Abstract
Purpose
Endometrial laminin subunit beta-3 (LAMB3) is a candidate gene whose expression distinguishes the endometrial window of receptivity (WOR) in human. This study aims to examine endometrial LAMB3 levels in patients with repeated implantation failure (RIF), in order to assess the ability of LAMB3 to predict pregnancy outcome.
Methods
Endometrial biopsies were taken during the WOR from 21 healthy volunteers in natural menstrual cycles and from 50 RIF patients in mock cycles prior to frozen embryo transfer (FET) cycles. Immunohistochemistry (IHC) staining of LAMB3 was performed, and the H-score was correlated with the pregnancy outcome in subsequent FETs.
Results
In healthy volunteers, endometrial LAMB3 was demonstrated to be highly expressed during the WOR with the staining exclusively in the cytoplasm of the epithelial cells. In a discovery set of RIF patients, the LAMB3 expression level was found to be significantly higher in those who conceived compared to those who did not in subsequent FETs. A receiving operator characteristic (ROC) analysis revealed an area under the curve (AUC) of 0.7818 (95% confidence interval 59.92–96.44%) with an H-score cutoff of 4.129 to differentiate cases with positive or negative pregnancy outcomes. This cutoff achieved an accuracy of 75% in pregnancy prediction in a following validation set of RIF patients, in which the pregnancy rate in subsequent FETs was three-fold higher when the mock cycle LAMB3 H-score was ≥ 4.129 compared to < 4.129.
Conclusions
IHC measurement of endometrial LAMB3 expression could be a promising prognostic method to predict pregnancy outcome for RIF patients undergoing FETs.
Keywords: Repeated implantation failure, Endometrial receptivity, Frozen embryo transfer, Pregnancy prediction, LAMB3
Introduction
Repeated implantation failure (RIF) is an emerging problem that affects up to 15% of patients undergoing assisted reproductive technology (ART) treatment [1]. A consensus definition of RIF is still lacking, and the proposed definitions have ranged from a failure of ≥ 3 “high-quality” embryo transfers to a ≥ 10 cleavage-stage embryo transfers [2–4]. RIF has tremendous medical, psychosocial, and economic implications, and improving our ability to predict and optimize implantation would significantly improve the outcomes not only for these patients but also the general ART patient population.
Embryo competence is a prerequisite but insufficient on its own for successful implantation. The average implantation rate per embryo transferred in women under 40 years of age is approximately 33–50% [5], and even with the addition of preimplantation genetic testing for aneuploidy (PGT-A), a screened euploid embryo has an implantation rate of around 60% [5]. This underscores the critical importance of endometrial receptivity, which refers to a temporal and molecular state of the endometrium to allow embryo implantation. In a natural menstrual cycle, the endometrium is only receptive to implantation during the “window of receptivity” (WOR), which begins in the mid-secretory phase, and lasts from 7 to 11 days after the luteinizing hormone (LH) surge [6]. In ART, the WOR is recapitulated by either endogenously produced or exogenously administered estrogen and progesterone, and fresh or frozen embryo transfer occurs to coincide with this window. To assay the molecular microenvironment of the WOR, our group has developed a method of uterine fluid aspiration (UFA) that allows minimally invasive sampling of the endometrium for whole-genome expression profiling. In a previous study to determine transcriptomic markers of the physiologic WOR, we performed UFA on non-infertile, naturally cycling women and identified 245 endometrial genes that were at least four-fold differentially expressed during the receptive phase compared to the pre-receptive phase [7]. These genes determined by the unique UFA method were cross-referenced with 57 meta-signature genes detected by conventional endometrial biopsy (EB) method that distinguished the natural cycle WOR, which were summarized in a systemic review [8]. The comparison identified 22 overlapping genes, of which a small cohort of genes related to extracellular matrix (ECM) were of special interest as endometrial ECM remodeling is known to be an important component of implantation [9]. One of these genes was laminin subunit beta-3 (LAMB3). Interestingly, in a subsequent discovery study consisting of subjects undergoing ovarian stimulation, our group again performed UFA and found that endometrial LAMB3 was also over four-fold differentially expressed during the stimulated cycle WOR [10]. These findings suggest that LAMB3 is a “conserved” gene that reliably distinguishes the WOR and may serve as a diagnostic and/or prognostic biomarker of endometrial receptivity.
As impaired endometrial receptivity is implicated in many cases of RIF, this is a critical and optimal population to study the molecular basis of altered endometrial receptivity. The objective of this study was to describe the correlation between endometrial LAMB3 expression in patients with RIF and their subsequent reproductive outcomes. EB samples obtained from patients with RIF were immunostained for LAMB3. We report that the expression of LAMB3 is a promising candidate for the diagnosis of impaired endometrial receptivity and the prognosis of pregnancy outcome in these patients.
