Abstract
The genus Chromobacterium is widely distributed in the environment and is composed of Gram-negative, aerobic, or facultative anaerobic bacilli that occur in violet-colored colonies. These bacteria rarely cause infections, but when it occurs, it spreads quickly and has a high mortality. Because diseases are infrequent, the diagnosis is often delayed, and it takes time for suitable treatment to be initiated, leading to increased mortality due to the rapid progression of the disease. After the death of a cougar, serologically positive for feline leukemia virus, at the Center for Medicine and Research on Wild Animals of the Federal University of Mato Grosso, an autopsy was carried out, and fragments of its organs were sent for bacterial culture. Significant lesions were found, mainly in the liver and lungs, and upon bacterial isolation, violet-colored colonies were obtained from all of the referred organs, suggestive of C. violaceum, which was later confirmed by 16S DNA sequencing. The objective of this study was to report a case of death associated primarily with disseminated infection caused by C. violaceum in a FeLV-positive wild cougar in July 2018; no other occurrence in this species has yet been described.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-021-00460-z.
Keywords: Violet bacteria, Feline leukemia, Sepsis, Immunosuppression
Introduction
The genus Chromobacterium belongs to the family Neisseriaceae [1] and is characterized as a Gram-negative, mobile, facultative anaerobic, and positive oxidase coccobacillus [2–6]. C. violaceum [1] and C. haemolyticum [7, 8] have been associated with infections in humans [2]. According to Guevara et al. [3], despite being a rare pathogen in humans, it can cause severe and fatal infections.
Considered an environmental microorganism, it is found in water and soil and is widely distributed in several geographical regions [4]. Its pathogenicity has not yet been elucidated. Dzupova and Benes [9] describe it as being similar to Yersinia pestis or Bacillus anthracis. It is believed that when the C. violaceum reach the bloodstream, they spread quickly, developing sepsis and multiple metastatic abscesses [9].
Because C. violaceum is uncommon, diagnosis is often delayed, and adequate treatment takes time to be instituted, leading to high mortality due to the disease’s rapid progression [10]. Microbiological isolation can be performed on blood agar and MacConkey agar, with most strains producing violacein, a pigment that gives a purple color [5]. Kothari et al. [4] suggested that the treatment of C. violaceum infections can be challenging due to their resistance to different antibiotics [4]. Considering the emerging status of C. violaceum [4], the description of new cases and hosts allows a better understanding of the epidemiology of this disease. Therefore, this report describes a sepsis associated with C. violaceum in a captive cougar (Puma concolor) carrying the feline leukemia virus (FeLV).
Materials and methods
In 2018, a captive female puma (Puma concolor) at CEMPAS (Center for Medicine and Research on Wild Animals of the Federal University of Mato Grosso), senile and FeLV carrier with a history of discrete apathy and hyporexia, died. A post mortem examination was performed, registering morphological changes. During this analysis, samples were collected for histological examination and molecular analysis. For histopathological analysis, fragments from all organs were collected, fixed in 10% formalin, and subsequently subjected to cleavage and other routine procedures for inclusion in paraffin in the Veterinary Pathology Laboratory of the Veterinary Hospital of the Federal University of Mato Grosso (UFMT). Sections (2 μm) were obtained and hematoxylin and eosin (H&E) stained for histological analysis by light microscopy.
The heart, liver, lung, and abdominal and thoracic tissues were seeded aerobically in blood agar, MacConkey agar, and Sabouraud agar and incubated at 37 °C for 72 h. Subsequently, the colonies were subjected to Gram staining and biochemical tests for identification [11].
To identify the species, genomic DNA was extracted from the isolates. Colonies were inoculated into brain and heart infusion broth and incubated under agitation at 37 °C overnight. After centrifugation, 1 mL of lysis buffer (100 mM NaCl, 25 mM EDTA, 100 mM Tris-HCl pH 8.0, 0.5% SDS, 0.1 mg Proteinase K) was added to the precipitate, following the protocol with phenol-chloroform. The DNA was eluted in 50 μL of ultrapure water, and the integrity was verified through agarose gel analysis [12].
The extracted DNA was subjected to PCR of the 16S rRNA gene, which amplifies a 1512 bp, using the oligonucleotides 27F (AGAGTTTGATGCCCTCAG) [13] and 1492R (GGTTACCTT GTTACGACTT) [14]. The PCR reaction was performed with ten ng of genomic DNA, 0.4 pmol of each oligonucleotide, 0.2 mM of dNTPs, 3.0 mM of MgCl2, 1×10× PCR buffer (200 mM Tris-HCl, pH 8.4, and 500 mM KCl), 1 U of Taq DNA polymerase, and ultrapure water for a final volume of 25 μL.
