Abstract
Background
A plasmid-mediated mechanism of bacterial resistance to polymyxin is a serious threat to public health worldwide. The present study aimed to determine the occurrence of plasmid-mediated colistin resistance genes and to conduct the molecular characterization of mcr-positive Escherichia coli strains isolated from Polish poultry.
Methods
In this study, 318 E. coli strains were characterized by the prevalence of mcr1–mcr5 genes, antimicrobial susceptibility testing by minimal inhibitory concentration method, the presence of antimicrobial resistance genes was screened by PCR, and the biofilm formation ability was tested using the crystal violet staining method. Genetic relatedness of mcr-1-positive E. coli strains was evaluated by multilocus sequence typing method.
Results
Among the 318 E. coli isolates, 17 (5.35%) harbored the mcr-1 gene. High antimicrobial resistance rates were observed for ampicillin (100%), tetracycline (88.24%), and chloramphenicol (82.35%). All mcr-1-positive E. coli strains were multidrug-resistant, and as many as 88.24% of the isolates contained the blaTEM gene, tetracycline (tetA and tetB), and sulfonamide (sul1, sul2, and sul3) resistance genes. Additionally, 41.18% of multidrug-resistant, mcr-1-positive E. coli isolates were moderate biofilm producers, while the rest of the strains showed weak biofilm production. Nine different sequence types were identified, and the dominant ST was ST93 (29.41%), followed by ST117 (17.65%), ST156 (11.76%), ST 8979 (11.76%), ST744 (5.88%), and ST10 (5.88%). Moreover, the new ST was identified in this study.
Conclusions
Our results showed a low occurrence of mcr-1-positive E. coli strains isolated from Polish poultry; however, all the isolated strains were resistant to multiple antimicrobial agents and were able to form biofilms at low or medium level.
Keywords: Escherichia coli, Mcr-1, Multidrug resistance, MLST, Biofilm, Sequence type
Introduction
Antimicrobial resistance (AMR) has emerged as one of the most important global threats to human health in the last few decades. The increasing resistance of Gram-negative bacteria isolated from poultry is receiving high attention, especially in terms of public health protection, but also in the ability to successfully treat bacterial infections in birds. Resistant bacteria can be transmitted from animals to humans via direct contact between animals and humans, or through the food chain and the environment [1]. A crucial issue seems to be the more frequent isolation of Gram-negative strains resistant to colistin from slaughter animals, e.g., poultry, pigs, and calves [2–4].
In poultry production, colistin (polymyxin E) has been widely administered for the treatment and metaphylaxis of avian colibacillosis, gastroenteritis, and diarrhea to reduce high incidence and mortalities. Such overuse and/or misuse of antibiotics contribute to the development and spread of AMR among poultry strains and flocks, leading to the emergence of multidrug-resistant (MDR) pathogens [5].
The mechanism of action of colistin is the ability of the drug to bind to the surface structures of the bacterial cell membrane (phospholipids and lipopolysaccharides (LPS)), which increases its permeability and weakens the osmotic integrity of the cytoplasmic membrane, while resistance to colistin is an effect of the inefficient binding of polymyxins to the lipid A moiety of LPS due to the 4ʹ-phosphoethanolamine (PEA) modification of lipid A on the LPS [6]. Colistin resistance may be encoded chromosomally or by the mcr genes located on mobile genetic elements in plasmid DNA. Chromosomally mediated resistance to colistin is caused by single nucleotide polymorphism in pmrAB, phoPQ, mgrB, and/or pmrD genes, resulting in modification of lipid A [7]. In 2015, Liu et al. [8] reported the first case of a plasmid-mediated colistin resistance mechanism, designated mcr-1, in E. coli and Klebsiella pneumoniae. Since then, an increasing number of mcr genes have been identified. At present, 10 different mcr genes and their variants have been described, and these discoveries indicate a rapid evolution of plasmid-mediated colistin resistance gene family [3, 9–15]. The mcr-1, mcr-2, and mcr-3 genes were originally characterized on plasmids in Enterobacteriaceae, but have recently been identified on chromosomes in Moraxella spp. and Aeromonas veronii [16, 17]. Additionally, mcr-4, mcr-5, mcr-6, mcr-7, and mcr-8 genes, compared to those listed above, have been described relatively recently. In 2019, the novel mcr-9 homolog was detected in the clinical isolate of Salmonella Typhimurium in the USA [18]. The first case of identification of the latest variant mcr-10 in an Enterobacter roggenkamp strain was reported in 2020 [19]. Currently, mcr genes have been globally distributed, and an in silico analysis showed their presence on plasmids and their high prevalence among Enterobacteriaceae strains isolated from humans, animals, food, and environment [15]. Moreover, this resistance could be easily transferred to other bacterial cells during cell division or horizontal gene transfer (e.g., conjugation or transduction) [20, 21].
It is worth noting that the transferable plasmid-mediated genes that could rapidly spread between bacterial species and hosts, and the possible transmission of resistance genes due to cross-contamination between food-production chains, animals, and humans have raised worldwide concern in recent years [6, 8, 22].
In human medicine, polymyxins are used only for the emergence of MDR bacteria, which are responsible for severe infections and deaths, as a last resort antimicrobial agent against these “super bacteria.” The spread of diverse antimicrobial resistance genes in Enterobacteriaceae, e.g., colistin and quinolone resistance genes, is well known among the bacteria within this family [23, 24].
Currently, with the increase in resistance of bacteria to commonly used antimicrobial agents, polymyxins are also used as the last resort therapy for biofilm-related infections. Biofilm is a multicellular structure, which is defined as a community of cooperating bacteria that adhere to biological or nonbiological surfaces contained in the extracellular polymeric matrix [25]. Bacteria embedded in the inner layers of the biofilm may show less susceptibility to antibiotics due to increased horizontal gene transmission, modification of the antibiotic target or cell permeability, and the use of efflux pumps or the expression of hydrolyzing enzymes. Colistin can act against metabolically inactive bacterial cells in the inner layers of the biofilm. Because of this property, colistin is the subject of research in which combined antibiotic therapy is recommended as a treatment for biofilm-related infections caused by Gram-negative bacteria [26, 27].
In the last few years, several reports have been published on the detection and characterization of colistin-resistant E. coli strains isolated from slaughter animals [4, 8, 28–31]. Studies on colistin resistance and the prevalence of resistance-associated genes among bacterial strains from various sources have been conducted worldwide. In Poland, studies on colistin-resistant E. coli strains isolated from slaughter and wild animals were conducted by Wasyl et al. [32, 33], Zając et al. [34], and Majewski et al. [35]. Those studies were mainly focused on the antimicrobial resistance of E. coli strains, the presence of mcr genes, and molecular identification and characterization of resistance mechanisms. However, there is still scarce research on the relationship between AMR, genotypic characterization (AMR genes, multilocus sequence typing — MLST), and the ability of biofilm formation in mcr-1-positive E. coli strains isolated from poultry in Poland.
The present study aimed to assess the prevalence of the mcr genes among E. coli strains isolated from different types of poultry (broilers, laying hens, turkeys, geese, and ducks), to determine the antimicrobial susceptibility phenotypes of mcr-positive strains, and to evaluate the association of observed phenotypes with the presence of AMR genes, MLST sequence types, and the ability of biofilm production by these strains.
Material and methods
Isolate collection
A total of 318 E. coli isolates were obtained from the AGRO-VET Veterinary Laboratory in Wrocław, Poland. The strains were collected during 2016–2020 from different types of poultry: broilers (n = 161), turkeys (n = 72), breeder broilers (n = 37), laying hens (n = 20), ducks (n = 14), and geese (n = 14). Strains were isolated from organs with lesions or from cloacal swabs and identified using standard microbial and chromogenic media for coliform bacteria, especially those for selective isolation of E. coli.
DNA isolation
Total DNA of all 318 E. coli strains was isolated from 18- to 20-h culture of the strains in LB medium (BIOCORP, Warszawa, Poland) incubated at 37 °C. For DNA extraction, the commercial Genomic Mini® kit (A&A Biotechnology, Gdynia, Poland) was used and the procedure was performed according to the manufacturer’s protocol. The purity and concentration of the obtained DNA were assessed using a spectrophotometer, and the amount of DNA was estimated to be approximately 30 ng/µl. As good quality DNA, the A260/A280 ratio of 1.7–2.0 and A260/A230 greater than 1.5 were taken [36]. The obtained DNA of strains was protected and stored at − 20 °C until further tests.
