Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) comprises hepatic alterations with increased lipid accumulation (steatosis) without or with inflammation (nonalcoholic steatohepatitis, NASH) and/or fibrosis in the absence of other causes of liver disease. NAFLD is developing as a burgeoning health challenge, mainly due to the worldwide obesity and diabetes epidemics.
Scope of review
This review summarizes the knowledge on the pathogenesis underlying NAFLD by focusing on studies in humans and on hypercaloric nutrition, including effects of saturated fat and fructose, as well as adipose tissue dysfunction, leading to hepatic lipotoxicity, abnormal mitochondrial function, and oxidative stress, and highlights intestinal dysbiosis. These mechanisms are discussed in the context of current treatments targeting metabolic pathways and the results of related clinical trials.
Major conclusions
Recent studies have provided evidence that certain conditions, for example, the severe insulin-resistant diabetes (SIRD) subgroup (cluster) and the presence of an increasing number of gene variants, seem to predispose for excessive risk of NAFLD and its accelerated progression. Recent clinical trials have been frequently unsuccessful in halting or preventing NAFLD progression, perhaps partly due to including unselected cohorts in later stages of NAFLD. On the basis of this literature review, this study proposed screening in individuals with the highest genetic or acquired risk of disease progression, for example, the SIRD subgroup, and developing treatment concepts targeting the earliest pathophysiolgical alterations, namely, adipocyte dysfunction and insulin resistance.
Keywords: Fatty liver, Lipotoxicity, Inflammation, Fibrosis, Insulin resistance, Clinical trials
Abbreviations
- ACC
acetyl coenzyme A carboxylase
- apoB100
apolipoprotein B100
- ATP
adenosine triphosphate
- BMI
body mass index
- JNK
c-Jun N-terminal kinase
- ChREBP
carbohydrate regulatory element-binding protein
- CCR
chemokine receptors
- CD36
cluster of differentiation 36
- DNL
de novo lipogenesis
- DAG
diacylglycerol
- DPP-4i
dipeptidyl peptidase-4 (DPP-4) inhibitors
- ER
endoplasmic reticulum
- ECM
extracellular matrix
- FXR
farnesoid X receptor
- FA
fatty acids
- FATP
fatty acids transponders
- FGF
fibroblast growth factor
- FNDC5
fibronectin type III domain-containing protein 5
- F
fibrosis stage
- GLP-1 RA
glucagon-like peptide 1 (GLP-1) receptor agonists
- GCKR
glucokinase regulatory protein
- HSC
hepatic stellate cells
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- LSECs
liver sinusoidal endothelial cells
- LYPLAL1
lysophospholipase-like 1
- MRI-PDFF
magnetic resonance imaging–estimated proton density fat fraction
- MRS
magnetic resonance spectroscopy
- MTTP
microsomal triglyceride transfer protein
- NAFLD
nonalcoholic fatty liver (NAFL) disease
- NASH
nonalcoholic steatohepatitis
- PNPLA3
patatin-like phospholipase domain-containing protein 3
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acids
- PKCε
protein kinase Cε
- RCT
randomized controlled trial
- ROS
reactive oxygen species
- RNA
ribonucleic acid
- SIRD
severe insulin-resistant diabetes
- SGLT2i
sodium glucose cotransporter (SGLT)-2 inhibitors
- SREBP1c
sterol regulatory element-binding protein 1c
- SCD1
stearoyl-CoA desaturase 1
- THR
thyroid hormone receptor
- TNF-α
tumor necrosis factor-α
- TLR4
toll-like receptor-4
- TGF-β
transforming growth factor-β
- TM6SF2
transmembrane 6 superfamily member 2
- TAG
triacylglycerol
- T2DM
type 2 diabetes mellitus
- UPR
unfolded protein response
- VLDL
very low density lipoprotein
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide, with a prevalence approaching 25% in the general population [1] and mainly affecting individuals with obesity and type 2 diabetes mellitus (T2DM) [2]. The spectrum of NAFLD encompasses different abnormalities, ranging from a simple increase in intrahepatic lipid content (steatosis, nonalcoholic fatty liver, NAFL) to nonalcoholic steatohepatitis (NASH) with various degrees of necrotic inflammation, fibrosis, and ultimately, cirrhosis [3]. NAFLD is not only associated with an increased risk of hepatocellular carcinoma but also cardiovascular diseases and complications related to T2DM, such as nephropathy and neuropathy [[4], [5], [6]]. Thus, delineating the mechanisms underlying the pathogenesis of NAFLD is crucial to managing NAFLD and its comorbidities.
Concepts in the literature have suggested that NAFLD results from a double-hit lesion [7]. The first hit was considered the intrahepatic accumulation of fatty acids (FA), which increased the vulnerability of the hepatic cells to a variety of secondary insults, leading to inflammation and ultimately fibrosis [8]. Several observations have challenged this view, for example, by showing that inflammation may precede hepatic triacylglycerol (TAG) accumulation and that steatosis may protect from liver damage, suggesting multiple hits acting simultaneously rather than sequentially to drive NAFLD progression [9]. This review addresses mechanisms underlying the development and progression of NAFLD from studies in humans. Furthermore, no pharmacological treatment has been approved for NAFLD [10], but recent evidence from well-designed randomized controlled trials (RCTs) points to the efficacy of certain pharmacological agents already in use to treat T2DM [[11], [12], [13]]. Thus, this review also summarizes current concepts to treat NAFLD in humans.
2. Populations at risk
Despite the general association among obesity, dysglycemia, and NAFLD, the presence of metabolic comorbidities coexisting with NAFLD varies substantially across populations. In a population-based cohort study of more than 4 million individuals, with a median follow-up period of 4.7 years, overweight and obese individuals without further metabolic abnormalities including diabetes, hypertension, and dyslipidemia, presented with a 3.3- and an almost 7-fold higher risk of incident NAFLD, respectively, than their normal-weight counterparts [14]. These findings underline the key role of body weight, before the development of further metabolic abnormalities. By contrast, obesity cannot be regarded as the sole criterion for the screening of NAFLD, because the prevalence of nonobese NAFLD varies in the general population from 25% to 50% [15] and the presence of ≥1 metabolic abnormalities even in the absence of obesity doubles the risk for NAFLD [14]. However, even among individuals with known diabetes, the prevalence of NAFLD may differ markedly.
A recent analysis of the German Diabetes Study (GDS) confirmed the presence of 5 diabetes subgroups (clusters) [16] by comprehensive phenotyping of 1105 humans with newly diagnosed diabetes. These subgroups comprised in addition to severe autoimmune diabetes, age-related diabetes, mild obesity-related diabetes, severe insulin-deficient diabetes, and severe insulin-resistant diabetes (SIRD), classically termed T2DM [4]. Members of the SIRD are not only characterized by high body mass index (BMI) and the highest degree of whole-body and adipose tissue insulin resistance but also the highest hepatocellular TAG content (assessed using magnetic resonance spectroscopy, MRS) at times of diagnosis. Notably, the SIRD group also shows evidence of mild fibrosis at the 5-year follow-up [4]. This subgroup further exhibits a more frequent prevalence of the rs738409(G) polymorphism in the patatin-like phospholipase domain-containing protein 3 (PNPAL3) gene, which was associated with augmented adipose tissue lipolysis and insulin resistance [17]. This points to a complex interaction between genetic and lifestyle factors in the development of NAFLD.
The importance of discriminating between populations at risk is further underlined by differences in the risk of long-term complications, specifically when comparing NAFLD cohorts presenting with or without obesity and metabolic syndrome. For instance, humans with NAFLD and metabolic syndrome have a greater risk for incident T2DM and cardiovascular events as well as a higher left ventricular mass index during a follow-up of 16 years, than humans with hepatic steatosis only do [18]. In line with these findings, a recent meta-analysis reported significantly lower rates of hypertension, lower uric acid, and fasting plasma glucose and a higher level of high-density lipoprotein among humans who are lean/nonobese with NAFLD than for those who are obese with NAFLD [19]. When body composition is categorized according to BMI and waist circumference, a higher risk for cardiovascular mortality is observed in humans with NAFLD who have a normal BMI but are characterized as obese by waist circumference than in those who are overweight (by BMI) with NAFLD with normal waist circumference [20]. Nevertheless, a high risk of new-onset cardiovascular disease of 18.7 per 1000 persons-years was reported in individuals with lean or nonobese NAFLD, underlining the severity of the condition even in the absence of obesity [15].
3. Gene variants
Human genetic analyses have provided evidence for some degree of heritability of NAFLD, which could at least partly explain the broad variability in phenotypes and risk of disease progression [21]. The most commonly described genetic variants associated with NAFLD have been identified in the PNPLA3, transmembrane 6 superfamily member 2 (TM6SF2), and glucokinase regulatory protein (GCKR) genes (Table 1) [[22], [23], [24], [25], [26]]. Different PNPLA3 gene alleles have been shown to either confer susceptibility (rs738409[G], encoding I148M, Hispanics), or protection from NAFLD (rs6006460[T], encoding S453I, African-American populations) [27]. Importantly, the presence of the mutant I148M seems to increase NAFLD risk, specifically in the context of body weight gain [28]. Many candidate gene studies of ethnic groups have reported on the association with histological severity, including hepatic fibrosis [23,[29], [30], [31], [32], [33], [34], [35]]. A cross-sectional study of 42 pairs of monozygotic twins and 18 pairs of dizygotic twins was conducted on the heritability of hepatic steatosis and hepatic fibrosis; the assessment used imaging techniques in humans with and without the PNPLA3 risk genotype and revealed no association, which might be because of low statistical power [21]. In another study, humans who were homozygotes for the 148M allele had higher steatosis scores (33.3% ± 4.0%) than those who were heterozygotes for the 148IM allele (26.3% ± 3.5%) and 148I allele (14.9% ± 3.9%) [36]. Human studies have suggested that the PNPLA3-148M allele remodels liver TAG by transferring polyunsaturated FA (PUFA) from diacylglycerol (DAG) species to feed phosphatidylcholine synthesis [37] (Figure 1). By contrast, the I148 wild type protein hydrolyses triglycerides from lipid droplets and therefore reduces their size [26], whereas the E434K variant was reported to downregulate PNPLA3 expression, reducing both the effect of I148M and the protective I148 variant on lipid droplets [26]. Recent studies have suggested an even more complex relationship between the PNPLA3 in individuals with diabetes and NAFLD. In GDS, the G allele associated positively with hepatic triglycerides, independent of age, sex, and BMI, as aforementioned [17]. Although the PNPLA3 polymorphism did not directly associate with insulin sensitivity, the G allele carriers also featured greater adipose tissue insulin resistance, which could contribute to NAFLD via excessive lipolysis and subsequently augmented FA flux to the liver.
Table 1.
Studies targeting specific genetic polymorphisms and large epidemiological genome-wide association studies, which evaluated the association of gene variants with histological severity of human NAFLD.
| Gene | Function | Polymorphism | MAF | Protein variant | Hepatic effect of variant | Steatosis | Inflammation | Fibrosis | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| PNPLA3 | Hepatic TAG composition | rs738409 | 0.230 | 148M | ↑ PUFA in TG | ↑ | ↑ | ↑ | [24,27,[30], [31], [32], [33], [34], [35],37] |
| rs2294918 G > A | 0.370 | E434K | PNPLA3 downregulation | ↑ (when combined with 148M) | ↑ (when combined with 148M) | NR | [26] | ||
| TM6SF2 | Extrahepatic TG transport | rs58542926 C > T | 0.067 | E167K | ↑ TG | ↑ | ↑ | ↑ | [25,35,38] |
| GCKR | Hepatocyte specific glucokinase (GK) | rs1260326 C > T | 0.293 | P446L | ↑ GK activity ↑ DNL |
↔/↑ | ↔/↑ | ↑ | [44] |
| IRS1 | Insulin signaling | rs1801278 | 0.053 | Gly972Arg | ↓ Akt Thr308 (27%)/Ser473Px (21%) | NR | ↔ | ↑ | [49] |
| ENPP1 | Insulin receptor activity | rs1044498 | 0.342 | Lys121Gln | ↓ Akt Thr308 (31%)/Ser473Px (46%) | NR | ↔ | ↑ | [49] |
| MBOAT7 | PIPn acyl-chain remodeling | rs641738 C > T | 0.40 | ↑ PIPn acyl-chain remodeling ↑ AA |
↑ | ↑ | ↑ | [47,48,55] | |
| HSD17B13 | Enzymatic activity against bioactive lipid species | rs72613567:TA | 0.180 | Hepatic HSD17B13 | ↓ HSD17B13 enzymatic activity ↓ PNPLA3 mRNA expression |
↑ | ↓ | ↓ | [56,57] |
| rs6834314 G/G rs72613567-A |
0.194 | ↓HSD17B13 enzymatic activity | ↑ | ↓ | ↔ | [58] | |||
| SOD2 | Protection from oxidative stress | rs4880 C > T | 0.411 | C47T | ↓ MnSOD activity ↑ oxidative stress |
↑ | ↔ | ↑ | [50] |
| UCP2 | Regulation of mitochondrial lipid fluxes and ROS production | rs695366 G > A | 0 264 | ↑ UCP2 | ↓ (but not DM/pre-DM) | ↓ | ↔ | [54] | |
| MERTK | HSC activation | rs4374383 G > A | 0.41 | ↓ HSC migration and procollagen expression ↑ HSC apoptosis |
↓ | ↓ | ↓ | [51,53] | |
| LYPLAL1 | TG catabolism | rs12137855 C > T | 0.164 | ? | ↑ | NR | NR | [59] | |
| FNDC5 | HSC activation | rs3480 A > G rs3480 G |
0.41 | ↓ irisin expression in HSC ↓ FNDC5 mRNA stability |
↔ ↑ |
↔ ↔ |
↓ ↔ |
[60] [61] |
Studies that provided no evidence of association (either positive or negative) between genetic determinants and liver disease (either steatosis, inflammation or fibrosis); studies addressing fibrogenesis, interferon and tumor necrosis factor production, nuclear receptors and hepatic iron deposition; and studies examining humans with NAFLD-related hepatocellular carcinoma and genetic determinants with a minor allele frequency <0.0001 were excluded from this analysis.
AA: arachidonic acid, APOC3: apolipoprotein C3, DM: diabetes mellitus, DNL: de novo lipogenesis, dys.:dysfunctional, ENPP1: ectoenzyme nucleotide pyrophosphate phosphodiesterase, FNDC5: fibronectin type III domain-containing protein 5, GCKR: glucokinase regulatory protein, DNL: de novo lipogenesis, HSC: hepatic stellate cells, HSD17B13: hydroxysteroid 17-β dehydrogenase 13, Ins-S: insulin sensitivity,intracell: intracellular, IRS1: insulin receptor substrate 1, LDs: lipid droplets, LYPLAL1: lysophospholipase-like 1, MBOAT7: membrane-bound O-acyltransferase domain-containing 7 gene, MERTK: myeloid-epithelial-reproductive tyrosine kinase, metab: metabolism, MnSOD: manganese-dependent superoxide dismutase, mRNA: messenger ribonucleic acid, NR: not reported, PIPn: phosphatidylinositol, PNPLA3: patatin-like phospholipase domain-containing protein 3, Px: phosphorylation, Ref.: references, Ser: serine, SOD2: superoxide dismutase 2, TAG: triacylglycerol, Thr: threonine, TM6SF2: transmembrane 6 superfamily member 2, TG, triglycerides, UCP:, uncoupling protein 2.
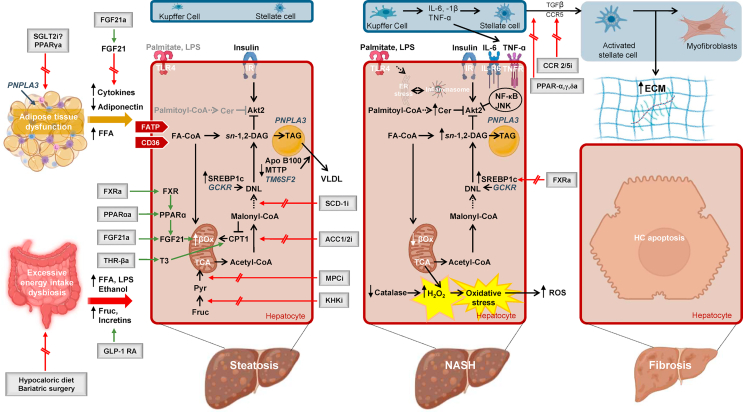
Figure 1.
