Abstract
Bombyx mori gloverin A2 (BMGlvA2) is an induced antimicrobial insect protein isolated from Bombyx mori. This study was conducted to explore the effect and potential mechanisms of BMGlvA2 on inflammatory responses and cellular functions in intestinal epithelial cells (IPEC-J2) exposure to enterotoxigenic E. coli (ETEC). IPEC-J2 cells pretreated with or without BMGlvA2 (12.5 μg/mL) were challenged by ETEC K88 (1×106 CFU/well) or culture medium. We show that BMGlvA2 pretreatment increased the cell viability and improved the distribution and abundance of tight junction protein ZO-1 in IPEC-J2 cells exposure to ETEC (P < 0.05). Interestingly, BMGlvA2 not only decreased the expression levels of inflammatory cytokines such as the tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), but also decreased the expression level of Caspase3 and the apoptosis rate in the ETEC-challenged cells (P < 0.05). Importantly, BMGlvA2 decreased the protein abundances of two critical inflammation-associated signaling proteins, phosphorylated nuclear factor-kappa-B inhibitor alpha (p-IκBα) and phosphorylated nuclear factor-kappa B (p-NF-κB), in the ETEC-challenged cells. These results indicate that BMGlvA2 attenuates ETEC-induced inflammation in the IPEC-J2 cells by regulating the NF-κB signaling pathway, resulting in decreased secretion of inflammatory cytokine and reduced cell apoptosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00532-0.
Keywords: Antimicrobial peptide, BMGlvA2, ETEC, Inflammation, IPEC-J2 cells
Introduction
Microbial infection increases diarrhea rate and mortality of neonatal animals. A variety of antibiotics have been used to treat most bacteria-induced infections; however, the emergence of antibiotic resistance not only decreases the effectiveness of traditionally used antibiotics, but also leads to their residues in animal products and causes various toxic effects, including allergy, immunopathological effects, carcinogenicity, mutagenicity, nephropathy (gentamicin) and bone marrow toxicity (chloramphenicol) and even anaphylactic shock in humans [1–5]. Thus, there is an urgent need to develop novel substitutes for antibiotics [6].
Antimicrobial peptides (AMPs), also known as host defense peptides, are a large class of small cationic polypeptide molecules, which widely exist in various organisms to protect themselves from the infection of bacteria, fungi, viruses, etc. [7, 8]. Previous study indicated that AMPs exert their roles through destroying bacterial cell membrane, modulating immune response, and regulating inflammation [9]. For instance, amphoteric AMPs destroy membrane integrity through hydrophobic and electrostatic interactions, resulting in membrane leakage and death of cancer cells [10, 11]. Cecropin B has potential anticancer activity against leukemia cells, gastric cancer, and lung cancer [12]. A variety of amphibian AMPs, including magainins, aureins, tryptophyllins, demaseptin, phylloseptin, bombinins (BLP-7), and bombinin (H-BO), showed antiproliferative activities [13]. BMGlvA2, an induced antimicrobial insect protein isolated from Bombyx mori (B. mori), is a small cationic linear α-helical peptide belonging to cecropin family [14]. In previous study, BMGlvA2 was found to inhibit the growth of bacteria and increase the permeability of bacterial membrane by binding to the lipopolysaccharide (LPS) components on the surface of bacterial membrane [15]. However, it has no toxic effects on mammalian cells [14]. Although increasing evidences have shown that the AMPs can serve as a critical regulator for diverse biological events including immune responses [16–19], the mechanism behind the BMGlvA2-regulated immunity is just beginning to be explored.
In our previous study, the BMGlvA2 was successfully expressed and purified by using a heterologous system, and the recombinant BMGlvA2 was found to show a moderate antibacterial activity against various gram-negative and gram-positive bacteria [20]. In this study, we explored its protective effect and potential mechanisms of BMGlvA2 on inflammatory responses and cellular functions in intestinal epithelial cells (IPEC-J2) exposure to ETEC K88.
Materials and methods
Expression and purification of BMGlvA2
The engineered strain L Orgami B (DE3)-harboring the recombinant plasmid (pet32a-gloverin A2) was previously constructed in our laboratory [20]. The protein expression was induced by 1.0 mM isopropyl β-d-1 thiogalactoside (IPTG). After incubation for 8 h at 28 °C, bacterial cells were harvested by centrifugation at 8000 r/min for 15 min at 4℃, 20 mL phosphate-buffered solution (PBS) was added to washing the precipitation, centrifuged at 8000 r/min for 15 min at 4℃, and the supernatant was discarded. Then, 20 mL lysis buffer was added and incubated at 4 °C overnight. Then, schizolytic cells were sonicated (4-s pulse and 8-s interval; 30 cycles; Sonics-Vibra cell, USA). The supernatant was harvested by centrifugation at 8000 r/min for 20 min at 4 °C. The supernatant obtained above was filtered by 0.22-μm filter, and then applied to Ni2+–IDA column (Sangon Biotech, China) and purified according specification. Protein concentration was quantified by the BCA assay (Beyotime, China).
Cell culture
Intestinal porcine epithelial cells (IPEC-J2) were cultured in a 75-cm2 cell culture flask in DMEM-F12 with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. 1 × 105 cells/well were seeded in 12-well plates and grown to ~ 60% confluence at 37 °C in a CO2 incubator (5% v/v). On reaching 80–90% confluence, the cells were routinely passaged at a 1:3 split ratio (approximately every 2–3 days).
E. coli K88 challenge
IPEC-J2 cells were seeded in 12-well cell culture plates (2 × 105 cells/well), cultured overnight to make cells adhere and then incubated with different concentrations of E. coli K88 (0, 105, 106, 107, and 108 CFU/well) for 0.5, 1, 2, and 3 h to choose the suitable E. coli K88 treatment dose and duration for inducing inflammatory injury. Finally, the cells were collected for gene expression analysis.
BMGlvA2 pretreatment
IPEC-J2 cells were seeded in 12-well cell culture plates at a density of 2 × 105 cells/well and then maintained at 37 °C in a CO2 incubator (5% v/v). After reaching sub-confluence, the cells were pretreated with different concentrations of BMGlvA2 (0, 12.5, 25, 50, and 100 μg/mL) for 24 h. Finally, the cells were collected for gene expression analysis.