PCR-based screening of colistin resistance genes
Multiplex PCR was used to amplify part of the five colistin resistance genes mcr-1, mcr-2, mcr-3, mcr-4, and mcr-5 in all 318 E. coli strains according to published protocol [37] with minor modification. Each PCR reaction was performed in 25 µl total volume consisting of 2.5 µl 10 × DreamTaq Green Buffer (Thermo Fisher Scientific, Waltham, USA), each primer at 0.2 µM final concentration (Genomed, Warszawa, Poland), 0.2 mM nucleotide mix (Thermo Fisher Scientific), 1 U of DreamTaq Green Polymerase (Thermo Fisher Scientific), and 1 µl DNA template. The thermal profile included initial denaturation at 95 °C for 10 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 63 °C for 90 s, elongation at 72 °C for 60 s, and final elongation at 72 °C for 10 min. The PCR products were run on 1.5% agarose gel with Midori Green DNA Stain (Nippon Genetics Europe GmbH, Dueren, Germany) at 100 V. PCR products with the expected base pair size (320, 715, 929, 1116, and 1644 bp, respectively) were subsequently sequenced and then analyzed using BioEdit (v. 7.2.5) software and GenBank database to confirm the test results.
As positive controls for mcr genes (mcr-1, mcr-2, mcr-3, mcr-4, mcr-5) detection, the following strains were used in this study: E. coli KP81 and E. coli KP37 for the detection of mcr-1 and mcr-2 genes, respectively. Strains were given by Christine Lammens from the University of Antwerp in Belgium. Moreover, E. coli SQ352, E. coli DH5α, and Salmonella paratyphi B-SA01718 were used as positive controls for mcr-3, mcr-4, and mcr-5 gene detection, respectively. These strains were obtained from the European Union Reference Laboratory for Antimicrobial Resistance at the National Food Institute, Technical University in Denmark.
Antimicrobial susceptibility test
The examination of antimicrobial susceptibility to selected antimicrobial agents was performed by determination of minimal inhibitory concentration (MIC) using the commercial system MIC Sensititre EU Surveillance Salmonella/E. coli EUVSEC Plate (Thermo Fisher Scientific, WalthamAZI, USA) according to the manufacturer’s instructions. Resistance breakpoints to fourteen antimicrobial agents, namely gentamicin (GEN), ampicillin (AMP), cefotaxime (CTX), ceftazidime (CAZ), meropenem (MEM), nalidixic acid (NAL), ciprofloxacin (CIP), chloramphenicol (CHL), azithromycin (AZM), colistin (CST), tetracycline (TET), tigecycline (TGC), sulfamethoxazole (SMX), and trimethoprim (TMP), were determined for mcr-1-positive strains. The tested E. coli strains were classified as susceptible (S) or resistant (R) based on EUCAST guidelines, version 10.0, 2020 [38]. In the case of absence of limit values for selected antimicrobials, the guidelines of the Clinical Laboratory Standards Institute (CLSI) were used to analyze the results [39]. The reference strain of E. coli ATCC 25,922 was used as test control. In addition, the investigated isolates were categorized as multidrug resistant (MDR), when they were simultaneously resistant to at least three antimicrobial agents from different classes of antimicrobial agents [30].
Detection of antimicrobial resistance genes
PCR amplification of the genes related to resistance to beta-lactams (blaCTX-M, blaSHV, and blaTEM), quinolones (qnrA, qnrB, qnrC, qnrD, qnrS, qepA, and aac(6′)-Ib-cr), phenicols (cat1, cat2, and cat3), tetracyclines (tetA, tetB, tetC, and tetD), and sulfonamides (sul1, sul2, and sul3) was performed for the E. coli isolates carrying the mcr-1 gene [30, 31, 40]. These genes were chosen as a molecular resistance mechanism of E. coli to the selected antimicrobials used for antimicrobial susceptibility tests in this study. Sequenced PCR products of resistance genes, collected during previous studies, were used as positive controls in this study [41].
Biofilm formation by mcr-1-positive E. coli strains
Biofilm formation was tested in 96-well flat polystyrene microtiter plates (Corning Inc., New York, USA), based on a modified protocol previously described [26, 42]. The mcr-1-positive E. coli strains were cultured overnight in LB medium, adjusted to a density of 0.5 McFarland units, and then diluted in a proportion of 1:9 with fresh LB medium. A volume of 200-µl aliquots of each dilution was then dispensed into a microtiter plate well, and each bacterial suspension was inoculated in 6 wells. Negative controls for the test were uninoculated LB medium. To compare biofilm formation, the results of mcr-1-positive E. coli isolates, E. coli ATCC 25,922, and three collected E. coli strains without mcr-1 were used. The microtiter plate was incubated for 24 h in aerobic conditions at 37 °C without shaking.
After incubation, the supernatant was discarded, and the microtiter plate was washed three times gently with 250 µl of phosphate buffer solution (PBS). Microplates were then stained with 200 µl of 0.1% crystal violet for 15 min, washed thrice with PBS, and dried for 30 min. Adherent cells were solubilized with 200 µl of 95% ethanol. OD590 (optical density) was measured using an automated microplate reader Spark (Tecan Group Ltd., Männedorf, Switzerland). Based on the OD produced by bacterial biofilms and established cut-off value (ODc), the strains were classified into the following categories: no biofilm former — OD ≤ ODc, weak biofilm former — ODc < OD ≤ 2ODc, moderate biofilm former — 2ODc < OD ≤ 4ODc, or strong biofilm former — 4ODc < OD [25, 43].
MLST of the mcr-1-positive strains
The mcr-1-positive E. coli isolates were analyzed using the MLST method to determine the sequence types (STs). Depending on the alleles of seven basic metabolism genes (housekeeping genes), namely adk, fumC, gyrB, icd, mdh, purA, and recA, the Achtman MLST method was performed using the EnteroBase (v.1.1.2) database (http://enterobase.warwick.ac.uk), and the protocol described by Wirth et al. [44]. Sequences of seven housekeeping genes were concatenated for each isolate using BioEdit (v. 7.2.5), and then the phylogenetic tree was reconstructed by the neighbor-joining method with 1000 bootstrap trials, and Kimura’s correction using MEGA 6.0 software [45–47].
Results
PCR-based screening of colistin resistance genes
Among all the tested E. coli strains (n = 318), 17 isolates (5.35%) were mcr-1 positive, whose presence was confirmed by the 100% of nucleotide identity of the amplicons when they were sequenced (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The mcr-2, mcr-3, mcr-4, and mcr-5 genes were not detected in any of the collected E. coli strains. Most of the mcr-1 harboring E. coli strains were isolated from turkeys (9; 52.94%), followed by seven strains (41.18%) from broilers and one strain (5.88%) from goose.
