Abstract
Background
Non-alcoholic fatty liver disease, or as recently proposed ‘metabolic-associated fatty liver disease’ (MAFLD), is characterized by pathological accumulation of triglycerides and other lipids in hepatocytes. This common disease can progress from simple steatosis to steatohepatitis, and eventually end-stage liver diseases. MAFLD is closely related to disturbances in systemic energy metabolism, including insulin resistance and atherogenic dyslipidemia.
Scope of review
The liver is the central organ in lipid metabolism by secreting very low density lipoproteins (VLDL) and, on the other hand, by internalizing fatty acids and lipoproteins. This review article discusses recent research addressing hepatic lipid synthesis, VLDL production, and lipoprotein internalization as well as the lipid exchange between adipose tissue and the liver in the context of MAFLD.
Major conclusions
Liver steatosis in MAFLD is triggered by excessive hepatic triglyceride synthesis utilizing fatty acids derived from white adipose tissue (WAT), de novo lipogenesis (DNL) and endocytosed remnants of triglyceride-rich lipoproteins. In consequence of high hepatic lipid content, VLDL secretion is enhanced, which is the primary cause of complex dyslipidemia typical for subjects with MAFLD. Interventions reducing VLDL secretory capacity attenuate dyslipidemia while they exacerbate MAFLD, indicating that the balance of lipid storage versus secretion in hepatocytes is a critical parameter determining disease outcome. Proof of concept studies have shown that promoting lipid storage and energy combustion in adipose tissues reduces hepatic lipid load and thus ameliorates MAFLD. Moreover, hepatocellular triglyceride synthesis from DNL and WAT-derived fatty acids can be targeted to treat MAFLD. However, more research is needed to understand how individual transporters, enzymes, and their isoforms affect steatosis and dyslipidemia in vivo, and whether these two aspects of MAFLD can be selectively treated. Processing of cholesterol-enriched lipoproteins appears less important for steatosis. It may, however, modulate inflammation and consequently MAFLD progression.
Keywords: Lipoprotein, NAFLD, Adipose tissue, Triglycerides, De novo lipogenesis, Liver
Highlights
• Genetic and environmental factors cause development of metabolic-associated fatty liver disease (MAFLD).
• MAFLD promotes dyslipidemia via secretion of large VLDL particles.
• Dysbalance of VLDL secretion and hepatic lipid synthesis causes MAFLD.
• Adipose tissue - liver crosstalk influences MAFLD via fatty acids and endocrine factors.
1. Lipoproteins in systemic lipid metabolism
Plasma lipids are transported within lipoproteins, a heterogeneous group of mixed micelles differing by size, lipid composition, apolipoprotein (APO) content, and tissue of origin. APOs confer multiple specific functions that are important for the metabolism and regulation of the various lipoprotein classes. Lipoproteins are characterized by a monolayer of membrane lipids at the surface, predominantly phosphatidylcholine and unesterified cholesterol, while the hydrophobic lipoprotein core contains mostly triglycerides, cholesterol esters and the lipophilic vitamins A, D, E, and K.
1.1. Metabolism of APOB-containing lipoproteins
Lipoproteins from various classes serve distinct roles in systemic lipid metabolism. The large, triglyceride-rich chylomicrons are assembled around APOB48 in enterocytes of the small intestine and carry dietary lipids [1]. The smaller very low density lipoproteins (VLDL) contain APOB100, the full length version of APOB, and are produced in hepatocytes [2]. Both types of triglyceride-rich lipoproteins (TRL) serve to transport fatty acids to peripheral organs, in particular adipose tissues, skeletal muscle, and the heart (Figure 1). In the capillary lumen of these organs, triglycerides are hydrolyzed by the enzyme lipoprotein lipase (LPL) that requires APOC2 as an essential cofactor to generate free fatty acids (FFA). These are taken up by the parenchymal cells and re-esterified for storage or oxidized for energy production [3,4]. By removal of triglycerides, the particles are transformed to so-called remnant lipoproteins that are smaller and cholesterol-enriched. These are taken up by hepatocytes through receptor-mediated endocytosis [5]. Importantly, a significant portion of the VLDL remnants, also known as intermediate density lipoprotein (IDL), is not degraded but the particles further lose triglycerides by action of intravascular lipolytic and transfer enzyme activities including hepatic lipase (HL), cholesterol ester transfer protein (CETP) and phospholipid transfer protein (PLTP) to become the cholesterol-rich low density lipoproteins (LDL) [6]. LDL provide peripheral organs with cholesterol and part of it circles back to the liver, ensuring efficient control of systemic sterol metabolism by various hepatic pathways [5]. Next to this physiological role, however, together with remnant lipoproteins, they can passively accumulate in arterial walls, causing atherosclerosis. Accordingly, high concentrations of LDL and APOB-containing remnant lipoproteins are strongly associated with cardiovascular disease and death. Conversely, therapeutic lowering of cholesterol-rich APOB-containing lipoproteins confers a clinically relevant reduction in cardiovascular disease risk [7,8].
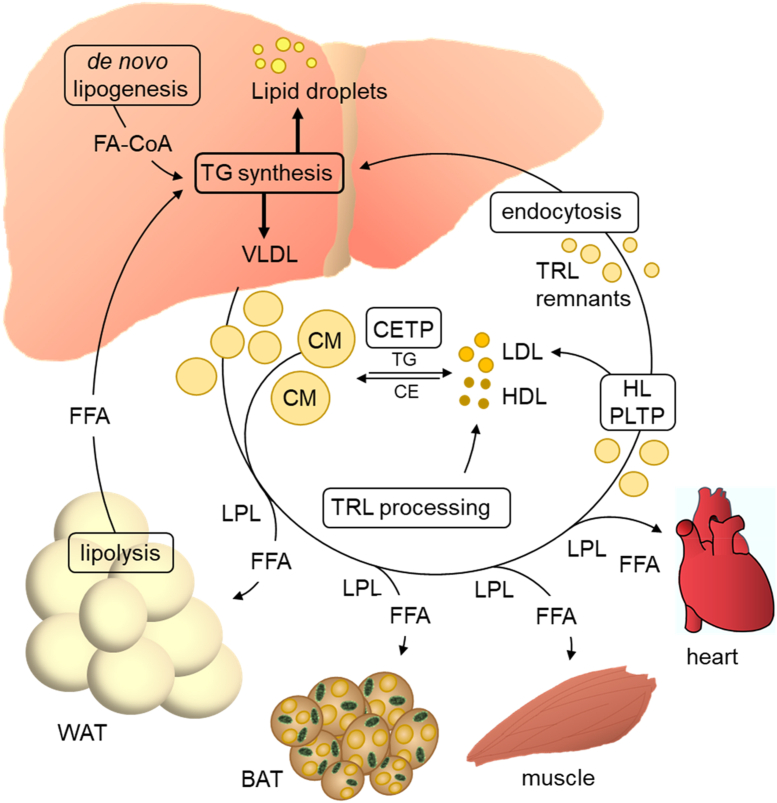
Figure 1.
The liver as a central organ of lipoprotein metabolism. The liver internalizes lipids from the circulation including FFA released from WAT, TRL remnants and cholesterol esters from HDL (not shown). The fatty acids entering the hepatocytes together with fatty acids derived from de novo lipogenesis are used for the synthesis of triglycerides and other complex lipids (not shown). The triglycerides are in part stored in lipid droplets and in part used for the assembly of VLDL that are released into the circulation. Here, VLDL together with intestinal-derived chylomicrons (CM) are processed in the capillaries of adipose tissues, the heart, and skeletal muscle by the enzyme LPL. LPL hydrolyzes triglycerides and thereby generates FFA that are taken up by the cells of the respective organ for storage or energy generation. Through LPL-dependent triglyceride hydrolysis, TRL become smaller and are thus transformed to cholesterol-enriched TRL remnants that are eventually taken up by hepatocytes through endocytosis and digested in the endo-lysosomal compartment. Part of the VLDL remnants are further processed by other intravascular enzymes including hepatic lipase (HL) and phospholipid transfer protein (PLTP) to become LDL. HDL particles are generated from lipid-poor APOA1 secreted from the liver (not shown) and during TRL processing (surface remnants containing APOA1). Both LDL and HDL exchange cholesterol ester for triglyceride with TRL in a reaction catalyzed by CETP.
1.2. Metabolism of high density lipoproteins
High density lipoproteins (HDL) are the smallest lipoproteins containing APOA1 as the defining structural protein that has multiple functions. The best studied one is reverse cholesterol transport [9]. In this pathway, APOA1 stimulates cholesterol efflux through the ATP-dependent transporter ABCA1 with concomitant absorption of free cholesterol from the cellular surface by the nascent HDL particle [10]. Next, APOA1 activates the enzyme lecithin-cholesterol acyl transferase (LCAT) and thus the synthesis of cholesterol esters [11]. By formation of a hydrophobic core, this allows growth of the particles and also involves a second cholesterol export pump, ABCG1 [10]. Eventually, the mature HDL deliver cholesterol esters to hepatocytes either directly via scavenger receptor B1 (SR-B1) or by delivering them first to APOB-containing lipoproteins through the action of CETP. CETP is a plasma protein that transfers cholesterol ester from HDL (or LDL) to TRL in exchange for triglyceride. Although low HDL concentrations are associated with cardiovascular disease, it is still debated to what extent reverse cholesterol transport by HDL particles is causal in preventing atherosclerosis [9]. Of note, in addition to APOA1, various other proteins can be associated with HDL particles, in particular such with anti-inflammatory and anti-oxidative function [12,13]. How these HDL-associated activities influence cardiovascular function and the immune system is currently under investigation.
2. Metabolic-associated fatty liver disease and dyslipidemia
A hallmark of non-alcoholic fatty liver disease [[14], [15], [16], [17]] is the expansion of hepatocyte lipid droplets (LDs) that contain triglycerides, cholesterol esters, and other lipid species. To better reflect the metabolism-related etiology, a recent consensus was achieved to change the nomenclature of this disease to metabolic-associated fatty liver disease (MAFLD) [18]. At the tissue level, fat accumulation can be benign, occurring without inflammation (hereafter referred to as metabolic-associated fatty liver; MAFL). It can also progress to a state of chronic inflammation accompanied by tissue damage (steatohepatitis). In particular, the latter condition is frequently associated with fibrosis of varying degree, a condition characterized by collagen production through transformation of hepatic stellate cells to fibroblast-like cells. Although steatohepatitis is not directly life-threatening, the patients have an increased risk of developing advanced liver disease (fibrosis, cirrhosis, and hepatocellular carcinoma) and cardiovascular disease, the latter being the major cause of death in patients with MAFLD [17,19,20].
2.1. Lipotoxicity in MAFLD
One important aspect of MAFLD progression is lipotoxicity caused by hepatic accumulation of lipids that can trigger cellular stress responses. In MAFL, lipid accumulation is generally benign because inert lipid species such as triglycerides and cholesterol esters prevail at this stage. Conversely, in steatohepatitis, elevated concentrations also of free cholesterol, diacylglycerols, and/or ceramides are frequently observed [21]. Free cholesterol and ceramides can promote cellular stress responses, inflammation, cell death, and fibrosis in the liver [22,23]. Ceramides and diacylglycerol species can induce insulin resistance [24]. Thus, there is strong evidence for the notion that an excess of lipids and its derivatives not safely stored as neutral lipids in LDs can contribute causally to MAFLD progression.
2.2. MAFLD-associated dyslipidemia
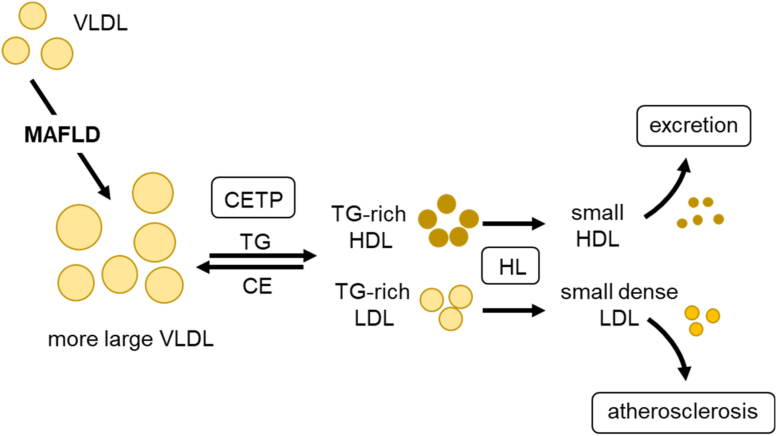
Most patients with MAFLD exhibit at least some features of the metabolic syndrome, in particular, overweight, insulin resistance, and atherogenic dyslipidemia characterized by high plasma triglyceride concentrations [25,26]. Accordingly, plasma lipoprotein patterns observed in MAFLD are similar or identical to those typically associated with metabolic syndrome and type 2 diabetes. The following plasma lipid traits are hallmarks of MAFLD-associated dyslipidemia: hypertriglyceridemia due to large VLDL particles (VLDL1), elevated concentrations of small dense LDL, and low HDL cholesterol [27,28]. This lipoprotein profile typical for MAFLD can be explained by CETP activity in the circulation. Under hypertriglyceridemic conditions, increased exchange of cholesterol ester and triglyceride molecules between LDL or HDL on the one side, and TRL particles on the other side, takes place (Figure 2). This CETP-catalyzed lipid exchange, which is driven solely by mass action, leads to a gain of triglycerides associated with LDL and HDL, making these lipoprotein particles a better substrate of HL. Consequently, they shrink through HL-mediated hydrolysis of triglycerides, explaining the generation of small dense LDL and small HDL particles (Figure 2). The latter are cleared by the kidney explaining low overall HDL cholesterol levels. Thus, the increased concentration of large TRL determines alterations in small dense LDL and HDL profiles in subjects with MAFLD [28]. Together with accumulation of TRL remnants (see section 6), this change in LDL and HDL is regarded as a major reason for increased cardiovascular risk of individuals with MAFLD [29]. Mechanistically, small dense LDL are particularly harmful, as they can easily enter the vascular intima, which accelerates cholesterol deposition in atherosclerotic plaques. In case of HDL, the lower particle number observed in MAFLD subjects may impair cholesterol homeostasis. Recent studies indicate that the capacity of HDL-induced cholesterol efflux from cultured macrophages is lower in serum from MAFLD patients compared to controls [30,31]. Conversely, a small but detailed study found the opposite, namely increased cholesterol efflux from macrophages and higher HDL turnover in vivo in subjects MAFLD [32]. This study also found alterations in other HDL functions. For instance, HDL-associated lipid peroxidation was increased, whereas the activity of the anti-oxidative HDL-associated enzyme paraoxonase-1 (PON-1) was diminished in patients with MAFLD. Contrariwise, diminished PON-1 activity was not observed in a large population-based study that did, however, use an indirect method to diagnose MAFLD [33]. Overall, more work needs to be done to better understand the association of cholesterol-, inflammation-, and oxidation-related HDL functions with MAFLD and to determine the relevance for disease development.
Figure 2.
