Abstract
Four hundred endophytic fungi isolates with different colony morphologies were isolated from roots of Hordeum vulgare L. collected from un-engineered landfills (the measured cadmium was 0.9 mg kg−1) of Kermanshah province in West Iran. Based on morphology and phylogeny of DNA sequence data for the internal transcribed spacer (ITS) rDNA and comparing the sequences with that available in NCBI database, 11 isolates are identified as dark septate endophytes (DSE) including Alternaria alternata, Microdochium bolleyi, Bipolaris zeicola, Alternaria sp., and Pleosporales sp., and the other nine are not dark septate endophytes (non-DSE) including Fusarium redolens, Fusarium tricinctum, Fusarium monliforme, Clonostachys rosea, and Epicoccum nigrum. Tolerance of DSE and non-DSE strains for Cd were investigated in potato dextrose agar medium. Minimum inhibitory concentrations (MIC) of Cd from nitrate salt source (Cd (NO3)2) and EC50 were determined. The means of MIC and EC50 values for DSE fungi species were 1254.5 and 209.74 mg/kg, compared to 800 and 150.3 mg/kg for non-DSEs. Among the endophytic fungi isolated, Alternaria sp. (TBR5) and Bipolaris zeicola (Tw26) showed the highest tolerance to Cd with a MIC value of 2000 mg/L and 1800 mg/L, respectively. Barley plants were inoculated with TBR5 and Tw26 in Cd-added sands (0, 10, 30, 60 mg Cd/kg sand). In terms of Cd accumulation, our results showed that TBR5 and Tw26 inoculation increased the amount of Cd in the barley roots. TBR5 and Tw26 significantly improved (p < 0.05) plant growth in the presence of Cd by enhancing plant growth attributes such as chlorophyll content, root weight, plant length, fresh weight, and dry weight of plants. This is the first study on the abundance and identification of endophytic root fungi of barley in a cadmium-contaminated soil in Iran. The results of this study showed that DSE and non-DSE have the potential to improve the efficiency of phytoremediation.
Keywords: Internal transcribed spacer (ITS) rDNA, Dark septate endophytes (DSE), Heavy metals, Minimum inhibitory concentration (MIC), Phytoremediation
Introduction
Contamination of the farming soil with heavy metals (HM) has an immensely hazardous effect on human and animal health as these metals can enter the food chain through crops. Besides, they are extremely persistent in the environment because of being non-biodegradable [1, 2]. Cadmium (Cd), one of the most toxic HMs, has a very high mobility in soil-plant systems, and even low levels of Cd can harm for plants and microorganisms [3, 4]. It has been approved that absorbing Cd through nutrition is the main source of Cd to be exposed by human. In point of fact, contamination of food chain with Cd is started by its transition from soil to plant [5]. In the recent years, human activities such as the landfill of municipal wastes cause a remarkable increase in the level of heavy metals in soils, especially in agricultural lands [6]. Farmlands contaminated with HMs have a hazardous effect on animals and human health since HMs enter to food chain through agricultural products [7, 8].
Recently, phytoremediation has been proposed as an efficient in situ method for treatment of HM-contaminated soils, which is both more environmentally friendly and cost-effective than conventional technologies [1]. Plant species used for phytoremediation must have the ability to accumulate a higher concentration of HM and also be able to tolerate toxic metals; most of these plants are known as hyperaccumulators [2]. Several studies indicated that grass species such as corn, oat, and barley have an ability of significant HMs tolerance and hyperaccumulation [9–12].
There are several evidences that endophytic fungi (fungi that infect living plant tissue without causing symptom) play various important roles in host plant growth [13]. In nature, almost all plants are associated with endophytic fungi, and they are taxonomically highly diverse [13]. It has been demonstrated that endophytic fungi infection can improve plant fitness, the growth of their host plant, biomass production, and plant tolerance to pollutant. It means that they can enhance the productivity of phytoremediation of contaminated soils [14, 15]. For instance, Soleimani et al. [12] reported that Neotyphodium endophytes enhanced biomass production and accumulation of Cd in roots and shoots of two grass species (Festuca arundinacea and Festuca pratensis) [12]. In addition, Solanum nigrum inoculated with Glomerella truncate, and Phomopsis fukushii fungal endophytes showed higher production of biomass and Cd accumulation than plants not inoculated with endophytes in various Cd-contaminated soils. These studies indicated that HM resistant endophytic fungi can be successfully used in phytoremediation. [16]. These studies indicated that HM resistant endophytic fungi can be successfully used in phytoremediation. Assessment of root endophytic fungal diversity seems to be essential in the HM-contaminated ecosystems, owing to their important roles on in tolerating stressful condition of host plants [17]. Resulting from several studies indicated that a group of endophytic fungi, dark septate endophytes, was observed frequently in HM-contaminated soils [17].
Dark septate endophytes (DSE) are a polyphyletic assemblage of fungi that ubiquitous in various stressful environmental conditions [18]. They are ascomycetous fungi belong to class 4 of non-clavicipitaceous endophytes that colonize living plant roots. DSE fungi are frequently isolated from roots of plants growing on heavy metal–contaminated soils [19, 20]. Thus, DSE may contribute to metal tolerance and nutrient acquisition on these sites. Cheshmehsefid landfill, located in Kermanshah, West of Iran, is the largest site of domestic garbage disposal in Kermanshah. Due to its high amount of leachate production and its geographic situation, can pollute the soils of nearby farmland with HMs [21]. Thus far, there has not been adequate information available concerning the amount of Cd and also endophytic fungi isolated from barley in contaminated soils in Iran. Hence, the purposes of this study were to (1) measure the concentration of Cd in the un-engineered landfills in Kermanshah province, Iran; (2) isolate and identify endophytic fungi in the cadmium hyperaccumulator barley; (3) determinate the minimum inhibitory concentration (MIC) and EC50 of Cd for each strain; (4) compare the Cd tolerance between DSE and non-DSE isolates; and (5) investigation the interactions among isolates with the host plant, Hippuris vulgaris, in a Cd-contaminated environment.
