Highlights
-
•
Dox induces kidney damage.
-
•
Dox leads to a decrease in antioxidant defense mechanism.
-
•
Diosmin administration restores antioxidant properties.
Keywords: Doxorubicin, Oxidative stress, Apoptosis, Inflammation, Nephrotoxicity, Diosmin
Abstract
Doxorubicin (Dox) is an anthracycline antibiotic that is primarily used for treating various solid tumors including that of pulmonary, ovary, breast, uterine, cervix, and several blood cancers. However, nephrotoxicity associated with Dox treatment limits its clinical use. Administration of Dox in combination with compounds exhibiting antioxidant properties are being used to minimize the side effects of Dox. Diosmin is a flavonoid glycoside with numerous beneficial properties that is found in the pericarp of many citrus fruits. Diosmin has demonstrated antioxidant, anti-inflammatory, and anti-apoptotic effects in response to various insults, although the exact mechanism remains unknown. Therefore, this study was designed to evaluate the effect of diosmin in preventing kidney damage in response to Dox treatment. Male Wistar rats were randomly divided into four groups: control group, Dox group (20 mg/kg, i.p.), Dox plus low-dose diosmin group (100 mg/kg orally), and Dox plus high-dose diosmin group (200 mg/kg orally). A single intraperitoneal injection of Dox resulted in kidney damage as evidenced by significant alterations in kidney markers, histological abnormalities, and the attenuation of antioxidant defense mechanisms (GSH, SOD, and CAT). Moreover, Dox treatment significantly altered the expression of oxidative stress, inflammatory, and anti-apoptotic protein markers. Diosmin pretreatment alleviated Dox-induced nephrotoxicity by ameliorating the antioxidant mechanism, decreasing inflammation and apoptosis, and restoring kidney architecture. In conclusion, our results indicate that diosmin is a promising therapeutic agent for the prevention of nephrotoxicity associated with DOX.
1. Introduction
Doxorubicin (DOX) is an anthracycline antibiotic that is primarily used to treat various solid tumors including lung, ovary, breast, uterine, cervix, and several blood cancers (Khames et al., 2019). Dox exerts its cytotoxic effect through DNA intercalation and topoisomerase II (TOP2) inhibition in rapidly dividing cancer cells (Sanajou et al., 2019). Dox produces a quinone moiety that is reduced to a semiquinone, thus contributing to the production of radical superoxide (O2•−). These O2•− molecules steadily destroy cell components as the concentration of reactive oxygen species (ROS) increases (Kamble and Patil, 2018). Although Dox has been considered an effective anticancer medication due to its unprecedented potency and broad therapeutic effects, organ toxicity, especially nephrotoxicity, restricts its overall usefulness. While the specific mechanism of nephrotoxicity induced by Dox remains unknown, published reports suggest that Dox induces free radical generation, oxidative damage to biological macromolecules, and peroxidation to membrane lipids that result in the degradation of the cell membrane (Tulubas et al., 2015). Moreover, several recent studies have shown that apoptosis and inflammation play an important role in Dox-mediated nephrotoxicity (Khames et al., 2019, ENTEZARI HERAVI et al., 2018).
Inflammation is an immune reaction triggered by many factors including harmful chemicals, pathogens, and cells that are injured (Chen et al., 2018). Macrophages are immune cells that perform various functions during inflammation including altered production of pro-inflammatory and anti-inflammatory cytokines, interleukins, tumor necrosis factor-alpha (TNF-α), and inducible nitric oxide synthase (i-NOS) (Rehman et al., 2014). Several studies have reported a relationship between Dox and nuclear factor kappa B (NF-kB), a transcriptional factor that regulates genes that encode apoptosis and inflammatory cytokines (Imam et al., 2018).
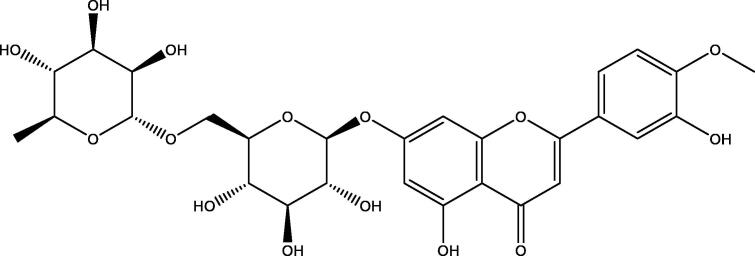
Flavonoids are polyphenolic tricyclic secondary metabolites that are abundant in plants. Diosmin (Fig. 1) is a flavone glycoside that is present in the pericarp of many citrus fruits (Rashid et al., 2019, Kumar and Pandey, 2013, ERASLAN et al., 2017). It has many biological properties, such as antioxidant (Ağır and Eraslan, 2019), anti-apoptotic (Shalkami et al., 2018), anti-mutagenic (Bear and Teel, 2000) and anti-inflammatory activity (Abdel-Daim et al., 2017). Several reports indicate that diosmin has preventative effects on liver damage, kidney injury, myocardial infarction, hepatocarcinogenesis, and hypertension (Shalkami et al., 2018, Bear and Teel, 2000, AĞıR, M. S. & ERASLAN, G, 2019, Abdel-Daim et al., 2017, Perumal et al., 2018, Silambarasan and Raja, 2012).
Fig. 1.
Structure of diosmin.
The nephroprotective effects of diosmin against some chemotherapeutic agents and other toxicants have been demonstrated previously (Abdel-Daim et al., 2017, Elhelaly et al., 2019); however, to our knowledge, there is no evidence that shows the protective effect against Dox-induced kidney damage. Hence, this study was conducted to investigate the protective properties of diosmin against Dox-induced nephrotoxicity. In this study, we report that Dox treatment resulted in increased expression of kidney damage markers, depletion of antioxidants, elevation of inflammatory and apoptotic mediators, and alteration of kidney histology. Diosmin pretreatment diminishes these harmful consequences and results in nephroprotection.
2. Materials and methods
2.1. Drugs and chemicals
Doxorubicin and diosmin were 98% pure and were purchased from AK Scientific, Inc. USA and all other chemicals used in the study were purchased from Sigma Aldrich St. Louis, USA, E. Merck, Darmstadt, Germany were highly pure.
2.2. Animals
The animals used in this study were obtained from the animal facility of the College of Pharmacy, King Saud University (KSU), Riyadh, Saudi Arabia. All the animals were kept under standard room temperature (25 ± 1 °C) with 12 h of light/dark cycle and were given free access to water and standard diet. All procedures and protocols used for the animal studies were approved by the KSU Local Institutional Study Ethics Committee (REC) (approval # KSU-SE-19-121).
