Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is defined by the abundance of lipid droplets (LDs) in hepatocytes. While historically considered simply depots for energy storage, LDs are increasingly recognized to impact a wide range of biological processes that influence cellular metabolism, signaling, and function. While progress has been made toward understanding the factors leading to LD accumulation (i.e. steatosis) and its progression to advanced stages of NAFLD and/or systemic metabolic dysfunction, much remains to be resolved.
Scope of review
This review covers many facets of LD biology. We provide a brief overview of the major pathways of lipid accretion and degradation that contribute to steatosis and how they are altered in NAFLD. The major focus is on the relationship between LDs and cell function and the detailed mechanisms that couple or uncouple steatosis from the severity and progression of NAFLD and systemic comorbidities. The importance of specific lipids and proteins within or on LDs as key components that determine whether LD accumulation is linked to cellular and metabolic dysfunction is presented. We discuss emerging areas of LD biology and future research directions that are needed to advance our understanding of the role of LDs in NAFLD etiology.
Major conclusions
Impairments in LD breakdown appear to contribute to disease progression, but inefficient incorporation of fatty acids (FAs) into LD-containing triacylglycerol (TAG) and the consequential changes in FA partitioning also affect NAFLD etiology. Increased LD abundance in hepatocytes does not necessarily equate to cellular dysfunction. While LD accumulation is the prerequisite step for most NAFLD cases, the protein and lipid composition of LDs are critical factors in determining the progression from simple steatosis. Further defining the detailed molecular mechanisms linking LDs to metabolic dysfunction is important for designing effective therapeutic approaches targeting NAFLD and its comorbidities.
Keywords: Lipid droplets, Lipotoxicity, NAFLD, NASH, Perilipins
HIGHLIGHTS
-
•
Numerous pathways contribute to the synthesis and degradation of hepatic LDs.
-
•
The protein and lipid composition of LDs largely dictate whether steatosis is coupled or uncoupled from local or systemic metabolic dysfunction.
-
•
Genetic variants provide insights into mechanisms that link LDs to NAFLD etiology.
-
•
The spatial regulation of LD metabolism is poorly understood despite known heterogeneity in LD accumulation with NAFLD.
1. Introduction
Hepatic steatosis refers to the accumulation of neutral lipids, typically TAG, in LDs and is commonly known as NAFLD when alcohol or viral infections are not contributing factors. NAFLD can comprise simple steatosis or more advanced non-alcoholic steatohepatitis (NASH), which is characterized by inflammation and hepatocyte injury that is often accompanied by fibrosis. While NAFLD can occur as a consequence of diseases such as obesity and type 2 diabetes, NAFLD increases the risk of type 2 diabetes, dyslipidemia, hypertension, cardiovascular disease, chronic kidney disease, liver cirrhosis, hepatocellular carcinoma, and mortality [[1], [2], [3], [4], [5]]. It has recently been proposed to change the nomenclature from NAFLD/NASH to metabolic-associated fatty liver disease (MAFLD) to account for the tight association with metabolic dysfunction and disease heterogeneity [6]. Regardless of the name, LD accumulation is the defining characteristic of NAFLD and alcoholic fatty liver disease. Historically considered to be inert and simply a marker of disease, LDs are increasingly recognized as etiological factors in numerous liver diseases as well as having important non-pathological roles in cell signaling and function [7]. These dynamic properties of LDs are highly regulated by hundreds of proteins that coat the LD droplet surface to control lipid trafficking and flux. Although NAFLD is commonly identified by LD abundance in hepatocytes, LD accumulation in other hepatic cell types may also contribute to disease development and progression. In the sections below, we highlight the major pathways of lipid metabolism that influence LD accumulation and explore key proteins and lipids that regulate LD dynamics and link LDs to cellular dysfunction and liver disease.
2. Lipid droplet formation
2.1. Sources of FAs
Uptake from the blood, de novo lipogenesis (DNL), and endocytotic recycling of lipoprotein remnants are the major sources of FAs that serve as substrates for TAG synthesis in the liver. Under most conditions, uptake from the blood provides the major source of FAs used for esterification into TAG [8]. This process is also dynamic with increased rates of FA uptake during fasting conditions or insulin resistance when adipocyte lipolysis is high. In contrast, during the post-prandial period, there is a decreased reliance from adipose-derived FAs, which coincides with an increase in the contribution from spillover of FAs during peripheral lipoprotein hydrolysis, lipoprotein remnant uptake, and DNL. The contribution of FAs from DNL is markedly increased in subjects with NAFLD and can account for 30–40% of hepatic FAs [9,10]. Not surprisingly, numerous clinical trials are targeting DNL as a potential treatment for NASH [11]. In addition, a reduction in serum free FAs and their subsequent uptake into the liver is thought to contribute to improvements in NAFLD observed with insulin-sensitizing drugs [12]. Thus, decreasing hepatic FA levels through reductions in their synthesis or uptake has therapeutic potential to prevent or treat NAFLD.
2.2. TAG synthesis and LD biogenesis/expansion
Upon uptake, a major branchpoint in metabolism of FAs is their partitioning between oxidative and esterification pathways. Numerous long-chain acyl-CoA synthetases (ACSLs) catalyze the conversion of FAs to their respective CoAs and influence their downstream fates. Differences in subcellular location, substrate preferences, and enzyme kinetics underlie the differential effects of these enzymes on FA channeling. In the liver, ACSL5 is thought to be the major isoform contributing to TAG synthesis [13], whereas ACSL4 regulates arachidonic acid metabolism, which in turn influences phospholipid composition and VLDL secretion [14]. While ACSL1 has major effects on FA oxidation in numerous tissues, ablation of hepatic ACSL1 has little effect on FA oxidation [15]; the ACSL isoform that drives FA oxidation in hepatocytes has yet to be identified. The key gatekeeping enzymes carnitine palmitoyltransferase and glycerol-3-phosphate acyltransferase (GPAT) commit the acyl-CoAs into the pathways of β oxidation or glycerolipid synthesis, respectively. Once FAs are esterified to glycerol-3-phosphate, the newly formed lysophosphatidic acid can then continue through the Kennedy pathway, yielding TAG or phospholipids (reviewed in [16]). Each of the 3 families of acyltransferase enzymes in this pathway have numerous isoforms with different substrate specificity, which dictates the FA composition of the downstream lipid products. GPAT1 shows substrate specificity for saturated FAs, thereby contributing to the high percentage of saturated FAs found in the sn-1 position of TAG [17]. Additionally, diacylglycerol acyltransferase 1 (DGAT1) preferentially channels exogenous FAs into TAG, whereas DGAT2 is more selective to saturated and monounsaturated FAs (MUFAs) derived from DNL [[18], [19], [20]]. Thus, the relative activity levels of these various isoforms dictate the acyl composition of TAG. However, it should be noted that some or complete compensation can occur when one of the DGAT isoforms is inhibited in mouse or human hepatocytes [21]; see [22] for a comprehensive review of DGAT biology.
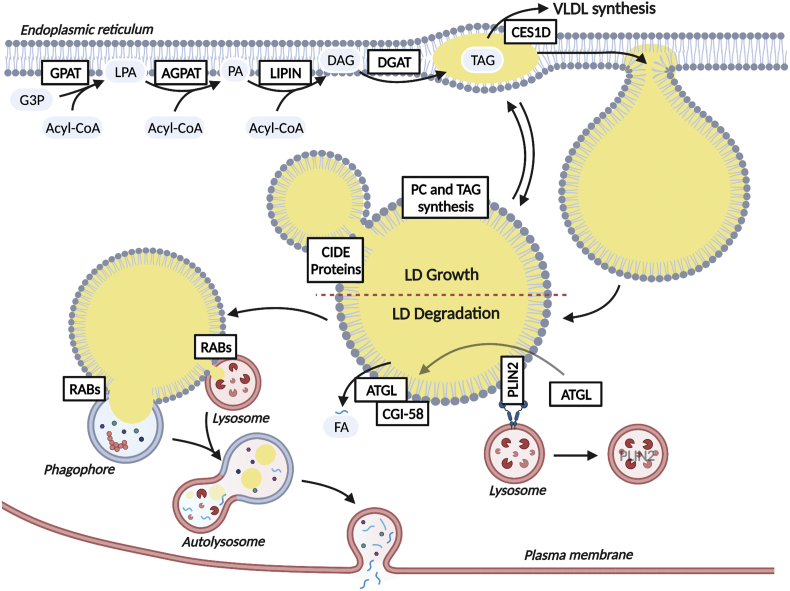
LDs form in the endoplasmic reticulum (ER), where neutral lipids accumulate within the leaflet of the membrane bilayer (Figure 1). ER-localized DGAT1, which has its active site within the ER bilayer [23], catalyzes the formation of TAG needed for LD biogenesis. This nascent LD and associated phospholipid monolayer eventually bud from the ER to form a cytosolic LD, a process that has been thoroughly reviewed and is beyond the scope of this review [24,25]. Once formed, small nascent LDs may expand by undergoing fusion, a process in which cell death-inducing DFFA-like effector proteins (CIDE) are intimately involved. While CIDEB is the most highly expressed isoform under basal conditions, CIDEA and CIDEC are induced with fasting and during steatosis to promote LD fusion [26,27]. In addition to fusion, it is speculated that LDs may also expand as a result of lipid synthesis occurring directly on the surface of LDs or at newly formed LD-ER membrane bridges. The LD monolayer membrane contains numerous enzymes involved in TAG synthesis, such as acyl-CoA synthetases as well as the acyltransferases GPAT4 and DGAT2, which allow the expansion of preexisting LDs [28]. The phospholipid monolayer must also expand with the concomitant addition of TAG to the LD core and numerous but not all required enzymes involved in the synthesis of phospholipids are present on LDs [29]. As such, it is unclear whether LDs can independently synthesize phospholipids or whether association with the ER and protein-mediated transfer of phospholipids occurs.
Figure 1.
Overview of pathways involved in TAG/LD formation and breakdown. ER-localized enzymes catalyze the synthesis of TAG for incorporation into de novo LDs. LDs may also grow through local synthesis of TAG and phospholipids on the LD surface, lipid exchange with the ER, or through CIDE protein-mediated fusion of two LDs. Ces1d directs LDs from the ER to the VLDL synthetic pathway. CMA-mediated degradation of PLIN2 is an initial event in the breakdown of LDs. Subsequently, ATGL has access to the LD surface and can bind its co-activator CGI-58 to initiate TAG hydrolysis. Lipophagy, which is initiated by numerous RAB proteins, is thought to be responsible for the hydrolysis of size-reduced LDs. Upon lysosomal degradation, FAs are largely effluxed at the plasma membrane. Solid arrows represent metabolite flux and dashed arrows represent signaling.
3. Lipid droplet catabolism
3.1. Lipolysis
The degradation of LDs is complex and involves the coordinated catabolism of surface proteins, phospholipid membranes, and core neutral lipids. Numerous proteins directly or indirectly influence this process and, as a result, impact LD levels and the subsequent partitioning of metabolites produced from these catabolic pathways. The perilipins (PLINs), which consists of 5 members, were the first characterized LD proteins. The PLIN2 isoform is highly expressed in the liver and is induced in response to FA loading in cells and NAFLD/NASH in both humans and rodents [30,31]. While perilipins may serve numerous functions, they have historically been viewed as LD coat proteins that inhibit access of lipases to the LD surface [32]. To allow for robust lipolysis during lipid mobilization, PLIN2 undergoes chaperone-mediated autophagy (CMA), resulting in its degradation and the subsequent access of lipases to the LD surface [33]. In support of these data, ablation of PLIN2 promotes LD catabolism while its overexpression has the opposite effect [34]. PLIN2 is also phosphorylated in response to AMPK activation, which has been postulated to enhance CMA-mediated degradation [35], although studies detailing the exact functional consequences of PLIN2 phosphorylation have yet to be conducted.
Of the lipases that contribute to hepatic LD turnover, adipose triglyceride lipase (ATGL) encoded by the patatin-like phospholipase domain-containing protein 2 (PNPLA2) gene has received the most attention. ATGL ablation in the liver leads to LD accumulation and reduced FA oxidation, while ATGL overexpression alleviates hepatic steatosis and increases FA oxidation without impacting VLDL secretion [[36], [37], [38]]. The subsequent enzymes in the lipolytic pathway classically accepted to be hormone-sensitive lipase and monoacylglycerol lipase (MAGL) have been less studied in regard to their roles in regulating hepatic TAG catabolism. Hormone-sensitive lipase is expressed at low levels in mouse liver [39], and its contribution to TAG catabolism in the liver has not been detailed [40]. Hormone-sensitive lipase does not appear to be expressed in human hepatocytes based on single cell sequencing data, further highlighting the lack of understanding of the lipolytic pathway in the liver [41]. MAGL contributes to hepatic MAG hydrolysis and influences endocannabinoid signaling through its metabolism of the endocannabinoid sn-2-20:4-glycerol (2-AG), which is discussed in Section 4.2.3.
Numerous proteins directly interact with ATGL to influence its lipolytic activity. CGI-58 is widely accepted as the primary co-activator of ATGL [42]. Ablation of hepatic CGI-58 leads to steatosis and progression to NASH [43]. While CGI-58 likely works in part through ATGL activity, it clearly has functions beyond ATGL, as ablation of CGI-58 results in a large (∼50-fold) increase in liver TAG and worsens NASH development compared to a more modest ∼2–3 fold increase in TAGs following ATGL ablation [37,44]. CGI-58 can also modulate LD catabolism independent of ATGL, suggesting, at least in the liver, a more complicated role of this protein in LD turnover [45]. A host of other proteins directly interact with and antagonize ATGL. G0/G1 switch gene 2 (G0S2) is a potent inhibitor of ATGL that influences hepatic energy metabolism. Hepatic overexpression of G0S2 promotes LD accumulation and reduces FA oxidation, while ablation of hepatic G0S2 does the opposite [[46], [47], [48]]. Other interacting proteins, including hypoxia-inducible lipid droplet associated [49] and CIDEC [50], inhibit ATGL activity, whereas pigment epithelial-derived factor promotes ATGL-catalyzed lipolysis [51].
The carboxylesterase (CES) family of enzymes also contributes to turnover of hepatic LDs. In particular, Ces1d (also known as triglyceride hydrolase, TGH), which colocalizes to ER luminal LDs, plays a role in trafficking lipids to VLDL assembly. Ablation of Ces1d reduces VLDL secretion and decreases the size but increases the number of cytosolic LDs [52,53]. Similarly, the human homologue of Ces1d, CES1, also enhances LD size, steatosis, and VLDL secretion [54,55]. Ces1d/CES1 appears to act as a potential ER branch point for shuttling lipids toward packaging in VLDL at the expense of cytosolic LD production [52]. Ces1d ablation also reduces DNL and increases FA oxidation, which may reduce steatosis through decreasing the FA pool size available for esterification into TAG [56]. While other mouse CES proteins have been studied, their relevance to human hepatic metabolism is unclear given that orthologues have not been identified [57].
