Abstract
Macroalgae comprise a vast group of aquatic organisms known for their richness in phytochemicals. In this sense, the lipophilic profile of five Antarctic seaweed species was characterized by chromatographic and spectroscopic analysis and their antioxidant and antimicrobial potential was evaluated. Results showed there were 31 lipophilic substances, mainly fatty acids (48.73 ± 0.77 to 331.91 ± 10.79 mg.Kg−1), sterols (14.74 ± 0.74 to 321.25 ± 30.13 mg.Kg−1), and alcohols (13.07 ± 0.04 to 91.87 ± 30.07 mg.Kg−1). Moreover, Desmarestia confervoides had strong antioxidant activity, inhibiting 86.03 ± 1.47% of the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical at 1 mg.mL−1. Antimicrobial evaluation showed that extracts from Ulva intestinalis, Curdiea racovitzae, and Adenocystis utricularis inhibited the growth of Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and Salmonella typhimurium (ATCC 14028) from concentrations of 1.5 to 6 mg.mL−1. Therefore, the evaluated brown, red, and green macroalgae contained several phytochemicals with promising biological activities that could be applied in the pharmaceutical, biotechnological, and food industries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00475-6.
Keywords: Lipophilic extract, Bioactive compounds, Macroalgae, Antioxidant activity, Antimicrobial activity
Introduction
The marine environment is known for its biodiversity and for being a source of more than 25,000 natural products [1]. Among photosynthetic organisms that inhabit aquatic ecosystems are macroalgae, which can be divided into green, brown, and red algae depending on morphological and biochemical aspects [2, 3]. Given their biological, chemical, and breeding properties, seaweeds are becoming increasingly important marine resources. Indeed, their harvesting is growing approximately 15% per year, with capture or aquaculture reaching 25 million metric tons in 2014 [4, 5].
The high degree of adaptation of macroalgae allows these organisms to inhabit many environments, running the gamut of tropical, subtropical, temperate and polar regions [4, 6, 7]. In complex habitats that include the Antarctic Peninsula, seaweeds are subjected to several abiotic parameters, such as low water temperature, restricted nutrient availability, high exposure to ultraviolet radiation, limited photoperiod, and high water salinity [3, 8]. These extreme environmental conditions cause aquatic organisms to develop defense and survival strategies, including activation of biochemical processes related to the production of metabolites [9].
Secondary metabolites found in aquatic ecosystems have unique structural and chemical moieties that are not commonly found in natural products from terrestrial plants [1]. According to previous reports, approximately 15,000 secondary metabolites have been identified in macroalgae, including, for instance, fatty acids (FAs), sterols, polysaccharides, amino acids, flavonoids, and terpenoids [3, 8]. In this sense, seaweeds compose an important reserve of possible bioactive compounds that can be used for antimicrobial or antioxidant purposes [2].
The search for novel bioactive compounds is important to minimize antimicrobial resistance. Due to the increasing demand for novel therapeutic drugs, there is growing interest in metabolites found in marine organisms. Several algal species have been reported to produce bactericidal or bacteriostatic substances. In this sense, macroalgal extracts could provide important bioactive compounds for use in biotechnological and pharmaceutical areas, among others [1].
Indeed, macroalgae comprise an almost unlimited reserve of potential biochemical compounds that could be employed in industrial applications. However, although more than 10,000 species of seaweeds have been identified, only a few representatives have been chemically characterized to screen them for molecules with potential applications. In recent years, our research group has analyzed lipophilic components of sub-Antarctic and Antarctic macroalgae including FAs [4, 6] and sterols [10] and successfully indicated their biological applications [11]. The aims of this work were to evaluate fatty acids, sterols, and carboxylic, dicarboxylic, and tricarboxylic acids, among other lipophilic constituents, of five extracts of Antarctic macroalgae and to evaluate their antioxidant and antibacterial activities.
Materials and methods
Sampling
Approximately 5 to 10 individuals of each species of brown, red, and green Antarctic macroalgae were manually collected in the eulittoral or infralittoral zone in several locations of the Antarctic Peninsula between November and December 2015 (Table 1) as part of Brazil’s Thirty-Fourth Antarctic Expedition. The samples were washed with seawater and further cleaned with distilled water to remove impurities, microorganisms, and salt. After morphological identification, the specimens were lyophilized, milled, and stored in hermetically sealed bags at −20°C before analyses.
Table 1.
Sampling information of the brown, red, and green Antarctic macroalgae
| Species | Collection site | Coordinates | Collection date |
|---|---|---|---|
| Ochrophyta | |||
| Adenocystis utricularis | Greenwich Island | 62° 29′ S × 59° 47′ W | December, 2015 |
| Desmarestia confervoides | Hennequim Point | 62° 7′ S × 58° 23′ W | November, 2015 |
| A.1.1.1.1. Rhodophyta | |||
| Curdiea racovitzae | Punta Plaza | 62° 5′ S × 58° 24′ W | November, 2015 |
| Myriogramme manginii | Snow Island | 62° 46′ S × 61° 31′ W | December, 2015 |
| A.1.1.1.2. Chlorophyta | |||
| Ulva intestinalis | Robert Island | 62° 22′ S × 59° 41′ W | December, 2015 |
Chemicals and materials
Pyridine, N-methyl-N-(trimethylsilyl)-trifluoroacetamide, 2,2-diphenyl-1-picrylhydrazyl-hydrate (DPPH), methyl nonadecanoate, cholesterol, and 1-decanolwere were purchased from Sigma-Aldrich (St. Louis, USA), while n-hexane and methanol were obtained from J.T. Baker (Radnor, USA). All other solvents and reagents were analytical grade
Extraction
The lipophilic fraction of each macroalgal sample (5 g) was extracted using n-hexane by means of a Soxhlet apparatus for 6 h after the sample was soaked with solvent overnight. Subsequently, the lipophilic extract was dried under reduced pressure. The procedure was performed in triplicate (n=3) and followed the modified method of Santos et al. (2015), replacing dichloromethane with n-hexane.
Chemical composition
Hydrolysis and derivatization
Briefly, 10 mg of the lipophilic extract and 10 mL of a 0.5-M solution of sodium hydroxide in methanol:water (50:50, v/v) were constantly mixed and refluxed for 1 h. Afterwards, the system was cooled and acidified to pH 2 by the gradual addition of a 1-M solution of hydrochloric acid. Furthermore, samples were extracted three times with 5 mL of dichloromethane. Lipophilic layers were combined and dried under reduced pressure. The hydrolyzed extracts were reconstituted in 100 μL of chloroform and further derivatized with 100 μL of N-methyl-N-(trimethylsilyl)-trifluoroacetamide and 100 μL of pyridine at 70°C for 30 min. Procedures were performed in triplicate (n=3) and followed the method of Santos et al. (2015).