2.3. Experimental design
In this study, 4–6 week old male Wistar rats (n = 32, 180–200 g) were randomly divided into four groups (eight rats per group). Experimental design and groups are illustrated in Fig. 2. The animals were allowed to acclimatize for 1 week prior to the start of the experiments. Animals in group I received a vehicle (normal saline) orally. Group II received a single dose of Dox (20 mg/kg i.p.) on day 17. Groups III and IV were treated with diosmin (100 and 200 mg/kg p.o., respectively) for 18 days and a single dose of Dox (20 mg/kg i.p.) on the 17th day (Rehman et al., 2014, Mahgoub et al., 2020).
Fig. 2.
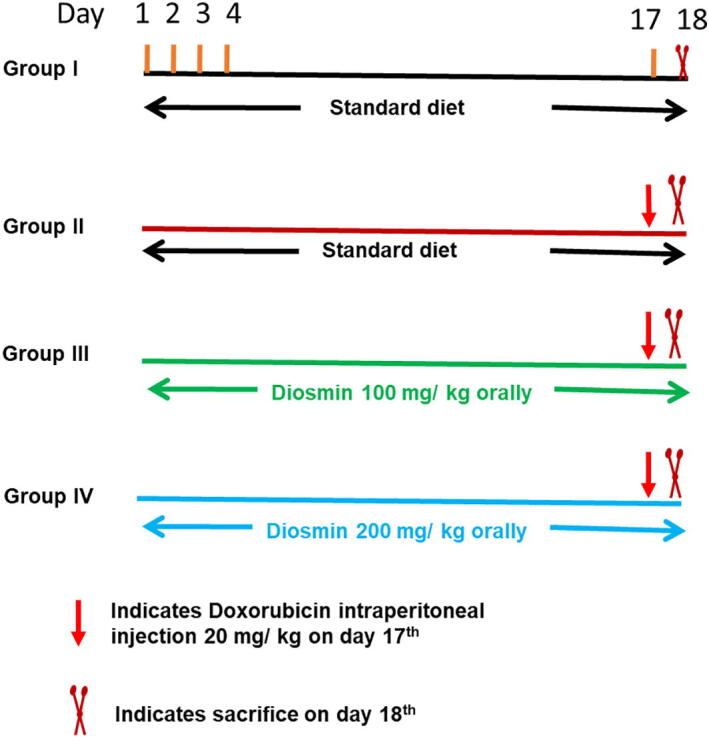
Illustration of the experimental design.
At the end of the study, blood was collected and both kidneys from each rat were detached and directly placed into liquid nitrogen for biochemical assays and western blot analysis. The remaining kidneys were perfused with ice-cold PBS followed by 4% paraformaldehyde (PFA) and fixed for histological analysis. No mortality occurred in any group.
2.4. Assessment of kidney markers
Blood samples obtained at the time of sacrifice were centrifuged at 5000 g for 10 min using a cooling centrifuge for serum isolation. The serum was then used for the measurement of creatinine, albumin, and nitrogen urea in the tissues (BUN). The values were calculated by the autoanalyzer (Dimension® RXL MAXTM, Siemens, USA.)
2.5. Measurement of lipid peroxidation
Lipid peroxidation was measured in tissues using the method described by Ohkawa et al. (Ohkawa et al., 1979). Briefly, tissue homogenates containing thiobarbituric acid (TBA) and trichloroacetic acid (TCA) were incubated in a shaking water bath at 90 °C for 30 min. The samples were placed on ice for 10 min and then centrifuged for 15 min at 3000 g in a refrigerated centrifuge. The absorbance of the supernatants was measured at 540 nm. The resulting values were expressed as nmol of MDA formed per mg of protein.
2.6. Measurement of reduced glutathione (GSH)
The amount of GSH in tissues was measured by the method of Sedlak and Lindsay (Sedlak and Lindsay, 1968). Briefly, 5,5′-dithiobis(3-nitrobenzoic acid) was added to the reaction mixture and the absorbance was immediately recorded at 412 nm. The GSH values were expressed as nmol/mg of protein.
2.7. Measurement of catalase activity (CAT)
The post-mitochondrial supernatant (PMS) from kidney tissue was used to estimate CAT activity by the Claiborne method (Claiborne, 1985). Briefly, the reaction mixture consisted of 1.95 ml (0.1 M, pH 7.4) phosphate buffer, 1 ml (0.019 M) hydrogen peroxide, and 0.05 ml PMS for a total volume of 3 ml. The absorbance was recorded at 240 nm for 5 min at an interval of 1 min. A difference in the absorbance was used to calculate the activity of CAT as the amount of moles of H2O2 changed per min per mg of protein.
2.8. Western blot analysis
Western blot analysis was done as previously described (AlAsmari et al., 2020). Protein extracts were prepared from kidney tissues and equivalent amounts of protein (20–50 μg) were resolved on 10–12% SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked with 5% non-fat dried milk for 1 h and incubated with primary antibodies specific to iNOS, Nox-4 (Novus Biologicals, USA, dilution 1:1000), NfkB p65, IL-6, IL-10, TNF- α (Santa Cruz, USA, dilution 1:500), SOD, Bax, Bcl-2, cleaved caspase-3, and β-actin (ABclonal Technology, USA, dilution 1:1000) proteins overnight at 4 °C while rocking. The membranes were then washed and incubated for 1 h with the appropriate secondary antibody conjugated with HRP (ABclonal Technology, USA, dilution 1:5000). The membranes were visualized using ECL reagent (ABclonal Technology, USA) and images were collected using a Bio-Rad gel imaging instrument.
2.9. Histopathology studies
The PFA-fixed kidney tissues from each group were processed and embedded in paraffin to prepare blocks. A microtome was used to cut 3 µm sections. The paraffin from the kidney sections was removed and the slides were stained with hematoxylin and eosin (H&E) and visualized under an optical microscope. The images were recorded using an Olympus BX microscope fitted with DP72 camera.