3.2. Lipophagy
Autophagy represents a well-characterized degradative mechanism through which cellular organelles, protein aggregates, and macronutrients are disassembled during times of energy scarcity. A seminal discovery in the field is that autophagy contributes to the catabolism of LDs, a process now referred to as lipophagy [58]. In addition to the indirect effects of CMA on lipolysis noted above, both macroautophagy and microautophagy also contribute to the broader lipophagic process [59]. Macrolipophagy is the process through which autophagosomes bud off part of the LD prior to fusing with lysosomes to form autolysosomes, which can then degrade the lipids via lysosomal acid lipase. In contrast, microlipophagy is the direct physical interaction and transfer of lipids between LDs and lysosomes. These processes are highly regulated through many nutrient sensing nodes including sirtuin 1 (SIRT1) and AMPK as well as numerous transcriptional networks as recently reviewed in [60].
Given the capacity for both cytosolic lipases and lipophagy to degrade hepatic LDs, several recent studies have explored the interplay between these two pathways. As noted above, the CMA-mediated degradation of perilipin proteins is an important initial event allowing for access of cytosolic lipases and possibly autophagic machinery to the LD surface. ATGL appears to be critical for the initial hydrolysis of TAG and works upstream of lipophagy through two known mechanisms. First, ATGL-catalyzed lipolysis promotes SIRT1 signaling (discussed in detail in Section 4.2.2), a major nutrient-sensing node that promotes deacetylation of numerous proteins directly involved in autophagy/lipophagy resulting in enhanced degradation of LDs [61]. Consistent with these studies, ablation of PLIN2 also induces autophagy/lipophagy, which is required for breakdown of hepatic TAGs [62], suggesting that lipophagy plays a major role in TAG turnover. Second, recent studies showed that ATGL hydrolyzes large LDs until they are sufficiently reduced in size to a point where lipophagy commences and catalyzes the remaining degradation [63]. Thus, one could speculate that the size of LDs dictates the relative contribution of these two processes to LD degradation. While these studies suggest some linearity in the LD degradation process, LC3, a key component of autophagosomes, also has been shown to directly interact with ATGL. Deletion of the LC3-interacting region on ATGL prevents it from localizing to LDs, suggesting that lipolysis and autophagy/lipophagy are highly intertwined [64]. While some of the proteins critical for lipophagy have been identified, especially the RAB family of GTPases, much remains to be learned regarding the detailed mechanisms through which hepatic LDs are degraded. A long-standing question in the field has been how FAs generated from LD degradation in lysosomes are subsequently trafficked to downstream pathways as there are no known FA transporters present on lysosomes. A recent study showed that FAs generated from lysosomal lipolysis can be effluxed from cells as a result of lysosomal fusion to the plasma membrane and expulsion of luminal contents [65]. These effluxed FAs are then available for reuptake in the same or adjacent cells for further metabolism [65]. These data are consistent with previous studies documenting FA exchange between hepatocytes [66], although the significance of this in vivo has yet to be characterized. In addition to genetic manipulation of various proteins to enhance hepatic autophagy in mice, many diet-derived small molecules reduce hepatic steatosis in animal models through increases in lipophagy [[67], [68], [69], [70], [71]]. Given that autophagy/lipophagy are reduced in human NAFLD [72], these preclinical studies highlight the potential of promoting autophagy/lipophagy as a therapeutic approach to treat NAFLD/NASH in humans [73].
4. Mechanisms linking LDs to cell signaling and function
4.1. Are LDs causal or consequential to hepatic dysfunction?
It is established that steatosis, under most circumstances, precedes NASH, and both are risk factors for advanced liver morbidities such as cirrhosis and hepatocellular carcinoma (HCC). The development and progression of NAFLD also correlates with the incidence of numerous metabolic diseases and can be both a cause or consequence of these diseases [74,75]. How steatosis per se is linked to these hepatic and extrahepatic morbidities remains a major question. Despite some studies that suggest a linear correlation between hepatic steatosis and metabolic morbidities including hepatic and/or peripheral insulin resistance [[76], [77], [78], [79]], other studies suggest that a variable threshold of liver fat must be reached before hepatic or peripheral insulin resistance is impaired [80]. Whether the degree of steatosis predicts progression to NASH or advanced liver disease is still debated [80,81]. One possibility that may help explain these discrepancies is that simple steatosis is perhaps not so simple. Whether diagnosed by MRS, histology, or enzymatic assays, the relative amount of TAG/LDs may not be the most accurate predictor of cellular dysfunction and disease development. This is evidenced by numerous animal models and genetic variants present in humans that uncouple steatosis from NAFLD progression and comorbidities. Intricacies in the composition of LDs or flux through the various LD-related metabolic pathways likely underlie NAFLD etiology as discussed in the following sections (see Figure 2 for an overview).
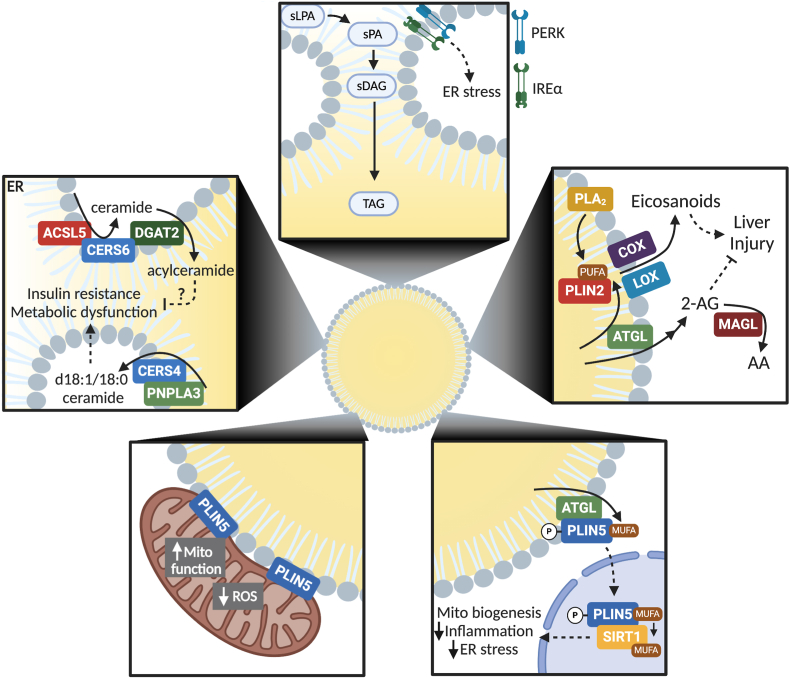
Figure 2.
Overview of mechanisms through which LDs influence cell signaling and function. (Top) Accumulation of the saturated intermediates lysophosphatidic acid (sPLA), phosphatidic acid (sPA), and diacylglycerol (sDAG) within the ER are sensed by PERK and IREα to initiate an ER stress response when flux through the TAG synthetic pathways cannot accommodate the FA substrates. (Upper right) Lipolytic products from PLA2 or ATGL can be used for eicosanoid signaling to promote inflammation or regulate the levels of endocannabinoid 2-arachidonylglycerol (2-AG), which prevents liver injury. (Bottom right) MUFAs released in response to ATGL-catalyzed lipolysis can bind phosphorylated PLIN5, which translocates them to the nucleus for their subsequent transfer to and activation of SIRT1. In turn, SIRT1 promotes mitochondrial biogenesis and oxidative metabolism and suppresses inflammation and ER stress. (Bottom left) PLIN5 facilitates the interaction of mitochondria and LDs that is associated with improved mitochondrial function and cell health. (Upper left) Enzyme complexes at the ER-LD interface regulate the production of ceramides to promote metabolic dysfunction or the conversion of ceramides to acylceramides to potentially alleviate the negative effects of ceramide signaling.
4.1.1. Lipotoxicity of glycerolipid intermediates
During chronic and excess caloric intake, the liver is inundated with FAs as a result of elevated rates of DNL and increased serum free FAs, the latter of which is a consequence of insulin resistance and increased adipose-derived free FAs. This deluge of FAs and their subsequent negative effects on cell metabolism and signaling can result in lipotoxicity, a term first coined by Dr. Roger Unger nearly 30 years ago [82]. While lipotoxicity generally refers to the toxic effects of a wide range of lipid metabolites, exposure of cells to relatively high doses of palmitic acid is a commonly used lipotoxicity model that disrupts metabolic homeostasis and induces cell damage [83]. Numerous studies showed that the addition of unsaturated FAs along with palmitic acid can increase rates of TAG synthesis and alleviate lipotoxicity [[84], [85], [86]]. These data are consistent with an inverse relationship between rates of FA esterification and lipotoxicity [87]. While it may seem paradoxical that increasing TAG synthesis and LD accumulation would be beneficial, an established body of literature shows that reduced flux through the TAG synthetic pathway contributes to lipotoxicity. Lipidomic analysis of cells exposed to palmitic acid reveals increased levels of the saturated glycerolipid intermediates lysophosphatidic acid, phosphatidic acid, and diacylglycerol [88]. The accumulation of these metabolites is likely due to the substrate selectivity of the acyltransferase and phosphatase enzymes involved in this pathway. The first enzyme in the pathway, GPAT1 or GPAT4, prefers saturated FAs as substrates to form lysophosphatidic acid [89,90]. However, the downstream enzymes LIPIN and the DGATs, which catalyze the last two steps in TAG synthesis, have reduced activity toward di-saturated substrates [88,91]. As a result, in the presence of low amounts of unsaturated FAs, palmitic acid has low rates of flux into TAG, resulting in the accumulation of di-saturated intermediates. In support of saturated glycerolipid intermediates playing a pathological role, inhibition of ER-localized GPAT3 and 4 reduces saturated intermediates and prevents palmitate-mediated lipotoxicity, whereas inhibition of DGAT1 has the opposite effect [88]. These results highlight the importance of acyltransferases and di-saturated intermediates as mediators of lipotoxicity [91]. Taken together, this work suggests that LDs play a key role in sequestering saturated FAs into TAG to prevent lipotoxicity.
DGAT2, which preferentially catalyzes the esterification of de novo synthesized FAs [19], has been the most widely studied TAG synthetic enzyme in the context of NAFLD. Hepatic overexpression of DGAT2 promotes steatosis, but does not promote insulin resistance [92]. Initial studies found that db/db mice fed a methionine choline-deficient diet and administered DGAT2 anti-sense oligonucleotides have transiently reduced steatosis, but increased inflammation, oxidative stress, and fibrosis compared to mice given control oligonucleotides [93]. Liver-specific DGAT2 knockout mice have reduced steatosis, but show similar inflammation and fibrosis in mice fed a NASH-promoting diet [94]. Small molecule inhibition of DGAT2 also reduces hepatic lipids in rodent models of NAFLD but not primates [95]. Other studies reported that chemical inhibition of DGAT2 improves markers of NASH in mice and shows promise in improving steatosis and circulating liver enzymes in a phase II clinical trial in humans [96]. Intuitively, it may seem that blockade of DGAT2 would lead to the accumulation of glycerolipid intermediates and associated lipotoxicity. However, several studies show a robust compensatory downregulation of DNL and increase in FA oxidation with ablation of DGAT2 [94,97], which likely contributes to the observed phenotypes. While the effects of targeting DGAT2 are somewhat mixed, perhaps due to different experimental models and conditions, numerous clinical trials are underway to determine the efficacy of DGAT2 inhibitors in NAFLD/NASH [98].
Because TAG synthesis and LD biogenesis occurs within the ER, it not surprising that ER stress is a consequence of an impairment in TAG synthesis and LD formation. Indeed, saturated FAs induce hepatic ER stress both in vitro and in vivo [99,100]. While the unfolded protein response is known to sense and regulate protein homeostasis, it can also respond to lipid-induced ER stress. Two major ER stress sensors, inositol-requiring enzyme 1 (IRE1) and double-stranded RNA-activated protein kinase-like ER kinase (PERK), respond to alterations in ER membrane FA saturation to initiate the unfolded protein response [101,102]. Both these sensors use amphipathic transmembrane domains, which are distinct from unfolded protein-sensing mechanisms, to monitor acyl chain enrichment in the ER and induce downstream lipotoxic responses including reactive oxygen species (ROS) generation, disruption of calcium homeostasis, inflammation, and apoptosis that occur with sustained ER stress [103]. Consistent with these data, markers of ER stress are upregulated in animal models and patient liver biopsies of NAFLD/NASH [[104], [105], [106]].
4.1.2. Lipotoxicity from ceramides
As a consequence of an imbalance between FA supply and flux through the glycerolipid pathway, FAs can “overflow” into additional pathways such as sphingolipid synthesis including ceramides [86,107]. Consistent with the benefits of incorporating FAs into TAG, blocking DGAT1 to suppress TAG synthesis also causes increased 16:0 ceramide [108]. Serine palmitoyl-CoA transferase is the rate-limiting enzyme of ceramide synthesis and has high substrate specificity for palmitoyl-CoA. Thus, high levels of palmitic acid can be readily incorporated into ceramides [109]. Reducing ceramides prevents steatosis, inflammation, and fibrosis in a NASH-promoting diet [110,111]. Ceramides are also tightly linked to insulin resistance in numerous tissues including the liver [112]. It was recently shown that deletion of dihydroceramide desaturase 1, which inserts a conserved double bond into the backbone of ceramides, resolves hepatic steatosis and insulin resistance in mice [113], suggesting that the composition of ceramides is an important mediator of NAFLD. In support, several metabolomic studies identified ceramides and other sphingolipids as predictive markers for the transition from steatosis to NASH [114,115].
Numerous recent studies also show that LDs may be directly involved in ceramide synthesis. Lipidomic analysis of LDs revealed that they contain ceramides [116]. Ceramide synthase 4 (CS4), which is highly expressed in the liver, interacts with PNPLA3 at the LD-ER interface to facilitate ceramide production [117]. This interaction leads to the production of d18:1/18:0 ceramide, which is attributed to the transacylase activities of PNPLA3 [118]. The increase in d18:0/18:0 ceramide drives protein phosphatase 2 activity to antagonize insulin signaling [117]. DGAT2 also catalyzes the conversion of ceramides to acylceramides, which are subsequently stored in LDs [119]. CS6 interacts with ACSL5 and DGAT2 to traffic FAs into acylceramides at ER/LD junctions. This conversion of ceramides into acylceramides and their subsequent storage into LDs reduces free ceramide levels, which could be speculated to serve a protective role against lipotoxicity [119]. These studies provide new insights into the complex biochemistry underlying ceramide synthesis and highlight an important role of LDs as storage pools and mediators of ceramide signaling. Despite these advances, the significance of LDs in ceramide metabolism in the context of NAFLD remains to be determined.