Materials and methods
Soil sampling
The study area has a temperate climate, annual precipitation of approximately 408 mm, and the mean annual temperature is 22.6 °C. In spring 2014, twenty soil samples were collected from the depth of 0–20 cm depth, from un-engineered landfills in Kermanshah province, Iran (34° 14′ N and 47 ° 01′ E). Moreover, four soil samples as natural fields of the region (34° 32′ N and 47 ° 09′ E), from distant area which were not affected by low cost, were taken to investigate the extent of pollution. The geographic characteristics of the sites were recorded using GPSMAP device model 76CSx. These soil samples, weighing about 2 kg each, were brought to the central laboratory in the Department of Plant Protection of Razi University, Kermanshah, for extraction of total concentration of metals.
Measuring total concentration of Cd in soil
The total concentration of Cd was done using the method described by McGrath and Cunliffe, 1985. The concentrations of Cd were determined by inductively coupled plasma spectroscopy (ICP) (Varian SpectrAA 220). The pollution index (PI) was used to determine the level of soil pollution with Cd, which is obtained by the equation PI = Cis/Cin [22].
Collection of plant sample
During the spring to autumn of 2014, healthy barley plants were collected from the contaminated site. Healthy plants were carefully uprooted and immediately transferred to the laboratory (in the Department of Plant Protection of Razi University, Kermanshah) in plastic bags under cold conditions for further examination.
Isolation of endophytic fungi from roots
The processing of samples in the laboratory took place using the method described by Larran et al. [23]. The root samples were rinsed under running tap water to completely remove soil and debris. Roots pieces (5 mL) were surface sterilized by 96% ethanol for 1 min, soaking for 3 min in sodium hypochlorite (5% available chlorine v/v) and 96% ethanol for 30 s, and finally rinsing three times in sterile distilled water to remove surface sterilization agents after which were dry-blotted onto the sterilized filter paper under sterilized conditions. Next, nine pieces of root samples were placed in potato dextrose agar (PDA) medium amended with chloramphenicol (50 mg/L), and dishes were incubated at 25 °C for 9 days.
Morphological and molecular identification
Micromorphological characteristics of reproductive structures of each isolate were studied using a light microscope (Olympus model BH2), and then, they were divided to DSE and non-DSE as described by Sieber and Grünig [24]. Images were captured with a camera (Canon Powershot model SX10). Fifty measurements of the observed fungal structures, including phialides, chlamydospores, and spore, were made using the BioloMICS software [25].
For molecular study, genomic DNA was extracted from the 10-day-old fungal mycelium from 18 isolates cultured on PDA using the methods described by Gardes and Bruns [26]. PCR amplification carried out by using primer pairs ITS1 (5-CCGTAGGTGAACCTGCGC-3) and ITS4 (5-TCCTCCGCTTATTGATATGC-3) [27] corresponding to the ITS1 + 5.8S+ITS2 of the ribosomal RNA in a final volume of 25 μL, by the following program: an initial denaturation step at 94 °C for 3 min; then 30 cycles, consisting of denaturation (30 s at 94 °C), annealing (30 s at 50 °C), and extension (2 min at 72 °C); and a final extension step of 10 min was allowed at 72 °C before cooling or removing the tubes. The amplified DNA were sequenced (Macrogene, South Korea), and sequence similarity searching was performed using BLAST service in NCBI (http://blast.ncbi.nlm.nih.gov). Poorly aligned positions and divergent regions of the sequences were eliminated using Gblocks software version 0.91b [28]. Homologous fungal ITS regions were retrieved from NCBI and a phylogenetic tree was constructed using the neighbour-joining method in MEGA5 [29], with 1000 bootstrap resamplings [30].
Examining the Cd tolerance of fungi isolates
Cadmium tolerance of the isolates was examined with minimum inhibitory concentration (MIC) and the effective concentration which inhibits 50% of mycelial growth (EC50). For this purpose, the PDA medium was prepared containing Cd from nitrate salt source (Cd (NO3)2) with 0 (control), 100, 200, 400, 600, 800, 1000, 1200, 1400, 1600, 1800, and 2000 mg Cd L−1. A PDA agar plugs containing 7-day-old fungal mycelium of isolates were placed in the center of petri dishes of 9 cm in size and were incubated on a 12-h:12-h light:dark cycle in a 25 °C incubator for 7–10 days. Subsequently, the diameter of each colony was measured, and MIC was recorded. Next, the inhibition percentage (IP) of Cd was measured for each endophytic isolate by using of equation IP = ((Ci – Ti)/Ci) ×100. In this equation, IP indicates inhibition percentage, Ci indicates the diameter of control colony, and Ti indicates diameter of colony in different treatments of Cd. Finally, the amounts of EC50 were calculated according to IP data (SPSS version 16.0). Significance of difference between DSE and non-DSE in both MIC and EC50 was determined by independent-sample T-tests at the 95 % confidence level (SPSS version 16.0).
Interaction between barley and Alternaria sp. (TBR5) and Bipolaris zeicola (Tw26) under Cd stress
Commercial sand (particle size 1.0 mm, pH = 7) was spiked with a Cd(NO3)2 aqueous solution to achieve the final concentrations of 0, 10, 30, and 60 mg Kg−1 as described by Khan et al. [16]. All concentrations of Cd-spiked sand were stabilized under greenhouse conditions for 2 weeks. In the meanwhile, barley (Sararood cultivar) also was colonized with TBR5 and TW26 as described by Khan et al. [16]. Next, plastic pots were filled with 300 g of the stabilized Cd spiked (experimental) and non-spiked (control) sand, after which seedlings were transplanted into plastic pots (three seedlings in each pot). This experiment consisted of two treatments: (i) control plants with four concentrations of Cd (0, 10, 30, and 60 mg Cd kg−1 sand); (ii) plants inoculated with TBR5 and Tw26 with four concentrations of Cd (0, 10, 30, and 60 mg Cd kg−1 sand). Five replicates were used for each treatment, and a total of 60 pots were randomly placed in the greenhouse (temperature 26 °C, relative humidity 50%, 14-h daylight) for 2 months.
