Highlights
-
•
Biological ZnONPs have considerable bactericidal against pathogenic fish bacteria.
-
•
Biological ZnONPs reduced the bacterial load in water and fish tissues.
-
•
Biological ZnONPs enhanced FCR feeding and swimming behaviors.
-
•
Biological ZnONPs stimulated fish health and production.
Keywords: ZnONPs, Biological, Physiological changes, Antimicrobial properties, Nile tilapia
Abstract
This work aims to evaluate the antibacterial activity of biological zinc nanoparticles (BIO-ZnONPs) against pathogenic fish bacteria and assess the effect of BIO-ZnONPs on the performance, behavior, and immune response in Nile tilapia (Oreochromis niloticus) as compared to chemical zinc nanoparticles (CH- ZnONPs). Aspergillus niger TS16 fabricated the BIO-ZnONPs were spherical shape with the average size of 45 nm and net charge of −27.23 mV. Generally, the results indicate that BIO-ZnONPs were more effective than CH- ZnONPs in enhancing the performance properties of Nile tilapia. Five experimental groups of Nile tilapia (initial body weight of 20.2 g) were treated with two concentrations of 0.5 and 1 mg L−1 from biological and chemical ZnONPs, while the fifth group was served as a control. After ten weeks of treated water with ZnONPs, the performance, feed efficiency parameters, feeding, and swimming behaviors significantly improved in BIO-ZnONPs treated groups (P < 0.05). The liver function, LYZ activity, and NBT values were significantly enhanced in the 0.5 mg L−1 BIO-ZnONPS group compared to CH- ZnONPs group and control (P < 0.05). Furthermore, the lowest cortisol and the highest testosterone and growth hormone levels were recorded in 1 mg L−1 BIO-ZnONPs group. Regarding the antibacterial effects, BIO-ZnONPs displayed the lower total bacterial loads in water and fish tissues (intestine, gills, skin, and muscle) and the maximum antibacterial properties against pathogenic bacteria (Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Aeromonas hydrophila). Our study exemplifies novel findings of BIO-ZnONPs in the promotion of fish health and production and its antibacterial properties in Nile tilapia.
1. Introduction
Aquaculture is a fast-growing agricultural sector to cover the increasing demand for worldwide animal protein, and it is linked to the natural environment through diversified relationships (FAO, 2020). Nile tilapia (Oreochromis niloticus) is an ideal model for aquaculture since its high rate of reproduction and growth, and tolerance to different environmental conditions and increasing demand in the market (El-Sayed, 2006). One of the most important environmental factors affecting fish production is the quantity, quality, and water source. Water source must be containing a high level of dissolved oxygen and optimum temperature throughout the culture period (Ngugi et al., 2007). Nanotechnology plays an important role in aquaculture through a wide range of applications such as water treatment, sterilization of culture ponds, effective delivery of hormones and vaccines to enhance the ability of fish to absorb these nutrients, moreover, controlling aquatic diseases (Bhattacharyya et al., 2015). Also, nanotechnology provides core applications for water treatment to provide a safe habitat for fish breeding. The zinc oxide nanoparticles (ZnONPs) are the most commonly utilized nanomaterials (NMs). Globally, ZnONPs have gained special attention because of their environmentally friendly characters with various excellent applications such as anticancer, antimicrobial, and photocatalysis, etc. (Jiang et al., 2018).
Moreover, it is classified as generally recognized as safe “GRAS” substance by the US FDA (He et al., 2014). Commonly, several studies have been reported on the influences of ZnONPs on the growth performance, behavior, blood biochemistry, oxidative stress, immunity, hormones, and antioxidant enzymes of animals and fish (Yusof et al., 2019, Taherian et al., 2019, Mohammady et al., 2021). Besides good influences on the genetic properties of fish (Abdel-Khalek et al., 2020). ZnONPs can be synthesized using several methods such as physical, chemical, and biological methods (by microbes or plants). The biological synthesis of NPs is safe, clean, biocompatible, and eco-friendly besides, the fast reduction of metal ions at ambient conditions of temperature unlike physical or chemical methods, they consume extensive energy or use toxic solvents, respectively (El-Saadony et al., 2020a, Akl et al., 2020, El-Saadony et al., 2019, El-Saadony et al., 2021a, El-Saadony et al., 2021b). From the biological methods used for the synthesis of NPs, fungus-mediated procedure gained great importance (Thakkar et al., 2010). Fungal biosynthesis of ZnONPs, especially using Aspergillus niger is a widely used method to produce monodispersed NPs with a broad spectrum of chemical compositions, high yield of chemically and thermally stable NPs, because fungi produce a large amount of protein and enzymes causing the stability (Kalpana et al., 2018), besides these nanoparticles can be commercially produced since the scalability of fungi. Furthermore, fungi can tolerate greater metal concentrations, have tremendous binding ability, and can reduce the larger amount of metal ions into metal NPs via the secretion of a high number of extra-cellular redox proteins and enzymes (Yusof et al., 2019). The ZnONPs can be extensively utilized as compared to bulk ZnO because of their unique antimicrobial characteristics (Padmavathy and Vijayaraghavan, 2008, Reda et al., 2021).
