Abstract
Marine microbes are potential source for novel metabolites. They are efficient in producing these metabolites utilizing agrowastes. Protease is one of the enzymes which find wide industrial applications. In the present study, protease producing bacteria was isolated from marine sediments and the organism was identified as Bacillus halodurans. The organism was subjected to protease production under solid state fermentation (SSF) using different agrowastes as substrates. Among the substrates used, wheat bran yielded maximum quantity of protease. The fermentation process was carried out under different cultural conditions to optimize the parameters influencing the enzyme production. The results of the stain removal studies by the enzyme revealed the increased efficiency of the microbial enzyme than the commercial detergent.
Keywords: Sold state fermentation, Agrowastes, Protease, Stain removal
1. Introduction
The extromophiles are organisms found in diverse and extreme environmental conditions and are noteworthy for the yield of compounds with bioactive principles which are prominent in various biotechnological applications (Kumar and Takagi, 1999). The extraction of enzymes with novel activity from the extremophiles provide the catalysts for the manufacturing of chemicals in various industries such as textile industry, pharmaceutical industry, paper industry and food industry so as to mitigate the pollution problem (Jani et al., 2012). The marine microbial strains because they survive in extreme environmental conditions possess some of the peculiar adaptations to overcome extreme saline, temperature, pressure etc and thus capable of yielding distinctive proteins with novel potential. The bioactive components present in these organisms may not found in their terrestrial counterparts (Shanmugapriya et al., 2008, Sarkar et al., 2010). Singh et al. (2017) stated that the screened microorganisms are cultured in fermenters under optimized media compositions in order to produce the industrially significant products. Microbial protease is an economically significant enzyme because they exhibit wide range of applications in different industries (Kandasamy et al., 2016, Das and Prasad, 2010). Gupta et al., 2002, Moo-young and Chisti, 1994 have documented production of protease by various bacterial strains of marine origin.
The enzyme alkaline protease is composed of 60 – 65% of global enzyme market (Zanphorlin et al., 2010, Widsten and Kandelbauer, 2008). Among the bacterial strains, species of Bacillus are notable producer of bioactive compounds in industrial sector. Several species of Bacillus represent both terrestrial as well as marine environments (Pham et al., 2019, Sekhon, 2010). B. subtilis is largely exploited for the yield of biochemicals of different industrial applications especially as contact lens cleaner as well as the effective remover of necrotic material of skin ulceration (Sjodahl et al., 2002, Chi et al., 2007). The solid state fermentation is a potential tool for the production industrially important compounds through bioconversion of nutrient rich agrowastes to value-added products (Esakkiraj et al., 2011). In this study, marine sediment sample was screened for bacterial strains producing protease. The isolated strain was characterized and the results of the production of protease under different conditions in SSF with agrowastes are presented and discussed.
2. Materials and methods
2.1. Screening of bacterial strains
The experimental sediment sample was collected from the Kanniyakumari coast, Southern Tamil Nadu, India in sterilized containers and transported to the laboratory under aseptic conditions. The sediment sample was serially diluted and were plated on the skim milk agar containing casein enzymic hydrolysate 5 g; yeast extract 2.5 g; dextrose 1 g; skim milk powder 28 g; agar 15 g in 1 L of water and with pH 7. Then, the plates were incubated at 37 °C for 24 hrs. After incubation, the colonies with clear zone clearance due to the hydrolysis of casein were selected. The CFU shown maximum zone of hydrolysis was selected for further studies.
2.2. Characterization of the strain
The selected strain was grown on Zobell marine agar to study the colony characteristics. Gram staining, various biochemical tests recommended by Smibert and Krieg (1994) were carried out and Bergy’s Manual of Determinative Bacteriology (Bergey et al., 1984) was referred for the biochemical characterization of the isolate. Additionally, the genomic identification was done on the basis of 16S rRNA gene sequencing technique. Genomic content of the candidate organism was purified (Redburn and Patel, 1993) for the gene amplification. The following primers P1 (5′-AGAGTTTGATCATCCTGGCTCAG-3′) and P2 (5′-ACGGCTACCTTGTTACGACTT3′) were used for the gene amplification of the candidate organism (Edwards et al., 1989). Initial denaturation at 94 °C for 3 min followed by 35 cycles of 94 °C for 30 s, 51 °C for 30 s, 72 °C for 3 min and final extension at 72 °C for 10 min for the amplification was followed. Sequence alignment of the candidate strain was accomplished by BLAST program and the multiple sequence alignment program CLUSTAL-W was used to align the 16S rRNA gene sequence.
