Abstract
The colonisation and development of the gut microbiota has been implicated in paediatric metabolic disorders via its powerful effect on host metabolic and immune homeostasis. Here we summarise the evidence from human studies on the early gut microbiota and paediatric overweight and obesity. Manipulation of the early gut microbiota may represent a promising target for countering the burgeoning metabolic disorders in the paediatric population, provided the assembly patterns of microbiota and their health consequences can be decoded. Therefore, in this review, we pay particular attention to the important ecological drivers affecting the community dynamics of the early gut microbiota. We then discuss the knowledge gaps in commonly studied exposures linking the gut microbiota to metabolic disorders, especially regarding maternal factors and antibiotic use. This review also attempts to give directions for future studies aiming to identify predictive and corrective measures for paediatric metabolic disorders based on the gut microbiota.
Gut microbiota; Metabolism; Paediatric overweight and obesity; Ecological driver; Dynamics; Infants
1. Introduction
The collection of bacteria, archaea, viruses, fungi, and eukarya residing in the human intestinal tract, known as the gut microbiota, represents one of the most significant features contributing to physiological inter-individual variability [1]. The developing gut microbiota is inextricably and interdependently involved in the concurrent maturation of the endocrine, immune and metabolic pathways during early life [2]. Considerable attention has been given to prevalent diseases at the interface between metabolism and immunity, including obesity, diabetes mellitus, and non-alcoholic fatty liver disease (NAFLD). While these diseases are manifested in later life, they are affected by metabolic programming occurring in early life and thus may be mitigated or averted via early correction of risk factors [3,4]. Accordingly, research in experimental models that demonstrates microbiota-dependent transmissibility of host disease phenotype provides some evidence that colonisation history, developmental trajectories, and disturbance in the early gut microbiota are implicated in metabolic and immune health later in life [5], [6], [7], [8]. To date, several prospective studies in humans have demonstrated links between deviations in the early life microbiota and paediatric overweight and obesity. Therefore, by understanding pivotal ecological drivers and environmental factors contributing to shifts in microbial equilibrium during the critical window of development, further deviations from the normal development may be forestalled. In this review, we discuss recent advances in understanding the connection between the early-life bacterial gut microbiota and metabolic health in children and adolescents, with a special focus on childhood obesity that has become a global public health crisis in the past decades [9].
2. Ecological perspective to the development of the human gut microbiota
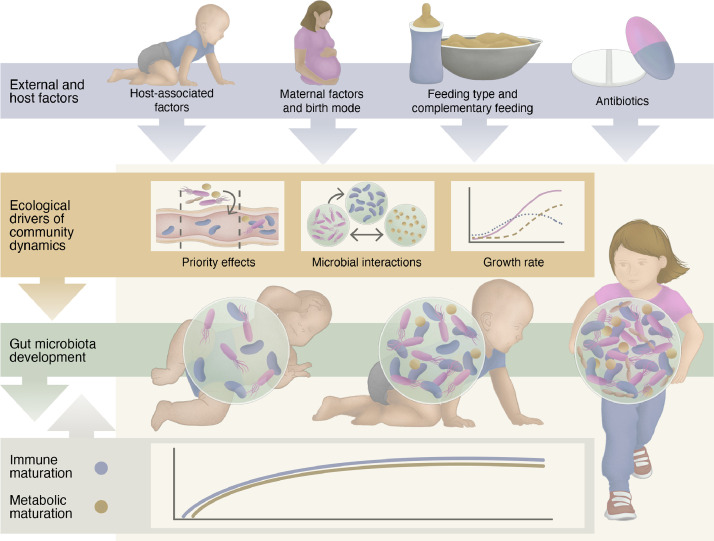
It is generally recognised that the main events of microbial colonisation in infants occur during and after birth and include vertical (from the mother) and horizontal (from the immediate environment) transfers [10,11]. During the last decade, several descriptive sequencing studies reporting the potential existence of microbial communities in the placenta and amniotic fluid point to a possibility of in utero gut colonisation, although the available scientific evidence is insufficient to assert this hypothesis currently [11]. Colonisation is followed by relatively reproducible successional dynamics of the early microbiota that continues to mature into adolescence, as has been extensively reviewed previously [2,10,12]. Recent studies have shown that the maturation of the infant gut microbiota into an adult-like one is an intricate and continuous process involving phylogenetic [13] and functional [14] convergence. The convergence is governed by forces such as host diet and drugs, hormonal and metabolic status, genotype, and immune system that contribute to shaping the gut microbes into a highly individualised and relatively stable community. The true impact of potential stochastic forces [15] has not been reliably quantified, as most of the observed stochasticity is likely caused by unmeasured deterministic forces. The effects of individual external factors, such as birth mode, antibiotic use, and availability of breast milk on shaping the early gut microbiota are relatively well studied [16]. On the other hand, the combinatory (cumulative or counteractive) effects of these common exposures remain relatively unexplored. Similarly, the mechanisms by which the host regulates the gut microbial communities in infancy, such as secretions of mucus glycans, immunoglobulin A (IgA) and bile acids, are poorly understood. Moreover, little is known regarding the ecological drivers of early microbial community dynamics, including colonisation history [17], bacterial growth or replication rates [18], and interactions between the community members [19], which may equally have long-lasting health implications (Fig. 1).
Fig. 1.
An overview of important host and external factors and ecological drivers affecting the infant's immune, metabolic and gut microbiota development.
A recent murine study demonstrated that the colonisation order of a seed community determines the resulting community composition, known as a priority effect. Priority effects may provide a link between certain microbial signatures observed in obese children and their early microbial exposures, such as decreased proportions of Bifidobacterium [20] that are often associated with caesarean (C-) section delivery, not being breastfed, and antibiotic use [16]. Priority effects partly dictate subsequent microbe-microbe interactions. For example, the lack of Bifidobacterium promotes the colonisation of streptococci [21], which have been linked to increased BMI in antibiotic-exposed infants [22]. On the other hand, the depletion of Bifidobacterium spp. could inhibit the colonisation of other bacteria key to infants’ growth due to the absence of trophic interactions [23].
Bacterial growth rate (the rate of change in the number of cells in a given habitat over time) represents an independent feature of the effect of the gut microbiota on the host and has been significantly correlated with several inflammatory and metabolic disorders in adults [18]. A higher bacterial replication rate as a measure of bacterial growth rate was observed before the diagnosis of necrotising enterocolitis in preterm infants [24]. Bacterial growth rates are highly relevant for gut bacteria that possess flexible metabolic capacity and short doubling times, such as many pro-inflammatory Proteobacteria that are initial colonisers and critical in education of host immunity [25]. Moreover, changes in the growth rates of particular bacteria e.g. catalytic [26] or keystone [27] species in response to environmental signals may herald the deviation of the gut microbiota development, leading to the collapse of normal gut communities and eventual dysbiosis-related disorders.
To study these ecological drivers that could provide understandings on disease aetiology and potential corrective approaches, it is necessary to quantify changes in the absolute abundance of biotic community members over time [19]. This has been largely overlooked in the preponderance of current microbiota studies based on a single snapshot of the developing microbiota, where sequencing-derived relative abundances of microbial taxa were analysed. On one hand, the use of relative abundance is problematic as its compositional nature (changes of abundances are mutually dependent due to a constant sum constraint) can mask true community dynamics [19] and lead to high false discovery rates [28]. On the other hand, absolute quantitation of the gut microbes provides richer insights into their immune and metabolic impact, as the magnitude of the immune responses or the concentrations of microbial metabolites largely depend on cell quantity [29]. Of note, growth rate measurement requires estimating cell density at a series of time points, which can be readily achieved by integrating absolute quantitation into widely used next-generation sequencing (NGS), termed quantitative microbiome profiling (QMP) [28,30]. QMP has been recently applied to understand health and disease in adults [30], but not yet in paediatric populations where the gut microbial interactions are highly dynamic. Importantly, no studies to date have comprehensively surveyed the changes of the gut microbes in absolute abundance during early development.