Materials and methods
Study population
This study was conducted at a university-affiliated, hospital-based fertility clinic with an institutional research ethics board-approved protocol and written informed consent obtained from each subject.
Archived EB samples collected from healthy controls were used to demonstrate the expression pattern of LAMB3 in endometrial tissue during the WOR. These samples were from 21 healthy female volunteers under 40 years of age with regular menstrual cycles, normal uterine cavities, and no history of infertility. These volunteers were recruited in our previous research study, in which we have shown elevated endometrial LAMB3 transcript levels by UFA on day LH + 7 (7 days after the LH surge) compared to day LH + 2 [7]. Each EB sample was obtained using an endometrial Pipelle (Wallach Surgical) on day LH + 7, within the WOR of a physiologic natural cycle.
We then examined archived EB samples from patients with RIF at our academic fertility clinic between 2014 and 2019. The population consisted of 50 women receiving frozen embryo transfers (FETs), in which the first 26 were included as the discovery set and the other 24 as the validation set. Subjects were between 29 and 44 years of age and had normal uterine cavities according to saline sonohysterography or hysteroscopy and a history of RIF defined at our clinic as failure to achieve pregnancy after transfer of at least 3 good-quality blastocysts in a minimum of 2 consecutive fresh or frozen cycles. All subjects were undergoing a mock hormonal replacement cycle for endometrial preparation prior to their actual FET cycle. The mock cycle was for the purpose of performing an endometrial receptivity analysis (ERA, Igenomix), and consenting patients had a portion of their EB sample used for this study. Endometrial preparation in the mock cycle involved the administration of micronized 17β-estradiol (Estrace, Acerus Pharmaceuticals) 4 mg twice daily orally or per vagina starting on day 2 of the menstrual cycle and continued for a minimum of 12 days until the endometrial lining measured ≥ 8 mm by transvaginal ultrasound. Micronized progesterone (Prometrium, Merck) 200 mg three times daily per vagina was then started. After 5–7 full days of progesterone treatment (P + 5–P + 7), which was within the presumptive WOR of the HR cycle, an EB sample was obtained using an endometrial Pipelle. In the subsequent FET cycles, endometrial preparation protocol was the same as the mock cycle with the day of ultrasound-guided embryo transfer “personalized” according to the ERA report. All transferred embryos were frozen-thawed blastocyst-stage embryos that had been cultured to day 5 or 6. Six patients in the discovery set received a transfer of euploid embryos screened by PGT-A. All the other patients received the embryo transfer based on conventional morphological assessment by Gardner’s scoring system [11]. Decisions regarding whether or not to have PGT-A were based on patient preference after consultation with the physician. Serum β-hCG levels were assessed 9 days after FET to determine if implantation occurred.
Immunohistochemistry (IHC) staining
EB samples were fixed in 10% neutral buffered formalin and paraffin embedded. Tissue sections (4 μm) were obtained from EB paraffin blocks and deparaffinized. Tissue sections were submitted to heat-induced epitope retrieval which consisted of immersing the sections in citrate buffer (pH 6.0). The immersed sections were heated for 7 min in a pressure cooker. The sections were then removed and allowed to stand for 10 min before being rinsed in distilled water for 5 min. The sections were then placed in 0.3% hydrogen peroxide in methanol for 15 min to quench endogenous peroxidase. Non-specific antibody binding was blocked by incubating tissue sections with 2.5% normal horse serum (Vector) for 1 h, prior to overnight incubation in mouse anti-human LAMB3 (Atlas, AMAb91161, 1:100). After washing, the sections were incubated for 30 min with ImmPRESS HRP horse anti-mouse IgG polymer (Vector), and immunoreactive staining was visualized by diaminobenzidine reagent (Abcam). The sections were counterstained with hematoxylin. A negative control without primary antibody incubation was also performed. Whole slide images (WSI) were scanned with a VS-120 scanner (Olympus).
QuPath image analysis
The observer was blinded to the pregnancy outcome after FET when analyzing the images. All digital-scanned images were analyzed by QuPath software (v0.1.2) [12], the use of which for IHC staining analysis in human endometrium has been verified before [13]. We performed QuPath analysis based on a published protocol with modifications [14]. Representative areas exhibiting typical morphology of the endometrium were selected for cell detection with the settings in QuPath as follows: requested pixel size 0.5 μm, background radius 8 μm, sigma 1.5 μm, minimum area 10 μm2, maximum area 400 μm2, intensity threshold 0.01, maximum background intensity 2, and cell expansion 4 μm. The detected cells were annotated as epithelial or stromal cells and included to train a Random Trees machine learning classifier to distinguish epithelial and stromal cells. The classifier was built based on 33 measurements automatically extracted from the cells in the training set, such as area, perimeter, circularity, and staining OD. The built-in auto-update tool in QuPath allowed real-time reassurance of training efficiency and accuracy. The accuracy of the classifier was then manually confirmed by the observer. This classifier was applied on each WSI in which a minimum of 10 areas were randomly selected for automated cell classification followed by H-score measurement. By creating a script to define staining intensity based on cell DAB optical density as follows: < 0.1 = weak, 0.2–0.3 = moderate, and > 0.3 = strong, the built-in QuPath algorithm automatically calculated the H-score of each area (H-score = 0 × % negative cells + 1 × % weakly stained cells + 2 × % moderately stained cells + 3 × % strongly stained cells) [12]. This H-score system has been previously validated as a semiquantitative assay for immunohistochemical staining [15]. The H-score results were exported for statistical analyses. The mean H-score of all selected areas on a WSI was determined as the final H-score of the image.