Antimicrobial susceptibility
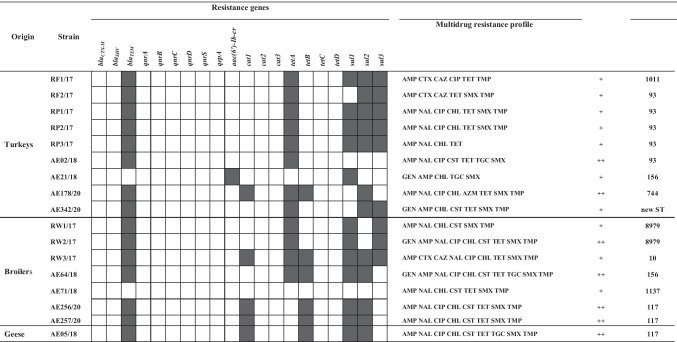
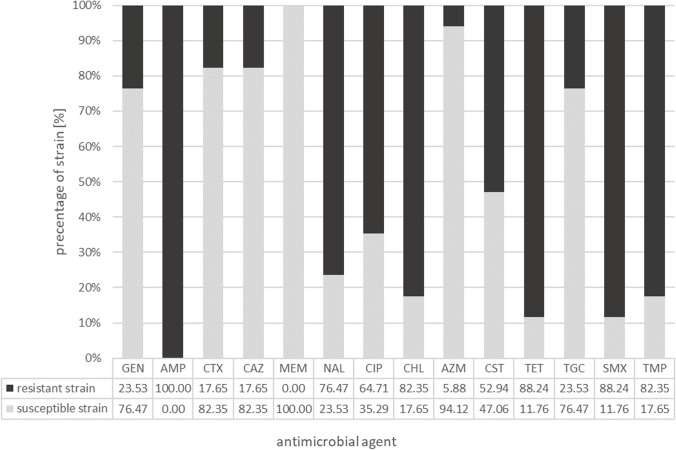
Antimicrobial susceptibility of the E. coli strains carrying the detected mcr-1 gene is shown in Table 2 Occurrence of resistance genes to antimicrobial agents, resistance profiles, biofilm formation, and sequence types (STs) in mcr-1-positive Escherichia coli strains (n = 17)[Display Image Removed]White square — lack of resistance gene; black square — presence of resistance gene.GEN, gentamicin; AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; MEM, meropenem; NAL, nalidixic acid; CIP, ciprofloxacin; CHL, chloramphenicol, AZM, azithromycin; CST, colistin; TET, tetracycline; TGC, tigecycline; SMX, sulfamethoxazole; TMP, trimethoprim.Biofilm formation: + + + — strong biofilm former, + + — moderate biofilm former, + — weak biofilm former. Table 1 and in Fig. 1. All mcr-1-positive E. coli strains (n = 17) were susceptible (100%) to meropenem (MIC value 0.03 µg/ml). In addition, most strains were susceptible to azithromycin (94.12%, MIC range of 2–8 µg/ml), ceftazidime (82.35%, MIC range of 0.5–1 µg/ml), cefotaxime (82.35%, MIC range of 0.25–1 µg/ml), and tigecycline (76.47%, MIC range of 0.25–0.5 µg/ml). Similarly, the percentage of strains susceptible to gentamicin was relatively high (76.47%; MIC range of 0.5–1 µg/ml).
Table 2.
Occurrence of resistance genes to antimicrobial agents, resistance profiles, biofilm formation, and sequence types (STs) in mcr-1-positive Escherichia coli strains (n = 17)
White square — lack of resistance gene; black square — presence of resistance gene.
GEN, gentamicin; AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; MEM, meropenem; NAL, nalidixic acid; CIP, ciprofloxacin; CHL, chloramphenicol, AZM, azithromycin; CST, colistin; TET, tetracycline; TGC, tigecycline; SMX, sulfamethoxazole; TMP, trimethoprim.
Biofilm formation: + + + — strong biofilm former, + + — moderate biofilm former, + — weak biofilm former.
Table 1.
Minimal inhibitory concentration (MIC) (μg/ml) of mcr-1-positive Escherichia coli strains (n = 17)
| Origin | Strain | MIC value (µg/ml) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GEN | AMP | CTX | CAZ | MEM | NAL | CIP | CHL | AZM | CST | TET | TGC | SMX | TMP | ||
| Turkeys | RF1/17 | 0.5 | > 64 | 4 | 4 | < 0.03 | 4 | 8 | 8 | 8 | 1 | > 64 | 0.5 | 8 | 8 |
| RF2/17 | 1 | > 64 | 4 | 4 | < 0.03 | 4 | 0.015 | 8 | 8 | 1 | > 64 | 0.5 | > 1024 | 32 | |
| RP1/17 | 1 | > 64 | 0.25 | 0.5 | < 0.03 | > 128 | > 8 | 32 | 2 | 1 | > 64 | 0.25 | > 1024 | > 32 | |
| RP2/17 | 1 | > 64 | 0.25 | 0.5 | < 0.03 | > 128 | > 8 | 32 | 2 | 1 | > 64 | 0.25 | > 1024 | > 32 | |
| RP3/17 | 0.5 | > 64 | 0.25 | 0.5 | < 0.03 | > 128 | 0.25 | > 128 | 8 | 1 | > 64 | 0.5 | 8 | 0.25 | |
| AE02/18 | 1 | > 64 | < 0.25 | < 0.5 | < 0.03 | > 128 | 8 | < 8 | 4 | 8 | > 64 | 1 | > 1024 | < 0.25 | |
| AE21/18 | > 32 | > 64 | < 0.25 | < 0.5 | < 0.03 | < 4 | 0.03 | 64 | < 2 | 2 | 4 | 2 | > 1024 | 1 | |
| AE178/20 | < 0.5 | > 64 | < 0.25 | < 0.5 | < 0.03 | > 128 | 8 | 128 | 16 | < 1 | > 64 | < 0.25 | > 1024 | > 32 | |
| AE342/20 | 32 | > 64 | < 0.25 | < 0.5 | < 0.03 | < 4 | < 0.015 | 32 | < 2 | 4 | 64 | < 0.25 | > 1024 | > 32 | |
| Broilers | RW1/17 | 0.5 | > 64 | 0.5 | 0.5 | < 0.03 | > 128 | 0.25 | 64 | 2 | 8 | 2 | 0.5 | > 1024 | > 32 |
| RW2/17 | > 32 | > 64 | 1 | 0.5 | < 0.03 | > 128 | > 8 | 128 | 8 | 8 | > 64 | 0.5 | > 1024 | > 32 | |
| RW3/17 | 8 | > 64 | > 4 | 4 | < 0.03 | > 128 | > 8 | 128 | < 2 | 2 | > 64 | < 0.25 | > 1024 | > 32 | |
| AE64/18 | > 32 | > 64 | < 0.25 | 1 | < 0.03 | > 128 | > 8 | > 128 | 8 | 8 | > 64 | 2 | > 1024 | > 32 | |
| AE71/18 | 1 | > 64 | < 0.25 | < 0.5 | < 0.03 | > 128 | 0.25 | 64 | 4 | 8 | 64 | < 0.25 | > 1024 | > 32 | |
| AE256/20 | < 0.5 | > 64 | < 0.25 | < 0.5 | < 0.03 | > 128 | 4 | 128 | < 2 | 8 | > 64 | < 0.25 | > 1024 | > 32 | |
| AE257/20 | < 0.5 | > 64 | < 0.25 | < 0.5 | < 0.03 | > 128 | 4 | 128 | < 2 | 8 | > 64 | < 0.25 | > 1024 | > 32 | |
| Geese | AE05/18 | 1 | > 64 | < 0.25 | < 0.5 | < 0.03 | > 128 | 8 | 128 | 4 | 8 | > 64 | 1 | > 1024 | > 32 |
Bold — resistance to an antimicrobial agent; italics — susceptible to an antimicrobial agent.
GEN, gentamicin; AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; MEM, meropenem; NAL, nalidixic acid; CIP, ciprofloxacin; CHL, chloramphenicol; AZM, azithromycin; CST, colistin; TET, tetracycline; TGC, tigecycline; SMX, sulfamethoxazole; TMP, trimethoprim.
Fig. 1.
Antimicrobial susceptibility of the mcr-1 harboring E. coli strains (n = 17). GEN, gentamicin; AMP, ampicillin; CTX, cefotaxime; CAZ, ceftazidime; MEM, meropenem; NAL, nalidixic acid; CIP, ciprofloxacin; CHL, chloramphenicol; AZM, azithromycin; CST, colistin; TET, tetracycline; TGC, tigecycline; SMX, sulfamethoxazole; TMP, trimethoprim
On the other hand, all strains (100%) were resistant to ampicillin (MIC > 64 µg/ml). The occurrence of resistant isolates to tetracycline (88.24%), chloramphenicol (82.35%) nalidixic acid (76.47%), and ciprofloxacin (64.71%) was also high. MIC values for tetracycline-resistant strains (n = 15) were in the range of 64 µg/ml (2 strains) and > 64 µg/ml (13 strains). The ranges of MIC values for chloramphenicol-resistant strains were varied and were as follows: 32 µg/ml (n = 3), 64 µg/ml (n = 3), 128 µg/ml (n = 8), and > 128 µg/ml (n = 2). Among the E. coli strains resistant to nalidixic acid, the MIC value for all strains was > 128 µg/ml, while the MIC value for ciprofloxacin ranged between 4 and ≥ 8 µg/ml.