3. Impact of major perinatal factors on the development of early microbiota and metabolic health
3.1. Maternal obesity and diet
Maternal vaginal and faecal microbes represent the first and potentially the most numerous sources of bacteria the infant is exposed to. Since vaginal bacteria do not persistently colonise the infant gut [31], the maternal gut microbiota is the main source of colonisation in vaginally born infants [31,32]. The vaginal microbiota may yet have a substantial impact on the infant's early immunity considering the neonate's exposure to high quantities of bacteria during vaginal birth (up to 109 bacteria per gram of vaginal secretions) [33], although this has been understudied.
Maternal obesity could introduce microbiota aberrations in the early-life microbiota relevant for metabolic health at least via two mechanisms. First, through the direct vertical microbiota transfer and priority effects, the maternal obesity-associated microbiota results in intergenerational effects [34,35] and possibly the development of metabolic disorders [35]. While dietary habits within the family as well as genetic and epigenetic factors are known to contribute to transgenerational obesity, maternal transmission of an altered gut microbiota has been attributed as one potential mechanism in offspring obesity and NAFLD in animal models [36]. The effect of maternal obesity on the infant's gut microbiota is less clear in humans [37], likely due to multiple confounding factors. Indeed, there are some data supporting the effect of obesity or metabolic disease on the maternal gut microbiota during pregnancy [38]. Interestingly, two studies reported that the gut microbiota in infants born to obese mothers had a reduced proportion of Proteobacteria [35,39] that are normally abundant during the first weeks of life [12]. Colonisation of germ-free mice with the gut microbes from the infants born to obese mothers yielded a persistently compromised innate immune cell function compared to controls, which was thought to exacerbate the development of NAFLD and excessive weight gain in the mice on an obesogenic diet [39]. Second, maternal overweight and obesity is a main risk factor for C-section delivery [40], which alters the early microbiota development as discussed in the next section. C-section delivered infants of overweight mothers were reported to have a higher risk of overweight at one year compared to vaginally delivered infants [35], stressing the importance of microbial colonisation of the newborn and priority effects in determining later microbiota and host development.
Maternal diet during the periconceptional period is one of the critical and modifiable factors on child health [41], effects of which could be partly driven by the gut microbiota. In mice, gut microbiota–derived metabolites represented by short-chain fatty acids have been shown to reach the embryos via the maternal liver and blood stream, shaping embryonic energy metabolism and subsequently postnatal energy homeostasis [42]. Garcia-Mantrana et al. reported that the maternal microbiota associated with a specific maternal dietary pattern (lower intakes of fibre and n-3 fatty acids, and higher intakes of animal protein and saturated fats) along with other perinatal factors shaped the neonatal gut microbiota that may predispose infants to overweight during the first 18 months of life [43]. A recent study found that maternal consumption of artificially sweetened beverages during pregnancy was associated with the depletion of Bacteroides spp. in the infant gut microbiota as well as elevated levels of urine succinate that correlated with infant BMI [44]. Exposure to maternal high-fat diet, associated with increased intrahepatic triglyceride levels in the offspring in experimental models [36], altered the neonatal gut microbiota in humans [45]. Short-term high-fat diet (saturated fats specifically), possibly through increases in bile acid secretion [46], induces specific compositional changes in the gut microbiota, such as increased pro-inflammatory Bilophila/Proteobacteria that are associated with liver steatosis [47] and hence may contribute to the intergenerational transmission of NAFLD and obese phenotypes.
To date, most dietary interventions in women at child-bearing age aiming to improve maternal health have not accounted for the diet-induced changes in the maternal gut microbiota that could positively or negatively influence metabolic outcomes in the children. For example, a low-energy diet used for pre-conception weight loss [48] is frequently linked to reduced Bifidobacterium [49] due to the insufficient amount of dietary fibre and starch. Whether the reduced abundance of Bifidobacterium would imprint in neonates’ gut microbiota and mediate potential metabolic consequences later in life is unknown. With the burgeoning trend of alternative diet regimes, such as ketogenic diets, future studies are needed to investigate their impact on the maternal and neonatal gut microbiota in relation to intergenerational transmission of metabolic outcomes.
3.2. Birth mode
Mode of delivery is one of the foremost contributors to sculpturing the infant's microbiota. The first bacteria populating the gut of naturally born infants come primarily from the mother via faecal-oral route [31,32]. Since the first strain-resolved metagenomic studies documenting the disruption of mother-to-neonate microbiota transmission in infants born by C-section [50], the more recent studies have also addressed the functional implications on the community and strain levels [51], [52], [53]. In C-section born infants, bacteria from the hospital environment colonise the gut and gradually become replaced by bacteria better adapted to the intestinal environment [10,52,53]. As a result, the assembly and dynamics of intestinal bacteria in C-section babies differ appreciably from those in the vaginally born, typified by the lack or decrease of Bacteroidetes, the decrease of bifidobacteria and respective increases in Proteobacteria [10]. Some studies have reported this imprint to last up to 18 months [54].
C-section delivery has been implicated in various immune disorders [55] as well as associated with an increased risk of offspring obesity in two earlier meta-analyses of 24 studies [56,57], which may be mediated by delayed maturation of the gut microbiota [58,59]. Nevertheless, some recent studies have found null associations between C-section and offspring corpulence, citing that the earlier studies failed to account for maternal BMI and prenatal factors [60].
Subtle differences in the infants born by elective C-section (no labour) versus emergency C-section (labour) have been observed in some studies [61], which are attributable to the lack of immune responses within the uterine cavity normally induced by labour [62]. It remains unknown whether these differences are pertinent to the different risks of childhood overweight and obesity between elective and emergency C-section [63,64].
3.3. Feeding type
After birth, breastfeeding becomes one of the most important and modifiable determinants of infant gut microbial colonisation. The differences in the gut microbiota composition between breastfed and formula-fed infants are well-established [65]. Human milk is a natural modifier of gut microbial composition via the prebiotic properties of human milk oligosaccharides (HMOs), its immunoglobulins and antimicrobial compounds that select for certain bacteria, and acting as a potential source of microbial inoculum that may play a role in infant growth [66]. The selective capacity for utilisation of HMOs favours the growth of Bifidobacteriaceae in the breastfed infant's gut [67]. The HMO composition in breast milk has been associated with infant growth and body composition [68,69], and theorised to contribute to the protective effects of breastfeeding against childhood overweight and obesity [70] and type 2 diabetes [71] via the gut microbiota [72]. Breastfeeding has been shown to partially restore the disruptions in the infant's gut microbiota caused by C-section, specifically the depletion of bifidobacteria, potentially mediated by the abundant amount of α1-2 fucosylated oligosaccharides in the breast milk of mothers with a functional FUT2 allele [73,74].
In contrast, a study in 1087 infants suggested that formula feeding stimulated changes in the gut microbiota (higher microbiota diversity and increased relative abundance of Lachnospiraceae) that partially explained the increased risk of overweight at one year [75]. A meta-analysis including seven studies across three continents reported that the gut microbial functions in non-exclusively breastfed neonates were characterised by increased carbohydrate metabolism and reduced lipid metabolism/homeostasis as well as decreases in detoxification pathways [76]. Early complementary feeding is a risk factor for childhood obesity [77] and NAFLD in adolescents [78], and has been linked to the altered abundances of Roseburia and Bilophila wasworthia [79].
3.4. Antibiotics
Early-life antibiotic exposure is well-known for disrupting gut microbiota homeostasis, giving rise to elevated proportions of Proteobacteria and reduced Bifidobacterium in the infant's gut microbiota [80,81]. Antibiotic use in infancy increases the risk of childhood overweight and obesity depending on gender and exposure timing [82]. In a large Finnish cohort of healthy children, antibiotic exposure before six months of age, or repeatedly during infancy, was associated with increased body mass at the age of 24 months [83]. There is some evidence suggesting that antibiotics alter host metabolism by reshaping bile acid profiles [80,84].