Cell treatment
IPEC-J2 cells were cultured in a 75-cm2 cell culture flask in DMEM-F12 with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. 1 × 105 cells/well were seeded in 12-well plates and grown to ~ 60% confluence at 37 °C in a CO2 incubator (5% v/v). After incubated with antimicrobial peptides BMGlvA2 (12.5 μg/mL) for 24 h or BAY11-7081(1 μM) for 2 h, then cells were challenged with 1 × 106 CFU/well ETEC K88 for 1 h or 2.5 h (only for assessment of apoptosis). It is worth noting that when challenged with ETEC K88, the cells were cultured in DMEM F12 medium supplemented with 2% FBS (without any antibiotics).
Cell viability assay
Cytotoxic effects on cell viability were measured by the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium] assay as previously described (Beyotime, Shanghai, China) after treatment as designed. After treatment, the supernatants were removed and cells were gently washed three times with phosphate-buffered saline (PBS, pH 7.4) to remove non-adherent bacteria. Then, 10 μL of MTT solution (5 mg/mL) was added to each well and plates were incubated for 4 h at 37 °C. After incubation, 150 μL of DMSO (dimethyl sulfoxide) was added into each well and incubated in a cell incubator for 10 min. The absorbance of the plate was determined at 570 nm by a UV-1100 spectrophotometer (ShangHai, China). All experiments were performed in triplicate.
RNA extraction and RT-PCR
Total RNA was isolated from IPEC-J2 cells using RNAiso Plus reagent (TaKaRa, Dalian, China). The quantity and quality of the isolated RNA were determined by absorbance at 260 and 280 nm [21]. And then, cDNA was synthesized using a reverse transcriptase kit (Takara, Dalian, China). Briefly, quantitative PCR was performed by QuanStudio 6 Flex Real-Time PCR detection system (Applied Biosystems, Foster City, CA, USA) with a total of 10 µL of assay solution containing 5 µL SYBR Green mix (Takara), 0.2 µL Rox, 3 µL deionized H2O, 1 µL cDNA template, and 0.4 µL each of forward and reverse primers (Qingke, China). The relative gene expressions compared with the housekeeping gene β-actin were calculated by 2−ΔΔCt [22].
Total protein extraction and western blot analysis
Protein samples were extracted using lysis buffer (Beyotime, Shanghai, China) according to the manufacturer’s recommendations. Western blot analysis was performed as before [23]. Briefly, proteins (25 μg/lane) were resolved by electrophoresis on 12% SDS-poly-acrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Richmond, CA, USA). The PVDF membranes were blocked (1 h at room temperature) in 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) before incubation with primary antibody. After thoroughly rinsing with TBS-T, the membranes were respectively incubated with rabbit anti-Tight Junction Protrin 1 (ZO1) (at 1:500 dilution, Novus Biologicals), rabbit anti-phospho-nuclear factor-κB (pNF-κB) p65 (at 1:500 dilution, Cell Signalling Technology, Inc., Danvers, MA, USA), mouse anti-NF-κB (at 1:500 dilution, Cell Signalling Technology), mouse anti-phospho-Iκb alpha (pIκbα) (at 1:500 dilution, Invitrogen), mouse anti-Iκbα (at 1:500 dilution, Cell Signalling Technology), and rabbit anti-GAPDH (at 1:15,000 dilution, Abcam plc) antibodies, with gentle agitation overnight at 4℃. Subsequently, the membranes were rinsed several times with TBS-T and then incubated with HRP-conjugated goat anti-rabbit IgG or anti-mouse IgG secondary antibody, at room temperature for 1 h (at 1:5000 dilution; Abcam plc). Finally, the membranes were rinsed several times with the TBS/T buffer at room temperature for 10 min each time. Blots were developed using a Clarity™ Western ECL Substrate (Bio-Rad Laboratories, Inc). The bands were visualized by exposure to X-OMAT BT films (Beyotime Institute of Biotechnology, Shanghai, China) for 1 min, and were quantified by using Quantity One software (Bio-Rad Laboratories, Inc). The relative abundance of each target protein was expressed as the ratio of targeted protein to GAPDH protein.
Immunofluorescence
Preparation of cell climbing slice: add 50 μL culture medium into the 6-well plate, put the treated with concentrated sulfuric acid coverslips, and press the coverslips with the gun head to remove the bubbles. Then, 500 μL of cell suspension was added to the coverslips. After 6 h, 1.5 mL medium was added to each well.
The cells were washed with PBS (pH7.4) three times after treatment according to the experimental design. Add 1 mL formaldehyde into each well and stand for 20 min at room temperature. Wash three times with PBS (pH 7.4) for 2 min each time, and then seal with 3% BSA (Beyotime Institute of Biotechnology, Shanghai, China) at room temperature for 30 min. Wash three times with PBS (pH 7.4) for 2 min each time, and then, samples were incubated overnight with the primary antibody at 4℃ and kept dark from light (rabbit anti-ZO-1; Novus). The samples were washed three times with PBS (pH 7.4) for 2 min each time and incubated for 2 h at room temperature with the appropriate secondary antibody (Alexa Fluor 488 conjugated goat anti-rabbit immunoglobulin (CST)). Samples with the biotinylated secondary antibody were incubated with Avidin Alexa Fluor 488 (CST) for visualization of the secondary antibody. Then, the nuclei were counterstained with DAPI (Sigma-Aldrich). Samples were imaged using a confocal scanning microscope (NIKON ECLIPSE TI-SR).