Growth parameters of barley
After 8 weeks, the chlorophyll contents were measured by a chlorophyll meter (SPAD-502 Minolta, Japan). Subsequently, the harvested plant samples were washed with distilled water, and their roots were removed carefully from the sand and then separated into roots and shoots; at this point, the lengths and fresh weight of shoots and roots were measured. Finally, the samples were oven-dried (60 °C), and their weights were determined before Cd analysis.
Cd analysis and determination of related parameters
All harvested plants were digested for inductively coupled plasma spectroscopy (ICP) (Varian SpectrAA 220). The amounts of dry plant materials were so small for usual methods, and because of that, we searched to find the best method for digestion. Finally, 1 mg or 50 mg dry plants were weighted and then added 1 mL of HNO3 trace metal grade. Samples stayed overnight and then heated to 60–70 °C for 2 h, then 0.7 mL H2O2 added and heated again for 2 h to become colorless. The final volume of solutions after digesting was 15 mL, approximately. For preparing the samples for ICP, the volume of samples was raised to 2 mL first and after vortex, then 0.25 mL of solutions were pipette in new tubes, and then 1.25 mL of water was added too. The dilution factor was 1:6.
The translocation factor (TF) was evaluated using the following formula:
The Cd tolerance index (TI) was calculated by the following formula:
Cd removal from each pot by plants was determined as:
Statistical analysis
In general, this experiment was conducted twice, and significant differences (p < 0.05) among the mean values of different treatments were calculated and evaluated using Duncan’s multiple range test (SPSS 16.0).
Results
Concentration of Cd in soil
The mean concentrations of Cd of 20 selected soil samples were 0.90 mg/kg (min = 0.42 and max = 1.57), and Cd concentration in natural soils equal to 0.3 mg/kg. In order to determinate the level of soil pollution these numbers were placed in the PI = Cis/Cin.
Isolation and identification of endophytic fungi
In the present study, 400 fungal isolates were obtained from barley roots. The results of morphological study showed that, from 400 endophytic fungal isolates obtained from barley roots, 70% were DSEs and remaining non-DSEs. Among the identified genera, Alternaria (35%) represented the dominant genus followed by Fusarium (34%), and Microdochium (10%).
Sequencing and phylogenetic analyses
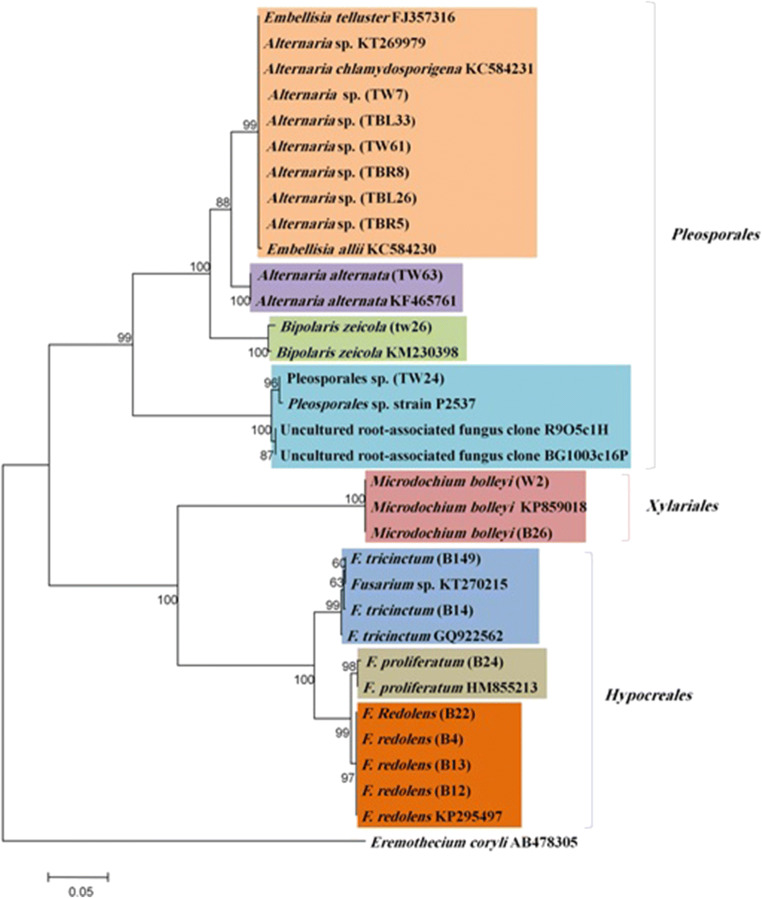
An average amplicon size of about 550 bp was obtained for the isolates. Sequencing analysis of these isolates showed 99–100% homology with other authentic endophytic fungi previously identified and deposited in GeneBank using the BLAST program (Table 1). According to these results, Alternaria alternatae (one isolate), Microdochium bolleyi (two isolates), Fusarium redolens (four isolates), Fusarium tricinctum (two isolates), Fusarium monliforme (one isolate), Bipolaris zeicola (one isolate), six isolates at the genus level from Alternaria sp., and one isolate at the order level from Pleosporales were identified. Phylogenetic tree analyses based on ITS regions allowed us to establish the precise taxonomic placement of each of the species. Phylogenetic tree was drawn using neighbor-joining (NJ) method based on the sequence of ITS region along with other authentic isolates obtained from GenBank (Fig. 1). Based on ITS1/ITS2 sequences, our isolates were clustered in distinct monophyletic clades with their known relatives with a high bootstrap value. Moreover, our isolates showed high genetic relatedness within the group (96–100%). All Alternaria sp. isolates placed within the Embellisia clade (Fig. 1). These results support the NCBI based identification. The Embellisia comprises three species including Alternaria chlamydosporigena, Alternaria embellisia, and Alternaria tellustris [31].