Role of VLDL in MAFLD-associated dyslipidemia. MAFLD is caused by a chronically positive energy balance that leads to elevated triglyceride synthesis in the liver. Part of the surplus triglycerides is funneled into synthesis of larger VLDL particles. Consequently, the concentration of these large triglyceride-rich VLDL particles in the circulation rises, a hallmark of MAFLD-associated dyslipidemia. Other important characteristics of MAFLD-dyslipidemia are the presence of small dense LDL and low HDL cholesterol levels. This is primarily caused by the high concentration of VLDL in the blood in combination with intravascular action of CETP and HL. In the presence of a high number of large VLDL particles, this leads to the generation of both triglyceride-rich LDL and HDL. These lipoproteins are further processed leading to small dense LDL and small HDL. Small dense LDL are more atherogenic than normal-sized LDL, because they pass endothelia more easily promoting plaque formation in the arterial wall. Because of their reduced size, small HDL particles exhibit an increased propensity for excretion via the kidneys and thus have a shorter half-life in the circulation explaining low HDL levels.
2.3. Dyslipidemia in the course of MAFLD progression
Dyslipidemia is not uniform across the stages of MAFLD. In general, plasma concentrations of large VLDL particles (VLDL1) and small dense LDL particles are higher in subjects with MAFL compared to non-steatotic controls [[34], [35], [36]]. Some studies show a further, modest increase in the transition from MAFL to steatohepatitis [34,36,37]. Progression to severe fibrosis or cirrhosis, however, is associated with a decrease in the plasma concentrations of large APOB-containing lipoproteins [34,35,38], a relationship supported by a large population-based study utilizing a non-invasive algorithm to estimate fibrosis [39]. Interestingly, a study reported decrease in ‘normal-sized’ VLDL2 particle from MAFL to steatohepatitis, and further to severe fibrosis [37], possibly reflecting the progressive loss of healthy, non-steatotic hepatocytes. Along these lines, a reduction of the liver-derived atherogenic lipoprotein Lp(a) was observed in advanced fibrotic stages of MAFLD [40]. Taken together, MAFLD dyslipidemia appears to be most pronounced in MAFL or mild steatohepatitis, stages characterized by pronounced liver steatosis, and it wanes in more advanced, fibrotic stages. This dependence on MAFLD stage is likely to be explained by dynamic changes in VLDL production and disposal, as will be discussed further below.
3. VLDL production in MAFLD
A major function of hepatocytes in systemic lipid homeostasis is to internalize circulating lipids, process them, and incorporate fatty acids not required for cellular metabolism into triglycerides, cholesterol esters and membrane lipids that are then released into the blood stream as VLDL. The fatty acids used for VLDL lipids enter the hepatocytes either as FFA through the plasma membrane (see section 7) or as lipoprotein-borne lipids via endocytosis (see section 6). In addition, hepatocytes synthesize fatty acids from glucose and other non-lipid precursors by de novo lipogenesis (DNL) (see section 4). Liver steatosis develops when hepatocyte triglyceride synthesis exceeds VLDL triglyceride secretion. Consequently, the development of MAFLD is tightly linked to VLDL production (Figure 3A). Disturbances in VLDL secretion can not only cause the accumulation of triglycerides, but equally that of lipotoxic lipids carried by VLDL [41], and thereby the development and progression of the disease. Conversely, excessive lipid storage in MAFLD promotes the secretion of VLDL and hence dyslipidemia.
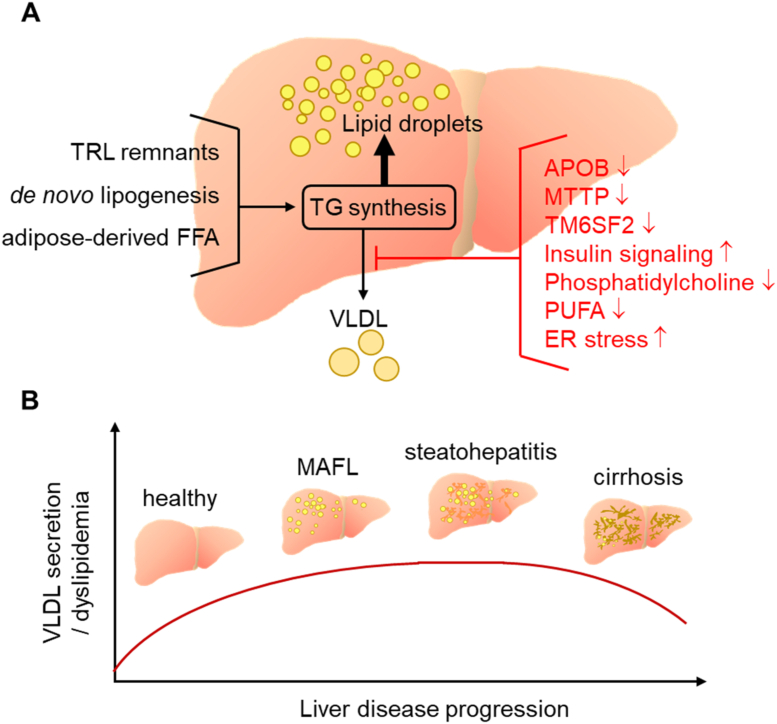
Figure 3.
Relationship of liver steatosis with the VLDL secretory pathway. One important feature of hepatocytes is the ability to efficiently export excess triglycerides and other lipids as VLDL. (A) If the rate of VLDL assembly and secretion is limiting, triglycerides are diverted to lipid droplets and the extent of steatosis increases. Impairment of VLDL secretion can be caused by naturally occurring mutations in proteins pivotal for VLDL production (APOB, MTTP, TM6SF2), by insulin action, lack of the major VLDL surface lipid phosphatidylcholine and possibly by reduced polyunsaturated fatty acids (PUFA) or by endoplasmic reticulum stress. (B) Clinical studies report that VLDL secretion, and in parallel dyslipidemia, increase with progression of MAFLD along with liver steatosis. A plateau is reached at the MAFL or the steatohepatitis stage, probably because the VLDL secretory capacity has reached a limit. In advanced liver disease such as cirrhosis VLDL secretion is reduced again, likely reflecting the loss of functional hepatocytes.
3.1. Cellular mechanism of VLDL secretion
The first step of VLDL synthesis is co-translational lipidation of the large APOB100 protein (in mouse liver also APOB48), a process mediated by microsomal triglyceride transfer protein (MTTP) within the endoplasmic reticulum (ER). Then, the nascent VLDL particle grows, at least in part by receiving lipid from lipid droplets located in the ER lumen that could be transferred by fusion [42] or intraluminal triglyceride hydrolysis and subsequent re-esterification [43]. Further expansion of the VLDL particle takes place after transfer to the Golgi apparatus through mechanisms not well defined [[44], [45], [46]]. APOB-containing nascent VLDL are transferred from the endoplasmic reticulum to the Golgi apparatus in coat protein complexes (COP II) vesicles [47,48], in a process that depends on the specific cargo receptor SURF4 in mice and humans [49]. VLDL production is a variable and regulated process. One major factor controlling the rate of VLDL production is the availability of the major lipid component triglyceride. It is well established that triglyceride accumulation in hepatocytes is positively associated with plasma triglyceride concentrations [26] and with the rate of VLDL triglyceride secretion [[50], [51], [52], [53]].
3.2. Genetic polymorphisms modulating VLDL secretion
On the other hand, when VLDL secretion is impaired, this favors hepatic lipid build-up, as demonstrated by severe liver steatosis in subjects with defective VLDL lipidation and secretion due to rare, loss-of function mutations in MTTP (abetalipoproteinemia) or in APOB (homozygous hypobetalipoproteinemia) [54]. Next to these extreme cases, common genetic polymorphisms in humans have confirmed the close link of hepatocyte VLDL secretory capacity with liver steatosis. This is particularly evident in the case of transmembrane 6 superfamily 2 (TM6SF2), an ER- and Golgi-resident protein. A loss-of-function variant of TM6SF2 was discovered to be associated with MAFLD in genetic association studies [[55], [56], [57]]. Accordingly, liver-specific knockdown of TM6SF2 in mice increased liver fat while it decreased the secretion of VLDL [55]. Similarly, knockdown in human hepatoma cells led to lipid droplet growth and suppressed VLDL release [56]. Consistent with ameliorated dyslipidemia, the TM6SF2 variant was found to be associated with reduced cardiovascular risk [[58], [59], [60]]. Subsequent research showed that livers of Tm6sf2 knockout mice exhibit expanded lipid droplets and reduced secretion of VLDL-associated triglyceride but no change in APOB secretion, suggesting that TM6SF2 is important for proper VLDL lipidation but not VLDL secretion per se [61]. The mechanisms underlying TM6SF2-dependent lipidation have not been elucidated. Carriers of the loss-of-function allele were reported to have reduced hepatic expression of several genes related to triglyceride synthesis, for example diacylglycerol acyl transferase-1 (DGAT1) and DGAT2 [62]. These enzymes catalyze the final step in triglyceride synthesis, and DGAT1 appears to be especially important for VLDL production [63]. This could explain the reduced VLDL triglyceride secretion, however, not the increase in liver fat, as mice lacking DGAT1 or DGAT2 have less steatotic livers [64,65]. Another interesting observation around the TM6SF2 polymorphism is a reduced percentage of polyunsaturated fatty acids (PUFA) in hepatic phosphatidylcholine species [62,66]. This alteration may be mechanistically relevant, as it could influence physical properties of the ER and/or nascent VLDL particles. Moreover, it has been known for a long time that hepatic phosphatidylcholine availability is very important for proper VLDL production and consequently the prevention of MAFLD, as shown by numerous human and rodent intervention studies using choline-deficient diets and knockouts of enzymes critical for phosphatidylcholine synthesis [67]. Of note, decreased PUFA content in phosphatidylcholine was also demonstrated for a genetic polymorphism (Ile148Met) of patatin-like phospholipase domain-containing protein 3 (PNPLA3) [68], the most common NAFLD-predisposing genetic polymorphism in humans [69] that is like the TM6SF2 polymorphism associated with reduced VLDL secretion [70]. Enzymatic activities described for PNPLA3 include glycerolipid hydrolase [71] and lysophosphatidic acid acyl transferase [72]. Furthermore, accumulation of the mutated protein at the surface of lipid droplets has been observed [73]. As transgenic mouse experiments have produced conflicting results [74], it is currently not clear whether decreased phosphatidylcholine PUFA content, disturbed fatty acid transfer, a mechanism involving disturbed mobilization of lipids from the lipid droplets or other mechanisms underlie the MAFLD predisposition of the PNPLA3 Ile148Met polymorphism.
3.3. Impact of insulin signaling and cellular stress on VLDL secretion
Next to genetic predisposition, insulin signaling is an important factor determining the balance of VLDL secretion and MAFLD. Insulin is an established negative regulator of VLDL triglyceride and APOB secretion [2,75]. Next to suppressing flux of the major VLDL triglyceride substrate FFA to the liver by inhibiting white adipose tissue lipolysis (see section 7), high insulin concentrations cause reduced expression and activity of MTTP [[76], [77], [78]]. They also diminish the translation of APOB mRNA [79] and promote the degradation of APOB through poorly defined mechanisms [75,80]. Physiologically, these insulin effects serve the purpose to transiently suppress lipid output from the liver to allow efficient disposal of gut-derived dietary fat in peripheral organs in the postprandial state. In individuals with MAFLD, basal VLDL production is increased [50,52,53]) and insulin-dependent suppression of VLDL secretion is impaired [51,81]. Individuals with MAFLD typically exhibit both hepatic and peripheral insulin resistance, making it difficult to estimate the relative contribution of the liver versus other insulin target tissues. The importance of liver insulin signaling was demonstrated by liver-specific insulin receptor knockout mice that exhibit constitutively increased VLDL secretion despite the absence of steatosis [82]. In summary, insulin resistance causes increased VLDL secretion in MAFLD compared to metabolically healthy states, a mechanism that counteracts the build-up of hepatic lipid stores, especially that of lipotoxic species, and is thus protective for the liver. This adaptation is, however, limited, as shown by studies indicating that VLDL secretion rates reach a plateau in mild to moderate steatosis and may even decrease upon progression to severe steatosis [52,83] and/or steatohepatitis [84,85]. Furthermore, experimental support for impaired VLDL secretion in severe steatosis was provided by cell culture studies showing that low exogenous fatty acids promote APOB secretion whereas high exogenous fatty acids reduce it through lipid-induced endoplasmic reticulum stress [86,87], which can for example be triggered by excessive saturated FFA and ceramides [23]. The endoplasmic reticulum is a major site of cellular lipid synthesis and pivotal for lipid sensing [88,89]. Endoplasmic reticulum stress, when unresolved, contributes to the development of insulin resistance, inflammation and cell death associated with fatty liver [90,91]. Thus, it does not only affect VLDL secretion but also many other disease aspects of MAFLD.
Taken together, both lipid-mediated stress as well as impaired insulin signaling affect the ratio of hepatic triglyceride storage versus lipoprotein secretion. Overall, a picture emerges where VLDL secretion follows a biphasic pattern in the course of MAFLD (Figure 3B). It is stimulated by increased liver lipid availability and insulin resistance in the early stages of the disease. With further progression, VLDL secretion drops due to hepatocyte stress responses or more general loss of tissue function. Throughout the process, the balance of lipid secretion and lipid storage in hepatocytes depends on the VLDL secretory capacity, which is determined by both genetics and cellular resilience.
4. De novo lipogenesis and lipoproteins in MAFLD
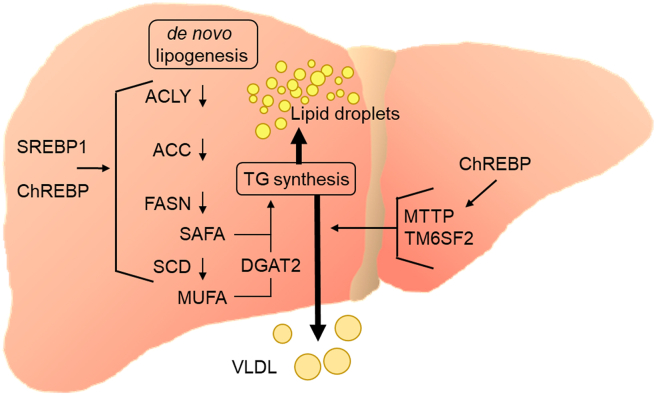
De novo lipogenesis (DNL), the metabolic pathway synthesizing saturated fatty acids (SAFAs) and monounsaturated fatty acids (MUFAs) from acetyl-CoA, typically accounts for only a small fraction of fatty acids (ca. 5%) in the liver and in VLDL [92,93] of lean, healthy humans. In MAFLD, however, the rate of hepatic DNL is strongly increased and more than 25% of VLDL triglycerides can stem from DNL [92,94,95]. This increase in MAFLD compared to healthy subjects can be explained to a large extent by induction of DNL enzymes [96,97], which occurs in particular through sterol regulatory element binding protein-1 (SREBP1) and carbohydrate response element-binding protein (ChREBP). These two transcription factors are activated by elevated glucose flux as well as insulin signaling. Accordingly, hyperglycemic and hyperinsulinemic conditions caused by energy excess promote DNL by chronic SREBP1 and ChREBP activation in the liver (Figure 4) [[98], [99], [100]].
