the day of triggering in patients undergoing PGT-A was not correlated with euploidy rate or other embryo quality variables in GnRH antagonist ICSI cycles. This initial approach of P shows that its serum value on the day of ovulation trigger does not act as a surrogate marker of low embryo quality. Despite the well-established influence of premature P elevation on endometrial receptivity, its association with oocyte and embryo quality cannot be underestimated. Our study provides more information about this association. More studies, especially in poor responder patients, are needed to fully understand the effect of P elevation on reproductive outcomes.
Authors’ contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Maria José De los Santos, Mar Nohales, and Maria Luisa Pardiñas. The first draft of the manuscript was written by Maria Luisa Pardiñas, and all authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The data that support the findings were stored in the IVI RMA Valencia computer system. The data collected are coded and pseudonymized so that no personal and clinical information of each patient can be identified.
Declarations
Ethics approval
This study was conducted retrospectively using data obtained for clinical purposes. It was approved by the Ethical Committee of Clinical Research IVI Valencia (1503-VLC-017-AM).
Consent to participate
This is research of general scientific interest and will be carried out in the same center where the data was obtained; therefore, it is not considered necessary to obtain the informed consent of the subjects. There is no opposition from the subjects to the use of the data for the intended purposes.s
Consent for publication
Authors are responsible for the correctness of the statements provided in the manuscript.
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maria Luisa Pardiñas, Email: marialuisa.pardinas@ivirma.com.
Mar Nohales, Email: mar.nohales@ivirma.com.
Elena Labarta, Email: elena.labarta@ivirma.com.
José María De los Santos, Email: josemaria.delossantos@ivirma.com.
Amparo Mercader, Email: amparo.mercader@ivirma.com.
José Remohí, Email: remohi@ivirma.com.
Ernesto Bosch, Email: ernesto.bosch@ivirma.com.
Maria José De los Santos, Email: mariajose.delossantos@ivirma.com.
References
- 1.Bosch E, Labarta E, Crespo J, Simón C, Remohí J, Jenkins J, Pellicer A. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25:2092–2100. doi: 10.1093/humrep/deq125. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Nieto C, Lee JA, Alkon-Meadows T, Luna-Rojas M, Mukherjee T, Copperman AB, Sandler B. Late follicular phase progesterone elevation during ovarian stimulation is not associated with decreased implantation of chromosomally screened embryos in thaw cycles. Hum Reprod. 2020;35:1889–1899. doi: 10.1093/humrep/deaa123. [DOI] [PubMed] [Google Scholar]
- 3.Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19:433–457. doi: 10.1093/humupd/dmt014. [DOI] [PubMed] [Google Scholar]
- 4.Schoolcraft W, Sinton E, Schlenker T, Huynh D, Hamilton F, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril. 1991;55:563–566. doi: 10.1016/S0015-0282(16)54186-7. [DOI] [PubMed] [Google Scholar]
- 5.Fleming R, Jenkins J. The source and implications of progesterone rise during the follicular phase of assisted reproduction cycles. Reprod BioMed Online. 2010;21:446–449. doi: 10.1016/j.rbmo.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Embryo cryopreservation rescues cycles with premature luteinization. Fertil Steril. 2010;93:636–641. doi: 10.1016/j.fertnstert.2009.01.134. [DOI] [PubMed] [Google Scholar]
- 7.Healy MW, Yamasaki M, Patounakis G, Richter KS, Devine K, DeCherney AH, et al. The slow growing embryo and premature progesterone elevation: compounding factors for embryo-endometrial asynchrony. Hum Reprod. 2017;32:362–367. doi: 10.1093/humrep/dew296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labarta E, MartÍnez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, Simón C, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26:1813–1825. doi: 10.1093/humrep/der126. [DOI] [PubMed] [Google Scholar]
- 9.Xiong Y, Wang J, Liu L, Chen X, Xu H, Li TC, et al. Effects of high progesterone level on the day of human chorionic gonadotrophin administration in in vitro fertilization cycles on epigenetic modification of endometrium in the peri-implantation period. Fertil Steril. 2017;108:269–276.e1. doi: 10.1016/j.fertnstert.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Huang B, Ren X, Wu L, Zhu L, Xu B, Li Y, et al. Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. Sun Q-Y, editor. PLoS One. 2016;11:e0145895. doi: 10.1371/journal.pone.0145895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racca A, Santos-Ribeiro S, De Munck N, Mackens S, Drakopoulos P, Camus M, et al. Impact of late-follicular phase elevated serum progesterone on cumulative live birth rates: is there a deleterious effect on embryo quality? Hum Reprod. 2018;33:860–868. doi: 10.1093/humrep/dey031. [DOI] [PubMed] [Google Scholar]