Chromatographic analysis
Chromatographic analysis followed the method of Santos et al. (2015) and was performed in a GCMS-QP2010 system (Shimadzu, Kyoto, Japan) followed by the injection of 1 μL in split mode (1:33) of the derivatized material into an Rtx-5MS capillary column (30 m × 0.25 mm × 0.25 μm; Restek, Bellefonte, USA) with helium gas flow of 1.50 mL.min−1. The injection port and interface operated at 250 and 290°C, respectively. The initial oven temperature was set at 80°C, maintained for 5 min, and increased at 4°C.min−1 to 260°C and then at 2°C.min−1 until the final oven temperature of 285°C, which was maintained for 8 min.
The mass spectrometer operated using electron ionization at 70 eV with the ion source scanned from m/z 30 to m/z 550. Identification of compounds was performed using the NIST08s spectral library. Quantitation followed the method of Santos et al. (2015) and was performed using pure reference standards as representatives of the major lipophilic families (methyl nonadecanoate, cholesterol, and 1-decanol) in solutions of 1, 0.500, 0.250, 0.125, 0.625, and 0.312 mg.mL−1 in n-hexane, injected in triplicate (n=3).
Spectroscopic analysis
Approximately 10 μL of each lipophilic extract was analyzed using attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR) with a Shimadzu Prestige 21 FTIR spectrometer (Kyoto, Japan) operating from 4000 to 600 cm−1 with resolution of 4 cm−1.
Antioxidant activity
Concentrations of 1, 0.500, 0.250, and 0.125 mg.mL−1 of lipophilic extracts were evaluated for antioxidant activity by mixing them with 300 μL of DPPH radical methanolic solution (0.394 mg.mL−1) and 3 mL of methanol. Samples were incubated at room temperature for 15 min in the dark and analyzed by spectrophotometry (UV-M51; Bel, Piracicaba, Brazil) at 517 nm. Positive controls were performed using ascorbic acid at the same concentrations of the tested samples. An analytical blank using 300 μL of DPPH radical mixed with 2.7 mL of methanol was also tested. All experiments were performed in triplicate (n=3) and followed the method of Pellati et al. [12]. Inhibition of the DPPH radical at different concentrations of the lipophilic extracts was measured by Eq. 1.
| 1 |
where ADPPH is the absorbance of the DPPH radical without sample, AExtract is the absorbance of lipophilic extracts mixed with DPPH radical, and ABlank is the absorbance of methanol.
Antibacterial activity
Test organisms
Antimicrobial activity was measured using the gram-positive standard strains Staphylococcus aureus (ATCC 25923) and Enterococcus faecalis (ATCC 51299) as well as the gram-negative standard strains Escherichia coli (ATCC 25922) and Salmonella typhimurium (ATCC 14028). Microorganisms were provided by the Oswaldo Cruz Foundation (FIOCRUZ). The evaluated strains were maintained in Mueller-Hinton agar at 4°C and reactivated prior to antimicrobial evaluation.
Minimum inhibitory concentration
The minimum inhibitory concentrations (MICs) were determined according to the broth microdilution method following the Clinical and Laboratory Standards Institute (CLSI [13]) guidelines. First, n-hexane extracts from Antarctic macroalgae were diluted in brain-heart infusion (BHI) broth at concentrations ranging from 6 to 0.0078 mg.mL−1 in 5% of ethanol. As negative control, 100 μL of BHI broth was used, while the positive control consisted of 50 μL of bacterial suspension and 50 μL of BHI broth.
Microorganisms were cultured in BHI broth and standardized to 0.5 on the McFarland scale, resulting in optical density between 0.08 and 0.1 at 630 nm. Afterwards, 50 μL of each cultured sample was diluted in 4950 μL of BHI broth, and 50 μL of each suspension was inserted in a well, resulting in a final concentration of microorganisms of 3.104 CFU.mL−1. The MIC values were investigated in triplicate (n=3) and plates were incubated at 37°C for 24 h. Subsequently, 20 μL of 2,3,5-triphenyl tetrazolium chloride (0.5%, w/v) was placed in each well and further incubated for 20 min at 37°C. Finally, bacterial growth was evaluated by color development.
Minimum microbicidal concentration
The minimum microbicidal concentration (MMC) was determined for all samples that had antimicrobial activity. Briefly, 5-μL aliquots were placed in Mueller-Hinton agar plates and incubated at 37°C for 24 h. Afterwards, the presence or absence of bacterial growth was evaluated for the determination of bacteriostatic or bactericidal activity. The experiments were performed in triplicate (n=3).
Statistical analysis
Two-way analysis of variance (ANOVA) followed by the Tukey test (p<0.05) was applied to determine significant differences between the constituents of the samples using GraphPad version 7 (La Jolla, USA). Principal component analysis (PCA) was used to evaluate similarities in the lipophilic composition of the algal extracts, employing the Minitab software version 17 (State College, USA).
Results
Chemical evaluation
Lipophilic yields of the studied Antarctic macroalgae (Table 2) were generally in the range of 0.189 ± 0.005 to 0.356 ± 0.038% DW in brown seaweed; 0.134 ± 0.005 to 0.138 ± 0.003% DW in red seaweed; and 0.187 ± 0.021% DW in U. intestinalis (green seaweed). Generally, brown macroalgae had the highest amounts of extracted lipophilic compounds, reaching as much as 0.356 ± 0.038% DW in D. confervoides. On the other hand, the lowest lipophilic yield was observed in M. manginii, of 0.134 ± 0.005% DW.
Table 2.
Lipophilic extract yields in dry weight (% DW) of brown, red, and green Antarctic macroalgae
| Sample | Extract yield (DW %) |
|---|---|
| U. intestinalis | 0.187 ± 0.021abc |
| D. confervoides | 0.356 ± 0.038bc |
| A. utricularis | 0.189 ± 0.005c |
| M. manginii | 0.134 ± 0.005a |
| C. racovitzae | 0.138 ± 0.003a |
Results expressed as mean ± standard deviation of triplicates (n=3)
Different superscript letters indicate significant difference (p<0.05)
Evaluation of chemical composition of the lipophilic extracts from Antarctic macroalgae (Table 3) showed that together the macroalgae had 31 distinct compounds, identified as FAs, alcohols, sterols, ketones, aldehydes, hydrocarbons, and other chemical classes. The highest number of compounds was found in Ochrophyta representatives such as D. confervoides and A. utricularis, which had 24 and 23 constituents, respectively, while U. intestinalis had the lowest variety, reaching 19 compounds.
Table 3.