Pathogenetic mechanisms driving the progression of human NAFLD and key pathways of therapeutic interventions. High caloric intake induces changes in the gut microbiota and enlargement of the adipose tissue. Diet and bariatric surgery are the main treatment strategies in this context. Gut dysbiosis is associated with disruption of intercellular tight junctions [87], which permit the translocation of bacterial lipopolysaccharide (LPS) into the systemic circulation and increase alcohol-producing bacteria [85]. The patatin-like phospholipase domain-containing protein 3 (PNPLA3) genetic variant increases adipose tissue lipolysis [17], increasing the flux of substrates and signaling molecules to the liver [92]. Adipose tissue–liver cross talk is further mediated through the secretion of exosomes [97] and adipokines [94]. Steatotic hepatocytes are characterized by a potentially upregulated uptake of FFA. Additionally, enhanced DNL converts acetyl-CoA to new fatty acids [109,112]. The genetic variant of the gene glucokinase regulatory protein (GCKR) contributes to elevated DNL, through increase in substrates availability [43]. In this context, mitochondrial fatty acid oxidation is upregulated [121]. Export of triglycerides as VLDL particles is compromised through reduced lipidation of microsomal triglyceride transfer protein (MTTP), the enzyme that catalyzes the lipidation of apolipoprotein B100 (apoB100) [138]. The transmembrane 6 superfamily member 2 (TM6SF2) genetic variant further attenuates the ability of hepatocytes to mobilize neutral lipids for the VLDL assembly [38]. Mitochondrial pyruvate carrier inhibitors (MPCi) decrease carbon flow into the tricarboxylic acid (TCA) cycle and therefore alter the ability of hepatic mitochondria to fuel DNL. A number of further pharmacological agents including fibroblast growth factor agonists (FGF21a), peroxisome proliferator-activated receptor (PPAR) ɑ agonists (PPARαa), thyroid hormone receptor-β agonists (THR-βa), and farnesoid X receptor agonists (FXRa) mainly aim to augment fatty acid oxidation, and DNL is targeted by ketohexokinase inhibitors (KHKi), stearoyl-CoA desaturase 1 inhibitors (SCD-1i), mitochondrial pyruvate carrier inhibitors (MPCi), and FXRa and acetyl coenzyme A carboxylase inhibitors (ACC1/2i). Following progression to NASH, production of sn-1,2-DAG and sphingolipids is favored. In NASH, the sn-1,2-DAG–PKCε pathway tightly correlates with hepatic insulin resistance [115]; hepatic dihydroceramides correlate with hepatic oxidative stress and inflammation [118]. Following progression to NASH, mitochondrial flexibility is lost, leading to decreased fatty acid oxidation [121] and oxidative stress and then inflammation [118], leading to hepatocytes apoptosis. Resident macrophage cells in the liver, the Kupffer cells, are increased and release proinflammatory cytokines [143]. Finally, HSC activation is regarded as a key initiating event in hepatic fibrogenesis, with activated HSC being characterized by enhanced extracellular matrix (ECM) production. Among other factors, the transforming growth factor β (TGF- β) initiates ECM gene expression in quiescent HSC [148]. Chemokine receptor (CCR2/5) antagonists and pan-PPARa mainly exert antifibrotic properties. ACC 1/2i, acetyl coenzyme A carboxylase 1/2 inhibitors; a: agonists, apoB100, apolipoprotein B100; βox, β oxidation; CER, ceramides; ChREBP, carbohydrate regulatory element binding protein; CCR, chemokine receptor; CD36, Cluster of differentiation 36; CPT1, carnitine palmitoyltransferase 1; DAG, diacylglycerols; DNL, de novo lipogenesis; ER, endoplasmic reticulum; ECM, extracellular matrix; Fa-CoA, fatty acyl-CoA; FATP, fatty acid transporters; FGF, fibroblast growth factor; FFA, free fatty acids; fruc, fructose; FXR, farnesoid X receptor; GCKR, glucokinase regulatory protein; GLP-1 RA, glucagon-like peptide 1 receptor agonists (GLP-1 RA); HC, hepatocyte; HSCs, hepatic stellate cells; i, inhibitors; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-6R, interleukin 6 receptro; IR, insulin receptor; JNK, c-Jun N-terminal kinase; KHKi, ketohexokinase inhibitors; LD, lipid droplet; LPS, lipopolysaccharide; MPCi, mitochondrial pyruvate carrier inhibitors; MTTP, microsomal triglyceride transfer protein; NF-κB: nuclear factor κ-light-chain-enhancer of activated B cells; PNPLA3, patatin-like phospholipase domain-containing protein 3; PPAR, peroxisome proliferator-activated receptor; pyr, pyruvate, SCD-1i, stearoyl-CoA desaturase 1 inhibitors; SGLT-2i, sodium glucose cotransporter-2 inhibitors; SREBP1c, sterol regulatory element binding protein 1c; TAG, triacylglycerol; TCA, tricarboxylic acid; TGF-β, transforming growth factor-β; THR, thyroid hormone receptor; TLR-4, toll-like receptor-4; TNF-α, tumor necrosis factor-α; TNFR, tumor necrosis factor receptor; TM6SF2, transmembrane 6 superfamily member 2; VLDL, very low density lipoprotein.
Among other genes, the most widely studied TM6SF2 variant, rs58542926 [C > T] encoding E167K, is associated with histological severity of steatosis and all the components of the NAFLD activity score [[38], [39], [40]], but in one study [35], not with fibrosis. This variant leads to an inability to mobilize neutral lipids for the very low density (VLDL) lipoprotein assembly in hepatocytes, resulting in increased TAG levels and lipid droplet formation [38,39] (Figure 1). Notably, recent data point to a greater proportion of lean humans with histologically proven NAFLD carrying the TM6SF2 5854296 T allele than overweight humans with NAFLD, despite similar PNPLA3 rs738409 GG genotype distribution. This difference persisted upon adjustment for multiple confounding factors, supporting the relevance of the genetic background even in the absence of adiposity [41]. The GCKR gene plays a central role in hepatic lipid metabolism through modulation of activity and intracellular localization of glucokinase [42]. The rs1260326 C > T polymorphism (P446L variant) enhances glucokinase activity in the liver in response to fructose-6-phosphate [43] and was shown to correlate with the severity of liver fibrosis but not with other NAFLD histological features [44]. In another study, the GCKR rs1260326 and rs780094 allele T were associated with susceptibility to NAFLD, NASH, and NASH with relevant fibrosis [45], and the GCKR rs1260326 variant explained 9.2% of the percentage of hepatic TAG content variance in Caucasians [46].
The genetics underlying the pathogenesis of NAFLD, ranging from inflammation to altered mitochondrial function, in oxidative stress and impaired insulin sensitivity, is less well defined [[47], [48], [49], [50], [51], [52], [53], [54]] (Table 1). Genetic variants influencing insulin receptor activity point to an independent association with fibrosis but not with NASH with the ectoenzyme nucleotide pyrophosphate phosphodiesterase (ENPP1) 121Gln and insulin receptor substrate-1 (IRS-1) 972Arg polymorphisms [49]. The membrane-bound O-acyltransferase domain-containing 7 gene (MBOAT7) rs641738 T allele was shown to increase the risk of steatosis, necroinflammation, and fibrosis stage (F) 2–4 [47,48]. MBOAT7, also known as lysophosphatidylinositol acyltransferase 1, and is an enzyme involved in the phospholipid acyl-chain remodeling, which transfers PUFA, such as arachidonoyl-CoA, to lysophosphatidylinositol and other lysophospholipids. An independent association of the MBOAT7 genotype across the entire spectrum of NAFLD-related liver damage was reported after adjustment for BMI [47], and further evidence underlined the association of this gene variant with fibrosis independent of the presence of histological inflammation [55]. Each copy of the T allele was reported to increase the odds of hepatic steatosis by 20% after correction for demographic and anthropometric, clinical, and genetic factors [47]. It was suggested that lower hepatic protein expression of MBOAT7 mediates the association with NAFLD severity via changes in the hepatic phosphatidylinositols acyl-chain remodeling [47,48].
Furthermore, the splice variant (rs72613567:TA) encoding the hepatic lipid droplet protein hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13), which has enzymatic activity against several bioactive lipid species implicated in lipid-mediated inflammation, reduced the risk of fibrosis [56] and NASH but not steatosis [57]. Each copy of the minor G allele of the rs6834314 splice variant of the HSD17B13 gene further increased the risk of liver TAG content ≥33% by 32% [58]. Carriage of the superoxide dismutase 2 (SOD2) C47T polymorphism, the gene encoding manganese-dependent superoxide dismutase, which protects cells from oxidative stress, was also associated with steatosis and more advanced fibrosis in NASH [50], and variants on the lysophospholipase-like 1 (LYPLAL1) were reported to increase hepatic steatosis, potentially because of a reduced triglyceride breakdown [59]. Uncoupling protein 2 (UCP2) rs695366 G genotype correlates with reduced steatosis and NASH risk, particularly in humans with fasting normoglycemia [54], whereas myeloid-epithelial-reproductive tyrosine kinase (MERTK) rs4374383 polymorphism decreased the risk of steatosis, NASH, and fibrosis [51,53]. The rs3480 A > G polymorphism of fibronectin type III domain-containing protein 5 (FNDC5) gene, which encodes irisin, was further associated with protection from clinically significant fibrosis in patients with NAFLD, in a study reporting an increased expression of irisin in activated human hepatic stellate cells (HSC), where irisin promoted the expression of proinflammatory and fibrogenic markers [60]; however, a more recent study reported an increased risk of steatosis with the same allele and a lack of association with other histological features [61]. The reasons for this discrepancy remain elusive. Other genes with links to autophagy are also being investigated [62,63]. Unfortunately, some of these studies have examined the association only with certain NAFLD stages [49] or were limited by the lack of validation in ethnic populations [44,47].
4. Nutrition
Excessive caloric intake, a nutritional pattern rich in saturated fat, carbohydrates, and sugar-sweetened beverages have all been implicated in the development of NAFLD [1,[64], [65], [66], [67], [68]] (Figure 1). (Table 2) Hypercaloric and high-fat diets seem to increase intrahepatic fat content [69,70]. Although a single monounsaturated fat load does not affect hepatic steatosis among glucose-tolerant volunteers [71], a single oral lipid load rich in saturated fat augments hepatic gluconeogenesis, induces insulin resistance and tends to augment hepatic lipid accumulation in healthy lean men [69], effects potentially ascribed to the lipid-mediated inhibition of insulin signaling [72].
Table 2.
Effects of dietary interventions in randomized controlled and randomized parallel group clinical trials demonstrating increases in intrahepatic fat content by magnetic resonance methods.
| Diet | Design, Ref. Cohort N total (males), % NAFLD BMI (kg/m2) |
Intervention Duration |
Δ body mass vs BL vs CTRL |
Liver TAG | Metabolic effects |
|---|---|---|---|---|---|
| Monounsaturated fat | R/C, [71] 14 (14), 0% 22±1 d |
Canola oil vs VCL Once |
↔ ↔ |
Canola oil: ↑ by 33% from -120 min to 240 min (↔ vs -120 min) VCL: ↑ by 7% from -120 min to 240 min (↔ vs -120 min+ BG) |
↔ WB- IS (HEC) |
| Polyunsaturated fat | R/P, [64] 37 (26), 0% 18–27 |
Habitual diet + high SFA vs high-n-6 PUFA muffins 7 w |
SFA: +1.6 kg PUFA: +1.6 kg ↔ |
SFA: ↑ 58% a PUFA: ↑ 53% a,f MRI |
PUFA: ↑ 0.22 of HOMA-IR SFA: ↑ 0.18 of HOMA-IR (↔BG) NR vs BL |
| R/P, [65] 61 (21) , NR PUFA: 30.3 ± 3.7 SFA: 31.3 ± 3.9 |
n-6 PUFA enriched (15% TE) SFA enriched (15% TE) 10 w |
↔ ↔ |
n-6 PUFA: ↓ 0.9% b SFA: ↑ 0.3% b,f MRI/1H-MRS |
Both: ↔ SAT + VAT mass (MRI) BG + vs BL |
|
| Saturated fat | R/P, [80] 38 (17), NR 31 ± 1 |
SFA (60% TE) UFA (59% TE) CARB (24% TE) 3 w |
All: +1.4% e ↔ BG |
SFA: ↑ 55%e,f UFA: ↑ 15%e CARB: ↑ 33%e 1H-MRS |
SFA: ↓ IS e (HEC) |
| High fat/high protein diets | R/C, [70] 10 (10), NR 22.4 ± 0.6 |
Hypercal+HF (+100% fat intake) Hypercal+HFHP (+100% fat +>100% protein intake) Isocal 4 d |
HF: ↔ HFHP: +1.1 kg e ↔ BG |
HF: ↑ 90% e HFHP: ↑ 68% a,e,f 1H-MRS |
↔ in the fEGP ↔EGP (2-step HEC) |
| High fat diet | R/P, [78] 20 (20), NR LF: 29.3 ± 0.6 HF: 28.3 ± 0.5 |
LF (20%TE) HF (55% TE) → LF to HF after 3 w 6 w |
NR | LF: ↓ 13% HF: ↑ 17% f NR vs BL 1H-MRS |
↔ IMCLC BG (histology) ↔ IS BG (HEC) NR vs BL |
| R/C, [79] 10 (0), NR 33 ± 4 |
LF (16% TE) HF (56% TE) 2 w |
↔ ↔ |
LF: ↓ 20% ± 9% e HF: ↑ 35% ± 21% e,f 1H-MRS |
↔ VAT + SAT mass (MRI) BG + vs BL |
|
| Energy-dense diet | R/P, [66] 36 (36), 0% 19.5–24.5 |
Habitual diet + 3 L/HF HS Habitual diet + HS beverage, consumed 3x/d with meals or 2–3 h after meal 6 w |
All: +2.5 kg c,e ↔ BG |
↔ in groups consuming beverages with meals, HF HS: ↑ 45%e HS: ↑ 110%e when consumed between meals (↔ in between meals groups) 1H-MRS |
↔ in WB-IS (2-step HEC) BG+ vs BL |
| Fructose | R/P, [67] 32 (32), NR Fru 30 ± 1.4 d Glu 28.9 ± 1.7 d |
StdD + Glu (25% TE) StdD + Fru (25% TE) 2 w |
Fru: +1.0 kg e Glu: +0.6 kg e ↔ BG |
Fru: ↑ 24% a,e Glu: ↑ 26% a,e (↔ BG) 1H-MRS |
Fru: ↑ 0.8 f HOMA-IR Glu: ↑ 0.1f HOMA-IR |
| R/C, [73] 10 (10), NR 19–25 |
StdD+Fru (3.5 g/kg/FFM) StdD+Glu (3.5 g/kg/FFM) 7 d |
Fru: +0.6 kga,e Glu: +1 kga,e |
Fru: ↑ 52% ± 13% e Glu: ↑ 58% ± 23% a ↔ BG 1H-MRS |
↔ FG + Ins Glu: ↑IMCLC (1H-MRS) e,f Fru: ↔IMCLC |
|
| R/C, [74] 9 (9), NR 22.6 ± 0.5 |
StdD + high Fru (3 g/kg)+PLC (6.6 g/day maltodextrin) StdD + high Fru (3 g/kg) + AAs (20.3 g/day) 6 d |
↔ ↔ |
High-Fru + PLC: ↑ 115% a,e High-Fru+ AAs: ↑ 81% a,e,f 1H-MRS |
↔ for glycemia 9 h after oral fructose loading | |
| Beverages | R/P, [68] 47 (17) , 0% 26–40 |
StdD + 1 L/d of cola, semi-skimmed milk, diet cola, or water 24 w |
NR ↔ BG |
Cola: ↑ 132%–143% vs other groups e ↔ milk, diet cola or water 1H-MRS |
Cola: ↑ 24%–31% VAT (dxa) vs other groupse (Significant difference between regular cola + milk) Cola: ↑ IMCLC (MRS) vs other groupse |
Single group assignment studies and non-randomized parallel group studies were excluded.
Subject data presented as mean (±SEM) unless otherwise stated.