The reactions were amplified with an initial denaturation program of 5 min at 95 °C, followed by 35 denaturation cycles of 45 s at 95 °C, hybridization for 60 s at 52 °C, 90 s at 72 °C extension, and a final extension cycle at 72 °C for 7 min. The amplicons were subjected to electrophoresis on 1.0% agarose gel (10 V/cm), stained with Gel Red, and visualized on a photo-documenter.
The PCR products were purified and subsequently sequenced with 27F primer in an automatic Sanger sequencer. The obtained sequences were compared with the GenBank database using BLAST on the NCBI server (www.ncbi.nlm.nih.govBLAST). Phylogenetic analysis based on partial 16S RNA alignment on muscle, maximum likelihood method by PhyML, and the phylogenetic tree was performed with TreeDyn (v198.3) to analyze relationship to other Chromobacterium species.
Results
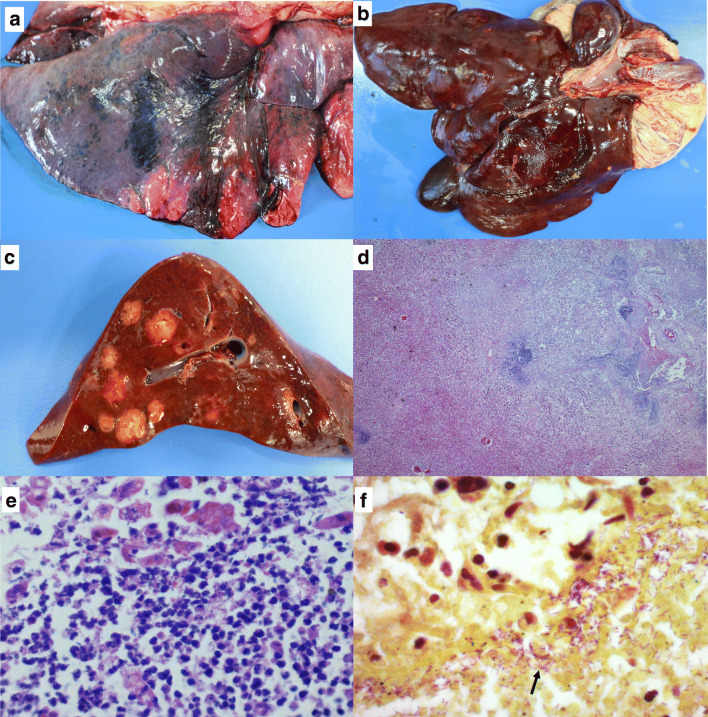
On autopsy, several petechiae, suffusions, and hematomas were diffusely observed, mainly on the parietal and visceral pleural surfaces as well as on the lungs. The right caudal, the accessory, and the right cranial lobes had a diffusely purple firm surface, and the parenchyma was hypocreptant upon palpation (Fig. 1). The liver had a dark red capsular surface with an evident lobular pattern, and there were multiple random areas, between 0.5 and 3 cm in size, and soft, irregular, yellow, and well-defined, which were observed on the cutting surface, replacing approximately 30% of the hepatic parenchyma (Fig. 1). A small deposit of yellowish serous fluid was noted in the abdominal cavity and the pericardial sac, and the stomach mucosa was intensely blackish red and had light red streaks.
Fig. 1.