Assessment of apoptosis by flow cytometry
The proportion of apoptotic cells in IPEC-J2 cells was determined by flow cytometry (CytoFlex, Beckman Coulter, Inc., Brea, CA, USA) using PE Annexin V Apoptosis Detection Kit I (Becton, Dickinson and Company, BD Biosciences, San Jose, CA, USA). When IPEC-J2 cells were grown to ~ 60% confluence at 37 °C in a CO2 incubator (5% v/v), they were incubated with BMGlvA2 for 24 h (BMGlvA2, BMGlvA2 + E. coli K88). When IPEC-J2 cells were grown to ~ 90% confluence at 37 °C in a CO2 incubator (5% v/v), they were incubated with Bay for 2 h (Bay, Bay + E. coli K88). Cells were challenged with 1 × 106 CFU/well E. coli K88 for 2.5 h (BMGlvA2 + E. coli K88, E. coli K88, Bay + E. coli K88) before sample collection; control cells were cultured in a culture medium without any treatment. Treated cells were harvested and labeled with an anti-Annexin V-FITC Apoptosis Detection Kit (BD Biosciences, USA). Floating cells were collected; then, attached cells were washed with 0.01 M PBS and digested with trypsin for 2 min. Finally, the digested cells and floating cells were added together to centrifugate at 350 g for 10 min; then, the cells were stained with 2 µL of Annexin V-FITC fluorescent dye at 4 °C in the dark. After 10 min, add 2 µL of PI staining for 5 min at 4 °C in the dark. Finally, detection of apoptotic cells was completed within 1 h after the addition of 400 µL Annexin V binding buffer (1 ×).
Statistics analysis
All statistical analysis was performed using SPSS 21.0 software. Data were expressed as the mean ± standard error of the mean (SEM). Statistical analysis of treatment of IPEC-J2 was carried out using two-way ANOVA followed by Duncan’s multiple comparisons test. Image production was carried using GraphPad Prism software (Version 7. GraphPad Software Inc., CA, USA).
Results
Influences of enterotoxigenic Escherichia coli challenge on viability and inflammatory response of IPEC-J2 cells
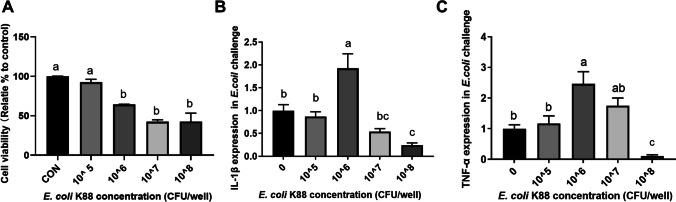
To explore the influence of enterotoxigenic Escherichia coli (ETEC) challenge on cell viability, IPEC-J2 cells were treated by ETEC at different final concentrations (1 × 105, 1 × 106, 1 × 107, and 1 × 108 CFU/well) for 3 h. As shown in Fig. 1A, the cell viability was significantly decreased upon ETEC challenge at a dose higher than 1 × 105 CFU/well. Moreover, the expression levels of IL-1β and TNF-α were significantly increased in the IPEC-J2 cells exposure to ETEC at a dose of 1 × 106 CFU/well. But a higher dose (1 × 107 and 1 × 108) led to downregulation of the IL-1β and TNF-α expression in the IPEC-J2 cells (Fig. 1B, C). Hence, the ETEC treatment dose used in further experiments was 1 × 106 CFU/well.
Fig. 1.
ETEC challenge affects cell viability and inflammatory gene expression in IPEC-J2 cells. IPEC-J2 cells (1 × 105 cells/well) were treated with E. coli K88 at different final concentrations (1 × 105, 1 × 106, 1 × 107, and 1 × 108 CFU/well) for 3 h. A, cell viability; B, the expression of IL-1β; C, the expression of TNF-α. Data are presented as mean ± SEM. a–bValues within a column differ if they do not share a common superscript (P < 0.05)
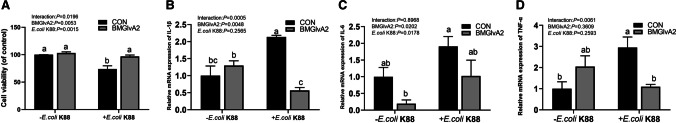
Effect of BMGlvA2 on cell viability and inflammatory responses of IPEC-J2 cells upon ETEC challenge
In this study, the BMGlvA2 has been successfully expressed and purified (Fig. S1). To explore the influence of BMGlvA2 on cell viability, the IPEC-J2 cells were treated with BMGlvA2 at different concentrations (0, 4, 20, and 100 μg/mL) for 24 h. As shown in Fig. 2, BMGlvA2 had no negative influence (toxic effect) on cell viability in the range from 4 to 100 μg/mL (P > 0.05). Therefore, a moderate dose of 12.5 μg/mL was used for further studies. As shown in Fig. 3A, ETEC challenge decreased the viability in the IPEC-J2 cells. However, pretreatment of the cells with 12.5 μg/mL BMGlvA2 significantly increased the cell viability upon ETEC challenge (P < 0.05). Moreover, ETEC challenge significantly elevated the expression levels of inflammatory cytokines such as the IL-1β, IL-6, and TNF-α in the IPEC-J2 cells (P < 0.05). However, BMGlvA2 pretreatment significantly downregulated the expression levels of IL-1β and TNF-α in the IPEC-J2 cells exposure to ETEC (Fig. 3B–D).
Fig. 2.
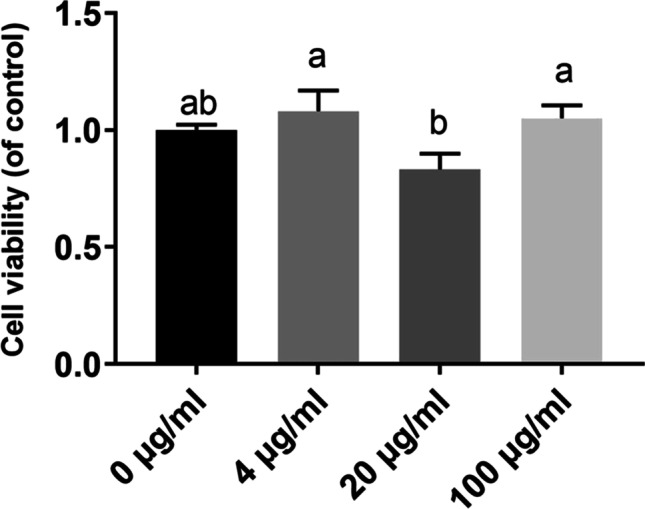
The influence of BMGlvA2 on cell viability in IPEC-J2 cells. IPEC-J2 cells were pretreated with various concentrations of BMGlvA2 (0, 4, 20, and 100 μg/mL) for 24 h. Data are presented as mean ± SEM. a–bValues within a column differ if they do not share a common superscript (P < 0.05)
Fig. 3.