AAs: amino acids, ALA: alpha-linolenic acid, BW: body weight, BG: between groups, BL: baseline, BMI: body mass index, CARB: simple sugars, CTRL: control, d: day, DHA: docosahexaenoic acid, dxa: dual-energy X-ray absorptiometry, EA: eicosapentaenoic acid, EGP: endogenous glucose production, fEGP: fasting endogenous glucose production, FFM: free fat mass, FG: fasting glucose, Fru: fructose, g: grams, Glu: glucose, HbA1c: glycated hemoglobin, HEC: hyperinsulinemic euglycemic clamp, HF: high fat, HFHP: high fat high protein, HOMA-IR: homeostasis model assessment of insulin resistance, 1H-MRS: proton magnetic resonance spectroscopy, HS: high sugar, Hypercal: hypercaloric, IMCLC: intramyocellular lipid content, Ins: insulin, IS: insulin sensitivity, isocal: isocaloric, kg: kilograms, LF: low fat, min: minutes, MRI: magnetic resonance imaging, MD: Mediterranean diet, N: number, NA: not assessed, NAFLD: non-alcoholic fatty liver disease, NASH: non-alcoholic steatohepatitis, NEFA: non esterified fatty acids, NR: not reported, PLC: placebo, PUFAs: polyunsaturated fatty acids, Ref: references, R/C: randomized controlled, R/P: randomized parallel group, SAT: subcutaneous adipose tissue, SG: single group assignment study, SFA: saturated fatty acids, StdD: standard diet, TAG: triacylglycerol, T2DM: type 2 diabetes mellitus, TE: total energy, TG: triglycerides, VAT: visceral adipose tissue, UFA: unsaturated fatty acids, VCL: vehicle, vs: versus, w: weeks, WB: whole body.
Estimated from table/graph.
Median.
Pooled data.
mean ± SD.
Significant effect of intervention (p < 0.05) according to the manuscript.
Significant difference between groups (p < 0.05) according to the manuscript.
Furthermore, robust effects of fructose consumption on hepatic steatosis were reported in healthy male humans [73,74] (Table 2). In a nonrandomized crossover study, a 7-day hypercaloric fructose diet increased intrahepatic TAG content and augmented hepatic steatosis more than an isocaloric diet did, with more potent stimulation of hepatic de novo lipogenesis (DNL) in 16 healthy males with a family history of T2DM than in 8 controls [75]. This finding indicates that fructose induces its detrimental effects, especially under hypercaloric conditions. In support, a systematic review and meta-analysis of studies in healthy humans revealed a fructose-induced increase in hepatic TAG content only in the context of excessive caloric intake and concluded that isocaloric exchange of fructose for other carbohydrates would not induce NAFLD at least in healthy participants [76]. Intriguingly, the available evidence points to similar detrimental effects of glucose and fructose in hypercaloric diets on liver fat content and liver enzymes in healthy men [77]. In long-term studies (Table 2), a high-fat diet [78,79], particularly rich in saturated fat [64,65,80], a high caloric intake [66], fructose [67], and beverage [68] consumption, all increase hepatic steatosis (Table 2), and further cohort studies point to the involvement of red and processed meat [81]. Evidence for a potential role of insufficient dietary fiber intake was also presented [82]. Notably, few dietary interventions have evaluated the effects on liver fibrosis in detail.
5. Gut microbiota
The body of evidence supporting an association between gut microbiome and NAFLD (Figure 1) is increasing, as illustrated by a surprisingly robust diagnostic accuracy (area under the curve 0.936) of gut microbiota-derived signature for predicting the presence of advanced fibrosis F3 or F4 in 86 well-characterized individuals with biopsy-proven NAFLD [83]. Moreover, individuals who are obese and have NASH seem to exhibit a different microbiota signature, especially pertaining to the taxa of Proteobacteria, Enterobacteriaceae, and Escherichia, than obese and lean individuals without NASH [84]. The NASH microbiome can be characterized by an increased abundance of alcohol-producing bacteria along with higher blood ethanol levels in individuals with NASH [84]. This endogenously produced alcohol is immediately transported through the portal blood to the liver and could thereby contribute to the generation of ROS and hepatic inflammation [85]. Enhanced presence of alcohol-producing bacteria was also found in advanced NAFLD, suggesting a role of these bacteria in disease progression [83]. The gut is also a major site of gut bacterial fermentation of dietary carbohydrates, leading to the production of short chain FA. Although these FA are generally considered to exert beneficial effects on glucose and lipid metabolism, they may stimulate nutrient absorption, favoring obesity and steatosis [86]. Altered gut microbiota composition has been further associated with increased intestinal permeability and disruption of intercellular tight junctions in human NAFLD [87], permitting translocation of bacterial lipopolysaccharide (LPS) into the circulation (Figure 1). Animal studies have demonstrated the operation of this mechanism facilitating hepatic FA accumulation and NASH progression [88].
In human studies, intrahepatic localization of LPS was reported to correlate with liver inflammation, through the interaction with the toll-like receptor-4 (TLR4)-mediated pathway [88]. Liver sinusoidal endothelial cells (LSEC) and tissue resident macrophages would be expected to eliminate blood-borne pathogens including gut-derived bacteria and other components entering the liver [89]. Thus, the mechanisms of impaired defense in NAFLD are unknown. A recent study identified differences in liver tissue 16S ribosomal RNA gene bacterial metataxonomic signatures between humans with NAFLD who are severe and moderately obese [90]. Reduction in liver bacterial DNA from the Lachnospiraceae family was associated with histological disease severity [90]. This study did not assess the origin of the detected microbiome. Nevertheless, the biological relevance of liver bacterial DNA fragments, the potential contribution of particular taxa, and finally, the causality of microbiota alterations for NAFLD progression in humans require dedicated studies.
Furthermore, among other intestinal actions, primary bile acids are metabolized in the small intestine into secondary bile acids. These are reabsorbed through the portal circulation back to the liver, where they act as signaling molecules by interacting with receptors, for example, the farnesoid X receptor (FXR) and members of the G-protein-coupled receptor superfamily, which self-modulate their synthesis, glucose and lipid metabolism, and energy expenditure (Figure 1). For instance, interaction with FXR represses de novo FA synthesis in the liver and stimulates FA oxidation [86]. Finally, glucagon-like peptide-1 is an incretin secreted postprandially by L-cells in the small intestine, stimulating insulin secretion and inhibiting glucagon secretion, with positive effects on body weight loss and cardiovascular outcomes [13].
6. Adipose tissue dysfunction
Adipose tissue is the principal sink of TAG and the main source of circulating FA during fasting because of lipolysis [72]. A systematic analysis of 798 humans undergoing bariatric surgery demonstrated that the percentage of body fat decreases while the ratio of trunk-to-limb fat increases with the progression from healthy liver to NASH (activity score >2) [91]. This points to limited subcutaneous adipose tissue expandability as an important mediator of the excess lipid fluxes to ectopic tissues, including the liver. Furthermore, subcutaneous adipose tissue hypertrophic adipocytes, which are more exposed to lipolysis and the production of proinflammatory mediators, correlated with NAFLD severity at least among female participants. Insulin-resistant states are specifically associated with blunted insulin-mediated restriction of adipose tissue lipolysis, which increases the flux of FFA and glycerol to the liver [13], where they serve as substrates for re-esterification, generation of lipid mediators (e.g., DAG) and DNL (Figure 1), or allosterically stimulate gluconeogenesis [72]. A recent study employing [1,1,2,3,3-2H5]glycerol in obese volunteers showed that insulin-mediated suppression of the rate of glycerol appearance and circulating FFA strongly correlates with cytosolic DAG concentration [92]. Obesity-related insulin resistance is further accompanied by macrophage infiltration and local increase in inflammatory cytokines in the adipose tissue [93]. However, adipose tissue insulin resistance can exist without concomitant adipose tissue inflammation, because oral lipid ingestion acutely induced adipose tissue insulin resistance without a concomitant increase in the expression and secretion of inflammatory cytokines including tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) from subcutaneous adipose tissue of healthy humans [69].
Nevertheless, long-standing insulin resistance and NAFLD generally associate with circulating proinflammatory cytokines, contributing to the adipose tissue–liver cross talk. The adipokines adiponectin and leptin contribute to this cross talk and glucose production, lipogenesis, and FA oxidation (Figure 1). Although higher levels of serum leptin and decreases in the soluble leptin receptor have been reported in human biopsy-proven NAFLD [94], no association has been observed between the leptin and fibrosis stage, after a correction for the important confounder, age, gender, BMI, diabetes, and insulin resistance [95]. By contrast, adiponectin was found to be reduced during NAFLD progression [96], probably reflecting the increasing insulin resistance. In addition to endocrine acting factors, adipocytes release small extracellular vesicles, exosomes, containing among others small noncoding RNA possibly serving as novel signals of interorgan communication. Severely obese and lean humans displayed more than 55 exosomal microRNA derived from visceral adipose tissue, differentially expressed in 2 cohorts. These microRNA (miR −23b, miR-140-3p, miR-148b, and miR-182) may regulate liver fibrosis through the upregulation of transforming growth factor-β (TGF-β), which activates the ECM secreting fibroblasts [97].
7. Hepatic lipid accumulation, insulin resistance, and mitochondrial adaptation
Net hepatic TAG accumulation is the result of hepatic lipid supply and demand. Circulating FFA and glycerol serve as lipid sources for hepatic re-esterification and DNL, and hepatic TAG can be disposed of through oxidation or export as VLDL particles [98]. The FA transporters (FATP2 and FATP5) and the cluster of differentiation 36 (CD36) [98] facilitate hepatocyte FFA uptake (Figure 1). Studies have reported conflicting findings on the expression patterns of FATP2 and CD36 in humans with steatosis and NASH [[99], [100], [101]]. Recently, evidence was provided for an inverse association between hepatic FATP5 messenger RNA expression and histologic changes in NAFLD, including ballooning and fibrosis, suggesting reduced FATP5-mediated TAG accumulation during NASH progression [101].
DNL, namely, the conversion of acetyl-CoA over malonyl-CoA via acetyl coenzyme A carboxylase (ACC) and finally to palmitate (Figure 1), was independently associated with hepatic TAG in individuals with NAFLD [102]. Comprehensive isotopic tracer studies have highlighted that DNL accounts for up to 26% of intrahepatic palmitate production in humans with NAFLD [[102], [103], [104]] and up to 10% in humans without steatosis [102,[105], [106], [107]]. Prolonged ingestion of 2H2O over 3–5 weeks, with correction for adipose tissue FA synthesis, revealed an even higher contribution of 38% of hepatic DNL to hepatic TAG among obese glucose-intolerant individuals with NAFLD, compared with the 19% and 11% in glucose-tolerant obese and lean individuals without NAFLD, compared with the prior appreciations [108]. Moreover, hepatic DNL negatively correlated with hepatic and skeletal muscle insulin sensitivity. DNL is regulated by the transcription factors sterol regulatory element-binding protein 1c (SREBP1c), which is activated by insulin and liver X receptor a (LXRa), and carbohydrate regulatory element-binding protein (ChREBP), mainly activated by carbohydrates [72] (Figure 1). Enhanced expression of SREBP1c has been demonstrated in human NAFLD [109]. From mouse studies, SREBP1c-stimulated DNL has been related to selective insulin resistance, by which insulin would continue to maintain elevated DNL despite its inability to suppress hepatic gluconeogenesis [72,110]. Subsequent mouse studies, however, provided evidence that chronic excessive lipid supply rather than insulin increases hepatic DNL [72]. ChREBP stimulates the expression of glycolytic genes, augmenting substrate availability for DNL, and induces the expression of stearoyl-CoA desaturase 1 (SCD1), the enzyme responsible for the conversion of saturated to monounsaturated FA, increasing TAG accumulation [111]. Elevated expression levels of ChREBP were found in human NASH [111], which decline with severe insulin resistance. Individuals with NAFLD may exhibit downregulated ChREBP more than healthy humans do [112].
Lipid metabolites also relate to hepatic insulin resistance in the context of NALFD. Liver of obese humans presents with increased hepatic DAG and activation of the novel protein kinase C (PKC) ε isoform, which also negatively relate to their insulin sensitivity [[113], [114], [115]]. In liver, PKCε was shown to induce serine phosphorylation, inhibiting insulin receptor kinase activity [116,117]. Ongoing research has addressed the role of specific DAG isoforms and their localization in subcellular compartments [92,113]. Interestingly, other lipid metabolites, including ceramides or acylcarnitines, or markers of endoplasmic reticulum (ER) stress or inflammation, were not increased or did not relate to hepatic insulin sensitivity [113,114,118]. However, specific ceramides and other sphingolipids were elevated only in obese individuals with NASH, not those with or without steatosis [118,119]. Interestingly, some sphingolipids correlated with markers of hepatic oxidative stress and inflammatory pathways, suggesting an important role of these lipid metabolites for NAFLD progression [118]. Studies have also highlighted the ability of insulin to induce connective tissue growth factor expression of the HSC in liver biopsies of patients with NASH (Figure 1), inferring that insulin resistance-associated hyperinsulinemia may directly accelerate fibrogenesis during NAFLD development [120].
Any increase in lipid overload is expected to stimulate its disposal. Mitochondrial oxidative capacity, as assessed with high-resolution respirometry, is up to 5-fold higher in human liver biopsies of obese insulin-resistant participants without or with histologically proven NAFLD than for lean humans [121]. Compared with the steatosis group, the NASH group had higher mitochondrial mass but a 31%–40% lower maximal respiration associated with mitochondrial uncoupling, leaking activity, augmented hepatic oxidative stress, and oxidative DNA damage [121]. Accordingly, recovery from hepatic adenosine triphosphate (ATP) depletion was shown to be severely impaired among humans who were both obese and had NASH [122,123]. Notably, individuals with T2DM feature reductions in fasting and postprandial absolute concentrations of γ-ATP and inorganic phosphate in hepatic ATP turnover were compared with age- and body mass index-matched and lean young humans [[124], [125], [126]]. This suggests that abnormal energy metabolism could contribute to the accelerated progression of NAFLD known for T2DM.
The aforementioned evidence of oxidative DNA damage in NASH highlights the importance of effective anti-oxidant mechanisms [121]. Oxidative stress occurs when the production of reactive oxygen species (ROS) exceeds the scavenging capacity of the antioxidative mechanisms [127] (Figure 1). Production of ROS, including major free radicals such as the superoxide anion radicals (O2−) and the major nonradical species hydrogen peroxide (H2O2), occurs continuously through energy metabolism in the intracellular environment of liver cells [128]. ROS that are maintained in normal values through clearance pathways (e.g., catalase, glutathione peroxidase, and peroxiredoxin), act as signaling molecules and are involved in many vital mechanisms for cellular survival [129]. Increased ROS levels have been demonstrated by using cell lines to modulate lipid metabolism, through downregulation of peroxisome proliferator-activated receptor (PPAR) α activity, a key transcriptional factor of FA oxidation, leading to subsequent disturbed lipid homeostasis of human liver cells exposed to H2O2 [130]. In NASH, augmented hepatic oxidative stress (H2O2, lipid peroxides) and oxidative DNA damage was paralleled by reduced anti-oxidant defense capacity and increased inflammatory response [121].
Finally, export via water-soluble VLDL particles represents another way to remove hepatic TAG [131,132] (Figure 1). Although VLDL secretion is increased in human NAFLD [[132], [133], [134]], it plateaus when hepatic TAG accumulation exceeds 10% [132]. Formation of VLDL particles occurs in the ER through lipidation of the apolipoprotein B100 (apoB100), catalyzed by microsomal triglyceride transfer protein (MTTP) [135]. There is evidence that apoB100 synthesis rates are lower in humans with NASH than in obese and lean humans without NASH [136]. Additionally, hepatic MTTP levels are lower in humans with NAFLD with hepatic TAG content exceeding 30% than in healthy humans [137]. Humans with NASH have lower hepatic messenger RNA levels of apoB100 and MTTP and serum VLDL-triglycerides than those without NASH do, suggesting a potential contribution of the compromised VLDL export to NAFLD progression [138]. Nevertheless, another study reported similar expression levels of MTTP and apoB100 between humans without/with steatosis or NASH [139]. Thus, NAFLD heterogeneity might also result from the variability of the individual plateau of ER stress-related exposure to FA, but this needs to be further investigated.
8. Intrahepatic mechanisms driving inflammation and fibrosis
The mechanistic concepts of intrahepatic inflammation and fibrosis have been recently reviewed [140,141]. Gut-derived LPS, translocated gut bacteria, certain metabolites (e.g., saturated FA, cholesterol), and injured hepatocytes activate the resident hepatic macrophage cells [142], the Kupffer cells, which release proinflammatory cytokines including interleukin-1β (IL-1β), IL-6 and TNF-α via TLR4 activation [143] (Figure 1). Experimental mouse models have revealed that the release of chemokines from the injured liver cells promotes the infiltration of circulating C–C chemokine receptors (CCR) 2 monocytes, which differentiate into macrophages. These cells further contribute to NASH progression by maintaining an inflammatory environment and activating HSC, releasing CCR5 and thereby regulating their activation and proliferation [144]. HSC are the main source of extracellular matrix (ECM) proteins and considered the main mediators of fibrosis development in human NAFLD [145]. Lipid accumulation in human-injured hepatocytes may trigger a profibrogenic response of HSC [146,147], activated among other mediators by the transforming growth factor-β (TGF-β) to express ECM gene expression [148]. Fibrosis progresses in parallel to altered angiogenesis, eventually driving the architectural changes characterizing the development of cirrhosis [149], with recent evidence showing that mechanical stretching of human hepatic endothelial cells suffices to trigger angiocrine signals that induce the release of hepatocyte growth-promoting signals [150]. Further evidence from human studies points to enhanced ER stress (increased expression of the ER stress markers Chop and Grp78) and inflammasome activation in obese patients with NASH than in those with/without NAFL, with a positive correlation between ER stress and level of inflammasome transcripts [151].