Sepsis in cougar (Puma concolor) associated with Chromobacterium violaceum: a Lung with an intensely dark red pleural surface associated with acute hemorrhage and congestion; b capsular surface of the liver with irregular nodules, multifocal to coalescent, yellowish, 0.5 to 2 cm; c cut surface of the liver with homogeneously yellowish, friable, well-demarcated nodules and with irregularly apparent capsule; d photomicrograph of the liver section with intense disorganization of the lobular structure with liquefactive hepatocellular necrosis and multifocal, random and extensive disappearance of parenchyma replaced by an intense suppurative infiltrate. H&E stain, ×2.5; e amplification of the anterior image showing an intense infiltration of neutrophils. H&E stain, ×63; and f section of the liver with marked pinkish purple bacillary structures (arrows). Brown-Hopps (Gram) stain, ×100
Microscopically, the main changes were pointed out in the liver and lung. In sections of the liver, especially in areas that corresponded to the yellowish nodules observed in the post mortem examination, there was intense disorganization of the lobular structure with liquefactive hepatocellular necrosis and multifocal, random, and extensive disappearance of parenchyma, which was replaced by a marked predominant infiltrate of neutrophils and a smaller population of macrophages with foamy cytoplasm. Coccobacillary bacteria were predominantly distributed on the margins of the necrotic areas (Fig. 1). Surrounding these areas, there was a slight proliferation of fibroblasts that sometimes extended between hepatocyte strands. Additionally, in border areas, there was congestion and moderate and acute hemorrhage. There was marked, diffuse, and acute congestion in the lung, and the lumen of the alveoli, bronchioles, and bronchi were filled irregularly with amorphous hyaline content (edema) occasionally with acute hemorrhage. Less frequently, there was chronic alveolar emphysema, and the bronchus-associated lymphoid tissue (BALT) was rarefied. In the kidney, there was mild proliferative glomerulonephritis and mild multifocal interstitial nephritis. There was moderate and random multifocal necrosis of the mucosa in the stomach with marked lymphoplasmacytic, histiocytic, and neutrophilic infiltrate. In the brain, the white matter had mild and diffuse vacuolization with increased perineuronal and perivascular space (status spongiosus).
In all tissue, similar purple colonies, catalase-positive, were isolated and characterized as Gram-negative coccobacilli. In the triple sugar iron agar test, there was no fermentation of sugars. The absence of black color in a 24-h period indicated that the bacteria did not produce hydrogen sulfide (H2S). In the sulfite, indole, and motility (SIM) test, the bacterium was classified as mobile and indole-negative (without degradation of tryptophan). The oxidation-fermentation test was classified as fermentative, and citrate was not used as a carbon source. In the gelatinase test, the bacillus was able to hydrolyze gelatin. There was no use of sugars, mannitol, urea, lactose, sucrose, glucose, or fructose, compatible with C. violaceum.
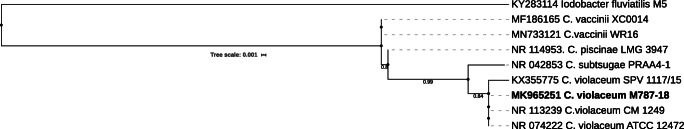
In the sequencing of the 16S rRNA PCR product, the sequence partial obtained was compared with the GenBank database using the BLAST program on the NCBI server (www. ncbi. nlm nih. gov/BLAST). The sequence extracted from the liver sample showed homology between nucleotides of 100% identity with C. violaceum. This sequence was deposited in GenBank under the number MK965251. Phylogenetic analysis showed that the Chromobacterium isolated from the cougar were clustered with others C. violaceum (Fig. 2) isolate from environment and animal.
Fig. 2.
Phylogenetic tree inferred of partial 16S rRNA (658 bp) of the Chromobacterium violaceum isolated from cougar (bold). The tree was constructed using maximum likelihood method (ML) and with Iodobacter fluviatilis as an outgroup. Bootstrap values obtained from 1000 resampling of the data set are given at the nodes
Discussion
Despite being a rare occurrence in Brazil, C. violaceum infection is associated with tropical and subtropical areas [15]. It is characterized as an opportunistic microorganism related to severe infections in mammalian hosts and is generally associated with immunosuppression [1]. In this case, the cougar could have been in a state of continuous stress, which would have increased corticosteroids’ levels and directly influences the host’s immune response [16]. Due to the conditions of captivity, it is suspected that the animal has suffered thermal stress damage caused by the sharp drop, falling 18°C in temperature in the city. Besides, the feline carried the FeLV, an immunosuppressive retrovirus that can exacerbate secondary agents’ clinical signs [17].
In animals, descriptions of this bacterium have been reported in cases of infection in humans [27], pigs [18], cattle [19], canines [20], non-human primates [22], and equines [24], and more recently, a case was reported in a calf in Brazil [26]. Besides, this report corroborates the literature, in which severe conditions lead to septicemia. Animal reports include suppurative pneumonia, abscesses, and necrosis in the lungs and liver [20, 21, 23, 24]. Macroscopic and microscopic lesions observed in the liver and lungs have been reported to be similar to those observed during septicemia in other animals [19, 21, 23].