2.10. Statistical analysis
The data are presented as the mean ± SD for the individual groups. The differences among groups were determined by one-way analysis of variance (ANOVA) followed by the Tukey comparison test using GraphPad Prism software 5 (CA, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Diosmin attenuates Dox-induced kidney damage
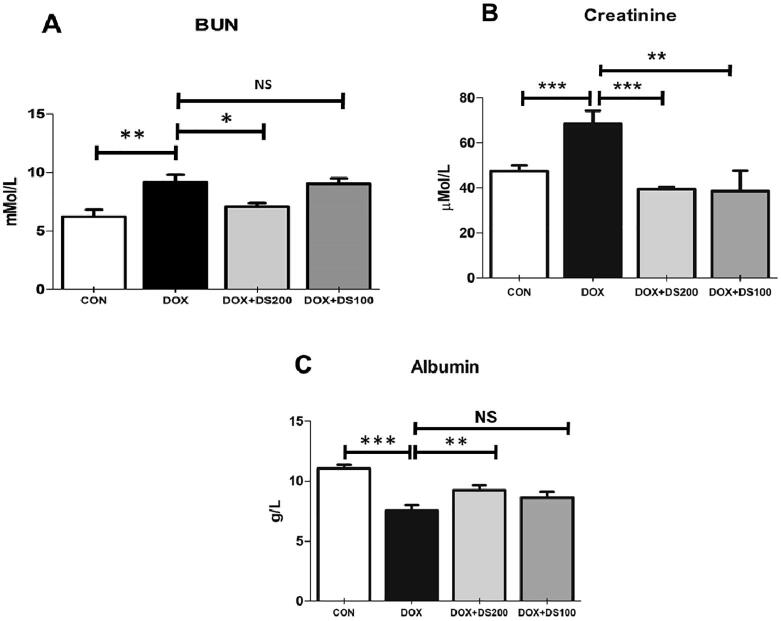
To confirm that Dox treatment is associated with kidney injury, serum BUN, creatinine, and albumin were measured. As expected, a single dose of Dox resulted in a significant rise in BUN, creatinine, and albumin levels (Fig. 3A–C). However, diosmin pretreatment diminished the increase in BUN, albumin, and creatinine levels suggesting that it protected against Dox-induced kidney damage.
Fig. 3.
Pretreatment with diosmin mitigated the altered serum levels of BUN (A), creatinine (B), and albumin (C) resulting from Dox treatment. Data are presented as the mean ± SD (n = 5) where ***p < 0.001, **p < 0.0, *p < 0.05, and NSp> 0.05. [CON, control; DOX, doxorubicin; DOX + DS100, doxorubicin plus diosmin 100 mg/kg; and DOX + DS200, doxorubicin plus diosmin 200 mg/kg, n = number of animals].
3.2. Diosmin attenuates Dox-induced lipid peroxidation and oxidative stress
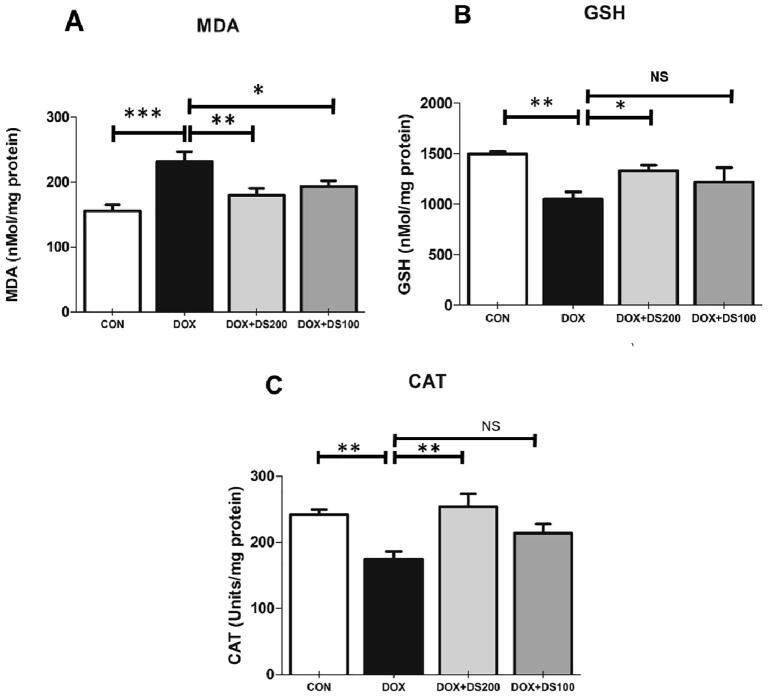
To determine whether diosmin treatment can mitigate oxidative stress and antioxidant status caused by Dox, we measured MDA levels, GSH content, and CAT activity in kidney tissue. The results indicated that a single Dox injection (20 mg/kg) resulted in a significant increase in MDA and a decrease in GSH content and CAT activity in kidney tissues compared with the control group. However, in rats pretreated with diosmin at 100 and 200 mg/kg, the observed increase in MDA and decline in GSH content and CAT activity were restored in a significant and dose-dependent manner. These results demonstrated the potential antioxidant activity of diosmin (Fig. 4A–C).
Fig. 4.
Pretreatment of diosmin diminishes the oxidative stress induced by Dox. Biochemical analysis of MDA (A), GSH (B), and CAT (C). Data are presented as the mean ± SD (n = 5) where **p < 0.0, *p < 0.05, and NSp > 0.05. [CON, control; DOX, doxorubicin; DOX + DS100, doxorubicin plus diosmin 100 mg/kg; and DOX + DS200, doxorubicin plus diosmin 200 mg/kg, n = number of animals].
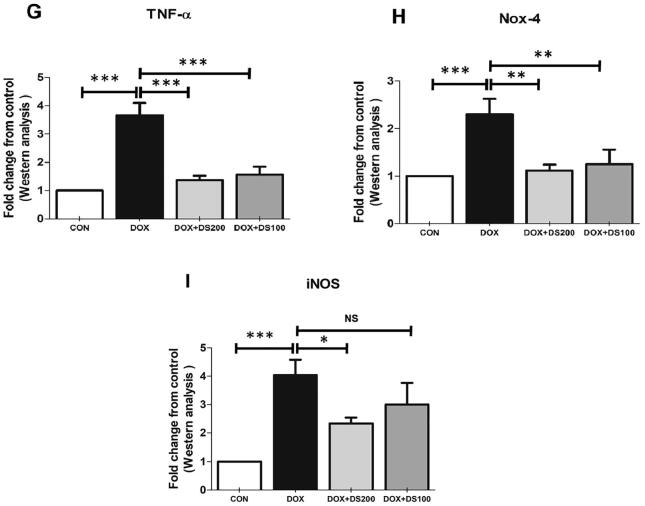
3.3. Diosmin mitigates Dox-induced alterations in inflammatory mediators
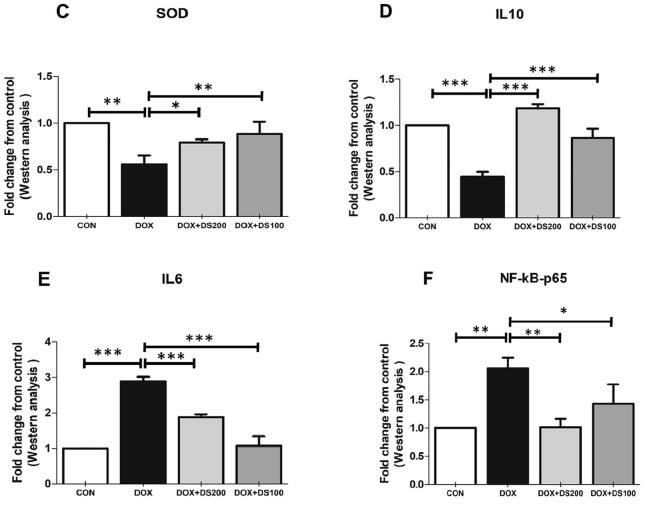
Studies published by Shalkami and co-workers have demonstrated the antioxidant and anti-inflammatory properties of diosmin (Shalkami et al., 2018). Therefore, to evaluate the antioxidant and anti-inflammatory properties of diosmin, the expression of SOD, NfkB, IL-10 IL-6, TNF-α, iNOS, and Nox-4 proteins were measured by western blot analysis. After Dox injection, the expression of SOD and IL-10 decreased, whereas the expression of NfkB, IL-6, TNF-α, Nox-4, and iNOS were significantly increased (Fig. 5A–I). Interestingly, the rats treated with diosmin exhibited a restoration of the Dox-induced alterations in protein expression involved in maintaining antioxidant status (SOD), oxidative stress (NfkB), and regulating inflammation (TNF-α, IL-6, iNOS, Nox-4, and IL-10) (Fig. 5A–I). These results demonstrate the antioxidant and anti-inflammatory properties of diosmin.