Statistical analyses
Student t test, Mann–Whitney test, and Fisher’s exact test were used for statistical analysis as appropriate. Receiving operator characteristic (ROC) analysis was performed by GraphPad Prism (v8.4.3).
Results
Characterization of LAMB3 in endometrial tissue
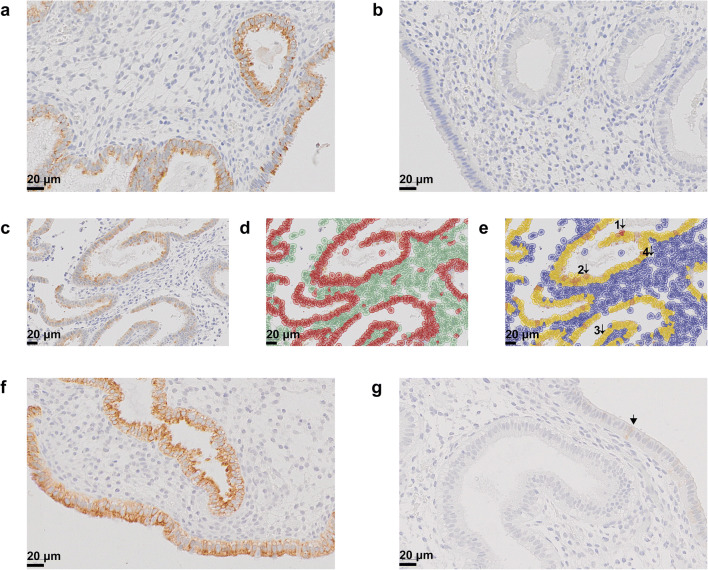
The immunostaining pattern and localization of LAMB3 in endometrial tissue was characterized using archived EB samples taken from healthy volunteers. During the physiologic WOR, LAMB3 was highly expressed in the endometrium, with the positive staining exclusively in the cytoplasm of the epithelial cells. This was apparent in all of the 21 EB samples obtained from healthy volunteers (Fig. 1a). This LAMB3 staining pattern was in line with the LAMB3 distribution reported in the Human Protein Atlas (HPA) project [16, 17].
Fig. 1.
Immunohistochemistry staining of endometrial LAMB3. a A representative EB sample taken on day LH + 7 from a healthy volunteer. Positive staining of LAMB3 (brown) was exclusively in the cytoplasm of epithelial cells. b Secondary antibody-only negative control. c, d, e The trained classifier and quantification of staining intensity by QuPath. Cells in c were detected by the software and highlighted in red or green for epithelial cells or stromal cells in d, respectively. Cells with strong, moderate, weak, and negative staining were highlighted in dark brown (1), light brown (2), yellow (3), and blue (4) in e, respectively. f A representative EB sample with high expression of LAMB3 from a patient in pregnant group of the discovery set. g A representative EB sample with low expression of LAMB3 from a patient in non-pregnant group of the discovery set. Arrowhead represented a positively stained cell
Discovery set: expression of endometrial LAMB3 correlates with pregnancy outcome
Staining of LAMB3 was then performed on EB samples taken during the WOR from patients with RIF. This was first carried out in a discovery set consisting of 26 subjects. The characteristics of the study population in the discovery set are summarized in Table 1. In this set, we followed the patients’ pregnancy outcome for up to 3 subsequent FET cycles after the mock HR cycle. Based on the outcome of the FETs, the subjects were categorized into pregnant (positive β-hCG, n = 11) or non-pregnant (negative β-hCG, n = 15) group. In the pregnancy group, 8 patients conceived in the first subsequent FET cycle, and the other 3 conceived in the second FET attempt. Age, body mass index (BMI), anti-Müllerian hormone (AMH), antral follicle count (AFC), and history of total failed cycles/embryos were similar between the two groups.
Table 1.