In humans, the infection can cause abscesses in the skin and visceral organs such as the liver, lungs, kidneys, spleen, lymph nodes, and brain. The rapid spread can lead to fulminant septicemia, with lesions in multiple organs, causing death [24, 25]. According to Dzupova and Benes [9], C. violaceum has an affinity for specific organs due to its ability to survive in macrophages that carry phagocytized bacteria in lymphoid tissues.
Microbiological and phenotypic characteristics corroborated the descriptions by Antunes [3] and Quinn et al. [11]. Therefore, the diagnosis of C. violaceum infection is based mainly on the culture of blood, abscesses, exudates, and tissues, in addition to routine biochemical tests for proper identification of the microorganism [19].
Also, the PCR of the 16S rRNA gene, sequencing, and phylogenetic analysis were essential in confirming the species as in other case reports in animals [22; 26] and humans [7]; this is a crucial complementary diagnostic method. Therefore, this first report of the detection of C. violaceum in Puma concolor confirms this microorganism’s ability to infect different species of mammals.
Conclusions
In this case, the cougar died primarily due to an aggressive infection linked to the bacterium C. violaceum, and FeLV probably contributed to worsening the clinical outcome. It was also found that it is a bacterium with rapid spread and evolution, corroborating what has been described in other reports concerning the same organism.
Supplementary information
Chromobacterium violaceum isolate from cougar sepsis. Dark purple colonies isolate in Nutrient Agar. (JPEG 87 kb)
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Batista JH, Silva Neto JF. Chromobacterium violaceum pathogenicity: updates and insights from genome sequencing of novel Chromobacterium species. Front Microbiol. 2017;8:2213. doi: 10.3389/fmicb.2017.02213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong CY, Lam MS. Case report and review of Chromobacterium sepsis a gram-negative sepsis mimicking melioidosis. Med J. 1997;38(6):263–265. [PubMed] [Google Scholar]
- 3.Antunes AA (2006). Chromobacterium violaceum: characterization, cultural, biochemical, molecular and detection of the production of polyhydroxyalkanoate-PHA. Master's Thesis, Federal University of Pernambuco, Recife. 85p.
- 4.Kothari V, Sharma S, Padia D. Recent research advances on Chromobacterium violaceum. Asian Pac J Trop Med. 2017;10(8):744–752. doi: 10.1016/j.apjtm.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Frawley AA, Powell L, McQuiston JR, Gulvik CA, Bégué R. Necrotizing pneumonia Caused by Chromobacterium violaceum: report of a rare human pathogen causing disease in an immunodeficient child. Am J Trop Med Hyg. 2018;99(1):164–167. doi: 10.4269/ajtmh.18-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jedruszczak A, Wegrzyn-Bak M, Budzynska-Nosal R, Maciejewski M, Marczewski K. Sepsis caused by Chromobacterium violaceum - probably the first case in Europe. Agric Environ Med. 2018;26(3):508–510. doi: 10.26444/aaem/99295. [DOI] [PubMed] [Google Scholar]
- 7.Okada M, Inokuchi R, Shinohara K, Matsumoto A, Ono Y, Narita M, Ishida T, Kazuki C, Nakajima S, Yahagi N. Chromobacterium haemolyticum-induced bacteremia in a healthy young man. BMC Infect Dis. 2013;13:406. doi: 10.1186/1471-2334-13-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miki T, Okada N. Draft genome sequence of Chromobacterium haemolyticum causing human bacteremia infection in Japan. Genome Annouc. 2014;2(e):1047–1014. doi: 10.3389/fmicb.2017.02213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dzupova O, Benes J. Serious imported infections: a focus on Chromobacterium violaceum. Bratisl Lek Listy. 2019;120(10):730–733. doi: 10.4149/BLL_2019_121. [DOI] [PubMed] [Google Scholar]
- 10.Guevara A, Salomon M, Oliveiros M, Guevara E, Guevara M, Medina L. Sepsis by Chromobacterium violaceum pigmentado y no pigmentados-Sepsis caused by pigmented and no pigmented Chromobacterium violaceum. Rev. Chil.infectol. 2007;24:402–406. doi: 10.4067/S0716-10182007000500010. [DOI] [PubMed] [Google Scholar]
- 11.Quinn PJ, Markey BK, Carter ME, Donelly WJ, Leonard FC. Cat-scratch disease bacterium. In: Quinn PJ, Markey BK, Carter ME, Donelly WJ, Leonard FC, editors. Clinical Veterinary microbiology. Porto Alegre: Elsevier; 2004. pp. 307–309. [Google Scholar]
- 12.Sambrook J, Russell DW (2001). Molecular cloning a laboratory manual. 3ed. New York: Cold Spring Harbor Laboratory. P. 6.4 - 6.11.