Fig. 5.
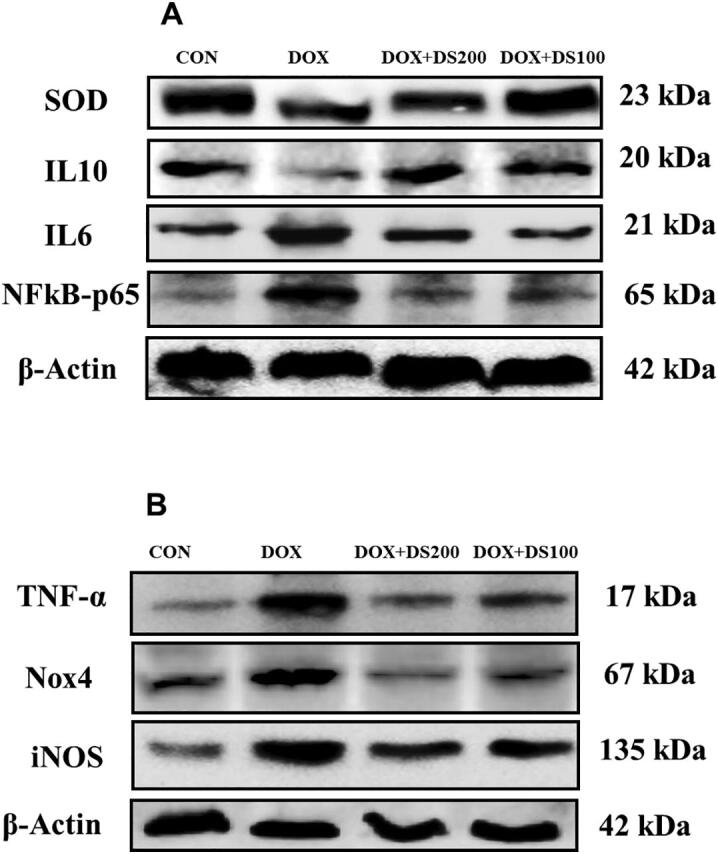
Diosmin reduces oxidative stress and inflammatory protein expression and induces antioxidant proteins. (A & B) Immunoblot representation of SOD, IL-10, IL-6, NfkB-p65, TNF-α, Nox-4, and iNOS. (C–I) Graphical representation of SOD, IL-10, IL-6, NfkB-p65, TNF-α, Nox-4, and iNOS. Results are shown as the mean ± SD (n = 5) where ***P < 0.001, **P < 0.01, *P < 0.05, and NSP > 0.05. [CON, control; DOX, doxorubicin; DOX + DS100, doxorubicin plus diosmin 100 mg/kg; and DOX + DS200, doxorubicin plus diosmin 200 mg/kg, n = number of animals].
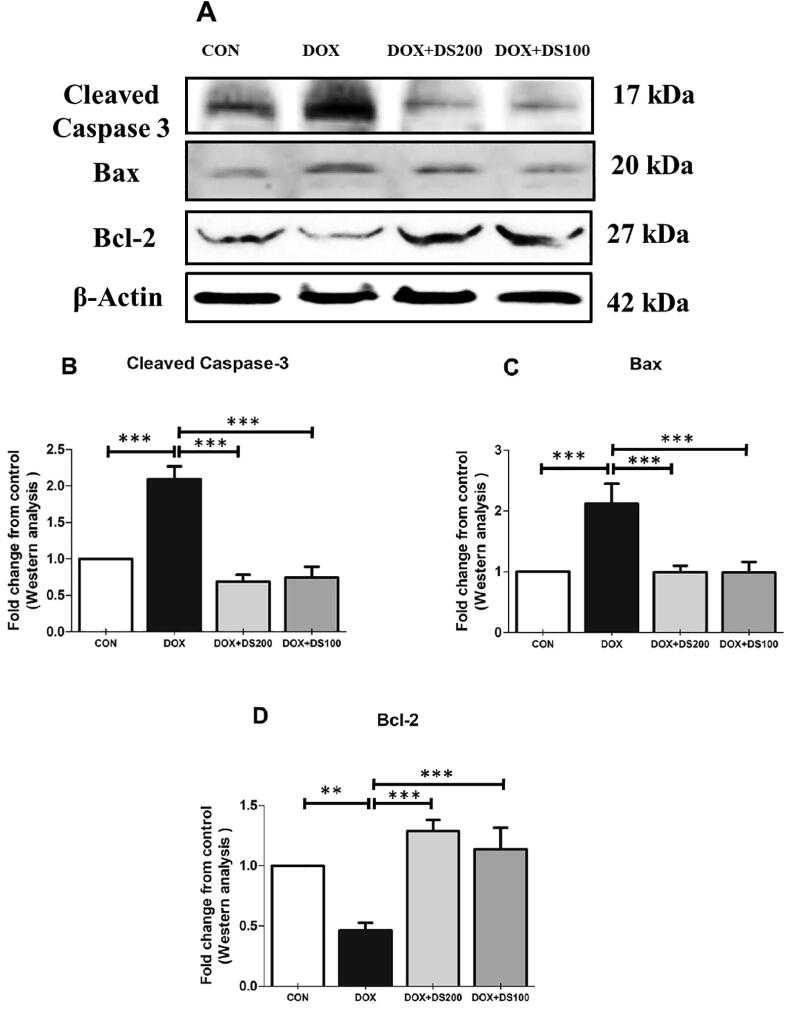
3.4. Diosmin attenuates Dox-induced apoptosis
To evaluate whether diosmin prevents apoptosis caused by Dox, we measured the expression of pro-apoptotic and anti-apoptotic protein markers. A single dose of Dox resulted in a significant rise in the expression of pro-apoptotic proteins (Bax and cleaved caspase-3) and a dramatic decrease in the anti-apoptotic protein, Bcl-2, in renal tissues compared with the control group. Rats treated with diosmin exhibited a significantly decreased expression of pro-apoptotic proteins and increased expression of anti-apoptotic proteins (Fig. 6A–D). These results indicate that pretreatment with diosmin attenuated apoptosis induced by Dox in rat kidneys.