Characteristics of the population in discovery set based on β-hCG outcome
| Characteristic | Pregnant (n = 11) | Non-pregnant (n = 15) | P value |
|---|---|---|---|
| Age (y), mean ± SD | 34 ± 4 | 35 ± 3.4 | 0.32a |
| BMI (kg/m2), median (range) | 23.6 (20.6–39.1) | 24.4 (20.7–30.4) | 0.93b |
| AMH (pmol/L), median (range) | 18.3 (5.96–45.9) | 22 (1.3–45) | 0.21b |
| AFC, median (range) | 14 (12–37) | 21 (10–38) | 0.21b |
| Total failed fresh and frozen cycles | 4 (2–8) | 5 (3–11) | 0.27b |
| Total embryos transferred in failed cycles | 4 (3–13) | 6 (3–11) | 0.75b |
| Subsequent FET characteristic | |||
| Number of embryos transferred in subsequent FET cycles, median (range) | 2 (1–3) | 2 (1–5) | 0.08b |
| Number of patients having PGT-A euploid embryo transfer, n (%) | 2 (2/11 = 18.2%) | 4 (4/15 = 26.7%) | > 0.99c |
| LAMB3 H-score, median (range) | 7.37 (0.59–70.39) | 0.25 (0.02–15.72) | 0.015b |
SD, standard deviation; BMI, body mass index; AMH, anti-Müllerian hormone; AFC, antral follicle count; PGT-A, preimplantation genetic testing for aneuploidy
aStudent t test, bMann-Whitney test, cFisher’s exact test
To measure the expression level of LAMB3 in endometrial tissue, a classifier for epithelial cells and stromal cells was built by QuPath software, and the staining intensity of LAMB3 was quantified by H-score (Fig. 1 c–e). Only the H-score results of the epithelial cells were exported for analysis as the stromal cells were not stained with LAMB3.
The H-score of epithelial LAMB3 expression was plotted for every patient in the discovery set in Fig.2 a. There was a significantly higher expression of endometrial LAMB3 in the pregnant group compared to non-pregnant group (median H-score 7.37 vs. 0.25, P = 0.015, Mann–Whitney test; Table 1). The difference in LAMB3 expression between the two groups yielded an area under the curve (AUC) of 0.7818 by ROC analysis (95% confidence interval 59.92–96.44%, P = 0.0158; Fig. 2 b). We proceeded to find an H-score cutoff of 4.129 which provided the highest likelihood ratio of 3.636 for positive pregnant outcome (sensitivity 72.73%, specificity 80%; Fig. 2 b). The cutoff was presented as the dotted line in Fig. 2 a.
Fig. 2.
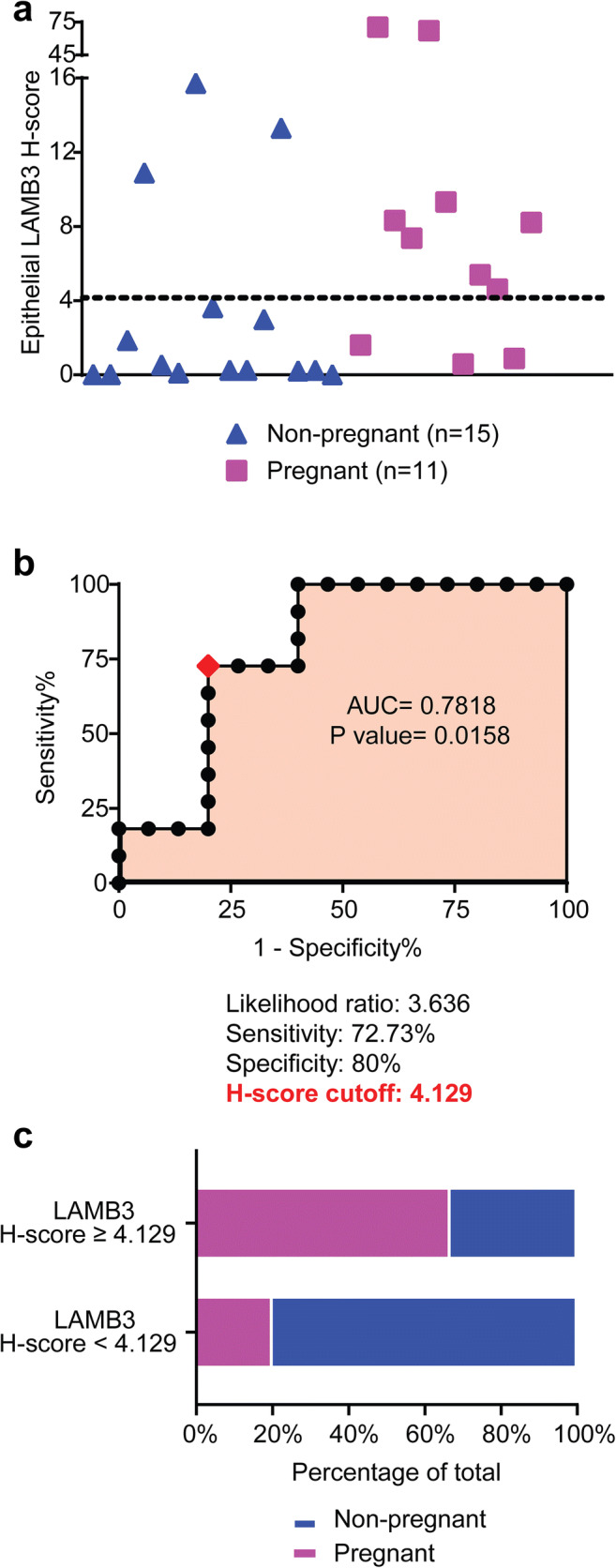
The H-score cutoff of endometrial LAMB3. a H-score values of endometrial epithelial LAMB3 in the discovery set. The dotted line represented ROC-determined H-score cutoff of 4.129. b The ROC analysis comparing LAMB3 H-scores between pregnant group and non-pregnant group in the discovery set. c The stacked bar graph showing the proportions of pregnant and non-pregnant subjects in the validation set. Subjects were grouped by LAMB3 H-score, and pregnant outcomes were based on serum β-hCG after subsequent FETs
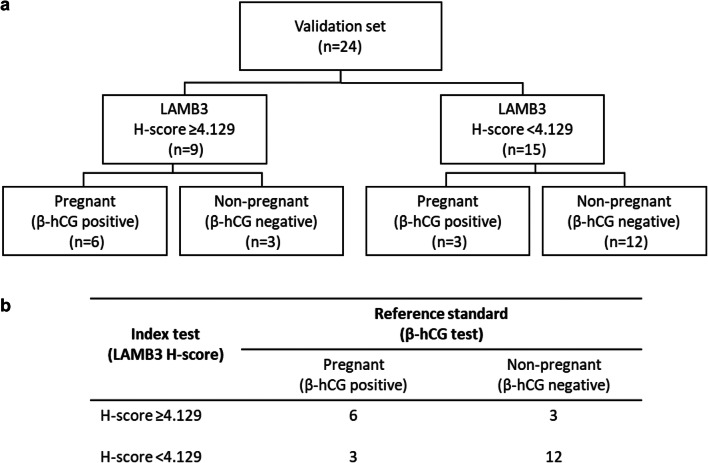
Validation set: evaluation of the H-score cutoff