There is no information about the effects of ZnONPs on enhancing the microbial load in the fish gut; however, little information about the Bio-ZnONPs impact on the growth performance and physiological responses of Nile tilapia fish up to the present time. Therefore, this study aims to estimate the antibacterial activity of BIO-ZnONPs against pathogenic fish bacteria and determine the effect of these nanoparticles on reducing the microbial load in fish organs as compared to CHE-ZnONPs. Also, evaluate the effects of both BIO-ZnONPs and CHE-ZnONPs treated water on the growth performance parameters, serum biochemical parameters, and changes of fish behaviors, immune and physiological responses of Nile tilapia.
2. Materials and methods
2.1. Isolation, screening, and identification of Zinc-tolerant fungus
The stock solutions of Zinc Nitrate (Zn (NO3)2·6H2O) (Sigma-Aldrich Germany) were prepared at 5 mM concentration as follows: 1.49 g of Zn (NO3)2·6H2O was dissolved in 1Liter of sterilized deionized water. Zinc Nitrate (1 mM) was obtained by the dilution of 200 mL of stock solution (5 mM) to liter with sterilized deionized water, and 400 mL of stock solution (5 mM) was diluted to 1 L with sterilized deionized water to obtain 2 mM concentration. The prepared solutions were stored for use in the experiments. Soil samples were gathered from the rhizospheric soils of plants grown at Abu-Hammad City, Wady El-Moulak village, Sharkia governorate, Egypt. Ten gram of soil samples were well stirred (Remi Magnetic Stirrer, 1MLH, India) in 90 mL sterile saline solution for 15 min to obtain 10−1. 1 mL of previous dilution (1 0 −1) was transferred to 9 mL saline solution to obtain 10−2, 1 mL of previous dilution (1 0 −2) was transferred to 9 mL saline solution to obtain 10−3, etc., until 10−7. 100 µL of each dilution was spread over potato dextrose agar plates supplemented with different levels of Zinc Nitrate (1, 2, and 5 mM). The previous PDA plates were incubated at 28 °C for 96 h (Majeed et al., 2018). The Zinc-tolerant fungus was identified using morphological and cultural properties, i.e., Surface topography [flat, raised, heaped, folded, domed, radial grooved], Surface texture [glabrous, suede-like, powdery, granular, fluffy, downy, cottony], Surface pigmentation [white, cream, yellow, brown, pink, grey, black etc.], Reverse pigmentation [none, yellow, brown, red, black, etc.] besides, comparing them with confirmed representatives of different species (Akintobi et al., 2011). The microscope identification was carried out as follows. The loop of fungi mycelium was placed onto a clean slide containing a drop of 5% potassium permanganate. The fungi portion was teased onto the potassium permanganate stain using a sterile needle, then gently covered to avoid air bubbles. The slide was then mounted and viewed under the microscope with the 10x and 40x objective lens (Mailafia et al., 2017). The obtained image of fungus was compared with a documented book of fungi (Sarah and Watkinson, 2017). And the identification was confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (Brurker Daltonics, Bremen, Germany) (Hunt et al., 2004).
2.2. Biosynthesis and optimization of Zinc oxide nanoparticles
The biological synthesis of ZnONPs was following the method of Yehia and Ahmed (2013) briefly. The isolated Zinc-tolerant fungus was grown on mineral salt medium (MSM) that composed of K2HPO4, 2.0 g; KH2PO4, 7.0 g; (NH4)2SO4, 1.0 g; MgSO4·7H2O, 0.1 g; Yeast extract, 0.6 g and glucose, 10.0 g/L, pH value was maintained at 6.2 ± 0.2. Then incubated in an orbital shaker (Lab-line® Incubator-Shaker-ORBIT, USA) at 30 °C/180 rpm for 96 h. The fungal culture was filtered using filter paper (Whatman no.1) to obtain the supernatant. The fungal supernatant (20 mL) was homogenized in 80 mL of Zinc nitrate (5 mM) and incubated at a shaking incubator at 30 °C/180 rpm for 96 h. The resultant white precipitate was indicated the biotransformation of ZnONPs. The reaction mixture was centrifuged at 15,000 rpm for 15 min to obtain the precipitate. Conical flasks with either fungal filtrate or Zinc nitrate were incubated at the same previous conditions served as a positive and negative control, respectively. BIO-ZnONPs size was optimized by several parameters one at a time, i.e., temperature (15, 20, 25, 30, and 35 °C), pH (3, 4, 5, 6, and 7), fungal supernatant: Zn (NO3)2.6H2O (v/v) (1:9, 2:8, 3:7, 4:6, and 5:5), and reaction time (1, 2, 3, 4, and 5 days). Also, various used medium (Potato dextrose broth (PDB), Sabouraud’s broth (SB), Mineral salt medium (MSM), Richard medium (RM), and Malt Glucose-Yeast Extract-Peptone (MGYP), and agitation speed (100, 140, 180, 220, and 260 rpm). The particle size of the BIO-ZnONPs was estimated using the Dynamic light scattering (DLS) technique.