2.3. Substrate pre-treatment
Agro wastes such as paddy husk, rice bran, paddy straw, fruit waste, vegetable waste, wheat bran and saw dust were tested for the substrate suitability in the production of proteinase. These substrates were collected from the local market, Madurai and were washed thoroughly to remove dirt and fungal spores. The substrates were pulverized to fine powder and treated by the addition of 1% (w/v) sodium hydroxide solution for 1 h. Then the materials were washed with distilled water to attain neutral pH and kept in room temperature for dehydration. Then the materials were autoclaved at 121 °C for an hour.
2.4. Solid state fermentation (SSF) process
Five grams of wheat brawn was taken in a 250 ml Erlenmeyer flask, moistened with salt solution (% w/v calcium chloride 0.1, magnesium sulphate 0.2) to achieve the desired moisture content. The contents were sterilized at 121 °C for 15mts and cooled. The sterilized substrate was inoculated with 1 ml of 24 hrs old isolated bacterial culture and incubated. After fermentation, crude extract was obtained from the culture medium by centrifugation which served as enzyme source (Kalaiarasi and Sunitha, 2009, Sumantha et al., 2006). The same procedure was adopted for all the substrates.
2.5. Assay of protease
The protease activity of cell free supernatant was carried out by the method given by Keay and Wildi (1970). 1 ml of 2% casein and 1 ml of enzyme source was mixed with tris buffer (1. 25 ml) and incubated at 35 °C for 20 min. The reaction was terminated by the addition of 2 ml of 0.4 M trichloroacetic acid and after the incubation, the reaction mixture was filtered through Whatman No.1 filter paper. To 1 ml of cell free content, 5 ml of sodium bicarbonate (0.4 M) and 1 ml of Folin phenol reagent (0.5 M) were added and incubated at 35 °C for 15 min. OD was made at 660 nm by using spectrophotometer. Amount of protease production was evaluated with the help of tyrosin standard graph.
2.6. Production optimization
In order to optimize the enzyme production, the fermentation process was carried out under SSF using various agrwastes (paddy husk, rice bran, paddy straw, fruit waste, vegetable waste, wheat bran and saw dust) with different experimental conditions such as various pH (5–10), temperature (10–60 °C), carbon as well as nitrogen sources (1%), sodium chloride concentrations (2–6%). Different pH was maintained using appropriate buffers; 0.1 M citrate buffer (pH 4–5), 0.2 M phosphate (pH 6–8) and phosphate-NaOH buffer (pH 8–11). The various carbon sources such as fructose, lactose, sucrose, cellulose, galactose, rhamnose and maltose were tested for their impact on enzyme yield. Urea, ammonium sulphate, ammonium nitrate, sodium nitrate, yeast extract, peptone, beef extract and skim milk powder were provided to assess the influence of nitrogen source on the yield of protease.
2.7. Influence of moisture content
Impact of moisture content on protease production was evaluated by adjusting the water contents of the fermentation media to 40, 50, 60, 70 and 80%.
2.8. Impact of metal ions
Impact of different metal ions on protease productions from the candidate species was studied by the addition of metal ions such as manganese chloride, calcium chloride, magnesium sulphate, copper sulphate, and zinc sulphate to the fermentation medium.
2.9. Washing performance of enzyme source
The washing performance of the enzyme was determined by adopting the procedure described by Adinarayana et al. (2003) with slight modifications. Pieces of white cotton cloths of 5 cm2 size were stained with blood and treated under the following conditions.
-
1.
Petri plate with distilled water (20 ml) + stained material.
-
2.
Petri plate with distilled water (20 ml) + stained material + 1 ml of commercial washing powder at 5 mg/ml.
-
3.
Petri plate with distilled water (20 ml) + stained cloth + 2 ml of enzyme solution
The above plates were properly maintained at 40 °C for 30 min. After incubation, the stained material was cleaned with water and dried. Visual examination was carried out to assess the stain removal activity of the enzyme. Blood stained untreated cloth piece was considered as control.
3. Results
3.1. Screening of protease producing organism
The inoculation of serially diluted marine sediment sample resulted in four morphologically distinct colonies. Then the colonies were screened for enzyme producing ability on skim milk agar by observing zone of clearance due to the hydrolysis of casein (Fig. 1). Among the four different isolates, one which produced maximum zone of clearance was subjected to further experimental work.