Table 1.
List of endophytic fungal isolates, homology, accession numbers, and potential of strains in Cd-contaminated medium growth. The values show the minimum inhibitory concentration (MIC) and EC50 of Cd
| Strain code |
DSE/non-DSE | Closest related species |
GenBank accession number |
Closest related species |
% Similarity | MIC (mg/L) | EC50 | |
|---|---|---|---|---|---|---|---|---|
| EC50 (mg L−1) | p-value | |||||||
| TBR8 | DSE | Alternaria sp. | KX061185 | Alternaria sp. (KT270173) | 99% | 1400 | 264.272 | 0.700 |
| TBL26 | DSE | Alternaria sp. | KX061184 | Alternaria sp. (KT269979) | 99% | 1400 | 266.055 | 0.558 |
| TBR5 | DSE | Alternaria sp. | KX061190 | Alternaria sp. (KT269979) | 99% | 2000 | 282.379 | 0.725 |
| TBL33 | DSE | Alternaria sp. | KX061186 | Alternaria sp. (KT270237) | 99% | 1000 | 204.33 | 0.538 |
| B26 | DSE | M. bolleyi | KX343032 | Microdochium (KY430557) | 99% | 1400 | 414.457 | 0.588 |
| Tw24 | DSE | pleosporales | KX061191 | Pleosporales sp. (KT269765) | 99% | 1200 | 90.15 | 0.972 |
| Tw26 | DSE | B. zeicola | KT833867 | B. zeicola strain (GQ253958) | 98% | 1800 | 264.145 | 0.579 |
| Tw7 | DSE | Alternaria sp. | KX061187 | Alternaria sp. (KT270237) | 100% | 1200 | 181.97 | 0.672 |
| Tw63 | DSE | A.alternata | KX061189 | A. alternata (KY026592) | 100% | 1200 | 89.679 | 0.840 |
| Tw61 | DSE | Alternaria sp. | KX061188 | Alternaria sp. (KT269865) | 100% | 600 | 116.14 | 0.806 |
| W2 | DSE | M. bolleyi | KX343031 | Microdochium (KY430557) | 99% | 600 | 133.540 | 0.195 |
| B12 | Non-DSE | F. redolens | KX343030 | F. redolens (KU527805) | 99% | 800 | 139.255 | 0.293 |
| B22 | Non-DSE | F. redolens | KY550714 | F. redolens (KX008376) | 99% | 1000 | 242.598 | 0.612 |
| B13 | Non-DSE | F. redolens | KY550713 | F. redolens (KU350708) | 99% | 800 | 139.255 | 0.293 |
| B4 | Non-DSE | F. redolens | KY550712 | F. redolens (KU350704) | 99% | 1000 | 242.598 | 0.612 |
| B24 | Non-DSE | F. monliforme | KX343028 | F. proliferatum (KY426426) | 100% | 600 | 61.102 | 0.695 |
| B14 | Non-DSE | F. tricinctum | KX343029 | F. tricinctum (KU350728) | 99% | 400 | 55.86 | 0.680 |
| B149 | Non-DSE | F. tricinctum | KX343033 | F. tricinctum (KU350729) | 99% | 600 | 55.640 | 0.821 |
| B5 | Non-DSE | C. rosea | - | - | - | 1000 | 209.464 | 0.538 |
| B3 | Non-DSE | E. nigrum | - | - | - | 1000 | 206.912 | 0.928 |
Fig. 1.
Phylogenetic analysis of the isolated endophytic fungal strains. The phylogenetic tree was constructed using the sequence data generated through analyzing the ITS regions of rDNA. A neighbor-joining algorithm was used with bootstrap values > 50% (1000 replicates) are shown next to the branches. Eremothecium coryli was included as out group
The Cd tolerance of fungi isolates
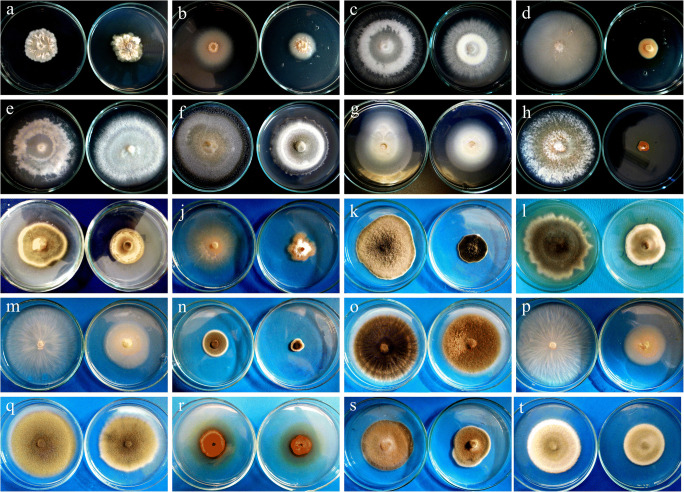
In this study, 20 isolates (11 DSEs and 9 non-DSEs) were used for Cd tolerance on Cd-supplemented PDA medium, and the MIC was determined. MIC values and EC50 are listed in Table 1. Among 20 isolates examined in this study, Alternaria sp. (TBR5) and B. zeicola (TW26) had the highest tolerance to Cd with a MIC value of 2000 and 1800 mg/L respectively. Additionally, M. bolleyi showed higher tolerance to Cd. The EC50 value of Alternaria sp. (TBR5) and B. zeicola (TW26) were 282.379 and 264.145 mg mL−1, respectively. The EC50 value of M. bolleyi (414.457 mg mL−1) was higher than other strains. The B14 strain from F. tricinctum was highly sensitive and was not able to grow at the lowest concentration of Cd with an MIC value of 400 mg/L. The means of MIC and EC50 values for DSE fungi species were 1254.5 and 209.74 mg kg−1 in turn, compared to 800 and 150.3 mg kg−1 for non-DSE (Fig. 2). Although, the means of MIC and EC50 values in DSE fungi species were higher than non-DSE, the differences between them were not significant (p ˃ 0.05).