It is worth emphasizing that among the 17 mcr-1-positive E. coli isolates, only nine strains (52.94%) were resistant to colistin; among these strains, the MIC value was 8 µg/ml for eight strains (88.89%). In all E. coli strains resistant to sulfamethoxazole (15; 88.24%), the MIC value exceeded 1024 µg/ml, while in strains resistant to trimethoprim (14; 82.35%), the MIC value ranged from 8 to ≥ 32 µg/ml.
Interestingly, among the 17 mcr-1-positive E. coli strains isolated from different poultry types, the resistance to the 13 tested antimicrobial agents was similar. An exception was the resistance to colistin, wherein six of strains were derived from broilers (35.29%), two from turkeys (11.76%), and one E. coli isolate (5.88%) from goose.
On the basis of the interpretation of MIC breakpoint values, according to EUCAST recommendations [38], 15 resistance profiles of the isolated E. coli strains were described. All the investigated isolates were MDR. The 15 resistance profiles obtained in this study are presented in Table 2.
The most common resistance profile consisted of six classes of antimicrobial agents: AMP + NAL + CIP + CHL + CST + TET + SMX + TMP and was observed in two strains of E. coli (AE256/20 and AE257/20) isolated from the internal organs of broilers. Another two E. coli strains isolated from the organs of turkey (RP1/17 and RP2/17) had a resistance profile of five classes of antimicrobial agents: AMP + NAL + CIP + CHL + TET + SMX + TMP; their resistance profiles (even for 7 classes of antimicrobial agents) were noted in single strains of E. coli isolated from different sources and places (turkeys, broilers, and geese; organs and cloacal swabs).
It is worth noting that among the mcr-1-positive E. coli strains isolated from chicken broilers, two strains (11.76%) exhibited resistance profile to as many as 7 classes of antimicrobial agents, four strains (23.53%) to 6 classes, and one strain (5.88%) to 5 classes of antimicrobial agents. In contrast, among isolates from turkeys, most strains showed a resistance profile to 5 classes of antimicrobial agents (5 strains, 29.41%). The remaining E. coli isolates showed resistance profiles to 6 classes (2 strains; 11.76%) and 4 classes (2 strains, 11.76%) of antimicrobial agents. The isolate from the goose was resistant to antimicrobial agents from 6 classes.
Occurrence of resistance genes and biofilm formation
The occurrence of the selected resistance genes among the mcr-1-positive E. coli strains isolated from poultry is shown in Table 2. The results showed the frequent presence of one of the beta-lactam resistance genes (blaTEM) (88.24%) in the isolated E. coli strains, with the simultaneous absence of the other two resistance genes to this class of antibiotics (blaSHV and blaCTX-M). The percentage of strains that harbored the sulfonamide resistance genes was as follows: sul1 — 70.59%, sul2 — 70.59%, and sul3 — 52.94%. All the three genes were detected in 29.41% of mcr-1-positive E. coli strains. In addition, 70.59% of E. coli isolates showed the presence of the tetA gene and 35.29% had the tetB gene. None of the investigated isolates harbored the tetC or tetD gene. The phenicol resistance gene cat1 was detected only in five isolates (29.41%), and it was one of the three resistance genes tested for this class of antimicrobial agents. In contrast, among the seven fluoroquinolone resistance genes tested (qnrA, qnrB, qnrC, qnrD, qnrS, qepA, and aac(6′)-Ib-cr), only one E. coli strain (5.88%) isolated from turkeys had aac(6′)-Ib-cr. No differences were observed in the presence or absence of the selected resistance genes depending on the source of E. coli.
The results of biofilm assay are presented in Table 2. The results showed that all the 17 E. coli strains isolated from poultry origin produced biofilms, although at different levels of intensity. Under the assessed incubation conditions, most strains isolated from turkeys (41.18%) produced biofilm weakly, while the remaining strains (11.76%) were found to be moderate biofilm formers. In contrast, 23.53% of broiler isolates were medium biofilm producers and 17.65% isolated were weak biofilm producers. The only goose E. coli strain showed moderate biofilm production. It should be noted that none of the tested strains showed a strong biofilm formation.
MLST
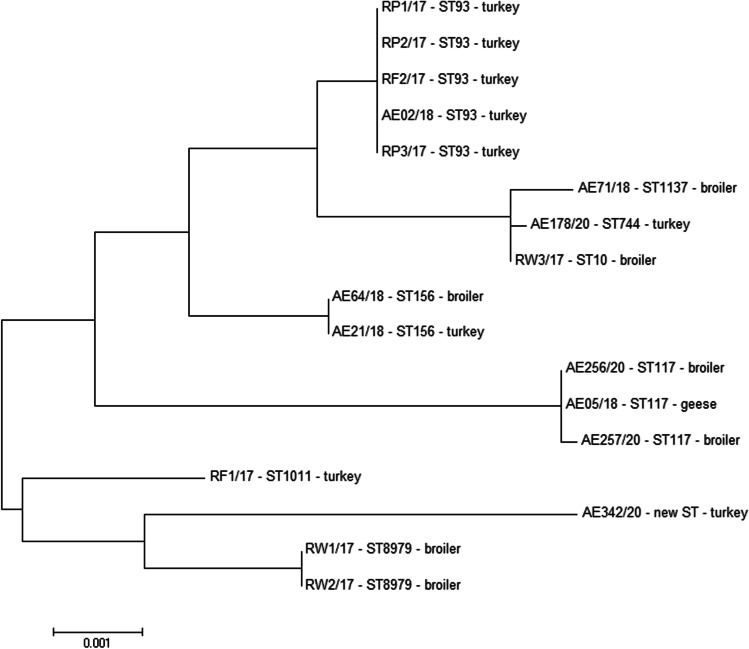
On the basis of the combination of allelic profiles of the tested housekeeping genes, all 17 E. coli strains were assigned to the ST (Fig. 2). All loci showed four or more alleles among the 17 tested strains. The adk allele showed the least genetic variability (4 different alleles), whereas gyrB was the most genetically diverse (8 different alleles) among all investigated housekeeping genes.
Fig. 2.
Sequence types (STs) and MLST-based phylogenetic tree of mcr-1-positive E. coli isolates. The phylogenetic tree was constructed using the neighbor-joining method in MEGA 6.0 software
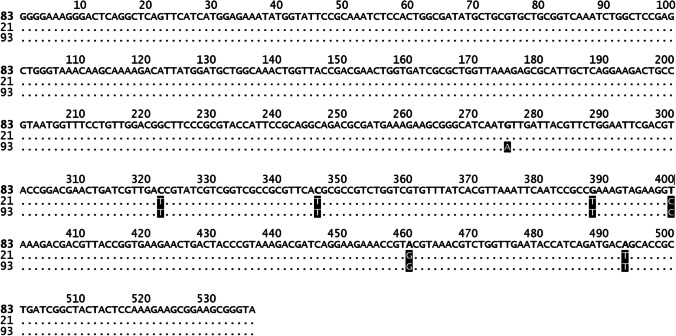
The MLST analysis showed the occurrence of nine STs, among which the most frequent were ST93 and ST117 (five and three isolates, respectively). The ST93 was observed in turkey isolates, while the ST117 in isolates obtained from broilers and from one goose. Moreover, the new ST was identified, which was related to the ST69 clonal complex. This complex includes the ST69 and ST408, which show the difference in adk allele (21 and 93, respectively). The new ST has an adk 83 allele, which was different in 6 nucleotides in comparison to adk 21 allele, and in 7 nucleotides in comparison to adk 93 allele. The details of the differences between adk alleles are presented in Fig. 3.
Fig. 3.
The nucleotide differences in adk allele between the new ST and the ST69 clonal complex (ST69 and ST408)
The phylogenetic relationship showed that the strains of the same sequence types, such as ST93, ST117, ST156, and ST8979, despite their various origins, were assigned to the same clusters, and had the closest relationship with each other. Moreover, the closely related strains exhibited very similar profiles of carried resistance genes and the level of biofilm formation. All ST93 strains were isolated from turkeys and 60% (3/5) of them showed the same resistance gene profile and weak ability to form biofilms. All of the ST117 strains included isolates that harbored the blaTEM, cat1, tetB, sul1, and sul2 genes, and they were moderate biofilm producers.
