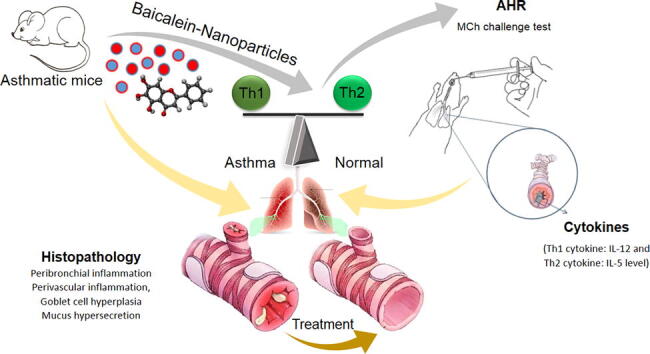
Graphical abstract
Keywords: Nano, Inflammation, Flavonoid, Airway
Abbreviations: AB, alcian blue; AHR, airway hyperresponsiveness; AP-1, activator protein 1; BALf, bronchoalveolar lavage fluid; BBB, blood–brain barrier; COX, cyclooxygenase; E-B-NP, encapsulated-Baicalein-nanoparticles; ELISA, the enzyme-linked immunosorbent assay; FT-IR, fourier-transform infrared spectroscopy; H&E, hematoxylin and eosin; IL, interleukin; iNOS, inducible nitric oxide synthase; IP, intraperitoneal; IT, intratracheal; L-B-NP, loaded-Baicalein-nanoparticles; MAP, mitogen-activated protein; MCh, methacholine; MTT, The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; mV, millivolt; NF-κB, the nuclear factor-κB; nm, nanometer; OVA, ovalbumin; PAS, periodic acid–schiff; PG, prostaglandin; Th, T lymphocyte helper; TNF, tumor necrosis factor
Abstract
Asthma as chronic airway disease has high prevalence in children and imbalance of Th1/Th2 is a critical mechanism in pathogenesis of the asthma. Baicalein as a cell protective and anti-inflammatory flavonoid may have anti-asthma effect. Therefore, for better using lung, baicalein was used in chitosan-nanoparticle as anti-asthma treatment.
Baicalein was loaded and encapsulated in chitosan nanoparticle. The morphology, physical characters (particle size, zeta potential and FT-IR) were analyzed. Drug encapsulation and loading capacity, accumulative release-time were studied. After asthma model producing, the mice were treated with L-B-NP and E-B-NP. At least, MCh challenge test, Cytokines measurement and Lung Histopathology were done.
Nanoparticles had average size 285 ± 25 nm with negative charge −2.5 mV. The L-B-NP decreased penh value and E-B-NP decreased inflammation. Both nanoparticles increased IL-12 and decreased IL-5. Also, L-B-NP decreased mucus secretion in bronchi.
L-B-NP and E-B-NP control immune-allergo-inflammatory response of asthma. L-B-NP controlled AHR and E-B-NP controlled inflammation that can be used as controlling anti-asthma drug.
1. Introduction
Asthma as a complicated chronic inflammatory airway disease has high prevalence in children, but all ages can be suffered from asthma in worldwide. The asthma main symptoms are bronchoconstriction, mucus over secretion and airway inflammation, which lead to airways narrowing and remodeling, breathlessness, wheezing, cough and chest tightness. The main reason of asthma is unregulated immune response and allergic reactions. Imbalance of Th1/Th2 is a critical mechanism in asthma pathogenesis. IL-4 is main cytokine in IgE production, IL-5 has important role in activation of eosinophils and airway inflammation and also, IL-13 is great trigger of the mucus production in asthma that are Th2 cytokines. These pathologic reactions can be attenuated by IL-12 as Th1 cytokine (Athari et al., 2017, Athari and Athari, 2014). Also, bronchial inflammation is main pathological occurrence and controlling of inflammation can have curable effect of asthma attck and remodeling. It can be harnessed by herbal flavonoids (Athari et al., 2017, Athari and Athari, 2014, de Oliveira et al., 2015).
Baicalein (5,6,7-Trihydroxy-2-phenyl-chromen-4-one, C15H10O5) as a flavonoid, isolated from the roots of Scutellaria baicalensis and has cell protective, anti-inflammatory and myorelaxation effects (de Oliveira et al., 2015, Dinda et al., 2017, Yin et al., 2018).