The influence of BMGlvA2 on cell viability and inflammatory responses in IPEC-J2 cells induced by ETEC. IPEC-J2 cells were pretreated with 12.5 μg/mL BMGlvA2 for 24 h, followed by co-treatment with E. coli K88 (1 × 106 CFU/well) for another 1 h. A, cell viability; B, the expression of IL-1β; C, the expression of IL-6; D, the expression of TNF-α. Data are presented as mean ± SEM. a–bValues within a column differ if they do not share a common superscript (P < 0.05)
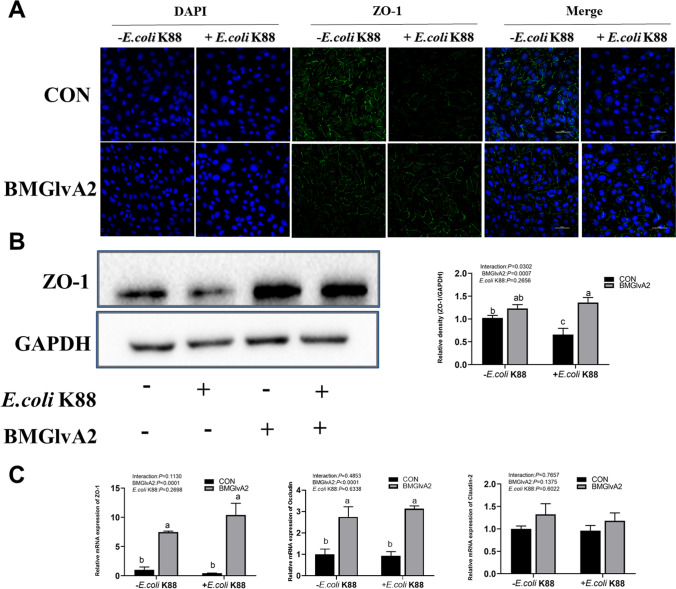
Effect of BMGlvA2 on tight junction protein distribution and abundance in IPEC-J2 cells upon ETEC challenge
We explored the distribution and abundance of zonula occludens-1 (ZO-1), one of the major tight junction-related proteins located in the IPEC-J2 cells by immunofluorescence analysis. We found that the ZO-1 staining in IPEC-J2 cells was diffuse due to ETEC challenge, with less staining in the tight intercellular junction area, indicating disruption of the tight junction upon ETEC infection. However, BMGlvA2 pretreatment attenuated the ETEC-induced disruption by improving the localization and abundance of the ZO-1 proteins in IPEC-J2 cells (Fig. 4A). The abundance of ZO-1 protein was validated by using western blot. We found that ETEC challenge significantly decreased the protein abundance of ZO-1 in the IPEC-J2 cells (Fig. 4B). However, BMGlvA2 pretreatment significantly elevated the protein abundance of ZO-1 in the ETEC-challenged cells (P < 0.05). Moreover, BMGlvA2 pretreatment significantly increased the expression levels of major tight junction-related proteins ZO-1 and Occludin in the ETEC-challenged cells (Fig. 4C) (P < 0.05).
Fig. 4.
The influence of BMGlvA2 on tight junction protein distribution and abundance in IPEC-J2 cells induced by ETEC. IPEC-J2 cells were pretreated with 12.5 μg/mL BMGlvA2 for 24 h, followed by co-treatment with E. coli K88 (1 × 106 CFU/well) for another 1 h. A, immunofluorescence of ZO-1; B, western blot analysis of protein expression of ZO-1; C, tight junction-related proteins. Representative immunofluorescent images for detection of ZO-1 (green) and DAPI (blue). Scale bar = 50 μm. Data are presented as mean ± SEM. a–cValues within a column differ if they do not share a common superscript (P < 0.05)
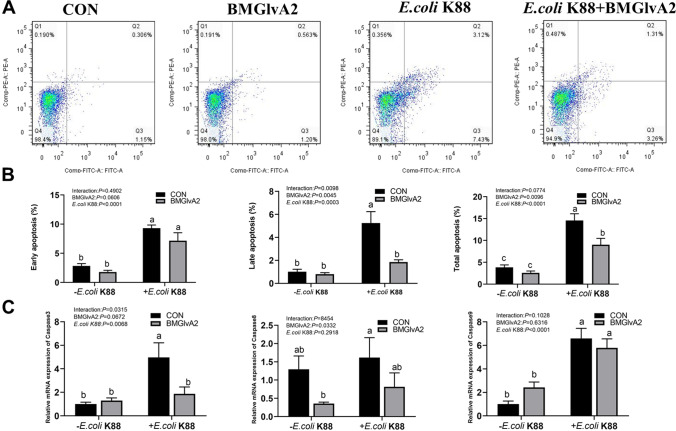
Effect of BMGlvA2 on apoptosis of IPEC-J2 cells upon ETEC challenge
As shown in Fig. 5A and B, as compared to the control group, ETEC challenge significantly elevated the early, late and total apoptosis rates in the IPEC-J2 cells (P < 0.05). However, BMGlvA2 pretreatment significantly decreased the late apoptosis and total apoptosis rates in the ETEC-challenged cells (P < 0.05). Additionally, ETEC challenge significantly elevated the expression levels of critical apoptosis-related genes such as the caspase 3, caspase 8, and caspase 9 in the cells (Fig. 5C). BMGlvA2 pretreatment significantly downregulated the expression level of apoptosis-related gene caspase3 in the ETEC-challenged cells (P < 0.05).
Fig. 5.