Figure 4.
De novo lipogenesis drives both VLDL secretion and liver steatosis. Hepatic expression of the DNL enzymes depends on nutritional state. After food intake, increased glucose metabolism and elevated insulin signaling increase the activity of the transcription factors ChREBP and SREBP1, respectively. Under conditions of chronic energy excess and hyperinsulinemia prevalent in MAFLD, both lipogenic transcription factors and thus de novo lipogenesis are constantly active, which leads to elevated triglyceride synthesis from saturated fatty acids (SAFA) and monounsaturated fatty acids (MUFA). Stearoyl-CoA desaturase (SCD) catalyzes the generation of MUFA-CoA that are efficiently incorporated into triglycerides via diacylglycerol acyl transferase-2 (DGAT2) that directly associates with SCD. Of note, induction of SCD was observed to go along with increased VLDL secretion, and ChREBP promotes expression of proteins important for VLDL assembly (MTTP, TM6SF2). Together these findings indicate a strong link of DNL to VLDL secretion and dyslipidemia.
4.1. DNL drives VLDL secretion
DNL is not only an important factor contributing to liver steatosis but is also intimately linked to VLDL production. This is demonstrated for example by the observation that ChREBP potently transactivates the genes of MTTP and TM6SF2 along with those of the DNL enzymes [101]. Further supporting a link to VLDL production, pharmacological stimulation of another lipogenic transcription factor, liver X receptor-α (LXRα), increases VLDL size but not particle number [98], suggesting that DNL feeds preferentially into the growth of VLDL after initial lipidation of APOB [102]. These results were confirmed in another study showing that LXRα activation affects VLDL particle size indirectly through inducing SREBP1c [103]. Further evidence for the link between DNL and VLDL production was provided by studies investigating stearoyl-CoA desaturase (SCD), the DNL enzyme that catalyzes a late step in DNL, namely the generation of MUFA. Hepatic SCD activity is increased in human MAFLD, as demonstrated by the selective increase of the MUFA palmitoleate in VLDL lipids of steatotic compared to non-steatotic individuals [104]. In rats, physiological stimuli that elevate the hepatic expression of the rodent orthologue SCD1 were shown to acutely stimulate VLDL secretion and plasma hypertriglyceridemia [105,106], whereas Scd1 knockdown was found to reduce liver triglyceride content, VLDL secretion, and plasma VLDL levels in MAFLD mouse models [107,108]. Interestingly, SCD1 was shown to physically interact with DGAT2 [109], which preferably incorporates DNL fatty acids into triglycerides [63,110]. Knockout or inhibition of DGAT2 results in a lower VLDL secretion rate in rodents [[110], [111], [112]]. In sum, these experiments indicate that SCD1 and DGAT2 together facilitate and control the release of lipids derived from DNL into the circulation.
4.2. Effects of DNL inhibition on MAFLD and dyslipidemia
Enzymes catalyzing earlier steps in DNL have also been investigated with regard to MAFLD and dyslipidemia. As these DNL steps are also involved in other pathways of lipid metabolism [113,114], it is not surprising that intervention studies produced complex results [[115], [116], [117], [118], [119]]. Inhibition of fatty acid synthase (FASN), the enzyme synthesizing palmitate from acetyl-CoA and malonyl-CoA, using a small molecule compound was found to reduce liver steatosis in some mouse models whereas it had no effect in others [117,120]. The variable results on liver triglyceride content, also observed in liver-specific Fasn knockout mice [121], might be related to dual action on fatty acid synthesis and oxidation which are both reduced upon FASN inhibition. The effect on fatty acid oxidation is explained by the accumulation of malonyl-CoA that allosterically inhibits carnitine-palmitoyl transferase-1, the rate-limiting enzyme of mitochondrial fatty acid oxidation [113]. It is thus not clear whether FASN inhibition is a valid approach for treating MAFLD or associated dyslipidemia. An enzyme that has been more carefully studied with regard to lipoprotein metabolism is acetyl-CoA carboxylase (ACC), the enzyme synthesizing malonyl-CoA. As predicted, pharmacological inhibition of ACC was found to reduce liver steatosis in rodents and humans. However, concurrently, the intervention led to elevated plasma triglycerides in both the pre-clinical models [115,116] as well as in the humans investigated in these studies [116,122]. The mechanism could be narrowed down in the rodent models to the activation of SREBP1c and subsequent induction of its target genes such as glycerol phosphate acyl transferase-1 (GPAT1) that promote VLDL secretion. The unexpected activation of SREBP1c could be linked to a decrease in PUFAs [115,116], which are well-known endogenous inhibitors of SREBP1c [123,124]. The PUFA reduction in the ACC inhibition experiments was probably caused by a decrease of malonyl-CoA and thus increased mitochondrial fatty acid oxidation [113]. Of note, these results are in apparent conflict with the above-mentioned hypothesis that reduced phosphatidylcholine PUFA secondary to TM6SF2 and PNPLA3 mutations (see section 3) cause a reduction in VLDL secretion [62,68]. However, in those studies, PUFA showed opposite regulations in hepatic FFA [62] and in triglycerides [68], suggesting that TM6SF2 and PNPLA3 polymorphisms do not cause general PUFA deficiency, which may explain the diverging effects on VLDL secretion. Another relevant DNL enzyme is ATP citrate lyase (ACLY). ACLY has been primarily targeted as a drug target for atherogenic dyslipidemia therapy. The enzyme produces cytosolic acetyl-CoA and therefore provides the main substrate for both cholesterol synthesis and DNL. The most advanced ACLY inhibitor, bempedoic acid, is liver-selective and efficiently lowers plasma LDL cholesterol in humans [125]. In hyperlipidemic mice on an atherogenic high fat diet, bempedoic acid suppressed hepatic DNL, corrected hyperlipidemia and reduced liver steatosis [119,126]. Surprisingly, liver-specific knockdown of Acly led to increased liver triglycerides in mice on high-fat diet [118] but had the opposite effect in leptin signaling-deficient db/db mice [127]. Although ACLY inhibition has the potential to treat MAFLD in humans, more work needs to be done to better understand the impact of ACLY inhibition on hepatic lipid homeostasis.
In summary, hepatic DNL is generally increased in MAFLD and contributes to both accumulation of lipids within hepatocytes and secretion of VLDL lipids into the circulation. More studies are needed to better understand the complex effects of selective DNL inhibitors on lipid metabolism and thus to explore their safety and efficacy as medication to treat MAFLD and MAFLD-related dyslipidemia.
5. Peripheral lipoprotein processing in MAFLD
In addition to secreting VLDL into the circulation, the liver regulates the disposal of VLDL and chylomicrons in peripheral organs by secreting factors that affect peripheral lipoprotein processing through modulating the activity of LPL. In this section, we will discuss the evidence indicating whether or how these factors are related to MAFLD.
5.1. APOC3
APOC3 is a protein involved both in peripheral and hepatic lipoprotein processing. It is expressed principally by the liver and is a determinant of plasma triglyceride levels in humans as shown by loss-of-function APOC3 variants causing lower triglycerides and gain-of-function having the opposite effect [[128], [129], [130], [131]]. Rodent studies have identified several mechanisms explaining the association of APOC3 with circulating triglycerides. Next to the well-established inhibition of LPL and thus attenuation of peripheral TRL disposal conferred by APOC3 [4,132,133], stimulation of VLDL secretion and inhibition of remnant endocytosis have been reported as APOC3 effects in the liver [132,134,135]. In support of LPL-independent mechanisms, APOC3 antisense therapy efficiently lowered triglycerides in patients with familial chylomicronemia that have very low or no residual LPL activity [136]. APOC3 is positively associated with insulin resistance, which can be explained by positive regulation of APOC3 expression by glucose and fatty acids, and negative regulation by insulin [134]. Studies employing transgenic mice overexpressing APOC3 have consistently reported plasma hypertriglyceridemia; however, conflicting results with regard to MAFLD. One study showed that these mice, when kept on a high-fat diet, were more insulin resistant and exhibited more pronounced liver steatosis than control mice, which was attributed to increased lipoprotein uptake in the liver that was not accompanied by increased VLDL secretion [137]. Another study confirmed insulin resistance and more pronounced steatohepatitis in APOC3 transgenic mice on high-fat diet; however, they reported accelerated VLDL secretion [138]. Yet another study observed no effect on liver steatosis in these mice (derived from same strain as that used by [137]) on various diets but a clear induction of VLDL secretion [139]. Thus, the steatosis-inducing effects of APOC3 overexpression are context-dependent and the studies suggest that APOC3 is not an independent risk factor for MAFLD. Consistent with this notion, one human study reported that gain-of-function APOC3 promoter mutations are associated with increased plasma triglycerides, insulin resistance and liver steatosis [140]. However, this finding could not be confirmed for the same genetic polymorphisms in subsequent studies investigating other populations [[141], [142], [143]]. Furthermore, large population-based studies found no association of APOC3 loss-of-function mutations with liver fat content or plasma markers predicting MAFLD [128,129]. Taken together, despite a close link to hepatic and peripheral lipoprotein metabolism, APOC3 appears not to be a major determinant of MAFLD development.
5.2. APOA5
APOA5 is an apolipoprotein that is expressed predominantly in the liver and an important regulator of TRL metabolism [[144], [145], [146]]. In the human population, genetic polymorphisms in the APOA5 gene are important genetic determinants of plasma triglycerides with loss-of-function alleles promoting hypertriglyceridemia [[147], [148], [149]]. Circulating APOA5 is bound to VLDL, chylomicrons as well as HDL [150] and lowers plasma triglycerides primarily by stimulating LPL-dependent hydrolysis of TRL [[151], [152], [153], [154]], with a preference for large particles [103]. It also facilitates hepatic endocytosis of lipoprotein remnants [155,156]. The important role of APOA5 in triglyceride metabolism suggests an association with MAFLD. Evidence for this was provided by a study describing higher APOA5 expression in the livers of MAFLD patients [157] and concomitant reduction of hepatic APOA5 expression and fat content upon weight loss in another clinical study [158]. Overexpression of APOA5 in hepatoma cells increased intracellular lipid storage [159,160] and APOA5 was found on the surface of lipid droplets under this condition [161]. Confirming these results in vivo, overexpression of APOA5 in liver of mice moderately elevated the liver triglyceride content in mice [162]. Conversely, APOA5 knockdown in hepatoma cells decreased cellular triglyceride content [158]. Along these lines, hepatic APOA5 knockdown in mice led to reduced peripheral and hepatic TRL clearance, which causes hypertriglyceridemia and at the same time lower liver triglyceride content [163]. Whether these findings can be translated to humans is an open question. A recent genetic study found no clear link of APOA5 polymorphisms to MAFLD diagnosed by ultrasonography [164]. Given the strong impact of APOA5 mutations on plasma triglyceride concentrations in humans and the observed association of an APOA5 mutant with hepatic lipid droplets [148,149,165], surprisingly few clinical studies have been conducted to determine the relevance of APOA5 for the development of MAFLD.
5.3. Angiopoietin-like proteins
Three members of the angiopoietin-like (ANGPTL) family, ANGPTL3, ANGPTL4 and ANGPTL8, regulate peripheral TRL processing. Both ANGPTL3 and ANGPTL4 exert their intravascular function by inhibiting LPL in a tissue-specific manner. ANGPTL8 is not an LPL inhibitor by itself but is a modulatory factor that physically interacts with the two other isoforms [[166], [167], [168], [169]]. ANGPTL8 activates ANGPTL3, thereby inhibiting LPL-dependent TRL disposal in heart and muscle [170,171]. Conversely, ANGPTL8 inhibits ANGPTL4 in adipose tissue and thereby activates LPL [170,171]. ANGPTL8 is expressed at very low levels during fasting and is strongly induced in the postprandial state [[172], [173], [174], [175]]. Thus, the overall role of ANGPTL8 appears to be to promote the storage of dietary fat in adipose tissue after a meal and divert it from oxidative tissues under this condition. Consistent with their effects on LPL, ANGPTLs are major determinants of plasma lipid levels in humans. Genetic studies found that carriers of ANGPTL3 [176,177]) or ANGPTL4 [[178], [179], [180]] loss-of-function mutations have lower triglycerides levels. Similarly, lowering of ANGPTL3 [176] or ANGPTL4 [178,181] by use of monoclonal antibodies or antisense oligonucleotides (ASOs) led to reduced plasma triglycerides in humans. Despite this clear impact on triglyceride metabolism, it appears that ANGPTL3 plays a minor role in the development of MAFLD. One study reported higher serum ANGPTL3 in steatohepatitis as compared to MAFL or non-steatotic control subjects [182]. However, neither genetic ANGPTL3 deficiency [183,184] nor pharmacological lowering of ANGPTL3 [181,185,186] in humans and mice had an effect on hepatic steatosis. Circulating ANGPTL8 levels are positively associated to liver steatosis [[187], [188], [189]], which may, however, simply reflect the association of plasma ANGPTL8 with insulin resistance [190,191]. Of note, ANGPTL8 KO mice had unaltered liver triglycerides [170]. Similarly, the administration of an anti-ANGPTL8 antibody decreased plasma triglycerides but had no effect on liver steatosis [192]. However, ANGPTL8 ASO treatment of rats on high-fat diet reduced liver fat, increased WAT uptake of fatty acids from TRL and selectively improved hepatic insulin resistance [191]. Whether ANGPTL4 affects MAFLD in humans is not known. Of note, adipose tissue-specific knockout in mice reduces liver steatosis while improving plasma lipids and glucose metabolism [193]. Thus, channeling fat away from the liver by ANGPTL4 inhibition in adipose tissue is a promising approach to counteract MAFLD in the context of the metabolic syndrome that warrants further investigation.
6. Hepatic lipoprotein processing in MAFLD
The majority of the TRL remnants generated through LPL-dependent peripheral triglyceride hydrolysis are subsequently returned to the liver, endocytosed and digested by hepatocytes to make lipids available for re-secretion as VLDL, or alternatively for excretion via the bile [194]. Humans with MAFLD have higher remnant lipoprotein concentrations [195,196] and liver fat content correlates with postprandial lipemia [197]. This leads to the question whether hepatic uptake of chylomicron remnants and IDL is involved in the development of MAFLD. Here, we focus on the role of lipoprotein receptors and co-receptors. The important aspect of endocytic trafficking for lipid accumulation and lipoprotein secretion was reviewed recently [5,46].
The major receptor for cholesterol-enriched APOB-containing remnant lipoproteins in hepatocytes is the LDL receptor (LDLR). Loss of function mutations of the protein can cause severe hypercholesterolemia and have adverse effects on cardiovascular health in humans [198]. Mouse experiments indicate that lack of LDLR also promotes MAFLD development. LDLR knockout mice when fed a high fat high cholesterol diet exhibit exaggerated liver steatosis [199] and accelerated progression to steatohepatitis [200]. To our best knowledge, however, the role of LDLR in the development and progression of MAFLD is unclear in humans.