A single course of amoxicillin, the most commonly used antibiotic in neonates, profoundly biased the maturation of the early gut microbiota in two- to three-month-old infants toward low abundance of bifidobacteria and increased abundance of clostridia [81]. In older children (two- to seven-year-olds), exposure to macrolides was shown to profoundly after the gut microbiota, specifically reducing bile-salt hydrolase producing bacteria including bifidobacteria. The changes were linked to increased BMI potentially via altered bile acid metabolism [80]. Uzan-Yulzari et al. recently reported that neonatal antibiotic exposure was associated with reduced growth during the first six years of life particularly in boys, whereas antibiotic use after the neonatal period was associated with excessive childhood weight gain in both genders. These effects were likely mediated by the gut microbiota, specifically the decreased abundance and diversity of bifidobacteria, which remained detectable even 24 months after exposure [85]. Alarmingly, antibiotic exposure during the breastfeeding period may eliminate the beneficial metabolic effects of breastfeeding by altering the gut microbiota [86].
Antibiotics are among the most commonly prescribed medications to newborns and children. The first exposure may come through the umbilical cord following intrapartum antibiotic prophylaxis (IAP), the preventive antibiotics given to the vaginally delivering mothers who screen positive for group B streptococcus (GBS) as well as during C-section or assisted vaginal delivery. IAP administration has been linked to an increased risk of obesity and immune diseases in the child [10]. The gut microbiota composition of IAP-exposed infants appears to be similar to that of C-section-delivered infants [10], which nevertheless could have been confounded by several factors as the few available studies are of small sample size and high heterogeneity in study design [87]. IAP exposure, particularly to multiple antibiotic classes, led to year-long alterations in the development trajectory of the gut microbiota in vaginally born infants, suggesting the importance of antibiotic class and selectivity in reshaping the gut microbiota [88].
Antibiotic prescribing patterns in infants and childhood are extremely variable, likely emanating from differences in the physicians’ clinical experiences to some extent [89]. For instance, the duration of parenteral antibiotics for infants with bacteremic urinary tract infection varied from 1 to 24 days depending on the treatment location of the patient [90]. Recent studies have shown possible dose-response [91] and class-specific [92] effects of the antibiotic exposure on children's growth trajectories. There is, however, a general paucity of research exploring the impact of antibiotic course, class and timing of administration on the neonate's gut microbiota in relation to health consequences.
3.5. Associations between early-life microbiota and adiposity in later infancy
While early disturbances on the microbiota seem to be transient and largely return to normal after weaning [93], temporary perturbations in the ecosystem during the sensitive developmental period may still increase the propensity to later metabolic dysregulation as demonstrated in experimental models [6]. Ten prospective studies have examined the link between the early-life gut microbiota and subsequent development of adiposity in the same subjects (Table 1). These studies, albeit differences in methodologies and confounding factors, have shown a general trend where higher relative abundances or the presence of Bacteroides spp. and lower relative abundances of bifidobacteria in early infancy are associated with paediatric overweight and obesity. Staphylococcus spp. may also be predictive of childhood BMI, although the directionality of the association is inconsistent [94,95]. Interestingly, microbiota features have been reported to associate with child adiposity as early as in the meconium i.e. the earliest stool [96]. While these findings imply that changes in the gut microbiota are detectable much earlier than the manifestation of obesity, prospective long-term cohort studies as well as randomised controlled trials (when ethical) are needed to ultimately determine whether the microbiota features in infancy are a mere reflection of, or a casual contributor to the pathogenesis of childhood overweight and obesity. This is further complicated by the fact that the gut bacteria undergo rapid undulations during the first year of life, sensitive to multiple sources of perturbation that cannot be captured by one snapshot as done in most existing studies. Moreover, none of the abovementioned studies employed metagenomic sequencing that could unravel specific bacterial species, strains and functions as well as potential viruses or fungi involved in the development of paediatric obesity. As taxonomic units have inherent limitations in resolution, future studies employing metagenomic analysis may benefit from genome-based analysis frameworks, where individual genomes present in a sample are directly taxonomically and/or functionally annotated [97,98].
Table 1.
Summary of studies on associations between early-life gut microbiota and infant weight development or childhood overweight and obesity.
| Study | Country | Sample size | Age at collection of faecal sampling (d=day; w=week; m=month) | Age at assessment of adiposity (final time point; y=year) | Key findings on the gut microbiota | Potential confounders | References (PMID) |
|---|---|---|---|---|---|---|---|
| Kalliomäki et al. | Finland | 49 | 6m-12m | 7y | Increased bifidobacteria in children remaining normal weight; increased Staphylococcus aureus in children developing overweight. | Maternal BMI, IAP, CS type | 18326589 |
| Vael et al. | Belgium | 138 | 3w-52w | 3y | Increased Bacteroides fragilis and decreased Staphylococcus associated with obesity later in childhood. | IAP | 21605455 |
| Luoto et al. | Finland | 30 | 3m | 10y | Increased bifidobacteria in children remaining normal weight (non-significant; P=0.087). | IAP, CS type | 21150648 |
| White et al. | Norway | 218 | 4d-120d | 0•5y | Developmental trajectories of Staphylococcus species and Escherichia coli associated with expected growth; presence of Bacteroides spp. at one month associated with growth. | - | 23671411 |
| Scheepers et al. | The Netherlands | 909 | 1m | 10y | Presence of Bacteroides fragilis group at one month associated with a higher BMI later in childhood. | - | 25298274 |
| Dogra et al. | Singapore | 75 | 6m | 1•5y | Infants who acquired a profile high in Bifidobacterium and Collinsella, and low in Enterobacteriaceae at a later age associated with lower adiposity at 18 months of age. | Ethnicity, antibiotics, CS type | 25650398 |
| Korpela et al. | The Netherlands & Finland | 162 | 3m | 5-6y | At three months of age, Bifidobaterium spp. negatively and streptococci positively associated with BMI at five to six years. | - | 28253911 |
| Forbes et al. | Canada | 1087 | 3m-4m | 1y | Longitudinal associations between the composition of gut microbiota at three to four months of age and weight status at one year of age. | - | 29868719 |
| Stanislawski et al. | Norway | 165 | 10d-730d | 12y | Over 50% of the variation in obesity at 12 years of age explained by the pattern of types of gut microbiota at two years of age. | CS type | 30352933 |
| Korpela et al. | Finland | 212 | <1d | 3y | Increased Bacteroides fragilis in the meconium associated with overweight at the age of three years. | Maternal BMI, CS type | 32638554 |
BMI: body mass index; IAP: intrapartum antibiotic prophylaxis; CS: caesarean section.
4. Potential mechanisms linking the early-life microbiota to metabolic dysregulation
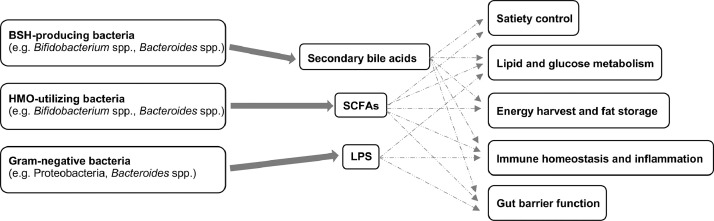
Evidence from previous studies, mainly in experimental models, suggests that the gut microbiota contributes to metabolic diseases via several mechanisms, including increased energy harvest and fat storage, regulation of lipid and glucose metabolism, gut barrier function, induction of low-grade inflammation, and to a lesser extent satiety control mediated by gut hormones as well as interactions with host genetics [99]. These mechanisms, chiefly ascribed to the actions of lipopolysaccharide (LPS), short-chain fatty acids (SCFAs) and bile acids, may work in parallel or synergistically to alter physiology in a developing newborn (Fig. 2). Additionally, recent studies have identified other microbiota-dependent metabolites that play a role in metabolic diseases mediated by pro-inflammatory pathways, such as the microbially produced histidine metabolite, imidazole propionate [100], and the microbial metabolite succinate [101].