4.1.3. Oxidative stress
Oxidative stress is a common indicator of metabolic dysfunction and a key etiological factor in NAFLD progression. Palmitic acid-induced ER stress can initiate p-JNK signaling, which in turn promotes mitochondrial production of ROS [120]. In addition to this mechanism, impairments in FA esterification lead to a diversion of FAs into mitochondrial β oxidation and the potential generation of ROS. For example, ablation of GPAT1 and FA esterification increases hepatic FA oxidation and oxidative stress [121]. Similarly, DGAT2 ablation also promotes FA oxidation and oxidative stress as previously noted [93], and DGAT1 is essential to prevent toxicity related to excess mitochondrial FA oxidation during fasting-induced autophagy in fibroblasts [108]. Oxidative stress results from an imbalance between FA supply vs mitochondrial capacity to oxidize incoming lipids and neutralize ROS that forms during this process. PLIN5 appears to be a key protein in regulating fatty acid oxidation and oxidative stress. Overexpression and knockdown studies revealed that PLIN5 protects against oxidative stress in hepatocytes [122,123] and other tissues [124]. Interestingly, PLIN5 is a pleiotropic protein that antagonizes lipolysis under basal/unstimulated conditions [125], facilitates LD-mitochondrial interactions [126], and signals via SIRT1 (discussed in Section 4.2.2); the contribution of each or all of these functions to its beneficial properties have not been elucidated. LDs can store oxidized TAG that accumulates near the LD phospholipid monolayer [127]. While the prevalence and importance of oxidized lipid storage in LDs during NAFLD remain to be determined, they are known to have detrimental effects on immune cell function [128].
4.2. LDs as a source of signaling molecules
The prior section highlighted the importance of sequestering FAs into TAGs, which are widely considered inert, to alleviate lipotoxicity. However, an important research area is to define whether and how LDs themselves, in the absence of exogenous FAs, can modulate downstream signaling pathways to influence cell function. This section details how lipids stored within LDs can be utilized as a substrate for numerous downstream lipid metabolism pathways. Many of these same lipid metabolites generated from LD metabolism are also potent signaling molecules and act as a bridge between LD metabolism and cell signaling networks that ultimately influence susceptibility and severity of NAFLD.
4.2.1. LDs and eicosanoid synthesis
Eicosanoids are oxidized lipids and potent signaling molecules involved in numerous pathways including inflammation. Hepatic and serum concentrations of eicosanoids are positively correlated with NAFLD progression [129,130]. Historically, it was viewed that phospholipases hydrolyze plasma membrane phospholipids to provide polyunsaturated FAs (PUFAs) as substrates for the eicosanoid synthetic pathway. However, a growing body of literature suggests that LDs are major contributors to eicosanoid synthesis in numerous cell types [131]. Many enzymes involved in eicosanoid synthesis such as cyclooxygenases and lipoxygenases are found on LDs [132,133]. In agreement with the presence of these enzymes, the surfaces of LDs are active sites of eicosanoid synthesis [134].
ATGL appears to be the primary supplier of hydrolyzed FAs from TAG-derived PUFAs in immune cells [[135], [136], [137]]. Mice with hepatic ablation of ATGL have similar or reduced inflammation markers compared to controls despite increased LD accumulation [37]. Lipid droplet proteins outside of ATGL can also influence inflammatory signaling. PLIN2 is known to antagonize ATGL-catalyzed lipolysis as noted above [32]. Thus, PLIN2 would be expected to prevent eicosanoid production and the ensuing inflammation. However, ablation of PLIN2 abolishes hepatic inflammatory signaling in response to either high-fat feeding or lipopolysaccharide administration in hepatocytes [138]. Because PLIN2 ablation decreases LD levels, it is difficult to determine if PLIN2 is directly involved in the production of inflammatory lipids such as eicosanoids or if it promotes inflammatory signaling simply by enlarging the LD pool. However, it is possible that PLIN2 plays a role in FA flux to pro-inflammatory enzymes on the LD surface as PLIN2 has been shown to bind pro-inflammatory FAs with high affinity [139]. Pro-inflammatory cytokines also promote LD accumulation in hepatocytes [140,141], further suggesting an intimate link between LDs and the inflammatory response. Given the prominent role of inflammation in NAFLD progression, studies attempting to further define the mechanisms linking LDs with inflammatory signaling are warranted.
4.2.2. Coupling LDs to nutrient-sensing nodes and oxidative metabolism
The prior examples suggested that lipolytic degradation of LDs may be detrimental to cell health. However, numerous studies show that lipolysis may have wide-ranging beneficial effects on hepatic function. Microarray analysis of ATGL knockout mice reveal that ATGL is required to maintain oxidative gene expression in numerous tissues such as heart, skeletal muscle, adipose tissue, and liver [142]. Hepatocytes overexpressing ATGL have increased peroxisome proliferator activated receptor α (PPARα) activity and expression of target genes, whereas ablation of hepatic ATGL in vivo suppresses PPARα signaling [34,36]. Because FAs are known ligands for PPARα, these data suggest that ATGL-mediated TAG hydrolysis provides an important source of PPARα ligands. However, administering the PPARα agonist fenofibrate to mice with suppressed hepatic ATGL did not increase the expression of PPARα target genes to levels in control mice treated with the same drug [143], implying that a more complicated mechanism may underlie this regulation. PPARα also drives the expression of genes involved in FA oxidation but not those involved in mitochondrial biogenesis or oxidative phosphorylation [144,145], which are commonly altered in response to manipulations of ATGL expression and/or activity. Additional studies revealed that ATGL promotes SIRT1 activity, which in turn deacetylates and activates peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α), the master regulator of mitochondrial gene expression [146]. Moreover, the inhibition of ATGL in hepatocytes prevents cAMP/PKA-mediated activation of PGC1α, suggesting that lipolysis is a key node in mediating hormonal induction of PGC1α and mitochondrial biogenesis/function [146].
More recent work detailed the mechanism through which ATGL affects SIRT1 activity. These studies showed that MUFAs released from ATGL-catalyzed lipolysis allosterically activate SIRT1 toward PGC1α and several other known SIRT1 protein substrates [147]. This activation is specific to MUFAs as other similar long-chain saturated and unsaturated FAs have no effect on SIRT1 activity. These data align with studies showing that ATGL preferentially hydrolyzes MUFAs from TAG [148]. Previous work shows that FA-binding protein 1, the major intracellular FA carrier in hepatocytes, is not required for ATGL-mediated changes in gene expression [143], thus providing a conundrum on how lipolysis-derived FAs activate SIRT1, which is primarily nuclear. In response to cAMP/PKA (i.e., lipolytic) signaling, PLIN5 becomes phosphorylated, which increases its translocation from LDs to the nucleus where it interacts with SIRT1 to enhance its activity [149]. Additional studies revealed that PLIN5 is a FA-binding protein that preferentially binds lipolysis-derived MUFAs, an effect that is enhanced with PKA-mediated phosphorylation of PLIN5, and traffics them to nuclear SIRT1 in hepatocytes [147]. As such, PLIN5 is required for ATGL-mediated activation of SIRT1 and PGC1α/PPARα. Collectively, these data unravel a novel signaling node involving PLIN5 trafficking of LD-derived MUFAs to the nucleus to activate SIRT1 and drive oxidative metabolism. These data are especially relevant to NAFLD as SIRT1 protein and activity are reduced in humans and mice with NAFLD, and genetic or small molecule activation of SIRT1 have potent therapeutic effects in preclinical models of NAFLD (reviewed in [150]).
4.2.3. Endocannabinoids
MAGL sits at an important branchpoint in TAG catabolism as it is responsible for the degradation of 2-arachidonoylglycerol, an endocannabinoid with potent signaling properties [151]. Global deletion of MAGL protects against diet-induced steatosis and hepatic injury in response to numerous types of insults including ischemia/reperfusion [152,153]. These effects are attributed to increased endocannabinoids and their subsequent signaling via the cannabinoid receptor type 2 and a concurrent reduction in eicosanoids. Genetic or chemical inhibition of MAGL results in resistance to fibrosis triggered from carbon tetrachloride injections or bile duct ligation [154]. MAGL expression is also a prognostic indicator of hepatocellular carcinoma [155]. While detailed studies involving diet-induced animal models of NASH or links to NAFLD development or progression in humans are lacking, studies to date suggest that MAGL inhibition may be a viable target toward NASH.
5. Genetic variants that impact LD biology and NAFLD
5.1. PNPLA3
The I148M variant of PNPLA3 is the single largest genetic driver of numerous liver diseases including NAFLD/NASH [156]. PNPLA3 is highly upregulated in response to high-carbohydrate diets, and the I148M variant exacerbates the detrimental effects of high-sucrose diets on NAFLD [157,158]. As PNPLA3 belongs to the same family as PNPLA2/ATGL, it was presumed that this mutation would lead to reduced lipolytic activity and steatosis. In contrast, ablation of PNPLA3 in mice does not cause steatosis, suggesting that a more complicated mechanism underlies its pathogenic effects [159]. Recent studies show that the PNPLA3 I148M mutant binds CGI-58 to reduce ATGL-catalyzed lipolysis [160]. The I148M variant is unable to further increase LD accumulation in cells or mice lacking CGI-58, indicating a critical role of this lipolytic activator in mediating the effects of the PNPLA3 mutant. In addition, the I148M variant is more resistant to ubiquitination and autophagic degradation, which results in higher protein abundance, compared to wild-type PNPLA3 (Figure 3A) [161,162]. Moreover, shRNA-mediated knockdown or expression of fusion constructs that target PNPLA3 I148M for proteasomal degradation reduce protein abundance and alleviate steatosis. In support, recent studies also show that knockdown of PNPLA3 in livers of mice homozygous for I148M largely negate the detrimental effects of a NASH-promoting diet [163]. Consistent with a role of ATGL-catalyzed lipolysis in driving autophagy/lipophagy, the PNPLA3 I148M variant suppresses numerous steps in the autophagy pathway, leading to reduced autophagic and lipophagic flux [164].
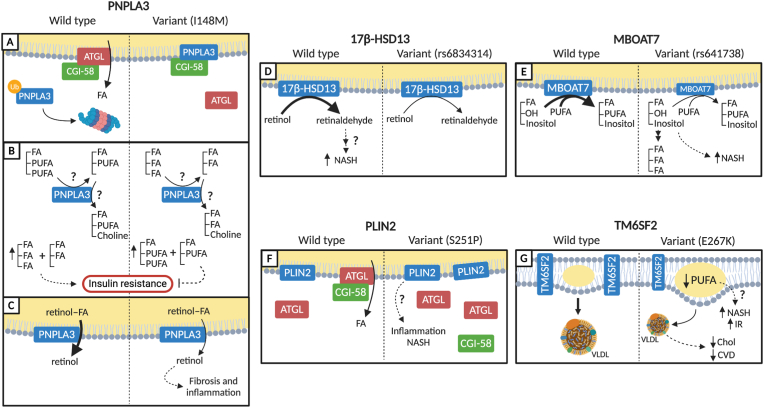
Figure 3.
Proposed mechanisms through which genetic variants influence NAFLD and its comorbidities. (A) The PNPLA3 I148M variant is resistant to ubiquitination, resulting in increased levels of PNPLA3, which can bind and sequester the ATGL co-activator CGI-58 to inhibit lipolysis. (B) The PNPLA3 I148M variant has reduced activity to transfer PUFA acyl chains from TAG or DAG. As a result, there is an enrichment of PUFAs in TAGs and DAGs, which may explain the uncoupling of steatosis from insulin resistance (IR) in carriers of the mutant. C) The I148M SNP also has reduced retinal ester hydrolase activity, which may serve to promote HSC-mediated pro-inflammatory and pro-fibrogenic signaling compared to wild-type PNPLA3. (D) The rs176834314 variant of 17β-HSD13 has reduced retinol dehydrogenase activity that renders carriers more resistant to NASH through unknown mechanisms. (E) The rs641738 variant of MBOAT7 leads to lower levels of the MBOAT7 protein, which has lysophosphatidylinositol acyltransferase activity. As a consequence, increased levels of lysophosphatidylinositol can promote TAG synthesis and cellular dysfunction, leading to NASH. (F) PLIN2 S251P potentially promotes steatosis through increased antagonism of ATGL. As PLIN2 regulates inflammation, it is speculated that this variant may enhance inflammatory signaling to promote NASH. (G) TM6SF2 resides in the ER where it is involved in the incorporation of PUFAs into TAG and phospholipids and VLDL secretion. The E267K variant reduces TM6SF2 protein levels and impairs PUFA enrichment and VLDL secretion. This variant increases NASH incidence but reduces CVD, likely as a result of reduced circulating cholesterol. Solid arrows represent enzymatic conversion or movement through the cell and dashed lines represent signaling. The line width represents flux through a given enzyme or pathway.
Despite its well-characterized effects on promoting NAFLD and numerous advanced liver diseases, the PNPLA3 I148M variant is not associated with systemic derangements in metabolism such as insulin resistance/type 2 diabetes or cardiovascular disease [165]. One explanation for this uncoupling of NAFLD from common comorbidities may be due to the effects of PNPLA3 on the composition of LDs, which may arise from PNPLA3's transacylase activities involving the transfer of acyl groups from TAG to phospholipids [118]. In particular, numerous studies show that compared to wild type, the PNPLA3 variant has reduced activity toward TAG substrates enriched with PUFAs, resulting in hepatic and circulating TAG with a higher PUFA composition relative to saturated and monounsaturated species (Figure 3B) [166,167]. In controlled labeling experiments, PNPLA3 I148M expression results in the accumulation of DAG-PUFAs and a subsequent reduction in PUFA-containing phosphatidylcholine (PC) [166]. As reductions in hepatic PUFAs and increases in saturated FAs in TAGs are associated with insulin resistance [168,169], it is plausible that reciprocal changes in these lipid species in subjects carrying the PNPLA3 I148M variant may underlie the dissociation of NAFLD from more systemic metabolic perturbations.
PNPLA3 may also contribute to NAFLD development and progression through mechanisms external to hepatocytes. PNPLA3 expression is robustly increased during hepatic stellate cell (HSC) activation [170]. Moreover, expression of the I148M variant potentiates the fibrogenic response of HSCs, whereas ablation of PNPLA3 attenuates HSC activation [170]. In contrast to hepatocyte LDs comprised primarily of TAGs, LDs in HSCs are enriched in retinol esters [171]. Recent studies revealed that recombinant PNPLA3 has retinyl ester hydrolase activity and promotes the release of retinol from HSCs [172]. The I148M variant has reduced retinyl palmitate lipase activity and more pro-inflammatory and pro-fibrogenic signaling compared to wild type (Figure 3C) [170,172]. These studies provided yet another clue into the multifaceted role of this PNPLA3 mutation in NAFLD etiology.