LSECs, the most abundant nonparenchymal liver cells, are involved in metabolite transfer via fenestrations, which can be lost during the transition from healthy to fibrotic conditions as a result of increasing liver stiffness due to a phenomenon called capillarization, as reported for human LSECs [152].
9. Clinical diagnosis of NAFLD
Understanding the results of clinical studies and the relevance of reported effects of treatments requires a short reflection on the different approaches to diagnose NAFLD in humans. Currently, liver biopsy remains the gold standard technique for the comprehensive diagnosis of NAFLD, because it allows for identifying inflammation and classifying fibrosis stages F0–4 [13]. Nevertheless, the invasive nature and—at least—risk of complications limits its widespread use. Liver biopsies have other methodological shortcomings, for example, access to a small volume of the liver and different classifications [13]. As a result, most—earlier—clinical studies applied ultrasonography, liver enzymes, or various indices for the diagnosis of NAFLD. With regard to steatosis, such approaches are inferior to 1H MRS and magnetic resonance imaging–estimated proton density fat fraction (MRI-PDFF), which allow for exact noninvasive quantification of hepatocellular TAG [[153], [154], [155]]. The NAFLD liver fat score and fatty liver index demonstrated modest diagnostic accuracy against 1H MRS [154] and offer limited potential to detect changes in hepatic TAG, especially when examining low-carbohydrate diets [156]. Additionally, indices to assess liver fibrosis, such as fibrosis-4 index, NAFLD fibrosis score, or aspartate aminotransferase to platelet ratio index, offer only moderate diagnostic efficacy, particularly in individuals with T2DM [157]. Imaging-based methods such as transient (ultrasound) or magnetic resonance elastography are promising novel tools [10] that require further testing in large prospective studies.
10. Treatment concepts
10.1. Strategies to induce body weight loss
10.1.1. Lifestyle modification
Hypocaloric nutrition in individuals who are overweight or obese and exercise training are considered essential to the nonpharmacological management of NAFLD [10] (Figure 1), with some evidence showing that even fibrosis and NASH resolution may be expected through lifestyle interventions [158]. There is evidence that a 10% weight loss can induce complete resolution of NAFLD in humans [159]. However, in the context of obesity and human NAFLD, the goal of 10% weight loss might be difficult to accomplish and even more difficult to sustain, with the majority of the studies reporting weight regain during the 2-year follow-up [159]. That said, there seem to be long-lasting beneficial effects of body weight loss on liver fat, despite body weight regain [159]. Another early study showed that a 12-week lifestyle intervention with an average of 8 kg of weight loss reversed hepatic steatosis [160]. Nevertheless, the study revealed no effects on whole-body insulin sensitivity or circulating markers of inflammation and adiponectin [160]. Subsequent studies have examined the role of different macronutrients, reporting beneficial effects of low carbohydrates [161], n-6 PUFA enriched fat [64,65], high protein [162,163], low fat [164], and Mediterranean diets [165,166], not n-3 PUFA enriched diets [167,168]. However, a recent meta-analysis showed little evidence that low-carbohydrate diets are more beneficial for NAFLD than differently composed diets with similar caloric intake [169]. Moderate-carbohydrate diets yielded changes in liver enzymes similar to those of low/moderate-fat diets [170]. In line with that finding, one RCT showed that the decrease in energy consumption rather than dietary composition, including modification of red meat, fiber, and caffeine, is the driver of the reduction of intrahepatic TAG accumulation, at least in individuals with T2DM and NAFLD [171]. By contrast, a moderate macronutrient shift, induced by substituting carbohydrates with protein and fat for 6 weeks reduced intrahepatic TAG content in weight-stable humans with T2DM [172]. Further lifestyle interventions have demonstrated decreases in liver TAG content independently of weight loss with supplementation of n-6 PUFA among abdominally obese participants [65] and high-protein diets in volunteers without [70] or with T2DM [163]. This may be of particular importance for lean humans with NAFLD. By contrast, some macronutrients may have deleterious effects, as reported with an excess of n-6 PUFA with regards to cardiovascular disease, cancer, and inflammatory and autoimmune diseases [173]. Additionally, increased consumption of animal protein has been linked to increased all-cause mortality [174].
Regular physical activity can also exert specific effects on NAFLD by various mechanisms, as reviewed recently [175]. For example, one 8-week resistance training study led to a robust decrease of 13% of hepatic TAG content in humans with NAFLD who maintained a stable body weight [176]. A meta-analysis including RCTs with 1073 patients with NAFLD revealed that exercise training improves transaminases and liver fat content, also irrespective of weight change [170]. There was no difference between aerobic and resistance exercise types, whereas moderate-to-high volume seems more beneficial than other exercise volumes. Nevertheless, interventions combining exercise and diet are more efficient to decrease transaminases (ALT) and to improve the NAFLD activity score (SMD = −0.61, 95% CI: −1.09 to −0.13) [170]. However, in the context of obesity, excessive lipid peroxidation, a known source of ROS, has been reported for acute intensive aerobic and resistance exercise sessions [177]. Even more, higher oxidative lipid damage as observed upon chronic combined (aerobic and resistance) exercise training in obese humans [177] suggests that not all individuals may equally benefit from certain modes of exercise.
10.1.2. Bariatric (metabolic) surgery
Because of its superior efficacy to cause sustained weight loss, bariatric surgery is another nonpharmacological therapeutic option in obese humans with NAFLD [13] (Figure 1). A recent systematic review and meta-analysis of 32 cohort studies comprising >3000 biopsy specimens reported a biopsy-proven resolution of steatosis in 66%, inflammation in 50%, ballooning in 76%, and fibrosis in 40% of all patients, yielding a significant decrease in mean NAFLD activity score [178]. Notably, 12% of the participants developed new or worsening features of NAFLD [178], but the lack of RCTs represents an important limitation of this meta-analysis. It remains unclear whether any worsening of NAFLD could be linked to the transient increase in lipolysis and circulating FFA, which also prevents early improvement in insulin resistance after bariatric surgery [179] but may be required for the subsequent beneficial epigenetic remodeling demonstrated in human skeletal muscle [179,180]. In addition, higher BMI, lower serum albumin, and an open surgical approach was independently associated with increased risk of complications, which include infections (mainly at the wound site), intra-abdominal fluid collections requiring drainage, and small bowel obstruction or stenosis of the surgical anastomosis [181].
10.2. Anti-hyperglycemic agents
Studies investigating the effects of anti-hyperglycemic drug classes in clinical trials to evaluate their efficacy for patients with NAFLD and with or without T2DM are discussed in this section, with a special focus on studies applying state-of-the art methods, such as 1H MRS and MRI-PDFF, for the quantification of TAG content.
10.2.1. Peroxisome proliferator-activated receptor agonists
PPAR agonists target nuclear transcription factors that primarily stimulate adipocyte differentiation and adipose tissue remodeling, modulating lipid fluxes to improve insulin signaling and glucose homeostasis [3,10]; although PPAR expression has further been reported in inactivated HSC, which declines upon HSC activation [182]. The best evidence for an improvement of histologically proven NAFLD has been reported for the PPARγ agonist, pioglitazone, which demonstrated reductions in hepatic steatosis and lobular inflammation, without significant effects on fibrosis scores after treatment for 96 weeks [183]. The results were replicated in smaller RCTs with a shorter duration [[184], [185], [186]] (Table 3). Importantly, some safety concerns such as body weight gain, decompensation of preexisting heart failure, and atypical bone fractures in women limit the broad use of pioglitazone [182]. Lobeglitazone, another PPARγ agonist, recently licensed in South Korea for the treatment of T2DM, also showed promising reduction of hepatic steatosis, assessed by controlled attenuation parameter with transient liver elastography [187], with an overall satisfactory safety profile, but still with an average body weight gain of 1.4 kg.
Table 3.
Randomized double-blinded placebo-controlled trials on drug treatment in adult patients with NAFLD with liver-related outcomes, as assessed by either liver histology or magnetic resonance methods, with >20 participants in the intervention arm.
| Class | Cohort, Ref. Method Duration |
Intervention | Primary outcome | Steatosis Inflammation Fibrosis |
Metabolic effects |
|---|---|---|---|---|---|
| PPARγ agonist | NASH [185] Histology, MRS 6 m |
PIO 45 mg/d (26) PLC (21) |
NR | ↓ B/I ↓ ↔ |
OGTT/[3H]glu/[14C] glu: Glu clearance ↑, Liver ↑, Adipose ↑ Adiponectin ↑, BW ↑ TG ↓ |
| NASH [183] Histology 96 w |
PIO 30 mg/d (80) PLC (83) |
NASH histology | ↓ B/I ↔/↓ ↔ |
HOMA-IR ↓ BW ↑ HDL ↑ |
|
| NASH [186] Histology, MRS 18 m (+open 18 m) |
PIO 45 mg/d (50) PLC (51) |
NAS ↓ >2 points without F worsening a | ↓ B/I ↓, NAS ↓ ↔,% F improv. ↑ |
Clamp: Rd ↑, Liver ↑, Adipose ↑ Adiponectin ↑, BW ↑ TG ↓, HDL ↑ |
|
| NASH [184] Histology 12 m |
PIO 30 mg/d (31 of 37) PLC (30 of 37) |
Hepatocyte injury (B) + F a | ↔ B/I ↓/↔ ↓ |
HOMA-IR ↓ TG ↔ |
|
| PPARα/δ agonist | NASH [189] Histology 52 w |
ELA 80 mg/d (93) ELA 120 mg/d (91) PLC (92) |
NASH resolution without F worsening b | ↔ ↓ ELA 120 mg:↓, ELA 80 mg: ↔ |
ELA 80 mg HOMA-IR ↔ ELA 120 mg HOMA-IR ↓ Both: LDL ↓, TG ↓ |
| SGLT2i | 79% NAFLD [11] MRS/MRI 24 w |
EMPA 25 mg/d (42) PLC (42) |
LFC a | ↓ NA NA |
Clamp/[2H]glu: M ↔, Liver ↔, Adipose ↔ Adiponectin ↑ Lipids ↔ |
| NR [196] MRS 24 w |
DAPA 10 mg/d (38 of 91) PLC (42 of 91) |
BW a Substudy: LFC b |
↔ NA NA |
Adiponectin ↔ Lipids NA |
|
| NAFLD [195] MRI 12 w |
DAPA 10 mg/d (21) Om3FA g/d (20) COMB (22) PLC (21) |
PDFF b | PDFF: DAPA ↔, Om3FA ↔, COMB ↓ NA NA |
HOMA-IR: DAPA ↓, Adipo-IR ↔ Adiponectin ↔ Lipids ↔ |
|
| 73% NAFLD [197] MRS 24 w |
CANA 300 mg/d (26) PLC (30) |
LFC b | ↔ NA NA |
Clamp/[2H]glu: Rd ↔, Liver ↑, Adipose ↔ Lipids NR |
|
| GLP-1 RA | NASH [12] Histology 48 w |
LIRA 1.8 mg /d (23 of 26) PLC (22 of 26) |
NASH resolution without F worsening a | ↔, % S improv. ↑ B/I ↔, NAS ↔ % F worsening ↓ |
HOMA-IR ↔, Adipo-IR ↔ HDL ↓ |
| NR [204] MRS 26 w |
LIRA 1.8 mg/d (24) PLC (26) |
Myocardial function a Substudy: LFC b |
↔ NA NA |
Ins-S NA Lipids ↔ |
|
| DPP-4i | NAFLD [209] MRI/MRS/MRE 24 w |
SITA 100 mg/d (25) PLC (25) |
PDFF b | ↔ NA NA |
HOMA-IR ↔ Lipids ↔ |
| NR [215] MRI 6 m |
VILDA 100 mg/d (22) PLC (22) |
INS-S b PDFF a |
↓ NA NA |
Clamp/[2H]glu: M/I ↔, Liver ↔ Lipids ↔ |
|
| Metformin | NAFLD [217] Histology (CT) 24 w |
MET 2.5/3.0 g/d (20 of 24) PLC (24) |
S b | ↔, % S improv. ↓ NA NA |
HOMA-IR ↔ Adiponectin ↔ LDL ↓ |
| FXR agonist | NASH [226] Histology 72 w |
OBCA 25 mg/d (141) PLC (142) |
NAS ↓ >2 points without F worsening a | ↓ B/I ↓ ↓ |
HOMA-IR ↑ LDL ↑, HDL ↓, TG ↔ |
| NASH [225] Histology 18 m (ongoing) |
OBCA 10 mg/d (312) OCA 25 mg/d (308) PLC (311) |
F improv. (≥1 stage) without NASH worsening a NASH resolution without F worsening b |
↓ B/I ↓ ↓ |
NA | |
| CCR 2/5 antagonist | NASH [228] Histology 2 years (arm A) 1 year (arm B) |
Arm A CVC 150 mg/d (145) Arm B PLC 1st year then CVC 150 mg/d (72) Arm C PLC (72) |
NAS ↓ >2 points with B/I ↓ ≥1 point without F worsening a | ↔ ↓ ↓ |
↔ |
| SCD1 inhibitor | NASH [229] Histology/MRS 52 w |
Aram 400 mg/d (101) Aram 600 mg/d (98) PLC (48) |
LFC a/b | Aram 400 ↓, Aram 600 ↔ Aram 400 ↔, Aram 600 ↓ ↔ |
HbA1c ↓ Ins-S NA Lipids NA |
| ACC ½ inhibitor | NAFLD [230] MRI/ MRE 12 w |
GS-0976 5 mg/d (51) GS-0976 20 mg/d (49) PLC (26) |
Safety a | GS-0976 20 ↓, GS-0976 5 ↔ NA NA |
HbA1c ↔ Ins-S NA LDL ↔, HDL ↔, TG ↑ |
| THR-β agonist | NASH [235] Histology/ MRI 36 w |
Res 80 mg/d (78 of 84) PLC (38 of 41) |
PDFF a | ↓ NAS ↔ ↓ |
Ins-S NA LDL ↓, HDL ↔, TG ↓ |
| MPC inhibitor | NASH [237] Histology 12 m |
MSDC-0602K 62.5 mg/d (99) MSDC-0602K 125 mg/d (98) MSDC-0602K 250 mg/d (101) PLC (94) |
NAS ↓ >2 points with B/I ↓ ≥1 point without F worsening b | MSDC-0602K 62.5 +125 ↔, MSDC-0602K 250 ↓ B/I ↔, MSDC-0602K 62.5 +125 NAS ↓, MSDC-0602K 250 NAS ↔ ↔ |
|
| FGF21 agonist | NASH [238] MRI 16 w |
Peg 10 mg/d (25) Peg 20 mg/w (24) PLC (26) |
Safety a PDFF a |
↓ NA NA |
INS-S NA Adiponektin ↑ Lipids NR |
ACC: acetyl coenzyme A carboxylase, Aram: aramchol, B: ballooning, BW: body weight, CANA: canagliflozin, COMB: combination, CCR: chemokine receptors, d: daily, CVC: cenicriviroc, DAPA: dapagliflozin, DM: diabetes mellitus, DPP-4i: dipeptidyl peptidase-4 inhibitors, ELA: elafibranor, EMPA: empagliflozin, FGF: fibroblast growth factor, FXR: farnesoid receptor X, GLP-1RA: glucagon-like peptide 1 receptor agonists, HbA1c: glycaeted haemoglobin, eHDL: high density lipoprotein, HOMA-IR: homeostatic model of assessment of insulin resistance, improv.: improvement, INS-S: insulin sensitivity, KHK: ketohexokinase, LDL: low density lipoprotein, LFC: liver fat content, LIRA: liraglutide, M, M/I, Rd, glu clearance = whole body (muscle) insulin sensitivity, m: months, MET: metformin, mg: miligrams, MRE: magnetic resonance elastography, MRI: magnetic resonance imaging, MRS: magnetic resonance spectroscopy, NAFLD: non-alcoholic fatty liver disease, NASH: non-alcoholic steatohepatitis, NA: not assessed, NR: not reported, OBCA: obeticholic acid, OGTT: oral glucose tolerance test, Om3FA: omega 3 fatty acids, Peg: pegbelfermin, PIO: pioglitazone, PLC: placebo, PDFF: protein density fat fraction, PPAR: peroxisome proliferator activated receptor, Res: resmetirom, Ref: references, SCD: stearoyl CoA desaturase, SGLT2i: sodium glucose co-transporter 2 inhibitor, SITA: sitagliptin, TG: triglycerides, THR: thyroid hormone receptor, VILDA: vildagliptin, w: weeks.