In recent years, many nanoparticles are used as carriers and drug delivery system. Chitosan is a polysaccharide, comprising copolymers of glucosamine and N-acetyl glucosamine consists of 2-amino-2-deoxy-d-glucose and 2-acetamido-2-deoxy-d-glucose units that are linked with beta bonds. Chitosan is extensively used for the drug delivery systems fabrication due to its mucus adhesion and penetration enhancing properties. Moreover, chitosan binds directly to mucus that is mainly composed of the negatively charge. Adhesive characteristic of the mucus allows to improve local drugs retention in the bronchi. Furthermore, chitosan is a biocompatibility and biodegradability material that can improve the drug permeability in the airway (Li et al., 2020, Babu and Kannan, 2012).
Therefore, for better using of this agent in respiratory system, baicalein was encapsulated in chitosan-nanoparticle and then, used as anti-asthma treatment. Due to the advantages of nano-herbal-component-agent administration, non-invasive, low side effect, cheap price, increase the lung absorption and improve the agent bioavailability, the research of new herbal drug carrier in this study has become the development trend. We produced chitosan nanoparticle that contains baicalein to control of inflammation in asthmatic bronchi.
2. Material and methods
2.1. Baicalein loaded nanoparticles
The baicalein loaded nanoparticles preparation was done according to Babu and Kannan (2012). Briefly; baicalein was dissolved in ethanol. Chitosan (100 mg) of was dissolved in 10 mL of 1% acetic acid, and 0.5 mL of cinnamaldehyde was added. Then aqueous phase was poured into oil phase. Finally, the whole mixture was washed and centrifuged at 12,000 rpm for 35 min and at least, nanoparticles were lyophilized.
2.2. Baicalein encapsulate nanoparticles
The baicalein encapsulate nanoparticles preparation was done according to Li et al. (2020). Briefly, Glyceryl monooleate (as lipid from), P407 (as a stabilizer) and baicalein were placed in an appropriate amount of anhydrous ethanol, and melted to dissolve in water bath at 55 °C, and thereafter the oil phase was obtained. The mixture was evaporated anhydrous ethanol at 45 °C. An appropriate amount of gelucire 44/14 (as an absorption enhancer) and trimethyl chitosan (cationic material) were dissolved in 30 mL Tris-HCl and then were heated to 55 °C. The water phase was added to dissolve the lipid film, and then dispersed by high speed shear, and the obtained colostrum in room temperature, was dispersed by ultrasound. Finally, the nanoparticles were lyophilized.
2.3. Characterization
The nanoparticle's morphology was observed using scanning electron microscopy, then viewed and photographed. The physical characters, particle size and zeta potential analysis were done zeta sizer zeta analysis. FT-IR spectra were performed by FT-IR spectrometer.
2.4. Quantitative analysis
Drug encapsulation and loading capacity were determined by Li et al., 2020, Babu and Kannan, 2012. Briefly, nanoparticles were dispersed and centrifuged for 30 min at 12,000 rpm. Then, the free drugs and total drugs were measured using high performance liquid chromatograph and ultraviolet absorption.
2.5. Drug release evaluation
Drug releasing was evaluated in physiological pH (~7.4) and also in bronchial pH (~6.6). To evaluation of drug releasing, briefly, the nanoparticles were dispersed in PBS (5 mL, pH = 7.4), putted in dialysis bags and incubated at 37◦C under slight shaking. At regular intervals, sample was removed and measured the ultraviolet absorption. The accumulative release-time curve was drowned according to the standard curve.
2.6. Animal and treatment schedule
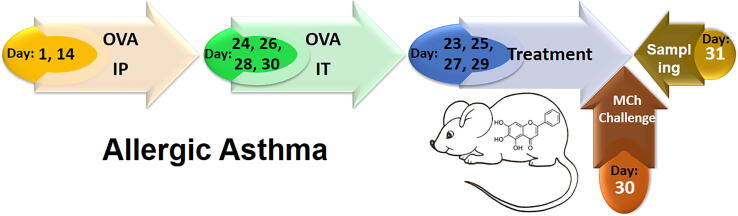
Female 6–8-week-old BALB/c mice were kept 1 week under standard conditions (12 h light–dark cycle, 24 ± 2 °C, 60 ± 10% humidity and free access to water and food) in animal house to adaptation. Mice were divided into 4 experimental groups (10 mice in each group) and in the3 groups, allergic asthma were inducted by OVA sensitization and challenging that was showed in Fig. 1 (Athari et al., 2016). One remained group (negative control) was sensitized and challenged with PBS without any treatment. Asthma groups includes: no treatment, treatment with loaded nanoparticles and treatment with encapsulated nanoparticles. All treatments were done via inhalation of nanoparticles (1 mg/ml for 30 min/day) by nebulizer. The mice were euthanized and then, the samples of mice were taken on day 31.