Fig. 1.

Zone of clearance produced by Bacillus halodurans on skim milk agar indicating the proteolytic activity.
3.2. Biochemical and molecular characterization of the isolate
The biochemical characteristic of the isolate is given in Table 1. The 16S rRNA gene of the isolate was amplified by PCR using the aforementioned primers and the strands were sequenced. The biochemical characteristics and the molecular sequencing revealed the organisms was phylogenetically related with B. halodurans and the sequence was submitted in Gen Bank, NCBI (Accession no. MK829640.1).
Table 1.
Biochemical characterization of Bacillus halodurans isolated from marine sediment.
| S.NO | Test | Result |
|---|---|---|
| 1 | Gram staining | + |
| 2 | Indole test | _ |
| 3 | MR test | + |
| 4 | VP test | _ |
| 5 | Catalase test | _ |
| 6 | Oxidase test | + |
| 7 | Citrate test | + |
| 8 | TSI test | + |
3.3. Optimization of protease production
The impact of different cultural conditions on the production of protease by the isolate B. halodurans was tested to optimize the production of the enzyme.
3.4. Effect of various waste substrates
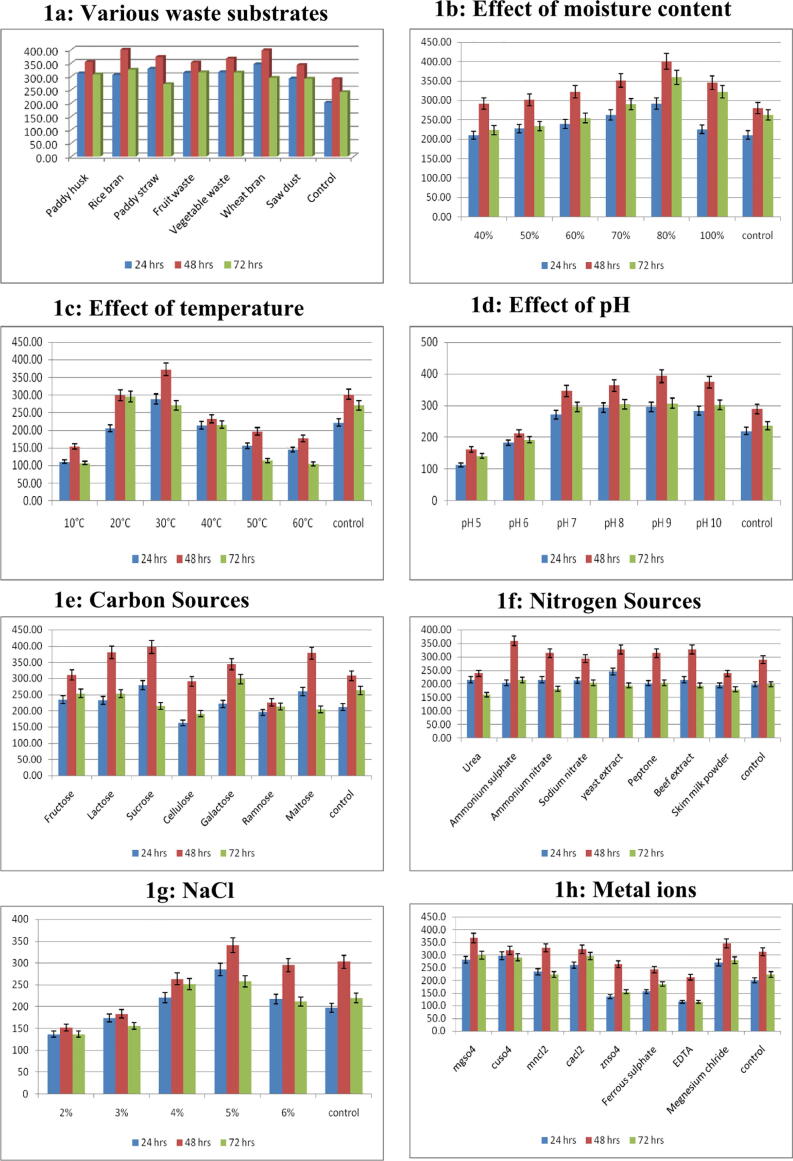
The impact of various waste substrates on protease production by B.s halodurans was carried out (Fig. 2a). Among the used substrates, the maximum quantity of enzyme yield (397.09 U/ml) and least (291.09 U/ml) were recorded in medium supplemented with wheat bran and saw dust respectively at 48 h of incubation.