Chemical constitution of the lipophilic extracts of green, brown, and red Antarctic macroalgae expressed as mg.kg−1 of dry material
| Compound | C. racovitzae | D. confervoides | M. manginii | U. intestinalis | A. utricularis |
|---|---|---|---|---|---|
| 2-Ethylhexanoic acid | 11.12 ± 5.48a | 49.65 ± 6.32b | 11.57 ± 0.48a | 22.12 ± 0.07c | 30.91 ± 0.30c |
| 5-Oxohexanoic acid | 4.69 ± 0.01a | 12.42 ± 0.18a | 4.50 ± 0.03a | 6.29 ± 0.05a | 6.47 ± 0.03a |
| Decanoic acid | nda | nda | nda | 10.36 ± 0.03b | nda |
| Dodecanoic acid | 5.27 ± 0.13ab | ndb | ndb | 10.89 ± 0.18a | 10.42 ± 0.09a |
| Tetradecanoic acid | 5.44 ± 0.14ac | 28.93 ± 0.38b | ndc | 8.24 ± 0.15ac | 14.00 ± 1.00a |
| Hexadecenoic acid | nda | 22.84± 0.62b | 5.08 ± 0.03a | 13.54 ± 0.00c | 8.69 ± 0.34c |
| Hexadecanoic acid | 10.10± 0.00ad | 49.01± 6.18b | 7.92 ± 0.09d | 18.92 ± 0.14ac | 24.73± 1.93c |
| Octadecanoic acid | 4.71 ± 0.00a | 20.97 ± 0.32b | 4.44 ± 0.01a | 8.27 ±0.25a | 7.82 ±0.60a |
| Octadecenoic acid | 5.19± 0.00a | 75.89± 1.55b | 5.86 ± 0.12a | 13.23 ± 0.19ac | 18.85 ± 0.38c |
| trans-Octadecenoic acid | 4.92± 0.11a | nda | 4.55 ± 0.02a | nda | nda |
| Octadecadienoic acid | 4.67 ± 0.13ac | 26.67 ± 0.01b | ndc | ndc | 10.92 ±1.60a |
| Octadecatrienoic acid | nda | nda | nda | 6.37 ± 0.02a | nda |
| Eicosapentaenoic acid | 4.96 ± 0.07ac | 18.49 ± 0.55b | 4.79 ± 0.02ac | ndc | 9.69 ±0.35a |
| Eicosatetraenoic acid | 4.63± 0.01ac | 27.00± 11.41b | ndac | ndac | 7.51 ± 0.37a |
| Fatty acids | 66.75 ± 5.01a | 331.91 ± 10.79b | 48.73 ± 0.77c | 118.28 ± 0.16d | 150.05 ± 4.29e |
| Hexanedioic acid | nda | nda | 5.20 ± 0.03a | nda | nda |
| Nonanedioic acid | nda | 25.23± 0.38b | nda | nda | nda |
| Benzoic acid | 4.77 ± 0.04a | nda | nda | nda | nda |
| Benzeneacetic acid | 4.69 ± 0.00a | nda | 4.47 ± 0.03a | nda | 7.07 ± 0.05b |
| Benzenedicarboxylic acid | nda | 13.67 ± 0.25b | 4.88 ± 0.03ab | nda | nda |
| Acetyltributylcitrate | 4.79 ± 0.06a | nda | 4.93 ± 0.08a | nda | nda |
| Carboxylic acids | 14.26 ± 0.10a | 38.90 ± 0.64b | 19.50 ± 0.17a | ndc | 7.07 ± 0.05d |
| Cyclopentanol | 5.17 ± 0.02ac | 13.29 ± 0.08ab | ndc | 6.95 ± 0.00ac | ndc |
| 2-Butoxyethanol | 30.98 ± 0.11a | 56.37 ± 26.20b | 7.48 ± 0.12c | 12.92 ± 0.20c | 55.14 ± 9.64b |
| Methylcyclohexenol | 7.81 ± 0.11a | 22.20 ± 3.79b | 5.59 ± 0.08ac | 8.28 ± 0.01ac | 12.55 ± 0.45ac |
| Alcohols | 43.97 ± 0.20a | 91.87 ± 30.07b | 13.07 ± 0.04c | 28.16 ± 0.22d | 67.70 ± 10.09e |
| Cholesterol | 28.82 ± 0.40a | 18.14 ± 0.07a | 14.74 ± 0.74b | 9.42 ± 0.12b | 11.88 ± 0.75b |
| Hydroxymethylcholesterol | nda | 20.15 ± 1.02bc | nda | nda | 12.83 ± 0.25c |
| Fucosterol | nda | 282.96 ± 29.02b | nda | 12.02 ± 0.00c | 41.60 ± 3.32d |
| Sterols | 28.82 ± 0.40a | 321.25 ± 30.13b | 14.74 ± 0.74c | 21.45± 0.11d | 66.32 ± 4.33e |
| α-Tocopherol | nda | 14.80 ± 0.36b | nda | nda | nda |
| Phytol | 5.66 ± 0.08a | 27.40 ± 1.08b | 7.23 ± 0.09a | 13.14 ± 0.11a | 10.05 ± 0.42a |
| L-cysteine | nda | 4.99 ± 0.09b | 4.81 ± 0.00b | nda | nda |
| Tetradecane | 5.08 ± 0.00a | 4.66 ± 0.09a | 4.84 ± 0.03a | 7.92 ± 0.14a | 6.51 ± 0.00a |
| Dihydroactinolide | nda | 13.45 ± 0.11b | nda | 8.09 ± 0.42c | 7.89 ± 0.35c |
| Benzaldehyde | nda | nda | 4.68 ± 0.10a | nda | nda |
| Hydroxymethylpentanone | 5.21 ± 0.04a | nda | nda | nda | nda |
| Trimethylbenzene | 5.27 ± 0.06a | 5.01 ± 0.07a | 4.56 ± 0.02a | 4.49 ± 0.00a | 6.51 ± 0.37a |
| Others | 21.22 ± 0.18a | 70.32 ± 1.61b | 26.16 ± 0.01ac | 33.65 ± 0.69c | 30.97 ± 0.43c |
Results expressed as mean ± standard deviation of triplicates (n=3)
Results without a common superscript letter are significant different (p<0.05)
As can be observed in Table 3, D. confervoides contained the most compounds found in higher concentrations compared to the other analyzed specimens. Generally, 2-ethylhexanoic acid (11.12 ± 5.48 to 49.65 ± 6.32 mg.kg−1), hexadecanoic acid (7.92 ± 0.09 to 49.01 ± 6.18 mg.kg−1), 2-butoxyethanol (7.48 ± 0.12 to 56.37 ± 26.20 mg.kg−1), and fucosterol (nd—282.96 ± 29.02 mg.kg−1) were the constituents found in the most relevant concentrations in the samples. The high presence of these compounds made the lipophilic extracts dominated by FAs (48.73 ± 0.77 to 331.91 ± 10.79 mg.kg−1), sterols (14.74± 0.74 to 321.25± 30.13 mg.kg−1), and alcohols (13.07 ± 0.04 to 51.87 ± 30.07 mg.kg−1).