Fig. 2.
Effect of Cd (100 mg/L) on Colony morphology (petri dish on the left side of each picture is control): a Clonostachys rosea, b Microdochium bolleyi (W2), c Fusarium redolens (B12), d Fusarium tricinctum (B149), e Fusarium redolens (B22), f Alternaria sp. (TW7), g Microdochium bolleyi (B26), h Fusarium tricinctum (B14), i Alternaria sp. (TBL33), j Fusarium moniliforme (B24), k Alternaria alternate (TW63), l Alternaria sp. (TW61), m Fusarium redolens (B4), n Pleosporales sp. (TW24), o Alternaria sp. (TBL26), p Fusarium redolens (B13), q Alternaria sp. (TBR8), r Epicoccum nigrum, s Bipolaris zeicola (TW26), t Alternaria sp. (TBR5)
Plant growth and biomass production
Re-isolation of Alternaria sp. (TBR5) and B. zeicola (TW26) indicated that the infection percentage of inoculated plants with these isolates was 100%, which was supported by microscopy (data not shown). The effects of fungal inoculations on plant growth under Cd contamination were determined by measuring barley growth attributes includes chlorophyll contents, root and shoot length, along with fresh and dry biomass. Our results clearly demonstrate that all these plant properties significantly decreased (p < 0.05) with increasing levels of Cd concentration (0–60 mg kg−1) in inoculated and non-inoculated plants. However, our results showed that inoculation of TBR5 and TW26 on barley roots significantly improved (p < 0.05) the growth parameters of the host plant under Cd stress and also alleviated Cd toxic effect in barley. A significant (p < 0.05) augmentation root growth of 13–31% and shoot growth of 6–20% were observed in inoculated plants as compared to non-inoculated plants. The shoot and root biomasses showed a similar trend. In our study, the plant chlorophyll content was reduced significantly (p < 0.05) by increasing the amount of Cd in the sand. In the research here, plants infected with the endophytic fungi showed higher chlorophyll contents (p < 0.05) than non-inoculated counterpart under Cd stress.
Cd accumulation and distribution in plant tissues influenced by fungal inoculation
In our test, the range of Cd content in the roots of barley was 262.5 to 1373.57 mg Kg−1 in different treatment (Table 2). Our results also reveal that fungal inoculation significantly elevated (p < 0.05) the concentrations of Cd in the roots of barley in an environment containing Cd treatment (Table 2). Cadmium content in shoots ranged from 29.80 to 75.22 mg kg−1 in various treatments. The contents of Cd accumulated in the aboveground portions of inoculated plants in high concentration of cadmium (30 and 60 mg kg−1) were significantly increased compared to non-inoculated plants (Table 2). To illustrate Cd distribution and translocation in plants, translocation factor (TF), Cd removal, and tolerance index (TI) were calculated (Table 2). In the study here, the values of TF, that indicate the plant’s ability to translocate Cd from roots to shoots, were generally small at 4.95 to 11.4%. The TI% was significantly higher in plants inoculated with TBR5 and TW26 in all Cd concentrations. Also, plants that were inoculated by TBR5 and TW26 showed the higher Cd removal factors (p < 0.05), compared to that of in non-inoculated control plants in the all environments containing Cd treatment.
Table 2.
Cadmium accumulation in shoot and root of barley inoculated with Alternaria sp. (TBR5) and Bipolaris zeicola (Tw26) treated with 0, 10, 30, and 60 mg Kg−1 Cd
| Cd treatment (mg kg−1 sand DW) | Association | Cd concentration (mg kg−1 plant DW) | TF% | Cd removal | TI% | |
|---|---|---|---|---|---|---|
| Root | Shoot | |||||
| 0 | Control | ND | ND | - | - | - |
| Tw26 | ND | ND | - | - | - | |
| TBR5 | ND | ND | - | - | - | |
| 10 | Control | 262.5 ± 9.52a | 29.80 ± 0.26a | 11.4 ± 0.51 | 0.229 ± 0.0096 | 70.83 |
| Tw26 | 486.0 ± 17.82b | 43.71 ± 2.22ab | 8.19 ± 0.71 | 0.303 ± 0.010 | 80.62 | |
| TBR5 | 540.0 ± 19.80c | 48.57 ± 2.47b | 9.1 ± 0.79 | 0.337 ± 0.010 | 89.58 | |
| 30 | Control | 504.5 ± 9.10c | 36.0 ± 4.50a | 7.1 ± 0.76 | 0.106 ± 0.0069 | 63.02 |
| Tw26 | 829.92 ± 35.1d | 52.28 ± 0.78c | 5.76 ± 0.32 | 0.125 ± 0.0047 | 63.74 | |
| TBR5 | 922.14 ± 39.0e | 58.42 ± 0.87c | 6.4 ± 0.36 | 0.139 ± 0.0047 | 70.83 | |
| 60 | Control | 768.6 ± 8.58f | 46.0 ± 4.07b | 6.0 ± 0.49 | 0.0465 ± 0.0004 | 44.79 |
| Tw26 | 1236.20 ± 37.80g | 67.70 ± 0.25cd | 4.95 ± 0.18 | 0.0600 ± 0.0031 | 45.93 | |
| TBR5 | 1373.57 ± 42.05h | 75.22 ± 0.28d | 5.5 ± 0.20 | 0.0680 ± 0.0031 | 51.04 | |
Values in the table are mean ± standard error (n = 5). The different letters within each column indicate a significant difference among treatments (p < 0.05) using Duncan’s multiple range test.