Fig. 1.
Mouse model of allergic Asthma. The mice were sensitized by OVA on days 1 and also day 14 via IP, and challenged on days 24, 26, 28 and 30 via IT. Treatment by produced nanoparticles was done on days 23, 25, 27, 29. MCh challenge test was done on day 30 and sampling was done on days 31.
2.7. MCH challenge test
MCh challenge test to determine AHR was done on day 30 in a manner described earlier (Athari et al., 2016). Briefly, MCh challenge test is assessed by determining enhanced pause (Penh value). The tracheotomized mice were connected to ventilator and exposed to MCh with a series of doubling concentrations (0, 1, 2, 4, 8, and 16 mg/ml).
2.8. Cytokines
The IL-12 level (as Th1 cytokine) and IL-5 level (as Th2 cytokine) were measured in BALf by ELISA kits according to the manufacturer’s instructions. Before measurement, BALf samples were taken on day 31 via tracheostomizing of mice (Athari et al., 2016).
2.9. Lung Histopathology
Lung of the mice were separated and pathological slides were produced and after staining with H&E, AB, PAS, and AB-PAS were evaluated by microscopy for eosinophil peribronchial and perivascular inflammation, goblet cell hyperplasia and mucus hypersecretion (Athari et al., 2016).
2.10. Statistical analysis
All data were presented as the mean of 3 replicates representative of 3 independent experiments. Version 19 of the SPSS was performed for statistical analyses. The data were presented as the mean ± SD and analyzed using ANOVA test and pearson’s method was used for correlation analysis. The P < 0.05 was considered significant. GraphPad prism software was used to drown the graphs.
3. Results
3.1. Size, morphology and surface charge
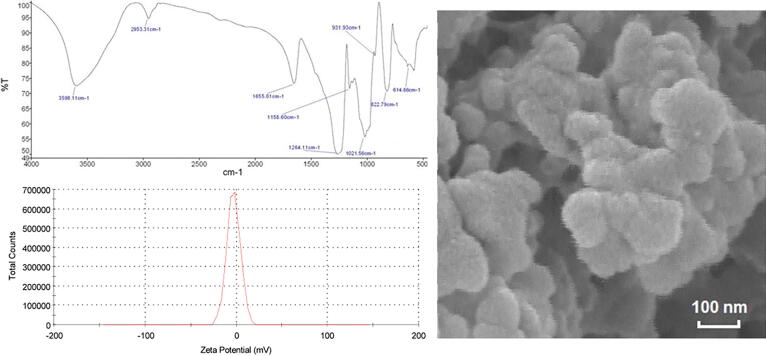
SEM (26 kV and 60 kilo X) was used to the morphology characterization of the nanoparticles and the average size was 285 ± 25 nm (Fig. 2). The result of zeta potential revealed that the nanoparticles had negative charge with a −10.5 mV zeta potential (Fig. 2). FT-IR characterization of nanoparticles shows changes of the FT-IR spectra of pure chitosan nanoparticles, baicalein loaded and encapsulated nanoparticles that approved loading and encapsulation of baicalein (Fig. 2).
Fig. 2.
Nanoparticle's characteristics. The morphological of the nanoparticle, zeta potential curve and FT-IR study were showed.
3.2. Baicalein encapsulation and loading capacity
The analysis described that loading and encapsulation efficiency were 74.2% and 96.1%, respectively.