Spectroscopic analysis of the lipophilic extracts (Table 1S) showed that samples mainly showed vibrations that corresponded to aliphatic carbon-hydrogen bonds (2963 to 2855 cm−1, stretching; 1457 to 1371 cm−1, bending), carbonyl groups (1742 to 1715 cm−1), and carbon-oxygen bonds (1230 to 1203 cm−1). Moreover, extracts from A. utricularis and D. confervoides had unsaturation (3009 to 3004 cm−1), while M. manginii and U. intestinalis had hydroxyl groups (3393 to 3357 cm−1). IR spectra and GC-MS chromatograms of the lipophilic extracts can be seen in the Supplementary Information section.
Antioxidant activity
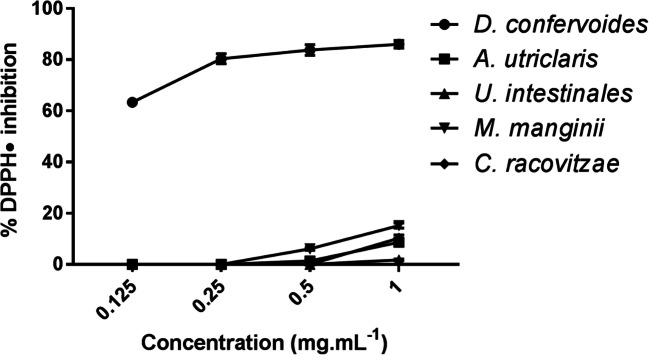
Evaluation of antioxidant activity of the lipophilic extracts (Fig. 1) showed that D. confervoides inhibited 86.03 ± 1.47% of the DPPH radical at 1 mg.mL−1 and maintained similar antioxidant potential at 0.500 and 0.250 mg.mL−1, which inhibited 83.79 ± 2.12 and 80.40 ± 1.94%, respectively. The lowest antioxidant capacity of the lipophilic extract was observed at 0.125 mg.mL−1, which had 63.39 ± 0.49% of inhibition of the DPPH radical. Moreover, comparison of the samples with the positive control revealed that all lipophilic extracts had lower antioxidant activity than ascorbic acid.
Fig. 1.
Antioxidant evaluation of lipophilic extracts from Antarctic macroalgae
As can be seen in Fig. 1, lipophilic extracts from A. utricularis, U. intestinalis, M. manginii, and C. racovitzae did not have significant antioxidant activity compared to D. confervoides. Generally, samples inhibited the DPPH radical in major proportions at 1 mg.mL−1, reaching 15.21 ± 1.01, 10.30± 1.26, and 8.70 ± 0.91% inhibition for M. manginii, C. racovitzae, and A. utricularis, respectively. However, at the maximum tested concentration, U. intestinalis had little antioxidant capacity, inhibiting 1.80 ± 0.36% of the DPPH radical. The lipophilic extracts mostly acted as antioxidants at 0.500 mg.mL−1, but their potential decreased or could not be observed at concentrations lower than 0.250 mg.mL−1.
Antimicrobial activity
Antimicrobial evaluation of n-hexane extracts from Antarctic macroalgae (Table 4) indicated the materials had activity against all the tested microorganisms except Enterococcus faecalis, which had bacterial growth in the evaluated concentrations (0.187 to 6 mg.mL−1). Desmarestia confervoides and M. manginii extracts did not have antimicrobial activity in the experimental conditions. In general, the lipophilic extracts had MICs that ranged from 6 to 1.5 mg.mL−1, varying according to the specimen and the microorganism. Furthermore, the MMC values of the samples indicated that concentrations that inhibited bacterial growth were bacteriostatic to the tested organisms.
Table 4.
Antimicrobial evaluation of n-hexane extracts from Antarctic macroalgae
| Microorganism | MIC of macroalgae extract (mg.mL-1) | ||
|---|---|---|---|
| U. intestinalis | C. racovitzae | A. utricularis | |
| Escherichia coli | 1.5 | 3 | 1.5 |
| Staphylococcus aureus | 3 | 6 | 3 |
| Salmonella Typhimurium | 3 | > 6 | 6 |
| Enterococcus faecalis | > 6 | 1.5 | > 6 |
Note: Minimum inhibitory concentration (MIC). Extracts from D. confervoides and M. manginii did not have antimicrobial activity in the tested microorganisms
According to the results seen in Table 4, growth of E. coli was inhibited at concentrations above 1.5 mg.mL−1 by A. utricularis and U. intestinalis extracts, while C. racovitzae had MIC of 3 mg.mL−1. In the case of Staphylococcus aureus, the MIC value of A. utricularis and U. intestinalis extracts was 3 mg.mL−1 while C. racovitzae inhibited bacterial growth at 6 mg.mL−1. Similar results were observed for Salmonella typhimurium, for which lipophilic extracts from C. racovitzae and A. utricularis had MIC of 6 mg.mL−1, while U. intestinalis had antimicrobial activity at concentrations above 3 mg.mL−1.
Multivariate analysis
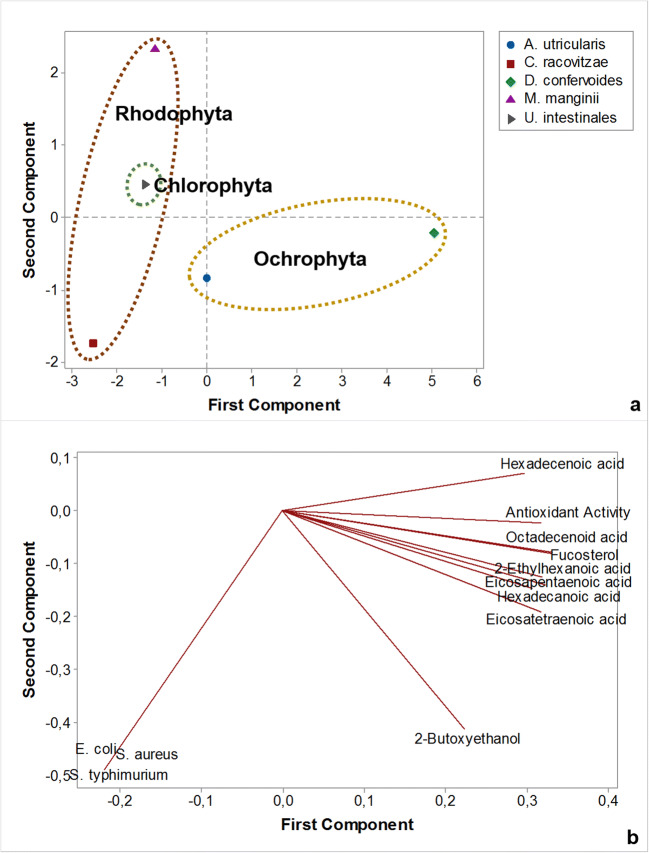
PCA was applied to evaluate the chemical composition, antioxidant activity, and antimicrobial activity among the studied specimens (Fig. 2). For the multivariate analysis, substances that caused significant variations including hexadecanoic, eicosatetraenoic, and eicosapentaenoic acids as well as fucosterol and 2-butoxyethanol were chosen. The obtained loading plot (Fig. 2b) indicated that most substances clustered along the positive axis of the first principal component (PC1) while those in the second principal component (PC2) varied from the negative to positive axis. On the other hand, the antimicrobial activity of the tested organisms was found to be negative in PC1 and PC2.