To test the performance of the H-score cutoff, we applied it to EB samples in a validation set consisting of 24 subjects where sample collection, preparation, and LAMB3 staining were performed identical to the discovery set. The flow of subjects in the validation set is summarized in Fig. 3 a. Based on the staining intensity of LAMB3, the subjects were categorized into LAMB3 testing positive (H-score ≥ 4.129, n = 9) or LAMB3 testing negative (H-score < 4.129, n = 15) group. The characteristics of the study population in the validation set are summarized in Table 2. Age, BMI, AMH, AFC, history of total failed cycles/embryos, and characteristics of subsequent FETs were similar between the two groups. A more than three-fold higher proportion of women in the H-score ≥ 4.129 group conceived after subsequent FETs compared to those in the H-score < 4.129 group (66.7% vs. 20%, P = 0.0361, Fisher’s exact test; Table 2; Fig. 2 c). Based on the contingency table in Fig. 3 b, the performance of this H-score cutoff as a pregnancy prediction test was calculated as follows: sensitivity 66.7%, specificity 80%, positive predictive value (PPV) 66.7%, negative predictive value (NPV) 80%, and accuracy 75%.
Fig. 3.
Validation set. a Flow of subjects in the validation set. b The 2 × 2 contingency table showing the performance of H-score cutoff
Table 2.
Characteristics of the population in validation set based on LAMB3 H-score
| Characteristic | LAMB3 H-score ≥ 4.129 (n = 9) | LAMB3 H-score < 4.129 (n = 15) | P value |
|---|---|---|---|
| Age (y), mean ± SD | 36 ± 3.5 | 37 ± 3.6 | 0.55a |
| BMI (kg/m2), median (range) | 23.8 (16.7–29.7) | 25.4 (18.1–29.7) | 0.92b |
| AMH (pmol/L), median (range) | 21.5 (4.03–37) | 19.2 (2.6–46.6) | 0.87b |
| AFC, median (range) | 15 (13–20) | 12 (4–40) | 0.56b |
| Total failed fresh and frozen cycles | 4 (3–8) | 4 (2–8) | 0.96b |
| Total embryos transferred in failed cycles | 4 (3–8) | 4 (3–8) | 0.80b |
| Subsequent FET characteristic | |||
| Number of embryos transferred in subsequent FET cycles, median (range) | 1 (1–2) | 1 (1–2) | 0.08b |
| Number of patients having PGT-A euploid embryo transfer, n (%) | 0 | 0 | N/A |
| Pregnant (β-hCG positive), n (%) | 6 (6/9 = 66.7%) | 3 (3/15 = 20%) | 0.0361c |
SD, standard deviation; BMI, body mass index; AMH, anti-Müllerian hormone; AFC, antral follicle count; PGT-A, preimplantation genetic testing for aneuploidy; N/A, not applicable
aStudent t test, bMann-Whitney test, cFisher’s exact test
Discussion
LAMB3 is a promising biomarker of endometrial receptivity. This study provides the first detailed examination of endometrial LAMB3 expression among a group of patients who have a history of RIF. The study results demonstrate a strong association between endometrial LAMB3 expression level and reproductive outcomes in FET cycles.