Discussion
Colistin resistance genes are widespread worldwide and have been found in Enterobacteriaceae from humans, food animals, and food, and among these resistance genes, the mcr-1 gene is the most frequently isolated one [48, 49]. In Poland, the first E. coli strain with the mcr-1 gene was isolated in 2015 from a 50-year-old patient with a urinary tract infection. The man had contact with farm animals, which may confirm the involvement of animals in the transmission of colistin-resistant strains [50]. Zhang et al. [51] revealed the prevalence of colistin resistance genes (mcr-1, mcr-2, and mcr-3) in various species of poultry, with the highest prevalence of the mcr-1 gene, which was obtained from 71.7% of geese, 34.6% of ducks, and 31.8% of broilers. Moreover, a serious concern was the presence of all three mcr genes in three separate E. coli isolates from broilers.
Our study showed a low occurrence (5.35%) of mcr-1-harboring E. coli strains isolated from poultry. The other mcr genes (mcr2–mcr5) were not detected in the all analyzed strains; this finding is similar to the results obtained by other authors [52–54]. According to the research conducted in China by Zhao et al. [55], the percentage of mcr-1-positive E. coli strains isolated from poultry was 15.3%, while it was only 0.34% in Japan [3]. Frequent use of antimicrobial agents in livestock production may lead to higher rate of resistant strain isolation, and commensal bacteria might serve as an indicator of antimicrobial usage for veterinary purposes [56]. A significantly higher percentage of colistin-resistant E. coli strains in Poland was confirmed by studies conducted in 2017–2018, where the presence of the mcr-1 gene occurring in the normal microbiota of chicken broilers, both treated and untreated with colistin sulfate, was tested. Isolates containing the mcr-1 gene were obtained in 11.27% of strains from untreated flocks and in 19.54% of isolates obtained from flocks treated with colistin [35]. In Europe, the prevalence of E. coli strains isolated from poultry and carrying the mcr-1 gene ranged from 1.5% in the Netherlands [28] to 13.95% in Portugal [54].
Irrgang et al. [57] showed that the prevalence of the mcr-1 gene depended on the type of poultry production. The highest prevalence of the mcr-1 gene was detected in turkeys (11.8%), followed by broilers (6.7%), and only three E. coli strains were mcr-1 positive in laying hens (3/1, 809 investigated isolates). In our present study, the proportions remained similar, i.e., the mcr-1 gene was most frequently present in turkeys (9/72; 12.50%), followed by broilers (7/161; 4.35%). Clemente et al. [54] revealed that as many as 27% of turkey strains and only 2% of the investigated broiler strains harbored the mcr-1 gene.
In the present study, the highest resistance profile was observed for ampicillin (100%), followed by sulfamethoxazole and tetracycline (88.24% for both), and trimethoprim and chloramphenicol (82.35% ex aequo); this finding is in accordance with other studies conducted in Brazil by Crecencio et al. [58] and in Portugal by Manageiro et al. [59]. Manageiro et al. revealed that a high percentage of E. coli strains isolated from broilers and turkeys were resistant to the following antimicrobial agents: ciprofloxacin (90.6% and 79.5%), nalidixic acid (88.6% and 73.5%), ampicillin (75.7% and 80%), sulfamethoxazole (69.3% and 71.9%), tetracycline (66.3% and 85.9%), trimethoprim (54.5% and 49.7%), and chloramphenicol (34.2% and 52.4%). Interestingly, multidrug resistance was observed in 81.3% of the isolates. Crecencio et al. [58] showed the highest resistance of retail chicken meat to beta-lactams (39.5%), followed by sulfonamide combined with trimethoprim (36.9%) and polymyxin (33.4%).
In our present study, regarding the obtained MIC values for the selected antimicrobial agents, all the investigated mcr-1-positive E. coli strains showed MDR profile (resistance to at least three antimicrobial classes). Similar results were obtained by other authors from Brazil, China, Argentina, and Poland [29–31, 35, 60]. Monte et al. [29] showed that most of the mcr-1-positive E. coli isolates exhibited an MDR phenotype and carried genes conferring resistance to aminoglycosides, quinolones, sulfonamides, and tetracyclines. In the present study as well as in the study of Haenni et al. [61], the association between mcr and other resistance elements such as beta-lactamases and the coexistence of the mcr-1 gene with sulfamethoxazole or tetracycline-resistance genes was observed. In our present study, the most frequently found resistance genes in the mcr-1-positive E. coli strains were as follows: blaTEM (resistance to beta-lactams), tetA and tetB (resistance to tetracyclines), and sul1, sul2, and sul3 genes (resistance to sulfonamides). These findings are in agreement with the results obtained by Zhao et al. [55] who showed that among mcr-1-positive E. coli strains, the blaTEM gene was the most prevalent (100%). β-lactamase encoding genes are usually localized on plasmids that facilitate their spread among Gram-negative bacilli via conjugation. Moreover, β-lactamase encoding plasmids often carry genes conferring resistance to other than β-lactam classes of antibiotics, limiting significantly the therapeutic options [62, 63]. It should be emphasized here that E. coli is an opportunistic pathogen that is capable of causing illness in animals and humans; therefore, the isolation of MDR bacteria from food animals is a worldwide public health problem because of potential transfer of resistant pathogens to humans [56] and the possibility of transmission of antimicrobial resistance genes among gut bacteria.
Resistance to fluoroquinolones is either a chromosomally mediated mechanism causing mutation in the quinolone resistance-determining region (QRDR) within the subunits constituting topoisomerases II (GyrA and GyrB) and IV (ParC and ParE) or is encoded by plasmid-mediated quinolone resistance genes (PMQR) [qnrA, qnrB, qnrC, qnrD, qnrS, qepA, oqxAB, and aac(6′)-Ib-cr], where the qepA and oqxAB genes encode an efflux pump that decreases intracellular drug levels [23, 64, 65]. Moreover, decreased accumulation of fluoroquinolones because of impermeability of the membrane and/or overexpression of the efflux pump systems has also been established [66]. In the present study, 76.47% and 64.71% of the mcr-1-positive E. coli strains were resistant to nalidixic acid and ciprofloxacin, respectively, while the presence of the aac(6′)-Ib-cr gene was detected in only one strain. This difference between phenotypic resistance to quinolone antibiotics and the presence of the resistance genes is probably due to other resistance mechanisms, listed above, which were not investigated in the present study.
Biofilm formation by E. coli strains is one of their mechanisms of virulence and is important in the development of antibiotic resistance. In the present study, most of the investigated strains were weak biofilm producers (58.82%), but a medium biofilm formation ability was observed in strains with multidrug resistance to 5, 6, or 7 classes of antimicrobial agents (41.18%). Also Pavlickova et al. [56] showed the correlation between the prevalence of antibiotic resistance and biofilm formation ability. Moreover, 71% of E. coli strains isolated from chicken exhibited weak and medium biofilm production ability, which is in agreement with the results of our present study. In comparison, Crecencio et al. [58] showed that as many as 70.44% of E. coli strains isolated from retail chicken meat had moderate to strong biofilm formation ability. These discrepancies may depend on strain properties, culture conditions, environmental factors, and methodology [67]. Regarding the poultry species, medium biofilm-producing E. coli strains were most frequently isolated from broilers (23.53%). This observation showed that on the one hand, multidrug resistance of these strains may enhance their virulence, especially in broiler isolates, and on the other hand, the general capacity of the mcr-1-positive E. coli strains to produce biofilms was at the medium and low level (no strong biofilm producers were observed in this study). Similar results were obtained by Barilli et al. [68], wherein E. coli strains isolated from retail meat products (including poultry) were weak biofilm producers. Although the tested in vitro strains did not show a strong biofilm production, it is worth noting that under appropriate in vivo conditions, with insufficient production hygiene, biofilm production may be more effective. Biofilm formation potential appears as an important virulence factor in ensuring the low penetration of antibiotics or disinfectants, and may lead to ineffective treatment.
In the present study, the MLST analysis revealed nine different E. coli STs. The most dominant sequence type was ST93 (29.41%), followed by ST117 (17.65%). Our study showed that eight obtained STs (ST93, ST1137, ST744, ST10, ST156, ST117, ST1011, and ST8979) have been isolated and previously identified among poultry in the USA, Europe, Asia, and Australia (data taken from EnteroBase). These STs were also noted in Poland, except for ST117 and ST1011, which were isolated for the first time from poultry source in our study. The newly identified ST was the most related to the ST69 clonal complex, which includes the ST69, widely distributed in the environment, and the ST408 which has been isolated from bovine in the USA.