5.2. 17β-HSD13
A proteomics screen of hepatic LDs identified 17β-hydroxysteroid dehydrogenase-13 (17β-HSD13) to be increased in liver biopsies from subjects with NAFLD [173]. Overexpression of 17β-HSD13 in the liver of mice increases steatosis, confirming a direct effect on disease etiology [173]. Several minor allele variants of 17β-HSD13 have been identified to affect liver disease. The rs72613567:TA allele results in a truncated and lower abundant protein associated with a reduction in numerous chronic liver diseases including NAFLD and non-alcoholic cirrhosis [174]. A minor allele variant (rs6834314) of 17β-HSD13 that results in loss of function is associated with increased steatosis but reduced markers of NASH such as inflammation, ballooning, and Mallory-Denk bodies [175]. Carriers of the rs6834314 variant have a lower risk of NASH and liver damage [176], reduced expression of inflammatory genes, are protected against fibrosis, and do not exhibit alterations in insulin sensitivity [177]. 17β-HSD13 was recently shown to possess retinol dehydrogenase activity, which depends on its targeting to LDs (Figure 3D) [175]. Thus, variants in both PNPLA3 and 17β-HSD13 influence retinol metabolism, but with divergent effects on disease progression. Interestingly, carrying the 17β-HSD13 rs6834314 or rs72613567:TA alleles protect against the detrimental effects of the PNPLA3 I148M genotype on advanced hepatic fibrosis [174,178]. These studies highlight an emerging role of retinol metabolism as an important etiological factor in NAFLD and suggest that an interplay of genetic variants regulates LD homeostasis to influence disease progression.
5.3. PLIN2
PLIN2 is the most abundant PLIN protein present on hepatic LDs and plays a key role in regulating LD turnover as discussed above. The presence of an rs35568725 variant of PLIN2 that encodes a S251P polymorphism results in a nearly 3-fold increase in NASH prevalence [179]. Overexpression of this variant increases LD numbers but reduces LD size in response to lipid loading. While the details on how this variant affects PLIN2 function are still unknown, the mutation resides in a key α-helix that regulates lipolysis in macrophages, suggesting that the variant may further antagonize TAG breakdown (Figure 3E) [180].
5.4. MBOAT7
Membrane-bound O-acyltransferase domain-containing 7 (MBOAT7), which is enriched in the ER and LDs [181,182], has lysophosphatidylinositol acyltransferase activities and contributes to the composition of phospholipids through the addition of PUFAs at the sn-2 position [183]. The rs641738 variant of MBOAT7 results in reduced mRNA and protein expression, leading to a more saturated phospholipid profile and increased lysophosphatidylinositol [181,182,184]. Consistent with a more detrimental effect of saturated lipids, the MBOAT7 variant is linked to NAFLD progression, HCC, and insulin resistance [181,182,185]. While the detailed mechanism of its effects on NAFLD etiology are not fully understood, ablation of MBOAT7 increases TAG synthesis via the non-canonical pathway of phosphatidylinositol breakdown to DAG [186]. The increase in 18:0 lysophosphatidylinositol observed in the MBOAT7 variant may also drive alterations in metabolism and facilitate NASH development (Figure 3F) [182]. These findings are supported by the recent characterization of liver-specific MBOAT7 knockout mice, which have reduced PUFA content specifically in phosphatidylinositol [187]. Because of the changes in phosphatidylinositol composition, DNL increases due to changes in processing of the lipogenic transcription factor sterol regulatory element-binding protein 1c, which is required for the development of steatosis in mice lacking hepatic MBOAT7 [187].
5.5. TM6SF2
A variant (E167K) of the transmembrane 6 superfamily member 2 (TM6SF2) is recognized to promote steatosis through reductions in VLDL secretion, as measured in human liver cells, resulting in reduced circulating TAG-rich lipoproteins [188]. The E167K mutant results in less TM6SF2 protein expression due to protein instability [189]. The TM6SF2 variant suppresses cholesterol synthesis and incorporation of PUFAs into hepatic PC and TAG [188,190]. Carriers of this variant have an increased risk of NAFLD and NASH and exhibit impaired glucose/insulin homeostasis but have a reduced risk for CVD likely due to lower circulating cholesterol (Figure 3G) [[191], [192], [193], [194], [195]]. Given that TM6SF2 differentially affects various metabolic outcomes, detailed studies in humans are needed to elucidate the intricacies of its complex biology.
6. Emerging concepts
6.1. Composition matters
6.1.1. Lipid composition
Cholesterol is rapidly emerging as a key metabolite in the etiology of NASH. Iannou et al. first observed the presence of cholesterol crystals in LDs from subjects with NASH but not in samples from subjects with steatosis [196]. This work was supported by other groups, showing that free cholesterol and rates of cholesterol synthesis are associated with NAFLD severity in humans and mice [[197], [198], [199], [200]]. Increasing dietary cholesterol intake in rodents also increases LD cholesterol crystals and promotes inflammation and fibrosis [201,202], although controlled studies in humans that dissect out specific contribution of dietary cholesterol to NAFLD are lacking. Activated Kupffer cells surround dead hepatocytes laden with cholesterol crystals, suggesting that cell death and signaling to immune cells may explain how cholesterol crystals promote inflammation [196]. Given that these crystals occur within the LD monolayer, it would seem likely that they also affect protein targeting to LDs. It is known that alterations in neutral lipid composition are sufficient to change LD targeting of the various perilipin family members including PLIN2, which has been shown to bind cholesterol, providing a possible mechanism for cholesterol accumulation in the LD monolayer [139,203]. Thus, a major thematic area of research moving forward will likely focus on how the accumulation of hepatic cholesterol crystals impacts LD surface proteins to facilitate NAFLD progression. In addition to cholesterol, the acyl profile of LDs may also impact NAFLD. Numerous studies confirm a reduction in n-3 PUFAs in NASH [130,204]. Results from clinical trials aimed at restoring n-3 PUFAs have been mixed, perhaps due to the heterogeneity of the disease [204]. As noted above, an enrichment of saturated fatty acyl groups in numerous lipid species exacerbates simple steatosis and links it to more advanced liver and systemic diseases. Indeed, saturated fat intake robustly promotes hepatic TAG accumulation, inflammation, and insulin resistance [205,206]. Levels of PC, which is the principal phospholipid in the LD monolayer, are reduced in subjects with NAFLD and NASH, which suggests that alterations in the LD monolayer could also influence disease progression [130]. In support, the relative amounts of PC to other phospholipids such as phosphatidylethanolamine dictate LD size and dynamics as well as protein binding [[207], [208], [209]]. There is also significant heterogeneity across LDs as large LDs have more saturated TAGs compared to smaller LDs in human samples of NAFLD [210]. As lipidomic approaches continue to advance, it is likely future analysis will quantify in more detail changes in LD lipid composition and how these changes link steatosis to its numerous comorbidities.
6.1.2. Protein composition
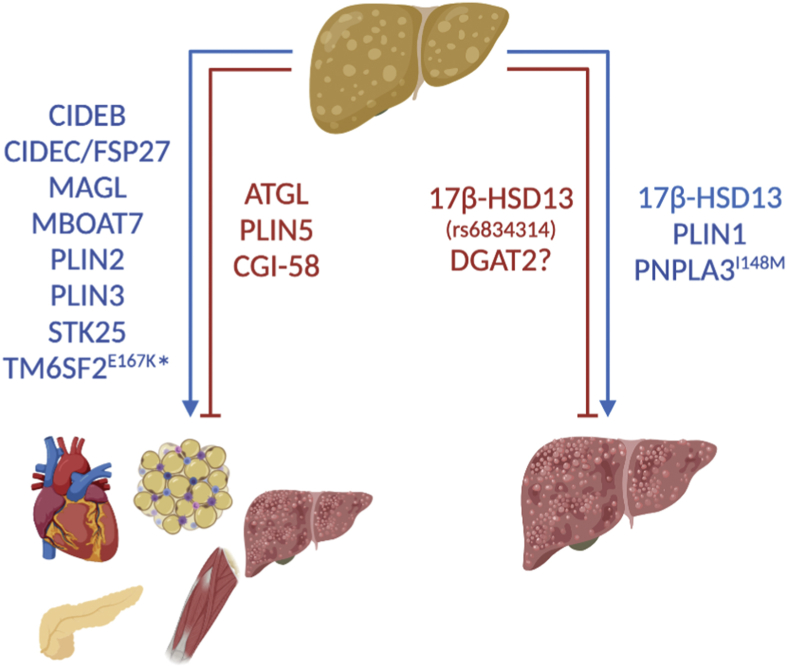
The surface of the LD is coated with hundreds of proteins whose presence and/or abundance are dynamic as evidenced by numerous previously described examples. While LD proteomics have been conducted in many cell types, several studies characterized the hepatic LD proteome under normal conditions or in NAFLD [173,[211], [212], [213]]. These studies expedited the discovery of novel proteins with pathological roles in liver disease. Figure 4 provides a list of proteins that drive NAFLD and couple or uncouple it from its comorbidities. PLIN2 and PLIN3 expression are increased in NAFLD [[214], [215], [216]], and consistent with their anti-lipolytic roles, liver-specific ablation of either protein prevents NAFLD [138,217,218]. While PLIN1 is normally expressed at low or undetectable levels in the liver, its expression increases in biopsies of subjects with NAFLD [214,219]. More specifically, PLIN1 increases with NASH and has been proposed to be used as a diagnostic marker for differentiating NASH from simple steatosis [214,215,219]. Despite its unique expression profile, it is unknown whether the increased abundance of PLIN1 is causal or consequential to NASH. Perilipins are also highly regulated through phosphorylation and potentially other post-translational modifications [32]. Thus, the identification of serine/threonine protein kinase 25 (STK25) as a resident LD protein in hepatocytes points to local regulation of protein modifications [220]. Overexpression of STK25 promotes steatosis, insulin resistance, inflammation, and fibrosis in mice, whereas knockdown prevents the same [220,221]. STK25 protein abundance also correlates with steatosis in humans [222]. While STK25's detailed mechanism of action remains to be elucidated, it is clearly a contributor to LD-linked development and progression of NAFLD. In addition to “classical” LD proteins such as perilipins, LD proteomic analyses reveal the presence of numerous classes of proteins including those involved in protein translation, transcriptional regulation including histones, vesicle trafficking, and cell signaling, among others [223]. While examples of some of these functions have been previously noted or characterized in the literature, the contribution of most of these proteins to LD biology remains largely uncharacterized.
Figure 4.
Proteins are shown that either promote or inhibit the development and progression of steatosis to NASH and systemic comorbidities (e.g., insulin resistance, CVD, etc.) or only facilitate the development of advanced liver diseases including NASH. ∗Carriers of the TM6SF2 E167K variant have an increased risk of NAFLD/NASH, insulin resistance, and type 2 diabetes, but are protected from CVD.
6.2. Location matters
6.2.1. Organelle interactions
A common hallmark of LD proteomic screens is the identification of proteins known to reside in other organelles. Multispectral time-lapse imaging of six organelles simultaneously reveals nutrient-regulated LD interactions with the ER, mitochondria, lysosomes, Golgi, and peroxisomes [224], although interactions with other organelles also exist. These interactions are thought to serve numerous functions including lipid and protein exchange as well as compartmentalization of signaling [225]. As noted above, the disruption of LD-mitochondrial interactions via deletion of PLIN5 is detrimental to cell health [125,147,[226], [227], [228]]. In addition, the disruption of LD interactions with lysosomes via RAB7 inhibition blocks lipophagy [59]. However, little is known about how LD interactions with other organelles change, or the importance of many such interactions in the context of NAFLD. With advances in proteomics and microscopic tools, defining these sites of compartmentalized metabolism and signaling may shed novel insights into NAFLD's etiology (Figure 5).
Figure 5.
While NAFLD is defined by LD accumulation, the location of LDs matters. (Upper right) LD size and composition can vary widely across hepatic sinusoids. (Lower right) In addition to hepatocytes, LDs can also accumulate in endothelial cells, HSCs, and Kupffer cells. (Lower left) LDs interact with numerous organelles such as the ER, mitochondria, and peroxisomes to coordinate lipid trafficking and compartmentalize cell signaling. LDs are also present in the nucleus under certain conditions, although the significance of this LD pool is largely undetermined.
6.2.2. Nuclear LDs
LDs have historically been accepted to reside in the cytosol with the exception of their transit through the ER or Golgi during lipoprotein secretion. However, recent studies show that hepatocytes may also contain nuclear LDs under certain conditions [229,230]. These nuclear LDs have distinct lipid compositions with reduced levels of TAGs and more cholesterol, cholesterol ester, and polar lipids compared with cytosolic LDs [229] and have a monolayer that is enriched in diacylglycerol and phosphatidic acid [231]. The inner nuclear membrane contains numerous proteins involved in LD biogenesis and is the source of nuclear LDs [231]. In support, deletion of the inner nuclear membrane protein lamina-associated polypeptide 1 triggers a robust accumulation of nuclear LDs [232]. Lipid-induced ER stress promotes nuclear LD formation that in turn regulates PC through the direct binding of the PC-synthesizing enzyme CTP phosphocholine cytidylyltransferase-α to nuclear LDs to promote PC synthesis and alleviate ER stress [233]. These studies identify a novel stress response mechanism that nuclear LDs specifically mediate. While only a handful of papers have focused on nuclear LDs, it is clear that this will be a major field of study moving forward given the potential myriad of mechanisms through which this unique LD pool can affect cell signaling and transcription.
6.2.3. Zonation
While heterogeneity of hepatocyte metabolism has been known for decades, the recent utilization of single cell-sequencing technologies has triggered a rapid advance in our understanding of hepatic zonation. It is established that adult subjects with NAFLD exhibit preferential accumulation of LDs in the pericentral region [81,234]. In contrast, children with NAFLD more commonly present with periportal steatosis [234]. In rodent models, diet composition appears to be a major determinant of zonal lipid accumulation [235]. Despite these differences, the significance of heterogeneous fat deposition is largely unknown. Given the diverse roles of LDs in regulating metabolism and cell signaling and the diversity of major metabolic pathways across the liver, studies characterizing the zonal aspects of LD accumulation are warranted.