Fig. 2.
Optimization of protease production (U/ml) with different parameters by Bacillus halodurans.
3.5. Effect of moisture content
Moisture is an essential parameter for SSF process. Therefore, the enzyme production was observed under different moisture content, for the strain B. halodurans, the optimal moisture content was 80% and activity was recorded at 401.18 U/ml (Fig. 2b).
3.6. Effect of temperature
The effect of temperature on the production of protease by B. halodurans was determined by fermenting at different temperatures ranging from 10 and 50 °C. The maximum quantity of protease production was obtained at 30 °C and it was 372.73 U/ml at 48 h of incubation. At the same time, the least amount of production was registered at 10 °C at 48 h of incubations (Fig. 2c).
3.7. Effect of pH
To study the impact of pH on the production of the enzyme, the production was quantified by incubating the fermentation process under different pH range. Among different pH tested, the maximum yield was recorded at pH 9 (393.538 U/ml) at 48 h and least production in pH 5 (Fig. 2d).
3.8. Effect of carbon sources
The impact of carbon source on the protease yield was studied by incubating culture with the supplement of different carbon sources. The maximum yield (387.64 U/ml) was recorded in sucrose supplemented medium at 48 hrs (Fig. 2e).
3.9. Effect of nitrogen sources
The influence of nitrogen source on the protease production was assessed by incubating the culture in medium containing different nitrogen sources. Among the tested sources, the maximum yield (359.27 U/ml) was recorded in medium containing ammonium sulphate at 48 h incubation and the least production was recorded in urea added medium (Fig. 2f).
3.10. Effect of NaCl
The effect of NaCl on enzyme production was determined by incubating the culture in medium added with different concentrations of NaCl. Among the tested concentration, 5% of NaCl maximized the production of protease enzyme at 48 h incubation (340.50 U/ml) and low level production was recorded in the medium containing 2% NaCl (Fig. 2g).
3.11. Effect of metal ions
Different metal ions namely magnesium sulphate, copper sulphate, manganese chloride, calcium chloride and zinc sulphate were amended to the production medium to determine the impact of metal ions. Among the tested metal ions, the magnesium sulphate yielded 366.9 U/ ml of protease at 48 h of incubation. The amendment of Zinc sulphate has yielded low quantity of the enzyme (Fig. 2h).
3.12. Stain removal
The protease enzyme produced by the marine isolate B. halodurans was tested for its suitability as an additive agent by subjecting blood stained cloth. The clearing efficacy of the enzyme was found to be higher (Fig. 3) when compared to the treatment with commercial powder.
Fig. 3.
Application of protease produced by Bacillus halodurans on the removal of blood stain.
4. Discussion
Proteases are one of the enzymes which find wide applications in industries. Among the microorganisms of different origin, marine microbes are competitive candidates with novel metabolites. Four different bacterial strains producing protease enzyme were isolated from marine sediment. Among the isolates, one strain was selected for further studies based on the maximum zone of clearance. The cultural, biochemical characteristics and 16S rRNA sequencing revealed that the isolate was B. halodurans. The sequence was deposited in Gen Bank, NCBI with Accession No. MK829640.1. Several Bacillus strains such as B. licheniformis, B. subtilis, B amyloliquifaciens, and B. mojavensis have been identified as potential sources for the protease production (Gupta et al., 2002). Different marine Bacillus species were also reported to produce protease (Yang et al., 2000, Olajuyigbe and Ogunyewo, 2013).
In the present study, agrowastes were used as substrates under SSF for the production of the enzyme protease. Esakkiraj et al. (2011) have utilized biowastes for the production of alkaline protease. Among the agrowastes tested wheat bran has yielded maximum protease in the present study. Utilizing wheat bran as substrate, Anandan et al. (2007) investigated the protease production by Aspergillus tamari under the SSF. Mahanta et al. (2008) also reported maximum amount of enzyme yield in wheat bran than other sources. Sumantha et al. (2006) have used rice bran as substrates for the SSF of protease enzyme. Oliveira et al. (2006) investigated that the Penicillium janthinellum CRC 87 and M-11S was able to produce protease under the SSF using agro residues.