Primary outcome measure achieved.
Primary outcome measure not achieved.
Several other PPAR agonists with different pharmacodynamic profiles are being tested in clinical trials [188]. Elafibranor, a dual PPARα/δ agonist, initially one of the most promising anti-NASH drugs, recently failed to fulfill the predefined primary endpoint of NASH resolution without worsening of fibrosis in a population of 1070 patients with NASH, according to the interim analysis of the phase III trial [189]. Results from saroglitazar, a dual PPARα/γ agonist, and lanifibranor, a pan-PPAR agonist, in phase II trials have not been reported [13]. Intriguingly, lanifibranor inhibited the activation and proliferation of human HSC [182]. The development of seladelpar, a PPARδ agonist, was suspended because of unexpected histological findings in a phase II trial of a NASH cohort [182].
10.2.2. Sodium glucose cotransporter (SGLT)-2 inhibitors (SGLT2i)
SGLT2i were initially developed because of their glucose-lowering effects, but empagliflozin, canagliflozin, and dapagliflozin later also showed reductions of cardiovascular endpoints in T2DM in large RCTs [190]. Recently, dapagliflozin treatment was associated with a significant risk reduction of worsening heart failure or cardiovascular mortality among heart failure patients, irrespective of the presence of T2DM [191]. The metabolic effects of SGLT2i resulting from renal glucosuria and energy loss with concomitant body weight loss include a shift of substrate utilization with increased ketogenesis and hepatic FFA use but might also attenuate oxidative stress and inflammatory pathways [192]. SGLT2i have an overall satisfactory safety profile, but some concerns are the risk of genitourinary tract infections and candida vulvovaginitis, bone fractures (particularly with canagliflozin), and normoglycemic ketoacidosis, mainly in the context of low body weight, severely impaired insulin secretion, and low carbohydrate intake [190]. A recent study showed that empagliflozin treatment also decreases circulating uric acid and significantly increases adiponectin secretion in individuals with T2DM, suggesting improvement of adipose tissue function [11]. Several RCTs have reported a decrease in hepatic fat content with empagliflozin [11], dapagliflozin [193,194] and the dual SGLT1/SGLT2i, licogliflozin [188], and some studies with dapagliflozin [195,196] and canagliflozin [197] have revealed no effect (Table 3). This could be explained by different efficacy to induce weight loss, but some studies have demonstrated a significant hepatic TAG reduction independently of any weight change [11]. Although no RCTs testing SGLT2i on histological features of NAFLD has been conducted, 2 small scale single-center studies reported some improvements in NAFLD activity from serial liver biopsies [198]. Preliminary data from a recent RCT also suggested that ipragliflozin may benefit ballooning and fibrosis in a small Japanese cohort [199]. Among humans without diabetes, a recent clinical study reported improvement in hepatic fibrosis and steatosis (assessed using transient elastography) with 24 weeks of empagliflozin treatment, compared with placebo with no major adverse events [200].
10.2.3. Incretin mimetics and co-agonists
GLP-1 agonists mimic the action of the endogenously produced GLP-1 [13,188] and associate with—mostly transient—gastrointestinal discomfort and a questionable risk of acute pancreatitis [201]. Liraglutide was associated with significant relative hepatic TAG reduction of 31%, as assessed by MRI-PDFF after 6 months of treatment in 68 humans with uncontrolled T2DM [202] and with higher rates of histological resolution of NASH without worsening of fibrosis than with placebo in 26 humans with and without T2DM after 48 weeks [12], with transient, mild, or moderate side effects including diarrhea, constipation, and loss of appetite. In a subgroup analysis, liraglutide was reported to reduce adipose tissue, as well as hepatic insulin resistance and DNL [203]. Notably, another pre-specified secondary analysis showed that liraglutide did not reduce hepatic TAG accumulation in individuals with T2DM, when compared with placebo treatment added to standard care [204] (Table 3).
Exenatide treatment showed a reduction of hepatic TAG content among obese humans with T2DM in comparison to reference treatment [205] or no significant histologic features in biopsy-proven NAFLD [201], with 2 out of the 8 participants in this study reporting abdominal discomfort. Studies with other GLP-1 agonists such as semaglutide and dulaglutide are ongoing [13].
In a 26-week phase IIb study of cotasutide, a dual glucagon receptor/GLP-1 agonist showed superior results pertaining to body weight reduction and transaminases improvement than with liraglutide among overweight humans with T2DM [188]. Similarly, the glucose-dependent insulinotropic polypeptide/GLP-1 combined agonist, tirzepatide, improved NASH-related biomarkers and increased circulating adiponectin at 26 weeks of treatment in humans with T2DM [206].
10.2.4. Dipeptidyl peptidase-4 (DPP-4) inhibitors (DPP-4i)
DPP4i inhibit the degradation of GLP-1, prolonging the action of the endogenously produced GLP-1 [207], and have a low risk of adverse effects such as upper respiratory tract infections and maybe acute pancreatitis [208]. Sitagliptin failed to ameliorate hepatic steatosis and fibrosis in several human RCTs [[209], [210], [211], [212], [213]] (Table 3). However, when combined with metformin, 26-week sitagliptin treatment was equally efficient as liraglutide and superior to insulin glargine to reduce intrahepatic lipids in individuals with T2DM and NAFLD [214]. Vildagliptin treatment for 6 months decreased hepatic steatosis without serious adverse events in a double-blind RCT involving 44 patients with well-controlled T2DM [215], but evidence from other DPP-4i remains scarce (Table 3).
10.2.5. Metformin
Metformin is the drug of choice for the first-line management of T2DM, with gastrointestinal intolerance being the most frequently reported side effect [216]. Despite its beneficial effects on body weight reduction, metformin was not associated with improvement of liver histology in a 6-month RCT examining participants with NAFLD [217] (Table 3). A systematic review and meta-analysis of 9 RCTs confirmed the lack of improvement of NAFLD-related histological features [218]. Importantly, retrospective studies have suggested a reduction in the rates of hepatocellular carcinoma in metformin-treated humans [10], but no RCTs demonstrating the effect of metformin on hepatocellular carcinoma have been published.
10.2.6. α-glycosidase inhibitors
Inhibition of intestinal α-glycosidases flattens the postprandial rise of blood glucose by delaying dietary carbohydrate absorption, which also accounts for dose-related gastrointestinal adverse effects [219].
Acarbose was reported to improve liver fat content, and histology, when combined with ezetimibe [220,221].
A 12-week treatment with acarbose decreased elevated hepatic fat content, measured with 1H MRS in humans with metabolic syndrome [222]. Likewise, a 12-month treatment with miglitol revealed significant improvement in steatosis, lobular inflammation, portal inflammation scores, and NAFLD activity score in 17 humans with T2DM and histologically confirmed NASH [223].
10.3. Anti-inflammatory drugs
10.3.1. FXR agonists
FXR agonists improve cholestatic liver disease and are one of the most promising drug classes to treat NAFLD, with animal studies pointing to FXR-induced inhibition of DNL [224]. Treatment with obeticholic acid, a potent semisynthetic and selective FXR agonist, for 18 months resulted in fibrosis resolution without worsening of NASH in humans with F 2–3 [225] and in resolution of NASH without worsening of fibrosis in humans with F1. In another 76-week multicenter, RCT, obeticholic acid also improved NASH histology but caused major dose-dependent adverse events including pruritus in 23% of the participants and dyslipidemia [226] (Table 3). Thus, and because only 1 of 4 humans with NASH responds to treatment with FXR agonists [188], combination treatments with other drugs are being explored. Ongoing studies are also investigating other FXR agonists, such as cilofexor, EDP-305, and EYP 001, in human NASH [188].
10.3.2. CCR 2/5 antagonists
The anti-inflammatory and anti-fibrotic properties of these agents were the basis for the investigation of cenicriviroc, an oral, dual CCR2/CCR5 receptor inhibitor in the phase IIb CENTAUR trial. Cenicriviroc showed no effect on the primary outcome measure, namely, a ≥2-point improvement in NAS with no worsening of fibrosis at 1 year. Nevertheless, the primary endpoint was achieved in 24% of patients who switched to cenicriviroc and in 17% of those who remained on placebo. Importantly, 60% of patients who achieved fibrosis improvement at year 1 maintained it during the next year, 30% in the placebo arm [227,228]. (Table 3). The frequency of adverse events was comparable to placebo [227], except for a higher frequency of nasopharyngitis [228]. The efficacy and safety of cenicriviroc are being tested in patients with NASH in a phase III clinical trial [188] and as a combination treatment with the FXR agonist tropifexor in patients with NASH and F 2–3 fibrosis.
10.4. Metabolic enzyme inhibitors
10.4.1. SCD1 inhibitors
Aramchol is a liver-directed SCD1 modulator that in a 52-week placebo RCT promoted NASH resolution and fibrosis stage reduction in a dose–response manner with a favorable biochemical and safety profile [229] (Table 3). Notably, pruritus was reported in 6.9% and 11.2% with aramchol 400 mg and 600 mg daily, respectively, and in 6.3% with placebo, without changes in body weight or circulating lipids. These findings led to further testing in a phase III clinical trial.
10.4.2. ACC 1/2 inhibitors
ACC is a rate-controlling enzyme of DNL such that inhibition of ACC decreases the synthesis of PUFA and increases beta-oxidation in mitochondria because the product of ACC2, malonyl-CoA, inhibits carnitine palmitoyltransferase 1, facilitating mitochondrial FA uptake. Firsocostat (GS-0976), a dual ACC 1/2 inhibitor, led to an almost 30% relative reduction of hepatic TAG content in 126 patients with NASH after 12 weeks but also to an increase in plasma triglycerides, particularly in individuals with preexisting hypertriglyceridemia [230] (Table 3). The most common adverse events included nausea, abdominal pain, diarrhea, and headache [230]. The second-generation liver-targeted ACC inhibitor, PF-05221304, is also associated with increased serum triglyceride levels in healthy humans but only at doses inhibiting fructose-stimulated hepatic DNL by >90% [231]. This finding has stimulated the further investigation of optimal dosing and combination treatments.
10.4.3. Ketohexokinase inhibitors
Ketohexokinase, the principal enzyme responsible for fructose metabolism, catalyzes the conversion of fructose to fructose-1-phosphate, which channels dietary sugar into DNL. PF-06835919, a ketohexokinase inhibitor, was evaluated in a phase IIa RCT for its safety, tolerability, and pharmacodynamics in once-daily administration for 6 weeks in 47 humans with NAFLD. The trial reported greater mean reduction of hepatic TAG content, by MRI-PDFF, than and a profile for PF-06835919 similar to placebo [232].
10.5. Modulation of energy metabolism
10.5.1. Thyroid hormone receptor (THR)-β agonists
Hepatic THR-β is involved in hepatic lipid metabolism. Thyroid hormones exert their catabolic actions on hepatic lipids mediated through mobilization and subsequent beta-oxidation of hepatic FFA stored as TAG [233]. The phase II study with the THR-β agonist, VK2809, reported >50% relative reduction in hepatic TAG content in individuals with NAFLD, with a transient increase in transaminases [234]. The THR-β agonist resmetirom (MGL-3196) was similarly effective to reduce hepatic TAG content in biopsy-proven NASH along with a NASH resolution of 27%, 6% in the placebo group, over 12 and 36 weeks [235] (Table 3). The most commonly reported adverse effects were diarrhea and nausea, with no changes in routine laboratory parameters from baseline and between treatment arms [235]. Both agents are being investigated in further clinical studies [188], which also address the safety profile of these agents, given the report of impaired skeletal muscle insulin sensitivity in animal studies [236].
10.5.2. Mitochondrial pyruvate carrier inhibitors
Recent evidence points to the mitochondrial pyruvate carrier as an important mediator of nutritional overload signals. MSDC-0602K, a mitochondrial pyruvate carrier inhibitor, is a second-generation insulin sensitizer designed to prevent side effects of PPARγ agonists. A 52-week phase IIb RCT revealed no significant superiority in the primary histological liver outcome in humans with biopsy-proven NASH with F1-3 and no difference for the incidence of hypoglycemia and PPARγ agonist-associated side effects [237]. Because of an improved NAFLD activity score and favorable effects on metabolic parameters [237] (Table 3), MSDC-0602K is undergoing a phase III RCT in individuals with TD2M and NASH [188].
10.5.3. Fibroblast growth factor (FGF) analogs
FGF 21 stimulates mitochondrial beta-oxidation in hepatocytes and modulates lipid and glucose metabolism. FGF21 and its related subfamily member, FGF19, are potent modulators of bile acid metabolism and cholesterol metabolism. Pegbelfermin (BMS-986036), a pegylated subcutaneously administered recombinant FGF21 analog, decreased hepatic fat fraction in a 16-week phase II study of 75 overweight/obese participants, with generally mild adverse events, who most frequently reported diarrhea and nausea [238] (Table 3). The efficacy and safety of pegbelfermin are currently being tested in advanced NAFLD, NASH with bridging fibrosis, and NASH with compensated cirrhosis [188].
NGM-282, an engineered FGF-19 analog, significantly reduced the NAFLD activity score and fibrosis score above one stage, without worsening of steatohepatitis in 43 humans with histologically proven NASH upon 12 weeks’ treatment [239]. Diarrhea occurred as a relevant side effect, which might have contributed to the beneficial effect on the liver via body weight loss.
11. Conclusions
During recent years, the awareness of NAFLD has markedly increased, leading to discoveries of pathogenetic mechanisms and innovative therapeutic opportunities. On the other hand, current treatments do not advance much beyond the recommendation of sustained body weight loss. The lack of efficacy or adverse effects of treatments are probably contributing to the proportional global increase in metabolic diseases and NAFLD, making them a progressive pandemic, which poses an important health and economic burden and significantly affects health-related quality of life [240]. A proposal is to screen individuals with the highest genetic or acquired risk of disease progression, for example, the SIRD subgroup, initiate early prevention and treatment, and develop therapeutic concepts, targeting the earliest pathophysiolgical alterations, namely, adipocyte dysfunction and insulin resistance.
Author's contribution
Idea and concept, MR, KP; Literature search, KP, MR; Manuscript writing, KP, MR; Manuscript editing, KP, MR.
Acknowledgments/Funding
The research of the authors is supported in part by the Ministry of Science and Research of the State of North Rhine-Westfalia (MIWF NRW) and the German Federal Ministry of Health (BMG), as well as by a grant of the Federal Ministry for Research (BMBF) to the German Center for Diabetes Research (DZD e.V.) and the German Research Foundation (DFG, CRC 1116/2).
Conflict of interest
KP declares no conflicts of interest. MR is on the scientific advisory boards of Bristol-Myers Squibb, Eli Lilly, Gilead Sciences, NovoNordisk, Servier Laboratories, Target Pharmasolutions, and Terra Firma and receives investigator-initiated support from Boehringer Ingelheim, Nutricia/Danone, and Sanofi–Aventis.