Discussion
Based on the obtained results, Cd has arithmetical mean concentrations of around 0.9 mg kg−1, despite the fact that Cd concentration in natural soil equal to 0.3 mg kg−1. Therefore, the soil was determined as moderate to significant polluted based on pollution index formula [22]. The World Health Organization has considered the maximum amount of Cd permitted for using in agricultural soils as to 0.2 mg kg−1 [32]. Additionally, the China Environmental Protection Agency has considered this amount less than or equal to 0.2 mg kg−1 [33]. In general, the concentration of Cd in the world agricultural soil measured from 0.05 (in India) to 13.5 (in USA) mg kg−1 [34].
In this research, the most obtained isolates were placed in Pleosporales, Hypocreales, and Xylariales orders, respectively. This result is consistent with some researches on the biodiversity of endophytic fungi of grass roots, and other plants [35–37]. Moreover, we found that Alternaria spp. represent the most frequent fungal isolates, followed by Fusarium spp. and Microdochium spp. It is shown that the most frequent genera identified as endophytic fungi of plant roots, especially in Gramineae, are Fusarium and Alternaria [16, 35, 38]. Six isolates were identified as Alternaria spp. MIC for an Alternaria genus (TBR5) was measured 2000 mg L−1, the highest levels of Cd tolerance among all strains, which was twice more than that of measured by Zafar et al. [39] for Alternaria, at 1000 mg L−1. Furthermore, four isolates identified as Fusarium redolens, which was the most frequent species of Fusarium that isolated from barley roots, but in previous studies, the F. oxysporum was more frequent than other species of Fusarium [40, 41]. Two isolates of Fusarium spp. isolated in our study were related to F. tricinctum [42]. Some isolates of Fusarium spp. in this research could tolerate 1000 mg L−1 Cd. However, in the research by Zafar et al. [39], the MIC for species of Fusarium genus was equal to 3000 mg L−1. Additionally, two isolates were identified as M. bolleyi. The M. bolleyi (syn. Idriella bolleyi) species is one of the most well-known DSE endophytes, due to its melanized cell walls and intra- and intercellular growth within the roots of healthy plants, and this fungus produces many inter- and intra-cellular melanized chlamydospores [43]. The M. bolleyi is a frequent and successful endophyte in cereal roots, such as barley (Hordeum vulgare L.), oats (Avena sativa L.), and native and invasive pasture grass and beach grasses [44]. In addition, one isolate was identified as B. zeicola. Previous studies have reported Bipolaris genus as endophytic fungi in grasses. For instance, Herrera et al. [36] isolated Bipolaris genus as a DSE fungus from the root of Bouteloua gracilis plant. The MIC for this fungus was 1800 mg L−1, indicating its high tolerance to cadmium.
The MIC and EC50 were selected to measure the sensitivity of the obtained isolates to tolarate Cd, which have been widely used for evaluation of heavy metal tolerance of filamentous fungi [8, 45]. Our results demonstrated that the DSEs have higher tolerance to Cd than non-DSEs, and a significant difference was observed between them in MIC and EC50 (Student’s t-test p >0.05). Likar and Regvar [46] suggested that the DSE may have an important role in host survival in these under-tension ecosystems. The MIC values suggest that the level of Cd tolerance differs among isolates of the same genus (Fusarium, Aternaria, and Micridochium) and even between isolates of the same species (M. Bolleyi). All 20 fungal isolates, which have been examined in this research, have reduced the growth rate of colonies; in addition, other morphological changes were also observed when exposure to Cd, and these changes have become more and more varied by increasing the concentration of Cd. Each of these changes indicates a special strategy to tolerate and resistant to heavy metal.
From 20 endophytic isolates, two isolates that were more resistant to cadmium were selected to investigate the effect of endophytes on plant and phytoremediation. Our results showed that inoculation of TBR5 and TW26 on barley roots significantly improved (p < 0.05) the growth parameters of the host plant under Cd stress, alleviating Cd toxic effect in barley. Khan et al. [14] indicated that inoculation of Glomerella truncata and Phomopsis fukushii on S. nigrum enhanced the host plant growth. They proposed that these fungi can induce biochemical pathways of their host plant which produce phytohormones or raise the availability of nutritional elements for the host plant [15]. The plants inoculated with the TBR5 and TW26 strains showed higher chlorophyll contents (p < 0.05) than non-inoculated counterpart under Cd stress. A number of previous reports have similarly shown that fungal inoculation reduced the negative effects of HM stress in their host plants such as Festuca arundinacea and Festuca pratensisv [12], Brassica napus [47], and S. nigrum [48]. Our results also reveal that TBR5 and TW26 inoculation significantly elevated (p < 0.05) the concentrations of Cd in the roots of barley in an environment containing Cd treatment (Table 2). Similarly, previous study indicated that endophytic fungi have increased the level of Cd accumulated in Lolium perenne L. [49] and S. nigrum [16]. The production of chelators such as phytochelatins and organic compounds like citric acid and oxalic acid by endophytic fungi may account for these effects. These chelators are important factors for Cd tolerance and accumulation by plants, as they form stable complexes with HMs, enhancing their solubilization in the soil [12, 16]. Hence, it can be suggested that TBR5 and TW26 can increase Cd availability to barley by enhancing Cd mobilization in the sand. Our results illustrated that, as Cd level concentrations in the sand were increased, there was a downward trend in Cd removal associated with a decreasing amount of biomass production. Expectedly, plants that were inoculated by TBR5 and TW26 showed the higher Cd removal factors (p < 0.05), compared to that of in non-inoculated control plants in the all environments containing Cd treatment, since it makes plants produce more biomass and uptakes higher Cd in their root than non-inoculated plants. Relatively higher accumulation capacities for Cd in roots of H. vulgare might also allow for the phytostabilization of Cd-contaminated soil.
Declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajkumar M, Ae N, Freitas H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere. 2009;77:153–160. doi: 10.1016/j.chemosphere.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 2.Rehman MZU, Rizwan M, Ali S, Ok YS, Ishaque W, Saifullah S, et al. Remediation of heavy metal contaminated soils by using Solanum nigrum: A review. Ecotoxicol Environ Saf. 2017;143:236–248. doi: 10.1016/j.ecoenv.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Perronnet K, Schwartz C, Gérard E, Morel JL. Availability of cadmium and zinc accumulated in the leaves of Thlaspi caerulescens incorporated into soil. Plant Soil. 2000;227:257–263. [Google Scholar]
- 4.Zhan F, He Y, Li T, Yang YY, Toor GS, Zhao Z. Tolerance and antioxidant response of a dark septate endophyte (DSE), Exophiala pisciphila, to cadmium stress. Bull Environ Contam Toxicol. 2015;94:96–102. doi: 10.1007/s00128-014-1401-8. [DOI] [PubMed] [Google Scholar]
- 5.Smolders E. Cadmium uptake by plants. Int J Occup Med Environ. 2001;14:177–183. [PubMed] [Google Scholar]
- 6.Tesfai M, Dresher S. Assessment of benefits and risks of landfill materials for agriculture in Eritrea. Waste Manag. 2009;29:851–858. doi: 10.1016/j.wasman.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Liao QL, Hua M, Wu XM, Bi KS, Yan CY, Chen B, Zhang XY. Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere. 2007;67:2148–2155. doi: 10.1016/j.chemosphere.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhang Y, Liu M, Shi X, Zhao Z. Dark septate endophyte (DSE) fungi isolated from metal polluted soils: their taxonomic position, tolerance, and accumulation of heavy metals in vitro. J Microbiol. 2008;46:624–632. doi: 10.1007/s12275-008-0163-6. [DOI] [PubMed] [Google Scholar]
- 9.Ebbs SD, Kochian LV. Phytoextraction of zinc by oat (Avena sativa), barley (Hordeum vulgare), and Indian mustard (Brassica juncea) Environ Sci Technol. 1998;32:802–806. [Google Scholar]
- 10.Shenker M, Fan T-M, Crowley D. Phytosiderophores influence on cadmium mobilization and uptake by wheat and barley plants. J Environ Qual. 2001;30:2091–2098. doi: 10.2134/jeq2001.2091. [DOI] [PubMed] [Google Scholar]
- 11.Tiryakioglu M, Eker S, Ozkutlu F, Husted S, Cakmak I. Antioxidant defense system and cadmium uptake in barley genotypes differing in cadmium tolerance. J Trace Elem Med Biol. 2006;20:181–189. doi: 10.1016/j.jtemb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Soleimani M, Hajabbasi MA, Afyuni M, Mirlohi A, Borggaard OK, Holm PE. Effect of endophytic fungi on cadmium tolerance and bioaccumulation by Festuca arundinacea and Festuca pratensis. Int J Phytoremediat. 2010;12:535–549. doi: 10.1080/15226510903353187. [DOI] [PubMed] [Google Scholar]
- 13.Arnold AE, Maynard Z, Gilbert GS, Coley PD, Kursar TA. Are tropical fungal endophytes hyperdiverse? Ecol Lett. 2000;3:267–274. [Google Scholar]
- 14.Khan AR, Ullah I, Waqas M, Park GS, Khan AL, Hong SJ, Ullah R, Jung BK, Park CE, Ur-Rehman S, Lee IJ, Shin JH. Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi. Ecotoxicol Environ Saf. 2017;136:180–188. doi: 10.1016/j.ecoenv.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Li H-Y, Li D-W, He C-M, Zhou Z-P, Mei T, Xu H-M. Diversity and heavy metal tolerance of endophytic fungi from six dominant plant species in a Pb–Zn mine wasteland in China. Fungal Ecol. 2012;5:309–315. [Google Scholar]
- 16.Khan AR, Waqas M, Ullah I, Khan AL, Khan MA, Lee I-J, Shin J-H. Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ Exp Bot. 2017;135:126–135. [Google Scholar]
- 17.Zhao D, Li T, Shen M, Wang J, Zhao Z. Diverse strategies conferring extreme cadmium (Cd) tolerance in the dark septate endophyte (DSE), Exophiala pisciphila: evidence from RNA-seq data. Microbiol Res. 2015;170:27–35. doi: 10.1016/j.micres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez RJ, White JF, Jr, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 19.Urban A, Puschenreiter M, Strauss J, Gorfer M. Diversity and structure of ectomycorrhizal and co-associated fungal communities in a serpentine soil. Mycorrhiza. 2008;18:339–354. doi: 10.1007/s00572-008-0189-y. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YY, Liu JH, Zhou YM, Gong TY, Wang J, Ge YL. Enhanced phytoremediation of mixed heavy metal (mercury)–organic pollutants (trichloroethylene) with transgenic alfalfa co-expressing glutathione S-transferase and human P450 2E1. J Hazard Mater. 2013;206:1100–1107. doi: 10.1016/j.jhazmat.2013.06.065. [DOI] [PubMed] [Google Scholar]
- 21.Shakeri A, Yousefi F. Source and health risk assessment of potentially toxic elements in the un-engineered landfills soil of Kermanshah province. J Eng Geol. 2018;12:11. [Google Scholar]
- 22.Liu C, Cui J, Jiang G, Chen X, Wang L, Fang C. Soil heavy metal pollution assessment near the largest landfill of China. Soil Sediment Contam. 2013;22:390–403. [Google Scholar]
- 23.Larran S, Perelló A, Simón MR, Moreno V. The endophytic fungi from wheat (Triticum aestivum L.) World J Microbiol Biotechnol. 2007;23:565–572. [Google Scholar]