The moisture content of the medium is important for the solid state fermentation of protease production. In our work, Bacillus halodurans has produced more enzyme (401.18 U/ml) under 85% moisture content among the other tested moisture conditions. The present study is supported by the earlier work of Esakkiraj et al. (2011) who recorded maximum protease yield in 75% of moisture by S. proteomaculans. Anandan et al., 2007, Mahanta et al., 2008 have reported 65% and 66.7% respectively as optimum moisture level.
The production of protease is chiefly affected by the factors such as pH and temperature in most of the organisms. Bajaj et al. (2013) stated that the secretion of protease varies in microorganisms and it is dependent on environmental factors. Prakash et al. (2011) reported that these factors are most probably inducing the production of enzyme by microbial isolate. In this work, the Bacillus halodurans was experimented for the maximizing the enzyme yield by optimizing pH as well as incubation temperature. The maximum protease production was recorded at pH 9 as the strain belongs to marine environment. Likewise, optimal protease yield was recorded at 40 °C from the candidate strain Bacillus halodurans. Similarly, Yanga et al. (2000) reported pH 8.0 as optimum for the secretion of more protease enzyme by Bacillus subtilis. Some of the earlier studies have quoted pH 9.0 and 37 °C as suitable pH and temperature for the optimal enzyme yield from the Bacillus subtilis (Rao et al., 1998, Qureshi et al., 2011).
Many researchers have investigated the effect different carbon sources on the enzyme secretion and the growth of bacterial strains (Gupta et al., 2002, Chi and Zhao, 2003). In our study, the maximum amount of protease yield was recorded in sucrose supplemented medium. Increased yield of alkaline protease production by the media supplemented with various carbon sources was examined by Malathis and Chakraborty (1991).
do Nascimento and Martins (2004) investigated the effect of nitrogen sources for the protease production and the optimal yield was registered with ammonium nitrate supplemented media when compared with other sources. In our study, the highest amount of protease yield was obtained in ammonium sulphate added medium. Similar observation was noticed by Sethi et al. (2013). They have reported that ammonium sulphate had induced the more enzyme production than the other sources.
The present study revealed that the Bacillus halodurans showed maximum production of protease in the medium augmented with magnesium sulfate as a metal ion source. Likewise, Mei and Jiang (2005) reported that barium chloride, manganese sulphate and magnesium chloride have enhanced protease production by Vibrio metschnikovii DL. The addition of metal ions like mercuric chloride and zinc chloride completely inhibited the protease production by the species of Baicllus (Maruthiah et al., 2014). Some of the earlier researchers have cited the influence of Ca2+ and Mg2+ ions in maximization of enzyme yield (Feng et al., 2001, Kalaiarasi and Sunitha, 2009).
Some of the Halomonas strain was able to grow in high saline conditions for their development or metabolic activity (Jiang et al., 2007). Halomonas salifordinae grew in the wide range of salt conditions (0.5–20%). Sankaralingam et al. (2017) studied protease production by Bacillus sonorensis BH3 using various concentrations of salinity and observed that maximum protease production at 3% NaCl supplemented medium in 48 hrs incubation. The marine bacterium Roseobacter sp. absolutely requires 3.0% of NaCl concentration for the growth and protease production (Shaheen et al., 2008). In the present study high yield by Bacillus halodurans was registered in 5% NaCl supplemented medium.
Protease are often tested for their employability as additives in cleaning the stains (Choudhary, 2012, Mothe and Sultanpuram, 2016). The experimental results on the washing performance of the protease secreted by Bacillus halodurans in removing the stains indicated that the enzyme showed better clearance of the blood stain than the commercial detergents. Banerjee et al. (1999) have removed blood stains using the enzyme produced by Bacillus brevis. Removal stain within 20 min by the purified proteinase was observed by Sathishkumar et al. (2014). The purification of the enzyme protease produced by Bacillus halodurans and further studies on the efficacy of the enzyme on the removal of various stains will reveal the potential of this enzyme for commercial application.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Authors gratefully acknowledge MHRD, Govt. of India for financial assistance and laboratory facilities through National Centre of Excellence, Thiagarajar College under FAST Scheme. The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group NO (RGP-271).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Chellapandian Balachandran, Email: bchandruji@gmail.com.
Kathirvelu Baskar, Email: suribaskar@hotmail.com.