Fig. 2.
Key gut bacteria, bacterial metabolites and mechanisms potentially involved in the development of paediatric metabolic disorders. BSH: bile salt hydrolase; HMO: human milk oligosaccharide; SCFAs: short-chain fatty acids; LPS: lipopolysaccharide.
During the critical time window in neonatal life, immune development is reliant on triggers provided by the neonatal microbiota as well as the maternal microbiota [102]. Maturation of innate immunity in return creates a selective pressure for the gut microbes, impelling their temporal development. The initially colonising Gram-negatives, mainly Proteobacteria and Bacteroides spp. are believed to play a role in this process via their structural component LPS, one of the best studied pathogen-associated molecular patterns (PAMPs) that represent targets of innate immune recognition [25]. A previous study demonstrated that vaginally-delivered mice acquired resistance to LPS shortly after birth, leading to immunotolerance [103]. While exposure to LPS during early postnatal development is required to educate the immune system, the sustained exposure to LPS with compromised gut barrier function results in the activation of pro-inflammatory pathways and subsequent metabolic dysregulation [104]. The relative abundances of Proteobacteria species are initially high and gradually decrease over time in full-term vaginally born neonates, which can be partly ascribed to the selective suppression of HMOs and secretory IgA in the human milk [105]. Neonatal antibiotic exposure, including IAP and postnatal antibiotic administration, has been associated with an increase in LPS-producing Proteobacteria [106]. The bacterial LPS structures between bacterial genera (e.g. between Escherichia and Bacteroides spp.) and even species can substantially differ in their inflammatory potential [107] and one study suggests that early colonization with the former may be needed for appropriate immune activation and endotoxin tolerance that decrease susceptibility to immune diseases [25]. Hence, the exposure to immunogenic LPS at the appropriate timing in early life may be part of normal development rather than harmful. However, the actual role of different LPS-producing bacteria in the early education of immunity as well as development of childhood obesity is currently unclear. It also remains unknown whether other PAMPs have a role in the pathophysiology of excessive weight gain in early life.
SCFAs (acetate, propionate and butyrate) are the primary microbial metabolites of HMO fermentation that contribute to energy homeostasis, modulate host adiposity, regulate immune functions, and alter gene expression of host satiety hormones [108]. Bifidobacteria spp. (producing acetate and lactate that can be converted into butyrate by other colonic bacteria), consistently enriched in vaginally born breast-fed infants, are major HMO utilisers that have been given credit for improved gut and systemic health in infants. The multifunctional effects of Bifidobacterium spp. include the amelioration of diet-induced endotoxemia and inflammation in the gut, liver and adipose tissue, and the deconjugation of bile acids influential to the host's lipid metabolism and energy expenditure [109,110]. Bifidobacterium infantis, a key early coloniser, has also been reported to directly correlate with the amount of secreted IgA antibodies [111], which are the centrepiece of protective humoral mucosal immunity. Therefore, the depletion of Bifidobacterium spp. may promote loss of immune- and metabolic homeostasis in infants and further, diseased states. Of note, a meta-analysis found a loss of bifidobacteria in healthy breastfed infants, indicated by increased faecal pH, over the past century (1926-2017) [112]. Similarly, a recent metagenomic study on the contemporary American infant gut microbiome reported reduced capacity for HMO utilisation and other ecosystem services provided by the gut microbiota to the host [113]. Interestingly, reduced levels of HMO-metabolising genes and the main Bifidobacterium species encoding them were not related to infant diet, alluding to the role of longer-term, potentially even generational influences on this early dysbiosis. Finally, as mentioned earlier, the scarcity of Bacteroides spp., the main propionate producers in the human gut, is a taxonomic signature of C-section born infants. While several mechanisms have been identified regarding how propionate can promote metabolic health [114], for now, very little is known about the physiological implications of reduced early colonisation by Bacteroides spp.
Bile acid metabolism is critical for the absorption of dietary fat and plays an important signalling role in metabolic regulation via the nuclear receptor FXR and the G-protein-coupled bile acid receptor-1, especially in the gut-to-liver axis [115]. Intestinal bacteria deconjugate primary conjugated bile acids by bile-salt hydrolase and modify them into secondary and tertiary forms [116]. Accordingly, the bile acid composition is largely shaped by the gut microbiota, and has been suggested to contribute to obesity susceptibility in both humans and mice [117]. On the other hand, bile acids pose a selective pressure on the gut microbes due to their toxicity for bacterial cells [116], rendering them influential for the early gut microbiota maturation based on a recent study in mice [118]. A recent longitudinal study suggested that neonates experience a drastic transition of the bile acid profiles during lactation that coincides with the transition of the microbiota profiles [119]. Perturbations of bile acid and/or microbiota profiles, such as antibiotic-driven depletion of bile-salt hydrolase producing bacteria [80], during this critical period of time may therefore have negative metabolic consequences. Currently, no studies have investigated the interactions between bile acids, the gut microbiota and host adiposity simultaneously in children.
5. Modelling gut microbiota dynamics in relation to metabolic health
Prediction and early intervention of excessive weight development and related metabolic dysregulation is the cornerstone of preventive healthcare to curb the epidemic of obesity. While changing dietary patterns and lifestyle of children is beneficial, another mode of intervention is reducing factors that increase the risk of diet-induced metabolic dysfunction. In this regard, the gut microbiota represents a promising target. A few studies have developed prognostic prediction models using known risk factors for childhood obesity e.g. maternal pre-pregnancy BMI and birth weight, achieving varying performances [120]. Recent studies employing machine learning algorithms have suggested comparable predictive values of the gut microbiota in childhood obesity, though external validation is lacking [96,121].
Reliable predictive models applied to dynamic systems, such as growing infants or developing gut microbiota, should have the ability to absorb changes in the environments upon their introduction. In terms of the gut microbiota, this requires a better understanding of the community dynamics of the gut microbiota, such as priority effects, intra- and interspecies interactions, and the consequences of alternative assembly patterns. Various mathematical models have been applied to predict the dynamic behaviours of the gut microbiota. For instance, the generalised Lotka-Volterra (gLV) model, which describes the change over time of a population of microbial members as a function of their intrinsic growth rates and pairwise interactions (e.g. predator-prey or mutualism), has become one of the most popular models [122]. Nevertheless, these simplistic pairwise interactions are insufficient to capture complex interaction networks occurring in a microbial community [123], calling into question the utility of the gLV model. To capture non-pairwise interactions mediated by environmental factors e.g. antibiotic perturbation, time series modeling from multiple sampling is essential [124]. Genome-scale metabolic models (GEMs) computationally describe gene-protein-reaction associations for entire metabolic genes in an organism [98] and have been utilised to accurately predict growth trajectories and changes in body compositions up to age six months in healthy neonates [125], which could be integrated with GEMs of the gut microbiota that contributes to a significant portion of daily metabolic fluxes. In addition, GEMs could prove a functional perspective with biologically relevant resolution taxonomically and prediction of metabolic interactions between microbial taxa in metagenomic studies, potentially revealing the “driver” microbes that contribute to the development of metabolic diseases.
6. Therapeutic potential of the early-life gut microbiota in paediatric metabolic disorders
The gut microbiota in infants is often regarded as having high plasticity due to its low diversity and rapid development, in comparison to the microbiota in adults, and has become an attractive target for modifications aiming to improve health in later life [126]. Microbiota-targeted modifications can be broadly categorised into three groups: depletion (e.g. antibiotics), modulation (e.g. pre- and probiotics or dietary intervention) and replacement (e.g. faecal microbiota transplantation (FMT)).