An alternative receptor for remnant uptake is LDLR-related protein-1 (LRP1) [201]. Hepatic knockout of Lrp1 accelerated steatohepatitis progression in mice on a high fat high cholesterol diet [202]. Similar effects on MAFLD parameters were observed in the liver of Lrp1 knockout mice on a diabetogenic high fat diet [203]. Of note, an opposite, protective effect in response to high-fat high-cholesterol diet feeding was observed in mice with a mutated distal NPxY motif in the intracellular domain of LRP1, a mutation that led to a compensatory upregulation of the LDLR [204]. For efficient internalization, both LDLR and LRP1 depend on APOE as co-ligand. APOE-deficient mice exhibited more pronounced steatohepatitis than wild type mice when fed chow or high-cholesterol diets [[205], [206], [207]]. Although APOE may also affect hepatocellular lipid balance by regulating VLDL secretion [208], these data are consistent with the notion that, despite increasing the lipid load of hepatocytes, efficient uptake of cholesterol-enriched remnant lipoproteins protects against diet-induced MAFLD. One explanation for the protective effect of remnant clearance may be reduced extracellular generation of oxidized lipoproteins [200], particles that can promote MAFLD progression after uptake into liver-resident Kupffer cells as well as infiltrating macrophages [209,210]. Other potential mechanisms are the induction of proteins mediating cholesterol removal via the biliary route [202] and facilitated uptake into hepatocytes of extracellular vesicles carrying the anti-inflammatory micro RNA species miR-223 [211]. It is important to note that lipid uptake as such does not protect from MAFLD but apparently favors it. This is indicated by the observation that knockout of the VLDL receptor (VLDLR), which predominantly mediates disposal of larger, triglyceride-rich particles [212], protects mice against high fat diet-induced MAFLD [213]. The VLDLR is expressed at low levels in healthy liver but increased upon development of MAFLD in mice and humans [214] and may thereby contribute to disease development. Interestingly, mice lacking proprotein convertase subtilisin/kexin type 9 (PCSK9) that display higher expression of the LDLR, VLDLR and also the fatty acid transporter CD36 in liver are more prone to develop steatosis [215]. Along these lines, humans with a loss of function PCSK9 mutation exhibit ectopic lipid accumulation in liver [216]. It is, however, of note that PCSK9-deficiency causes an impaired beta cell function and decreased plasma insulin levels, which could be a confounding factor influencing MAFLD development in PCSK9-deficiency[217]. In summary, more research is needed to dissect the pleiotropic functions of PCSK9, the contribution of different lipoprotein receptors and processing of internalized lipoproteins for MAFLD.
7. Adipose tissue - liver crosstalk in MAFLD
7.1. WAT – liver crosstalk
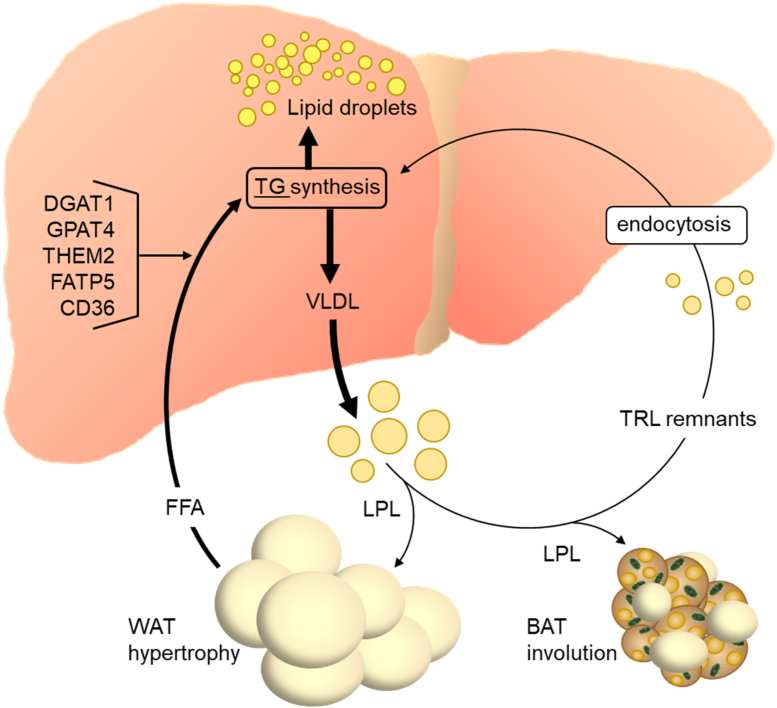
WAT and the liver form a metabolic unit that stores and handles fatty acids for use by other organs [194]. In white adipocytes, fatty acids are liberated by lipolysis during catabolic states and these FFA are the principal source for VLDL synthesis in the liver [95]. Importantly, too much fatty acid release from WAT causes hepatic steatosis, since the VLDL secretory pathway has limited capacity and is thus saturable [52,[83], [84], [85]]. In obese subjects, hypertrophic insulin resistant white adipocytes have elevated lipolysis and poorly stored fatty acids [218,219], which explains the connection between obesity and MAFLD [220,221]. Conversely, interventions reducing overweight and adipocyte hypertrophy such as physical exercise lead to an amelioration of MAFLD [222]. Importantly, FFA from WAT equally act as regulatory molecules in hepatocytes that, through activating the transcription factor peroxisome proliferator-activated receptor-α (PPARα), stimulate fatty acid oxidation and reduce inflammation [223,224]. Similarly, the adipokine adiponectin can counteract hepatic steatosis and inflammation by activating PPARα and AMP-dependent kinase [225,226]. Of note, hepatic PPARα is a major determinant of circulating fibroblast growth factor-21 (FGF21), an endocrine hormone that alleviates liver lipid load at least in part by stimulating the activity of lipid-combusting BAT [224,227]. Taken together, WAT has a major impact on the development of MAFLD by feeding the liver with fatty acids for lipid synthesis (Figure 5). In addition, it exerts several hormonal actions on the liver that have the potential to directly or indirectly influence MAFLD by cross-talk with other organs such as BAT.
Figure 5.
Impact of adipose tissues on liver steatosis and dyslipidemia. MAFLD is associated with hypertrophic WAT, which is characterized by increased flux of FFA to the liver. In hepatocytes, several transporter and enzymes (FATP5, CD36, THEM2, GPAT4, DGAT1) were found to be involved in the preferential incorporation of circulation-derived fatty acids into triglycerides. On the other hand, little evidence exists that these triglycerides are selectively used for VLDL, as plasma FFA-derived triglycerides are also efficiently incorporated into lipid droplet triglycerides. Interventions reverting WAT hypertrophy generally improve MAFLD by reducing the lipid flux to the liver. The mass and function of brown adipose tissue (BAT) is generally decreased in metabolically unhealthy states such as obesity and probably also in MAFLD, a process called BAT involution. As BAT can take up substantial amounts of lipid from the circulation and combust it, reactivation of BAT has the potential to divert lipid from the liver and thus ameliorate MAFLD.
Another parameter determining MAFLD is the metabolization of circulating FFA by the liver to generate triglycerides and phospholipids for VLDL synthesis. This process is initiated by fatty acid uptake into the hepatocytes. It has been reported that in the livers of humans with MAFLD, expression of the fatty acid transporters CD36, fatty acid transport protein-2 (FATP2) and FATP5 is elevated [228,229]. Importantly, silencing of the FATP5 gene (Slc27a5) protects mice from diet-induced MAFLD [230]. In humans, a gain-of-function promoter mutation of SLC27A5 is moderately associated with liver steatosis and postprandial plasma triglyceride concentrations [231]. Thus, facilitated uptake of fatty acids by hepatocytes appears to favor not only lipid accumulation but also VLDL secretion. Downstream of fatty acid uptake, several enzyme isoforms have been proposed to preferentially use plasma-derived fatty acids for triglyceride synthesis and subsequent incorporation into VLDL, including thioesterase superfamily member 2 (THEM2) [232], GPAT4 [233], and DGAT1 [234]. Of note, individual knockout of these enzymes reduced liver fat content in mice [64,235,236], indicating that they also synthesize lipids for storage in lipid droplets. In the case of DGAT1, elevated VLDL secretion rate was observed after overexpression of the enzyme in the liver of mice in two studies [237,238] but not in another one [239]. In all cases, DGAT1 overexpression also caused liver steatosis. Thus, it appears unlikely that these enzymes preferentially provide lipids for VLDL production and thereby affect the balance between lipid storage versus secretion.
7.2. BAT – liver crosstalk
In contrast to WAT, BAT does not release FFA for the use of other organs but burns them to generate heat [240]. In rodents exposed to cold environmental temperatures, BAT is activated and becomes a major sink of energy with fatty acids from TRL as the predominant energy source [241]. Consequently, BAT activation leads to lowering of plasma lipids [242] as well as accelerated flux of cholesterol-enriched TRL remnants and HDL to the liver [243,244]. The latter does, however, not cause hepatic lipid accumulation but rather increased conversion of cholesterol to bile acids and excretion from the liver [245]. Consistent with these alterations in lipid metabolism, BAT activation ameliorates MAFLD [246] and enhances beneficial effects of calorie restriction on steatohepatitis in mice [247]. Conversely, BAT inactivation by warm (thermoneutral) housing exacerbates MAFLD in mice [246]. Notably, activated BAT secretes the hormone neuregulin-4 (NRG4) which was reported to reduce DNL through ErbB3 and ErbB4 signaling in hepatocytes [248]. Thus, endocrine in addition to metabolic functions of BAT have the potential to ameliorate steatosis and dyslipidemia in MAFLD. In humans, cardiometabolic health as well as MAFLD are inversely associated with the presence and abundance of BAT [249,250] and BAT expresses lipoprotein-processing genes [251,252]. These data point toward a lipid-lowering and liver-protective role of BAT also in humans, even though their BAT is a less prominent organ compared to that of rodents. Of note, the contribution of human BAT to systemic processing and lipoprotein disposal has not been studied extensively, which may be explained by the lack of appropriate tracers used in positron emission tomography/computer tomography (PET/CT). One PET/CT study showed relatively low uptake of a chylomicron-borne fatty acid analogue into BAT of cold-stimulated humans [253]. This may be explained by the slow metabolization rate of the specific fatty acid analogue used in the experiments [254]. Alternative fatty acid analogues such as one reported recently [255] need to be tested in future studies to obtain better quantitative estimates of BAT lipoprotein uptake and lipid combustion, and thus the metabolic impact of BAT on the development of MAFLD in humans.
8. Conclusions and perspectives
The systemic metabolism of lipoproteins is closely linked to lipid homeostasis in the liver and, consequently, the development and progression of MAFLD. The accumulation of lipids in hepatocytes as the initiating event of MAFLD development is determined first by the efflux of lipids, predominantly through VLDL secretion, second through fatty acid and lipoprotein uptake, and third by de novo synthesis of fatty acids. Genetic polymorphisms and interventions demonstrate that functional alteration in single proteins of these pathways can be sufficient to influence MAFLD. A better understanding of the interaction between the branches of lipid metabolism within the liver is needed to predict outcomes of specific interventions. In particular, there are gaps in our knowledge regarding the subcellular compartmentalization of lipid synthesis, storage, and secretion.
Liver steatosis and damage have a substantial impact on plasma lipoprotein patterns, dyslipidemia, and consequently cardiovascular health. The effect of plasma lipoproteins on MAFLD is less clear and more research is warranted to understand how uptake of lipoprotein particles into hepatocytes and particularly into liver-resident or -infiltrating immune cells influences the progression of MAFLD. Along these lines, deciphering the effects of cell type-dependent lipoprotein processing on inflammation as compared to insulin resistance appears to be a promising research avenue towards better understanding the role of lipoprotein metabolism in the initiation and progression of MAFLD.
Funding
This work was supported by research grants of the German Research Foundation DFG (SFB 841: Liver Inflammation: Infection, Immune Regulation and Consequences, project B6 to J.H. and SCHE 522/4–1 to L.S.).
Contributor Information
Joerg Heeren, Email: heeren@uke.de.
Ludger Scheja, Email: l.scheja@uke.de.
Conflict of interest
None declared.