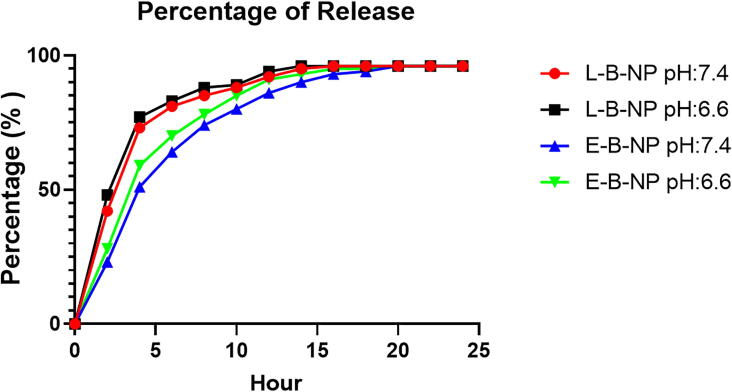
3.3. Baicalein release studies
The Baicalein releasing efficiency of loaded and encapsulated nanoparticles in different pH were shown in Fig. 3 and was found that releasing speed was higher in loaded nanoparticles (L-B-NP, pH 7.4; 2:42, 4:73, 6:81, 8:85, 10:88, 12:92, 14:95, 16–24:96 and pH 6.6; 2:48, 4:77, 6:83, 8:88, 10:89, 12:94, 14–24:96) than encapsulated nanoparticles (E-B-NP, pH 7.4; 2:23, 4:51, 6:64, 8:74, 10:80, 12:86, 14:90, 16:93, 18:94, 20–24:96 and pH 6.6; 2:28, 4:59, 6:70, 8:78, 10:85, 12:91, 14:93, 16:95, 18:95, 20–24: 96). Also, releasing was higher in pH 6.6 than 7.4 in both loaded and encapsulated nanoparticles.
Fig. 3.
Releasing time curve. The releasing of baicalein in various time of 24 h period from encapsulated/loaded chitosan-nanoparticle was evaluated.
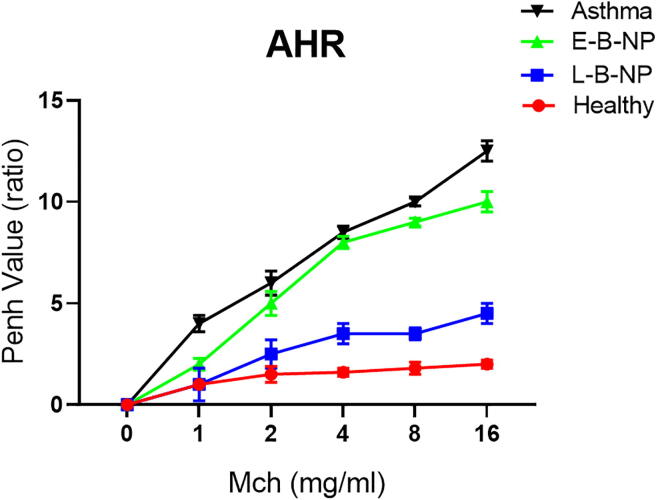
3.4. AHR (MCh challenge test)
In this study, the penh value was significantly increased in the asthma group (1: 4 ± 0.4, 2: 6 ± 0.6, 4: 8.5 ± 0.3, 8: 10 ± 0.2 and 16: 12.5 ± 0.5) compared with healthy group (1: 1 ± 0.2, 2: 1.5 ± 0.4, 4: 1.6 ± 0.2, 8: 1.8 ± 0.3 and 16: 2 ± 0.2) for all concentrations of MCh (p < 0.05). Treated asthma groups, displayed a reduced penh value significantly (p < 0.05) compared with the non-treated asthma group. This reduction was significant in L-B-NP received group (1: 1 ± 0.8, 2: 2.5 ± 0.7, 4: 3.5 ± 0.5, 8: 3.5 ± 0.3 and 16: 4.5 ± 0.5) (p < 0.05) compared with E-B-NP received group (1: 2 ± 0.3, 2: 5 ± 0.6, 4: 8 ± 0.3, 8: 9 ± 0.2 and 16: 10 ± 0.5) (Fig. 4).
Fig. 4.
The penh value of AHR in response to increase dose of MCh. Mice after anesthetization, were tracheotomized and then exposed to doubling concentrations series of aerosolized MCH to AHR changes.