The possible role of LAMB3 as a receptivity-related molecule was first documented by Carson et al., who observed a significant upregulation of LAMB3 transcript in endometrial tissue during the mid-secretory phase compared to the early-secretory phase among fertile volunteers in their natural menstrual cycles by microarray technique [18]. This finding in the natural cycles has been validated in other endometrial transcriptomic profiling studies, including our own [7, 19–22], and has also been observed in the controlled ovarian stimulation cycles [10, 23]. LAMB3 transcript is also included in the examination panel, among many other candidates, in some commercial EB-based endometrial receptivity tests, such as the ERA and the Window Implantation Test (Win-Test).
In the present study, we evaluated the expression of LAMB3 in EB samples at the protein level instead of the messenger RNA (mRNA) level. With the identification of LAMB3 as an endometrial protein highly expressed in epithelial cells during the receptive phase, we hypothesized that a biopsy-based LAMB3 staining test to assess functional endometrial receptivity might be feasible. To address this, we first compared endometrial LAMB3 levels in patients with a history of RIF who went on to have a positive pregnancy outcome versus those with a negative outcome. Significantly reduced LAMB3 expression levels were detected by semiquantitative H-score in patients who failed to conceive. After establishing that the AUC exceeded 0.5 in the ROC curve, we proceeded to find an H-score cutoff correlated with positive and negative pregnancy outcomes. Next, we validated the test in a second set of patients to demonstrate that endometrial LAMB3 can be used a potential prognostic marker for reproductive outcome.
The role of LAMB3 in cell adhesion may explain the association between low LAMB3 expression and poor pregnancy outcomes after FET. It is known that LAMB3, together with laminin subunit alpha-3 (LAMA3) and laminin subunit gamma-2 (LAMC2), forms laminin-332 (also known as laminin-5), which is a large secreted ECM glycoprotein. Binding of laminin-332 to cell–surface receptors such as integrins to stimulate cell–cell or cell–ECM adhesion and communication has been previously described, especially in cancer biology [24–27]. The α3β1 and α6β4 integrins, which are two known receptors for laminin-332, are expressed on the surface of the endometrial epithelial cells [28, 29]. Human preimplantation embryos also express α3β1 integrin [30]. During the receptive phase of the endometrium, it is plausible that laminin-332 interacts with integrins on the endometrial epithelium or the embryonic trophectoderm to facilitate the attachment of the conceptus to the endometrium.
The strength of our study is the blinding of the observer performing quantification of IHC staining, who did so without knowledge of the pregnancy outcomes of the subjects. In addition, the use of an automated software for IHC scoring reduced potential operator bias in data interpretation. A limitation of this study is the small sample size as EB sampling is not the standard of care at our clinic for patients undergoing ART treatment whether or not they have a history of RIF. Only RIF patients who were undergoing mock cycles for ERA and provided informed consent to donate their EB samples to research were included in this study. Therefore, power analysis was not performed prior to sample collection in this pilot study. Nevertheless, post hoc power analysis showed a power of 96.2% with great reliability to interpret the association between LAMB3 H-score and pregnancy outcome, and data from this preliminary study provide an H-score cutoff for future prospective clinical trials to determine the accuracy of LAMB3 as a prognostic maker of FET outcome. In addition, the majority of patients in this study underwent a transfer of embryos that were not screened by PGT-A. This may cause bias given that implantation failure in the non-pregnant patients may be attributed not only to defects in the endometrium but also to embryo ploidy. Another limitation is that this study is limited to describing LAMB3 protein levels in patients undergoing endometrial preparation for FET using exogenous hormone replacement. It was beyond the scope of this discovery and validation study to extend the analysis to patients undergoing fresh embryo transfer. A final limitation is that the quantification of LAMB3 occurred in a mock cycle, not in the actual embryo transfer cycle. This limitation is shared by all EB-based receptivity tests, including ERA. However, the endometrial gene expression has been shown to have some degree of inter-cycle reproducibility [31].