References
- 1.Dash S., Xiao C., Morgantini C., Lewis G.F. New insights into the regulation of chylomicron production. Annual Review of Nutrition. 2015;35:265–294. doi: 10.1146/annurev-nutr-071714-034338. [DOI] [PubMed] [Google Scholar]
- 2.Choi S.H., Ginsberg H.N. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends in Endocrinology and Metabolism. 2011;22(9):353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S.A., Kersten S., Qi L. Lipoprotein lipase and its regulators: an unfolding story. Trends in Endocrinology and Metabolism. 2021;32(1):48–61. doi: 10.1016/j.tem.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu D., Goldberg I.J. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Current Opinion in Lipidology. 2020;31(3):154–160. doi: 10.1097/MOL.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vos D.Y., van de Sluis B. Function of the endolysosomal network in cholesterol homeostasis and metabolic-associated fatty liver disease (MAFLD) Molecular Metabolism. 2021:101146. doi: 10.1016/j.molmet.2020.101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feingold K.R., Grunfeld C. Introduction to lipids and lipoproteins. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dungan K., editors. Endotext. 2000. South Dartmouth (MA) [Google Scholar]
- 7.Langsted A., Nordestgaard B.G. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology. 2019;51(2):131–141. doi: 10.1016/j.pathol.2018.09.062. [DOI] [PubMed] [Google Scholar]
- 8.Ference B.A., Ginsberg H.N., Graham I., Ray K.K., Packard C.J., Bruckert E. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. European Heart Journal. 2017;38(32):2459–2472. doi: 10.1093/eurheartj/ehx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouimet M., Barrett T.J., Fisher E.A. HDL and reverse cholesterol transport. Circulation Research. 2019;124(10):1505–1518. doi: 10.1161/CIRCRESAHA.119.312617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plummer A.M., Culbertson A.T., Liao M. The ABCs of sterol transport. Annual Review of Physiology. 2021;83:153–181. doi: 10.1146/annurev-physiol-031620-094944. [DOI] [PubMed] [Google Scholar]
- 11.Pavanello C., Calabresi L. Genetic, biochemical, and clinical features of LCAT deficiency: update for 2020. Current Opinion in Lipidology. 2020;31(4):232–237. doi: 10.1097/MOL.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 12.Brites F., Martin M., Guillas I., Kontush A. Antioxidative activity of high-density lipoprotein (HDL): mechanistic insights into potential clinical benefit. Biochimica et Biophysica Acta (BBA) Clinical. 2017;8:66–77. doi: 10.1016/j.bbacli.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nazir S., Jankowski V., Bender G., Zewinger S., Rye K.A., van der Vorst E.P.C. Interaction between high-density lipoproteins and inflammation: function matters more than concentration! Advanced Drug Delivery Reviews. 2020;159:94–119. doi: 10.1016/j.addr.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Haas J.T., Francque S., Staels B. Pathophysiology and mechanisms of nonalcoholic fatty liver disease. Annual Review of Physiology. 2016;78:181–205. doi: 10.1146/annurev-physiol-021115-105331. [DOI] [PubMed] [Google Scholar]
- 15.Friedman S.L., Neuschwander-Tetri B.A., Rinella M., Sanyal A.J. Mechanisms of NAFLD development and therapeutic strategies. Nature Medicine. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel V.T., Shulman G.I. Nonalcoholic fatty liver disease as a nexus of metabolic and hepatic diseases. Cell Metabolism. 2018;27(1):22–41. doi: 10.1016/j.cmet.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deprince A., Haas J.T., Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Molecular Metabolism. 2020;42:101092. doi: 10.1016/j.molmet.2020.101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eslam M., Sanyal A.J., George J., International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1991. [DOI] [PubMed] [Google Scholar]
- 19.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. New England Journal of Medicine. 2010;363(14):1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 20.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158(7):1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 22.Ioannou G.N. The role of cholesterol in the pathogenesis of NASH. Trends in Endocrinology and Metabolism. 2016;27(2):84–95. doi: 10.1016/j.tem.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Hajduch E., Lachkar F., Ferre P., Foufelle F. Roles of ceramides in non-alcoholic fatty liver disease. Journal of Clinical Medicine. 2021;10(4) doi: 10.3390/jcm10040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen M.C., Shulman G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends in Pharmacological Sciences. 2017;38(7):649–665. doi: 10.1016/j.tips.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jinjuvadia R., Antaki F., Lohia P., Liangpunsakul S. The association between nonalcoholic fatty liver disease and metabolic abnormalities in the United States population. Journal of Clinical Gastroenterology. 2017;51(2):160–166. doi: 10.1097/MCG.0000000000000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bril F., Barb D., Portillo-Sanchez P., Biernacki D., Lomonaco R., Suman A. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65(4):1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 27.Amor A.J., Pinyol M., Sola E., Catalan M., Cofan M., Herreras Z. Relationship between noninvasive scores of nonalcoholic fatty liver disease and nuclear magnetic resonance lipoprotein abnormalities: a focus on atherogenic dyslipidemia. Journal of Clinical Lipidology. 2017;11(2):551–561. doi: 10.1016/j.jacl.2017.02.001. e557. [DOI] [PubMed] [Google Scholar]
- 28.DeFilippis A.P., Blaha M.J., Martin S.S., Reed R.M., Jones S.R., Nasir K. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227(2):429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santos R.D., Valenti L., Romeo S. Does nonalcoholic fatty liver disease cause cardiovascular disease? Current knowledge and gaps. Atherosclerosis. 2019;282:110–120. doi: 10.1016/j.atherosclerosis.2019.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Fadaei R., Poustchi H., Meshkani R., Moradi N., Golmohammadi T., Merat S. Impaired HDL cholesterol efflux capacity in patients with non-alcoholic fatty liver disease is associated with subclinical atherosclerosis. Scientific Reports. 2018;8(1):11691. doi: 10.1038/s41598-018-29639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Berg E.H., Gruppen E.G., Ebtehaj S., Bakker S.J.L., Tietge U.J.F., Dullaart R.P.F. Cholesterol efflux capacity is impaired in subjects with an elevated Fatty Liver Index, a proxy of non-alcoholic fatty liver disease. Atherosclerosis. 2018;277:21–27. doi: 10.1016/j.atherosclerosis.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 32.McCullough A., Previs S.F., Dasarathy J., Lee K., Osme A., Kim C. HDL flux is higher in patients with nonalcoholic fatty liver disease. American Journal of Physiology. Endocrinology and Metabolism. 2019;317(5):E852–E862. doi: 10.1152/ajpendo.00193.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg E.H., Gruppen E.G., James R.W., Bakker S.J.L., Dullaart R.P.F. Serum paraoxonase 1 activity is paradoxically maintained in nonalcoholic fatty liver disease despite low HDL cholesterol. The Journal of Lipid Research. 2019;60(1):168–175. doi: 10.1194/jlr.P088997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannisto V.T., Simonen M., Soininen P., Tiainen M., Kangas A.J., Kaminska D. Lipoprotein subclass metabolism in nonalcoholic steatohepatitis. The Journal of Lipid Research. 2014;55(12):2676–2684. doi: 10.1194/jlr.P054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui M.S., Fuchs M., Idowu M.O., Luketic V.A., Boyett S., Sargeant C. Severity of nonalcoholic fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clinical Gastroenterology and Hepatology. 2015;13(5):1000–1008. doi: 10.1016/j.cgh.2014.10.008. e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujita K., Nozaki Y., Wada K., Yoneda M., Fujimoto Y., Fujitake M. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50(3):772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Z.G., Tapper E.B., Connelly M.A., Pimentel C.F., Feldbrugge L., Kim M. Steatohepatitis and liver fibrosis are predicted by the characteristics of very low density lipoprotein in nonalcoholic fatty liver disease. Liver International. 2016;36(8):1213–1220. doi: 10.1111/liv.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucero D., Miksztowicz V., Gualano G., Longo C., Landeira G., Alvarez E. Nonalcoholic fatty liver disease associated with metabolic syndrome: influence of liver fibrosis stages on characteristics of very low-density lipoproteins. Clinica Chimica Acta. 2017;473:1–8. doi: 10.1016/j.cca.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Jiang Z.G., Tsugawa Y., Tapper E.B., Lai M., Afdhal N., Robson S.C. Low-fasting triglyceride levels are associated with non-invasive markers of advanced liver fibrosis among adults in the United States. Alimentary Pharmacology & Therapeutics. 2015;42(1):106–116. doi: 10.1111/apt.13216. [DOI] [PubMed] [Google Scholar]
- 40.Konishi K., Miyake T., Furukawa S., Senba H., Kanzaki S., Nakaguchi H. Advanced fibrosis of non-alcoholic steatohepatitis affects the significance of lipoprotein(a) as a cardiovascular risk factor. Atherosclerosis. 2020;299:32–37. doi: 10.1016/j.atherosclerosis.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 41.Mucinski J.M., Manrique-Acevedo C., Kasumov T., Garrett T.J., Gaballah A., Parks E.J. Relationships between very low-density lipoproteins-ceramides, -diacylglycerols, and -triacylglycerols in insulin-resistant men. Lipids. 2020;55(4):387–393. doi: 10.1002/lipd.12244. [DOI] [PubMed] [Google Scholar]
- 42.Raabe M., Veniant M.M., Sullivan M.A., Zlot C.H., Bjorkegren J., Nielsen L.B. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. Journal of Clinical Investigation. 1999;103(9):1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian J., Nelson R., Lehner R. Carboxylesterases in lipid metabolism: from mouse to human. Protein Cell. 2018;9(2):178–195. doi: 10.1007/s13238-017-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos A.J., Nogueira C., Ortega-Bellido M., Malhotra V. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. The Journal of Cell Biology. 2016;213(3):343–354. doi: 10.1083/jcb.201603072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins J.A. Evidence that during very low density lipoprotein assembly in rat hepatocytes most of the triacylglycerol and phospholipid are packaged with apolipoprotein B in the Golgi complex. FEBS Letters. 1988;232(2):405–408. doi: 10.1016/0014-5793(88)80780-4. [DOI] [PubMed] [Google Scholar]
- 46.Seebacher F., Zeigerer A., Kory N., Krahmer N. Hepatic lipid droplet homeostasis and fatty liver disease. Seminars in Cell & Developmental Biology. 2020;108:72–81. doi: 10.1016/j.semcdb.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Gusarova V., Brodsky J.L., Fisher E.A. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. Journal of Biological Chemistry. 2003;278(48):48051–48058. doi: 10.1074/jbc.M306898200. [DOI] [PubMed] [Google Scholar]
- 48.Butkinaree C., Guo L., Ramkhelawon B., Wanschel A., Brodsky J.L., Moore K.J. A regulator of secretory vesicle size, Kelch-like protein 12, facilitates the secretion of apolipoprotein B100 and very-low-density lipoproteins--brief report. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(2):251–254. doi: 10.1161/ATVBAHA.113.302728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X., Wang H., Xu B., Huang D., Nie C., Pu L. Receptor-mediated ER export of lipoproteins controls lipid homeostasis in mice and humans. Cell Metabolism. 2021;33(2):350–366. doi: 10.1016/j.cmet.2020.10.020. e357. [DOI] [PubMed] [Google Scholar]
- 50.Adiels M., Taskinen M.R., Packard C., Caslake M.J., Soro-Paavonen A., Westerbacka J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49(4):755–765. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 51.Adiels M., Westerbacka J., Soro-Paavonen A., Hakkinen A.M., Vehkavaara S., Caslake M.J. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50(11):2356–2365. doi: 10.1007/s00125-007-0790-1. [DOI] [PubMed] [Google Scholar]
- 52.Fabbrini E., Mohammed B.S., Magkos F., Korenblat K.M., Patterson B.W., Klein S. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fabbrini E., Magkos F., Mohammed B.S., Pietka T., Abumrad N.A., Patterson B.W. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the U.S.A. 2009;106(36):15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welty F.K. Hypobetalipoproteinemia and abetalipoproteinemia: liver disease and cardiovascular disease. Current Opinion in Lipidology. 2020;31(2):49–55. doi: 10.1097/MOL.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 55.Kozlitina J., Smagris E., Stender S., Nordestgaard B.G., Zhou H.H., Tybjaerg-Hansen A. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahdessian H., Taxiarchis A., Popov S., Silveira A., Franco-Cereceda A., Hamsten A. TM6SF2 is a regulator of liver fat metabolism influencing triglyceride secretion and hepatic lipid droplet content. Proceedings of the National Academy of Sciences of the U.S.A. 2014;111(24):8913–8918. doi: 10.1073/pnas.1323785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu D.J., Peloso G.M., Yu H., Butterworth A.S., Wang X., Mahajan A. Exome-wide association study of plasma lipids in >300,000 individuals. Nature Genetics. 2017;49(12):1758–1766. doi: 10.1038/ng.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pirola C.J., Sookoian S. The dual and opposite role of the TM6SF2-rs58542926 variant in protecting against cardiovascular disease and conferring risk for nonalcoholic fatty liver: a meta-analysis. Hepatology. 2015;62(6):1742–1756. doi: 10.1002/hep.28142. [DOI] [PubMed] [Google Scholar]
- 59.Dongiovanni P., Petta S., Maglio C., Fracanzani A.L., Pipitone R., Mozzi E. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 60.Musso G., Cassader M., Paschetta E., Gambino R. TM6SF2 may drive postprandial lipoprotein cholesterol toxicity away from the vessel walls to the liver in NAFLD. Journal of Hepatology. 2016;64(4):979–981. doi: 10.1016/j.jhep.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 61.Smagris E., Gilyard S., BasuRay S., Cohen J.C., Hobbs H.H. Inactivation of Tm6sf2, a gene defective in fatty liver disease, impairs lipidation but not secretion of very low density lipoproteins. Journal of Biological Chemistry. 2016;291(20):10659–10676. doi: 10.1074/jbc.M116.719955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luukkonen P.K., Zhou Y., Nidhina Haridas P.A., Dwivedi O.P., Hyotylainen T., Ali A. Impaired hepatic lipid synthesis from polyunsaturated fatty acids in TM6SF2 E167K variant carriers with NAFLD. Journal of Hepatology. 2017;67(1):128–136. doi: 10.1016/j.jhep.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 63.Wurie H.R., Buckett L., Zammit V.A. Diacylglycerol acyltransferase 2 acts upstream of diacylglycerol acyltransferase 1 and utilizes nascent diglycerides and de novo synthesized fatty acids in HepG2 cells. FEBS Journal. 2012;279(17):3033–3047. doi: 10.1111/j.1742-4658.2012.08684.x. [DOI] [PubMed] [Google Scholar]