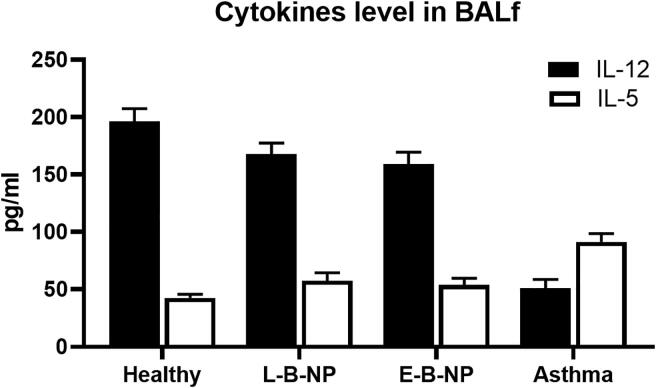
3.5. Cytokines
The level of IL-5 (91.40 ± 7.34 pg/ml) was increased in the asthma group compared with the healthy group (42.59 ± 3.04 pg/ml) and a reverse trend was found in IL-12 (asthma 51.09 ± 7.64 compared with healthy 196.20 ± 11.28 pg/ml) (P < 0.05). The level of IL-5 was significantly decreased in both treatment groups (L-B-NP: 57.63 ± 6.83, E-B-NP: 53.82 ± 5.9 pg/ml) compared with non-treated group and the IL-12 level was increased significantly (P < 0.05) in in both treatment groups (L-B-NP: 167.65 ± 9.76, E-B-NP: 159.26 ± 10.25 pg/ml) compared with non-treated group (Fig. 5).
Fig. 5.
Cytokines levels. The levels of IL-5, and IL-12 were measured in BALf's of the all groups of asthma.
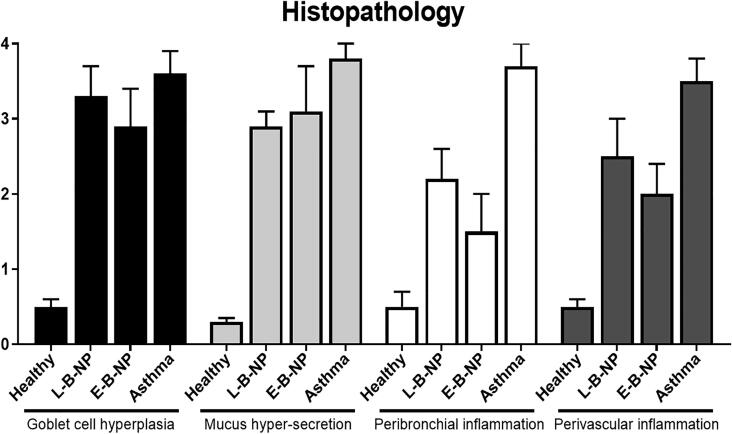
3.6. Lung Histopathology
Inflammation on the peribronchial and Perivascular, mucus hyper-secretion and goblet cell hyperplasia, were significantly increased (P < 0.05) in the non-treated asthma group (3.7 ± 0.3, 3.5 ± 0.3, 3.8 ± 0.2 and 3.6 ± 0.3 respectively) compared with healthy group (0.5 ± 0.2, 0.5 ± 0.1, 0.3 ± 0.05 and 0.5 ± 0.1 respectively). Goblet cell hyperplasia (L-B-NP: 3.3 ± 0.4, E-B-NP: 2.9 ± 0.5) and mucus hyper-secretion (L-B-NP: 2.9 ± 0.2, E-B-NP: 3.1 ± 0.6) were decreased in both treated groups compared with non-treated group but not significant (P> 0.05) (Fig. 6, Fig. 7). The peribronchial and Perivascular inflammation (L-B-NP: 2.2 ± 0.4, 2.5 ± 0.5; E-B-NP: 1.5 ± 0.5, 2 ± 0.4 respectively) were significantly decreased in treated asthma groups (P < 0.05) compared with non-treated group (Fig. 6, Fig. 7).
Fig. 6.
Histopathological study. The peribronchiolar and perivascular eosinophilic inflammation, goblet cell hyperplasia and mucus hypersecretion were evaluated.