The influence of BMGlvA2 on cell apoptosis in IPEC-J2 cells induced by ETEC. IPEC-J2 cells were pretreated with 12.5 μg/mL BMGlvA2 for 24 h, followed by co-treatment with E. coli K88 (1 × 106 CFU/well) for another 2.5 h. A, apoptosis of flow cytometry; B, cell apoptosis rate; C, apoptosis-related genes. Data are presented as mean ± SEM. a–cValues within a column differ if they do not share a common superscript (P < 0.05)
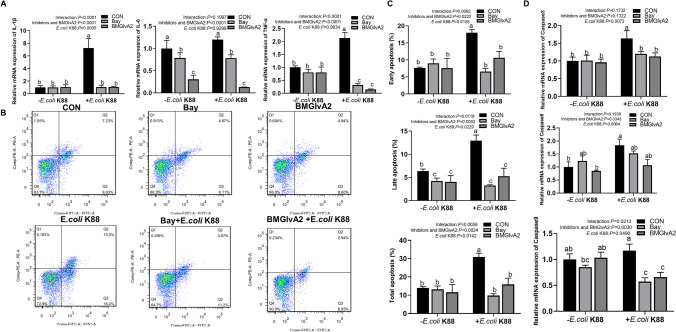
BMGlvA2 suppressed ETEC-induced cell apoptosis and inflammatory response via suppressing the IκBα/NF-κB signaling
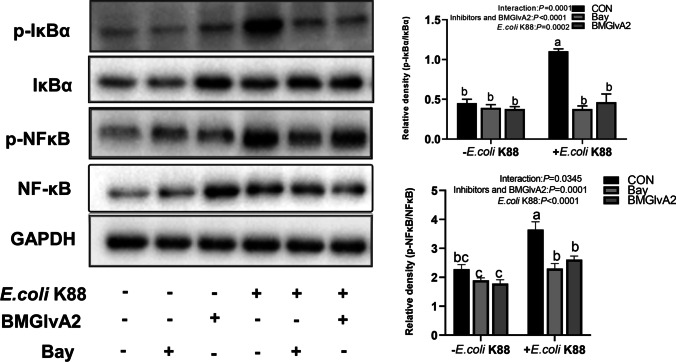
NF-κB transcription factor plays an important role in cell survival and apoptosis, and participates in a variety of inflammatory signal transduction. To further explore if the BMGlvA2-modulated inflammatory responses in the intestinal epithelial cells are associated with the NF-κB signaling pathway; the IPEC-J2 cells were pretreated with BMGlvA2 and NF-κB inhibitor and subsequently challenged by ETEC. To further explore whether the inflammatory response of intestinal epithelial cells regulated by BMGlvA2 is associated with NF-κB signaling pathway, IPEC-J2 cells were pretreated with BMGlvA2 and NF-κB inhibitor (Bay 11–7082) and subsequently challenged by ETEC. Both significantly inhibit the ETEC-induced inflammatory response, which is manifested as decreased apoptosis (such as early apoptosis, late apoptosis, and total apoptosis) and secretion of critical apoptosis-related genes (Caspase 3 and Caspase 9) and inflammatory cytokines (such as IL-1β, IL-6, and TNF-α) (Fig. 6). Meanwhile, BMGlvA2 and Bay pretreatment improved distribution and abundance of ZO-1 in the IPEC-J2 cells exposure to ETEC (Fig. 7A). Moreover, we detected the expression levels of key proteins in the signaling pathway by western blot analysis (Fig. 7B). The results showed that ETEC significantly elevated the abundance of phosphorylated IκBα (p-IκBα) and phosphorylated NF-κB (p-NF-κB) in the IPEC-J2 cells (P < 0.05). However, BMGlvA2 and Bay pretreatment significantly decreased their expression levels in the ETEC-challenged cells (P < 0.05).
Fig. 6.
BMGlvA2 inhibits ETEC-induced cell apoptosis and inflammation response by inhibiting the IκB-α/NF-κB signaling. IPEC-J2 cells were pretreated with 12.5 μg/mL BMGlvA2 for 24 h or Bay for 2 h, followed by co-treatment with E. coli K88 (1 × 106 CFU/well) for another 1 or 2.5 h. A, inflammatory mediator; B, apoptosis of flow cytometry; C, cell apoptosis rate; D, apoptosis-related genes. Data are presented as mean ± SEM. a–cValues within a column differ if they do not share a common superscript (P < 0.05)
Fig. 7.
BMGlvA2 improved ETEC-induced tight junction protein distribution and abundance by inhibiting the IκB-α/NF-κB signaling. IPEC-J2 cells were pretreated with 12.5 μg/mL BMGlvA2 for 24 h or Bay for 2 h, followed by co-treatment with E. coli K88 (1 × 106 CFU/well) for another 1 h. A Western blot analysis of protein expression of NF-κB signaling pathway. Data are presented as mean ± SEM. a–bValues within a column differ if they do not share a common superscript (P < 0.05)
Discussion
The integrity of intestinal epithelial barrier not only is the key to maintain the health and disease of the body [24], but also plays an important role in maintaining homeostasis of host intestinal epithelium [25]. Destruction of intestinal barrier function may lead to chronic immune activation, leading to local and systemic infections or diseases, including coeliac disease, colorectal cancer, inflammatory bowel disease (IBD), and metabolic disorders [26–28]. ETEC is a kind of common pathogenic bacteria, which produces heterogeneous fimbriae or non-fimbriae adhesins, attaches bacteria to the host receptor, and colonizes in the small intestine of animals [29]. Meanwhile, they transfer heat-labile and heat-stable enterotoxins to intestinal epithelial cells, destroy homoeostasis, disrupt homeostasis, and cause excessive fluid hyper secretion and watery diarrhea [30]. Previous studies have shown that Cecropins (e g., Cecropin A, Cecropin AD, and Mdc) alleviates intestinal inflammation in E. coli-induced animals by modulating the MyD88-NFκB signaling pathway [31–33].