It is worth noting that in the present study, two strains isolated from broilers were described as ST8979; according to the EnteroBase, this ST has been isolated only twice from the environment in the USA. The ST1137 was deposited in the EnteroBase in 12 cases, including three strains each isolated from poultry in the USA, France, and Kenya. Additionally, three of STs (ST10, ST93, and ST744), obtained in the present study, were detected in E. coli from samples of raw poultry meat and liver, which came from Poland and were retailed in the Czech Republic. This investigation suggests that it poses a significant threat to public health [69].
Zhao et al. [38, 55] showed that among the mcr-1-positive E. coli strains isolated from poultry farms in China, the dominant ST was ST93 (18.62%); this finding is in agreement with the results obtained in the present study. The ST93, ST117, and ST156 were also described in E. coli strains obtained from chicken broilers in Egypt [70]. In Switzerland, Zurfluh et al. [71] characterized the ST156 in an mcr-1-positive E. coli strain isolated from chicken meat, while Hassen et al. [72] revealed the presence of ST117 in a beta-lactamase-producing mcr-1-encoding E. coli strain isolated from chicken meat samples in Tunisia. The other STs, namely ST10 and ST117 were found in E. coli isolated from broilers at slaughter [73] and broiler breeders [74], respectively. In Poland, Zając et al. [34] described 49 STs among the mcr-1-positive E. coli strains isolated from chicken and turkeys, among which the most common types were ST354 and ST359, which were not observed in our present study. The common STs for both classes of strains, published by Zając et al. [34] and in the present study, were ST10, ST93, ST117, and ST1011. In addition, in the present study, 60.0% (3/5) of ST93 isolates carried the blaTEM, tetA, sul1, sul2, and sul3 genes, and they showed 100% similarity on the MLST phylogenetic tree; this finding may indicate clonal types of these strains.
Conclusion
In summary, the conducted research confirmed that poultry can be considered as an important reservoir of MDR E. coli isolates. A wide range of phenotypic resistance to both antibiotics used in veterinary and human medicine was identified and resistance to colistin, tetracycline, quinolones, and β-lactams was observed among the analyzed strains. Additionally, strains with multidrug resistance to 5, 6, or 7 classes of antimicrobial agents were medium biofilm producers. The co-resistance of plasmid-mediated colistin resistance encoded by mcr-1 gene and tet (A and B) or blaTEM genes was 88.24%, equally. Furthermore, our findings suggest the diversity in resistance determinants, which could be responsible for the high resistance profiles found in this study. This may pose a threat to public health due to the existing risk of spread of resistance genes among bacterial strains, including their potential ability to transfer antimicrobial resistance to humans.
Due to the fact that Poland is a significant poultry producer in Europe, research on this aspect should be widely conducted in Poland and constantly improved. It is essential to monitor colistin-resistant E. coli strains for understanding the prevalence of colistin resistance genes in both human and veterinary medicine, including poultry production.
Author contribution
Conception and design the study: K.Ć., A.WB., A.W.; Microbiology and molecular testing: K.Ć., M.Ka., M.S.; Data analysis: K.Ć., A.WB., A.W.; Draft paper: K.Ć., A.WB., M.Ka., M.S., A.W.; Review manuscript: K.Ć., A.WB., M.Ka., M.S., A.R.R., R.S.H., A.W.; Approve final manuscript: K.Ć., A.WB., M.Ka., M.S., C.L., A.R.R., R.S.H., M.Ku., M.CW., A.W.; Submission: A.WB.
Funding
The research is financed under the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
Declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Antimicrobial resistance: global report on surveillance. Geneva: WHO; 2014. [Google Scholar]
- 2.Apostolakos I, Piccirillo A. A review on the current situation and challenges of colistin resistance in poultry production. Avian Pathol. 2018;47:546–558. doi: 10.1080/03079457.2018.1524573. [DOI] [PubMed] [Google Scholar]
- 3.Kawanishi M, Abo H, Ozawa M, Uchiyama M, Shirakawa T, Suzuki S, Shima A, Yamashita A, Sekizuka T, Kato K, Kuroda M, Koike R, Kijima M. Prevalence of colistin resistance gene mcr-1 and absence of mcr-2 in Escherichia coli isolated from healthy food-producing animals in Japan. Antimicrob Agents Chemother. 2016;61:e02057–e2116. doi: 10.1128/AAC.02057-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umpiérrez A, Bado I, Oliver M, Acquistapace S, Etcheverría A, Padola NL, Vignoli R, Zunino P. Zoonotic potential and antibiotic resistance of Escherichia coli in neonatal calves in Uruguay. Microbes Environ. 2017;32:275–282. doi: 10.1264/jsme2.ME17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang H, Chen S, White DG, Zhao S, McDermott P, Walker R, Meng J. Characterization of multiple-antimicrobial-resistant Escherichia coli isolates from diseased chickens and swine in China. J Clin Microbiol. 2004;42:3483–3489. doi: 10.1128/JCM.42.8.3483-3489.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forde BM, Zowawi HM, Harris PNA, Roberts L, Ibrahim E, Shaikh N, Deshmukh A, Sid MA, Al Ahmed M , Cottrell K, Trembizki E, Sundac L, Yu HH, Li J, Schembri MA, Whiley DM, Paterson DL, Beatson S A. Discovery of mcr-1-Mediated Colistin Resistance in a Highly Virulent Escherichia coli Lineage. mSphere. 2018;3:e00486–18. doi: 10.1128/mSphere.00486-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Woo JH, Kim N, Kim MH, Kim SY, Son JH, Moon DC, Lim SK, Shin M, Lee JC. Characterization Of chromosome-mediated colistin resistance in Escherichia coli isolates from livestock in Korea. Infect Drug Resist. 2019;12:3291–3299. doi: 10.2147/IDR.S225383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–8. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 9.Hernández M, Iglesias MR, Rodríguez-Lázaro D, Gallardo A, Quijada N, Miguela-Villoldo P, Campos MJ, Píriz S, López-Orozco G, de Frutos C, Sáez JL, Ugarte-Ruiz M, Domínguez L, Quesada A. Co-occurrence of colistin-resistance genes mcr-1 and mcr-3 among multidrug-resistant Escherichia coli isolated from cattle, Spain, September 2015. Euro Surveill. 2017;22:30586. doi: 10.2807/1560-7917.ES.2017.22.31.30586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother. 2017;72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 11.Borowiak M, Baumann B, Fischer J, Thomas K, Deneke C, Hammerl JA, Szabo I, Malorny B. Development of a novel mcr-6 to mcr-9 multiplex PCR and assessment of mcr-1 to mcr-9 occurrence in colistin-resistant Salmonella enterica isolates from environment, feed, animals and food (2011–2018) in Germany. Front Microbiol. 2020;11:80. doi: 10.3389/fmicb.2020.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, Pezzotti G, Magistrali CF. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill. 2017;22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Garch F, Sauget M, Hocquet D, LeChaudee D, Woehrle F, Bertrand X. mcr-1 is borne by highly diverse Escherichia coli isolates since 2004 in food-producing animals in Europe. Clin Microbiol Infect. 2017;23:51.e1–51.e4. doi: 10.1016/j.cmi.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, Zhang S, Shen J, Shen Z, Wang Y. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect. 2018;7:122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Dai X, Zeng J, Gao Y, Zhang Z, Zhang L. Characterization of the global distribution and diversified plasmid reservoirs of the colistin resistance gene mcr-9. Sci Rep. 2020;10:8113. doi: 10.1038/s41598-020-65106-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kieffer N, Nordmann P, Poirel L. Moraxella species as potential sources of MCR-like polymyxin resistance determinants. Antimicrob Agents Chemother. 