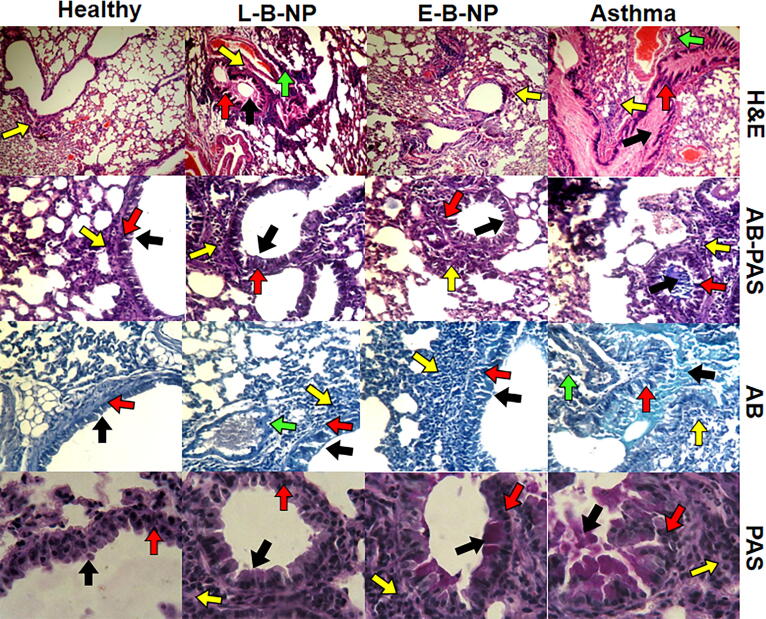
Fig. 7.
Histopathological sections. The histopathological sections of the lungs in all groups were stained with H&E, AB, PAS and AB-PAS. The goblet cell hyperplasia, perivascular and peribronchiolar inflammation and mucus secretion were evaluated in all groups. The peribronchiolar inflammation was showed with yellow arrow, the perivascular inflammation was showed with green arrow, the goblet cell hyperplasia was showed with red arrow, and the mucus secretion was showed with black arrow.
4. Discussion
Asthma is a chronic inflammatory lung disease that involve more than 350 million people globally. Immune response with inflammation lead to obstruction of airway and mucous production causes mechanical limitation of air flow. Herbal medicine and components are effective therapy for treatment of asthma. However, these should be used carefully for inappropriate dosages. Well-designed methods are needed to clarify the efficacy and safety of herbal base treatment before they can be used actively in clinical practice (Athari et al., 2017, Athari and Athari, 2014, Sudhakaran et al., 2018).
The delivery systems are useful in the drugs-controlled release. This system helps to more constant effects on the target tissues, protects rapid drugs degradation, and minimized drugs side effects. Chitosan has biocompatibility, biodegradability, and mucu-adhesive properties and is derived from the chitin that used extensively as drug delivery system. The stability and size of nanoparticles are important parameters in the preparation process, especially in lung and airway. Also, the toxicological property plays an important role in the medicine fields and in the body, immunetolerance against nanoparticles is main character. Chitosan has received much attention for biocompatible property with low or no adverse effect as a delivery vehicle for drugs (Razavi et al., 2019).
Chitosan as biodegradable molecule has suitable renal clearance. Babu et al., 2012, used chitosan nanoparticle as baicalein delivery system. They observed high encapsulation and controlled release of nanoparticles and presented that nanoparticles had very low cytotoxicity in MTT assay (Babu and Kannan, 2012).
Size of the nanoparticle is one of the most important parameters, which needs to be considered while developing an asthma-therapeutic nanoparticle. Particles less than 50 nm can be return outside with exhalation and more than 500 nm can sediment in upper respiratory system (Li et al., 2020). The baicalein-chitosan-nanoparticles size that was prepared in this study 285 ± 25 nm, which makes the nanoparticles suitable for treatment of asthma (50 < 285 ± 25 < 500 nm). Also, the release profiles of nanoparticles were better drug the first hours (under 5 h) and then had a slower rate in a sustained manner up to 10 h. It gives an additive advantage that leads to increased drug accessibility in bronchi. When drug is released immediately, it can have fast effect in the control of asthma attack in short periodic time. Because in most cases, the mucus has a negative charge, for better and stronger attaching of nanoparticles to mucus, it would be better that produced nanoparticles have positive charge. Our produced particles had negative charge, but it was very low potential (-2.5 mV). Therefore, charge regulation for suitable result, is recommended.