6.2.4. Hepatic cell types
While hepatocytes constitute the major cell type in the liver, HSC, Kupffer cells, and liver sinusoidal endothelial cells as well as numerous but less abundant immune cells play significant roles in liver function and NAFLD development. As noted above, HSCs possess abundant retinol-rich LDs, and metabolism of these LDs is a major determinant of HSC activation [236]. In response to high-fat feeding in mice, Kupffer cells accumulate LDs, which sensitizes their pro-inflammatory response to LPS [237]. LDs also accumulate in liver sinusoid endothelial cells in response to high-fat feeding or NASH-promoting diets in mice [238,239]. It is documented that LDs broadly regulate metabolism, signaling, and immunogenic response across a wide range of immune cell types [240]. Given these findings, it seems critical for future studies to assess how cell type-specific accumulation of LDs and their subsequent metabolism and signaling contribute to NAFLD etiology.
6.3. Timing matters
While differences in composition and localization of LDs undoubtedly impact hepatic function and NAFLD, the impact of time-dependent changes on LD metabolism and signaling are poorly understood as most studies focused on a single time point for assessing LDs. These changes included both normal circadian regulation of LD biology as well as responses to both the amount and composition of a meal. For example, many genes involved in hepatic LD metabolism and LD lipid composition are under circadian control [241], which is known to be disrupted in NAFLD [242]. In addition, a single study assessing the LD proteome in fasted and fasted/refed mice reveal abundant changes in LD proteins, implicating differences in organelle interactions and LD metabolism [243]. These initial studies provid a sound premise to more thoroughly investigate how feeding/refeeding, meal composition, and circadian patterns govern LD dynamics. Thus, in addition to spatial aspects of LD biology, temporal factors are likely to play important roles in regulating LD metabolism, and in coupling/uncoupling LD accumulation from disease development.
7. Conclusions
LDs are the distinguishing trait of NAFLD and play important roles in both the development and progression of this disease that lacks efficacious and approved treatments. Numerous factors including diet, genetics, and predisposing diseases (obesity, diabetes, etc.) can lead to NAFLD through different etiological paths. Understanding how alterations in the LD proteome and lipidome change in response to these factors to influence the development, severity, and unique subtypes of NAFLD will be a critical research area moving forward. Moreover, advancing our understanding of the heterogeneity and unique aspects of LDs within a given cell, across cell types, or across the entire liver will be required if we truly want to define the molecular mechanism of NAFLD. Recent studies have greatly strengthened our grasp of LD biology and, with the rapid emergence or more sensitive and powerful scientific tools, we expect that this field will continue its rapid expansion to further our progress in understanding and treating this prevalent and deleterious liver disease.
Acknowledgments
The author thanks Brian Finck, Sudha Biddinger, Lisa Chow, and members of the Mashek laboratory for their critical evaluation of this review. DGM is supported by NIH grants R01AG055452 and R01DK114401.
Conflict of interest
None declared.
References
- 1.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 2.Bonora E., Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nature Reviews Gastroenterology & Hepatology. 2012;9(7):372–381. doi: 10.1038/nrgastro.2012.79. [DOI] [PubMed] [Google Scholar]
- 3.Rafiq N., Bai C., Fang Y., Srishord M., McCullough A., Gramlich T. Long-term follow-up of patients with nonalcoholic fatty liver. Clinical Gastroenterology and Hepatology. 2009;7(2):234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Baffy G., Brunt E.M., Caldwell S.H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. Journal of Hepatology. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature Reviews Gastroenterology & Hepatology. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 6.Eslam M., Sanyal A.J., George J., Sanyal A.J., Neuschwander-Tetri B., Tiribelli C. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg A.S., Coleman R.A., Kraemer F.B., McManaman J.L., Obin M.S., Puri V. The role of lipid droplets in metabolic disease in rodents and humans. Journal of Clinical Investigation. 2011;121(6):2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrows B.R., Parks E.J. Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. Journal of Clinical Endocrinology & Metabolism. 2006;91(4):1446–1452. doi: 10.1210/jc.2005-1709. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly K.L., Margosian M.R., Sheth S.S., Lusis A.J., Parks E.J. Increased lipogenesis and fatty acid reesterification contribute to hepatic triacylglycerol stores in hyperlipidemic Txnip-/- mice. Journal of Nutrition. 2004;134(6):1475–1480. doi: 10.1093/jn/134.6.1475. [DOI] [PubMed] [Google Scholar]
- 10.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2019;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finck B.N. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes. 2018;67(12):2485–2493. doi: 10.2337/dbi18-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaffler A., Scholmerich J., Buchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue–emerging role in nonalcoholic fatty liver disease. Nature Clinical Practice Gastroenterology & Hepatology. 2005;2(6):273–280. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- 13.Bu S.Y., Mashek D.G. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. Journal of Lipid Research. 2010;51(11):3270–3280. doi: 10.1194/jlr.M009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh A.B., Kan C.F.K., Kraemer F.B., Sobel R.A., Liu J. Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet. American Journal of Physiology - Endocrinology And Metabolism. 2019;316(5):E880–E894. doi: 10.1152/ajpendo.00503.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L.O., Ellis J.M., Paich H.A., Wang S., Gong N., Altshuller G. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. Journal of Biological Chemistry. 2009;284(41):27816–27826. doi: 10.1074/jbc.M109.022467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman R.A., Mashek D.G. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chemical Reviews. 2011;111(10):6359–6386. doi: 10.1021/cr100404w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vancura A., Haldar D. Purification and characterization of glycerophosphate acyltransferase from rat liver mitochondria. Journal of Biological Chemistry. 1994;269(44):27209–27215. [PubMed] [Google Scholar]
- 18.Wurie H.R., Buckett L., Zammit V.A. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS Journal. 2012;279(17):3033–3047. doi: 10.1111/j.1742-4658.2012.08684.x. [DOI] [PubMed] [Google Scholar]
- 19.Qi J., Lang W., Geisler J.G., Wang P., Petrounia I., Mai S. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. Journal of Lipid Research. 2012;53(6):1106–1116. doi: 10.1194/jlr.M020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva C.J., Monetti M., Shih M., Zhou P., Watkins S.M., Bhanot S. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50(2):434–442. doi: 10.1002/hep.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C., Li L., Lian J., Watts R., Nelson R., Goodwin B. Roles of Acyl-CoA: diacylglycerol acyltransferases 1 and 2 in triacylglycerol synthesis and secretion in primary hepatocytes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35(5):1080–1091. doi: 10.1161/ATVBAHA.114.304584. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt-Wessel B., Jordan T.W., Miller J.H., Peng L. Role of DGAT enzymes in triacylglycerol metabolism. Archives of Biochemistry and Biophysics. 2018;655:1–11. doi: 10.1016/j.abb.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Qian H., Nian Y., Han Y., Ren Z., Zhang H. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020;581(7808):329–332. doi: 10.1038/s41586-020-2280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilfling F., Haas J.T., Walther T.C., Jr R.V.F. Lipid droplet biogenesis. Current Opinion in Cell Biology. 2014;29:39–45. doi: 10.1016/j.ceb.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pol A., Gross S.P., Parton R.G. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins, and sites. The Journal of Cell Biology. 2014;204(5):635–646. doi: 10.1083/jcb.201311051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu W., Wu L., Yu M., Chen F.-J., Arshad M., Xia X. Differential roles of cell death-inducing DNA fragmentation factor-α-like effector (CIDE) proteins in promoting lipid droplet fusion and growth in subpopulations of hepatocytes. Journal of Biological Chemistry. 2016;291(9):4282–4293. doi: 10.1074/jbc.M115.701094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langhi C., Baldán Á. CIDEC/FSP27 is regulated by peroxisome proliferator-activated receptor alpha and plays a critical role in fasting- and diet-induced hepatosteatosis. Hepatology. 2015;61(4):1227–1238. doi: 10.1002/hep.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilfling F., Wang H., Haas J.T., Krahmer N., Gould T.J., Uchida A. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Developmental Cell. 2013;24(4):384–399. doi: 10.1016/j.devcel.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao M., Huang X., Song B.L., Yang H. The biogenesis of lipid droplets: lipids take center stage. Progress in Lipid Research. 2019;75:100989. doi: 10.1016/j.plipres.2019.100989. [DOI] [PubMed] [Google Scholar]
- 30.Grasselli E., Voci A., Pesce C., Canesi L., Fugassa E., Gallo G. PAT protein mRNA expression in primary rat hepatocytes: effects of exposure to fatty acids. International Journal of Molecular Medicine. 2010;25(4):505–512. doi: 10.3892/ijmm_00000370. [DOI] [PubMed] [Google Scholar]
- 31.Imai Y., Varela G.M., Jackson M.B., Graham M.J., Crooke R.M., Ahima R.S. Reduction of hepatosteatosis and lipid levels by an adipose differentiation-related protein antisense oligonucleotide. Gastroenterology. 2007;132(5):1947–1954. doi: 10.1053/j.gastro.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 32.Sztalryd C., Brasaemle D.L. The perilipin family of lipid droplet proteins: gatekeepers of intracellular lipolysis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862(10 Pt B):1221–1232. doi: 10.1016/j.bbalip.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaushik S., Cuervo A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nature Cell Biology. 2015;17(6):759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapiro J.M., Mashek M.T., Greenberg A.S., Mashek D.G. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. Journal of Lipid Research. 2009;50(8):1621–1629. doi: 10.1194/jlr.M800614-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaushik S., Cuervo A.M. AMPK-dependent phosphorylation of lipid droplet protein PLIN2 triggers its degradation by CMA. Autophagy. 2016;12(2):432–438. doi: 10.1080/15548627.2015.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong K.T., Mashek M.T., Bu S.Y., Greenberg A.S., Mashek D.G. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2011;53(1):116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu J.W., Wang S.P., Alvarez F., Casavant S., Gauthier N., Abed L. Deficiency of liver adipose triglyceride lipase in mice causes progressive hepatic steatosis. Hepatology. 2011;54(1):122–132. doi: 10.1002/hep.24338. [DOI] [PubMed] [Google Scholar]
- 38.Turpin S.M., Hoy A.J., Brown R.D., Rudaz C.G., Honeyman J., Matzaris M. Adipose triacylglycerol lipase is a major regulator of hepatic lipid metabolism but not insulin sensitivity in mice. Diabetologia. 2011;54(1):146–156. doi: 10.1007/s00125-010-1895-5. [DOI] [PubMed] [Google Scholar]
- 39.Sekiya M., Osuga J.I., Yahagi N., Okazaki H., Tamura Y., Igarashi M. Hormone-sensitive lipase is involved in hepatic cholesteryl ester hydrolysis. Journal of Lipid Research. 2008;49(8):1829–1838. doi: 10.1194/jlr.M800198-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Holm C., Kirchgessner T.G., Svenson K.L., Fredrikson G., Nilsson S., Miller C.G. Hormone-sensitive lipase: sequence, Expression, and chromosomal localization to 19 cent-q13.3. Science. 1988;241(4872):1503–1506. doi: 10.1126/science.3420405. [DOI] [PubMed] [Google Scholar]
- 41.MacParland S.A., Liu J.C., Ma X.-Z., Innes B.T., Bartczak A.M., Gage B.K. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nature Communications. 2018;9(1):4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metabolism. 2006;3(5):309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Yang P., Wang Y., Tang W., Sun W., Ma Y., Lin S. Western diet induces severe nonalcoholic steatohepatitis, ductular reaction, and hepatic fibrosis in liver CGI-58 knockout mice. Scientific Reports. 2020;10(1):4701. doi: 10.1038/s41598-020-61473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo F., Ma Y., Kadegowda A.K.G., Xie P., Liu G., Liu X. Deficiency of liver Comparative Gene Identification-58 causes steatohepatitis and fibrosis in mice. Journal of Lipid Research. 2013;54(8):2109–2120. doi: 10.1194/jlr.M035519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord C.C., Ferguson D., Thomas G., Brown A.L., Schugar R.C., Burrows A. Regulation of hepatic triacylglycerol metabolism by CGI-58 does not require ATGL Co-activation. Cell Reports. 2016;16(4):939–949. doi: 10.1016/j.celrep.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y., Zhang Y., Qian H., Lu J., Zhang Z., Min X. The g0/g1 switch gene 2 is an important regulator of hepatic triglyceride metabolism. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0072315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W., Bu S.Y., Mashek M.T., O-Sullivan I., Sibai Z., Khan S.A. Integrated regulation of hepatic lipid and glucose metabolism by adipose triacylglycerol lipase and FoxO proteins. Cell Reports. 2016;15(2):349–359. doi: 10.1016/j.celrep.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugaya Y., Satoh H. Liver-specific G0/G1 switch gene 2 (G0s2) expression promotes hepatic insulin resistance by exacerbating hepatic steatosis in male Wistar rats. Journal of Diabetes. 2017;9(8):754–763. doi: 10.1111/1753-0407.12482. [DOI] [PubMed] [Google Scholar]
- 49.Das K.M.P., Wechselberger L., Liziczai M., Rodriguez M.D. la R., Grabner G.F., Heier C. Hypoxia-inducible lipid droplet-associated protein inhibits adipose triglyceride lipase. Journal of Lipid Research. 2018;59(3):531–541. doi: 10.1194/jlr.M082388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grahn T.H.M., Kaur R., Yin J., Schweiger M., Sharma V.M., Lee M.-J. Fat-specific protein 27 (FSP27) interacts with adipose triglyceride lipase (ATGL) to regulate lipolysis and insulin sensitivity in human adipocytes. Journal of Biological Chemistry. 2014;289(17):12029–12039. doi: 10.1074/jbc.M113.539890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung C., Doll J.A., Stellmach V.M., Gonzales J., Surapureddi S., Cornwell M. Pigment epithelium-derived factor is an angiogenesis and lipid regulator that activates peroxisome proliferator-activated receptor alpha. Advances in Experimental Medicine & Biology. 2008;617:591–597. doi: 10.1007/978-0-387-69080-3_61. [DOI] [PubMed] [Google Scholar]
- 52.Wang H., Wei E., Quiroga A.D., Sun X., Touret N., Lehner R. Altered lipid droplet dynamics in hepatocytes lacking triacylglycerol hydrolase expression. Molecular Biology of the Cell. 2010;21(12):1991–2000. doi: 10.1091/mbc.E09-05-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lian J., Wei E., Wang S.P., Quiroga A.D., Li L., Di Pardo A. Liver specific inactivation of carboxylesterase 3/triacylglycerol hydrolase decreases blood lipids without causing severe steatosis in mice. Hepatology. 2012;56(6):2154–2162. doi: 10.1002/hep.25881. [DOI] [PubMed] [Google Scholar]