2017;61:e00129–e217. doi: 10.1128/AAC.00129-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ling Z, Yin W, Li H, Zhang Q, Wang X, Wang Z, Ke Y, Wang Y, Shen J. Chromosome-mediated mcr-3 variants in Aeromonas veronii from chicken meat. Antimicrob Agents Chemother. 2017;61:e01272–e1317. doi: 10.1128/AAC.01272-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio. 2019;10:e00853–19. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect. 2020;9:508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Xie M, Lv J, Wai-Chi Chan E, Chen S. Complete genetic analysis of plasmids carrying mcr-1 and other resistance genes in an Escherichia coli isolate of animal origin. J Antimicrob Chemother. 2017;72:696–699. doi: 10.1093/jac/dkw509. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Liu P, Feng F, Zhang J, Li F, Wang M, Sun Y. Evaluation of potential ARG packaging by two environmental T7-like phage during phage-host interaction. Viruses. 2020;12:1060. doi: 10.3390/v12101060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye H, Li Y, Li Z, Gao R, Zhang H, Wen R, Gao GF, Hu Q, Feng Y. Diversified mcr-1-harbouring plasmid reservoirs confer resistance to colistin in human gut microbiota. mBio. 2016;7:e00177. doi: 10.1128/mBio.00177-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordmann P, Poirel L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother. 2005;56:463–469. doi: 10.1093/jac/dki245. [DOI] [PubMed] [Google Scholar]
- 24.Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villegas NA, Baronetti J, Albesa I, Polifroni R, Parma A, Etcheverría A, Becerra M, Padola N, Paraje M. Relevance of biofilms in the pathogenesis of Shiga-toxin-producing Escherichia coli infection. ScientificWorldJournal. 2013;2013:607258. doi: 10.1155/2013/607258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cepas V, López Y, Muñoz E, Rolo D, Ardanuy C, Martí S, Xercavins M, Horcajada JP, Bosch J, Soto SM. Relationship between biofilm formation and antimicrobial resistance in Gram-negative bacteria. Microb Drug Resist. 2019;25:72–79. doi: 10.1089/mdr.2018.0027. [DOI] [PubMed] [Google Scholar]
- 27.Lora-Tamayo J, Murillo O, Ariza J. Clinical use of colistin in biofilm-associated infections. Adv Exp Med Biol. 2019;1145:181–195. doi: 10.1007/978-3-030-16373-0_13. [DOI] [PubMed] [Google Scholar]
- 28.Kluytmans-van den Bergh MF, Huizinga P, Bonten MJ, Bos M, De Bruyne K, Friedrich AW, Rossen JW, Savelkoul PH, Kluytmans JA. Presence of mcr-1-positive Enterobacteriaceae in retail chicken meat but not in humans in the Netherlands since 2009. Euro Surveill. 2016;21:30149. doi: 10.2807/1560-7917.ES.2016.21.9.30149. [DOI] [PubMed] [Google Scholar]
- 29.Monte DF, Mem A, Fernandes MR, Cerdeira L, Esposito F, Galvão JA, Franco BDGM, Lincopan N, Landgraf M. Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 genes in South America. Antimicrob Agents Chemother. 2017;61:e02718–e2816. doi: 10.1128/AAC.02718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez JE, Redondo LM, Figueroa Espinosa RA, Cejas D, Gutkind GO, Chacana PA, Di Conza JA, Fernández Miyakawa ME. Simultaneous carriage of mcr-1 and other antimicrobial resistance determinants in Escherichia coli from poultry. Front Microbiol. 2018;9:1679. doi: 10.3389/fmicb.2018.01679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Yu L, Zhang Y, Dai Y, Wang P, Feng C, Liu M, Sun S, Xie Z, Wang F. Prevalence and characteristics of multidrug-resistant mcr-1-positive Escherichia coli isolates from broiler chickens in Tai’an, China. Poult Sci. 2020;99:1117–1123. doi: 10.1016/j.psj.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasyl D, Hoszowski A, Zając M, Szulowski K. Antimicrobial resistance in commensal Escherichia coli isolated from animals at slaughter. Front Microbiol. 2013;4:221. doi: 10.3389/fmicb.2013.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasyl D, Zając M, Lalak A, Skarżyńska M, Samcik I, Kwit R, Jabłoński A, Bocian Ł, Woźniakowski G, Hoszowski A, Szulowski K. Antimicrobial resistance in Escherichia coli isolated from wild animals in Poland. Microb Drug Resist. 2018;24:807–815. doi: 10.1089/mdr.2017.0148. [DOI] [PubMed] [Google Scholar]
- 34.Zając M, Sztromwasser P, Bortolaia V, Leekitcharoenphon P, Cavaco LM, Ziȩtek-Barszcz A, Hendriksen RS, Wasyl D. Occurrence and characterization of mcr-1-positive Escherichia coli isolated from food-producing animals in Poland, 2011–2016. Front Microbiol. 2019;10:1753. doi: 10.3389/fmicb.2019.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majewski M, Łukomska A, Wilczyński J, Wystalska D, Racewicz P, Nowacka-Woszuk J, Pszczola M, Anusz K. Colistin resistance of non-pathogenic strains of Escherichia coli occurring as natural intestinal flora in broiler chickens treated and not treated with colistin sulphate. J Vet Res. 2020;64:399–405. doi: 10.2478/jvetres-2020-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desjardins P, Conklin D. NanoDrop microvolume quantitation of nucleic acids. J Vis Exp. 2010;22:2565. doi: 10.3791/2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, Guerra B, Malorny B, Borowiak M, Hammerl JA, Battisti A, Franco A, Alba P, Perrin-Guyomard A, Granier SA, De Frutos EC, Malhotra-Kumar S, Villa L, Carattoli A, Hendriksen RS. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23:17–00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EUCAST (2020) The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0
- 39.CLSI (2019) Subcommittee on Antimicrobial Susceptibility Testing. M100 Performance Standards for Antimicrobial Susceptibility Testing. 29th Edition
- 40.Türkyılmaz S, Hazimoglu S, Bozdogan B. Antimicrobial susceptibility and resistance genes in Salmonella enterica serovar enteritidis isolated from Turkeys. Israel J Vet Med. 2009;64:72–77. [Google Scholar]
- 41.Ćwiek K, Korzekwa K, Tabiś A, Bania J, Bugla-Płoskońska G, Wieliczko A. Antimicrobial resistance and biofilm formation capacity of Salmonella enterica Serovar Enteritidis strains isolated from poultry and humans in Poland. Pathogens. 2020;9:643. doi: 10.3390/pathogens9080643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 43.Sun Y, Hu X, Guo D, Shi C, Zhang C, Peng X, Yang H, Xia X. Disinfectant resistance profiles and biofilm formation capacity of Escherichia coli isolated from retail chicken. Microb Drug Resist. 2019;25:703–711. doi: 10.1089/mdr.2018.0175. [DOI] [PubMed] [Google Scholar]
- 44.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol. 2006;60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 46.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 47.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo Q, Wang Y, Xiao Y. Prevalence and transmission of mobilized colistin resistance (mcr) gene in bacteria common to animals and humans. Biosafety and Health. 2020;2:71–78. doi: 10.1016/j.bsheal.2020.05.001. [DOI] [Google Scholar]