Fig. 2.
Multivariate analysis of the chemical composition and bioactivity of lipophilic extracts from Antarctic macroalgae
The resulting score plot (Fig. 2a) showed that the studied Antarctic macroalgae could be differentiated based on the chosen variables. In general lines, Ochrophyta representatives were found along the positive axis of PC1, while Rhodophyta and Chlorophyta species were observed along the negative axis of PC1. The results showed that bioactive potential can be associated with the three phyla of seaweeds.
Discussion
Chemical evaluation
Extractive yield and spectroscopic analysis
Previous reports in the literature indicate that lipophilic extracts of brown, green, and red macroalgae had values that ranged from 0.12 ± 0.01% DW to 1.74 ± 0.08% DW, which agree with the results found by us [4, 9]. Martins et al. (2018) analyzed Antarctic macroalgae and found small extractive yields in their specimens. Nonetheless, the extracts had antimicrobial and antifungal activities, indicating the presence of potential bioactive substances [11].
Spectroscopic evaluation of the lipophilic extracts indicated that the samples mainly consisted of lipid esters, since the main vibrations observed in the spectra corresponded to carbon-hydrogen bonds, carbon-oxygen bonds, and carbonyl groups [14]. Moreover, hydroxyl groups in the extracts of M. manginii and U. intestinalis were associated with non-esterified lipids and alcohols, as indicated in Table 3. Curiously, little information is available regarding the spectroscopic profiles of macroalgal extracts, despite the importance of fully characterizing these substances.
Fatty acids
FAs are among the vast lipid classes found in macroalgae, acting as membrane constituents (e.g., phosphoglycerides) or for energy storage (e.g., triacylglycerol). They generally have higher concentrations than other lipids, including sterols, hydrocarbons, and fatty alcohols, for instance [15–17]. In this sense, comparison between our results and those reported in the literature showed that the majority FAs found in U. intestinalis (hexadecanoic, hexadecanoic, and octadecenoic acids) were also identified by Martins et al. (2016), who analyzed the same species collected in the sub-Antarctic region [6]. Regarding C. racovitzae and A. utricularis, qualitative results were similar to the literature, but we found lower concentrations of PUFAs than reported by Pacheco et al. (2018). To the best of our knowledge, the FA profile of M. manginii and D. confervoides has not been previously reported in the literature. Nonetheless, representatives from the same order had similar patterns to those observed by us [7, 18].
Among the reasons explaining the variations between our results and those reported in the literature are the influences of the Antarctic environment, which include limited photoperiod and low water temperature, salinity, nutrient disposal, and pH [4, 19]. These abiotic parameters influence the production of FAs in seaweeds, primarily inducing the biosynthesis of PUFAs in order to maintain the integrity of membranes [18, 19]. Moreover, differences in the extraction approaches may also have influenced the overall results, since the extraction of FAs is generally performed using a solution of chloroform:methanol [20].
Carboxylic, dicarboxylic, and tricarboxylic acids
Carboxylic and dicarboxylic acids, including hexanedioic, octanedioic, nonanedioic, and undecanedioic acids, have been widely reported in the literature as constituents of seaweeds [3, 4]. These compounds have been associated with biological activities such as antimicrobial action, as well as for treatment of skin hyperpigmentation [21]. Previous research has identified the presence of carboxylic and dicarboxylic acids in macroalgae. Santos et al. (2016) detected octanedioic and 2-butenedioic acid as majority dicarboxylic acids in Undaria pinnatifida and Cystoseira tamariscifolia, with total amounts of 99.8 and 2.2 mg.kg−1 DW, respectively [3]. In turn, [9] reported that nonanedioic acid was found in higher concentrations in green seaweed species compared to the other phyla, while red macroalgae mainly contained octanedioic acid.
Benzoic acid derivatives comprise a class of aromatic carboxylic acids that are formed by the shikimate pathway [22]. We found benzoic, benzeneacetic, and benzenedicarboxylic acids in the green, red, and brown Antarctic macroalgae, although other compounds have been detected in seaweeds, such as salicylic, gentisic, vanilic and gallic acids [23]. Furthermore, benzeneacetic acid can be categorized as an auxin, which is a plant hormone related to root development [24]. To the best of our knowledge, this article is the first to report detection of carboxylic and dicarboxylic acids in the studied Antarctic macroalgae.
Sterols
Sterols include a vast number of compounds that play roles as membrane constituents and hormonal precursors in aquatic and terrestrial organisms [10]. According to the literature [23], cholesterol and its derivatives are generally predominant in red and green algae, while fucosterol and its derivatives are more commonly found in brown seaweeds. Our findings corroborate these reports. Sterols are known for their biological activities, which include antioxidant, antitumor, antibacterial, antiviral, antifungal, and antiulcerative, among others [23, 25].
According to the literature [10], specimens of A. utricularis and Desmarestia anceps collected on King George Island (Antarctica) also contained fucosterol as the main sterol [10]. Moreover, in that study, other steroidal components were also detected in small amounts, including stigmasterol, cholesterol, and ergosterol, which were not observed in our samples. Differences in extraction and analytical approaches may have influenced the results, since the specimens were collected under similar environmental conditions. Sterols were also found in relevant concentrations in two other studies that evaluated Ulva lactuta and Sargassum muticum collected along the Portuguese coast [3, 9].
Previous reports indicate that the content of steroids in macroalgae varies considerably during the year, reaching maximum values in winter and minimum levels in summer, showing that seasonal variations play an important role in the steroid biosynthesis of seaweeds [2, 26]. As can be observed in Table 1, our samples were collected in the Antarctic summer, which could have lowered the concentration of steroids compared to what would be observed in other seasons [26]. Moreover, it is thought that other abiotic conditions, including water temperature and growth stage, may also affect the production of sterols by macroalgae [26, 27].
Other constituents
Several other constituents were found in the macroalgae and distributed in various chemical classes, including ketones, aldehydes, hydrocarbons, and amino acids. The presence of these constituents can be related to several biochemical mechanisms in seaweeds to survive in the Antarctic environment, since these compounds are linked to defense against oxidant agents and ultraviolet radiation, as well as to chemical signaling among individuals [28, 29].