2.3. Synthesis ZnONPs by chemical method
1.5 g of Zn (NO3)2·6H2O was dissolved in 1000 mL of sterilized deionized water to obtain (5 mM) and then stirred at hot plate magnetic stirrer (70 °C) for 25 min, Then NaOH (10%) solution was slowly added drop by drop into the zinc nitrate beaker and stirred for 2 h at 70 °C. The mixed solution was warm out then filtered by Whatman filter paper. The filtered sample was dried in the oven at 160 °C for 210 min and ashed at 300 °C for 5 h in a muffle furnace following the method of Becheri et al. (2008) with some modifications.
2.4. Characterization of biological and chemical ZnONPs
The UV–visible absorption spectra of synthesized ZnONPs (biological or chemical form) were measured using UV–visible Laxco™ dual-beam spectrophotometer “model Laxco™, Alpha-1502 Alpha Series Spectrophotometer”, 200–1000 nm (Mettler-Toledo LLC., Columbus, OH, USA) (Forough and Fahadi, 2011). Besides, the Transmission electron microscopy images were obtained by (JEOL 1010, Japan) at an accelerated voltage of 200 kV for particle size and shape Aguilar-Méndez et al., 2011), also, Zeta sizer analyzer (Nano “Z2 Malven, Malvern Hills, UK”), for the size analysis and Zeta potential for estimating the charge as per El-Saadony et al. (2018). Fourier transform-infrared (FTIR) spectroscopy (“Bruker Tensor 37, Kaller”, Germany) was used to determine the active compounds in the BIO-ZnONPs at the wavelength range of 500–3500 cm−1 (Chattopadhyay et al., 2013).
2.5. In vitro assessment of antibacterial activities of ZnONPs
2.5.1. Bacterial strains
The pathogenic bacterial strains; Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Aeromonas hydrophila were used in this study.
2.5.2. Disc diffusion assay
A freshly prepared Mueller-Hinton agar (MHA) medium was used to assess the antibacterial activities of different concentrations of BIO-ZnONPs and CH-ZnONPs (50, 100, 150, 200, and 250 μg mL−1) against tested bacteria. An inoculum (500 µL) from each bacterium was cultured in 5 mL of Mueller-Hinton broth and incubated overnight at 37 °C for further use. 100 μL of the activated bacterial culture was then spread on the sterile Mueller-Hinton agar plate. Discs (5 mm) diameter of Whatman filter paper were soaked in biological and chemical ZnONPs solutions until saturation, then immediately placed on the Mueller-Hinton agar plates (Langfield et al., 2004). The MHA plates were incubated at 37 °C for 24 h, the resultant inhibition zones of ZnONPs were measured using a transparent ruler (mm) (Reda et al., 2020, Sheiha et al., 2020). The Zn (NO3)2·6H2O was served as the positive control.
2.5.3. Determination of MIC and MBC
A freshly prepared Mueller-Hinton (MH) broth medium was used for the determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of BIO-ZnONPs and CHE-ZnONPs against the tested bacterial strains. An inoculum of 500 µL (2.5 × 105 CFU mL−1) from each bacterial strain was added to 9 mL of MH broth medium supplemented with 500 µL of each concentration from BIO-ZnONPs and CH-ZnONPs (50,100,150, 200 and 250 μg mL−1). The culture of bacteria without ZnONPs was used as a negative control and Zn (NO3)2·6H2O was served as the positive control. The lower concentration inhibited bacterial growth was considered the MIC (El-Saadony et al., 2021b). The MBC was measured by sub-culturing the MIC levels onto sterile MH agar plates and incubated at 37 °C for 24 h. Then, the minimum concentration of ZnONPs that killed 100% of bacterial growth was considered MBC.
2.6. Fish management and experimental design
2.6.1. Ethical approval
All experimental procedures for the present study were conducted following the Local Experimental Animal Care Committee and subsequently approved by the Institutional Ethics Committee, Department of Animal Production, Faculty of Agriculture, Zagazig University, Zagazig, Egypt. The present work was performed at the Experimental Fish Wet Lab belonging to the Department of plant microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt.
2.6.2. Fish culture and maintenance
Nile tilapia (Oreochromis niloticus) in fry stage (20.2 ± 0.46 g) (n = 150) were obtained from the hatchery of Central Laboratory for Fish Research, belonging to the Ministry of Agriculture, Egypt. Fish were acclimated to the ambient laboratory conditions for two weeks in 15 glass aquaria (45 × 28 × 80 cm/each) containing 100 L dechlorinated water. Fish were grouped into five treatments with three replicates (15 aquaria) and each one includes ten fish.