References
- 1.Cotter T.G., Rinella M. Nonalcoholic fatty liver disease 2020: the State of the disease. Gastroenterology. 2020;158:1851–1864. doi: 10.1053/j.gastro.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 2.Roden M. Mechanisms of disease: hepatic steatosis in type 2 diabetes-pathogenesis and clinical relevance. Nature Clinical Practice Endocrinology & Metabolism. 2006;2:335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 3.Pafili K., Maltezos E., Papanas N. Ipragliflozin and sodium glucose transporter 2 inhibitors to reduce liver fat: will the prize we sought be won? Expert Opinion on Pharmacotherapy. 2018;19:185–187. doi: 10.1080/14656566.2017.1413346. [DOI] [PubMed] [Google Scholar]
- 4.Zaharia O.P., Strassburger K., Strom A., Bonhof G.J., Karusheva Y., Antoniou S. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. The Lancet Diabetes & Endocrinology. 2019;7:684–694. doi: 10.1016/S2213-8587(19)30187-1. [DOI] [PubMed] [Google Scholar]
- 5.Anstee Q.M., Targher G., Day C.P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nature Reviews Gastroenterology & Hepatology. 2013;10:330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed] [Google Scholar]
- 6.Targher G., Lonardo A., Byrne C.D. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nature Reviews Endocrinology. 2018;14:99–114. doi: 10.1038/nrendo.2017.173. [DOI] [PubMed] [Google Scholar]
- 7.Day C.P., James O.F. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 8.Imajo K., Yoneda M., Kessoku T., Ogawa Y., Maeda S., Sumida Y. Rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. International Journal of Molecular Sciences. 2013;14:21833–21857. doi: 10.3390/ijms141121833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tilg H., Moschen A.R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the L. European Association for the Study of D. European Association for the Study of O EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of Hepatology. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Kahl S., Gancheva S., Strassburger K., Herder C., Machann J., Katsuyama H. Empagliflozin effectively lowers liver fat content in well-controlled type 2 diabetes: a randomized, double-blind, phase 4, placebo-controlled trial. Diabetes Care. 2020;43:298–305. doi: 10.2337/dc19-0641. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 13.Dewidar B., Kahl S., Pafili K., Roden M. Metabolic liver disease in diabetes - from mechanisms to clinical trials. Metabolism. 2020;154299 doi: 10.1016/j.metabol.2020.154299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vusirikala A., Thomas T., Bhala N., Tahrani A.A., Thomas G.N., Nirantharakumar K. Impact of obesity and metabolic health status in the development of non-alcoholic fatty liver disease (NAFLD): a United Kingdom population-based cohort study using the health improvement network (THIN) BMC Endocrine Disorders. 2020;20:96. doi: 10.1186/s12902-020-00582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye Q., Zou B., Yeo Y.H., Li J., Huang D.Q., Wu Y. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2020;5:739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 16.Ahlqvist E., Storm P., Karajamaki A., Martinell M., Dorkhan M., Carlsson A. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. The Lancet Diabetes & Endocrinology. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 17.Oana P., Zaharia K.S., Knebel Birgit, Kupriyanova Yuliya, Karusheva Yanislava, Wolkersdorfer Martin, Bódis Kálmán. Role of patatin-like phospholipase domain–containing 3 gene for hepatic lipid content and insulin resistance in diabetes. Diabetes Care. 2020;43:2161–2168. doi: 10.2337/dc20-0329. [DOI] [PubMed] [Google Scholar]
- 18.Karajamaki A.J., Bloigu R., Kauma H., Kesaniemi Y.A., Koivurova O.P., Perkiomaki J. Non-alcoholic fatty liver disease with and without metabolic syndrome: different long-term outcomes. Metabolism. 2020;66:55–63. doi: 10.1016/j.metabol.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Wang Q, Sun Y, Zhao X, Kong Y, Ou X, et al. The prevalence of lean/nonobese nonalcoholic fatty liver disease: a systematic review and meta-analysis. Journal of Clinical Gastroenterology;54:378-387. [DOI] [PubMed]
- 20.Golabi P., Paik J.M., Arshad T., Younossi Y., Mishra A., Younossi Z.M. Mortality of NAFLD according to the body composition and presence of metabolic abnormalities. Hepatology Communications. 2020;4:1136–1148. doi: 10.1002/hep4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loomba R., Schork N., Chen C.H., Bettencourt R., Bhatt A., Ang B. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 2015;149:1784–1793. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sookoian S., Pirola C.J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 23.Danford C.J., Yao Z.M., Jiang Z.G. Non-alcoholic fatty liver disease: a narrative review of genetics. Journal of Biomedical Research. 2018;32:389–400. doi: 10.7555/JBR.32.20180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peter A., Kovarova M., Nadalin S., Cermak T., Konigsrainer A., Machicao F. PNPLA3 variant I148M is associated with altered hepatic lipid composition in humans. Diabetologia. 2014;57:2103–2107. doi: 10.1007/s00125-014-3310-0. [DOI] [PubMed] [Google Scholar]
- 25.Dongiovanni P., Petta S., Maglio C., Fracanzani A.L., Pipitone R., Mozzi E. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 26.Donati B., Motta B.M., Pingitore P., Meroni M., Pietrelli A., Alisi A. The rs2294918 E434K variant modulates patatin-like phospholipase domain-containing 3 expression and liver damage. Hepatology. 2016;63:787–798. doi: 10.1002/hep.28370. [DOI] [PubMed] [Google Scholar]
- 27.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishioji K., Mochizuki N., Kobayashi M., Kamaguchi M., Sumida Y., Nishimura T. The impact of PNPLA3 rs738409 genetic polymorphism and weight gain >/=10 kg after age 20 on non-alcoholic fatty liver disease in non-obese Japanese individuals. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seko Y., Yamaguchi K., Itoh Y. The genetic backgrounds in nonalcoholic fatty liver disease. Clinical Journal of Gastroenterology. 2018;11:97–102. doi: 10.1007/s12328-018-0841-9. [DOI] [PubMed] [Google Scholar]
- 30.Carpino G., Pastori D., Baratta F., Overi D., Labbadia G., Polimeni L. PNPLA3 variant and portal/periportal histological pattern in patients with biopsy-proven non-alcoholic fatty liver disease: a possible role for oxidative stress. Scientific Reports. 2017;7:15756. doi: 10.1038/s41598-017-15943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotman Y., Koh C., Zmuda J.M., Kleiner D.E., Liang T.J., Nash C.R.N. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H., Lee K.W., Lee K., Seo S., Park M.Y., Ahn S.W. Effect of PNPLA3 I148M polymorphism on histologically proven non-alcoholic fatty liver disease in liver transplant recipients. Hepatology Research. 2018;48:E162–E171. doi: 10.1111/hepr.12940. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi T., Sumida Y., Umemura A., Matsuo K., Takahashi M., Takamura T. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anstee Q.M., Darlay R., Cockell S., Meroni M., Govaere O., Tiniakos D. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort. Journal of Hepatology. 2020;73:505–515. doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Krawczyk M., Rau M., Schattenberg J.M., Bantel H., Pathil A., Demir M. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. The Journal of Lipid Research. 2017;58:247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sookoian S., Castano G.O., Burgueno A.L., Gianotti T.F., Rosselli M.S., Pirola C.J. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. The Journal of Lipid Research. 2009;50:2111–2116. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luukkonen P.K., Nick A., Holtta-Vuori M., Thiele C., Isokuortti E., Lallukka-Bruck S. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahdessian H., Taxiarchis A., Popov S., Silveira A., Franco-Cereceda A., Hamsten A. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proceedings of the National Academy of Sciences of the U S A. 2014;111:8913–8918. doi: 10.1073/pnas.1323785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smagris E., Gilyard S., BasuRay S., Cohen J.C., Hobbs H.H. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. Journal of Biological Chemistry. 2016;291:10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y.L., Reeves H.L., Burt A.D., Tiniakos D., McPherson S., Leathart J.B. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nature Communications. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen F., Esmaili S., Rogers G.B., Bugianesi E., Petta S., Marchesini G. Lean NAFLD: a distinct entity shaped by differential metabolic adaptation. Hepatology. 2020;71:1213–1227. doi: 10.1002/hep.30908. [DOI] [PubMed] [Google Scholar]
- 42.Raimondo A., Rees M.G., Gloyn A.L. Glucokinase regulatory protein: complexity at the crossroads of triglyceride and glucose metabolism. Current Opinion in Lipidology. 2015;26:88–95. doi: 10.1097/MOL.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beer N.L., Tribble N.D., McCulloch L.J., Roos C., Johnson P.R., Orho-Melander M. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Human Molecular Genetics. 2009;18:4081–4088. doi: 10.1093/hmg/ddp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petta S., Miele L., Bugianesi E., Camma C., Rosso C., Boccia S. Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan H.L., Zain S.M., Mohamed R., Rampal S., Chin K.F., Basu R.C. Association of glucokinase regulatory gene polymorphisms with risk and severity of non-alcoholic fatty liver disease: an interaction study with adiponutrin gene. Journal of Gastroenterology. 2014;49:1056–1064. doi: 10.1007/s00535-013-0850-x. [DOI] [PubMed] [Google Scholar]
- 46.Santoro N., Zhang C.K., Zhao H., Pakstis A.J., Kim G., Kursawe R. Variant in the glucokinase regulatory protein (GCKR) gene is associated with fatty liver in obese children and adolescents. Hepatology. 2012;55:781–789. doi: 10.1002/hep.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mancina R.M., Dongiovanni P., Petta S., Pingitore P., Meroni M., Rametta R. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150:1219–1230. doi: 10.1053/j.gastro.2016.01.032. e1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luukkonen P.K., Zhou Y., Hyotylainen T., Leivonen M., Arola J., Orho-Melander M. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. Journal of Hepatology. 2016;65:1263–1265. doi: 10.1016/j.jhep.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 49.Dongiovanni P., Valenti L., Rametta R., Daly A.K., Nobili V., Mozzi E. Genetic variants regulating insulin receptor signalling are associated with the severity of liver damage in patients with non-alcoholic fatty liver disease. Gut. 2010;59:267–273. doi: 10.1136/gut.2009.190801. [DOI] [PubMed] [Google Scholar]
- 50.Al-Serri A., Anstee Q.M., Valenti L., Nobili V., Leathart J.B., Dongiovanni P. The SOD2 C47T polymorphism influences NAFLD fibrosis severity: evidence from case-control and intra-familial allele association studies. Journal of Hepatology. 2012;56:448–454. doi: 10.1016/j.jhep.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 51.Petta S., Valenti L., Marra F., Grimaudo S., Tripodo C., Bugianesi E. MERTK rs4374383 polymorphism affects the severity of fibrosis in non-alcoholic fatty liver disease. Journal of Hepatology. 2016;64:682–690. doi: 10.1016/j.jhep.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 52.Petersen K.F., Dufour S., Hariri A., Nelson-Williams C., Foo J.N., Zhang X.M. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. New England Journal of Medicine. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musso G., Cassader M., De Michieli F., Paschetta E., Pinach S., Saba F. MERTK rs4374383 variant predicts incident nonalcoholic fatty liver disease and diabetes: role of mononuclear cell activation and adipokine response to dietary fat. Human Molecular Genetics. 2017;26:1747–1758. doi: 10.1093/hmg/ddw400. [DOI] [PubMed] [Google Scholar]
- 54.Fares R., Petta S., Lombardi R., Grimaudo S., Dongiovanni P., Pipitone R. The UCP2 -866 G>A promoter region polymorphism is associated with nonalcoholic steatohepatitis. Liver International. 2015;35:1574–1580. doi: 10.1111/liv.12707. [DOI] [PubMed] [Google Scholar]
- 55.Thangapandi V.R., Knittelfelder O., Brosch M., Patsenker E., Vvedenskaya O., Buch S. Loss of hepatic Mboat7 leads to liver fibrosis. Gut. 2020 doi: 10.1136/gutjnl-2020-320853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luukkonen P.K., Tukiainen T., Juuti A., Sammalkorpi H., Pan Haridas, Niemela O. Hydroxysteroid 17-beta dehydrogenase 13 variant increases phospholipids and protects against fibrosis in nonalcoholic fatty liver disease. JCI Insight. 2020;5 doi: 10.1172/jci.insight.132158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abul-Husn N.S., Cheng X., Li A.H., Xin Y., Schurmann C., Stevis P. A protein-truncating HSD17B13 variant and protection from chronic liver disease. New England Journal of Medicine. 2018;378:1096–1106. doi: 10.1056/NEJMoa1712191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y., Belyaeva O.V., Brown P.M., Fujita K., Valles K., Karki S. 17-Beta hydroxysteroid dehydrogenase 13 is a hepatic retinol dehydrogenase associated with histological features of nonalcoholic fatty liver disease. Hepatology. 2019;69:1504–1519. doi: 10.1002/hep.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Speliotes E.K., Yerges-Armstrong L.M., Wu J., Hernaez R., Kim L.J., Palmer C.D. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genetics. 2011;7 doi: 10.1371/journal.pgen.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petta S., Valenti L., Svegliati-Baroni G., Ruscica M., Pipitone R.M., Dongiovanni P. Fibronectin type III domain-containing protein 5 rs3480 A>G polymorphism, irisin, and liver fibrosis in patients with nonalcoholic fatty liver disease. Journal of Clinical Endocrinology & Metabolism. 2017;102:2660–2669. doi: 10.1210/jc.2017-00056. [DOI] [PubMed] [Google Scholar]
- 61.Metwally M., Bayoumi A., Romero-Gomez M., Thabet K., John M., Adams L.A. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3'UTR. Journal of Hepatology. 2019;70:494–500. doi: 10.1016/j.jhep.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 62.Schwerbel K., Kamitz A., Krahmer N., Hallahan N., Jahnert M., Gottmann P. Immunity-related GTPase induces lipophagy to prevent excess hepatic lipid accumulation. Journal of Hepatology. 2020 doi: 10.1016/j.jhep.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seitz S., Kwon Y., Hartleben G., Julg J., Sekar R., Krahmer N. Hepatic Rab24 controls blood glucose homeostasis via improving mitochondrial plasticity. Nature Metabolism. 2019;1:1009–1026. doi: 10.1038/s42255-019-0124-x. [DOI] [PubMed] [Google Scholar]
- 64.Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H.E., Larsson A. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- 65.Bjermo H., Iggman D., Kullberg J., Dahlman I., Johansson L., Persson L. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. American Journal of Clinical Nutrition. 2012;95:1003–1012. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 66.Koopman K.E., Caan M.W., Nederveen A.J., Pels A., Ackermans M.T., Fliers E. Hypercaloric diets with increased meal frequency, but not meal size, increase intrahepatic triglycerides: a randomized controlled trial. Hepatology. 2014;60:545–553. doi: 10.1002/hep.27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnston R.D., Stephenson M.C., Crossland H., Cordon S.M., Palcidi E., Cox E.F. No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology. 2013;145:1016–1025 e1012. doi: 10.1053/j.gastro.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Maersk M., Belza A., Stodkilde-Jorgensen H., Ringgaard S., Chabanova E., Thomsen H. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. American Journal of Clinical Nutrition. 2012;95:283–289. doi: 10.3945/ajcn.111.022533. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez E.A., Kahl S., Seelig A., Begovatz P., Irmler M., Kupriyanova Y. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. Journal of Clinical Investigation. 2017;27:695–708. doi: 10.1172/JCI89444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bortolotti M., Kreis R., Debard C., Cariou B., Faeh D., Chetiveaux M. High protein intake reduces intrahepatocellular lipid deposition in humans. American Journal of Clinical Nutrition. 2009;90:1002–1010. doi: 10.3945/ajcn.2008.27296. [DOI] [PubMed] [Google Scholar]
- 71.Sarabhai T., Kahl S., Szendroedi J., Markgraf D.F., Zaharia O.P., Barosa C. Monounsaturated fat rapidly induces hepatic gluconeogenesis and whole-body insulin resistance. JCI Insight. 2020;5 doi: 10.1172/jci.insight.134520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roden M., Shulman G.I. The integrative biology of type 2 diabetes. Nature. 2019;576:51–60. doi: 10.1038/s41586-019-1797-8. [DOI] [PubMed] [Google Scholar]
- 73.Ngo Sock E.T., Le K.A., Ith M., Kreis R., Boesch C., Tappy L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. British Journal of Nutrition. 2010;103:939–943. doi: 10.1017/S0007114509992819. [DOI] [PubMed] [Google Scholar]
- 74.Theytaz F., Noguchi Y., Egli L., Campos V., Buehler T., Hodson L. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. American Journal of Clinical Nutrition. 2012;96:1008–1016. doi: 10.3945/ajcn.112.035139. [DOI] [PubMed] [Google Scholar]
- 75.Le K.A., Ith M., Kreis R., Faeh D., Bortolotti M., Tran C. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. American Journal of Clinical Nutrition. 2009;89:1760–1765. doi: 10.3945/ajcn.2008.27336. [DOI] [PubMed] [Google Scholar]
- 76.Chiu S., Sievenpiper J.L., de Souza R.J., Cozma A.I., Mirrahimi A., Carleton A.J. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. European Journal of Clinical Nutrition. 2014;68:416–423. doi: 10.1038/ejcn.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chung M., Ma J., Patel K., Berger S., Lau J., Lichtenstein A.H. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. American Journal of Clinical Nutrition. 2014;100:833–849. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Herpen N.A., Schrauwen-Hinderling V.B., Schaart G., Mensink R.P., Schrauwen P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. Journal of Clinical Endocrinology & Metabolism. 2011;96:E691–E695. doi: 10.1210/jc.2010-2243. [DOI] [PubMed] [Google Scholar]