Among the detected substances were α-tocopherol and phytol, which can be found in lipid membranes and storage structures. According to the literature, the content of tocopherols is higher in brown macroalgae than the other classes of seaweeds. Our results corroborate those findings, since we only observed α-tocopherol in Ochrophyta specimens. Moreover, concentrations previously reported for α-tocopherol ranged from 9.6 to 14 μg.g−1 DW in Undaria pinnatifida, as also found by us [26]. It is worth noting that the consumption of α-tocopherol and phytol can have biological benefits due to the antioxidant and anticancer activities of these compounds [9, 26].
Evaluation of the lipophilic profile of Antarctic macroalgae showed the presence of trimethylbenzene, which is thought to derive from sugar or carotenoid degradation [30]. Other types of alkylbenzenes have also been detected in macroalgae, including, for instance, ethylbenzene, ethyltoluene, and tetramethylbenzene when analyzing volatile organic compounds of Oscillatoria perornata and Palmaria palmata [31, 32]. Tetradecane was also observed in small amounts in our specimens, and its presence can be associated with chemical signaling during the algal reproductive cycle [28].
Concerning other compounds found in the lipophilic profile, dihydroactinidiolide is a carotenoid derivative widely reported in the analysis of volatile compounds produced by macroalgae. It has been associated with the prevention of coronary diseases and tumors in humans [24]. Benzaldehyde was also detected in the specimens, and its presence can be associated to pathways of amino acid biosynthesis [33]. Finally, L-cysteine was observed in the samples, which can be related to defense mechanisms of seaweeds against antioxidant stress [34]. Studying the metabolites of macroalgae, Belghit et al. (2017) also detected relevant amounts of L-cysteine in brown and red seaweeds, corroborated by our results [29].
Antioxidant activity
Antioxidant evaluation showed that lipophilic extracts from Antarctic macroalgae have distinct levels of inhibiting DPPH radical scavenging. Generally, brown algae had greater antioxidant activity than red and green algae, in agreement with data reported in the literature [35]. In this sense, Paiva et al. (2016) indicated that extracts from Ulva compressa, Gelidium microdon, and Pterocladiella capillacea at concentrations of 2 mg.mL−1 inhibited DPPH radical activity by 40.21 ± 2.84, 47.73 ± 3.01, and 26.14 ± 1.90% [36]. We found that D. confervoides had greater antioxidant capacity than indicated in the literature, while the other specimens had lower antioxidant activity.
The presence of antioxidants in seaweeds can be associated with mechanisms of defense and survival against oxidative stress and abiotic parameters [37, 38]. Generally, the antioxidant capacity of extracts is related to certain constituents, such as phenolic compounds, carbohydrates, FAs, and sterols [39, 40]. Among the components of lipophilic extracts, octadecenoic and octadecadienoic acids as well as phytosterols have been highlighted as compounds with antioxidant activity [39]. Indeed, extracts of D. confervoides, which had higher amounts of these lipid components compared to the other samples, had higher inhibition of DPPH radical activity than the other macroalgal extracts.
Antimicrobial activity
Previous studies of the lipophilic extracts of macroalgae have identified several biological activities, including antimicrobial, anti-inflammatory, antifungal, anticoagulant, antitumor, and antioxidant properties [11, 35, 41]. In this sense, metabolites from seaweeds have been receiving increased attention for use in various areas, including cosmetics, foods, and pharmaceuticals, due to their wide possible applications [2].
According to previous research works, the antibacterial activity of macroalgal extracts can be associated with the presence of distinct FAs [42, 43]. Based on our results, these biomolecules were present in greater amounts in the samples compared to other biochemical classes. Despite generally having weak bioactivities when isolated, several FAs acting together can promote bacterial inhibition, probably due to synergistic effects [43]. Among the reasons that can explain the antimicrobial activity of FAs are the amphipathic features of these molecules, allowing them to interact and penetrate membranes, inducing damage, and allowing the diffusion of other molecules that can further affect other biological processes [11, 42].
The antimicrobial activity found in the extracts from U. intestinalis, C. racovitzae, and A. utricularis agrees with the previous results of previous studies of macroalgal extracts. In this sense, Shanmughapriya et al. (2008) studied methanol:toluene extracts of Sargassum wightii and found inhibition of E. faecalis, Staphylococcus epidermidis, E. coli, S. aureus, and Pseudomonas aeruginosa [44]. Moreover, Cortés et al. 2014 used dichloromethane extracts from Ceramium rubrum against Yersinia ruckeri and Syspastospora parasitica, finding MICs of approximately 0.5 and 2 mg.mL−1. Therefore, macroalgal extracts are potential antimicrobial agents with promising health applications.
Multivariate analysis
Evaluation of the PCA results showed that the Antarctic macroalgae could be distinguished according to their respective phyla according to parameters that involve biological activities and chemical composition. In this sense, D. confervoides was found along the positive axis of PC1, possibly due to its higher antioxidant capacity, while intermediate concentrations of bioactive compounds and antibacterial activity probably influenced the results of A. utricularis. Similarly, antibacterial activity was probably a key parameter for the presence of C. racovitzae and U. intestinalis along the negative axis of PC1. Finally, intermediate concentrations of FAs, sterols, and alcohols as well as little to no biological potential possibly influenced the results of M. manginii, with presence along the negative axis of PC1.
The results obtained in the multivariate analysis agree with those reported by Kumar et al. (2011), who evaluated more than 20 macroalgal species from Rhodophyta, Chlorophyta, and Ochrophyta phyla found along the Indian coast [45]. The study indicated that PCA was influenced by the lower antioxidant activity of red macroalgae compared to brown seaweeds, which we also observed. Moreover, the use of FAs and VOCs as variables in the multivariate analysis allowed the discrimination of phyla, as also accomplished by other studies in the area [45, 46].
Conclusion
The lipophilic profiles of n-hexane extracts from red, brown, and green Antarctic macroalgae were characterized, indicating the presence of various compounds, mainly sterols, fatty acids, and other carboxylic acids. Several constituents are biosynthesized within seaweeds as defense mechanisms against the extreme conditions of the Antarctic environment. PCA indicated that bioactive compounds and their biological activities were associated with the macroalgal phyla. Therefore, the analyzed species have noteworthy potential for use in the biotechnological, pharmaceutical, and food areas, since their lipophilic components are associated with beneficial biological activities including antibacterial, antifungal, and antioxidant.
Supplementary Information
(DOCX 1343 kb)
Authors’ contributions
Conceptualization: Dalila Venzke, Pio Colepicolo; Methodology: Ivandra S. de Santi; Formal analysis and investigation: Marco A. Z. ds Santos, Rodrigo de A. Vaucher; Writing (original draft preparation): Lucas M. Berneira; Writing (review and editing): Caroline C. da Silva; Funding acquisition: Claudio M. P. Pereira.