Fig. 6.
Effect of diosmin on pro-apoptotic and anti-apoptotic protein expression. Immunoblot representation of cleaved caspase-3, Bax, and Bcl-2 (A–D). Results are shown as the mean ± SD (n = 5) where ***P < 0.001 and **P < 0.01. [CON, control; DOX, doxorubicin; DOX + DS100, doxorubicin plus diosmin 100 mg/kg; and DOX + DS200, doxorubicin plus diosmin 200 mg/kg, n = number of animals].
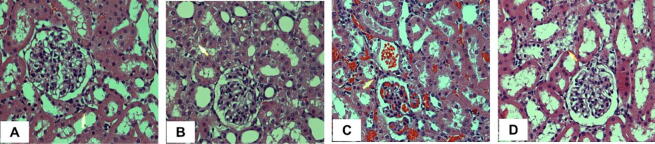
3.5. Diosmin restores kidney histology
To further confirm the biochemical and protein expression data described above, we examined the histology of kidney tissues in response to Dox treatment. Normal tubules and glomeruli were observed in rats of the control group (Fig. 7A). Dox administration caused damage to the usual architecture of the kidney as shown by glomerular congestion and tubular damage (Fig. 7B). However, treatment with diosmin reversed Dox-induced damage in a dose-dependent manner (Fig. 7C & D).
Fig. 7.
Light micrographs of kidney tissues using H&E staining. (A) The standard architecture of the kidney. (B) Disruption of the regular kidney architecture caused by Dox administration was observed as indicated by arrows. (C & D) diosmin treatment exhibited beneficial improvement in the glomeruli and tubules and tubular epithelial cell morphology at both doses.
4. Discussion
The kidney is a very complex and dynamic organ that takes part in the elimination of waste material, helps to maintain homeostasis, and controls acid-base balance. Toxicity to the kidney can impair its function (M, 2019). Dox-induced kidney damage has been well studied in the context of inflammation and apoptosis resulting from oxidative stress (El-Sayed et al., 2017a, Zhang et al., 2017). Naturally occurring nutritional substances are becoming popular worldwide due to their medicinal benefits that include antioxidant, anti-inflammatory, and anti-apoptotic effects (Wali et al., 2020). The present study was done to assess the protective effect of diosmin against Dox-induced kidney damage in rats.
Albumin, blood urea nitrogen (BUN), and creatinine are established markers for kidney dysfunction as a healthy kidney does not allow albumin to pass from the blood to urine, whereas BUN and creatinine are filtered by the kidney from the blood into urine. In the event of kidney dysfunction, however, these processes do not work properly and albumin passes into the urine resulting in low levels of serum albumin. Similarly, creatinine and BUN are not properly filtered by the kidneys, which results in high levels of serum creatinine and BUN (M, R, 2019, Zhang et al., 2017, El-Sayed et al., 2017b, Mansour et al., 1999). In this study, we observed a significant decrease in serum albumin and an increase in BUN and creatinine in the Dox-treated rat group. These variations correlated with Dox-induced kidney injury and are consistent with previously reported studies (Khames et al., 2019). Likewise, when rats were pretreated with diosmin, albumin, BUN, and creatinine levels were restored. This indicates that diosmin provides protection against kidney toxicity caused by Dox exposure.
Lipid peroxidation is a marker of oxidative stress and several published reports have documented that elevations in the amount of malondialdehyde (MDA), a lipid peroxidation product, have been reported following Dox treatment (Rashid et al., 2013, Rehman et al., 2014, Khames et al., 2019). In this study, we observed a dramatic alteration in the antioxidant status of the kidney in Dox-treated rats. In the Dox-treated group, a significantly high amount of MDA was observed. This suggests that Dox causes the formation of free radicals, oxidative injury to biological molecules, membrane lipid peroxidation, and protein oxidation (M, 2019). In contrast, diosmin pretreatment at both doses returns these changes to baseline. Our results validate the same pattern observed by Elhelaly et al. (Elhelaly et al., 2019).
Glutathione (GSH) is a low-molecular-weight tripeptide that functions as an endogenous cellular antioxidant in the detoxication of ROS generated by different stimuli that are required to maintain homeostasis for the normal functioning of cells (Rashid et al., 2017). Superoxide dismutase (SOD) is abundantly present in all cells and protects against oxidative injury by ROS. It converts O2•− to hydrogen peroxide (H2O2) that is later catalyzed by catalase (CAT) and glutathione peroxidase (GPx), scavenging O2•− is one of the essential defensive mechanisms occurring in different diseases (Mohan et al., 2010, Tu et al., 2010, Liu et al., 2007, Rašković et al., 2011, El-Shitany et al., 2008). In this study, we observed a substantial decrease in GSH levels, SOD protein expression, and CAT activity in the Dox-treated group than in the control group, which is consistent with previously reported findings (Rashid et al., 2013). However, diosmin pretreatment mitigated the changes in GSH content, SOD expression, and CAT activity, which emphasizes the renoprotective and antioxidant properties of this compound.
NADPH oxidase (Nox) is present in different isoforms in the kidney. However, it has been reported that Dox activates the Nox pathway that results in the production of ROS. This subsequently creates a disturbance in the antioxidant defense that may be responsible for the toxicity to various organs. Nox4 has been considered to be the most common of all Nox isoforms and is involved in kidney pathophysiology (Sedeek et al., 2013). Our findings revealed that the amount of Nox4 in the Dox-treated group increased compared with the control group, which is consistent with previously published reports (Elsherbiny and El-Sherbiny, 2014). Interestingly, diosmin pretreatment reduced Nox4 protein expression indicating that diosmin protects against Dox-induced nephrotoxicity.