References
- Adinarayana K., Ellaiah P., Prasad D.S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS Pharm. Sci. Tech. 2003;4(4):440–448. doi: 10.1208/pt040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandan D., Marmer W.N., Dudley R.L. Isolation, characterization and optimization of culture parameters for production of an alkaline protease isolated from Aspergillus tamari. J. Ind. Microbiol. Biotechnol. 2007;34(5):339–347. doi: 10.1007/s10295-006-0201-5. [DOI] [PubMed] [Google Scholar]
- Bajaj B.K., Sharma N., Singh S. Enhanced production of fibrinolytic protease from Bacillus cereus NS-2 using cotton seed cake as nitrogen source. Biocatal. Agric. Biotechnol. 2013;2(3):204–209. doi: 10.1016/j.bcab.2013.04.003. [DOI] [Google Scholar]
- Banerjee U.C., Sani R.K., Azmi W., Soni R. Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry additive. Proc. Biochem. 1999;35(1–2):213–219. doi: 10.1016/S0032-9592(99)00053-9. [DOI] [Google Scholar]
- Bergey, D.H., Krieg, N.R., Holt, J.G., 1984. Bergey's Manual of Systematic Bacteriology. Published, Baltimore, M.D., Williams and Wilkins, London.
- Chi Z., Ma C., Wang P., Li H.F. Optimization of medium and cultivation conditions for alkaline protease production by the marine yeast Aureobasidium pullulans. Bioresour. Technol. 2007;98(3):534–538. doi: 10.1016/j.biortech.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Chi Z., Zhao S. Optimization of medium and cultivation conditions for pullulan production by a new pullulan-producing yeast strain. Enzyme Microb. Technol. 2003;33(2–3):206–211. doi: 10.1016/S0141-0229(03)00119-4. [DOI] [Google Scholar]
- Choudhary V. Compatibility with commercial detergents and stain removal capability of Aspergillus versicolor protease. J. Acad. Indus. Res. 2012;1(6):301–305. [Google Scholar]
- Das G., Prasad M.P. Isolation, purification & mass production of protease enzyme from Bacillus subtilis. Int. Res. J. Microbiol. 2010;1(2):26–31. [Google Scholar]
- Do Nascimento W.C.A., Martins M.L.L. Production and properties of an extracellular protease from thermophilic Bacillus sp. Braz. J. Microbiol. 2004;35(1–2):91–96. [Google Scholar]
- Edwards U., Rogall T., Blocker H., Emde M., Bottger E.C. Isolation and direct complete nucleotide determination of entire genes: characterization of gene coding for 16S rRNA. Nucleic Acids Res. 1989;17(19):7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esakkiraj P., Sankaralingam S., Usha R., Palavesam A., Immanuel G. Solid-state protease production using anchovy waste meal by moderate halophile Serratia proteamaculans AP-CMST isolated from fish intestine. Ann. Microbiol. 2011;61:749–755. doi: 10.1007/s13213-010-0191-4. [DOI] [Google Scholar]
- Feng Y., Yang W., Ong S., Hu J., Ng W. Fermentation of starch for enhanced alkaline protease production by constructing an alkalophilic Bacillus pumilus strain. Appl. Microbiol. Biotechnol. 2001;57:153–160. doi: 10.1007/s002530100765. [DOI] [PubMed] [Google Scholar]
- Gupta R., Beg Q.K., Lorenz P. Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 2002;59(1):15–32. doi: 10.1007/s00253-002-0975-y. [DOI] [PubMed] [Google Scholar]
- Jani S.A., Chudasama C.J., Patel D.B., Bhat P.S., Patel H.N. Optimization of extracellular protease production from alkali thermo tolerant Actinomycetes: Saccharomonospora viridis SJ-21. Bullet. Environ. Pharmacol. Life Sci. 2012;1(6):84–92. [Google Scholar]
- Jiang J.J., Zeng Q.X., Zhu Z.W., Zhang L.Y. Chemical and sensory changes associated Yu-lu fermentation process-A traditional Chinese fish sauce. Food Chem. 2007;104(4):1629–1634. doi: 10.1016/j.foodchem.2007.03.024. [DOI] [Google Scholar]
- Kalaiarasi K., Sunitha P.U. Optimization of alkaline protease production from Pseudomonas fluorescens isolated from meat waste contaminated soil. Afr. J. Biotechnol. 2009;8(24):7035–7041. [Google Scholar]