- 54.Blais D.R., Lyn R.K., Joyce M.A., Rouleau Y., Steenbergen R., Barsby N. Activity-based protein profiling identifies a host enzyme, carboxylesterase 1, which is differentially active during hepatitis C virus replication. Journal of Biological Chemistry. 2010;285(33):25602–25612. doi: 10.1074/jbc.M110.135483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lian J., Bahitham W., Panigrahi R., Nelson R., Li L., Watts R. Genetic variation in human carboxylesterase CES1 confers resistance to hepatic steatosis. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2018;1863(7):688–699. doi: 10.1016/j.bbalip.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Lian J., Wei E., Groenendyk J., Das S.K., Hermansson M., Li L. Ces3/TGH deficiency attenuates steatohepatitis. Scientific Reports. 2016;6:25747. doi: 10.1038/srep25747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quiroga A.D., Lehner R. Pharmacological intervention of liver triacylglycerol lipolysis: the good, the bad and the ugly. Biochemical Pharmacology. 2018;155:233–241. doi: 10.1016/j.bcp.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schroeder B., Schulze R.J., Weller S.G., Sletten A.C., Casey C.A., McNiven M.A. The Small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology. 2015;61(6):1896–1907. doi: 10.1002/hep.27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulze R.J., Sathyanarayan A., Mashek D.G. Breaking fat: the regulation and mechanisms of lipophagy. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862(10) doi: 10.1016/j.bbalip.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sathyanarayan A., Mashek M.T., Mashek D.G. ATGL promotes autophagy/lipophagy via SIRT1 to control hepatic lipid droplet catabolism. Cell Reports. 2017;19(1):1–9. doi: 10.1016/j.celrep.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai T.-H., Chen E., Li L., Saha P., Lee H.-J., Huang L.-S. The constitutive lipid droplet protein PLIN2 regulates autophagy in liver. Autophagy. 2017;13(7):1130–1144. doi: 10.1080/15548627.2017.1319544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schott M.B., Weller S.G., Schulze R.J., Krueger E.W., Drizyte-Miller K., Casey C.A. Lipid droplet size directs lipolysis and lipophagy catabolism in hepatocytes. The Journal of Cell Biology. 2019;218(10):3320–3335. doi: 10.1083/jcb.201803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinez-Lopez N., Garcia-Macia M., Sahu S., Athonvarangkul D., Liebling E., Merlo P. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the Brown adipose tissue and liver. Cell Metabolism. 2015;23(1):113–127. doi: 10.1016/j.cmet.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui W., Sathyanarayan A., Lopresti M., Aghajan M., Chen C., Mashek D.G. Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis. Autophagy. 2020:1–16. doi: 10.1080/15548627.2020.1728097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herms A., Bosch M., Ariotti N., Reddy B.J.N., Fajardo A., Fernández-Vidal A. Cell-to-Cell heterogeneity in lipid droplets suggests a mechanism to reduce lipotoxicity. Current Biology. 2013;23(15):1489–1496. doi: 10.1016/j.cub.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinha R.A., Farah B.L., Singh B.K., Siddique M.M., Li Y., Wu Y. Caffeine stimulates hepatic lipid metabolism by the autophagy-lysosomal pathway in mice. Hepatology. 2014;59(4):1366–1380. doi: 10.1002/hep.26667. [DOI] [PubMed] [Google Scholar]
- 68.Huang Q., Wang T., Yang L., Wang H.-Y. Ginsenoside Rb2 alleviates hepatic lipid accumulation by restoring autophagy via induction of Sirt1 and activation of AMPK. International Journal of Molecular Sciences. 2017;18(5):1063. doi: 10.3390/ijms18051063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X., Chan H., Zhang L., Liu X., Ho I.H.T., Zhang X. The phytochemical polydatin ameliorates non-alcoholic steatohepatitis by restoring lysosomal function and autophagic flux. Journal of Cellular and Molecular Medicine. 2019;6:4290–4300. doi: 10.1111/jcmm.14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu X., Xiong T., Liu P., Guo X., Xiao L., Zhou F. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food and Chemical Toxicology. 2018;114:52–60. doi: 10.1016/j.fct.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 71.Parafati M., Lascala A., Morittu V.M., Trimboli F., Rizzuto A., Brunelli E. Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. The Journal of Nutritional Biochemistry. 2015;26(9):938–948. doi: 10.1016/j.jnutbio.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 72.Carotti S., Aquilano K., Zalfa F., Ruggiero S., Valentini F., Zingariello M. Lipophagy impairment is associated with disease progression in NAFLD. Frontiers in Physiology. 2020;11:850. doi: 10.3389/fphys.2020.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Allaire M., Rautou P.E., Codogno P., Lotersztajn S. Autophagy in liver diseases: time for translation? Journal of Hepatology. 2019;70(5):985–998. doi: 10.1016/j.jhep.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 74.Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. The Lancet Diabetes and Endocrinology. 2014:901–910. doi: 10.1016/S2213-8587(14)70032-4. [DOI] [PubMed] [Google Scholar]
- 75.Glass L.M., Hunt C.M., Fuchs M., Su G.L. Comorbidities and nonalcoholic fatty liver disease: the chicken, the egg, or both? Federal Practitioner. 2019;36(2):64–71. [PMC free article] [PubMed] [Google Scholar]
- 76.Korenblat K.M., Fabbrini E., Mohammed B.S., Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gastaldelli A., Cusi K., Pettiti M., Hardies J., Miyazaki Y., Berria R. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133(2):496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 78.Kotronen A., Juurinen L., Tiikkainen M., Vehkavaara S., Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 79.Kotronen A., Vehkavaara S., Seppälä-Lindroos A., Bergholm R., Yki-Järvinen H. Effect of liver fat on insulin clearance. American Journal of Physiology - Endocrinology And Metabolism. 2007;293(6):E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 80.Bril F., Barb D., Portillo-Sanchez P., Biernacki D., Lomonaco R., Suman A. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 81.Chalasani N., Wilson L., Kleiner D.E., Cummings O.W., Brunt E.M., Ünalp A. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2008;48(5):829–834. doi: 10.1016/j.jhep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee Y., Hirose H., Ohneda M., Johnson J.H., McGarry J.D., Unger R.H. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(23):10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ertunc M.E., Hotamisligil G.S. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. Journal of Lipid Research. 2016;57(12):2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Listenberger L.L., Han X., Lewis S.E., Cases S., Farese R.V., Jr., Ory D.S. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proceedings of the National Academy of Sciences. 2003;100(6):3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maedler K., Spinas G.A., Dyntar D., Moritz W., Kaiser N., Donath M.Y. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50(1):69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 86.Ricchi M., Odoardi M.R., Carulli L., Anzivino C., Ballestri S., Pinetti A. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. Journal of Gastroenterology and Hepatology. 2009;24(5):830–840. doi: 10.1111/j.1440-1746.2008.05733.x. [DOI] [PubMed] [Google Scholar]
- 87.Kharroubi I., Ladriere L., Cardozo A.K., Dogusan Z., Cnop M., Eizirik D.L. Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology. 2004;145(11):5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 88.Piccolis M., Bond L.M., Kampmann M., Pulimeno P., Chitraju C., Jayson C.B.K. Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Molecular Cell. 2019;74(1):32–44. doi: 10.1016/j.molcel.2019.01.036. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dircks L.K., Sul H.S. Mammalian mitochondrial glycerol-3-phosphate acyltransferase. Biochimica et Biophysica Acta. 1997;1348(1–2):17–26. doi: 10.1016/s0005-2760(97)00106-9. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y.Q., Kuo M.-S., Li S., Bui H.H., Peake D.A., Sanders P.E. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. Journal of Biological Chemistry. 2008;283(15):10048–10057. doi: 10.1074/jbc.M708151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masuda M., Miyazaki-Anzai S., Keenan A.L., Okamura K., Kendrick J., Chonchol M. Saturated phosphatidic acids mediate saturated fatty acid-induced vascular calcification and lipotoxicity. Journal of Clinical Investigation. 2015;125(12):4544–4558. doi: 10.1172/JCI82871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monetti M., Levin M.C., Watt M.J., Sajan M.P., Marmor S., Hubbard B.K. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metabolism. 2007;6(1):69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 93.Yamaguchi K., Yang L., McCall S., Huang J., Yu X.X., Pandey S.K. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 94.Gluchowski N.L., Gabriel K.R., Chitraju C., Bronson R.T., Mejhert N., Boland S. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology. 2019;70(6):1972–1985. doi: 10.1002/hep.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McLaren D.G., Han S., Murphy B.A., Wilsie L., Stout S.J., Zhou H. DGAT2 inhibition alters aspects of triglyceride metabolism in rodents but not in non-human primates. Cell Metabolism. 2018;27(6):1236–1248. doi: 10.1016/j.cmet.2018.04.004. e6. [DOI] [PubMed] [Google Scholar]
- 96.Amin N.B., Carvajal-Gonzalez S., Purkal J., Zhu T., Crowley C., Perez S. Targeting diacylglycerol acyltransferase 2 for the treatment of nonalcoholic steatohepatitis. Science Translational Medicine. 2019;11(520) doi: 10.1126/scitranslmed.aav9701. [DOI] [PubMed] [Google Scholar]
- 97.Choi C.S., Savage D.B., Kulkarni A., Yu X.X., Liu Z.-X., Morino K. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. Journal of Biological Chemistry. 2007;282(31):22678–22688. doi: 10.1074/jbc.M704213200. [DOI] [PubMed] [Google Scholar]
- 98.Esler W.P., Bence K.K. Metabolic targets in nonalcoholic fatty liver disease. Cellular and Molecular Gastroenterology and Hepatology. 2019:247–267. doi: 10.1016/j.jcmgh.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei Y., Wang D., Topczewski F., Pagliassotti M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. AJP - Endocrinology and Metabolism. 2006;291(2):E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 100.Wang D., Wei Y., Pagliassotti M.J. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 101.Halbleib K., Pesek K., Covino R., Hofbauer H.F., Wunnicke D., Hänelt I. Activation of the unfolded protein response by lipid bilayer stress. Molecular Cell. 2017;67(4):673–684. doi: 10.1016/j.molcel.2017.06.012. e8. [DOI] [PubMed] [Google Scholar]
- 102.Volmer R., Van Der Ploeg K., Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Malhi H., Kaufman R.J. Endoplasmic reticulum stress in liver disease. Journal of Hepatology. 2011;54(4):795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puri P., Mirshahi F., Cheung O., Natarajan R., Maher J.W., Kellum J.M. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 105.Lake A.D., Novak P., Hardwick R.N., Flores-Keown B., Zhao F., Klimecki W.T. The Adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicological Sciences. 2014;137(1):26–35. doi: 10.1093/toxsci/kft230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee S., Kim S., Hwang S., Cherrington N.J., Ryu D.Y. Dysregulated expression of proteins associated with ER stress, autophagy and apoptosis in tissues from nonalcoholic fatty liver disease. Oncotarget. 2017;8(38):63370–63381. doi: 10.18632/oncotarget.18812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shih L.-M., Tang H.-Y., Lynn K.-S., Huang C.-Y., Ho H.-Y., Cheng M.-L. Stable isotope-labeled lipidomics to unravel the heterogeneous development lipotoxicity. Molecules. 2018;23(11):2862. doi: 10.3390/molecules23112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen T.B., Louie S.M., Daniele J.R., Tran Q., Dillin A., Zoncu R. DGAT1-Dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Developmental Cell. 2017;42(1):9–21. doi: 10.1016/j.devcel.2017.06.003. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Konstantynowicz-Nowicka K., Harasim E., Baranowski M., Chabowski A. New evidence for the role of ceramide in the development of hepatic insulin resistance. PloS One. 2015;10(1) doi: 10.1371/journal.pone.0116858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kasumov T., Li L., Li M., Gulshan K., Kirwan J.P., Liu X. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PloS One. 2015;10(5) doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang M., Li C., Liu Q., Wang A., Lei M. Inhibiting ceramide synthesis attenuates hepatic steatosis and fibrosis in rats with non-alcoholic fatty liver disease. Frontiers in Endocrinology. 2019;10:665. doi: 10.3389/fendo.2019.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Summers S.A. Ceramides in insulin resistance and lipotoxicity. Progress in Lipid Research. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 113.Chaurasia B., Tippetts T.S., Monibas R.M., Liu J., Li Y., Wang L. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365(6451):386–392. doi: 10.1126/science.aav3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorden D.L., Myers D.S., Ivanova P.T., Fahy E., Maurya M.R., Gupta S. Biomarkers of NAFLD progression : a lipidomics approach to an epidemic. Journal of Lipid Research. 2015;56(3):722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alonso C., Fernández-Ramos D., Varela-Rey M., Martínez-Arranz I., Navasa N., Van Liempd S.M. Metabolomic identification of subtypes of nonalcoholic steatohepatitis. Gastroenterology. 2017;152(6):1449–1461. doi: 10.1053/j.gastro.2017.01.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Preuss C., Jelenik T., Bódis K., Müssig K., Burkart V., Szendroedi J. A new targeted lipidomics approach reveals lipid droplets in liver, muscle and heart as a repository for diacylglycerol and ceramide species in non-alcoholic fatty liver. Cells. 2019;8(3):277. doi: 10.3390/cells8030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matsuzaka T., Kuba M., Koyasu S., Yamamoto Y., Motomura K., Arulmozhiraja S. Hepatocyte Elovl6 determines ceramide acyl-chain length and hepatic insulin sensitivity in mice. Hepatology. 2019;71(5):1609–1625. doi: 10.1002/hep.30953. [DOI] [PubMed] [Google Scholar]