- 13.Lane DJ. 16S / 23S rRNA sequencing. Nucleic acid techniques in bacterial systematics. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematic. New York: Wiley; 1991. pp. 125–175. [Google Scholar]
- 14.Turner S, Pryer KM, Miao VPWAMP, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol. 1999;46(4):327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 15.Dias JP, Silvany C, Saraiva MM, Ruf HR, Guzman JD, Carmo EH. Chromobacteriosis in Ilhéus, Bahia: clinical and laboratory epidemiological investigation. See Soc Br Med Trop. 2005;38:503–506. doi: 10.1590/s0037-86822005000600011. [DOI] [PubMed] [Google Scholar]
- 16.Fedullo D (2001). Wild Animal Clinic: reptiles, primates and felines. Lecture given during Anclivepa, Porto Alegre.
- 17.Decaro N, Carmichael LE, Buonavoglia C. Viral reproductive pathogens of dogs and cats. Vet Clin. 2012;42(3):583–598. doi: 10.1016/j.cvsm.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu CH, Chu RM, Weng CN, Lin YL, Chi CS. An acute pleuropneumonia in a pig caused by Chromobacterium violaceum infections in 13 non-human primates. J Med Primatol. 1989;41:107–114. doi: 10.1111/j.1600-0684.2011.00529.x. [DOI] [Google Scholar]
- 19.Ajithdoss DK, Porter BF, Calise DV, Libal MC, Edwards JF. Septicemia in a neonatal calf associated witch Chromobacterium violaceum. Vet Pathol. 2009;46:71–74. doi: 10.1354/vp.46-1-71. [DOI] [PubMed] [Google Scholar]
- 20.Crosse PA, Soares K, Wheeler JL, Cooke KL, Adin CA, O’Kelley JJ, Levy JK. Chromobacterium violaceum infection in two dogs. J Am Anim Hosp Assoc. 2006;42(2):154–159. doi: 10.5326/0420154. [DOI] [PubMed] [Google Scholar]
- 21.Liu DX, Didier PJ, Plauche GB. Chromobacterium violaceum infections in 13 non-human primates. J Med Primatol. 2012;41:107–114. doi: 10.1111/j.1600-0684.2011.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammerschmitt ME, Rolim VM, Snel GGM, Siqueira FM, Driemeier D, Pavarini SP. Chromobacterium violaceum infection in a horse. J Comp Path. 2017;156:334–338. doi: 10.1016/j.jcpa.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco L, Astorga R, Mendez A. Acute pleuropneumonia in Barbaryeep (Amnotragus lervia) associated with Chromobacterium violaceum. Vet Rec. 1996;138:499–500. doi: 10.1136/vr.138.20.499. [DOI] [PubMed] [Google Scholar]
- 24.Siqueira IC, Dias J, Ruf H, Ramos EA, Maciel EA, Rolim A, Labur L, Vasconcelos L, Silvany C. Chromobacterium violaceum in siblings, Brazil. Emerg Infect Dis. 2005;11:1443–1445. doi: 10.3201/eid1109.050278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teoh AY, Hui M, Ngo KY, Wong J, Lee KF, Lai PB. Fatal septicemia from Chromobacterium violaceum: case reports and review of the literature. Med J. 2006;12(3):228–231. [PubMed] [Google Scholar]
- 26.Soares RL, Dias Neto NB, Guizelini CC, Araújo MA, Leal CRB, Möck TBM, Ramos CAN. Chromobacteriosis (Chromobacterium violaceum) in a calf from Brazil - case report. Arquivo Brasil Med Veter. 2019;71:1929–1933. doi: 10.1590/1678-4162-11063. [DOI] [Google Scholar]
- 27.Anah MU, Udo JJ, Ochigbo SO, Abia-Bassey LN. Neonatal septicaemia in Calabar, Nigeria. Trop Dr. 2008;38:136–128. doi: 10.1258/td.2006.006037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chromobacterium violaceum isolate from cougar sepsis. Dark purple colonies isolate in Nutrient Agar. (JPEG 87 kb)