Increased oxidative stress and depleted self-antioxidant status activate the immune response (Elsherbiny and El-Sherbiny, 2014, Ghosh et al., 2011, Zordoky et al., 2011). The generation of ROS is an important process that protects against anticancer drug-mediated toxicity. This occurs through the activation of NF-kB pathways that initiate apoptosis and inflammation (Rashid et al., 2017). Published data suggest that the activation of the inflammatory pathway is one of the causes of Dox-mediated nephrotoxicity (Zhang et al., 2017). Administration of Dox may create a microenvironment that supports the activation of NF-kB and pro-inflammatory cytokines, such as TNF-α and IL-6, followed by inhibition of anti-inflammatory cytokines such as IL-10 (Wali et al., 2020, Chen et al., 2007). Furthermore, inducible nitric oxide synthase (iNOS) is needed for the synthesis of nitric oxide (NO), which is also involved in the inflammatory process that links Dox toxicity with the level of NO (Sayed-Ahmed et al., 2001, Pacher et al., 2003, Ayla et al., 2011). In this study, we observed significantly high expression of NfkB, TNF-α, IL-6, and iNOS proteins and low expression of IL-10 in Dox-treated rats. However, diosmin exposure at two doses mitigated the altered expression of NfkB, TNF-α, IL-6, IL-10, and iNOS, which suggests an anti-inflammatory property of diosmin.
Oxidative stress is considered an essential mediator that triggers apoptotic pathways. Moreover, the mitochondrial membrane is damaged in response to organ toxicity due to the enormous production of ROS. This results in activation of the intrinsic apoptosis. Due to damage to the mitochondrial membrane, cytochrome c is released into the cytosol, which in turn activates several biochemical reactions that result in caspase-9 activation. Activated caspase-9 subsequently activates caspase-3. Bax is a pro-apoptotic protein that enhances the porosity of the membrane and facilitates the release of cytochrome c that is present between the intermembrane space, thus initiating the intrinsic apoptosis pathway. In contrast, Bcl-2 is an anti-apoptotic protein that is present in the outer mitochondrial membrane that maintains the integrity of the mitochondria and prevents the release of cytochrome c into the cytosol. Thus, the survival of the cell primarily depends on the balance between Bax and Bcl-2 (Kalyanaraman et al., 2002, Liu et al., 2004, SABBAH, H. N, 2000, Kunisada et al., 2002, Li and Cohen, 2005, CROMPTON, M, 2000, ELMORE, S, 2007, Ibrahim et al., 2020). In this study, Dox treatment resulted in a dramatic increase in cleaved caspase-3 and Bax expression and a significant decrease in Bcl-2 expression. These findings are similar to the previously published data by Ibrahim et al. (Ibrahim et al., 2020). The abnormal expression of apoptosis proteins was considerably restored by diosmin pretreatment.
To further confirm alterations in the biochemical parameters and abnormal expression of inflammatory and apoptotic proteins, we analyzed histological alterations in the kidney in response to Dox. Dox treatment distorted the architecture of the tubules of the kidney that is consistent with the earlier report by Rashid et al. (Rashid et al., 2013). Nonetheless, diosmin pretreatment restored the altered architecture of the kidney compared with the Dox group.
5. Conclusion
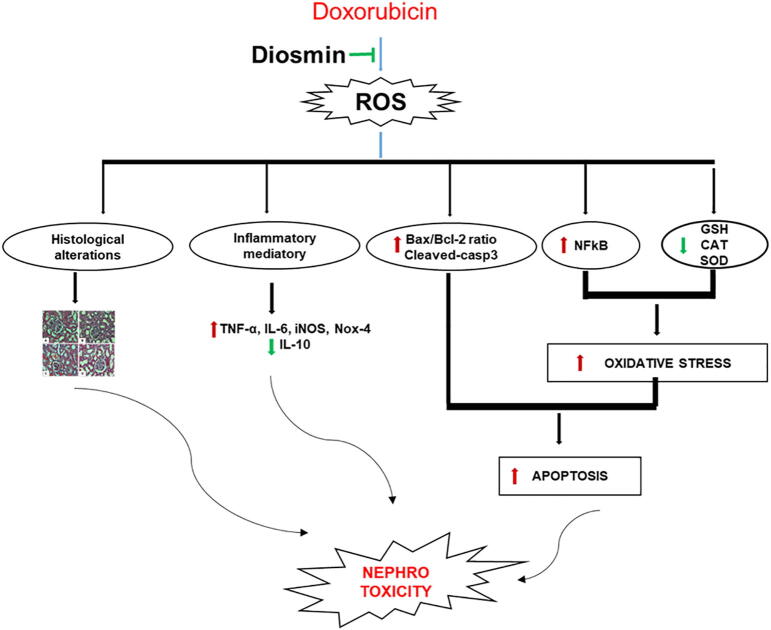
The data obtained from this study demonstrated that diosmin is a potential beneficial intervention against Dox-induced kidney injury. This likely occurs by mitigating oxidative stress, reversing the antioxidant imbalance, and ameliorating the damage in the kidney by suppressing inflammation and apoptosis (Fig. 8).
Fig. 8.
Schematic representation of doxorubicin-induced renal toxicity and underlying nephroprotective mechanism of diosmin.
CRediT authorship contribution statement
Nemat Ali: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - Original Draft, Writing - Review & Editing, Supervision, Funding acquisition. Abdullah F. AlAsmari: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing - Review & Editing, Supervision, Funding acquisition. Faisal Imam: Validation. Mohammad Z. Ahmed: Investigation. Faleh Alqahtani: Investigation. Metab Alharbi: Methodology. Mohammed AlSwayyed: Methodology. Fawaz AlAsmari: Formal analysis, Investigation. Mohammed Alasmari: . Abdulrahman Alshammari: Writing - Review & Editing. Omer I. Fantoukh: Writing - Review & Editing. Mohammed M. Alanazi: Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the Research Group (No. RG-1441-451).
Data availability statement’
All data used in this study have been included in the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Daim M.M., Khalifa H.A., Abushouk A.I., Dkhil M.A., Al-Quraishy S.A. Diosmin attenuates methotrexate-induced hepatic, renal, and cardiac injury: a biochemical and histopathological study in mice. Oxid. Med. Cell. Longev. 2017;2017:3281670. doi: 10.1155/2017/3281670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AĞıR, M. S. & ERASLAN, G., 2019. The effect of diosmin against liver damage caused by cadmium in rats. J. Food Biochem., 43, e12966. [DOI] [PubMed]
- Alasmari, A.F., Ali, N., Alasmari, F., Alanazi, W.A., Alshammari, M.A., Al-Harbi, N.O., Alhoshani, A., As Sobeai, H.M., Alswayyed, M., Alanazi, M.M., Alghamdi, N.S., 2020. Liraglutide attenuates gefitinib-induced cardiotoxicity and promotes cardioprotection through the regulation of MAPK/NF-κB signaling pathways. Saudi Pharm. J., 28, 509-518. [DOI] [PMC free article] [PubMed]
- Ayla S., Seckin I., Tanriverdi G., Cengiz M., Eser M., Soner B.C., Oktem G. Doxorubicin induced nephrotoxicity: protective effect of nicotinamide. Int. J. Cell. Biol. 2011;2011:390238. doi: 10.1155/2011/390238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear W.L., Teel R.W. Effects of citrus flavonoids on the mutagenicity of heterocyclic amines and on cytochrome P450 1A2 activity. Anticancer Res. 2000;20:3609–3614. [PubMed] [Google Scholar]
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y., Jungsuwadee, P., Vore, M., Butterfield, D.A., St Clair, D.K., 2007. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol. Interv., 7, 147-56. [DOI] [PubMed]
- Claiborne, A., 1985. Catalase activity. Handbook of Methods for Oxygen Radical Research, CRC Press, 2.