- 24.Sieber T, Grünig C. Fungal root endophytes. In: Eshel A, Beeckman T, editors. Plant Roots: The Hidden Half. Boca Raton: CRC Press, Taylor & Francis Group; 2013. pp. 31–49. [Google Scholar]
- 25.Robert V, Szoke S, Jabas B, Vu D, Chouchen O, Blom E, Cardinali G. BioloMICS Software: Biological data management, identification, classification and statistics. Open Appl Inform J. 2011;5:87–98. [Google Scholar]
- 26.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 27.White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics PCR protocols: a guide to methods and applications. 18:315–322
- 28.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 29.Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30:1229–1235. doi: 10.1093/molbev/mst012. [DOI] [PubMed] [Google Scholar]
- 30.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 31.Woudenberg JH, Groenewald JZ, Binder M, Crous PW. Alternaria redefined. Stud Mycol. 2013;75:171–212. doi: 10.3114/sim0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnoor JL. Australasian soil contamination gets attention. Environ Sci Technol. 2004;38(3):53A. doi: 10.1021/es040368m. [DOI] [PubMed] [Google Scholar]
- 33.Wong S, Li X, Zhang G, Qi S, Min Y. Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut. 2002;119:33–44. doi: 10.1016/s0269-7491(01)00325-6. [DOI] [PubMed] [Google Scholar]
- 34.Su C. A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environ Skept Crit. 2014;3:24. [Google Scholar]
- 35.Glynou K, Ali T, Buch AK, Haghi Kia S, Ploch S, Xia X, Çelik A, Thines M, Maciá-Vicente JG. The local environment determines the assembly of root endophytic fungi at a continental scale. Environ Microb. 2016;18:2418–2434. doi: 10.1111/1462-2920.13112. [DOI] [PubMed] [Google Scholar]
- 36.Herrera J, Khidir HH, Eudy DM, Porras-Alfaro A, Natvig DO, Sinsabaugh RL. Shifting fungal endophyte communities colonize Bouteloua gracilis: effect of host tissue and geographical distribution. Mycologia. 2010;102:1012–1026. doi: 10.3852/09-264. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez Márquez S, Bills GF, Acuña LD, Zabalgogeazcoa I. Endophytic mycobiota of leaves and roots of the grass Holcus lanatus. Fungal Divers. 2010;41:115–123. [Google Scholar]
- 38.Sieber TN, Waisel Y, Eshel A, Kafkafi U. Fungal root endophytes. Plant roots: the Hidden Half. Boca Raton: CRC Press, Taylor & Francis Group; 2002. pp. 887–917. [Google Scholar]
- 39.Zafar S, Aqil F, Ahmad I. Metal tolerance and biosorption /+potential of filamentous fungi isolated from metal contaminated agricultural soil. Bioresour Technol. 2007;98:2557–2561. doi: 10.1016/j.biortech.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 40.Macia-Vicente JG, Jansson HB, Abdullah SK, Descals E, Salinas J, Lopez-Llorca LV. Fungal root endophytes from natural vegetation in Mediterranean environments with special reference to Fusarium spp. FEMS Microbiol Ecol. 2008;64:90–105. doi: 10.1111/j.1574-6941.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 41.Vujanovic V, Hamel C, Yergeau E, St-Arnaud M. Biodiversity and biogeography of Fusarium species from northeastern North American asparagus fields based on microbiological and molecular approaches. Microb Ecol. 2006;51:242–255. doi: 10.1007/s00248-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 42.Yates I, Bacon C, Hinton D. Effects of endophytic infection by Fusarium moniliforme on corn growth and cellular morphology. Plant Dis. 1997;81:723–728. doi: 10.1094/PDIS.1997.81.7.723. [DOI] [PubMed] [Google Scholar]
- 43.Mandyam K, Loughin T, Jumpponen A. Isolation and morphological and metabolic characterization of common endophytes in annually burned tallgrass prairie. Mycologia. 2010;102:813–821. doi: 10.3852/09-212. [DOI] [PubMed] [Google Scholar]
- 44.David AS, Haridas S, LaButti K, Lim J, Lipzen A, Wang M, Barry K, Grigoriev IV, Spatafora JW, May G (2016) Draft genome sequence of Microdochium bolleyi, a dark septate fungal endophyte of beach grass. Genome Announc 4(2) [DOI] [PMC free article] [PubMed]
- 45.Ban Y, Tang M, Chen H, Xu Z, Zhang H, Yang Y. The response of dark septate endophytes (DSE) to heavy metals in pure culture. PLoS One. 2012;7(10):e47968. doi: 10.1371/journal.pone.0047968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Likar M, Regvar M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil. 2013;370:593–604. [Google Scholar]
- 47.Deng X, Chai L, Yang Z, Tang C, Tong H, Yuan P. Bioleaching of heavy metals from a contaminated soil using indigenous Penicillium chrysogenum strain F1. J Hazard Mater. 2012;233:25–32. doi: 10.1016/j.jhazmat.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 48.Khan AR, Ullah I, Khan AL, Park GS, Waqas M, Hong SJ, Jung BK, Kwak Y, Lee IJ, Shin JH. Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environ Sci Pollut Res. 2015;22:14032–14042. doi: 10.1007/s11356-015-4647-8. [DOI] [PubMed] [Google Scholar]
- 49.Alvarenga P, Goncalves AP, Fernandes RM, de Varennes A, Vallini G, Duarte E, Cunha-Queda AC. Evaluation of composts and liming materials in the phytostabilization of a mine soil using perennial ryegrass. Sci Total Environ. 2008;406:43–56. doi: 10.1016/j.scitotenv.2008.07.061. [DOI] [PubMed] [Google Scholar]