- 118.Mitsche M.A., Hobbs H.H., Cohen J.C. Patatin-like phospholipase domain-containing protein 3 promotes transfers of essential fatty acids from triglycerides to phospholipids in hepatic lipid droplets. Journal of Biological Chemistry. 2018;293(18):6958–6968. doi: 10.1074/jbc.RA118.002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Senkal C.E., Salama M.F., Snider A.J., Allopenna J.J., Rana N.A., Koller A. Ceramide is metabolized to acylceramide and stored in lipid droplets. Cell Metabolism. 2017;25(3):686–697. doi: 10.1016/j.cmet.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Win S., Than T.A., Le B.H.A., García-Ruiz C., Fernandez-Checa J.C., Kaplowitz N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. Journal of Hepatology. 2015;62(6):1367–1374. doi: 10.1016/j.jhep.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hammond L.E., Albright C.D., He L., Rusyn I., Watkins S.M., Doughman S.D. Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Experimental and Molecular Pathology. 2007;82(2):210–219. doi: 10.1016/j.yexmp.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan, Jin, Wang, Huang, Wu Ren. Perilipin 5 protects against cellular oxidative stress by enhancing mitochondrial function in HepG2 cells. Cells. 2019;8(10):1241. doi: 10.3390/cells8101241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma X., Cheng F., Yuan K., Jiang K., Zhu T. Lipid storage droplet protein 5 reduces sodium palmitate-induced lipotoxicity in human normal liver cells by regulating lipid metabolism-related factors. Molecular Medicine Reports. 2019;20(2):879–886. doi: 10.3892/mmr.2019.10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng P., Xie Z., Yuan Y., Sui W., Wang C., Gao X. Plin5 alleviates myocardial ischaemia/reperfusion injury by reducing oxidative stress through inhibiting the lipolysis of lipid droplets. Scientific Reports. 2017;7:42574. doi: 10.1038/srep42574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang C., Zhao Y., Gao X., Li L., Yuan Y., Liu F. Perilipin 5 improves hepatic lipotoxicity by inhibiting lipolysis. Hepatology. 2015;61(3):870–882. doi: 10.1002/hep.27409. [DOI] [PubMed] [Google Scholar]
- 126.Wang H., Sreenivasan U., Hu H., Saladino A., Polster B.M., Lund L.M. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. Journal of Lipid Research. 2011;52(12):2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mohammadyani D., Tyurin V.A., O'Brien M., Sadovsky Y., Gabrilovich D.I., Klein-Seetharaman J. Molecular speciation and dynamics of oxidized triacylglycerols in lipid droplets: mass spectrometry and coarse-grained simulations. Free Radical Biology and Medicine. 2014;76:53–60. doi: 10.1016/j.freeradbiomed.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jarc E., Petan T. A twist of FATe: lipid droplets and inflammatory lipid mediators. Biochimie. 2019;169:69–87. doi: 10.1016/j.biochi.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 129.Maciejewska D., Drozd A., Skonieczna-Zydecka K., Skórka-Majewicz M., Dec K., Jakubczyk K. Eicosanoids in nonalcoholic fatty liver disease (NAFLD) progression. Do serum eicosanoids profile correspond with liver eicosanoids content during NAFLD development and progression? Molecules. 2020;25(9):2026. doi: 10.3390/molecules25092026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puri P., Baillie R.A., Wiest M.M., Mirshahi F., Choudhury J., Cheung O. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 131.Dichlberger A., Schlager S., Lappalainen J., Käkelä R., Hattula K., Butcher S.J. Lipid body formation during maturation of human mast cells. Journal of Lipid Research. 2011;52(12):2198–2208. doi: 10.1194/jlr.M019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W., Wei S., Li L., Su X., Du C., Li F. Proteomic analysis of murine testes lipid droplets. Scientific Reports. 2015;5(1):12070. doi: 10.1038/srep12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen X., Xu S., Wei S., Deng Y., Li Y., Yang F. Comparative proteomic study of fatty acid-treated myoblasts reveals role of cox-2 in palmitate-induced insulin resistance. Scientific Reports. 2016;6(1):21454. doi: 10.1038/srep21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bozza P.T., Yu W., Penrose J.F., Morgan E.S., Dvorak A.M., Weller P.F. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. Journal of Experimental Medicine. 1997;186(6):909–920. doi: 10.1084/jem.186.6.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dichlberger A., Schlager S., Maaninka K., Schneider W.J., Kovanen P.T. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. Journal of Lipid Research. 2014;55(12):2471–2478. doi: 10.1194/jlr.M048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Riederer M., Lechleitner M., Köfeler H., Frank S. Reduced expression of adipose triglyceride lipase decreases arachidonic acid release and prostacyclin secretion in human aortic endothelial cells. Archives of Physiology and Biochemistry. 2017;123(4):249–253. doi: 10.1080/13813455.2017.1309052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schlager S., Goeritzer M., Jandl K., Frei R., Vujic N., Kolb D. Adipose triglyceride lipase acts on neutrophil lipid droplets to regulate substrate availability for lipid mediator synthesis. Journal of Leukocyte Biology. 2015;98(5):837–850. doi: 10.1189/jlb.3A0515-206R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Najt C.P., Senthivinayagam S., Aljazi M.B., Fader K.A., Olenic S.D., Brock J.R. Liver-specific loss of perilipin 2 alleviates diet-induced hepatic steatosis, inflammation, and fibrosis. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2016;310(9):G726–G738. doi: 10.1152/ajpgi.00436.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Najt C.P., Lwande J.S., McIntosh A.L., Senthivinayagam S., Gupta S., Kuhn L.A. Structural and functional assessment of perilipin 2 lipid binding domain(s) Biochemistry. 2014;53(45):7051–7066. doi: 10.1021/bi500918m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grunfeld C., Soued M., Adi S., Moser A.H., Dinarello C.A., Feingold K.R. Evidence for two classes of cytokines that stimulate hepatic lipogenesis: relationships among tumor necrosis factor, interleukin-1 and interferon-α. Endocrinology. 1990;127(1):46–54. doi: 10.1210/endo-127-1-46. [DOI] [PubMed] [Google Scholar]
- 141.Feingold K.R., Soued M., Serio M.K., Moser A.H., Dinarello C.A., Grunfeld C. Multiple cytokines stimulate hepatic lipid synthesis in vivo. Endocrinology. 1989;125(1):267–274. doi: 10.1210/endo-125-1-267. [DOI] [PubMed] [Google Scholar]
- 142.Pinent M., Hackl H., Burkard T.R., Prokesch A., Papak C., Scheideler M. Differential transcriptional modulation of biological processes in adipocyte triglyceride lipase and hormone-sensitive lipase-deficient mice. Genomics. 2008;92(1):26–32. doi: 10.1016/j.ygeno.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 143.Ong K.T., Mashek M.T., Davidson N.O., Mashek D.G. Hepatic ATGL mediates PPAR-alpha signaling and fatty acid channeling through a L-FABP independent mechanism. Journal of Lipid Research. 2014;55(5):808–815. doi: 10.1194/jlr.M039867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de la Rosa Rodriguez M.A., Sugahara G., Hooiveld G.J.E.J., Ishida Y., Tateno C., Kersten S. The whole transcriptome effects of the PPARα agonist fenofibrate on livers of hepatocyte humanized mice. BMC Genomics. 2018;19(1):443. doi: 10.1186/s12864-018-4834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rakhshandehroo M., Knoch B., Müller M., Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Research. 2010:1–20. doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khan S.A., Sathyanarayan A., Mashek M.T., Ong K.T., Wollaston-Hayden E.E., Mashek D.G. ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1α/PPAR-α signaling. Diabetes. 2015;64(2):418–426. doi: 10.2337/db14-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Najt C.P., Khan S.A., Heden T.D., Witthuhn B.A., Perez M., Heier J.L. Lipid droplet-derived monounsaturated fatty acids traffic via PLIN5 to allosterically activate SIRT1. Molecular Cell. 2019;77(4):810–824. doi: 10.1016/j.molcel.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eichmann T.O., Kumari M., Haas J.T., Farese R.V., Zimmermann R., Lass A. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. Journal of Biological Chemistry. 2012;287(49):41446–41457. doi: 10.1074/jbc.M112.400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gallardo-Montejano V.I., Saxena G., Kusminski C.M., Yang C., McAfee J.L., Hahner L. Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function. Nature Communications. 2016;7:12723. doi: 10.1038/ncomms12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nassir F., Ibdah J.A. Sirtuins and nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2016;22(46):10084–10092. doi: 10.3748/wjg.v22.i46.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tardelli M. Monoacylglycerol lipase reprograms lipid precursors signaling in liver disease. World Journal of Gastroenterology. 2020;26(25):3577–3585. doi: 10.3748/wjg.v26.i25.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tardelli M., Bruschi F.V., Claudel T., Fuchs C.D., Auer N., Kunczer V. Lack of monoacylglycerol lipase prevents hepatic steatosis by favoring lipid storage in adipose tissue and intestinal malabsorption. Journal of Lipid Research. 2019;60(7):1284–1292. doi: 10.1194/jlr.M093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cao Z., Mulvihill M.M., Mukhopadhyay P., Xu H., Erdélyi K., Hao E. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 2013;144(4):808–817. doi: 10.1053/j.gastro.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Habib A., Chokr D., Wan J.H., Hegde P., Mabire M., Siebert M. Inhibition of monoacylglycerol lipase, an anti-inflammatory and antifibrogenic strategy in the liver. Gut. 2019;68(3):522–532. doi: 10.1136/gutjnl-2018-316137. [DOI] [PubMed] [Google Scholar]
- 155.Zhang J., Liu Z., Lian Z., Liao R., Chen Y., Qin Y. Monoacylglycerol lipase: a novel potential therapeutic target and prognostic indicator for hepatocellular carcinoma. Scientific Reports. 2016;6:35784. doi: 10.1038/srep35784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davis J.N., Lê K.-A., Walker R.W., Vikman S., Spruijt-Metz D., Weigensberg M.J. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. American Journal of Clinical Nutrition. 2010;92(6):1522–1527. doi: 10.3945/ajcn.2010.30185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smagris E., BasuRay S., Li J., Huang Y., Lai K.V., Gromada J. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology. 2015;61(1):108–118. doi: 10.1002/hep.27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Basantani M.K., Sitnick M.T., Cai L., Brenner D.S., Gardner N.P., Li J.Z. Pnpla3/Adiponutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. Journal of Lipid Research. 2011;52(2):318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y., Kory N., BasuRay S., Cohen J.C., Hobbs H.H. PNPLA3, CGI-58, and inhibition of hepatic triglyceride hydrolysis in mice. Hepatology. 2019;69(6):2427–2441. doi: 10.1002/hep.30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.BasuRay S., Wang Y., Smagris E., Cohen J.C., Hobbs H.H. Accumulation of PNPLA3 on lipid droplets is the basis of associated hepatic steatosis. Proceedings of the National Academy of Sciences. 2019;116(19):9521–9526. doi: 10.1073/pnas.1901974116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.BasuRay S., Smagris E., Cohen J.C., Hobbs H.H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology. 2017;66(4):1111–1124. doi: 10.1002/hep.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lindén D., Ahnmark A., Pingitore P., Ciociola E., Ahlstedt I., Andréasson A.-C. Pnpla3 silencing with antisense oligonucleotides ameliorates nonalcoholic steatohepatitis and fibrosis in Pnpla3 I148M knock-in mice. Molecular Metabolism. 2019;22:49–61. doi: 10.1016/j.molmet.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Negoita F., Blomdahl J., Wasserstrom S., Winberg M.E., Osmark P., Larsson S. PNPLA3 variant M148 causes resistance to starvation-mediated lipid droplet autophagy in human hepatocytes. Journal of Cellular Biochemistry. 2018;120(1):343–356. doi: 10.1002/jcb.27378. [DOI] [PubMed] [Google Scholar]
- 165.Käräjämäki A.J., Bloigu R., Kauma H., Kesäniemi Y.A., Koivurova O.-P., Perkiömäki J. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metabolism - Clinical and Experimental. 2016;66:55–63. doi: 10.1016/j.metabol.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 166.Luukkonen P.K., Nick A., Hölttä-Vuori M., Thiele C., Isokuortti E., Lallukka-Brück S. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4(16) doi: 10.1172/jci.insight.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou Y., Orešič M., Leivonen M., Gopalacharyulu P., Hyysalo J., Arola J. Noninvasive detection of nonalcoholic steatohepatitis using clinical markers and circulating levels of lipids and metabolites. Clinical Gastroenterology and Hepatology. 2016;14(10):1463–1472. doi: 10.1016/j.cgh.2016.05.046. e6. [DOI] [PubMed] [Google Scholar]
- 168.Luukkonen P.K., Zhou Y., Sädevirta S., Leivonen M., Arola J., Orešič M. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2016;64(5):1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 169.Roumans K.H.M., Lindeboom L., Veeraiah P., Remie C.M.E., Phielix E., Havekes B. Hepatic saturated fatty acid fraction is associated with de novo lipogenesis and hepatic insulin resistance. Nature Communications. 2020;11(1):1891. doi: 10.1038/s41467-020-15684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bruschi F.V., Claudel T., Tardelli M., Caligiuri A., Stulnig T.M., Marra F. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology. 2017;65(6):1875–1890. doi: 10.1002/hep.29041. [DOI] [PubMed] [Google Scholar]
- 171.Blaner W.S., Li Y., Brun P.-J., Yuen J.J., Lee S.-A., Clugston R.D. Vitamin A absorption, storage and mobilization. Sub-Cellular Biochemistry. 2016;81:95–125. doi: 10.1007/978-94-024-0945-1_4. [DOI] [PubMed] [Google Scholar]
- 172.Pirazzi C., Valenti L., Motta B.M., Pingitore P., Hedfalk K., Mancina R.M. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Human Molecular Genetics. 2014;23(15):4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Su W., Wang Y., Jia X., Wu W., Li L., Tian X. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences. 2014;111(31):11437–11442. doi: 10.1073/pnas.1410741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P. A protein-truncating HSD17B13 variant and protection from chronic liver disease. New England Journal of Medicine. 2018;378(12):1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ma Y., Belyaeva O.V., Brown P.M., Fujita K., Valles K., Karki S. 17-Beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 2019;69(4):1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pirola C.J., Garaycoechea M., Flichman D., Arrese M., Martino J.S., Gazzi C. Splice variant rs72613567 prevents worst histologic outcomes in patients with nonalcoholic fatty liver disease. Journal of Lipid Research. 2019;60(1):176–185. doi: 10.1194/jlr.P089953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luukkonen P.K., Tukiainen T., Juuti A., Sammalkorpi H., Nidhina Haridas P.A., Niemelä O. Hydroxysteroid 17-β dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI Insight. 2020;5(5) doi: 10.1172/jci.insight.132158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Seko Y., Yamaguchi K., Tochiki N., Yano K., Takahashi A., Okishio S. Attenuated effect of PNPLA3 on hepatic fibrosis by HSD17B13 in Japanese patients with non-alcoholic fatty liver disease. Liver International. 2020;40(7):1686–1692. doi: 10.1111/liv.14495. [DOI] [PubMed] [Google Scholar]
- 179.Faulkner C.S., White C.M., Shah V.H., Jophlin L.L. A single nucleotide polymorphism of PLIN2 is associated with nonalcoholic steatohepatitis and causes phenotypic changes in hepatocyte lipid droplets: a pilot study. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2020;1865(5):158637. doi: 10.1016/j.bbalip.2020.158637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Magné J., Aminoff A., Sundelin J.P., Mannila M.N., Gustafsson P., Hultenby K. The minor allele of the missense polymorphism Ser251Pro in perilipin 2 (PLIN2) disrupts an α-helix, affects lipolysis, and is associated with reduced plasma triglyceride concentration in humans. Federation of American Societies for Experimental Biology Journal. 2013;27(8):3090–3099. doi: 10.1096/fj.13-228759. [DOI] [PubMed] [Google Scholar]