RIF is a particularly challenging clinical problem given the time and resources that have been invested in previous failed cycles. By the time patients meet the criteria for RIF, they usually have depleted their embryos available for transfer. Maximization of their pregnancy outcomes in subsequent embryo transfers is a major incentive for patients and physicians alike. However, the limiting step in the management of RIF has always been the diagnostic conundrum of determining if the etiology of failure is embryonic abnormality versus impaired endometrial receptivity. Central to this problem is the lack of reliable diagnostic tools available to assess endometrial receptivity. Commercial endometrial receptivity assays have demonstrated limited success in prognosticating pregnancy. For example, the ERA is one of the most commonly used transcriptomic-based assays, yet a study on ERA testing of RIF patients demonstrated an implantation rate of only 30% and pregnancy rate of only 46% in subsequent FET for RIF patients despite a “receptive” endometrial transcriptome by ERA testing [32].
Overall, our study for the first time correlates endometrial LAMB3 expression with pregnancy outcomes of subsequent FET among patients with a history of RIF. We report specific staining intensity H-score cutoffs for the prognostication of successful implantation. Our results suggest a role for endometrial LAMB3 staining as a diagnostic tool to distinguish whether or not the uterine microenvironment is potentially receptive to implantation. The LAMB3 H-score cutoff showed a NPV of 80% in our validation set. This high NPV is particularly important for the RIF population as it indicates that LAMB3 testing can be used to avoid unnecessary embryo transfer and save their precious embryos when the uterine microenvironment is suboptimal. Before implementing LAMB3 testing as a diagnostic tool, future prospective controlled studies are needed, ideally with PGT-screened euploid embryos, to assess if testing can lead to improved outcomes. Once validated, these findings have the potential to advance our management of the RIF population in new and unique ways. For example, if endometrial LAMB3 levels are favorable, patients may continue transferring the embryos until success is achieved with a competent embryo; alternatively, if LAMB3 levels are unfavorable, changing endometrial preparation protocol and delaying embryo transfer or gestational surrogacy may be considered. The findings of this study also open the possibility of endometrial therapy to augment receptivity (e.g., LAMB3 intrauterine supplementation), which would first require further functional studies. Lastly, the secreted nature of LAMB3 protein makes it particularly measurable in aspirated uterine fluid and paves the way for possible non-invasive tests in the future to predict FET outcome based on secreted LAMB3 without the need of a biopsy.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Busnelli A, Reschini M, Cardellicchio L, Vegetti W, Somigliana E, Vercellini P. How common is real repeated implantation failure? An indirect estimate of the prevalence. Reprod BioMed Online. 2020;40(1):91–97. doi: 10.1016/j.rbmo.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, Cutting R, Ong K, Sallam H, Li TC. Recurrent implantation failure: definition and management. Reprod BioMed Online. 2014;28(1):14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Simon A, Laufer N. Repeated implantation failure: clinical approach. Fertil Steril. 2012;97(5):1039–1043. doi: 10.1016/j.fertnstert.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Stern C, Chamley L, Norris H, Hale L, Baker HG. A randomized, double-blind, placebo-controlled trial of heparin and aspirin for women with in vitro fertilization implantation failure and antiphospholipid or antinuclear antibodies. Fertil Steril. 2003;80(2):376–383. doi: 10.1016/S0015-0282(03)00610-1. [DOI] [PubMed] [Google Scholar]
- 5.SART. Society for Assisted Reproductive Technology (SART) Preliminary National Summary Report for 2018. 2018. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2018. .
- 6.Elnashar AM, Aboul-Enein GI. Endometrial receptivity. Middle East Fertility Soc J. 2004;9:10–24. [Google Scholar]
- 7.Chan C, Virtanen C, Winegarden NA, Colgan TJ, Brown TJ, Greenblatt EM. Discovery of biomarkers of endometrial receptivity through a minimally invasive approach: a validation study with implications for assisted reproduction. Fertil Steril. 2013;100(3):810–7. e8. doi: 10.1016/j.fertnstert.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Altmäe S, Koel M, Võsa U, Adler P, Suhorutšenko M, Laisk-Podar T, et al. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci Rep. 2017;7(1):1–15. doi: 10.1038/s41598-017-10098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown JJ, Papaioannou VE. Extracellular matrix remodeling at implantation: role of hyaluronan. Molecular and cellular aspects of periimplantation processes. Springer. 1995:125–52.
- 10.Li T, Chan C, Greenblatt E. Minimally-invasive transcriptomic profiling of the endometrium to identify markers of endometrial receptivity. Canadian Fertility & Andrology Society (CFAS) Annual Meeting 2019; September 20, 2019; Ottawa, ON, Canada2019.