- Crompton, M., 2000. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr. Opin. Cell. Biol., 12, 414–419. [DOI] [PubMed]
- El-Sayed E.M., Mansour A.M., El-Sawy W.S. Alpha lipoic acid prevents doxorubicin-induced nephrotoxicity by mitigation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21940. [DOI] [PubMed] [Google Scholar]
- El-Sayed E.M., Mansour A.M., El-Sawy W.S. Protective effect of proanthocyanidins against doxorubicin-induced nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2017;31 doi: 10.1002/jbt.21965. [DOI] [PubMed] [Google Scholar]
- El-Shitany N.A., El-Haggar S., El-Desoky K. Silymarin prevents adriamycin-induced cardiotoxicity and nephrotoxicity in rats. Food Chem. Toxicol. 2008;46:2422–2428. doi: 10.1016/j.fct.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Elhelaly A.E., Albasher G., Alfarraj S., Almeer R., Bahbah E.I., Fouda M.M.A., Bungău S.G., Aleya L., Abdel-Daim M.M. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut. Res. Int. 2019;26:35151–35162. doi: 10.1007/s11356-019-06660-3. [DOI] [PubMed] [Google Scholar]
- Elmore, S., 2007. Apoptosis: a review of programmed cell death. Toxicol. Pathol., 35, 495–516. [DOI] [PMC free article] [PubMed]
- Elsherbiny N.M., El-Sherbiny M. Thymoquinone attenuates Doxorubicin-induced nephrotoxicity in rats: Role of Nrf2 and NOX4. Chem. Biol. Interact. 2014;223:102–108. doi: 10.1016/j.cbi.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Entezari Heravi, N., Hosseinian, S., Naji Ebrahimi Yazd, Z., Shafei, M.N., Ebrahimzadeh Bideskan, A., Shahraki, S., Samadi Noshahr, Z., Motejadded, F., Beheshti, F., Mohebbati, R., Parhizgar, S., Khajavi Rad, A., 2018. Doxorubicin-induced renal inflammation in rats: Protective role of Plantago major. Avicenna J. Phytomed., 8, 179-187. [PMC free article] [PubMed]
- Eraslan, G., Sarica, Z. S., Bayram, L., Tekeli, M.Y., Kanbur, M., Karabacak, M., 2017. The effects of diosmin on aflatoxin-induced liver and kidney damage. Environ. Sci. Pollut. Res. Int., 24, 27931-27941. [DOI] [PubMed]
- Ghosh J., Das J., Manna P., Sil P.C. The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis. Biomaterials. 2011;32:4857–4866. doi: 10.1016/j.biomaterials.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Ibrahim K.M., Mantawy E.M., Elanany M.M., Abdelgawad H.S., Khalifa N.M., Hussien R.H., El-Agroudy N.N., El-Demerdash E. Protection from doxorubicin-induced nephrotoxicity by clindamycin: novel antioxidant, anti-inflammatory and anti-apoptotic roles. Naunyn. Schmiedebergs Arch. Pharmacol. 2020;393:739–748. doi: 10.1007/s00210-019-01782-4. [DOI] [PubMed] [Google Scholar]
- Imam, F., Al-Harbi, N.O., Al-Harbi, M.M., Ansari, M.A., Al-Asmari, A.F., Ansari, M.N., Al-Anazi, W.A., Bahashwan, S., Almutairi, M.M., Alshammari, M., Khan, M.R., Alsaad, A.M., Alotaibi, M.R., 2018. Apremilast prevent doxorubicin-induced apoptosis and inflammation in heart through inhibition of oxidative stress mediated activation of NF-κB signaling pathways. Pharmacol. Rep., 70, 993–1000. [DOI] [PubMed]
- Kalyanaraman B., Joseph J., Kalivendi S., Wang S., Konorev E., Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol. Cell. Biochem. 2002;234–235:119–124. [PubMed] [Google Scholar]
- Kamble S.M., Patil C.R. Asiatic acid ameliorates doxorubicin-induced cardiac and hepato-renal toxicities with Nrf2 transcriptional factor activation in rats. Cardiovasc. Toxicol. 2018;18:131–141. doi: 10.1007/s12012-017-9424-0. [DOI] [PubMed] [Google Scholar]
- Khames, A., Khalaf, M.M., Gad, A.M., Abd El-Raouf, O.M., Kandeil, M.A., 2019. Nicorandil combats doxorubicin-induced nephrotoxicity via amendment of TLR4/P38 MAPK/NFκ-B signaling pathway. Chem. Biol. Interact., 311, 108777. [DOI] [PubMed]
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisada K., Tone E., Negoro S., Nakaoka Y., Oshima Y., Osugi T., Funamoto M., Izumi M., Fujio Y., Hirota H., Yamauchi-Takihara K. Bcl-xl reduces doxorubicin-induced myocardial damage but fails to control cardiac gene downregulation. Cardiovasc. Res. 2002;53:936–943. doi: 10.1016/s0008-6363(01)00506-5. [DOI] [PubMed] [Google Scholar]
- Li Y., Cohen R. Caspase inhibitors and myocardial apoptosis. Int. Anesthesiol. Clin. 2005;43:77–89. doi: 10.1097/01.aia.0000157492.14705.59. [DOI] [PubMed] [Google Scholar]
- Liu L.L., Li Q.X., Xia L., Li J., Shao L. Differential effects of dihydropyridine calcium antagonists on doxorubicin-induced nephrotoxicity in rats. Toxicology. 2007;231:81–90. doi: 10.1016/j.tox.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Liu X., Chua C.C., Gao J., Chen Z., Landy C.L., Hamdy R., Chua B.H. Pifithrin-alpha protects against doxorubicin-induced apoptosis and acute cardiotoxicity in mice. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H933–H939. doi: 10.1152/ajpheart.00759.2003. [DOI] [PubMed] [Google Scholar]
- M, R., 2019. Nephroprotective effect of Costus pictus extract against doxorubicin-induced toxicity on Wistar rat. Bangladesh J. Pharmacol., 14, 8.