In the present study, ETEC challenge significantly elevated the expression levels of inflammatory cytokines such as the IL-1β and TNF-α in the IPEC-J2 cells, which was consistent with previous studies using the same cell line [34, 35]. ETEC activates the innate immune system of host cells, producing pro-inflammatory and chemokines [36, 37]. However, BMGlvA2 pretreatment significantly decreased the expression levels of IL-1β and TNF-α in ETEC-induced cells. Intestinal epithelial integrity is regulated by the tight junction [38, 39]. Substantial evidence has shown that the pro-inflammatory cytokine TNF-α can reduce the expression of ZO-1 and Claudin-2, and destroy the structural integrity of intestinal epithelial cells [40–42]. In this study, we found that BMGlvA2 increased the expression of tight junction proteins and inhibited the decrease of ZO-1 abundance in IPEC-J2 cells induced by ETEC. These results showed that an imbalance in pro- and anti-inflammatory cytokine levels has been correlated with ETEC-induced intestinal epithelial cell injury. This is similar to previous studies [43, 44]. BMGlvA2 can increase the expression of tight junction protein in intestinal epithelial cells and improve the structural integrity of intestinal epithelial cells by inhibiting the expression of pro-inflammatory factors.
Apoptosis is the process of programmed cell death. Two pathways (intrinsic pathway and extrinsic pathway) are associated with apoptosis [45, 46]. In cells, apoptosis can be regulated by Bax and Bcl-2, which plays a role through p53 signal pathway and Caspase protein [29]. Caspase 3 directly promotes apoptosis through Caspase 8 of death receptor pathway and Caspase 9 of mitochondrial pathway [47]. In the present study, we detected the apoptosis of IPEC-J2 cells by flow cytometry. These results showed that the expression of Caspase 3, 8, and 9 were significantly upregulated in ETEC-induced cells, and their expressions were significantly decreased by BMGlvA2, which was consistent with previous research [48]. Moreover, ETEC significantly increased the early, late, and total apoptosis, while BMGlvA2 pretreatment significantly decreased the early, late, and total apoptosis. These results indicate that BMGlvA2 can inhibit the expression of Caspase gene and reduce apoptosis.
To further explore the anti-inflammatory mechanism of BMGlvA2, we used Bay11-7082 (specific inhibitors of NF-κB signaling pathway) to investigate whether NF-κB signaling pathway is involved. The NF-κB transcription factor can be activated by a series of stimuli (e.g., pathogenic derived substances, inflammatory cytokines, and a variety of enzymes) [49, 50], which play an important role in cell survival and apoptosis (Girard et al. 2009). Activation of NF-κB upregulated Bcl-Xs in rat hippocampal studies, and these effects were inhibited by IκBα [51]. Under physiological conditions, IκB proteins present in the cytoplasm inhibit NF-κB [52]. Phosphorylation of IκB leads to its degradation by proteasome, followed by release of NF-κB for nuclear translocation and gene transcription activation [53]. This pathway regulates the production of pro-inflammatory cytokines and recruitment of inflammatory cells, which contribute to the inflammatory response [54, 55]. In agreement with previous studies [56], elevated level of phosphorylated NF-κB and IκBα protein expression was documented in the IPEC-J2 cells induced by ETEC. However, BMGlvA2 inhibits the NF-κB and IκBα phosphorylation. The inhibition of phosphorylation of IκBα and NF-κB by BMGlvA2 was similar to that of inhibitor. Thus, we speculated that BMGlvA2 play a potential regulatory role by inhibiting IκBα/NF-κB pathway. Additionally, we also found that Bay11-7082 and BMGlvA2 had similar effects on inflammatory factors, tight junction proteins, and apoptosis, which further indicate that BMGlvA2 protects IPEC-J2 cells by inhibiting NF-κB signaling pathway.
In conclusion, BMGlvA2 can attenuate ETEC-induced inflammation and injury in the IPEC-J2 cells which was associated with decreased secretion of inflammatory cytokine, reduced cell apoptosis, and improved intestinal barrier functions. Moreover, the BMGlvA2 may act as a novel inhibitor of the NF-κB signaling pathway that can be tentatively used for the treatment of various inflammatory bowel diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Yaqiang Dai and Xiang Li for their help in the animal experiments. We also thank Huifen Wang and Quyuan Wang for purchasing consumables and reagents.
Author contribution
All authors contributed significantly to the work. JH conceived the study. QL designed and performed experiments and analyzed results with the help of QQF, GQS, DWC, BY, YHL, PZ, and XBM. QL wrote the manuscript with help of ZQH, JY, JQL, and HY.
Funding
This work was supported by the Key Research and Development Program of Sichuan Province (20ZDYF003), and the National Natural Science Foundation of China (31972599).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Darwish WS, Eldaly EA, Elabbasy MT, Ikenaka Y, Nakayama SMM, Ishizuka M. Antibiotic residues in food: the African scenario. Jpn J Vet Res. 2013;61(Suppl):S13–S22. doi: 10.1136/vr.f624. [DOI] [PubMed] [Google Scholar]
- 2.Nisha AR. Antibiotic residues - A global health hazard. Vet World. 2008;1:375–377. doi: 10.5455/vetworld.2008.375-377. [DOI] [Google Scholar]
- 3.Lin Q, Su G, Wu A, Chen D, He J (2019) Bombyx mori gloverin A2 alleviates enterotoxigenic Escherichia coli-induced inflammation and intestinal mucosa disruption. Antimicrob Resist Infect Control 8. 10.1186/s13756-019-0651-y [DOI] [PMC free article] [PubMed]
- 4.Merve B, Nurşen B. Importance of antibiotic residues in animal food. FCT. 2019;125:462–466. doi: 10.1016/j.fct.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Magana M, Pushpanathan M, Santos AL, Leanse L, Tegos GP. The value of antimicrobial peptides in the age of resistance. Lancet Infect Dis. 2020;20:e216–e230. doi: 10.1016/S1473-3099(20)30327-3. [DOI] [PubMed] [Google Scholar]
- 6.Deslouches B, Montelaro RC, Urish KL, Di YP. Engineered cationic antimicrobial peptides (eCAPs) to combat multidrug-resistant bacteria. Pharmaceutics. 2020;12:501. doi: 10.3390/pharmaceutics12060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umnyakova ES, Zharkova MS, Berlov MN, Shamova OV, Kokryakov VN. Human antimicrobial peptides in autoimmunity. Autoimmunity. 2020;53:137–147. doi: 10.1080/08916934.2020.1711517. [DOI] [PubMed] [Google Scholar]
- 8.Zhang LJ, Gallo RL. Antimicrobial peptides. Curr Biol. 2016;26:R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 9.Mookherjee N, Hancock REW. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64:922–933. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulicherla KK, Nelson R, Sobha K, Kotra SR, Peravali JB (2013) Antimicrobial peptides: an effective alternative for antibiotic therapy, Mintage J Pharm Med Sci 2.