Funding
We are grateful for the logistical support provided by the Brazilian Antarctic Program and for the financial support by the Office to Coordinate Improvement of University Personnel (CAPES—grant 99999.002378/2015-9), Rio Grande do Sul State Research Foundation (FAPERGS—grant 2010/50193-1), São Paulo State Research Foundation (FAPESP) and National Council for Scientific and Technological Development (CNPq—grant 407588/2013-2).
Declarations
Conflicts of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shobier AH, Abdel Ghani SA, Barakat KM. GC/MS spectroscopic approach and antifungal potential of bioactive extracts produced by marine macroalgae. Egypt J Aquat Res. 2016;42:289–299. doi: 10.1016/j.ejar.2016.07.003. [DOI] [Google Scholar]
- 2.Andrade PB, Barbosa M, Matos RP, Lopes G, Vinholes J, Mouga T, Valentão P. Valuable compounds in macroalgae extracts. Food Chem. 2013;138:1819–1828. doi: 10.1016/j.foodchem.2012.11.081. [DOI] [PubMed] [Google Scholar]
- 3.Santos SAO, Oliveira CSD, Trindade SS, Abreu MH, Rocha SSM, Silvestre AJD. Bioprospecting for lipophilic-like components of five Phaeophyta macroalgae from the Portuguese coast. J Appl Phycol. 2016;28:3151–3158. doi: 10.1007/s10811-016-0855-y. [DOI] [Google Scholar]
- 4.Santos MAZ, Colepicolo P, Pupo D, Fujii MT, de Pereira CMP, Mesko MF. Antarctic red macroalgae: a source of polyunsaturated fatty acids. J Appl Phycol. 2017;29:759–767. doi: 10.1007/s10811-016-1034-x. [DOI] [Google Scholar]
- 5.Passos LF, Berneira LM, Poletti T, Mariotti KC, Carreño NLV, Hartwig CA, Pereira CMP (2020) Evaluation and characterization of algal biomass applied to the development of fingermarks on glass surfaces. Aust J Forensic Sci:1–10. 10.1080/00450618.2020.1715478
- 6.Martins RM, dos Santos MAZ, Pacheco BS, et al. Fatty acid profile of the Chlorophyta species from Chile’s sub-Antarctic region. Acad J Sci Res. 2016;4:93–98. doi: 10.15413/ajsr.2015.0154. [DOI] [Google Scholar]
- 7.Schmid M, Kraft LGK, van der Loos LM, Kraft GT, Virtue P, Nichols PD, Hurd CL. Southern Australian seaweeds: a promising resource for omega-3 fatty acids. Food Chem. 2018;265:70–77. doi: 10.1016/j.foodchem.2018.05.060. [DOI] [PubMed] [Google Scholar]
- 8.Berneira L, da Silva C, Poletti T, Ritter M, dos Santos M, Colepicolo P, de Pereira CMP. Evaluation of the volatile composition and fatty acid profile of seven Antarctic macroalgae. J Appl Phycol. 2020;32:3319–3329. doi: 10.1007/s10811-020-02170-9. [DOI] [Google Scholar]
- 9.Santos SAO, Vilela C, Freire CSR, Abreu MH, Rocha SM, Silvestre AJD. Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem. 2015;183:122–128. doi: 10.1016/j.foodchem.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Pereira CMP, Nunes CFP, Zambotti-Villela L, Streit NM, Dias D, Pinto E, Gomes CB, Colepicolo P. Extraction of sterols in brown macroalgae from Antarctica and their identification by liquid chromatography coupled with tandem mass spectrometry. J Appl Phycol. 2017;29:751–757. doi: 10.1007/s10811-016-0905-5. [DOI] [Google Scholar]
- 11.Martins RM, Nedel F, Guimarães VBS, da Silva AF, Colepicolo P, de Pereira CMP, Lund RG. Macroalgae extracts from Antarctica have antimicrobial and anticancer potential. Front Microbiol. 2018;9:1–10. doi: 10.3389/fmicb.2018.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellati F, Benvenuti S, Magro L, Melegari M, Soragni F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. Pharm and Biomedical Anal. 2004;35:289–301. doi: 10.1016/S0731-7085(03)00645-9. [DOI] [PubMed] [Google Scholar]
- 13.CLSI, M07-A10: Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. CLSI (Clinical and Laboratory Standards Institute). 2015.
- 14.Tanniou A, Vandanjon L, Gonçalves O, Kervarec N, Stiger-Pouvreau V. Rapid geographical differentiation of the European spread brown macroalga Sargassum muticum using HRMAS NMR and Fourier-Transform Infrared spectroscopy. Talanta. 2015;132:451–456. doi: 10.1016/j.talanta.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Li-Beisson Y, Thelen JJ, Fedosejevs E, Harwood JL. The lipid biochemistry of eukaryotic algae. Prog Lipid Res. 2019;74:31–68. doi: 10.1016/j.plipres.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Loftsson T, Ilievska B, Asgrimsdottir GM, Ormarsson OT, Stefansson Fatty acids from marine lipids: Biological activity, formulation and stability. J Drug Deliv Sci Technol. 2016;34:71–75. doi: 10.1016/j.jddst.2016.03.007. [DOI] [Google Scholar]
- 17.Kumari P, Kumar M, Reddy CRK, Jha B. Algal lipids, fatty acids and sterols (2013) Functional Ingredients from Algae for Foods and Nutraceuticals. Woodhead Publishing 7-134. 10.1533/9780857098689.1.87
- 18.Graeve M, Kattner G, Wiencke C, Karsten U. Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. Mar Ecol Prog Ser. 2002;231:67–74. doi: 10.3354/meps231067. [DOI] [Google Scholar]
- 19.Becker S, Graeve M, Bischof K. Photosynthesis and lipid composition of the Antarctic endemic rhodophyte Palmaria decipiens: effects of changing light and temperature levels. Polar Biol. 2010;33:945–955. doi: 10.1007/s00300-010-0772-5. [DOI] [Google Scholar]
- 20.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Fitton A, Goa KL. Azelaic Acid. Drugs. 1991;41(5):780–798. doi: 10.2165/00003495-199141050-00007. [DOI] [PubMed] [Google Scholar]
- 22.Widhalm JR, Dudareva N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol Plant. 2015;8(1):83–97. doi: 10.1016/j.molp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Torres P, Santos JP, Chow F, dos Santos DYAC. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta) Algal Res. 2019;37:288–306. doi: 10.1016/j.algal.2018.12.009. [DOI] [Google Scholar]
- 24.Torres P, Novaes P, Ferreira LG, et al. Effects of extracts and isolated molecules of two species of Gracilaria (Gracilariales, Rhodophyta) on early growth of lettuce. Algal Res. 2018;32:142–149. doi: 10.1016/j.algal.2018.03.016. [DOI] [Google Scholar]