All groups were fed on a basal pelleted diet (20% soybean meal, corn 23.0%, fish meal 15.0%, alfalfa hay 14.0%, wheat bran 13.0%, Corn gluten meal 11.5%, sunflower oil 1%, 1% vitamin mixture, 0.5% mineral mixture and 1% carboxymethyl cellulose). The diet's proximate chemical composition was 31.12% crude protein, 5.40% ether extract, 5.73% crude fiber, and gross energy of 19.17 MJ per 100 g. All fish were fed daily to apparent satiation.
2.6.3. Experimental setup
Fish were exposed to 0.5 and 1 mg L−1 of both forms of chemically and biologically synthesized ZnONPs consecutive ten for weeks. The control group was left in aged tap water only. The concentrations used in the present study were selected according to the related literature of Hao and Chen (2012). The semi-static exposure in the experimental setup occurred through daily siphoning 60% of the water in the morning and 40% in the evening and then replaced by a fresh one. Later, following water change, sonicated ZnONPs were applied to the water at the same dose rate. The physicochemical parameters of water should be weekly measured, and the values were maintained as the temperature of 25.4 ± 0.3 °C, dissolved oxygen of 7.30 ± 0.13 mg L−1, pH 7.90 ± 0.06, and total hardness of 132 ± 4.4 mg CaCO3 L−1. The tap water was maintained as 0.4, 0.05, 0.6, and 0.8 mmol L−1 for Na+, K+, Mg+, and Ca++, respectively.
2.7. Physiological characteristics
2.7.1. Fish behaviors
2.7.1.1. Ingestive behavior
The ingestive behavior of fish was evaluated according to Colgan (1986). The feeding behavior has been assessed with the actual consumption of fish to food at the feeding time. Simultaneously, the meantime (sec) and the mean frequency of feeding have been recorded every three h. Additionally, the foraging behavior has been evaluated with searching for consuming food and the fish grazing on the aquarium's side or bottom or surface. While the meantime (sec) and the mean frequency of foraging have been recorded every three h.
2.7.1.2. Surfacing behavior
The surfacing behavior was measured as the frequency of the fish rises periodically to the surface to gulp air, according to Noga (2010). The mean time (sec) and the mean frequency of surfacing have been recorded every 3 h.
2.7.1.3. Swimming behavior
The swimming behavior of fish was measured in terms of the rapid or slow movement (up, middle, and down) in the aquarium without any behavior activity described by Anras and Lagardère (2004). Whereas the meantime (sec), the mean frequency, the site (surface, middle, or bottom) of swimming, have been recorded every 3 h.
2.7.1.4. Resting behavior
Resting behavior was evaluated when fish were inactive and motionless on the bottom with an open eye because they have no eyelids (Sekiguchi and Kohshima, 2003). The mean time (sec) and the mean frequency of resting have been recorded every 8 h.
2.7.1.5. Body care behavior
The body-shaking referred to the lateral shaking of the entire body two or three times in rapid succession (Sekiguchi and Kohshima, 2003). The mean time (sec) and the mean frequency of body shaking have been recorded every 8 h. On the other hand, scratching (chafing) referred to rubbing any part of the body against any object (Coppedge and Shaw, 1997). The mean time (sec) and the mean frequency of scratching have been recorded every 8 h.
2.7.1.6. Aggressive behavior
The aggressive behavior patterns have been defined and recorded according to Kramer and Bauer (45), whereas the meantime (sec.) and the mean frequency of aggression have been recorded every 8 h. The patterns of aggression include the following; Approach (direct movement of one fish toward another), chasing (the frequency of swim of fish vigorously after another), fin tugging (bites of fish the fin of another), biting (sharp mouth contact of one fish against any region of another fish), butting (the fish butt with the snout against genital papilla of another), fleeing (the swimming of fish away from the opponent), mouth pushing (frequency of two fish stands face to face with their opened mouth against each other), spreading of fins (expand or spread its all fins), and arousal frequency and duration (Sec) (in which fish has a locomotor activity).
2.8. Fish performance traits
The fish live productive traits such as weight gain (WG) between two successive weeks has been individually calculated according to the following formulas: WG (g) = W2- W1,
Specific growth rate (SGR; %/day) = 100 [Ln W2 (g) − Ln W1 (g)]/T, whereas W1 and W2 are the initial and final weight, respectively, and T is the number of rearing days during the feeding duration (70 days). Total feed intake (TFI) (g feed/fish) was calculated by partitioning the total amounts of the pelleted diets offered to fish throughout the whole experimental period on the total number of fish. Feed conversion ratio (FCR) = TFI/WG,
Survival rate (SR) (%) = (Fish numbers at the end of the experiment/ Fish number at the beginning of the experiment) × 100.