- 64.Villanueva C.J., Monetti M., Shih M., Zhou P., Watkins S.M., Bhanot S. Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology. 2009;50(2):434–442. doi: 10.1002/hep.22980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gluchowski N.L., Gabriel K.R., Chitraju C., Bronson R.T., Mejhert N., Boland S. Hepatocyte deletion of triglyceride-synthesis enzyme acyl CoA: diacylglycerol acyltransferase 2 reduces steatosis without increasing inflammation or fibrosis in mice. Hepatology. 2019;70(6):1972–1985. doi: 10.1002/hep.30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruhanen H., Nidhina Haridas P.A., Eskelinen E.L., Eriksson O., Olkkonen V.M., Kakela R. Depletion of TM6SF2 disturbs membrane lipid composition and dynamics in HuH7 hepatoma cells. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2017;1862(7):676–685. doi: 10.1016/j.bbalip.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 67.van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., Jacobs R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2017;1859(9 Pt B):1558–1572. doi: 10.1016/j.bbamem.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 68.Luukkonen P.K., Nick A., Holtta-Vuori M., Thiele C., Isokuortti E., Lallukka-Bruck S. Human PNPLA3-I148M variant increases hepatic retention of polyunsaturated fatty acids. JCI Insight. 2019;4(16) doi: 10.1172/jci.insight.127902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L.A. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pirazzi C., Adiels M., Burza M.A., Mancina R.M., Levin M., Stahlman M. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. Journal of Hepatology. 2012;57(6):1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 71.Huang Y., Cohen J.C., Hobbs H.H. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. Journal of Biological Chemistry. 2011;286(43):37085–37093. doi: 10.1074/jbc.M111.290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumari M., Schoiswohl G., Chitraju C., Paar M., Cornaciu I., Rangrez A.Y. Adiponutrin functions as a nutritionally regulated lysophosphatidic acid acyltransferase. Cell Metabolism. 2012;15(5):691–702. doi: 10.1016/j.cmet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.BasuRay S., Smagris E., Cohen J.C., Hobbs H.H. The PNPLA3 variant associated with fatty liver disease (I148M) accumulates on lipid droplets by evading ubiquitylation. Hepatology. 2017;66(4):1111–1124. doi: 10.1002/hep.29273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perez-Matos M.C., Sandhu B., Bonder A., Jiang Z.G. Lipoprotein metabolism in liver diseases. Current Opinion in Lipidology. 2019;30(1):30–36. doi: 10.1097/MOL.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 75.Haas M.E., Attie A.D., Biddinger S.B. The regulation of ApoB metabolism by insulin. Trends in Endocrinology and Metabolism. 2013;24(8):391–397. doi: 10.1016/j.tem.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim D.H., Zhang T., Lee S., Calabuig-Navarro V., Yamauchi J., Piccirillo A. FoxO6 integrates insulin signaling with MTP for regulating VLDL production in the liver. Endocrinology. 2014;155(4):1255–1267. doi: 10.1210/en.2013-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kamagate A., Dong H.H. FoxO1 integrates insulin signaling to VLDL production. Cell Cycle. 2008;7(20):3162–3170. doi: 10.4161/cc.7.20.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Allister E.M., Borradaile N.M., Edwards J.Y., Huff M.W. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54(6):1676–1683. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 79.Karimian Pour N., Adeli K. Insulin silences apolipoprotein B mRNA translation by inducing intracellular traffic into cytoplasmic RNA granules. Biochemistry. 2011;50(32):6942–6950. doi: 10.1021/bi200711v. [DOI] [PubMed] [Google Scholar]
- 80.Brown A.M., Gibbons G.F. Insulin inhibits the maturation phase of VLDL assembly via a phosphoinositide 3-kinase-mediated event. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(10):1656–1661. doi: 10.1161/hq1001.096640. [DOI] [PubMed] [Google Scholar]
- 81.Poulsen M.K., Nellemann B., Stodkilde-Jorgensen H., Pedersen S.B., Gronbaek H., Nielsen S. Impaired insulin suppression of VLDL-triglyceride kinetics in nonalcoholic fatty liver disease. Journal of Clinical Endocrinology & Metabolism. 2016;101(4):1637–1646. doi: 10.1210/jc.2015-3476. [DOI] [PubMed] [Google Scholar]
- 82.Biddinger S.B., Hernandez-Ono A., Rask-Madsen C., Haas J.T., Aleman J.O., Suzuki R. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metabolism. 2008;7(2):125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lytle K.A., Bush N.C., Triay J.M., Kellogg T.A., Kendrick M.L., Swain J.M. Hepatic fatty acid balance and hepatic fat content in humans with severe obesity. Journal of Clinical Endocrinology & Metabolism. 2019;104(12):6171–6181. doi: 10.1210/jc.2019-00875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charlton M., Sreekumar R., Rasmussen D., Lindor K., Nair K.S. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35(4):898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 85.Jiang Z.G., de Boer I.H., Mackey R.H., Jensen M.K., Lai M., Robson S.C. Associations of insulin resistance, inflammation and liver synthetic function with very low-density lipoprotein: the Cardiovascular Health Study. Metabolism. 2016;65(3):92–99. doi: 10.1016/j.metabol.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caviglia J.M., Gayet C., Ota T., Hernandez-Ono A., Conlon D.M., Jiang H. Different fatty acids inhibit apoB100 secretion by different pathways: unique roles for ER stress, ceramide, and autophagy. The Journal of Lipid Research. 2011;52(9):1636–1651. doi: 10.1194/jlr.M016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ota T., Gayet C., Ginsberg H.N. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. Journal of Clinical Investigation. 2008;118(1):316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lemmer I.L., Willemsen N., Hilal N., Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Molecular Metabolism. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Widenmaier S.B., Snyder N.A., Nguyen T.B., Arduini A., Lee G.Y., Arruda A.P. NRF1 is an ER membrane sensor that is central to cholesterol homeostasis. Cell. 2017;171(5):1094–1109. doi: 10.1016/j.cell.2017.10.003. e1015. [DOI] [PubMed] [Google Scholar]
- 90.Koo J.H., Han C.Y. Signaling nodes associated with endoplasmic reticulum stress during NAFLD progression. Biomolecules. 2021;11(2) doi: 10.3390/biom11020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Song M.J., Malhi H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacology & Therapeutics. 2019;203:107401. doi: 10.1016/j.pharmthera.2019.107401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diraison F., Moulin P., Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes & Metabolism. 2003;29(5):478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 93.Faix D., Neese R., Kletke C., Wolden S., Cesar D., Coutlangus M. Quantification of menstrual and diurnal periodicities in rates of cholesterol and fat synthesis in humans. The Journal of Lipid Research. 1993;34(12):2063–2075. [PubMed] [Google Scholar]
- 94.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2020;130(3):1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. Journal of Clinical Investigation. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kohjima M., Enjoji M., Higuchi N., Kato M., Kotoh K., Yoshimoto T. Re-evaluation of fatty acid metabolism-related gene expression in nonalcoholic fatty liver disease. International Journal of Molecular Medicine. 2007;20(3):351–358. [PubMed] [Google Scholar]
- 97.Softic S., Gupta M.K., Wang G.X., Fujisaka S., O'Neill B.T., Rao T.N. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. Journal of Clinical Investigation. 2018;128(3):1199. doi: 10.1172/JCI99009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Postic C., Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. Journal of Clinical Investigation. 2008;118(3):829–838. doi: 10.1172/JCI34275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Y., Viscarra J., Kim S.J., Sul H.S. Transcriptional regulation of hepatic lipogenesis. Nature Reviews Molecular Cell Biology. 2015;16(11):678–689. doi: 10.1038/nrm4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Eissing L., Scherer T., Todter K., Knippschild U., Greve J.W., Buurman W.A. De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nature Communications. 2013;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lei Y., Hoogerland J.A., Bloks V.W., Bos T., Bleeker A., Wolters H. Hepatic carbohydrate response element binding protein activation limits nonalcoholic fatty liver disease development in a mouse model for glycogen storage disease type 1a. Hepatology. 2020;72(5):1638–1653. doi: 10.1002/hep.31198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grefhorst A., Elzinga B.M., Voshol P.J., Plosch T., Kok T., Bloks V.W. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. Journal of Biological Chemistry. 2002;277(37):34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 103.Takanashi M., Kimura T., Li C., Tanaka M., Matsuhashi A., Yoshida H. Critical role of SREBP-1c large-VLDL pathway in environment-induced hypertriglyceridemia of apo AV deficiency. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(3):373–386. doi: 10.1161/ATVBAHA.118.311931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J.J., Lambert J.E., Hovhannisyan Y., Ramos-Roman M.A., Trombold J.R., Wagner D.A. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. American Journal of Clinical Nutrition. 2015;101(1):34–43. doi: 10.3945/ajcn.114.092262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lam T.K., Gutierrez-Juarez R., Pocai A., Bhanot S., Tso P., Schwartz G.J. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nature Medicine. 2007;13(2):171–180. doi: 10.1038/nm1540. [DOI] [PubMed] [Google Scholar]
- 106.Rojas J.M., Stafford J.M., Saadat S., Printz R.L., Beck-Sickinger A.G., Niswender K.D. Central nervous system neuropeptide Y signaling via the Y1 receptor partially dissociates feeding behavior from lipoprotein metabolism in lean rats. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(12):E1479–E1488. doi: 10.1152/ajpendo.00351.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brown J.M., Chung S., Sawyer J.K., Degirolamo C., Alger H.M., Nguyen T. Inhibition of stearoyl-coenzyme A desaturase 1 dissociates insulin resistance and obesity from atherosclerosis. Circulation. 2008;118(14):1467–1475. doi: 10.1161/CIRCULATIONAHA.108.793182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miyazaki M., Flowers M.T., Sampath H., Chu K., Otzelberger C., Liu X. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metabolism. 2007;6(6):484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 109.Man W.C., Miyazaki M., Chu K., Ntambi J. Colocalization of SCD1 and DGAT2: implying preference for endogenous monounsaturated fatty acids in triglyceride synthesis. The Journal of Lipid Research. 2006;47(9):1928–1939. doi: 10.1194/jlr.M600172-JLR200. [DOI] [PubMed] [Google Scholar]
- 110.Qi J., Lang W., Geisler J.G., Wang P., Petrounia I., Mai S. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. The Journal of Lipid Research. 2012;53(6):1106–1116. doi: 10.1194/jlr.M020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McLaren D.G., Han S., Murphy B.A., Wilsie L., Stout S.J., Zhou H. DGAT2 inhibition alters aspects of triglyceride metabolism in rodents but not in non-human primates. Cell Metabolism. 2018;27(6):1236–1248. doi: 10.1016/j.cmet.2018.04.004. e1236. [DOI] [PubMed] [Google Scholar]
- 112.Liu Y., Millar J.S., Cromley D.A., Graham M., Crooke R., Billheimer J.T. Knockdown of acyl-CoA:diacylglycerol acyltransferase 2 with antisense oligonucleotide reduces VLDL TG and ApoB secretion in mice. Biochimica et Biophysica Acta. 2008;1781(3):97–104. doi: 10.1016/j.bbalip.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Kerner J., Hoppel C. Fatty acid import into mitochondria. Biochimica et Biophysica Acta. 2000;1486(1):1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 114.Goedeke L., Fernandez-Hernando C. Regulation of cholesterol homeostasis. Cellular and Molecular Life Sciences. 2012;69(6):915–930. doi: 10.1007/s00018-011-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goedeke L., Bates J., Vatner D.F., Perry R.J., Wang T., Ramirez R. Acetyl-CoA carboxylase inhibition reverses NAFLD and hepatic insulin resistance but promotes hypertriglyceridemia in rodents. Hepatology. 2018;68(6):2197–2211. doi: 10.1002/hep.30097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim C.W., Addy C., Kusunoki J., Anderson N.N., Deja S., Fu X. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metabolism. 2017;26(3):576. doi: 10.1016/j.cmet.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 117.Wu M., Singh S.B., Wang J., Chung C.C., Salituro G., Karanam B.V. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proceedings of the National Academy of Sciences of the U.S.A. 2011;108(13):5378–5383. doi: 10.1073/pnas.1002588108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Q., Li S., Jiang L., Zhou Y., Li Z., Shao M. Deficiency in hepatic ATP-citrate lyase affects VLDL-triglyceride mobilization and liver fatty acid composition in mice. The Journal of Lipid Research. 2010;51(9):2516–2526. doi: 10.1194/jlr.M003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Samsoondar J.P., Burke A.C., Sutherland B.G., Telford D.E., Sawyez C.G., Edwards J.Y. Prevention of diet-induced metabolic dysregulation, inflammation, and atherosclerosis in ldlr(-/-) mice by treatment with the ATP-citrate lyase inhibitor bempedoic acid. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(4):647–656. doi: 10.1161/ATVBAHA.116.308963. [DOI] [PubMed] [Google Scholar]
- 120.Singh S.B., Kang L., Nawrocki A.R., Zhou D., Wu M., Previs S. The fatty acid synthase inhibitor platensimycin improves insulin resistance without inducing liver steatosis in mice and monkeys. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0164133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chakravarthy M.V., Pan Z., Zhu Y., Tordjman K., Schneider J.G., Coleman T. New" hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metabolism. 2005;1(5):309–322. doi: 10.1016/j.cmet.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 122.Alkhouri N., Lawitz E., Noureddin M., DeFronzo R., Shulman G.I. GS-0976 (Firsocostat): an investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH) Expert Opinion on Investigational Drugs. 2020;29(2):135–141. doi: 10.1080/13543784.2020.1668374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu J., Teran-Garcia M., Park J.H., Nakamura M.T., Clarke S.D. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. Journal of Biological Chemistry. 2001;276(13):9800–9807. doi: 10.1074/jbc.M008973200. [DOI] [PubMed] [Google Scholar]
- 124.Moon Y.A., Hammer R.E., Horton J.D. Deletion of ELOVL5 leads to fatty liver through activation of SREBP-1c in mice. The Journal of Lipid Research. 2009;50(3):412–423. doi: 10.1194/jlr.M800383-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng X., Zhang L., Xu S., Shen A.Z. ATP-citrate lyase (ACLY) in lipid metabolism and atherosclerosis: an updated review. Progress in Lipid Research. 2020;77:101006. doi: 10.1016/j.plipres.2019.101006. [DOI] [PubMed] [Google Scholar]
- 126.Pinkosky S.L., Newton R.S., Day E.A., Ford R.J., Lhotak S., Austin R.C. Liver-specific ATP-citrate lyase inhibition by bempedoic acid decreases LDL-C and attenuates atherosclerosis. Nature Communications. 2016;7:13457. doi: 10.1038/ncomms13457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Q., Jiang L., Wang J., Li S., Yu Y., You J. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology. 2009;49(4):1166–1175. doi: 10.1002/hep.22774. [DOI] [PubMed] [Google Scholar]
- 128.Tg, Hdl Working Group of the Exome Sequencing Project, N.H.L. Blood I., Crosby J., Peloso G.M., Auer P.L. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. New England Journal of Medicine. 2014;371(1):22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jorgensen A.B., Frikke-Schmidt R., Nordestgaard B.G., Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. New England Journal of Medicine. 2014;371(1):32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 130.Wulff A.B., Nordestgaard B.G., Tybjaerg-Hansen A. APOC3 loss-of-function mutations, remnant cholesterol, low-density lipoprotein cholesterol, and cardiovascular risk: mediation- and meta-analyses of 137 895 individuals. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(3):660–668. doi: 10.1161/ATVBAHA.117.310473. [DOI] [PubMed] [Google Scholar]
- 131.Silbernagel G., Scharnagl H., Kleber M.E., Hoffmann M.M., Delgado G., Stojakovic T. Common APOC3 variants are associated with circulating ApoC-III and VLDL cholesterol but not with total apolipoprotein B and coronary artery disease. Atherosclerosis. 2020;311:84–90. doi: 10.1016/j.atherosclerosis.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 132.Jong M.C., Hofker M.H., Havekes L.M. Role of ApoCs in lipoprotein metabolism: functional differences between ApoC1, ApoC2, and ApoC3. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19(3):472–484. doi: 10.1161/01.atv.19.3.472. [DOI] [PubMed] [Google Scholar]