- 11.Gardner D, Schoolcraft W. In vitro culture of human blastocysts. Toward reproductive certainty: fertility and genetics beyond. Jansen, R., Mortimer, D. Parthenon Publishing, Carnforth, UK; 1999.
- 12.Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Li M, Wei C, Tang L, Sheng Y, Liu Y, Li D, Ding D, Qiu J, Zhu X. TSP1-CD47-SIRPα signaling facilitates the development of endometriosis by mediating the survival of ectopic endometrium. Am J Reprod Immunol. 2020;83(6):e13236. doi: 10.1111/aji.13236. [DOI] [PubMed] [Google Scholar]
- 14.Zhang AW, McPherson A, Milne K, Kroeger DR, Hamilton PT, Miranda A, et al. Interfaces of malignant and immunologic clonal dynamics in ovarian cancer. Cell. 2018;173(7):1755–69. e22. doi: 10.1016/j.cell.2018.03.073. [DOI] [PubMed] [Google Scholar]
- 15.Goulding H, Pinder S, Cannon P, Pearson D, Nicholson R, Snead D, Bell J, Elston CWE, Robertson JF, Blamey RW, Ellis IO. A new immunohistochemical antibody for the assessment of estrogen receptor status on routine formalin-fixed tissue samples. Hum Pathol. 1995;26(3):291–294. doi: 10.1016/0046-8177(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 16.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347:6220. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 17.HRA. The human protein atlas. 2005. https://www.proteinatlas.org/ENSG00000196878-LAMB3/tissue/endometrium. Accessed March 06 2020.
- 18.Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, et al. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8(9):871–879. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 19.Riesewijk A, Martín J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simón C. Gene expression profiling of human endometrial receptivity on days LH+ 2 versus LH+ 7 by microarray technology. Mol Hum Reprod. 2003;9(5):253–264. doi: 10.1093/molehr/gag037. [DOI] [PubMed] [Google Scholar]
- 20.Talbi S, Hamilton A, Vo K, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 21.Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alamá P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95(1):50–60. e15. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 22.Haouzi D, Mahmoud K, Fourar M, Bendhaou K, Dechaud H, De Vos J, et al. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod. 2009;24(1):198–205. doi: 10.1093/humrep/den360. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Garcia J, Kolp L, Cheadle C, Rodriguez A, Vlahos NF. The impact of luteal phase support on gene expression of extracellular matrix protein and adhesion molecules in the human endometrium during the window of implantation following controlled ovarian stimulation with a GnRH antagonist protocol. Fertil Steril. 2010;94(6):2264–2271. doi: 10.1016/j.fertnstert.2010.01.068. [DOI] [PubMed] [Google Scholar]
- 24.Manda R, Kohno T, Niki T, Yamada T, Takenoshita S, Kuwano H, Yokota J. Differential expression of the LAMB3 and LAMC2 genes between small cell and non-small cell lung carcinomas. Biochem Biophys Res Commun. 2000;275(2):440–445. doi: 10.1006/bbrc.2000.3331. [DOI] [PubMed] [Google Scholar]
- 25.Marinkovich MP. Laminin 332 in squamous-cell carcinoma. Nat Rev Cancer. 2007;7(5):370–380. doi: 10.1038/nrc2089. [DOI] [PubMed] [Google Scholar]
- 26.Niessen C, Hogervorst F, Jaspars L, De Melker A, Delwel G, Hulsman E, et al. The α6β4 integrin is a receptor for both laminin and kalinin. Exp Cell Res. 1994;211(2):360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 27.Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65(4):599–610. doi: 10.1016/0092-8674(91)90092-D. [DOI] [PubMed] [Google Scholar]
- 28.Giannelli G, Sgarra C, Di Naro E, Lavopa C, Angelotti U, Tartagni M, et al. Endometriosis is characterized by an impaired localization of laminin-5 and α3β1 integrin receptor. Int J Gynecol Cancer. 2007;17(1). [DOI] [PubMed]
- 29.Lessey BA, Castelbaum AJ. Integrins in the endometrium. Reprod Med Rev. 1995;4(1):43–58. doi: 10.1017/S0962279900001058. [DOI] [PubMed] [Google Scholar]
- 30.Campbell S, Swann H, Seif M, Kimber S, Aplin J. Integrins and adhesion molecules: cell adhesion molecules on the oocyte and preimplantation human embryo. Hum Reprod. 1995;10(6):1571–1578. doi: 10.1093/HUMREP/10.6.1571. [DOI] [PubMed] [Google Scholar]
- 31.Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, Garrido N, Pellicer A, Simón C. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril. 2013;99(2):508–517. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, Gómez E, Fernández-Sánchez M, Carranza F, Carrera J, Vilella F, Pellicer A, Simón C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil Steril. 2013;100(3):818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]