2.9. Blood sampling and measured parameters
At the end of the experimentation period, 3 mL of blood was sampled in sterile Eppendorf tubes from the caudal veins using a sterile heparinized needle. The samples were then kept on ice. Serum was collected after centrifugation at 3000 rpm for 15 min (Abdelnour et al., 2020a, Abdelnour et al., 2020b). Blood glucose (GLU) level (Trinder, 1969) and serum transaminases (alanine transferase (ALT), and aspartate transferase (AST)) (Reitman and Frankel, 1957) were determined using specific kits. Respiratory burst activity was determined using nitro blue tetrazolium (NBT) reagent following Secombes and Olivier (1997). Serum lysozyme (LYZ) activity was evaluated using a microtiter plate procedure by ELISA reader at a wavelength of 520 nm as described by Soltani and Pourgholam (2007). The fish testosterone and progesterone (Cuisset et al., 1994), follicle-stimulating hormone (FSH) (Aizen et al., 2007), Growth Hormone (GH) (Nash et al., 2000), and cortisol (Tintos et al., 2006) were evaluated using commercially purchased enzyme-linked immune sorbent assay (ELISA) kits.
2.10. Total bacterial and Aeromonas spp. Count in water and fish tissues
Water samples from each aquarium at 1st, 4th, 8th, and 10th week of the experiment to evaluate the total bacterial count and the total count of Aeromonas spp. Similarly, three fish from each treated group were sampled (muscles, skins, gills, and intestines) to evaluate the total bacterial count and total Aeromonas spp. count. 10 mL of water samples were mixed in a conical flask containing 90 mL of saline peptone buffer water and serial dilution was done. Similarity: 10 g of fish organs were homogenized in a conical flask containing 90 mL of saline peptone buffer the water and serial dilution were done. 100 µL of each dilution was spread over plate count agar medium (PCA) used to enumerate total bacterial count after incubation at 30 °C for 48 h (Ashour et al., 2020, Abdelnour et al., 2020a, Alagawany et al., 2021). Aeromonas agar medium was used to calculate total Aeromonas spp., count after incubation at for 37 °C and 48 h. The presumptive green represented Aeromonas spp., with black centered colonies surrounded by clear yellow to a honey color zone. The total count of bacterial loads and Aeromonas spp., were expressed as log CFU ml−1 in water and fish samples (El-Saadony et al., 2020b).
2.11. Statistical analysis
All data were tested for normality and homogeneity by Kolmogorov–Smirnov’s test and Bartlett's test (Glass, 1966), respectively. The values were then statically analyzed by the ANOVA test at p ≤ 0.05 level and means compared by least significant difference (LSD) test by SPSS program version 20 (SPSS, Richmond, VA, USA).
3. Results
3.1. Isolation and identification of Zinc-tolerant fungus
Initially, 27 isolates were appeared in plates supplemented with 1 mM Zinc nitrate (Zn (NO3)2·6H2O). The isolates were coded from TS1-TS27. 13 isolates existed in 2 mM Zinc nitrate (Zn (NO3)2·6H2O) plates and only one fungus isolate (TS16) appeared in 5 mM Zinc nitrate (Zn (NO3)2·6H2O) supplemented plates. Based on light microscope examination, the selected zinc-tolerant fungus colonies on potato dextrose agar are initially white, quickly becoming black with conidial production indicate that isolate is Aspergills niger. MALDI-TOF MS confirmed the identification as Aspergills niger.
3.2. Physicochemical optimization of BIO-ZnONPs
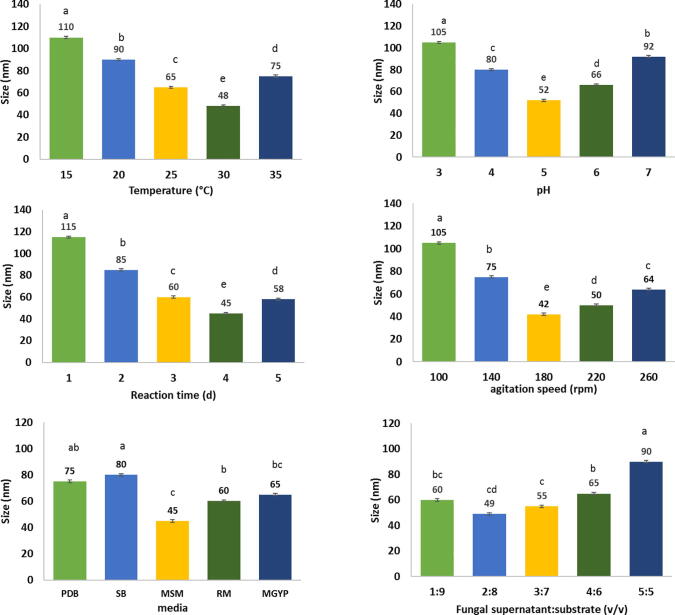
Fig. 1 showed the optimization of the biosynthesis parameters, i.e., temperature, pH incubation time, the ratio between fungal supernatant and Zinc nitrate, medium, and agitation speed to achieve the better size of obtained BIO-ZnONPs by DLS. The DLS analysis shows that the smallest size of BIO-ZnONPs of 48 nm occurred at 30 °C, and the size was significantly decreased (p ≤ 0.05) when temperature deviated from 15 to 30 °C, but, further temperature about 30 °C, the BIO-ZnONPs size was increased. Also, the size of BIO-ZnONPs was significantly decreased with the increment of reaction time from 1 to 4 days then the size was increased after that, the smallest size of 45 nm was achieved after 4 days incubation. The BIO-ZnONPs with the size of 52 nm occurred at pH 5. Moreover, the size of BIO-ZnONPs significantly decreased (p ≤ 0.05) by the increase of fungal supernatant. However, the best size of 49 nm was obtained in 20 mL of fungal supernatant to 80 mL of Zinc nitrate (2:8, v/v) ratio. Also, the best medium was MSM obtained the size of BIO-ZnONPs (45 nm). Besides, the best agitation speed was 180 rpm that gave BIO-ZnONPs with the size of 42 nm.