- 133.Kersten S. Physiological regulation of lipoprotein lipase. Biochimica et Biophysica Acta. 2014;1841(7):919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 134.Ramms B., Gordts P. Apolipoprotein C-III in triglyceride-rich lipoprotein metabolism. Current Opinion in Lipidology. 2018;29(3):171–179. doi: 10.1097/MOL.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 135.Ramms B., Patel S., Nora C., Pessentheiner A.R., Chang M.W., Green C.R. ApoC-III ASO promotes tissue LPL activity in the absence of apoE-mediated TRL clearance. The Journal of Lipid Research. 2019;60(8):1379–1395. doi: 10.1194/jlr.M093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Witztum J.L., Gaudet D., Freedman S.D., Alexander V.J., Digenio A., Williams K.R. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. New England Journal of Medicine. 2019;381(6):531–542. doi: 10.1056/NEJMoa1715944. [DOI] [PubMed] [Google Scholar]
- 137.Lee H.Y., Birkenfeld A.L., Jornayvaz F.R., Jurczak M.J., Kanda S., Popov V. Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology. 2011;54(5):1650–1660. doi: 10.1002/hep.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paiva A.A., Raposo H.F., Wanschel A.C., Nardelli T.R., Oliveira H.C. Apolipoprotein CIII overexpression-induced hypertriglyceridemia increases nonalcoholic fatty liver disease in association with inflammation and cell death. Oxidation Medicine Cell Longevity. 2017;2017:1838679. doi: 10.1155/2017/1838679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng X., Yamauchi J., Lee S., Zhang T., Gong Z., Muzumdar R. APOC3 protein is not a predisposing factor for fat-induced nonalcoholic fatty liver disease in mice. Journal of Biological Chemistry. 2017;292(9):3692–3705. doi: 10.1074/jbc.M116.765917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Petersen K.F., Dufour S., Hariri A., Nelson-Williams C., Foo J.N., Zhang X.M. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. New England Journal of Medicine. 2010;362(12):1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kozlitina J., Boerwinkle E., Cohen J.C., Hobbs H.H. Dissociation between APOC3 variants, hepatic triglyceride content and insulin resistance. Hepatology. 2011;53(2):467–474. doi: 10.1002/hep.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hyysalo J., Stojkovic I., Kotronen A., Hakkarainen A., Sevastianova K., Makkonen J. Genetic variation in PNPLA3 but not APOC3 influences liver fat in non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2012;27(5):951–956. doi: 10.1111/j.1440-1746.2011.07045.x. [DOI] [PubMed] [Google Scholar]
- 143.Verrijken A., Beckers S., Francque S., Hilden H., Caron S., Zegers D. A gene variant of PNPLA3, but not of APOC3, is associated with histological parameters of NAFLD in an obese population. Obesity. 2013;21(10):2138–2145. doi: 10.1002/oby.20366. [DOI] [PubMed] [Google Scholar]
- 144.Pennacchio L.A., Olivier M., Hubacek J.A., Cohen J.C., Cox D.R., Fruchart J.C. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294(5540):169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 145.Forte T.M., Ryan R.O. Apolipoprotein A5: extracellular and intracellular roles in triglyceride metabolism. Current Drug Targets. 2015;16(12):1274–1280. doi: 10.2174/1389450116666150531161138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Merkel M., Heeren J. Give me A5 for lipoprotein hydrolysis! Journal of Clinical Investigation. 2005;115(10):2694–2696. doi: 10.1172/JCI26712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dorfmeister B., Zeng W.W., Dichlberger A., Nilsson S.K., Schaap F.G., Hubacek J.A. Effects of six APOA5 variants, identified in patients with severe hypertriglyceridemia, on in vitro lipoprotein lipase activity and receptor binding. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(10):1866–1871. doi: 10.1161/ATVBAHA.108.172866. [DOI] [PubMed] [Google Scholar]
- 148.Wang J., Ban M.R., Kennedy B.A., Anand S., Yusuf S., Huff M.W. APOA5 genetic variants are markers for classic hyperlipoproteinemia phenotypes and hypertriglyceridemia. Nature Clinical Practice Cardiovascular Medicine. 2008;5(11):730–737. doi: 10.1038/ncpcardio1326. [DOI] [PubMed] [Google Scholar]
- 149.Triglyceride Coronary Disease Genetics, C., Emerging risk factors, C. Sarwar N., Sandhu M.S., Ricketts S.L., Butterworth A.S. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375(9726):1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O'Brien P.J., Alborn W.E., Sloan J.H., Ulmer M., Boodhoo A., Knierman M.D. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clinical Chemistry. 2005;51(2):351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 151.Fruchart-Najib J., Bauge E., Niculescu L.S., Pham T., Thomas B., Rommens C. Mechanism of triglyceride lowering in mice expressing human apolipoprotein A5. Biochemical and Biophysical Research Communications. 2004;319(2):397–404. doi: 10.1016/j.bbrc.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 152.Schaap F.G., Rensen P.C., Voshol P.J., Vrins C., van der Vliet H.N., Chamuleau R.A. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. Journal of Biological Chemistry. 2004;279(27):27941–27947. doi: 10.1074/jbc.M403240200. [DOI] [PubMed] [Google Scholar]
- 153.Merkel M., Loeffler B., Kluger M., Fabig N., Geppert G., Pennacchio L.A. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. Journal of Biological Chemistry. 2005;280(22):21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 154.Trevino-Villarreal J.H., Reynolds J.S., Bartelt A., Langston P.K., MacArthur M.R., Arduini A. Dietary protein restriction reduces circulating VLDL triglyceride levels via CREBH-APOA5-dependent and -independent mechanisms. JCI Insight. 2018;3(21) doi: 10.1172/jci.insight.99470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nilsson S.K., Lookene A., Beckstead J.A., Gliemann J., Ryan R.O., Olivecrona G. Apolipoprotein A-V interaction with members of the low density lipoprotein receptor gene family. Biochemistry. 2007;46(12):3896–3904. doi: 10.1021/bi7000533. [DOI] [PubMed] [Google Scholar]
- 156.Gonzales J.C., Gordts P.L., Foley E.M., Esko J.D. Apolipoproteins E and AV mediate lipoprotein clearance by hepatic proteoglycans. Journal of Clinical Investigation. 2013;123(6):2742–2751. doi: 10.1172/JCI67398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feng Q., Baker S.S., Liu W., Arbizu R.A., Aljomah G., Khatib M. Increased apolipoprotein A5 expression in human and rat non-alcoholic fatty livers. Pathology. 2015;47(4):341–348. doi: 10.1097/PAT.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ress C., Moschen A.R., Sausgruber N., Tschoner A., Graziadei I., Weiss H. The role of apolipoprotein A5 in non-alcoholic fatty liver disease. Gut. 2011;60(7):985–991. doi: 10.1136/gut.2010.222224. [DOI] [PubMed] [Google Scholar]
- 159.Blade A.M., Fabritius M.A., Hou L., Weinberg R.B., Shelness G.S. Biogenesis of apolipoprotein A-V and its impact on VLDL triglyceride secretion. The Journal of Lipid Research. 2011;52(2):237–244. doi: 10.1194/jlr.M010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gao X., Forte T.M., Ryan R.O. Influence of apolipoprotein A-V on hepatocyte lipid droplet formation. Biochemical and Biophysical Research Communications. 2012;427(2):361–365. doi: 10.1016/j.bbrc.2012.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shu X., Chan J., Ryan R.O., Forte T.M. Apolipoprotein A-V association with intracellular lipid droplets. The Journal of Lipid Research. 2007;48(7):1445–1450. doi: 10.1194/jlr.C700002-JLR200. [DOI] [PubMed] [Google Scholar]
- 162.Shu X., Nelbach L., Ryan R.O., Forte T.M. Apolipoprotein A-V associates with intrahepatic lipid droplets and influences triglyceride accumulation. Biochimica et Biophysica Acta. 2010;1801(5):605–608. doi: 10.1016/j.bbalip.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Camporez J.P., Kanda S., Petersen M.C., Jornayvaz F.R., Samuel V.T., Bhanot S. ApoA5 knockdown improves whole-body insulin sensitivity in high-fat-fed mice by reducing ectopic lipid content. The Journal of Lipid Research. 2015;56(3):526–536. doi: 10.1194/jlr.M054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu Y., Lu L.L., Liu S.S., Du S.X., Zhu H.L., Dong Q.J. Apolipoprotein A5 gene polymorphisms are associated with non-alcoholic fatty liver disease. Hepatobiliary and Pancreatic Diseases International. 2018;17(3):214–219. doi: 10.1016/j.hbpd.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 165.Albers K., Schlein C., Wenner K., Lohse P., Bartelt A., Heeren J. Homozygosity for a partial deletion of apoprotein A-V signal peptide results in intracellular missorting of the protein and chylomicronemia in a breast-fed infant. Atherosclerosis. 2014;233(1):97–103. doi: 10.1016/j.atherosclerosis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 166.Haller J.F., Mintah I.J., Shihanian L.M., Stevis P., Buckler D., Alexa-Braun C.A. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. The Journal of Lipid Research. 2017;58(6):1166–1173. doi: 10.1194/jlr.M075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chi X., Britt E.C., Shows H.W., Hjelmaas A.J., Shetty S.K., Cushing E.M. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Molecular Metabolism. 2017;6(10):1137–1149. doi: 10.1016/j.molmet.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kovrov O., Kristensen K.K., Larsson E., Ploug M., Olivecrona G. On the mechanism of angiopoietin-like protein 8 for control of lipoprotein lipase activity. The Journal of Lipid Research. 2019;60(4):783–793. doi: 10.1194/jlr.M088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen S., Feng M., Zhang S., Dong Z., Wang Y., Zhang W. Angptl8 mediates food-driven resetting of hepatic circadian clock in mice. Nature Communications. 2019;10(1):3518. doi: 10.1038/s41467-019-11513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D.M., Cohen J.C. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proceedings of the National Academy of Sciences of the U.S.A. 2013;110(40):16109–16114. doi: 10.1073/pnas.1315292110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oldoni F., Cheng H., Banfi S., Gusarova V., Cohen J.C., Hobbs H.H. ANGPTL8 has both endocrine and autocrine effects on substrate utilization. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.138777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ren G., Kim J.Y., Smas C.M. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. American Journal of Physiology. Endocrinology and Metabolism. 2012;303(3):E334–E351. doi: 10.1152/ajpendo.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochemical and Biophysical Research Communications. 2012;424(4):786–792. doi: 10.1016/j.bbrc.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 174.Quagliarini F., Wang Y., Kozlitina J., Grishin N.V., Hyde R., Boerwinkle E. Atypical angiopoietin-like protein that regulates ANGPTL3. Proceedings of the National Academy of Sciences of the U.S.A. 2012;109(48):19751–19756. doi: 10.1073/pnas.1217552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen Y.Q., Pottanat T.G., Siegel R.W., Ehsani M., Qian Y.W., Zhen E.Y. Angiopoietin-like protein 8 differentially regulates ANGPTL3 and ANGPTL4 during postprandial partitioning of fatty acids. The Journal of Lipid Research. 2020;61(8):1203–1220. doi: 10.1194/jlr.RA120000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dewey F.E., Gusarova V., Dunbar R.L., O'Dushlaine C., Schurmann C., Gottesman O. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. New England Journal of Medicine. 2017;377(3):211–221. doi: 10.1056/NEJMoa1612790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Minicocci I., Tikka A., Poggiogalle E., Metso J., Montali A., Ceci F. Effects of angiopoietin-like protein 3 deficiency on postprandial lipid and lipoprotein metabolism. The Journal of Lipid Research. 2016;57(6):1097–1107. doi: 10.1194/jlr.P066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dewey F.E., Gusarova V., O'Dushlaine C., Gottesman O., Trejos J., Hunt C. Inactivating variants in ANGPTL4 and risk of coronary artery disease. New England Journal of Medicine. 2016;374(12):1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Myocardial Infarction G., Investigators C.A.E.C., Stitziel N.O., Stirrups K.E., Masca N.G., Erdmann J. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. New England Journal of Medicine. 2016;374(12):1134–1144. doi: 10.1056/NEJMoa1507652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lim G.B. Genetics: polymorphisms in ANGPTL4 link triglycerides with CAD. Nature Reviews Cardiology. 2016;13(5):245. doi: 10.1038/nrcardio.2016.46. [DOI] [PubMed] [Google Scholar]
- 181.Gaudet D., Karwatowska-Prokopczuk E., Baum S.J., Hurh E., Kingsbury J., Bartlett V.J. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. European Heart Journal. 2020;41(40):3936–3945. doi: 10.1093/eurheartj/ehaa689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yilmaz Y., Ulukaya E., Atug O., Dolar E. Serum concentrations of human angiopoietin-like protein 3 in patients with nonalcoholic fatty liver disease: association with insulin resistance. European Journal of Gastroenterology and Hepatology. 2009;21(11):1247–1251. doi: 10.1097/MEG.0b013e32832b77ae. [DOI] [PubMed] [Google Scholar]
- 183.Minicocci I., Santini S., Cantisani V., Stitziel N., Kathiresan S., Arroyo J.A. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: a pooled analysis. The Journal of Lipid Research. 2013;54(12):3481–3490. doi: 10.1194/jlr.P039875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Inaba T., Matsuda M., Shimamura M., Takei N., Terasaka N., Ando Y. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. Journal of Biological Chemistry. 2003;278(24):21344–21351. doi: 10.1074/jbc.M213202200. [DOI] [PubMed] [Google Scholar]
- 185.Wang Y., McNutt M.C., Banfi S., Levin M.G., Holland W.L., Gusarova V. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proceedings of the National Academy of Sciences of the U.S.A. 2015;112(37):11630–11635. doi: 10.1073/pnas.1515374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gusarova V., Alexa C.A., Wang Y., Rafique A., Kim J.H., Buckler D. ANGPTL3 blockade with a human monoclonal antibody reduces plasma lipids in dyslipidemic mice and monkeys. The Journal of Lipid Research. 2015;56(7):1308–1317. doi: 10.1194/jlr.M054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mele C., Grugni G., Mai S., Vietti R., Aimaretti G., Scacchi M. Circulating angiopoietin-like 8 (ANGPTL8) is a marker of liver steatosis and is negatively regulated by Prader-Willi Syndrome. Scientific Reports. 2017;7(1):3186. doi: 10.1038/s41598-017-03538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.von Loeffelholz C., Pfeiffer A.F.H., Lock J.F., Lieske S., Docke S., Murahovschi V. ANGPTL8 (betatrophin) is expressed in visceral adipose tissue and relates to human hepatic steatosis in two independent clinical collectives. Hormone and Metabolic Research. 2017;49(5):343–349. doi: 10.1055/s-0043-102950. [DOI] [PubMed] [Google Scholar]
- 189.Hu W., Shao X., Guo D., Hao H., Zhang Y., Xia M. Relationship of serum betatrophin with nonalcoholic fatty liver in a Chinese population. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0170758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee Y.H., Lee S.G., Lee C.J., Kim S.H., Song Y.M., Yoon M.R. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Scientific Reports. 2016;6:24013. doi: 10.1038/srep24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vatner D.F., Goedeke L., Camporez J.G., Lyu K., Nasiri A.R., Zhang D. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia. 2018;61(6):1435–1446. doi: 10.1007/s00125-018-4579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gusarova V., Banfi S., Alexa-Braun C.A., Shihanian L.M., Mintah I.J., Lee J.S. ANGPTL8 blockade with a monoclonal antibody promotes triglyceride clearance, energy expenditure, and weight loss in mice. Endocrinology. 2017;158(5):1252–1259. doi: 10.1210/en.2016-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aryal B., Singh A.K., Zhang X., Varela L., Rotllan N., Goedeke L. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight. 2018;3(6) doi: 10.1172/jci.insight.97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scheja L., Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. Journal of Hepatology. 2016;64(5):1176–1186. doi: 10.1016/j.jhep.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 195.Chin J., Mori T.A., Adams L.A., Beilin L.J., Huang R.C., Olynyk J.K. Association between remnant lipoprotein cholesterol levels and non-alcoholic fatty liver disease in adolescents. JHEP Report. 2020;2(6):100150. doi: 10.1016/j.jhepr.2020.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pastori D., Baratta F., Novo M., Cocomello N., Violi F., Angelico F. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non-alcoholic fatty liver disease. Journal of Clinical Medicine. 2018;7(11) doi: 10.3390/jcm7110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Matikainen N., Manttari S., Westerbacka J., Vehkavaara S., Lundbom N., Yki-Jarvinen H. Postprandial lipemia associates with liver fat content. Journal of Clinical Endocrinology & Metabolism. 2007;92(8):3052–3059. doi: 10.1210/jc.2007-0187. [DOI] [PubMed] [Google Scholar]