There are several successful studies about classical molecular dynamics that provide useful insights to understand the chitosan and different drugs interaction. Metadynamic (an enhanced method to study the kinetics and interaction of small molecules and macromolecules) introduces many algorithms are successfully developed in different fields bio-molecular nano-medicine and is describing the free energy surface of molecular binding and unbinding, which can be used in nanoparticle design (Shadrack and Swai, 2019). A study showed that chitosan-alginate nanoparticle is promising drug carrier, which effectively encapsulated and stabilized drug degradation and drug encapsulation increased its intracellular retention. The loaded drug on the chitosan-alginate nanoparticles, leads to improve the effective capacity of drug (Yoncheva et al., 2020). After producing and preparation of two chitosan-nanoparticles that had loaded and encapsulated baicalein and treatment of asthmatic mice, it was observed that AHR (MCh challenge test) in baicalein-loaded-chitosan-nanoparticles treated group was controlled better than baicalein-encapsulated-chitosan-nanoparticles group. This change was significant especially in the increased doses. It may be influenced from speed of drug releasing time, because the loaded-chitosan released baicalein faster than encapsulated-chitosan and therefore, drug accessibility was acceptable and had notable effect in control of AHR and early response of airway. On the other hand, inflammation is late phase response and slowly baicalein releasing could control airway inflammation in the long time. Thus, encapsulated baicalein may be effective treatment to control of airway inflammation (long time treatment), and loaded baicalein may be effective treatment to control of airway hyperresponsiveness (short time treatment). Also loaded baicalein can treat inflammationm but not strongly of encapsulated baicalein treatment.
Baicalein has anti-inflammatory activity and antioxidant property. Since NF-κB, and AP-1 are important transcriptional processes, which regulate inflammation. Huang et al., 2008, represented that baicalein and its analogs (A ring 6th position modified) had strong effect on NF-κB with or without TNF-α and in the presence of TNF-α, maximum effect on NF-κB was observed and maybe the target site is located downstream of the TNF-α receptor. Moreover, they showed that analogs of the baicalein had not effect on the AP-1-mediated transcription and therefore, AP-1-mediated transcription is not be responsible for the anti-inflammatory activity of baicalein analogs (Huang et al., 2008). Baicalin and baicalein were used to treat inflammation of the respiratory tract with inhibition of the expression of pro-inflammatory mediators including COX-2, IL-1β, IL-6, iNOS, TNF-α, and PGE2 by downregulating NF-κB and the MAP kinase signaling pathway. Also, they have anti-allergic effect that can suppress histamine-induced allergy and anaphylaxis. Moreover, recently it was reported that baicalin and similar isomer of it, baicalein reduce the BBB permeability by increasing the claudin-5 and ZO-1 thigh Junction proteins expression in brain endothelial (Shin and Bae, 2013, Ardah et al., 2020). In this study, inflammation could be controlled by baicalein nanoparticles and E-B-NP had strongly effect in reduction of inflammation than L-B-NP. The two produced nanoparticles had no significant effect on goblet cell hyperplasia but, L-B-NP could reduce mucus secretion in the airway. The two produced nanoparticles enhanced IL-12 and strongly decreased IL-5 level. The E-B-NP and L-B-NP could reduce IL-5 level and therefore, they could control eosinophilic inflammation. Because, E-B-NP reduced IL-5 more than L-B-NP, so, E-B-NP controlled inflammation more than L-B-NP.
L-B-NP and E-B-NP can control asthma pathophysiology. L-B-NP can control AHR and early phase of immune-allergic response and E-B-NP can control inflammation and late phase of immune-inflammatory response in airways. It may better effect when L-B-NP and E-B-NP would be used together, that was not done in this study and may be effective asthma treatment in control of breathlessness and inflammation.