The growth promoting effect of antibiotics has been long appreciated in animal production [127] and exploited to increase growth rates in malnourished children in low-income countries [128]. Since specific antibiotic classes are highly selective against particular microbes, this property could be leveraged to manipulate their collateral effect on the microbiota in the future [129].
Probiotics have increasingly been administered to infants and children in recent years, and shown a larger impact on C-section-delivered infants compared to their vaginally born counterparts [130]. However, mixed results have been documented from few trials examining the effect of early administration of probiotics on excessive weight development in neonates. The perinatal use of Lactobacillus rhamnosus appeared to modify children's growth patterns by inhibiting excessive weight gain during the first few years of life [131], while other studies in 179 healthy, term infants using L. paracasei subsp. paracasei F19 found no short-term [132] and long-term [133] metabolic benefits. The disparities between the studies may be partially explained by priority effects arising from different probiotic strains administered at different time points and for different durations. It is also worth mentioning that there is insufficient evidence supporting the role of Lactobacillus spp., while being the most commonly used probiotic strains, in weight control in the infant age group, especially considering their low abundance and strain-specific effects [134]. Thus, hypothesis-driven probiotic interventions using the strains known to be involved in energy metabolism are urgently needed.
Replacement of the gut microbiota by FMT represents the most powerful option to re-direct the microbiota. A recent FMT trial in adolescents with obesity led to a slight but significant reduction in visceral adiposity [135]. A recent proof-of-concept study found that maternal FMT, not vaginal swabbing, corrected the C-section associated depletion in Bacteroides spp. and the delayed maturation of Bifidobacterium in neonates [31]. While the metabolic consequences in the treated infants are to be followed, this study highlights the importance of the vertical microbiota transfer on the colonisation.
7. Conclusion
Over the past decade, research in animal models and epidemiological research in humans has provided evidence linking the disruption of multifaceted microbiota-host dialogues in infancy to negative metabolic and immune health later in life. To move beyond associations, hypothesis-directed interventions in children during development are the necessary next step toward tapping into the therapeutic potential of the gut microbiota in paediatric metabolic disorders. Importantly, ecological drivers of early microbial community dynamics could provide new insight into immune and metabolic homeostasis as well as susceptibility to inflammatory diseases later in life. Therefore, we posit a need for novel approaches that enable studying host-microbe dynamics in a community ecology perspective, which can be utilised for devising effective diagnostic and therapeutic strategies.
8. Outstanding questions
While previous studies have generated ample evidence on the importance of the gut microbiota in the critical window of development, it remains unclear whether alterations in the gut microbiota occurring early in time would exert the same impact on the host as those lasting longer or arising later, which could vary across different metabolic outcomes as well. Therefore, longitudinal analysis of gut microbiota, host faecal and serum biomarkers and detailed background data are of paramount importance in understanding the time window of microbiota effects on host metabolism, which determines the timing of administrating corrective measures, as well as in inferring mechanisms. Based on this knowledge, hypothesis-driven interventions in children should be derived and longitudinal experiments designed as the ultimate way to establish causality.
There is currently scant information on the mechanisms of maternal effects, such as the role of the microbiota in vertically transmitted metabolic phenotypes, and the effects of maternal medication and nutrition on neonates during the periconceptional period and during lactation. These are imperative questions to answer, considering prenatal preventative interventions are required to break the cyclical process of intergenerational metabolic dysregulation.
9. Search and selection criteria
This review is based on a systematic search in PubMed and MEDLINE using the terms (microbiota OR microbiome) AND (metabolic health OR metabolism OR obesity OR overweight) as of February 10th, 2021. We limited our search to articles on full-term infants and written in English in the last 15 years.
Contributors
CJ conceptualised, conducted the literature search, designed figures and tables and wrote the draft with important inputs from KK, WMdV and AS. KK and AS provided critical feedback and revised the draft. NC conducted the literature search with the supervision from OH. OH provided resources and project administration. All authors have read and approved the final version of the manuscript. Any pharmaceutical or other company was not involved in any work related to writing of this article.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgements
The study was supported by the grants from the University of Helsinki (KK), Academy of Finland (grant 1308255 to WMdV and grant 1325103 to AS) and Biocodex Microbiota Foundation (AS, OH). The funders had no role in paper design data collection, data analysis, interpretation, writing of the paper. We thank Fabio Tuccillo for the illustration of Fig. 1.
Ethic committee approval
Not applicable.
References
- 1.Zhou W., Sailani M.R., Contrepois K. Longitudinal multi-omics of host-microbe dynamics in prediabetes. Nature. 2019;569(7758):663–671. doi: 10.1038/s41586-019-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson R.C., Manges A.R., Finlay B.B., Prendergast A.J. The Human Microbiome and Child Growth - First 1000 Days and Beyond. Trends Microbiol. 2019;27(2):131–147. doi: 10.1016/j.tim.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Giorgio V., Prono F., Graziano F., Nobili V. Pediatric non alcoholic fatty liver disease: old and new concepts on development, progression, metabolic insight and potential treatment targets. BMC Pediatr. 2013;13:40. doi: 10.1186/1471-2431-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dattilo A.M., Birch L., Krebs N.F., Lake A., Taveras E.M., Saavedra J.M. Need for early interventions in the prevention of pediatric overweight: a review and upcoming directions. J Obes. 2012;2012 doi: 10.1155/2012/123023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho I., Yamanishi S., Cox L. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488(7413):621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox L.M., Yamanishi S., Sohn J. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olszak T., An D., Zeissig S. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell S.L., Gold M.J., Hartmann M. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cesare M., Sorić M., Bovet P. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17(1):212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore R.E., Townsend S.D. Temporal development of the infant gut microbiome. Open Biol. 2019;9(9) doi: 10.1098/rsob.190128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter J., Hornef M.W. A philosophical perspective on the prenatal in utero microbiome debate. Microbiome. 2021;9(1):5. doi: 10.1186/s40168-020-00979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milani C., Duranti S., Bottacini F. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017;81(4) doi: 10.1128/MMBR.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Muinck E.J., Trosvik P. Individuality and convergence of the infant gut microbiota during the first year of life. Nat Commun. 2018;9(1):2233. doi: 10.1038/s41467-018-04641-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vatanen T., Plichta D.R., Somani J. Genomic variation and strain-specific functional adaptation in the human gut microbiome during early life. Nat Microbiol. 2019;4(3):470–479. doi: 10.1038/s41564-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprockett D., Fukami T., Relman D.A. Role of priority effects in the early-life assembly of the gut microbiota. Nat Rev Gastroenterol Hepatol. 2018;15(4):197–205. doi: 10.1038/nrgastro.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korpela K., de Vos W.M. Early life colonization of the human gut: microbes matter everywhere. Curr Opin Microbiol. 2018;44:70–78. doi: 10.1016/j.mib.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Martínez I., Maldonado-Gomez M.X., Gomes-Neto J.C. Experimental evaluation of the importance of colonization history in early-life gut microbiota assembly. eLife. 2018;7 doi: 10.7554/eLife.36521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korem T., Zeevi D., Suez J. Growth dynamics of gut microbiota in health and disease inferred from single metagenomic samples. Science. 2015;349(6252):1101–1106. doi: 10.1126/science.aac4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao C., Coyte K.Z., Bainter W., Geha R.S., Martin C.R., Rakoff-Nahoum S. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature. 2021;591(7851):633–638. doi: 10.1038/s41586-021-03241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Silva C.C., Monteil M.A., Davis E.M. Overweight and Obesity in Children Are Associated with an Abundance of Firmicutes and Reduction of Bifidobacterium in Their Gastrointestinal Microbiota. Child Obes. 2020;16(3):204–210. doi: 10.1089/chi.2019.0280. [DOI] [PubMed] [Google Scholar]