- 11.Guo Z, Peng H, Kang J, Sun D. Cell-penetrating peptides: Possible transduction mechanisms and therapeutic applications (Review) Biomed Rep. 2016;4:528–534. doi: 10.3892/br.2016.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha R, Shukla P (2018) Antimicrobial Peptides (AMPs): roles, functions and mechanism of action. Protein Peptide Lett 25. 10.1007/s10989-019-09946-9
- 13.Zhou C, Wang Z, Peng X, Liu Y, Lin Y, Zhang Z, Qiu Y, Jin M, Wang R, Kong D. Discovery of two bombinin peptides with antimicrobial and anticancer activities from the skin secretion of Oriental fire-bellied toad. Bombina orientalis Chem Biol Drug Des. 2017;91:50–61. doi: 10.1111/cbdd.13055. [DOI] [PubMed] [Google Scholar]
- 14.Axén A, Carlsson A, Engström Å, Bennich H. Gloverin, an antibacterial protein from the immune hemolymph of hyalophora pupae. Febs J. 2010;247:614–619. doi: 10.1111/j.1432-1033.1997.00614.x. [DOI] [PubMed] [Google Scholar]
- 15.Nesa J, Sadat A, Buccini DF, Kati A, Franco OL. Antimicrobial peptides from Bombyx mori: a splendid immune defense response in silkworm. RSC Adv. 2019;10:512–523. doi: 10.1039/C9RA06864C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarvari M, Mikani A, Mehrabadi M. The innate immune gene relish and caudal jointly contribute to the gut immune homeostasis by regulating antimicrobial peptides in galleria mellonella. Dev Comp Immunol. 2020;110:103732. doi: 10.1016/j.dci.2020.103732. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Zhang G, Zhou M (2020) Inhibitory and anti‐inflammatory effects of two antimicrobial peptides moronecidin and temporin‐1Dra against Propionibacterium acnes in vitro and in vivo. J Pept Sci 26. 10.1002/psc.3255 [DOI] [PubMed]
- 18.Taute H, Bester MJ, Gaspar ARM. The dual functionality of antimicrobial peptides Os and Os-C in human leukocytes. J Pept Sci. 2019;25:e3156. doi: 10.1002/psc.3156. [DOI] [PubMed] [Google Scholar]
- 19.Mansour SC, Pena OM, Hancock REW. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35:443–450. doi: 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Guoqi S, Feng T, Daiwen C, Bing Y, Zhiqing H, Yuheng L, Xiangbing M, Ping Z, Jie Y, Junqiu L. Expression, purification and characterization of a novel antimicrobial peptide: Gloverin A2 from Bombyx mori. Int J Pept Res Ther. 2018;25:827–833. doi: 10.1007/s10989-018-9732-7. [DOI] [Google Scholar]
- 21.Kusminski CM, Park J. Scherer PE MitoNEET-mediated effects on browning of white adipose tissue. Nat Commun. 2014;5:3962. doi: 10.1038/ncomms4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001. [DOI] [PubMed] [Google Scholar]
- 23.Xu M, Chen X, Huang Z, Wen W, Liu G. Prokaryotic expression, purification, polyclonal antibody preparation, and tissue distribution of porcine Six1. Turk J Biol. 2015;39:335–342. doi: 10.3906/biy-1408-66. [DOI] [Google Scholar]
- 24.Choi W, Yeruva S, Turner JR. Contributions of intestinal epithelial barriers to health and disease. Exp Cell Res. 2017;358:71–77. doi: 10.1016/j.yexcr.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Branca JJV, Gulisano M, Nicoletti C. Intestinal epithelial barrier functions in ageing. Ageing Res Rev. 2019;54:100938. doi: 10.1016/j.arr.2019.100938. [DOI] [PubMed] [Google Scholar]
- 26.He C, Yu T, Shi Y, Ma C, Yang W, Fang L, Sun M, Wu W, Xiao F, Guo F. MicroRNA 301A promotes intestinal inflammation and colitis-associated cancer ddevelopment by inhibiting BTG1. Gastroenterol. 2017;152:1434–1448. doi: 10.1053/j.gastro.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 27.Grivennikov S, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:61–64. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.JoR A, Tomas J, Brenner C, Sansonetti P. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie. 2017;141:97–106. doi: 10.1016/j.biochi.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Xia YY, Bin P, Liu SJ, Chen S, Yin J, Liu G. Enterotoxigenic Escherichia coli infection promotes apoptosis in piglets. Microb Pathog. 2018;125:290–294. doi: 10.1016/j.micpath.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Joffré E, Von Mentzer A, Svennerholm AM, Sjling S. Identification of new heat-stable (STa) enterotoxin allele variants produced by human enterotoxigenic Escherichia coli (ETEC) Int J Med Microbiol. 2016;306:586–594. doi: 10.1016/j.ijmm.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Gui S, Liang Z, Liu A, Chen Z, Tang Y, Xiao M, Chu F, Liu W, Jin X. Musca domestica Cecropin (Mdc) Musca domestica Cecropin (Mdc) alleviates Salmonella typhimurium-induced colonic mucosal barrier impairment: Associating with inflammatory and oxidative stress response, tight junction as well as intestinal flora. Front Microbiol. 2019;10:522. doi: 10.3389/fmicb.2019.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S, Zhang F, Huang Z, Liu H, Xie C, Zhang J, Thacker PA, Qiao S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides. 2012;35:225–230. doi: 10.1016/j.peptides.