- Mahgoub S., Sallam A.O., Sarhan H.K.A., Ammar A.A.A., Soror S.H. Role of Diosmin in protection against the oxidative stress induced damage by gamma-radiation in Wistar albino rats. Regul. Toxicol. Pharm. 2020;113:104622. doi: 10.1016/j.yrtph.2020.104622. [DOI] [PubMed] [Google Scholar]
- Mansour M.A., El-Kashef H.A., Al-Shabanah O.A. Effect of captopril on doxorubicin-induced nephrotoxicity in normal rats. Pharmacol. Res. 1999;39:233–237. doi: 10.1006/phrs.1998.0432. [DOI] [PubMed] [Google Scholar]
- Mohan M., Kamble S., Gadhi P., Kasture S. Protective effect of Solanum torvum on doxorubicin-induced nephrotoxicity in rats. Food Chem. Toxicol. 2010;48:436–440. doi: 10.1016/j.fct.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pacher P., Liaudet L., Bai P., Mabley J.G., Kaminski P.M., Virág L., Deb A., Szabó E., Ungvári Z., Wolin M.S., Groves J.T., Szabó C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- Perumal S., Langeshwaran K., Selvaraj J., Ponnulakshmi R., Shyamaladevi B., Balasubramanian M.P. Effect of diosmin on apoptotic signaling molecules in N-nitrosodiethylamine-induced hepatocellular carcinoma in experimental rats. Mol. Cell. Biochem. 2018;449:27–37. doi: 10.1007/s11010-018-3339-3. [DOI] [PubMed] [Google Scholar]
- Rashid M.I., Fareed M.I., Rashid H., Aziz H., Ehsan N., Khalid S., Ghaffar I., Ali R., Gul A., Hakeem K.R. Flavonoids and their biological secrets. Plant and Human Health. 2019;2:27. [Google Scholar]
- Rashid S., Ali N., Nafees S., Ahmad S.T., Arjumand W., Hasan S.K., Sultana S. Alleviation of doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in Wistar rats. Toxicol. Mech. Methods. 2013;23:337–345. doi: 10.3109/15376516.2012.759306. [DOI] [PubMed] [Google Scholar]
- Rashid, S., Nafees, S., Siddiqi, A., Vafa, A., Afzal, S.M., Parveen, R., Ali, N., Hasan, S.K., Barnwal, P., Shahid, A., Sultana, S., 2017. Partial protection by 18β Glycrrhetinic acid against Cisplatin induced oxidative intestinal damage in wistar rats: Possible role of NFkB and caspases. Pharmacol. Rep., 69, 1007-1013. [DOI] [PubMed]
- Rašković A., Stilinović N., Kolarović J., Vasović V., Vukmirović S., Mikov M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 2011;16:8601–8613. doi: 10.3390/molecules16108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M.U., Tahir M., Khan A.Q., Khan R., Oday O.H., Lateef A., Hassan S.K., Rashid S., Ali N., Zeeshan M., Sultana S. D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp. Biol. Med. (Maywood) 2014;239:465–476. doi: 10.1177/1535370213520112. [DOI] [PubMed] [Google Scholar]
- Sabbah, H.N., 2000. Apoptotic cell death in heart failure. Cardiovasc. Res., 45, 704-12. [DOI] [PubMed]
- Sanajou, D., Nazari Soltan Ahmad, S., Hosseini, V., Kalantary-Charvadeh, A., Marandi, Y., Roshangar, L., Bahrambeigi, S., Mesgari-Abbasi, M., 2019. β-Lapachone protects against doxorubicin-induced nephrotoxicity via NAD(+)/AMPK/NF-kB in mice. Naunyn Schmiedebergs Arch. Pharmacol., 392, 633-640. [DOI] [PubMed]
- Sayed-Ahmed M.M., Khattab M.M., Gad M.Z., Osman A.M. Increased plasma endothelin-1 and cardiac nitric oxide during doxorubicin-induced cardiomyopathy. Pharmacol. Toxicol. 2001;89:140–144. doi: 10.1034/j.1600-0773.2001.d01-148.x. [DOI] [PubMed] [Google Scholar]
- Sedeek M., Nasrallah R., Touyz R.M., Hébert R.L. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J. Am. Soc. Nephrol. 2013;24:1512–1518. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal. Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shalkami A.S., Hassan M., Bakr A.G. Anti-inflammatory, antioxidant and anti-apoptotic activity of diosmin in acetic acid-induced ulcerative colitis. Hum. Exp. Toxicol. 2018;37:78–86. doi: 10.1177/0960327117694075. [DOI] [PubMed] [Google Scholar]
- Silambarasan, T., Raja, B., 2012. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur. J. Pharmacol., 679, 81-9. [DOI] [PubMed]
- Tu H.K., Pan K.F., Zhang Y., Li W.Q., Zhang L., Ma J.L., Li J.Y., You W.C. Manganese superoxide dismutase polymorphism and risk of gastric lesions, and its effects on chemoprevention in a Chinese population. Cancer Epidemiol. Biomarkers Prev. 2010;19:1089–1097. doi: 10.1158/1055-9965.EPI-09-1174. [DOI] [PubMed] [Google Scholar]
- Tulubas F., Gurel A., Oran M., Topcu B., Caglar V., Uygur E. The protective effects of ω-3 fatty acids on doxorubicin-induced hepatotoxicity and nephrotoxicity in rats. Toxicol. Ind. Health. 2015;31:638–644. doi: 10.1177/0748233713483203. [DOI] [PubMed] [Google Scholar]
- Wali, A.F., Rashid, S., Rashid, S.M., Ansari, M.A., Khan, M.R., Haq, N., Alhareth, D.Y., Ahmad, A., Rehman, M.U., 2020. Naringenin regulates doxorubicin-induced liver dysfunction: impact on oxidative stress and inflammation. Plants (Basel), 9. [DOI] [PMC free article] [PubMed]
- Zhang Y., Xu Y., Qi Y., Xu L., Song S., Yin L., Tao X., Zhen Y., Han X., Ma X., Liu K., Peng J. Protective effects of dioscin against doxorubicin-induced nephrotoxicity via adjusting FXR-mediated oxidative stress and inflammation. Toxicology. 2017;378:53–64. doi: 10.1016/j.tox.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Zordoky B.N., Anwar-Mohamed A., Aboutabl M.E., El-Kadi A.O. Acute doxorubicin toxicity differentially alters cytochrome P450 expression and arachidonic acid metabolism in rat kidney and liver. Drug Metab. Dispos. 2011;39:1440–1450. doi: 10.1124/dmd.111.039123. [DOI] [PubMed] [Google Scholar]