- 25.Santos MAZ, Miguel R, Pereira CMP, Freitag RA, Bairros AV. Analysis of Phytosterols in Plants and Derived Products by Gas Chromatography – A Short Critical Review. Austin Publ Gr. 2014;1(5):1–4. [Google Scholar]
- 26.Boulom S, Robertson J, Hamid N, Ma Q, Lu J. Seasonal changes in lipid, fatty acid, α-tocopherol and phytosterol contents of seaweed, Undaria pinnatifida, in the Marlborough Sounds. New Zealand Food Chem. 2014;161:261–269. doi: 10.1016/j.foodchem.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Honya M, Kinoshita T, Ishikawa M, Mori H, Nisizawa K. Seasonal variation in the lipid content of cultured Laminaria japonica: fatty acids, sterols, β-carotene and tocopherol. J Appl Phycol. 1994;6:25–29. doi: 10.1007/BF02185900. [DOI] [Google Scholar]
- 28.de Alencar DB, Diniz JC, Rocha SAS, dos Santos Pires-Cavalcante KM, Freitas JO, Nagano CS, Sampaio AH, Saker-Sampaio S. Chemical composition of volatile compounds in two red seaweeds, Pterocladiella capillacea and Osmundaria obtusiloba, using static headspace gas chromatography mass spectrometry. J Appl Phycol. 2017;29(3):1571–1576. doi: 10.1007/s10811-016-1020-3. [DOI] [Google Scholar]
- 29.Bruckner CG, Heesch S, Liland N, et al. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017;26:240–249. doi: 10.1016/j.algal.2017.08.001. [DOI] [Google Scholar]
- 30.Sun SM, Chung GH, Shin TS. Volatile compounds of the green alga, Capsosiphon fulvescens. J Appl Phycol. 2012;24:1003–1013. doi: 10.1007/s10811-011-9724-x. [DOI] [Google Scholar]
- 31.Le Pape M-A, Grua-Priol J, Prost C, Demaimay M. Optimization of Dynamic Headspace Extraction of the Edible Red Algae Palmaria palmata and Identification of the Volatile Components. J Agric Food Chem. 2004;52:550–556. doi: 10.1021/jf030478x. [DOI] [PubMed] [Google Scholar]
- 32.Tellez MR, Schrader KK, Kobaisy M. Volatile components of the cyanobacterium Oscillatoria perornata (Skuja) J Agric Food Chem. 2001;49:5989–5992. doi: 10.1021/jf010722p. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Baldermann S, Yoshikawa K, Fujita A, Mase N, Watanabe N. Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci World J. 2014;2014:1–8. doi: 10.1155/2014/289780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakinuma M, Park CS, Amano H. Distribution of free L-cysteine and glutathione in seaweeds. Fish Sci. 2001;67:194–196. doi: 10.1046/j.1444-2906.2001.00223.x. [DOI] [Google Scholar]
- 35.Tenorio-Rodriguez PA, Murillo-Álvarez JI, Campa-Cordova ÁI, Angulo C. Antioxidant screening and phenolic content of ethanol extracts of selected Baja California Peninsula macroalgae. J Food Sci Technol. 2017;54:422–429. doi: 10.1007/s13197-016-2478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paiva L, Lima E, Isabel A, Marcone M, Baptista J. Health-promoting ingredients from four selected Azorean macroalgae. Food Res Int. 2016;89:432–438. doi: 10.1016/j.foodres.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Alves C, Pinteus S, Simões T, Horta A, Silva J, Tecelão C, Pedrosa R. Bifurcaria bifurcata: a key macro-alga as a source of bioactive compounds and functional ingredients. Int J Food Sci Technol. 2016;51:1638–1646. doi: 10.1111/ijfs.13135. [DOI] [Google Scholar]
- 38.Zubia M, Fabre MS, Kerjean V, Lann KL, Stiger-Pouvreau V, Fauchon M, Deslandes E. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 2009;116:693–701. doi: 10.1016/j.foodchem.2009.03.025. [DOI] [Google Scholar]
- 39.Fernandes F, Andrade PB, Ferreres F, Gil-Izquierdo A, Sousa-Pinto I, Valentão P. The chemical composition on fingerprint of Glandora diffusa and its biological properties. Arab J Chem. 2017;10:583–595. doi: 10.1016/j.arabjc.2015.01.012. [DOI] [Google Scholar]
- 40.Feller R, Matos ÂP, Mazzutti S, Moecke EHS, Tres MV, Derner RB, Oliveira JV, Junior AF. Polyunsaturated Ω-3 and Ω-6 fatty acids, total carotenoids and antioxidant activity of three marine microalgae extracts obtained by supercritical CO2 and subcritical n-butane. J Supercrit Fluids. 2017;133:437–443. doi: 10.1016/j.supflu.2017.11.015. [DOI] [Google Scholar]
- 41.Guedes EAC, dos Santos Araújo MA, Souza AKP, de Souza LIO, de Barros LD, de Albuquerque Maranhão FC, Sant’Ana AEG. Antifungal Activities of Different Extracts of Marine Macroalgae Against Dermatophytes and Candida Species. Mycopathologia. 2012;174:223–232. doi: 10.1007/s11046-012-9541-z. [DOI] [PubMed] [Google Scholar]
- 42.Pacheco BS, Dos Santos MAZ, Schultze E, et al. Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front Bioeng Biotechnol. 2018;6:185. doi: 10.3389/fbioe.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cortés Y, Hormazábal E, Leal H, Urzúa A, Mutis A, Parra L, Quiroz A. Novel antimicrobial activity of a dichloromethane extract obtained from red seaweed Ceramium rubrum (Hudson) (Rhodophyta:Florideophyceae) against Yersinia ruckeri and Saprolegnia parasitica agents that cause disease. Electron J Biotechnol. 2014;17(3):126–131. doi: 10.1016/j.ejbt.2014.04.005. [DOI] [Google Scholar]
- 44.Shanmughapriya S, Manilal A, Sujith S, Selvin J, Kiran GS. Antimicrobial activity of seaweeds extracts against multiresistant pathogens. Ann Microbiol. 2008;58:535–541. doi: 10.1007/BF03175554. [DOI] [Google Scholar]
- 45.Kumar M, Kumari P, Trivedi N, Shukla MK, Gupta V, Reddy CRK, Jha B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J Appl Phycol. 2011;23:797–810. doi: 10.1007/s10811-010-9578-7. [DOI] [Google Scholar]
- 46.Berneira LM, da Silva CC, Passos LF, Mansilla A, dos Santos MAZ, de Pereira CMP. Evaluation of volatile organic compounds in brown and red sub-Antarctic macroalgae. Braz J Bot. 2021;44:79–84. doi: 10.1007/s40415-020-00684-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 1343 kb)