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Zhenya Z, Xiaojun N, Chenglong J, Wenkai R, Jie L, Jinping D, Baichuan D, Yulong Y. Cecropin A modulates tight junction-related protein expression and enhances the barrier function of porcine intestinal epithelial cells by suppressing the MEK/ERK pathway. Int J Mol Sci. 2018;19:1941. doi: 10.3390/ijms19071941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Junning P, Daiwen C, Gang T, Jun H, Ping Z, Xiangbing M, Jie Y, Zhiqing H, Ling Z, Junqiu L. Protective effects of benzoic acid, Bacillus coagulans, and oregano oil on intestinal injury caused by enterotoxigenic Escherichia coli in weaned piglets. BioMed Res Int. 2018;2018:1–12. doi: 10.1155/2018/1829632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan J, Zhang J, Wu G, Chen D, Yu B, Huang Z, Luo Y, Zheng P, Luo J, Mao X, Yu J, He J. Amelioration of enterotoxigenic Escherichia coli-induced intestinal barrier disruption by low-molecular-weight chitosan in weaned pigs is related to suppressed intestinal inflammation and apoptosis. Int J Mol Sci. 2019;20:3485. doi: 10.3390/ijms20143485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang-Shieh YF, Wu CH, Chien HF, Wei IH, Chang ML, Shieh JY, Wen CY. Reactive changes of interstitial glia and pinealocytes in the rat pineal gland challenged with cell wall components from gram-positive and -negative bacteria. J Pineal Res. 2010;38:17–26. doi: 10.1111/j.1600-079X.2004.00170.x. [DOI] [PubMed] [Google Scholar]
- 37.Morris M, Li L. Molecular mechanisms and pathological consequences of endotoxin tolerance and priming. Arch Immunol Ther Ex. 2012;60:13. doi: 10.1007/s00005-011-0155-9. [DOI] [PubMed] [Google Scholar]
- 38.Kim JC, Hansen CF, Mullan BP, Pluske JR. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 2001;173:3–16. doi: 10.1016/j.anifeedsci.2011.12.022. [DOI] [Google Scholar]
- 39.Takuya S. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y, Zhou M, Yan J, Gong Z, Chen Y (2016) miR-200b inhibits TNFα-induced IL-8 secretion and tight junction disruption of intestinal epithelial cells in vitro. Ajp Gastrointest Liver Physiol 312. 10.1152/ajpgi.00316.2016 [DOI] [PubMed]
- 41.Ma TY, Iwamoto GK, Hoa NT, Akotia V, Said HM. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Ajp Gastrointest Liver Physiol. 2004;286:G367–376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- 42.Kan X, Shuting C, Lefei J. TGF-β1 protects intestinal integrity and influences Smads and MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate Immun. 2017;23:276–284. doi: 10.1177/1753425917690815. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Zhu C, Chen Z, Chen Z, Zhang W, Ma X, Wang L, Yang X, Jiang Z. Protective effects of Lactobacillus plantarum on epithelial barrier disruption caused by enterotoxigenic Escherichia coli in intestinal porcine epithelial cells. Vet Immunol Immunop. 2016;172:55–63. doi: 10.1016/j.vetimm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Haitao Y, Xiuliang D, Lijun S, Xiangfang Z, Hongbin L, Ning L, Shuo H, Yuming W, Gang W, Shuang C. Protective ability of biogenic antimicrobial peptide microcin J25 against enterotoxigenic Escherichia coli-induced intestinal epithelial dysfunction and inflammatory responses IPEC-J2 cells. Front Cell Infect Mi. 2018;8:242. doi: 10.3389/fcimb.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Bio. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 46.Mccracken JM, Allen LAH. Regulation of human neutrophil apoptosis and lifespan in health and disease. J Cell Death. 2014;7:15–23. doi: 10.4137/JCD.S11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goilav B, Satlin LM, Wilson PD. Pathways of apoptosis in human autosomal recessive and autosomal dominant polycystic kidney diseases. Pediatr Neurol. 2008;23:1473–1482. doi: 10.1007/s00467-008-0851-9. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Tang H, Zhang Y, Zhu F, Chen K. Research progress on the immune mechanism of the silkworm Bombyx mori. Physiol Entomol. 2017;43:159–168. doi: 10.1111/phen.12241. [DOI] [Google Scholar]
- 49.Pasparakis M, Luedde T, Schmidt-Supprian M (2006) Dissection of the NF-κB signalling cascade in transgenic and knockout mice. Cell Death Differ. 861-872. 10.1038/sj.cdd.4401870 [DOI] [PubMed]
- 50.Basak S, Kim H, Kearns JD, Tergaonkar V, O"Dea E, Werner SL, Benedict CA, Ware CF, Ghosh G, Verma IM, A fourth IκB protein within the NF-κB signaling module. Cell. 2007;128:369–381. doi: 10.1016/j.cell.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shou Y, Li N, Li L, Borowitz JL, Isom GE. NF-kappaB-mediated up-regulation of Bcl-X(S) and Bax contributes to cytochrome release in cyanide-induced apoptosis. J Neurochem. 2002;81:842–852. doi: 10.1046/j.1471-4159.2002.00880.x. [DOI] [PubMed] [Google Scholar]
- 52.Kadhim HJ, Tabarki B, Verellen G, Prez CD, Sébire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/WNL.56.10.1278. [DOI] [PubMed] [Google Scholar]
- 53.Hayden MS, Ghosh S. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol. 2011;224:245–260. doi: 10.1002/path.2858. [DOI] [PubMed] [Google Scholar]
- 55.Linlin C, Huidan D, Hengmin C, Jing F, Zhicai Z, Junliang D, Yinglun L, Xun W, Ling Z. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roselli M, Finamore A, Hynonen U, Palva A, Mengheri E. Differential protection by cell wall components of Lactobacillus amylovorus DSM 16698(T)against alterations of membrane barrier and NF-kB activation induced by enterotoxigenic F4(+) Escherichia coli on intestinal cells. BMC Microbiol. 2016;16:226. doi: 10.1186/s12866-016-0847-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.