- 21.Aloisio I., Mazzola G., Corvaglia L.T. Influence of intrapartum antibiotic prophylaxis against group B Streptococcus on the early newborn gut composition and evaluation of the anti-Streptococcus activity of Bifidobacterium strains. Appl Microbiol Biotechnol. 2014;98(13):6051–6060. doi: 10.1007/s00253-014-5712-9. [DOI] [PubMed] [Google Scholar]
- 22.Korpela K., Zijlmans M.A., Kuitunen M. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5(1):26. doi: 10.1186/s40168-017-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duranti S., Longhi G., Ventura M., van Sinderen D., Turroni F. Exploring the Ecology of Bifidobacteria and Their Genetic Adaptation to the Mammalian Gut. Microorganisms. 2020;9(1) doi: 10.3390/microorganisms9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olm M.R., Bhattacharya N., Crits-Christoph A. Necrotizing enterocolitis is preceded by increased gut bacterial replication, Klebsiella, and fimbriae-encoding bacteria. Sci Adv. 2019;5(12):eaax5727. doi: 10.1126/sciadv.aax5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vatanen T., Kostic A.D., d'Hennezel E. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165(6):1551. doi: 10.1016/j.cell.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 26.Warren P.H., Law R., Weatherby A.J. Mapping the assembly of protist communities in microcosms. Ecology. 2003;84(4):1001–1011. [Google Scholar]
- 27.Trosvik P., de Muinck E.J. Ecology of bacteria in the human gastrointestinal tract–identification of keystone and foundation taxa. Microbiome. 2015;3:44. doi: 10.1186/s40168-015-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian C., Luukkonen P., Yki-Järvinen H., Salonen A., Korpela K. Quantitative PCR provides a simple and accessible method for quantitative microbiota profiling. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamburini S., Shen N., Wu H.C., Clemente J.C. The microbiome in early life: implications for health outcomes. Nat Med. 2016;22(7):713–722. doi: 10.1038/nm.4142. [DOI] [PubMed] [Google Scholar]
- 30.Vandeputte D., Kathagen G., D'Hoe K. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551(7681):507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 31.Korpela K., Helve O., Kolho K.L. Maternal Fecal Microbiota Transplantation in Cesarean-Born Infants Rapidly Restores Normal Gut Microbial Development: A Proof-of-Concept Study. Cell. 2020;183(2) doi: 10.1016/j.cell.2020.08.047. 324-34.e5. [DOI] [PubMed] [Google Scholar]
- 32.Ferretti P., Pasolli E., Tett A. Mother-to-Infant Microbial Transmission from Different Body Sites Shapes the Developing Infant Gut Microbiome. Cell Host Microbe. 2018;24(1):133–145. doi: 10.1016/j.chom.2018.06.005. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kervinen K., Kalliala I., Glazer-Livson S., Virtanen S., Nieminen P., Salonen A. Vaginal microbiota in pregnancy: Role in induction of labor and seeding the neonate''s microbiota? J Biosci. 2019;44(5) [PubMed] [Google Scholar]
- 34.Blaser M.J. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat Rev Immunol. 2017;17(8):461–463. doi: 10.1038/nri.2017.77. [DOI] [PubMed] [Google Scholar]
- 35.Tun H.M., Bridgman S.L., Chari R. Roles of Birth Mode and Infant Gut Microbiota in Intergenerational Transmission of Overweight and Obesity From Mother to Offspring. JAMA Pediatr. 2018;172(4):368–377. doi: 10.1001/jamapediatrics.2017.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson M.D. Developmental Programming of NAFLD by Parental Obesity. Hepatol Commun. 2020;4(10):1392–1403. doi: 10.1002/hep4.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanislawski M.A., Dabelea D., Wagner B.D., Sontag M.K., Lozupone C.A., Eggesbø M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 2017;5(1):113. doi: 10.1186/s40168-017-0332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou L., Xiao X. The role of gut microbiota in the effects of maternal obesity during pregnancy on offspring metabolism. Biosci Rep. 2018;38(2) doi: 10.1042/BSR20171234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soderborg T.K., Clark S.E., Mulligan C.E. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9(1):4462. doi: 10.1038/s41467-018-06929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu S.Y., Kim S.Y., Schmid C.H. Maternal obesity and risk of cesarean delivery: a meta-analysis. Obes Rev. 2007;8(5):385–394. doi: 10.1111/j.1467-789X.2007.00397.x. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson J., Heslehurst N., Hall J. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet. 2018;391:1830–1841. doi: 10.1016/S0140-6736(18)30311-8. 10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura I., Miyamoto J., Ohue-Kitano R. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science. 2020;367 doi: 10.1126/science.aaw8429. 6481. [DOI] [PubMed] [Google Scholar]
- 43.García-Mantrana I., Selma-Royo M., González S., Parra-Llorca A., Martínez-Costa C., Collado M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes. 2020;11(4):962–978. doi: 10.1080/19490976.2020.1730294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laforest-Lapointe I., Becker A.B., Mandhane P.J. Maternal consumption of artificially sweetened beverages during pregnancy is associated with infant gut microbiota and metabolic modifications and increased infant body mass index. Gut Microbes. 2021;13(1):1–15. doi: 10.1080/19490976.2020.1857513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirpuri J. Evidence for maternal diet-mediated effects on the offspring microbiome and immunity: implications for public health initiatives. Pediatr Res. 2021;89(2):301–306. doi: 10.1038/s41390-020-01121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy B.S. Diet and excretion of bile acids. Cancer Res. 1981;41:3766–3768. 9 Pt 2. [PubMed] [Google Scholar]
- 47.Jian C., Luukkonen P., Sädevirta S., Yki-Järvinen H., Salonen A. Impact of short-term overfeeding of saturated or unsaturated fat or sugars on the gut microbiota in relation to liver fat in obese and overweight adults. Clin Nutr. 2021;40(1):207–216. doi: 10.1016/j.clnu.2020.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Sim K.A., Partridge S.R., Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes Rev. 2014;15(10):839–850. doi: 10.1111/obr.12217. [DOI] [PubMed] [Google Scholar]
- 49.Lane M., Howland G., West M. The effect of ultra-processed very low-energy diets on gut microbiota and metabolic outcomes in individuals with obesity: A systematic literature review. Obes Res Clin Pract. 2020;14(3):197–204. doi: 10.1016/j.orcp.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Bäckhed F., Roswall J., Peng Y. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17(5):690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Wampach L., Heintz-Buschart A., Fritz J.V. Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat Commun. 2018;9(1):5091. doi: 10.1038/s41467-018-07631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shao Y., Forster S.C., Tsaliki E. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. 2019;574(7776):117–121. doi: 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Podlesny D., Fricke W.F. Strain inheritance and neonatal gut microbiota development: A meta-analysis. Int J Med Microbiol. 2021;311(3) doi: 10.1016/j.ijmm.2021.151483. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell C.M., Mazzoni C., Hogstrom L. Delivery Mode Affects Stability of Early Infant Gut Microbiota. Cell Rep Med. 2020;1(9) doi: 10.1016/j.xcrm.2020.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sevelsted A., Stokholm J., Bønnelykke K., Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–e98. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]
- 56.Darmasseelane K., Hyde M.J., Santhakumaran S., Gale C., Modi N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS One. 2014;9(2):e87896. doi: 10.1371/journal.pone.0087896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li H.T., Zhou Y.B., Liu J.M. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond) 2013;37(7):893–899. doi: 10.1038/ijo.2012.195. [DOI] [PubMed] [Google Scholar]