Fig. 1.
Optimization of the physiochemical parameters for BIO-ZnONPs fabricated by A. niger TS16. Data in the figure represent means ± SD. Different lowercases above the columns indicate significant differences between the treatments at p ≤ 0.05.
3.3. Characterization of BIO-ZnONPs and CHE-ZnONPs
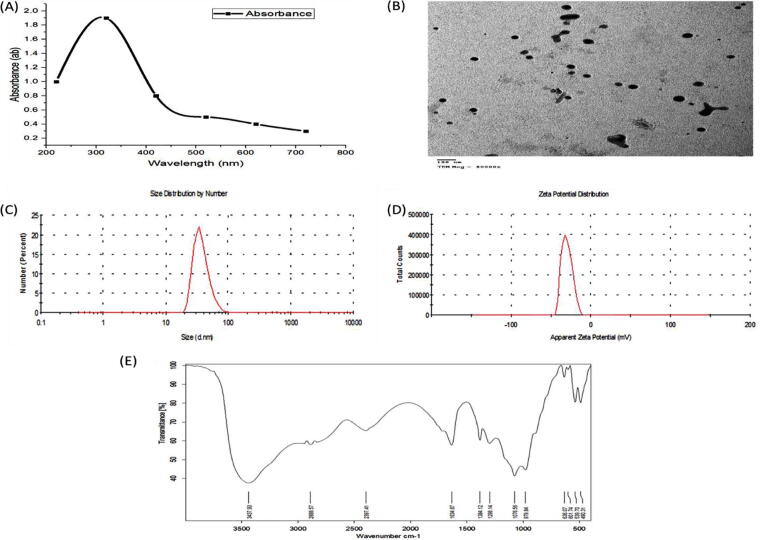
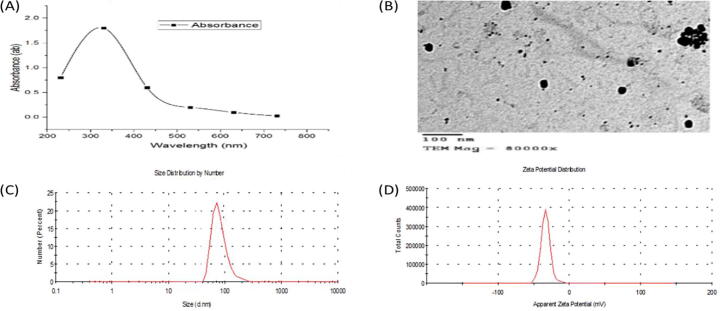
The addition of fungal supernatant to Zinc nitrate solution under optimum conditions led to the change of the mixture color to white, confirming the transformation of BIO-ZnONPs. The UV absorbance of this mixture was in the range of 300–380 nm and the maximum absorbance at 320 nm (Fig. 2a). TEM image showed that the synthesized BIO-ZnONPs were spherical in shape and in a size range of 10–65 nm (Fig. 2b). The FTIR spectrum of BIO-ZnONPs witnessed twelve bands at 3437.90, 2888.75, 2397.41, 1634.87, 1384.12, 1298.14, 1076.56, 979.84, 636.07, 601.74, 539.70, and 490.31-cm−1. These bands are responsible for several functional groups, e.g., alcohol, primary and secondary amines, amides, phenols (Fig. 2e). Also, (Fig. 2c, d) showed the DLS analysis of the monodispersed BIO-ZnONPs with the average size of 45 nm obtained by zeta sizer and net charge of −27.23 mV obtained by zeta potential. On the other hand, CHE-ZnONPs solution was absorbed the U.V. spectrum in the range of 300–380 nm and the maximum absorbance at 330 nm (Fig. 3a), and they are spherical with the size range of 13–76 nm obtained by TEM (Fig. 3b). The average size and net charge of CHE-ZnONPs estimated by zeta sizer and zeta potential were 75 nm and −25.44 mV, respectively (Fig. 3c, d).
Fig. 2.
Characterzation of BIO-ZnONPs (A-E): (A) UV–Visible spectrum of biological ZnONPs, (B) Transmission Electron Microscope (TEM), (C) the accurate size of BIO-ZnONPs by zeta sizer, (D) the net charge of BIO-ZnONPs by zeta potentional, (E) FT-IR analysis for BIO-ZnONPs.