- 198.Brown M.S., Goldstein J.L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 199.Karavia E.A., Papachristou D.J., Kotsikogianni I., Giopanou I., Kypreos K.E. Deficiency in apolipoprotein E has a protective effect on diet-induced nonalcoholic fatty liver disease in mice. FEBS Journal. 2011;278(17):3119–3129. doi: 10.1111/j.1742-4658.2011.08238.x. [DOI] [PubMed] [Google Scholar]
- 200.Bieghs V., Van Gorp P.J., Wouters K., Hendrikx T., Gijbels M.J., van Bilsen M. LDL receptor knock-out mice are a physiological model particularly vulnerable to study the onset of inflammation in non-alcoholic fatty liver disease. PLoS One. 2012;7(1) doi: 10.1371/journal.pone.0030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rohlmann A., Gotthardt M., Hammer R.E., Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. Journal of Clinical Investigation. 1998;101(3):689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hamlin A.N., Chinnarasu S., Ding Y., Xian X., Herz J., Jaeschke A. Low-density lipoprotein receptor-related protein-1 dysfunction synergizes with dietary cholesterol to accelerate steatohepatitis progression. Journal of Biological Chemistry. 2018;293(25):9674–9684. doi: 10.1074/jbc.RA118.001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ding Y., Xian X., Holland W.L., Tsai S., Herz J. Low-density lipoprotein receptor-related protein-1 protects against hepatic insulin resistance and hepatic steatosis. EBioMedicine. 2016;7:135–145. doi: 10.1016/j.ebiom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jaeschke A., Haller A., Cash J.G., Nam C., Igel E., Roebroek A.J.M. Mutation in the distal NPxY motif of LRP1 alleviates dietary cholesterol-induced dyslipidemia and tissue inflammation. The Journal of Lipid Research. 2020 doi: 10.1194/jlr.RA120001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bartelt A., Orlando P., Mele C., Ligresti A., Toedter K., Scheja L. Altered endocannabinoid signalling after a high-fat diet in Apoe(-/-) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia. 2011;54(11):2900–2910. doi: 10.1007/s00125-011-2274-6. [DOI] [PubMed] [Google Scholar]
- 206.Schierwagen R., Maybuchen L., Zimmer S., Hittatiya K., Back C., Klein S. Seven weeks of Western diet in apolipoprotein-E-deficient mice induce metabolic syndrome and non-alcoholic steatohepatitis with liver fibrosis. Scientific Reports. 2015;5:12931. doi: 10.1038/srep12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wagner T., Bartelt A., Schlein C., Heeren J. Genetic dissection of tissue-specific apolipoprotein E function for hypercholesterolemia and diet-induced obesity. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mensenkamp A.R., Van Luyn M.J., Havinga R., Teusink B., Waterman I.J., Mann C.J. The transport of triglycerides through the secretory pathway of hepatocytes is impaired in apolipoprotein E deficient mice. Journal of Hepatology. 2004;40(4):599–606. doi: 10.1016/j.jhep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 209.Ho C.M., Ho S.L., Jeng Y.M., Lai Y.S., Chen Y.H., Lu S.C. Accumulation of free cholesterol and oxidized low-density lipoprotein is associated with portal inflammation and fibrosis in nonalcoholic fatty liver disease. Journal of Inflammation. 2019;16:7. doi: 10.1186/s12950-019-0211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.McGettigan B., McMahan R., Orlicky D., Burchill M., Danhorn T., Francis P. Dietary lipids differentially shape nonalcoholic steatohepatitis progression and the transcriptome of kupffer cells and infiltrating macrophages. Hepatology. 2019;70(1):67–83. doi: 10.1002/hep.30401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.He Y., Rodrigues R.M., Wang X., Seo W., Ma J., Hwang S. Neutrophil-to-hepatocyte communication via LDLR-dependent miR-223-enriched extracellular vesicle transfer ameliorates nonalcoholic steatohepatitis. Journal of Clinical Investigation. 2020 doi: 10.1172/JCI141513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takahashi S., Sakai J., Fujino T., Hattori H., Zenimaru Y., Suzuki J. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. Journal of Atherosclerosis and Thrombosis. 2004;11(4):200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- 213.Jo H., Choe S.S., Shin K.C., Jang H., Lee J.H., Seong J.K. Endoplasmic reticulum stress induces hepatic steatosis via increased expression of the hepatic very low-density lipoprotein receptor. Hepatology. 2013;57(4):1366–1377. doi: 10.1002/hep.26126. [DOI] [PubMed] [Google Scholar]
- 214.Zarei M., Barroso E., Palomer X., Dai J., Rada P., Quesada-Lopez T. Hepatic regulation of VLDL receptor by PPARbeta/delta and FGF21 modulates non-alcoholic fatty liver disease. Molecular Metabolism. 2018;8:117–131. doi: 10.1016/j.molmet.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lebeau P.F., Byun J.H., Platko K., Al-Hashimi A.A., Lhotak S., MacDonald M.E. Pcsk9 knockout exacerbates diet-induced non-alcoholic steatohepatitis, fibrosis and liver injury in mice. JHEP Report. 2019;1(6):418–429. doi: 10.1016/j.jhepr.2019.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Baragetti A., Balzarotti G., Grigore L., Pellegatta F., Guerrini U., Pisano G. PCSK9 deficiency results in increased ectopic fat accumulation in experimental models and in humans. European journal of preventive cardiology. 2017;24:1870–1877. doi: 10.1177/2047487317724342. [DOI] [PubMed] [Google Scholar]
- 217.Da Dalt L., Ruscica M., Bonacina F., Balzarotti G., Dhyani A., Di Cairano E. PCSK9 deficiency reduces insulin secretion and promotes glucose intolerance: the role of the low-density lipoprotein receptor. European Heart Journal. 2019;40:357–368. doi: 10.1093/eurheartj/ehy357. [DOI] [PubMed] [Google Scholar]
- 218.Verboven K., Wouters K., Gaens K., Hansen D., Bijnen M., Wetzels S. Abdominal subcutaneous and visceral adipocyte size, lipolysis and inflammation relate to insulin resistance in male obese humans. Scientific Reports. 2018;8(1):4677. doi: 10.1038/s41598-018-22962-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yang J., Eliasson B., Smith U., Cushman S.W., Sherman A.S. The size of large adipose cells is a predictor of insulin resistance in first-degree relatives of type 2 diabetic patients. Obesity. 2012;20(5):932–938. doi: 10.1038/oby.2011.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tchernof A., Despres J.P. Pathophysiology of human visceral obesity: an update. Physiological Reviews. 2013;93(1):359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 221.Bosy-Westphal A., Braun W., Albrecht V., Muller M.J. Determinants of ectopic liver fat in metabolic disease. European Journal of Clinical Nutrition. 2019;73(2):209–214. doi: 10.1038/s41430-018-0323-7. [DOI] [PubMed] [Google Scholar]
- 222.Shojaee-Moradie F., Cuthbertson D.J., Barrett M., Jackson N.C., Herring R., Thomas E.L. Exercise training reduces liver fat and increases rates of VLDL clearance but not VLDL production in NAFLD. Journal of Clinical Endocrinology & Metabolism. 2016;101(11):4219–4228. doi: 10.1210/jc.2016-2353. [DOI] [PubMed] [Google Scholar]
- 223.Montagner A., Polizzi A., Fouche E., Ducheix S., Lippi Y., Lasserre F. Liver PPARalpha is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 2016;65(7):1202–1214. doi: 10.1136/gutjnl-2015-310798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Fougerat A.S., Polizzi A., Regnier M., Wagner C., Smati S., Fougeray T. ATGL-dependent white adipose tissue lipolysis controls hepatocyte PPARα activity. bioRxiv. 2021 doi: 10.1101/2021.01.28.428684. [DOI] [PubMed] [Google Scholar]
- 225.Stern J.H., Rutkowski J.M., Scherer P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metabolism. 2016;23(5):770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Shabalala S.C., Dludla P.V., Mabasa L., Kappo A.P., Basson A.K., Pheiffer C. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomedicine & Pharmacotherapy. 2020;131:110785. doi: 10.1016/j.biopha.2020.110785. [DOI] [PubMed] [Google Scholar]
- 227.BonDurant L.D., Potthoff M.J. Fibroblast growth factor 21: a versatile regulator of metabolic homeostasis. Annual Review of Nutrition. 2018;38:173–196. doi: 10.1146/annurev-nutr-071816-064800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Miquilena-Colina M.E., Lima-Cabello E., Sanchez-Campos S., Garcia-Mediavilla M.V., Fernandez-Bermejo M., Lozano-Rodriguez T. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut. 2011;60(10):1394–1402. doi: 10.1136/gut.2010.222844. [DOI] [PubMed] [Google Scholar]
- 229.Zhu L., Baker S.S., Liu W., Tao M.H., Patel R., Nowak N.J. Lipid in the livers of adolescents with nonalcoholic steatohepatitis: combined effects of pathways on steatosis. Metabolism. 2011;60(7):1001–1011. doi: 10.1016/j.metabol.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 230.Doege H., Grimm D., Falcon A., Tsang B., Storm T.A., Xu H. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. Journal of Biological Chemistry. 2008;283(32):22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Auinger A., Valenti L., Pfeuffer M., Helwig U., Herrmann J., Fracanzani A.L. A promoter polymorphism in the liver-specific fatty acid transport protein 5 is associated with features of the metabolic syndrome and steatosis. Hormone and Metabolic Research. 2010;42(12):854–859. doi: 10.1055/s-0030-1267186. [DOI] [PubMed] [Google Scholar]
- 232.Alves-Bezerra M., Li Y., Acuna M., Ivanova A.A., Corey K.E., Ortlund E.A. Thioesterase superfamily member 2 promotes hepatic VLDL secretion by channeling fatty acids into triglyceride biosynthesis. Hepatology. 2019;70(2):496–510. doi: 10.1002/hep.30411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wendel A.A., Cooper D.E., Ilkayeva O.R., Muoio D.M., Coleman R.A. Glycerol-3-phosphate acyltransferase (GPAT)-1, but not GPAT4, incorporates newly synthesized fatty acids into triacylglycerol and diminishes fatty acid oxidation. Journal of Biological Chemistry. 2013;288(38):27299–27306. doi: 10.1074/jbc.M113.485219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Irshad Z., Chmel N., Adya R., Zammit V.A. Hepatic VLDL secretion: DGAT1 determines particle size but not particle number, which can be supported entirely by DGAT2. The Journal of Lipid Research. 2019;60(1):111–120. doi: 10.1194/jlr.M089300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Kang H.W., Niepel M.W., Han S., Kawano Y., Cohen D.E. Thioesterase superfamily member 2/acyl-CoA thioesterase 13 (Them2/Acot13) regulates hepatic lipid and glucose metabolism. The FASEB Journal. 2012;26(5):2209–2221. doi: 10.1096/fj.11-202853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Vergnes L., Beigneux A.P., Davis R., Watkins S.M., Young S.G., Reue K. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. The Journal of Lipid Research. 2006;47(4):745–754. doi: 10.1194/jlr.M500553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yamazaki T., Sasaki E., Kakinuma C., Yano T., Miura S., Ezaki O. Increased very low density lipoprotein secretion and gonadal fat mass in mice overexpressing liver DGAT1. Journal of Biological Chemistry. 2005;280(22):21506–21514. doi: 10.1074/jbc.M412989200. [DOI] [PubMed] [Google Scholar]
- 238.Monetti M., Levin M.C., Watt M.J., Sajan M.P., Marmor S., Hubbard B.K. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metabolism. 2007;6(1):69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 239.Millar J.S., Stone S.J., Tietge U.J., Tow B., Billheimer J.T., Wong J.S. Short-term overexpression of DGAT1 or DGAT2 increases hepatic triglyceride but not VLDL triglyceride or apoB production. The Journal of Lipid Research. 2006;47(10):2297–2305. doi: 10.1194/jlr.M600213-JLR200. [DOI] [PubMed] [Google Scholar]
- 240.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84(1):277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 241.Heine M., Fischer A.W., Schlein C., Jung C., Straub L.G., Gottschling K. Lipolysis triggers a systemic insulin response essential for efficient energy replenishment of activated Brown adipose tissue in mice. Cell Metabolism. 2018;28(4):644–655. doi: 10.1016/j.cmet.2018.06.020. e644. [DOI] [PubMed] [Google Scholar]
- 242.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K. Brown adipose tissue activity controls triglyceride clearance. Nature Medicine. 2011;17(2):200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 243.Berbee J.F., Boon M.R., Khedoe P.P., Bartelt A., Schlein C., Worthmann A. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nature Communications. 2015;6:6356. doi: 10.1038/ncomms7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bartelt A., John C., Schaltenberg N., Berbee J.F.P., Worthmann A., Cherradi M.L. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nature Communications. 2017;8:15010. doi: 10.1038/ncomms15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Worthmann A., John C., Ruhlemann M.C., Baguhl M., Heinsen F.A., Schaltenberg N. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nature Medicine. 2017;23(7):839–849. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 246.Giles D.A., Moreno-Fernandez M.E., Stankiewicz T.E., Graspeuntner S., Cappelletti M., Wu D. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nature Medicine. 2017;23(7):829–838. doi: 10.1038/nm.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Poekes L., Gillard J., Farrell G.C., Horsmans Y., Leclercq I.A. Activation of brown adipose tissue enhances the efficacy of caloric restriction for treatment of nonalcoholic steatohepatitis. Laboratory Investigation. 2019;99(1):4–16. doi: 10.1038/s41374-018-0120-x. [DOI] [PubMed] [Google Scholar]
- 248.Wang G.X., Zhao X.Y., Meng Z.X., Kern M., Dietrich A., Chen Z. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature Medicine. 2014;20(12):1436–1443. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yilmaz Y., Ones T., Purnak T., Ozguven S., Kurt R., Atug O. Association between the presence of brown adipose tissue and non-alcoholic fatty liver disease in adult humans. Alimentary Pharmacology & Therapeutics. 2011;34(3):318–323. doi: 10.1111/j.1365-2036.2011.04723.x. [DOI] [PubMed] [Google Scholar]
- 250.Becher T., Palanisamy S., Kramer D.J., Eljalby M., Marx S.J., Wibmer A.G. Brown adipose tissue is associated with cardiometabolic health. Nature Medicine. 2021;27(1):58–65. doi: 10.1038/s41591-020-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chondronikola M., Volpi E., Borsheim E., Porter C., Saraf M.K., Annamalai P. Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metabolism. 2016;23(6):1200–1206. doi: 10.1016/j.cmet.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fischer A.W., Jaeckstein M.Y., Gottschling K., Heine M., Sass F., Mangels N. Lysosomal lipoprotein processing in endothelial cells stimulates adipose tissue thermogenic adaptation. Cell Metabolism. 2020 doi: 10.1016/j.cmet.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 253.Blondin D.P., Tingelstad H.C., Noll C., Frisch F., Phoenix S., Guerin B. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nature Communications. 2017;8:14146. doi: 10.1038/ncomms14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Labbe S.M., Grenier-Larouche T., Croteau E., Normand-Lauziere F., Frisch F., Ouellet R. Organ-specific dietary fatty acid uptake in humans using positron emission tomography coupled to computed tomography. American Journal of Physiology. Endocrinology and Metabolism. 2011;300(3):E445–E453. doi: 10.1152/ajpendo.00579.2010. [DOI] [PubMed] [Google Scholar]
- 255.Paulus A., Drude N., Nascimento E.B.M., Buhl E.M., Berbee J.F.P., Rensen P.C.N. [(18)F]BODIPY-triglyceride-containing chylomicron-like particles as an imaging agent for brown adipose tissue in vivo. Scientific Reports. 2019;9(1):2706. doi: 10.1038/s41598-019-39561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]