In this study, there were some limitations. There are other allergic related factors (such as IgE) and cytokines (such as IL-13, IL-33) that may have effect in asthma pathophysiology and were not studied that should be noted in further researches. We did not measure the other allergic related immunoglobulins. Also, using of baicalein with other nanoparticles is recommended.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ardah Mustafa T., Ghanem Simona S., Abdulla Sara A., Lv Guohua, Emara Mohamed M., Paleologou Katerina E., Vaikath Nishant N., Jia-Hong Lu, Li Min, Vekrellis Konstantinos, Eliezer David, El-Agnaf Omar M.A. Inhibition of alpha-synuclein seeded fibril formation and toxicity by herbal medicinal extracts. BMC Complement. Med. Therapies. 2020;20:73. doi: 10.1186/s12906-020-2849-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Athari Seyyed Shamsadin, Athari Seyyed Moehyadin. The importance of eosinophil, platelet and dendritic cell in asthma. Asian Pacific J. Trop. Dis. 2014;4(1):S41–S47. [Google Scholar]
- Athari Seyyed Shamsadin, Pourpak Zahra, Folkerts Gert, Garssen Johan, Moin Mostafa, Adcock Ian M., Movassaghi Masoud, Ardestani Mehdi Shafiee, Moazzeni Seyed Mohammad, Mortaz Esmaeil. Conjugated Alpha-Alumina nanoparticle with vasoactive intestinal peptide as a Nano-drug in treatment of allergic asthma in mice. Eur. J. Pharmacol. 2016;791:811–820. doi: 10.1016/j.ejphar.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Athari Seyyed Shamsadin, Athari Seyyede Masoume, Beyzay Fateme, Movassaghi Masoud, Mortaz Esmaeil, Taghavi Mehdi. Critical role of Toll-like receptors in pathophysiology of allergic asthma. Eur. J. Pharmacol. 2017;808:21–27. doi: 10.1016/j.ejphar.2016.11.047. [DOI] [PubMed] [Google Scholar]
- Babu Varukattu Nipun, Kannan Soundarapandian. Enhanced delivery of baicalein using cinnamaldehyde cross-linked chitosan nanoparticle inducing apoptosis. Int. J. Biol. Macromol. 2012;51:1103–1108. doi: 10.1016/j.ijbiomac.2012.08.038. [DOI] [PubMed] [Google Scholar]
- de Oliveira Marcos Roberto, Nabavi Seyed Fazel, Habtemariam Solomon, Erdogan Ilkay, Orhan, Daglia Maria, Mohammad Nabavi Seyed. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol. Res. 2015;100:296–308. doi: 10.1016/j.phrs.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Dinda Biswanath, Dinda Subhajit, DasSharma Saikat, Banik Rajarshi, Chakraborty Ankita, Dinda Manikarna. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017;131:68e80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- Huang Sheng-Teng, Lee Yashang, Gullen Elizabeth A., Cheng Yung-Chi. Impacts of baicalein analogs with modification of the 6th position of A ring on the activity toward NF-jB-, AP-1-, or CREB-mediated transcription. Bioorg. Med. Chem. Lett. 2008;18:5046–5049. doi: 10.1016/j.bmcl.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Jinjing, Jin Xin, Yang Yang, Zhang Lingling, Liu Rui, Li Zheng. Trimethyl chitosan nanoparticles for ocular baicalein delivery: Preparation, optimization, in vitro evaluation, in vivo pharmacokinetic study and molecular dynamics simulation. Int. J. Biol. Macromol. 2020;156:749–761. doi: 10.1016/j.ijbiomac.2020.04.115. [DOI] [PubMed] [Google Scholar]
- Razavi Shahnaz, Seyedebrahimi Reihaneh, Jahromi Maliheh. Biodelivery of nerve growth factor and gold nanoparticles encapsulated in chitosan nanoparticles for schwann-like cells differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2019;513:681e687. doi: 10.1016/j.bbrc.2019.03.189. [DOI] [PubMed] [Google Scholar]
- Shadrack Daniel M., Swai Hulda S. Solvent effects on molecular encapsulation of Toussantine-A by chitosan nanoparticle: A metadynamics study. J. Mol. Liq. 2019;292 [Google Scholar]
- Shin Hee Soon, Bae Min-Jung. Sun Young Jung, Dong-Hwa Shon. Inhibitory effect of skullcap (Scutellaria baicalensis) extract on ovalbumin permeation in vitro and in vivo. Food Chem. 2013;140:22–30. doi: 10.1016/j.foodchem.2013.01.042. [DOI] [PubMed] [Google Scholar]
- Sudhakaran P., Hayhoe S., Aung S.K., Barnes M.A., Cayir Y., D'alessandro E.G., Lin C.A., Pai H.J., Imai K., Imai M., Hisajima T. How Do You Treat Asthma in Your Practice? Med. Acupunct. 2018;30(2):100–112. doi: 10.1089/acu.2018.29078.cpl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Huafeng, Huang Lihao, Ouyang Ting, Chen Lvyi. Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2018;55:55–62. doi: 10.1016/j.intimp.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Yoncheva K., Tzankov B., Yordanov Y., Spassova I., Kovacheva D., Frosini M., Valoti M., Tzankova V. Encapsulation of doxorubicin in chitosan-alginate nanoparticles improves its stability and cytotoxicity in resistant lymphoma L5178 MDR cells. J. Drug Delivery Sci. Technol. 2020;59 [Google Scholar]