- 79.Westerbacka J., Lammi K., Hakkinen A.M., Rissanen A., Salminen I., Aro A. Dietary fat content modifies liver fat in overweight nondiabetic subjects. Journal of Clinical Endocrinology & Metabolism. 2005;90:2804–2809. doi: 10.1210/jc.2004-1983. [DOI] [PubMed] [Google Scholar]
- 80.Luukkonen P.K., Sadevirta S., Zhou Y., Kayser B., Ali A., Ahonen L. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zelber-Sagi S., Ivancovsky-Wajcman D., Fliss Isakov N., Webb M., Orenstein D., Shibolet O. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. Journal of Hepatology. 2018;68:1239–1246. doi: 10.1016/j.jhep.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 82.Cantero I., Abete I., Monreal J.I., Martinez J.A., Zulet M.A. Fruit fiber consumption specifically improves liver health status in obese subjects under energy restriction. Nutrients. 2017;9 doi: 10.3390/nu9070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loomba R., Seguritan V., Li W., Long T., Klitgord N., Bhatt A. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metabolism. 2017;25:1054–1062. doi: 10.1016/j.cmet.2017.04.001. e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu L., Baker S.S., Gill C., Liu W., Alkhouri R., Baker R.D. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–609. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 85.Lieber C.S. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968-1998)-a review. Alcoholism: Clinical and Experimental Research. 1999;23:991–1007. [PubMed] [Google Scholar]
- 86.Zhang X., Ji X., Wang Q., Li J.Z. New insight into inter-organ crosstalk contributing to the pathogenesis of non-alcoholic fatty liver disease (NAFLD) Protein Cell. 2018;9:164–177. doi: 10.1007/s13238-017-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miele L., Valenza V., La Torre G., Montalto M., Cammarota G., Ricci R. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 88.Carpino G., Del Ben M., Pastori D., Carnevale R., Baratta F., Overi D. Increased liver localization of lipopolysaccharides in human and experimental NAFLD. Hepatology. 2019;72:470–485. doi: 10.1002/hep.31056. [DOI] [PubMed] [Google Scholar]
- 89.Bashiardes S., Shapiro H., Rozin S., Shibolet O., Elinav E. Non-alcoholic fatty liver and the gut microbiota. Molecular Metabolism. 2016;5:782–794. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sookoian S., Salatino A., Castano G.O., Landa M.S., Fijalkowky C., Garaycoechea M. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut. 2020;69:1483–1491. doi: 10.1136/gutjnl-2019-318811. [DOI] [PubMed] [Google Scholar]
- 91.Bedossa P., Tordjman J., Aron-Wisnewsky J., Poitou C., Oppert J.M., Torcivia A. Systematic review of bariatric surgery liver biopsies clarifies the natural history of liver disease in patients with severe obesity. Gut. 2017;66:1688–1696. doi: 10.1136/gutjnl-2016-312238. [DOI] [PubMed] [Google Scholar]
- 92.Ter Horst K.W., Gilijamse P.W., Versteeg R.I., Ackermans M.T., Nederveen A.J., la Fleur S.E. Hepatic diacylglycerol-associated protein kinase cepsilon translocation links hepatic steatosis to hepatic insulin resistance in humans. Cell Reports. 2017;19:1997–2004. doi: 10.1016/j.celrep.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Journal of Clinical Investigation. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang X.D., Fan Y., Zhang H., Wang P., Yuan J.P., Li M.J. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World Journal of Gastroenterology. 2008;14:2888–2893. doi: 10.3748/wjg.14.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Angulo P., Alba L.M., Petrovic L.M., Adams L.A., Lindor K.D., Jensen M.D. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. Journal of Hepatology. 2004;41:943–949. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 96.Polyzos S.A., Kountouras J., Mantzoros C.S. Adipokines in nonalcoholic fatty liver disease. Metabolism. 2016;65:1062–1079. doi: 10.1016/j.metabol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Ferrante S.C., Nadler E.P., Pillai D.K., Hubal M.J., Wang Z., Wang J.M. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatric Research. 2015;77:447–454. doi: 10.1038/pr.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ipsen D.H., Lykkesfeldt J., Tveden-Nyborg P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cellular and Molecular Life Sciences. 2018;75:3313–3327. doi: 10.1007/s00018-018-2860-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu L., Baker S.S., Liu W., Tao M.H., Patel R., Nowak N.J. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 2011;60:1001–1011. doi: 10.1016/j.metabol.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Miquilena-Colina M.E., Lima-Cabello E., Sanchez-Campos S., Garcia-Mediavilla M.V., Fernandez-Bermejo M., Lozano-Rodriguez T. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60:1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 101.Enooku K., Tsutsumi T., Kondo M., Fujiwara N., Sasako T., Shibahara J. Hepatic FATP5 expression is associated with histological progression and loss of hepatic fat in NAFLD patients. Journal of Gastroenterology. 2020;55:227–243. doi: 10.1007/s00535-019-01633-2. [DOI] [PubMed] [Google Scholar]
- 102.Lambert J.E., Ramos-Roman M.A., Browning J.D., Parks E.J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726–735. doi: 10.1053/j.gastro.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Diraison F., Moulin P., Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes & Metabolism. 2003;29:478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 105.Diraison F., Yankah V., Letexier D., Dusserre E., Jones P., Beylot M. Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. The Journal of Lipid Research. 2003;44:846–853. doi: 10.1194/jlr.M200461-JLR200. [DOI] [PubMed] [Google Scholar]
- 106.Hellerstein M.K., Christiansen M., Kaempfer S., Kletke C., Wu K., Reid J.S. Measurement of de novo hepatic lipogenesis in humans using stable isotopes. Journal of Clinical Investigation. 1991;87:1841–1852. doi: 10.1172/JCI115206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marques-Lopes I., Ansorena D., Astiasaran I., Forga L., Martinez J.A. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. American Journal of Clinical Nutrition. 2001;73:253–261. doi: 10.1093/ajcn/73.2.253. [DOI] [PubMed] [Google Scholar]
- 108.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2020;130:1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Caballero F., Fernandez A., De Lacy A.M., Fernandez-Checa J.C., Caballeria J., Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. Journal of Hepatology. 2009;50:789–796. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 110.Brown M.S., Goldstein J.L. Selective versus total insulin resistance: a pathogenic paradox. Cell Metabolism. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 111.Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. Journal of Clinical Investigation. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Higuchi N., Kato M., Shundo Y., Tajiri H., Tanaka M., Yamashita N. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatology Research. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 113.Kumashiro N., Erion D.M., Zhang D., Kahn M., Beddow S.A., Chu X. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Magkos F., Su X., Bradley D., Fabbrini E., Conte C., Eagon J.C. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142:1444–1446. doi: 10.1053/j.gastro.2012.03.003. e1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luukkonen P.K., Zhou Y., Sadevirta S., Leivonen M., Arola J., Oresic M. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. Journal of Hepatology. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 116.Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. Journal of Clinical Investigation. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jelenik T., Kaul K., Sequaris G., Flogel U., Phielix E., Kotzka J. Mechanisms of insulin resistance in primary and secondary nonalcoholic fatty liver. Diabetes. 2017;66:2241–2253. doi: 10.2337/db16-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Apostolopoulou M., Gordillo R., Koliaki C., Gancheva S., Jelenik T., Filippo E.D. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steatohepatitis. Diabetes Care. 2018;41:1235–1243. doi: 10.2337/dc17-1318. [DOI] [PubMed] [Google Scholar]
- 119.Yki-Jarvinen H. Ceramides: a cause of insulin resistance in nonalcoholic fatty liver disease in both murine models and humans. Hepatology. 2020;71:1499–1501. doi: 10.1002/hep.31095. [DOI] [PubMed] [Google Scholar]
- 120.Paradis V., Perlemuter G., Bonvoust F., Dargere D., Parfait B., Vidaud M. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738–744. doi: 10.1053/jhep.2001.28055. [DOI] [PubMed] [Google Scholar]
- 121.Koliaki C., Szendroedi J., Kaul K., Jelenik T., Nowotny P., Jankowiak F. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metabolism. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 122.Cortez-Pinto H., Chatham J., Chacko V.P., Arnold C., Rashid A., Diehl A.M. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA. 1999;282:1659–1664. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 123.Traussnigg S., Kienbacher C., Gajdosik M., Valkovic L., Halilbasic E., Stift J. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver International. 2017;37:1544–1553. doi: 10.1111/liv.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szendroedi J., Chmelik M., Schmid A.I., Nowotny P., Brehm A., Krssak M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology. 2009;50:1079–1086. doi: 10.1002/hep.23093. [DOI] [PubMed] [Google Scholar]
- 125.Fritsch M., Koliaki C., Livingstone R., Phielix E., Bierwagen A., Meisinger M. Time course of postprandial hepatic phosphorus metabolites in lean, obese, and type 2 diabetes patients. American Journal of Clinical Nutrition. 2015;102:1051–1058. doi: 10.3945/ajcn.115.107599. [DOI] [PubMed] [Google Scholar]
- 126.Schmid A.I., Szendroedi J., Chmelik M., Krssak M., Moser E., Roden M. Liver ATP synthesis is lower and relates to insulin sensitivity in patients with type 2 diabetes. Diabetes Care. 2011;34:448–453. doi: 10.2337/dc10-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lake A.D., Novak P., Hardwick R.N., Flores-Keown B., Zhao F., Klimecki W.T. The adaptive endoplasmic reticulum stress response to lipotoxicity in progressive human nonalcoholic fatty liver disease. Toxicological Sciences. 2014;137:26–35. doi: 10.1093/toxsci/kft230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takaki A., Kawai D., Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) International Journal of Molecular Sciences. 2013;14:20704–20728. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Forrester S.J., Kikuchi D.S., Hernandes M.S., Xu Q., Griendling K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circulation Research. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li J.L., Wang Q.Y., Luan H.Y., Kang Z.C., Wang C.B. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. Journal of Biomedical Sciences. 2012;19:32. doi: 10.1186/1423-0127-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the U S A. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adiels M., Taskinen M.R., Packard C., Caslake M.J., Soro-Paavonen A., Westerbacka J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 135.Kawano Y., Cohen D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. Journal of Gastroenterology. 2013;48:434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Charlton M., Sreekumar R., Rasmussen D., Lindor K., Nair K.S. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 137.Higuchi N., Kato M., Tanaka M., Miyazaki M., Takao S., Kohjima M. Effects of insulin resistance and hepatic lipid accumulation on hepatic mRNA expression levels of apoB, MTP and L-FABP in non-alcoholic fatty liver disease. Experimental and Therapeutic Medicine. 2011;2:1077–1081. doi: 10.3892/etm.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fujita K., Nozaki Y., Wada K., Yoneda M., Fujimoto Y., Fujitake M. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology (Baltimore, Md.) 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 139.Nagaya T., Tanaka N., Suzuki T., Sano K., Horiuchi A., Komatsu M. Down-regulation of SREBP-1c is associated with the development of burned-out NASH. Journal of Hepatology. 2010;53:724–731. doi: 10.1016/j.jhep.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 140.Ignat S.R., Dinescu S., Hermenean A., Costache M. Cellular interplay as a consequence of inflammatory signals leading to liver fibrosis development. Cells. 2020;9:461. doi: 10.3390/cells9020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kazankov K., Jorgensen S.M.D., Thomsen K.L., Moller H.J., Vilstrup H., George J. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nature Reviews Gastroenterology & Hepatology. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 143.Das S., Alhasson F., Dattaroy D., Pourhoseini S., Seth R.K., Nagarkatti M. NADPH oxidase-derived peroxynitrite drives inflammation in mice and human nonalcoholic steatohepatitis via TLR4-lipid raft recruitment. American Journal of Pathology. 2015;185:1944–1957. doi: 10.1016/j.ajpath.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwabe R.F., Bataller R., Brenner D.A. Human hepatic stellate cells express CCR5 and RANTES to induce proliferation and migration. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2003;285:G949–G958. doi: 10.1152/ajpgi.00215.2003. [DOI] [PubMed] [Google Scholar]
- 145.Kawada N. Cytoglobin as a marker of hepatic stellate cell-derived myofibroblasts. Frontiers in Physiology. 2015;6:329. doi: 10.3389/fphys.2015.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wobser H., Dorn C., Weiss T.S., Amann T., Bollheimer C., Buttner R. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Research. 2009;19:996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- 147.Zehra M., Curry J.C., Pillai S.S., Lakhani H.V., Edwards C.E., Sodhi K. Elucidating potential profibrotic mechanisms of emerging biomarkers for early prognosis of hepatic fibrosis. International Journal of Molecular Sciences. 2020;21:4737. doi: 10.3390/ijms21134737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu L., Hui A.Y., Albanis E., Arthur M.J., O'Byrne S.M., Blaner W.S. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schuppan D., Surabattula R., Wang X.Y. Determinants of fibrosis progression and regression in NASH. Journal of Hepatology. 2018;68:238–250. doi: 10.1016/j.jhep.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 150.Lorenz L., Axnick J., Buschmann T., Henning C., Urner S., Fang S. Mechanosensing by beta1 integrin induces angiocrine signals for liver growth and survival. Nature. 2018;562:128–132. doi: 10.1038/s41586-018-0522-3. [DOI] [PubMed] [Google Scholar]
- 151.Lebeaupin C., Proics E., de Bieville C.H., Rousseau D., Bonnafous S., Patouraux S. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death & Disease. 2015;6 doi: 10.1038/cddis.2015.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ford A.J., Jain G., Rajagopalan P. Designing a fibrotic microenvironment to investigate changes in human liver sinusoidal endothelial cell function. Acta Biomaterialia. 2015;24:220–227. doi: 10.1016/j.actbio.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 153.De Lucia Rolfe E., Brage S., Sleigh A., Finucane F., Griffin S.J., Wareham N.J. Validity of ultrasonography to assess hepatic steatosis compared to magnetic resonance spectroscopy as a criterion method in older adults. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kahl S., Strassburger K., Nowotny B., Livingstone R., Kluppelholz B., Kessel K. Comparison of liver fat indices for the diagnosis of hepatic steatosis and insulin resistance. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Idilman I.S., Keskin O., Celik A., Savas B., Elhan A.H., Idilman R. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiologica. 2016;57:271–278. doi: 10.1177/0284185115580488. [DOI] [PubMed] [Google Scholar]
- 156.Kabisch S., Bather S., Dambeck U., Kemper M., Gerbracht C., Honsek C. Liver fat scores moderately reflect interventional changes in liver fat content by a low-fat diet but not by a low-carb diet. Nutrients. 2018;10 doi: 10.3390/nu10020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bril F., McPhaul M.J., Caulfield M.P., Clark V.C., Soldevilla-Pico C., Firpi-Morell R.J. Performance of plasma biomarkers and diagnostic panels for nonalcoholic steatohepatitis and advanced fibrosis in patients with type 2 diabetes. Diabetes Care. 2020;43:290–297. doi: 10.2337/dc19-1071. [DOI] [PubMed] [Google Scholar]
- 158.Yki-Jarvinen H. Nutritional modulation of non-alcoholic fatty liver disease and insulin resistance. Nutrients. 2015;7:9127–9138. doi: 10.3390/nu7115454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romero-Gomez M., Zelber-Sagi S., Trenell M. Treatment of NAFLD with diet, physical activity and exercise. Journal of Hepatology. 2017;67:829–846. doi: 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 160.Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haufe S., Engeli S., Kast P., Bohnke J., Utz W., Haas V. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology. 2011;53:1504–1514. doi: 10.1002/hep.24242. [DOI] [PubMed] [Google Scholar]
- 162.Chen Y., Feng R., Yang X., Dai J., Huang M., Ji X. Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. American Journal of Clinical Nutrition. 2019;109:1611–1619. doi: 10.1093/ajcn/nqy358. [DOI] [PubMed] [Google Scholar]
- 163.Markova M., Pivovarova O., Hornemann S., Sucher S., Frahnow T., Wegner K. Isocaloric diets high in animal or plant protein reduce liver fat and inflammation in individuals with type 2 diabetes. Gastroenterology. 2017;152:571–585 e578. doi: 10.1053/j.gastro.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 164.Properzi C., O'Sullivan T.A., Sherriff J.L., Ching H.L., Jeffrey G.P., Buckley R.F. Ad libitum mediterranean and low-fat diets both significantly reduce hepatic steatosis: a randomized controlled trial. Hepatology. 2018;68:1741–1754. doi: 10.1002/hep.30076. [DOI] [PubMed] [Google Scholar]