- 58.Martinez K.A., 2nd, Devlin J.C., Lacher C.R. Increased weight gain by C-section: Functional significance of the primordial microbiome. Sci Adv. 2017;3(10) doi: 10.1126/sciadv.aao1874. eaao1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mueller N.T., Bakacs E., Combellick J., Grigoryan Z., Dominguez-Bello M.G. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–117. doi: 10.1016/j.molmed.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahlqvist V.H., Persson M., Magnusson C., Berglind D. Elective and nonelective cesarean section and obesity among young adult male offspring: A Swedish population-based cohort study. PLoS Med. 2019;16(12) doi: 10.1371/journal.pmed.1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoang D.M., Levy E.I., Vandenplas Y. The impact of Caesarean section on the infant gut microbiome. Acta Paediatr. 2021;110(1):60–67. doi: 10.1111/apa.15501. [DOI] [PubMed] [Google Scholar]
- 62.Stinson L.F., Payne M.S., Keelan J.A. A Critical Review of the Bacterial Baptism Hypothesis and the Impact of Cesarean Delivery on the Infant Microbiome. Front Med (Lausanne) 2018;5:135. doi: 10.3389/fmed.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masukume G., O'Neill S.M., Baker P.N., Kenny L.C., Morton S.M.B., Khashan A.S. The Impact of Caesarean Section on the Risk of Childhood Overweight and Obesity: New Evidence from a Contemporary Cohort Study. Sci Rep. 2018;8(1):15113. doi: 10.1038/s41598-018-33482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai M., Loy S.L., Tan K.H. Association of Elective and Emergency Cesarean Delivery With Early Childhood Overweight at 12 Months of Age. JAMA Netw Open. 2018;1(7) doi: 10.1001/jamanetworkopen.2018.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Sullivan A., Farver M., Smilowitz J.T. The Influence of Early Infant-Feeding Practices on the Intestinal Microbiome and Body Composition in Infants. Nutr Metab Insights. 2015;8:1–9. doi: 10.4137/NMI.S29530. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laursen M.F., Larsson M.W., Lind M.V. Intestinal Enterococcus abundance correlates inversely with excessive weight gain and increased plasma leptin in breastfed infants. FEMS Microbiol Ecol. 2020;96(5) doi: 10.1093/femsec/fiaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirmiz N., Robinson R.C., Shah I.M., Barile D., Mills D.A. Milk Glycans and Their Interaction with the Infant-Gut Microbiota. Annu Rev Food Sci Technol. 2018;9:429–450. doi: 10.1146/annurev-food-030216-030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagström H., Rautava S., Ollila H. Associations between human milk oligosaccharides and growth in infancy and early childhood. Am J Clin Nutr. 2020;111(4):769–778. doi: 10.1093/ajcn/nqaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alderete T.L., Autran C., Brekke B.E. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am J Clin Nutr. 2015;102(6):1381–1388. doi: 10.3945/ajcn.115.115451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan J., Liu L., Zhu Y., Huang G., Wang P.P. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267. doi: 10.1186/1471-2458-14-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horta B.L., Loret de Mola C., Victora C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):30–37. doi: 10.1111/apa.13133. [DOI] [PubMed] [Google Scholar]
- 72.Maessen S.E., Derraik J.G.B., Binia A., Cutfield W.S. Perspective: Human Milk Oligosaccharides: Fuel for Childhood Obesity Prevention? Adv Nutr. 2020;11(1):35–40. doi: 10.1093/advances/nmz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Korpela K., Salonen A., Hickman B. Fucosylated oligosaccharides in mother's milk alleviate the effects of caesarean birth on infant gut microbiota. Sci Rep. 2018;8(1):13757. doi: 10.1038/s41598-018-32037-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tonon K.M., Morais T.B., Taddei C.R. Gut microbiota comparison of vaginally and cesarean born infants exclusively breastfed by mothers secreting α1-2 fucosylated oligosaccharides in breast milk. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Forbes J.D., Azad M.B., Vehling L. Association of Exposure to Formula in the Hospital and Subsequent Infant Feeding Practices With Gut Microbiota and Risk of Overweight in the First Year of Life. JAMA Pediatr. 2018;172(7) doi: 10.1001/jamapediatrics.2018.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ho N.T., Li F., Lee-Sarwar K.A. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(1):4169. doi: 10.1038/s41467-018-06473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pearce J., Taylor M.A., Langley-Evans S.C. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond) 2013;37(10):1295–1306. doi: 10.1038/ijo.2013.99. [DOI] [PubMed] [Google Scholar]
- 78.Ayonrinde O.T., Oddy W.H., Adams L.A. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol. 2017;67(3):568–576. doi: 10.1016/j.jhep.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 79.Differding M.K., Benjamin-Neelon S.E., Hoyo C., Østbye T., Mueller N.T. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020;20(1):56. doi: 10.1186/s12866-020-01723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Korpela K., Salonen A., Virta L.J. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. 2016;7:10410. doi: 10.1038/ncomms10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Korpela K., Salonen A., Saxen H. Antibiotics in early life associate with specific gut microbiota signatures in a prospective longitudinal infant cohort. Pediatr Res. 2020;88(3):438–443. doi: 10.1038/s41390-020-0761-5. [DOI] [PubMed] [Google Scholar]
- 82.Miller S.A., Wu R.K.S., Oremus M. The association between antibiotic use in infancy and childhood overweight or obesity: a systematic review and meta-analysis. Obes Rev. 2018;19(11):1463–1475. doi: 10.1111/obr.12717. [DOI] [PubMed] [Google Scholar]
- 83.Saari A., Virta L.J., Sankilampi U., Dunkel L., Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135(4):617–626. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 84.Vrieze A., Out C., Fuentes S. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60(4):824–831. doi: 10.1016/j.jhep.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 85.Uzan-Yulzari A., Turta O., Belogolovski A. Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization. Nat Commun. 2021;12(1):443. doi: 10.1038/s41467-020-20495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korpela K., Salonen A., Virta L.J., Kekkonen R.A., de Vos W.M. Association of Early-Life Antibiotic Use and Protective Effects of Breastfeeding: Role of the Intestinal Microbiota. JAMA Pediatr. 2016;170(8):750–757. doi: 10.1001/jamapediatrics.2016.0585. [DOI] [PubMed] [Google Scholar]
- 87.Dierikx T.H., Visser D.H., Benninga M.A. The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: A systematic review. J Infect. 2020;81(2):190–204. doi: 10.1016/j.jinf.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Coker M.O., Hoen A.G., Dade E. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG. 2020;127(2):217–227. doi: 10.1111/1471-0528.15799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poole N.M., Shapiro D.J., Fleming-Dutra K.E., Hicks L.A., Hersh A.L., Kronman M.P. Antibiotic Prescribing for Children in United States Emergency Departments: 2009-2014. Pediatrics. 2019;143(2) doi: 10.1542/peds.2018-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leva N.V., Copp H.L. An End in Sight: Shorter Duration of Parenteral Antibiotics in Neonates. Pediatrics. 2019;144(3) doi: 10.1542/peds.2019-1611. [DOI] [PubMed] [Google Scholar]
- 91.Dawson-Hahn E.E., Rhee K.E. The association between antibiotics in the first year of life and child growth trajectory. BMC Pediatr. 2019;19(1):23. doi: 10.1186/s12887-018-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aversa Z., Atkinson E.J., Schafer M.J. Association of Infant Antibiotic Exposure With Childhood Health Outcomes. Mayo Clin Proc. 2021;96(1):66–77. doi: 10.1016/j.mayocp.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stinson L.F. Establishment of the early-life microbiome: a DOHaD perspective. J Dev Orig Health Dis. 2020;11(3):201–210. doi: 10.1017/S2040174419000588. [DOI] [PubMed] [Google Scholar]
- 94.Vael C., Verhulst S.L., Nelen V., Goossens H., Desager K.N. Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathog. 2011;3(1):8. doi: 10.1186/1757-4749-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kalliomäki M., Collado M.C., Salminen S., Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 96.Korpela K., Renko M., Vänni P. Microbiome of the first stool and overweight at age 3 years: A prospective cohort study. Pediatr Obes. 2020;15(11):e12680. doi: 10.1111/ijpo.12680. [DOI] [PubMed] [Google Scholar]
- 97.Zhu Q, Huang S, Gonzalez A. OGUs enable effective, phylogeny-aware analysis of even shallow metagenome community structures. bioRxiv 2021: 2021.04.04.438427.