- Kandasamy S., Muthusamy G., Balakrishnan S., Duraisamy S., Thangasamy S., Seralathan K.K., Chinnappan S. Optimization of protease production from surface-modified coffee pulp waste and corncobs using Bacillus sp. by SSF. 3. Biotech. 2016;6(167):11. doi: 10.1007/s13205-016-0481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay L., Wildi B.S. Proteinases of the genus Bacillus. I. Neutral proteinases. Biotechnol Bioeng. 1970;12(2):179–212. doi: 10.1002/bit.260120205. [DOI] [PubMed] [Google Scholar]
- Kumar C.G., Takagi H. Microbial alkaline proteases: from a bioindustrial viewpoint. Biotechnol. Adv. 1999;17(7):561–594. doi: 10.1016/s0734-9750(99)00027-0. [DOI] [PubMed] [Google Scholar]
- Mahanta N., Gupta A., Khare S.K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa PseA in solid-state fermentation using Jatropha curcas seed cake as substrate. Bioresour. Technol. 2008;99(6):1729–1735. doi: 10.1016/j.biortech.2007.03.046. [DOI] [PubMed] [Google Scholar]
- Malathis S., Chakraborty Production of alkaline protease by a new Aspergillus flavus isolate under solid-substrate fermentation conditions for use as a depilation agent. Appl. Environ. Microbiol. 1991;57(3):712–716. doi: 10.1128/aem.57.3.712-716.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthiah T., Esakkiraj P., Immanuel G., Palavesam A. Alkaline serine protease from marine Bacillus flexus APCMST-RS2P purification and characterization. Curr. Biotechnol. 2014;3(3):238–243. doi: 10.2174/2213529401666140528002909. [DOI] [Google Scholar]
- Mei C., Jiang X. A novel surfactant and oxidation-stable alkaline protease from Vibrio metschnikovii DL 33–51. Proc. Biochem. 2005;40(6):2167–2172. doi: 10.1016/j.procbio.2004.08.007. [DOI] [Google Scholar]
- Moo-young M., Chisti Y. Biochemical engineering in biotechnology. Pure Appl. Chem. 1994;66(1):117–136. [Google Scholar]
- Mothe T., Sultanpuram V.R. Production, purification and characterization of a thermotolerant alkaline serine protease from a novel species Bacillus caseinilyticus. 3. Biotech. 2016;6(1):53. doi: 10.1007/s13205-016-0377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajuyigbe F.M., Ogunyewo O.A. Enhanced production and physicochemical properties of a commercially viable alkaline protease from Bacillus amyloliquefaciens PFB-01. Curr. Biotechnol. 2013;2(1):73–80. doi: 10.2174/2211550111302010012. [DOI] [Google Scholar]
- Oliveira L.A., Porto A.L.F., Tambourgi E.B. Production of xylanase and protease by Penicillium janthinellum CRC 87M–115 from different agricultural wastes. Bioresour. Technol. 2006;97(6):862–867. doi: 10.1016/j.biortech.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Pham J.V., Yilma M.A., Feliz A., Majid M.T., Maffetone N., Walker J.R., Kim E., Cho H.J., Reynolds J.M., Song M.C., Park S.R., Yoon Y.J. A review of the microbial production of bioactive natural products and biologics. Front. Microbiol. 2019;10:1404. doi: 10.3389/fmicb.2019.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Kannapiran E., Ramasubburayan R., Iyapparaj P., Ananthi S., Palavesam A., Immanuel G. Production and partial purification of protease by selected bacterial strains using raw milk as substrate. Malay. J. Microbiol. 2011;7(4):192–200. [Google Scholar]
- Qureshi A.S., Bhutto M.A., Khushk I., Dahot M.U. Optimization of cultural conditions for protease production by Bacillus subtilis EFRL 01. Afric. J. Biotechnol. 2011;10(26):5173–5181. doi: 10.5897/AJB09.1574. [DOI] [Google Scholar]