- 165.Abenavoli L., Greco M., Milic N., Accattato F., Foti D., Gulletta E. Effect of mediterranean diet and antioxidant formulation in non-alcoholic fatty liver disease: a randomized study. Nutrients. 2017;9 doi: 10.3390/nu9080870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ryan M.C., Itsiopoulos C., Thodis T., Ward G., Trost N., Hofferberth S. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. Journal of Hepatology. 2013;59:138–143. doi: 10.1016/j.jhep.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 167.Sanyal A.J., Abdelmalek M.F., Suzuki A., Cummings O.W., Chojkier M., Group E.A.S. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–384 e371. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 168.Nogueira M.A., Oliveira C.P., Ferreira Alves V.A., Stefano J.T., Rodrigues L.S., Torrinhas R.S. Omega-3 polyunsaturated fatty acids in treating non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. Clinical Nutrition. 2016;35:578–586. doi: 10.1016/j.clnu.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 169.Ahn J., Jun D.W., Lee H.Y., Moon J.H. Critical appraisal for low-carbohydrate diet in nonalcoholic fatty liver disease: review and meta-analyses. Clinical Nutrition. 2019;38:2023–2030. doi: 10.1016/j.clnu.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 170.Katsagoni C.N., Georgoulis M., Papatheodoridis G.V., Panagiotakos D.B., Kontogianni M.D. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism. 2017;68:119–132. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 171.Nowotny B., Zahiragic L., Bierwagen A., Kabisch S., Groener J.B., Nowotny P.J. Low-energy diets differing in fibre, red meat and coffee intake equally improve insulin sensitivity in type 2 diabetes: a randomised feasibility trial. Diabetologia. 2015;58:255–264. doi: 10.1007/s00125-014-3457-8. [DOI] [PubMed] [Google Scholar]
- 172.Skytte M.J., Samkani A., Petersen A.D., Thomsen M.N., Astrup A., Chabanova E. A carbohydrate-reduced high-protein diet improves HbA1c and liver fat content in weight stable participants with type 2 diabetes: a randomised controlled trial. Diabetologia. 2019;62:2066–2078. doi: 10.1007/s00125-019-4956-4. [DOI] [PubMed] [Google Scholar]
- 173.Perdomo C.M., Fruhbeck G., Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11 doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Etemadi A., Sinha R., Ward M.H., Graubard B.I., Inoue-Choi M., Dawsey S.M. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. doi: 10.1136/bmj.j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.El-Agroudy N.N., Kurzbach A., Rodionov R.N., O'Sullivan J., Roden M., Birkenfeld A.L. Are lifestyle therapies effective for NAFLD treatment? Trends in Endocrinology and Metabolism. 2019;30:701–709. doi: 10.1016/j.tem.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 176.Hallsworth K., Fattakhova G., Hollingsworth K.G., Thoma C., Moore S., Taylor R. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60:1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pesta D., Roden M. The Janus head of oxidative stress in metabolic diseases and during physical exercise. Current Diabetes Reports. 2017;17:41. doi: 10.1007/s11892-017-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee Y., Doumouras A.G., Yu J., Brar K., Banfield L., Gmora S. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clinical Gastroenterology and Hepatology. 2019;17:1040–1060 e1011. doi: 10.1016/j.cgh.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 179.Gancheva S., Ouni M., Jelenik T., Koliaki C., Szendroedi J., Toledo F.G.S. Dynamic changes of muscle insulin sensitivity after metabolic surgery. Nature Communications. 2019;10:4179. doi: 10.1038/s41467-019-12081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ahrens M., Ammerpohl O., von Schonfels W., Kolarova J., Bens S., Itzel T. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metabolism. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 181.Ribeireiro T., Swain J., Sarr M., Kendrick M., Que F., Sanderson S. NAFLD and insulin resistance do not increase the risk of postoperative complications among patients undergoing bariatric surgery-a prospective analysis. Obesity Surgery. 2011;21:310–315. doi: 10.1007/s11695-010-0228-6. [DOI] [PubMed] [Google Scholar]
- 182.Boeckmans J., Natale A., Rombaut M., Buyl K., Rogiers V., De Kock J. Anti-NASH drug development hitches a lift on PPAR agonism. Cells. 2019;9:37. doi: 10.3390/cells9010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. New England Journal of Medicine. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Aithal G.P., Thomas J.A., Kaye P.V., Lawson A., Ryder S.D., Spendlove I. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–1184. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 185.Belfort R., Harrison S.A., Brown K., Darland C., Finch J., Hardies J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. New England Journal of Medicine. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 186.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C. Long-Term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Annals of Internal Medicine. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 187.Lee Y.H., Kim J.H., Kim S.R., Jin H.Y., Rhee E.J., Cho Y.M. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. Journal of Korean Medical Science. 2017;32:60–69. doi: 10.3346/jkms.2017.32.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dufour J.F., Caussy C., Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut. 2020;69:1877–1884. doi: 10.1136/gutjnl-2019-319104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ratziu V., Harrison S.A., Francque S., Bedossa P., Lehert P., Serfaty L. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-alpha and -delta, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology. 2016;150:1147–1159. doi: 10.1053/j.gastro.2016.01.038. e1145. [DOI] [PubMed] [Google Scholar]
- 190.Pafili K., Maltezos E., Papanas N. The potential of SGLT2 inhibitors in phase II clinical development for treating type 2 diabetes. Expert Opinion on Investigational Drugs. 2016;25:1133–1152. doi: 10.1080/13543784.2016.1216970. [DOI] [PubMed] [Google Scholar]
- 191.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Kober L., Kosiborod M.N., Martinez F.A. Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 192.Pafili K., Papanas N. Sodium-glucose cotransporter-2 inhibitors in type 2 diabetes: a magic potion to reduce heart failure? Expert Review of Clinical Pharmacology. 2019;12:693–695. doi: 10.1080/17512433.2019.1635453. [DOI] [PubMed] [Google Scholar]
- 193.Latva-Rasku A., Honka M.J., Kullberg J., Mononen N., Lehtimaki T., Saltevo J. The SGLT2 inhibitor dapagliflozin reduces liver fat but does not affect tissue insulin sensitivity: a randomized, double-blind, placebo-controlled study with 8-week treatment in type 2 diabetes patients. Diabetes Care. 2019;42:931–937. doi: 10.2337/dc18-1569. [DOI] [PubMed] [Google Scholar]
- 194.Shimizu M., Suzuki K., Kato K., Jojima T., Iijima T., Murohisa T. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes, Obesity and Metabolism. 2019;21:285–292. doi: 10.1111/dom.13520. [DOI] [PubMed] [Google Scholar]
- 195.Eriksson J.W., Lundkvist P., Jansson P.A., Johansson L., Kvarnstrom M., Moris L. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923–1934. doi: 10.1007/s00125-018-4675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bolinder J., Ljunggren O., Kullberg J., Johansson L., Wilding J., Langkilde A.M. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. Journal of Clinical Endocrinology & Metabolism. 2012;97:1020–1031. doi: 10.1210/jc.2011-2260. [DOI] [PubMed] [Google Scholar]
- 197.Cusi K., Bril F., Barb D., Polidori D., Sha S., Ghosh A. Effect of canagliflozin treatment on hepatic triglyceride content and glucose metabolism in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2019;21:812–821. doi: 10.1111/dom.13584. [DOI] [PubMed] [Google Scholar]
- 198.Khan R.S., Newsome P.N. Fat and fibrosis: does empagliflozin impair the progression of nonalcoholic steatohepatitis in patients with type 2 diabetes mellitus? Digestive Diseases and Sciences. 2020;65:342–344. doi: 10.1007/s10620-019-05573-y. [DOI] [PubMed] [Google Scholar]
- 199.Takahashi H. EASL dILC2020. 2020. Resolution of non-alcoholic steatohepatitis and hepatic fibrosis by sodium glucose transporter-2 inhibitor, ipragliflozin; a multicenter randomized controlled trial. 2020. [Google Scholar]
- 200.Taheri H., Malek M., Ismail-Beigi F., Zamani F., Sohrabi M., Reza Babaei M. Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Advances in Therapy. 2020;37:4697–4708. doi: 10.1007/s12325-020-01498-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kenny P.R., Brady D.E., Torres D.M., Ragozzino L., Chalasani N., Harrison S.A. Exenatide in the treatment of diabetic patients with non-alcoholic steatohepatitis: a case series. American Journal of Gastroenterology. 2010;105:2707–2709. doi: 10.1038/ajg.2010.363. [DOI] [PubMed] [Google Scholar]
- 202.Petit J.M., Cercueil J.P., Loffroy R., Denimal D., Bouillet B., Fourmont C. Effect of liraglutide therapy on liver fat content in patients with inadequately controlled type 2 diabetes: the lira-NAFLD study. Journal of Clinical Endocrinology & Metabolism. 2017;102:407–415. doi: 10.1210/jc.2016-2775. [DOI] [PubMed] [Google Scholar]
- 203.Armstrong M.J., Hull D., Guo K., Barton D., Hazlehurst J.M., Gathercole L.L. Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. Journal of Hepatology. 2016;64:399–408. doi: 10.1016/j.jhep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bizino M.B., Jazet I.M., de Heer P., van Eyk H.J., Dekkers I.A., Rensen P.C.N. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia. 2020;63:65–74. doi: 10.1007/s00125-019-05021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dutour A., Abdesselam I., Ancel P., Kober F., Mrad G., Darmon P. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes, Obesity and Metabolism. 2016;18:882–891. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 206.Hartman M.L., Sanyal A.J., Loomba R., Wilson J.M., Nikooienejad A., Bray R. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43:1352–1355. doi: 10.2337/dc19-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Omar B., Ahren B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes. 2014;63:2196–2202. doi: 10.2337/db14-0052. [DOI] [PubMed] [Google Scholar]
- 208.Gallwitz B. Clinical use of DPP-4 inhibitors. Frontiers in Endocrinology (Lausanne) 2019;10:389. doi: 10.3389/fendo.2019.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cui J., Philo L., Nguyen P., Hofflich H., Hernandez C., Bettencourt R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. Journal of Hepatology. 2016;65:369–376. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Joy T.R., McKenzie C.A., Tirona R.G., Summers K., Seney S., Chakrabarti S. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World Journal of Gastroenterology. 2017;23:141–150. doi: 10.3748/wjg.v23.i1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Smits M.M., Tonneijck L., Muskiet M.H., Kramer M.H., Pouwels P.J., Pieters-van den Bos I.C. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016;59:2588–2593. doi: 10.1007/s00125-016-4100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Deng X.L., Ma R., Zhu H.X., Zhu J. Short article: a randomized-controlled study of sitagliptin for treating diabetes mellitus complicated by nonalcoholic fatty liver disease. European Journal of Gastroenterology and Hepatology. 2017;29:297–301. doi: 10.1097/MEG.0000000000000780. [DOI] [PubMed] [Google Scholar]
- 213.Alam S., Ghosh J., Mustafa G., Kamal M., Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepatic Medicine. 2018;10:23–31. doi: 10.2147/HMER.S158053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yan J., Yao B., Kuang H., Yang X., Huang Q., Hong T. Liraglutide, sitagliptin, and insulin glargine added to metformin: the effect on body weight and intrahepatic lipid in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease. Hepatology. 2019;69:2414–2426. doi: 10.1002/hep.30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Macauley M., Hollingsworth K.G., Smith F.E., Thelwall P.E., Al-Mrabeh A., Schweizer A. Effect of vildagliptin on hepatic steatosis. Journal of Clinical Endocrinology & Metabolism. 2015;100:1578–1585. doi: 10.1210/jc.2014-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Papanas N., Maltezos E., Mikhailidis D.P. Metformin: diamonds are forever. Expert Opinion on Pharmacotherapy. 2009;10:2395–2397. doi: 10.1517/14656560903176453. [DOI] [PubMed] [Google Scholar]
- 217.Haukeland J.W., Konopski Z., Eggesbo H.B., von Volkmann H.L., Raschpichler G., Bjoro K. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scandinavian Journal of Gastroenterology. 2009;44:853–860. doi: 10.1080/00365520902845268. [DOI] [PubMed] [Google Scholar]
- 218.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomedical Reports. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.van de Laar F.A., Lucassen P.L., Akkermans R.P., van de Lisdonk E.H., Rutten G.E., van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 220.Nozaki Y., Fujita K., Yoneda M., Wada K., Shinohara Y., Takahashi H. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. Journal of Hepatology. 2009;51:548–556. doi: 10.1016/j.jhep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 221.Lieber C.S., Leo M.A., Mak K.M., Xu Y., Cao Q., Ren C. Acarbose attenuates experimental non-alcoholic steatohepatitis. Biochemical and Biophysical Research Communications. 2004;315:699–703. doi: 10.1016/j.bbrc.2004.01.116. [DOI] [PubMed] [Google Scholar]
- 222.Rudovich N.N., Weickert M.O., Machann J., Pfeiffer A.F. Combination of acarbose and ezetimibe prevents non-alcoholic fatty liver disease: a break of intestinal insulin resistance? Journal of Hepatology. 2010;52:952–953. doi: 10.1016/j.jhep.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 223.Komatsu M., Tanaka N., Kimura T., Fujimori N., Sano K., Horiuchi A. Miglitol attenuates non-alcoholic steatohepatitis in diabetic patients. Hepatology Research. 2018;48:1092–1098. doi: 10.1111/hepr.13223. [DOI] [PubMed] [Google Scholar]
- 224.Jadhav K., Xu Y., Xu Y., Li Y., Xu J., Zhu Y. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Molecular Metabolism. 2018;9:131–140. doi: 10.1016/j.molmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 226.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Friedman S.L., Ratziu V., Harrison S.A., Abdelmalek M.F., Aithal G.P., Caballeria J. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology. 2018;67:1754–1767. doi: 10.1002/hep.29477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ratziu V., Sanyal A., Harrison S.A., Wong V.W., Francque S., Goodman Z. Cenicriviroc treatment for adults with nonalcoholic steatohepatitis and fibrosis: final analysis of the phase 2b CENTAUR study. Hepatology. 2020 doi: 10.1002/hep.31108. [DOI] [PubMed] [Google Scholar]
- 229.Ratziu V., de Guevara L., Safadi R., Poordad F., Fuster F., Flores-Figueroa J. One-year results of the global phase 2b randomized placebo-controlled arrest trial of aramchol, a stearoyl CoA desaturase inhibitor, in patients with NASH. Hepatology. 2018;68 [Google Scholar]
- 230.Loomba R., Kayali Z., Noureddin M., Ruane P., Lawitz E.J., Bennett M. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155:1463–1473. doi: 10.1053/j.gastro.2018.07.027. e1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bergman A., Carvajal-Gonzalez S., Tarabar S., Saxena A.R., Esler W.P., Amin N.B. Safety, tolerability, pharmacokinetics, and pharmacodynamics of a liver-targeting acetyl-CoA carboxylase inhibitor (PF-05221304): a three-Part Randomized phase 1 study. Clinical Pharmacology in Drug Development. 2020;9:514–526. doi: 10.1002/cpdd.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Calle R., Bergman A., Somayaji V., Chidsey K., Kazierad D. PS-110-Ketohexokinase inhibitor PF-06835919 administered for 6 weeks reduces whole liver fat as measured by magnetic resonance imaging-proton density fat fraction in subjects with non-alcoholic fatty liver disease. Journal of Hepatology. 2019;70:e69–e70. [Google Scholar]
- 233.Ritter M.J., Amano I., Hollenberg A.N. Thyroid hormone signaling and the liver. Hepatology. 2020;72:742–752. doi: 10.1002/hep.31296. [DOI] [PubMed] [Google Scholar]
- 234.Esler W.P., Bence K.K. Metabolic targets in nonalcoholic fatty liver disease. Cellular and Molecular Gastroenterology and Hepatology. 2019;8:247–267. doi: 10.1016/j.jcmgh.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Harrison S.A., Bashir M.R., Guy C.D., Zhou R., Moylan C.A., Frias J.P. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394:2012–2024. doi: 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 236.Vatner D.F., Weismann D., Beddow S.A., Kumashiro N., Erion D.M., Liao X.H. Thyroid hormone receptor-beta agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. American Journal of Physiology. Endocrinology and Metabolism. 2013;305:E89–E100. doi: 10.1152/ajpendo.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Harrison S.A., Alkhouri N., Davison B.A., Sanyal A., Edwards C., Colca J.R. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase IIb study. Journal of Hepatology. 2020;72:613–626. doi: 10.1016/j.jhep.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 238.Sanyal A., Charles E.D., Neuschwander-Tetri B.A., Loomba R., Harrison S.A., Abdelmalek M.F. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392:2705–2717. doi: 10.1016/S0140-6736(18)31785-9. [DOI] [PubMed] [Google Scholar]
- 239.Harrison S.A., Rossi S.J., Paredes A.H., Trotter J.F., Bashir M.R., Guy C.D. NGM282 improves liver fibrosis and histology in 12 Weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–1212. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Younossi Z.M. Non-alcoholic fatty liver disease - a global public health perspective. Journal of Hepatology. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]