- 49.Savin M, Bierbaum G, Blau K, Parcina M, Sib E, Smalla K, Schmithausen R, Heinemann C, Hammerl JA, Kreyenschmidt J. Colistin-resistant Enterobacteriaceae isolated from process waters and wastewater from German poultry and pig slaughterhouses. Front Microbiol. 2020;11:575391. doi: 10.3389/fmicb.2020.575391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izdebski R, Baraniak A, Bojarska K, Urbanowicz P, Fiett J, Pomorska-Wesołowska M, Hryniewicz W, Gniadkowski M, Żabicka D. Mobile MCR-1-associated resistance to colistin in Poland. J Antimicrob Chemother. 2016;71:2331–2333. doi: 10.1093/jac/dkw261. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Chen L, Wang J, Yassin AK, Butaye P, Kelly P, Gong J, Guo W, Li J, Li M, Yang F, Feng Z, Jiang P, Song C, Wang Y, You J, Yang Y, Price S, Qi K, Kang Y, Wang C. Molecular detection of colistin resistance genes (mcr-1, mcr-2 and mcr-3) in nasal/oropharyngeal and anal/cloacal swabs from pigs and poultry. Sci Rep. 2018;8:3705. doi: 10.1038/s41598-018-22084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lima Barbieri N, Nielsen DW, Wannemuehler Y, Cavender T, Hussein A, Yan SG, Nolan LK, Logue CM. mcr-1 identified in Avian Pathogenic Escherichia coli (APEC) PLoS One. 2017;12(3):e0172997. doi: 10.1371/journal.pone.0172997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang YQ, Li YX, Song T, Yang YX, Jiang W, Zhang AY, Guo XY, Liu BH, Wang YX, Lei CW, Xiang R, Wang HN. Colistin resistance gene mcr-1 and its variant in Escherichia coli isolates from chickens in China. Antimicrob Agents Chemother. 2017;61(5):e01204–e1216. doi: 10.1128/AAC.01204-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clemente L, Manageiro V, Correia I, Amaro A, Albuquerque T, Themudo P, Ferreira E, Caniça M. Revealing mcr-1-positive ESBL-producing Escherichia coli strains among Enterobacteriaceae from food-producing animals (bovine, swine and poultry) and meat (bovine and swine), Portugal, 2010–2015. Int J Food Microbiol. 2019;296:37–42. doi: 10.1016/j.ijfoodmicro.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Zhao X, Liu Z, Zhang Y, Yuan X, Hu M, Liu Y. Prevalence and molecular characteristics of avian-origin mcr-1-harboring Escherichia coli in Shandong Province. China Front Microbiol. 2020;11:255. doi: 10.3389/fmicb.2020.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavlickova S, Klancnik A, Dolezalova M, Mozina SS, Holko I. Antibiotic resistance, virulence factors and biofilm formation ability in Escherichia coli strains isolated from chicken meat and wildlife in the Czech Republic. J Environ Sci Health B. 2017;52:570–576. doi: 10.1080/03601234.2017.1318637. [DOI] [PubMed] [Google Scholar]
- 57.Irrgang A, Roschanski N, Tenhagen BA, Grobbel M, Skladnikiewicz-Ziemer T, Thomas K, Roesler U, Käsbohrer A. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010–2015. PloS One. 2016;11:e0159863. doi: 10.1371/journal.pone.0159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crecencio RB, Brisola MC, Bitner D, Frigo A, Rampazzo L, Borges KA, Furian TQ, Salle CTP, Moraes HLS, Faria GA, Da Silva AS, Stefani LM. Antimicrobial susceptibility, biofilm formation and genetic profiles of Escherichia coli isolated from retail chicken meat. Infect Genet Evol. 2020;84:104355. doi: 10.1016/j.meegid.2020.104355. [DOI] [PubMed] [Google Scholar]
- 59.Manageiro V, Clemente L, Graça R, Correia I, Albuquerque T, Ferreira E, Caniça M. New insights into resistance to colistin and third-generation cephalosporins of Escherichia coli in poultry, Portugal: Novel blaCTX-M-166 and blaESAC genes. Int J Food Microbiol. 2017;263:67–73. doi: 10.1016/j.ijfoodmicro.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Zhuge X, Ji Y, Tang F, Sun Y, Jiang M, Hu W, Wu Y, Xue F, Ren J, Zhu W, Dai J. Population structure and antimicrobial resistance traits of avian-origin mcr-1-positive Escherichia coli in Eastern China, 2015 to 2017. Transbound Emerg Dis. 2019;66:1920–1929. doi: 10.1111/tbed.13222. [DOI] [PubMed] [Google Scholar]
- 61.Haenni M, Poirel L, Kieffer N, Châtre P, Saras E, Métayer V, Dumoulin R, Nordmann P, Madec JY. Co-occurrence of extended spectrum β lactamase and MCR-1 encoding genes on plasmids. Lancet Infect Dis. 2016;16:281–282. doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 62.Wu C, Wang Y, Shi X, Wang S, Ren H, Shen Z, Wang Y, Lin J, Wang S. Rapid rise of the ESBL and mcr-1 genes in Escherichia coli of chicken origin in China, 2008–2014. Emerg Microbes Infect. 2018;7:30. doi: 10.1038/s41426-018-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shafiq M, Huang J, Shah JM, Ali I, Rahman SU, Wang L. Characterization and resistant determinants linked to mobile elements of ESBL-producing and mcr-1-positive Escherichia coli recovered from the chicken origin. Microb Pathog. 2021;150:104722. doi: 10.1016/j.micpath.2020.104722. [DOI] [PubMed] [Google Scholar]
- 64.Hansen LH, Jensen LB, Sørensen HI, Sørensen SJ. Substrate specificity of the OqxAB multidrug resistance pump in Escherichia coli and selected enteric bacteria. J Antimicrob Chemother. 2007;60:145–147. doi: 10.1093/jac/dkm167. [DOI] [PubMed] [Google Scholar]
- 65.Poirel L, Madec J-Y, Lupo A, Schink A-K, Kieffer N, Nordmann P, Schwarz S (2018) Antimicrobial resistance in Escherichia coli. Microbiol Spectrum 6(4):ARBA-0026-2017. 10.1128/microbiolspec.ARBA-0026-2017 [DOI] [PMC free article] [PubMed]
- 66.Ruiz J. Mechanisms of resistance to quinolones: target alterations, decreased accumulation and DNA gyrase protection. J Antimicrob Chemother. 2003;51:1109–1117. doi: 10.1093/jac/dkg222. [DOI] [PubMed] [Google Scholar]
- 67.Naves P, del Prado G, Huelves L, Gracia M, Ruiz V, Blanco J, Rodríguez-Cerrato V, Ponte MC, Soriano F. Measurement of biofilm formation by clinical isolates of Escherichia coli is method-dependent. J Appl Microbiol. 2008;105:585–590. doi: 10.1111/j.1365-2672.2008.03791.x. [DOI] [PubMed] [Google Scholar]
- 68.Barilli E, Vismarra A, Frascolla V, Rega M, Bacci C. Escherichia coli strains isolated from retail meat products: evaluation of biofilm formation ability, antibiotic resistance, and phylogenetic group analysis. J Food Prot. 2020;14:233–240. doi: 10.4315/0362-028X.JFP-19-330. [DOI] [PubMed] [Google Scholar]
- 69.Gelbíčová T, Baráková A, Florianová M, Jamborová I, Zelendová M, Pospíšilová L, Koláčková I, Karpíšková R. Dissemination and comparison of genetic determinants of mcr-mediated colistin resistance in Enterobacteriaceae via retailed raw meat products. Front Microbiol. 2019;10:2824. doi: 10.3389/fmicb.2019.02824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hussein AH, Ghanem IA, Eid AA, Ali MA, Sherwood JS, Li G, Nolan LK, Logue CM. Molecular and phenotypic characterization of Escherichia coli isolated from broiler chicken flocks in Egypt. Avian Dis. 2013;57:602–611. doi: 10.1637/10503-012513-Reg.1. [DOI] [PubMed] [Google Scholar]
- 71.Zurfluh K, Nüesch-Inderbinen M, Klumpp J, Poirel L, Nordmann P, Stephan R. Key features of mcr-1-bearing plasmids from Escherichia coli isolated from humans and food. Antimicrob Resist Infect Control. 2017;6:91. doi: 10.1186/s13756-017-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hassen B, Abbassi MS, Ruiz-Ripa L, Mama OM, Hassen A, Torres C, Hammami S. High prevalence of mcr-1 encoding colistin resistance and first identification of blaCTX-M-55 in ESBL/CMY-2-producing Escherichia coli isolated from chicken faeces and retail meat in Tunisia. Int J Food Microbiol. 2020;318:108478. doi: 10.1016/j.ijfoodmicro.2019.108478. [DOI] [PubMed] [Google Scholar]
- 73.Castellanos LR, Donado-Godoy P, León M, Clavijo V, Arevalo A, Bernal JF, Timmerman AJ, Mevius DJ, Wagenaar JA, Hordijk J. High heterogeneity of Escherichia coli sequence types harbouring ESBL/AmpC genes on IncI1 plasmids in the Colombian poultry chain. PLoS One. 2017;12:e0170777. doi: 10.1371/journal.pone.0170777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landman WJ, Buter GJ, Dijkman R, van Eck JH. Molecular typing of avian pathogenic Escherichia coli colonies originating from outbreaks of E. coli peritonitis syndrome in chicken flocks. Avian Pathol. 2014;43:345–356. doi: 10.1080/03079457.2014.935291. [DOI] [PubMed] [Google Scholar]