Fig.3.
The characterzation of CH-ZnONPs (A-D): (A) UV–Visible spectrum; (B) Transmission Electron Microscope (TEM), (C) the accurate size of CH-ZnONPs by zeta sizer, (D) the net charge of CH-ZnONPs by zeta potentional.
3.4. Antibacterial activities of ZnONPs
The antibacterial activity of BIO-ZnONPs and CHE- ZnONPs were tested in vitro by measuring the diameter of the inhibition zone against six bacterial pathogens: Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Aeromonas hydrophila using the disc diffusion method (Table 1). Both forms of ZnONPs displayed excellent antibacterial activities against all the tested bacteria.
Table 1.
Antibacterial activity of chemically synthesized (CH-ZnONPs) and biological synthesized (BIO-ZnONPs) against pathogenic bacterial strains expressed by inhibition zone (mm) produced by concentration, MIC, and MBC.
| Bacterial strains |
CH-ZnONPs Concentration (μg mL−1) |
BIO-ZnONPs Concentration (μg mL−1) |
CH |
BIO |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | 50 | 100 | 150 | 200 | 250 | MIC | MBC | MIC | MBC | |
| L. monocytogenes | 12 ± 0.6bE | 16 ± 0.3aD | 19 ± 0.5aC | 22 ± 0.2bcBC | 24 ± 0.2bB | 16 ± 0.5aD | 19 ± 0.2aC | 22 ± 0.1aBC | 24 ± 0.4bB | 29 ± 0.2bA | 65d | 105e | 50d | 90e |
| B. cereus | 13 ± 0.5aEF | 15 ± 0.4bE | 18 ± 0.1bD | 23 ± 0.1bB | 25 ± 0.6abB | 15 ± 0.1abE | 17 ± 0.3bD | 20 ± 0.2bC | 25 ± 0.3abB | 27 ± 0.2bA | 70 cd | 120d | 60 cd | 100d |
| S. aureus | 15 ± 0.4aE | 17 ± 0.2aD | 20 ± 0.2aCD | 25 ± 0.4aBC | 28 ± 0.7aB | 16 ± 0.2aD | 20 ± 0.4aCD | 22 ± 0.2aC | 26 ± 0.2aBC | 31 ± 0.1aA | 65d | 110e | 55d | 95e |
| E. coli | 10 ± 0.6cE | 13 ± 0.1cD | 17 ± 0.5bC | 21 ± 0.2cBC | 23 ± 0.8bB | 13 ± 0.4bD | 15 ± 0.3cD | 18 ± 0.3cC | 22 ± 0.4cBC | 26 ± 0.3cA | 75c | 130c | 65c | 110c |
| P. aeruginosa | 11 ± 0.4bE | 14 ± 0.2bD | 16 ± 0.1cC | 20 ± 0.6cBC | 22 ± 0.1cB | 13 ± 0.6bD | 16 ± 0.3cC | 19 ± 0.5bcBC | 23 ± 0.4cB | 27 ± 0.2cA | 80b | 150b | 70b | 130b |
| A. hydrophila | 9 ± 0.6cEF | 12 ± 0.4cE | 15 ± 0.3cD | 19 ± 0.4dBC | 21 ± 0.2cB | 12 ± 0.3cE | 14 ± 0.2cdD | 17 ± 0.4cC | 21 ± 0.5cB | 24 ± 0.5cdA | 90a | 160a | 80a | 140a |
Values means ± SD, means with different lowercases in same column indicate a significant difference in the effect of each NPs concentration on bacterial strains, means with different blue uppercases in same raw indicate a significant difference between inhibition zones of CH and BIO-NPs concentration P < 0.05.
Table 1 showed all bacterial strains affected by BIO-ZnONPs and CH-ZnONPs levels (50, 100, 150, 200, and 250 µg/mL). The inhibition zones diameter (IZD) of BIO-ZnONPs significantly p ≤ 0.05 increased with a relative increase of about 10% over CHE-ZnONPs IZD. Zinc nitrate 50 μg mL−1 (control) gave zones <8 mm against some tested bacteria (data not shown). S. aureus, B. cereus, and P. aeruginosa were the most sensitive bacteria towards BIO- ZnONPs (250 µg/mL), i.e., 31, 29, and 28, moreover, CHE- ZnONPs (250 µg/mL), i.e., 28, 25 and 23 mm, respectively. The bacterial susceptibility towards both forms of ZnONPs is obvious in G+ bacteria than G− bacteria because of their cell wall structure.