- 98.Gu C., Kim G.B., Kim W.J., Kim H.U., SY L.e.e. Current status and applications of genome-scale metabolic models. Genome Biol. 2019;20(1):121. doi: 10.1186/s13059-019-1730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun L., Ma L., Ma Y., Zhang F., Zhao C., Nie Y. Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell. 2018;9(5):397–403. doi: 10.1007/s13238-018-0546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koh A., Molinaro A., Ståhlman M. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell. 2018;175(4):947–961. doi: 10.1016/j.cell.2018.09.055. e17. [DOI] [PubMed] [Google Scholar]
- 101.Serena C., Ceperuelo-Mallafré V., Keiran N. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018;12(7):1642–1657. doi: 10.1038/s41396-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez de Agüero M., Ganal-Vonarburg S.C., Fuhrer T. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351(6279):1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 103.Lotz M., Gütle D., Walther S., Ménard S., Bogdan C., Hornef M.W. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203(4):973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cani P.D., Amar J., Iglesias M.A. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 105.Mulligan C.M., Friedman J.E. Maternal modifiers of the infant gut microbiota: metabolic consequences. J Endocrinol. 2017;235(1) doi: 10.1530/JOE-17-0303. R1-R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fouhy F., Guinane C.M., Hussey S. High-throughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother. 2012;56(11):5811–5820. doi: 10.1128/AAC.00789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chilton P.M., Embry C.A., Mitchell T.C. Effects of Differences in Lipid A Structure on TLR4 Pro-Inflammatory Signaling and Inflammasome Activation. Front Immunol. 2012;3:154. doi: 10.3389/fimmu.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chambers E.S., Preston T., Frost G., Morrison D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7(4):198–206. doi: 10.1007/s13668-018-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cani P.D., Neyrinck A.M., Fava F. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 110.Grill J.P., Manginot-Dürr C., Schneider F., Ballongue J. Bifidobacteria and probiotic effects: action of Bifidobacterium species on conjugated bile salts. Curr Microbiol. 1995;31(1):23–27. doi: 10.1007/BF00294629. [DOI] [PubMed] [Google Scholar]
- 111.Sjögren Y.M., Tomicic S., Lundberg A. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39(12):1842–1851. doi: 10.1111/j.1365-2222.2009.03326.x. [DOI] [PubMed] [Google Scholar]
- 112.Henrick B.M., Hutton A.A., Palumbo M.C. Elevated Fecal pH Indicates a Profound Change in the Breastfed Infant Gut Microbiome Due to Reduction of Bifidobacterium over the Past Century. mSphere. 2018;3(2) doi: 10.1128/mSphere.00041-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Casaburi G., Duar R.M., Brown H. Metagenomic insights of the infant microbiome community structure and function across multiple sites in the United States. Sci Rep. 2021;11(1):1472. doi: 10.1038/s41598-020-80583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El Hage R., Hernandez-Sanabria E., Calatayud Arroyo M., Props R., Van de Wiele T. Propionate-Producing Consortium Restores Antibiotic-Induced Dysbiosis in a Dynamic in vitro Model of the Human Intestinal Microbial Ecosystem. Front Microbiol. 2019;10:1206. doi: 10.3389/fmicb.2019.01206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Di Ciaula A., Garruti G., Lunardi Baccetto R. Bile Acid Physiology. Ann Hepatol. 2017;16 doi: 10.5604/01.3001.0010.5493. (Suppl. 1: s3-105.): s4-s14. [DOI] [PubMed] [Google Scholar]
- 116.Molinero N., Ruiz L., Sánchez B., Margolles A., Delgado S. Intestinal Bacteria Interplay With Bile and Cholesterol Metabolism: Implications on Host Physiology. Front Physiol. 2019;10:185. doi: 10.3389/fphys.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wei M., Huang F., Zhao L. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.van Best N., Rolle-Kampczyk U., Schaap F.G. Bile acids drive the newborn's gut microbiota maturation. Nat Commun. 2020;11(1):3692. doi: 10.1038/s41467-020-17183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tanaka M., Sanefuji M., Morokuma S. The association between gut microbiota development and maturation of intestinal bile acid metabolism in the first 3 y of healthy Japanese infants. Gut Microbes. 2020;11(2):205–216. doi: 10.1080/19490976.2019.1650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butler É.M., Derraik J.G.B., Taylor R.W., Cutfield W.S. Prediction Models for Early Childhood Obesity: Applicability and Existing Issues. Horm Res Paediatr. 2018;90(6):358–367. doi: 10.1159/000496563. [DOI] [PubMed] [Google Scholar]
- 121.Stanislawski MA, Dabelea D, Wagner BD. Gut Microbiota in the First 2 Years of Life and the Association with Body Mass Index at Age 12 in a Norwegian Birth Cohort. mBio 2018; 9(5). [DOI] [PMC free article] [PubMed]
- 122.Gonze D., Coyte K.Z., Lahti L., Faust K. Microbial communities as dynamical systems. Curr Opin Microbiol. 2018;44:41–49. doi: 10.1016/j.mib.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 123.Sundarraman D., Hay E.A., Martins D.M., Shields D.S., Pettinari N.L., Parthasarathy R. Higher-Order Interactions Dampen Pairwise Competition in the Zebrafish Gut Microbiome. mBio. 2020;11(5) doi: 10.1128/mBio.01667-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de Muinck E.J., Lundin K.E.A., Trosvik P. Linking Spatial Structure and Community-Level Biotic Interactions through Cooccurrence and Time Series Modeling of the Human Intestinal Microbiota. mSystems. 2017;2(5) doi: 10.1128/mSystems.00086-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nilsson A., Mardinoglu A., Nielsen J. Predicting growth of the healthy infant using a genome scale metabolic model. NPJ Syst Biol Appl. 2017;3:3. doi: 10.1038/s41540-017-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Derrien M., Alvarez A.S., de Vos W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019;27(12):997–1010. doi: 10.1016/j.tim.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 127.Chattopadhyay M.K. Use of antibiotics as feed additives: a burning question. Front Microbiol. 2014;5:334. doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gough E.K., Moodie E.E., Prendergast A.J. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348 doi: 10.1136/bmj.g2267. g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cani P.D., Van Hul M., Lefort C., Depommier C., Rastelli M., Everard A. Microbial regulation of organismal energy homeostasis. Nat Metab. 2019;1(1):34–46. doi: 10.1038/s42255-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 130.Davis E.C., Dinsmoor A.M., Wang M., Donovan S.M. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig Dis Sci. 2020;65(3):706–722. doi: 10.1007/s10620-020-06092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luoto R., Kalliomäki M., Laitinen K., Isolauri E. The impact of perinatal probiotic intervention on the development of overweight and obesity: follow-up study from birth to 10 years. Int J Obes (Lond) 2010;34(10):1531–1537. doi: 10.1038/ijo.2010.50. [DOI] [PubMed] [Google Scholar]
- 132.Chorell E., Karlsson Videhult F., Hernell O., Antti H., West C.E. Impact of probiotic feeding during weaning on the serum lipid profile and plasma metabolome in infants. Br J Nutr. 2013;110(1):116–126. doi: 10.1017/S0007114512004618. [DOI] [PubMed] [Google Scholar]
- 133.Karlsson Videhult F., Öhlund I., Stenlund H., Hernell O., West C.E. Probiotics during weaning: a follow-up study on effects on body composition and metabolic markers at school age. Eur J Nutr. 2015;54(3):355–363. doi: 10.1007/s00394-014-0715-y. [DOI] [PubMed] [Google Scholar]
- 134.Koleva P.T., Bridgman S.L., Kozyrskyj A.L. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. 2015;7(4):2237–2260. doi: 10.3390/nu7042237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Leong K.S.W., Jayasinghe T.N., Wilson B.C. Effects of Fecal Microbiome Transfer in Adolescents With Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.30415. e2030415. [DOI] [PMC free article] [PubMed] [Google Scholar]