- 12.Vanni VS, Somigliana E, Reschini M, Pagliardini L, Marotta E, Faulisi S, et al. Top quality blastocyst formation rates in relation to progesterone levels on the day of oocyte maturation in GnRH antagonist IVF/ ICSI cycles. Kim S, editor. PLoS One. 2017;12:e0176482. doi: 10.1371/journal.pone.0176482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ubaldi F, Smitz J, Wisanto A, Joris H, Schiettecatte J, Derde MP, Borkham E, van Steirteghem A, Devroey P. Oocyte and embryo quality as well as pregnancy rate in intracytoplasmic sperm injection are not affected by high follicular phase serum progesterone. Hum Reprod. 1995;10:3091–3096. doi: 10.1093/oxfordjournals.humrep.a135864. [DOI] [PubMed] [Google Scholar]
- 14.Melo MAB, Meseguer M, Garrido N, Bosch E, Pellicer A, Remohí J. The significance of premature luteinization in an oocyte-donation programme. Hum Reprod. 2006;21:1503–1507. doi: 10.1093/humrep/dei474. [DOI] [PubMed] [Google Scholar]
- 15.Lahoud R, Kwik M, Ryan J, Al-Jefout M, Foley J, Illingworth P. Elevated progesterone in GnRH agonist down regulated in vitro fertilisation (IVFICSI) cycles reduces live birth rates but not embryo quality. Arch Gynecol Obstet. 2012;285:535–540. doi: 10.1007/s00404-011-2045-0. [DOI] [PubMed] [Google Scholar]
- 16.Xu B, Li Z, Zhang H, Jin L, Li Y, Ai J, et al. Serum progesterone level effects on the outcome of in vitro fertilization in patients with different ovarian response: an analysis of more than 10,000 cycles. Fertil Steril. 2012;97:1321–1327. doi: 10.1016/j.fertnstert.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 17.Racca A, De Munck N, Santos-Ribeiro S, Drakopoulos P, Errazuriz J, Galvao A, et al. Do we need to measure progesterone in oocyte donation cycles? A retrospective analysis evaluating cumulative live birth rates and embryo quality. Hum Reprod. 2020;35:167–174. doi: 10.1093/humrep/dez238. [DOI] [PubMed] [Google Scholar]
- 18.Fanchin R, Righini C, Olivennes F, De Ziegler D, Selva J, Frydman R. Premature progesterone elevation does not alter oocyte quality in in vitro fertilization. Fertil Steril. 1996;65:1178–1183. doi: 10.1016/S0015-0282(16)58335-6. [DOI] [PubMed] [Google Scholar]
- 19.Shufaro Y, Sapir O, Oron G, Ben Haroush A, Garor R, Pinkas H, Shochat T, Fisch B. Progesterone-to-follicle index is better correlated with in vitro fertilization cycle outcome than blood progesterone level. Fertil Steril. 2015;103:669–674. doi: 10.1016/j.fertnstert.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Ovarian Stimulation TEGG on. Bosch E, Broer S, Griesinger G, Grynberg M, Humaidan P, et al. ESHRE guideline: ovarian stimulation for IVF/ICSI. Hum Reprod Open. 2020;2020:1–13. doi: 10.1093/hropen/hoaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 22.de los Santos MJ, Diez Juan A, Mifsud A, Mercader A, Meseguer M, Rubio C, et al. Variables associated with mitochondrial copy number in human blastocysts: what can we learn from trophectoderm biopsies? Fertil Steril. 2018;109:110–117. doi: 10.1016/j.fertnstert.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Silverberg KM, Martin M, Olive DL, Burns WN, Schenken RS. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration in in vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril. 1994;61:508–513. doi: 10.1016/S0015-0282(16)56584-4. [DOI] [PubMed] [Google Scholar]
- 24.Urrego R, Herrera-Puerta E, Chavarria NA, Camargo O, Wrenzycki C, Rodriguez-Osorio N. Follicular progesterone concentrations and messenger RNA expression of MATER and OCT-4 in immature bovine oocytes as predictors of developmental competence. Theriogenology. 2015;83:1179–1187. doi: 10.1016/j.theriogenology.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Fair T, Lonergan P. The role of progesterone in oocyte acquisition of developmental competence. Reprod Domest Anim. 2012;47:142–147. doi: 10.1111/j.1439-0531.2012.02068.x. [DOI] [PubMed] [Google Scholar]
- 26.Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C, van Steirteghem A, Devroey P. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril. 2009;91:949–952. doi: 10.1016/j.fertnstert.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 27.Roque M, Valle M, Guimarães F, Sampaio M, Geber S. Freeze-all cycle for all normal responders? J Assist Reprod Genet. 2017;34:179–185. doi: 10.1007/s10815-016-0834-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue Y, Tong X, Zhu H, Li K, Zhang S. Freeze-all embryo strategy in poor ovarian responders undergoing ovarian stimulation for in vitro fertilization. Gynecol Endocrinol. 2018;34:680–683. doi: 10.1080/09513590.2018.1427715. [DOI] [PubMed] [Google Scholar]
- 29.Roque M, Nuto Nóbrega B, Valle M, Sampaio M, Geber S, Haahr T, Humaidan P, Esteves SC. Freeze-all strategy in IVF/ICSI cycles: An update on clinical utility. Panminerva Med. 2019;61:52–57. doi: 10.23736/S0031-0808.18.03492-4. [DOI] [PubMed] [Google Scholar]
- 30.Lawrenz B, Labarta E, Fatemi H, Bosch E. Premature progesterone elevation: targets and rescue strategies. Fertil Steril. 2018;109:577–582. doi: 10.1016/j.fertnstert.2018.02.128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings were stored in the IVI RMA Valencia computer system. The data collected are coded and pseudonymized so that no personal and clinical information of each patient can be identified.