- Rao M.B., Tanksale A.M., Ghatge M.S., Deshpande V.V. Molecular and biotechnological aspects of microbial proteases. Microbiol. Molec. Biol. Rev. 1998;62(3):597–635. doi: 10.1128/mmbr.62.3.597-635.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redburn A.C., Patel B.K.C. Phylogenetic analysis of Desulfotomaculum thermobenzoicum using polymerase chain reaction-amplified 16S rRNA-specific DNA. FEMS Microbiol. Lett. 1993;113(1):81–86. doi: 10.1111/j.1574-6968.1993.tb06492.x. [DOI] [PubMed] [Google Scholar]
- Sankaralingam S., Harinathan B., Palpperumal S., Kathiresan D., Rajendran S., Shankar T., Prabhu D., Sivakumar N. Optimization of culture conditions for the production of halophilic protease by newly isolated Bacillus sonorensis. Am.-Eur. J. Agric. Environ. Sci. 2017;17(4):293–299. doi: 10.5829/idosi.aejaes.2017.293.299. [DOI] [Google Scholar]
- Sarkar S., Pramanik A., Mitra A., Mukherjee J. Bioprocessing data for the production of marine enzymes. Mar Drugs. 2010;8(4):1323–1372. doi: 10.3390/md8041323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar R., Ananthan G., Raghunathan C. Production and characterization of haloalkaline protease from ascidian associated Virgibacillus halodenitrificans RSK CASi using marine wastes. Ann. Microbiol. 2014;65:1481–1493. doi: 10.1007/s13213-014-0987-8. [DOI] [Google Scholar]
- Sekhon B.S. Food nanotechnology–an overview. Nanotechnol. Sci. Appl. 2010;3(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Datta A., Gupta B.L., Gupta S. Optimization of cellulase production from bacteria isolated from soil. ISRN Biotechnol. 2013:7. doi: 10.5402/2013/985685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen M., Shah A.A., Hameed A.A., Hasan F. Influence of culture conditions on production and activity of protease from Bacillus subtilis BS1. Pak. J. Bot. 2008;40(5):2161–2169. [Google Scholar]
- Shanmugapriya S., Krishnareni J., Selvin J., Gandhimathi R., Arunkumar M., Thangavelu T., Kiran S.G., Natarajaseenivasan K. Optimization of extracellular thermotolerant alkaline protease produced by marine Roseobacter Sp (MMD040) Bioprocess Biosyst. Eng. 2008;31(5):427–433. doi: 10.1007/s00449-007-0179-z. [DOI] [PubMed] [Google Scholar]
- Singh V., Haque S., Niwas R., Srivastava A., Pasupuleti M., Tripathi C.K. Strategies for fermentation medium optimization: an in-depth review. Front Microbiol. 2017;7:2087. doi: 10.3389/fmicb.2016.02087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodahl J., Emmer A., Vincent J., Roeraade J. Characterization of proteinases from Antarctic krill (Euphausia superba) Protein Expr. Purif. 2002;26(1):153–161. doi: 10.1016/S1046-5928(02)00519-3. [DOI] [PubMed] [Google Scholar]
- Smibert R.M., Krieg N.R. Phenotypic characterization. In: Gerhardt P., Murray R.G.E., Wood W.A., Krieg N.R., editors. Methods for General and Molecular Bacteriology. American Society for Microbiology; Washington, DC: 1994. pp. 607–654. [Google Scholar]
- Sumantha A., Deepa P., Sandhya C., Szakacs G., Soccol C.R., Pandey A. Rice bran as a substrate for proteolytic enzyme production. Braz. Arch. Biol. Technol. 2006;49(5):843–851. doi: 10.1590/S1516-89132006000600019. [DOI] [Google Scholar]
- Widsten P., Kandelbauer A. Laccase applications in the forest products industry: a review. Enzyme Microbial. Technol. 2008;42(4):293–307. doi: 10.1016/j.enzmictec.2007.12.003. [DOI] [Google Scholar]
- Yang J.K., Shih I.L., Tzeng Y.M., Wang S.L. Production and purification of protease from a Bacillus subtilis that can deproteinase crustacean wastes. Enzyme Microb. Technol. 2000;26(5–6):406–413. doi: 10.1016/S0141-0229(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Zanphorlin L.M., Facchini F.D.A., Vasconcelos F., Bonugli-Santos R.C., Rodrogues A., Sette L.D., Gomes E., Bonilla-Rodriguez G.O. Production, partial characterization, and immobilization in alginate beads of an alkaline protease from a new thermophilic fungus Myceliophthora sp. J. Microbiol. 2010;48:331–336. doi: 10.1007/s12275-010-9269-8. [DOI] [PubMed] [Google Scholar]