- 181.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230. doi: 10.1053/j.gastro.2016.01.032. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Helsley R.N., Varadharajan V., Brown A.L., Gromovsky A.D., Schugar R.C., Ramachandiran I. Obesity-linked suppression of membrane-bound o-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. ELife. 2019;8:1–69. doi: 10.7554/eLife.49882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee H.C., Inoue T., Sasaki J., Kubo T., Matsuda S., Nakasaki Y. LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Molecular Biology of the Cell. 2012;23(24):4689–4700. doi: 10.1091/mbc.E12-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Luukkonen P.K., Zhou Y., Hyötyläinen T., Leivonen M., Arola J., Orho-Melander M. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. Journal of Hepatology. 2016;65(6):1263–1265. doi: 10.1016/j.jhep.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 185.Meroni M., Longo M., Fracanzani A.L., Dongiovanni P. EBioMedicine; 2020. MBOAT7 down-regulation by genetic and environmental factors predisposes to MAFLD; p. 102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tanaka Y., Shimanaka Y., Caddeo A., Kubo T., Mao Y., Kubota T. LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. Gut. 2020 doi: 10.1136/gutjnl-2020-320646. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xia M., Chandrasekaran P., Rong S., Fu X., Mitsche M.A. Hepatic deletion of Mboat7 (Lpiat1) causes activation of SREBP-1c and fatty liver. Journal of Lipid Research. 2020 doi: 10.1194/jlr.RA120000856. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mahdessian H., Taxiarchis A., Popov S., Silveira A., Franco-Cereceda A., Hamsten A. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(24):8913–8918. doi: 10.1073/pnas.1323785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjærg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Luukkonen P.K., Zhou Y., Nidhina Haridas P.A., Dwivedi O.P., Hyötyläinen T., Ali A. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. Journal of Hepatology. 2017;67(1):128–136. doi: 10.1016/j.jhep.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 191.Dongiovanni P., Petta S., Maglio C., Fracanzani A.L., Pipitone R., Mozzi E. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 192.Zhou Y., Llauradó G., Orešič M., Hyötyläinen T., Orho-Melander M., Yki-Järvinen H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. Journal of Hepatology. 2015;62(3):657–663. doi: 10.1016/j.jhep.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 193.Musso G., Cipolla U., Cassader M., Pinach S., Saba F., De Michieli F. TM6SF2 rs58542926 variant affects postprandial lipoprotein metabolism and glucose homeostasis in NAFLD. Journal of Lipid Research. 2017;58(6):1221–1229. doi: 10.1194/jlr.M075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sookoian S., Castaño G.O., Scian R., Mallardi P., Fernández Gianotti T., Burgueño A.L. Genetic variation in transmembrane 6 superfamily member 2 and the risk of nonalcoholic fatty liver disease and histological disease severity. Hepatology. 2015;61(2):515–525. doi: 10.1002/hep.27556. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y.L., Reeves H.L., Burt A.D., Tiniakos D., McPherson S., Leathart J.B.S. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nature Communications. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ioannou G.N., Haigh W.G., Thorning D., Savard C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. Journal of Lipid Research. 2013;54(5):1326–1334. doi: 10.1194/jlr.M034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Min H.K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metabolism. 2012;15(5):665–674. doi: 10.1016/j.cmet.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Van Rooyen D.M., Larter C.Z., Haigh W.G., Yeh M.M., Ioannou G., Kuver R. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 2011;141(4):1393–1403. doi: 10.1053/j.gastro.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ioannou G.N., Landis C.S., Jin G., Haigh W.G., Farrell G.C., Kuver R. Cholesterol crystals in hepatocyte lipid droplets are strongly associated with human nonalcoholic steatohepatitis. Hepatology Communications. 2019;3(6):776–791. doi: 10.1002/hep4.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang L., Xu M., Jones O.D., Li Z., Liang Y., Yu Q. Nonalcoholic fatty liver disease experiences accumulation of hepatic liquid crystal associated with increasing lipophagy. Cell & Bioscience. 2020;10(1):55. doi: 10.1186/s13578-020-00414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ioannou G.N., Subramanian S., Chait A., Haigh W.G., Yeh M.M., Farrell G.C. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. Journal of Lipid Research. 2017;58(6):1067–1079. doi: 10.1194/jlr.M072454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Savard C., Tartaglione E.V., Kuver R., Haigh W.G., Farrell G.C., Subramanian S. Synergistic interaction of dietary cholesterol and dietary fat in inducing experimental steatohepatitis. Hepatology. 2013;57(1):81–92. doi: 10.1002/hep.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hsieh K., Lee Y.K., Londos C., Raaka B.M., Dalen K.T., Kimmel A.R. Perilipin family members preferentially sequester to either triacylglycerol-specific or cholesteryl-esterspecific intracellular lipid storage droplets. Journal of Cell Science. 2012;125(17):4067–4076. doi: 10.1242/jcs.104943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Spooner M.H., Jump D.B. Omega-3 fatty acids and nonalcoholic fatty liver disease in adults and children: where do we stand? Current Opinion in Clinical Nutrition and Metabolic Care. 2019;22(2):103–110. doi: 10.1097/MCO.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hernández E.A., Kahl S., Seelig A., Begovatz P., Irmler M., Kupriyanova Y. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. Journal of Clinical Investigation. 2017;127(2):695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luukkonen P.K., Sädevirta S., Zhou Y., Kayser B., Ali A., Ahonen L. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41(8):1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2017;1859(9):1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 208.Krahmer N., Guo Y., Wilfling F., Hilger M., Lingrell S., Heger K. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metabolism. 2011;14(4):504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Listenberger L., Townsend E., Rickertsen C., Hains A., Brown E., Inwards E. Decreasing phosphatidylcholine on the surface of the lipid droplet correlates with altered protein binding and steatosis. Cells. 2018;7(12):230. doi: 10.3390/cells7120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Alamri H., Patterson N.H., Yang E., Zoroquiain P., Lazaris A., Chaurand P. Mapping the triglyceride distribution in NAFLD human liver by MALDI imaging mass spectrometry reveals molecular differences in micro and macro steatosis. Analytical and Bioanalytical Chemistry. 2018;411(4):885–894. doi: 10.1007/s00216-018-1506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Khan S.A., Wollaston-Hayden E.E.E., Markowski T.W.T.W., Higgins L., Mashek D.G. Quantitative analysis of the murine lipid droplet-associated proteome during diet-induced hepatic steatosis. Journal of Lipid Research. 2015;56(12):2260–2272. doi: 10.1194/jlr.M056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu M., Ge R., Liu W., Liu Q., Xia X., Lai M. Differential proteomics profiling identifies LDPs and biological functions in high-fat diet-induced fatty livers. Journal of Lipid Research. 2017;58(4):681–694. doi: 10.1194/jlr.M071407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Krahmer N., Najafi B., Schueder F., Quagliarini F., Steger M., Seitz S. Organellar proteomics and phospho-proteomics reveal subcellular reorganization in diet-induced hepatic steatosis. Developmental Cell. 2018;47(2):205–221. doi: 10.1016/j.devcel.2018.09.017. e7. [DOI] [PubMed] [Google Scholar]
- 214.Straub B.K., Stoeffel P., Heid H., Zimbelmann R., Schirmacher P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology. 2008;47(6):1936–1946. doi: 10.1002/hep.22268. [DOI] [PubMed] [Google Scholar]
- 215.Carr R.M., Dhir R., Mahadev K., Comerford M., Chalasani N.P., Ahima R.S. Perilipin staining distinguishes between steatosis and non-alcoholic steatohepatitis in adults and children. Clinical Gastroenterology and Hepatology. 2017;15(1):145–147. doi: 10.1016/j.cgh.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Straub B.K., Herpel E., Singer S., Zimbelmann R., Breuhahn K., Macher-Goeppinger S. Lipid droplet-associated PAT-proteins show frequent and differential expression in neoplastic steatogenesis. Modern Pathology. 2010;23(3):480–492. doi: 10.1038/modpathol.2009.191. [DOI] [PubMed] [Google Scholar]
- 217.Imai Y., Boyle S., Varela G.M., Caron E., Yin X., Dhir R. Effects of perilipin 2 antisense oligonucleotide treatment on hepatic lipid metabolism and gene expression. Physiological Genomics. 2012;44(22):1125–1131. doi: 10.1152/physiolgenomics.00045.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Carr R.M., Peralta G., Yin X., Ahima R.S. Absence of perilipin 2 prevents hepatic steatosis, glucose intolerance and ceramide accumulation in alcohol-fed mice. PloS One. 2014;9(5) doi: 10.1371/journal.pone.0097118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pawella L.M., Hashani M., Eiteneuer E., Renner M., Bartenschlager R., Schirmacher P. Perilipin discerns chronic from acute hepatocellular steatosis. Journal of Hepatology. 2014;60(3):633–642. doi: 10.1016/j.jhep.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 220.Amrutkar M., Cansby E., Nuñez-Durán E., Pirazzi C., Ståhlman M., Stenfeldt E. Protein kinase STK25 regulates hepatic lipid partitioning and progression of liver steatosis and NASH. Federation of American Societies for Experimental Biology Journal. 2015;29(4):1564–1576. doi: 10.1096/fj.14-264937. [DOI] [PubMed] [Google Scholar]
- 221.Nuñez-Durán E., Aghajan M., Amrutkar M., Sütt S., Cansby E., Booten S.L. Serine/threonine protein kinase 25 antisense oligonucleotide treatment reverses glucose intolerance, insulin resistance, and nonalcoholic fatty liver disease in mice. Hepatology Communications. 2018;2(1):69–83. doi: 10.1002/hep4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Amrutkar M., Kern M., Nuñez-Durán E., Ståhlman M., Cansby E., Chursa U. Protein kinase STK25 controls lipid partitioning in hepatocytes and correlates with liver fat content in humans. Diabetologia. 2016;59(2):341–353. doi: 10.1007/s00125-015-3801-7. [DOI] [PubMed] [Google Scholar]
- 223.Bersuker K., Peterson C.W.H., To M., Sahl S.J., Savikhin V., Grossman E.A. A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Developmental Cell. 2018;44(1):97–112. doi: 10.1016/j.devcel.2017.11.020. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Valm A.M., Cohen S., Legant W.R., Melunis J., Hershberg U., Wait E. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature. 2017;546(7656):162–167. doi: 10.1038/nature22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Schuldiner M., Bohnert M. A different kind of love – lipid droplet contact sites. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862(10):1188–1196. doi: 10.1016/j.bbalip.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 226.Montgomery M.K., Watt M.J., Keenan S.N., Meex R.C., Lo J.C.Y., Ryan A. Perilipin 5 deletion in hepatocytes remodels lipid metabolism and causes hepatic insulin resistance in mice. Diabetes. 2019;68(3):543–555. doi: 10.2337/db18-0670. [DOI] [PubMed] [Google Scholar]
- 227.Ma S., Sun K., Zhang M., Zhou X., Zheng X., Tian S. Disruption of Plin5 degradation by CMA causes lipid homeostasis imbalance in NAFLD. Liver International. 2020 doi: 10.1111/liv.14492. in press. [DOI] [PubMed] [Google Scholar]
- 228.Zhang E., Cui W., Lopresti M., Mashek M.T., Najt C.P., Hu H. Hepatic PLIN5 signals via SIRT1 to promote autophagy and prevent inflammation during fasting. Journal of Lipid Research. 2020;61(3):338–350. doi: 10.1194/jlr.RA119000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Layerenza J.P., González P., García de Bravo M.M., Polo M., Sisti M.S., Ves-Losada A. Nuclear lipid droplets: a novel nuclear domain. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2012;1831(2):327–340. doi: 10.1016/j.bbalip.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 230.Uzbekov R., Roingeard P. Nuclear lipid droplets identified by electron microscopy of serial sections. BMC Research Notes. 2013;6(1):386. doi: 10.1186/1756-0500-6-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Romanauska A., Köhler A. The inner nuclear membrane is a metabolically active territory that generates nuclear lipid droplets. Cell. 2018;174(3):700–715. doi: 10.1016/j.cell.2018.05.047. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shin J.Y., Hernandez-Ono A., Fedotova T., Östlund C., Lee M.J., Gibeley S.B. Nuclear envelope–localized torsinA-LAP1 complex regulates hepatic VLDL secretion and steatosis. Journal of Clinical Investigation. 2019;129(11):4885–4900. doi: 10.1172/JCI129769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sołtysik K., Ohsaki Y., Tatematsu T., Cheng J., Fujimoto T. Nuclear lipid droplets derive from a lipoprotein precursor and regulate phosphatidylcholine synthesis. Nature Communications. 2019;10(1):473. doi: 10.1038/s41467-019-08411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yeh M.M., Brunt E.M. Pathological features of fatty liver disease. Gastroenterology. 2014:754–764. doi: 10.1053/j.gastro.2014.07.056. [DOI] [PubMed] [Google Scholar]
- 235.Wu W., Tsuchida H., Kato T., Niwa H., Horikawa Y., Takeda J. Fat and carbohydrate in western diet contribute differently to hepatic lipid accumulation. Biochemical and Biophysical Research Communications. 2015;461(4):681–686. doi: 10.1016/j.bbrc.2015.04.092. [DOI] [PubMed] [Google Scholar]
- 236.Shmarakov I.O., Jiang H., Liu J., Fernandez E.J., Blaner W.S. Hepatic stellate cell activation: a source for bioactive lipids. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2019;1864(5):629–642. doi: 10.1016/j.bbalip.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Leroux A., Ferrere G., Godie V., Cailleux F., Renoud M.L., Gaudin F. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. Journal of Hepatology. 2012;57(1):141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 238.Szafraniec E., Kus E., Wislocka A., Kukla B., Sierka E., Untereiner V. Raman spectroscopy–based insight into lipid droplets presence and contents in liver sinusoidal endothelial cells and hepatocytes. Journal of Biophotonics. 2019;12(4) doi: 10.1002/jbio.201800290. [DOI] [PubMed] [Google Scholar]
- 239.Xiong X., Kuang H., Ansari S., Liu T., Gong J., Wang S. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Molecular Cell. 2019;75(3):644–660. doi: 10.1016/j.molcel.2019.07.028. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.den Brok M.H., Raaijmakers T.K., Collado-Camps E., Adema G.J. Lipid droplets as immune modulators in myeloid cells. Trends in Immunology. 2018;39(5):380–392. doi: 10.1016/j.it.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 241.Adamovich Y., Rousso-Noori L., Zwighaft Z., Neufeld-Cohen A., Golik M., Kraut-Cohen J. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metabolism. 2014;19(2):319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Reinke H., Asher G. Circadian clock control of liver metabolic functions. Gastroenterology. 2016;150(3):574–580. doi: 10.1053/j.gastro.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 243.Kramer D.A., Quiroga A.D., Lian J., Fahlman R.P., Lehner R. Fasting and refeeding induces changes in the mouse hepatic lipid droplet proteome. Journal of Proteomics. 2018;181:213–224. doi: 10.1016/j.jprot.2018.04.024. [DOI] [PubMed] [Google Scholar]