3.5. MIC and MBC estimation
The minimum inhibitory concentration (MIC) is the lowest concentration of BIO- ZnONPs and CHE- ZnONPs that inhibited bacterial growth, but the lowest concentration that killed the tested bacteria is the minimum bactericidal concentration (MBC). The diverse BIO-ZnONPs levels displayed strong concentration-dependent antibacterial activities as compared to Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Aeromonas hydrophila, where MIC values of BIO-ZnONPs in the range of 50–80 µg mL−1 while MBC ranged from 90 to 140 µg mL−1 against tested bacterial strains, while the MIC levels of CHE-ZnONPs were larger than BIO-ZnONPs with relative increase 15% and the MBC levels in the range of 105–160 µg mL−1 for CHE-ZnONPs indicating the antibacterial efficiency of BIO-ZnONPs (Table 1).
3.6. Growth performance
Growth performance in terms of the final body weight (FBW), weight gain (WG), specific growth rate (SGR), total feed intake (TFI), survival rate percentage (SR%), and feed conversion ratio (FCR) of Nile tilapia in response to different doses of CH-ZnONPs and BIO-ZnONPs were illustrated in (Table 2). There were significant differences in FBW, TFI, WG, SGR, FCR, and SR% (P < 0.05) among different groups. The maximum values of FBW, TFI, WG, SGR, and SR% and the lowest values FCR were recorded in both groups of BIO-ZnONPs compared with CH-ZnONPs and control groups. Meanwhile, their lowest levels were recorded in fish groups treated with 1 mg L−1 CH-ZnONPs.
Table 2.
Growth performance of Nile tilapia (Oreochromis niloticus) treated with different levels of chemically synthesized (CH-ZnONPs) and bio-fabricated zinc oxide nanoparticles (BIO-ZnONPs) for 10 weeks (means ± SE).
| Parameters | Treatment (mg L−1) |
P value | ||||
|---|---|---|---|---|---|---|
| CTR0.0 | CH-ZnONPs |
BIO-ZnONPs |
||||
| 0.5 | 1 | 0.5 | 1 | |||
| IBW (g) | 20.2 ± 0.46 | 19.8 ± 0.21 | 20.4 ± 0.38 | 19.9 ± 0.24 | 20.2 ± 0.22 | 0.856 |
| FBW (g) | 43.0 ± 0.21c | 47.4 ± 0.94b | 40.2 ± 0.25d | 51.5 ± 0.18a | 50.8 ± 0.23a | 0.005 |
| WG (g) | 22.8 ± 0.34c | 27.6 ± 0.98b | 19.8 ± 0.26d | 31.6 ± 0.31a | 30.6 ± 0.43a | <0.001 |
| SGR (%/day) | 1.05 ± 0.02c | 1.21 ± 0.031b | 0.94 ± 0.01d | 1.32 ± 0.012a | 1.28 ± 0.02ab | 0.023 |
| TFI (g feed/fish) | 33.6 ± 0.24c | 44.01 ± 1.13b | 29.79 ± 0.30d | 40.34 ± 0.21a | 39.03 ± 0.27a | < 0.001 |
| FCR | 1.47 ± 0.04b | 1.59 ± 0.05c | 1.50 ± 0.08a | 1.28 ± 0.019d | 1.27 ± 0.042d | < 0.001 |
| SR (%) | 100 ± 0.09a | 90 ± 0.23c | 96.6 ± 0.53b | 100 ± 0.09a | 96.7 ± 0.22b | 0.012 |
IBW: Initial body weight, FBW: Final body weight, WG: Weight gain, SGR: Specific growth rate, TFI: Total feed intake, FCR: Feed conversion ratio, SR: Survival rate. Means having different superscript letters in the same row are significantly different at P < 0.05.
3.7. Reproductive and feeding behaviors
Behaviors of Nile tilapia treated in water with different CH-ZnONPs and BIO-ZnONPs levels for ten weeks were illustrated in Table 3. The feeding behavior (time and frequency) was significantly improved (P < 0.05) with the addition of 1 mg L−1 BIO-ZnONPs. In another trend, the treatment of fish with 1 mL L−1 CH-ZnONPs induced a significant adverse effect (P < 0.05). Besides, the fish took a long time to swim with 1 mg L−1 BIO-ZnONPs (P < 0.05). Regarding the resting and arousal behavior (time and frequency), their values were significantly increased with 1 mg L−1 CH-ZnONPs treatment (P < 0.05). However, both exposure doses of BIO-ZnONPs in water-induced significantly reduced motility and activity of treated fish. Considering the foraging behavior and sensitivity of fish to food stimuli, the fish group treated with 0.5 mg L−1 BIO-ZnONPs rise to the water surface. Concerning the aggression (time and frequency), the aggression was increased with 0.5 mg L−1 CH-ZnONPs treatment if compared with the BIO-ZnONPs treatment. Meanwhile, it was suppressed in comparison with the control group. The sexual behavior was significantly improved with BIO-ZnONPs treatment and decreased with CH-ZnONPs treatment, although the differences were non-significant.
Table 3.
Different behavior traits of Nile tilapia (Oreochromis niloticus) treated with different levels of chemically synthesized (CH-ZnONPs) and biological zinc oxide nanoparticles (BIO-ZnONPs) for 10 weeks.
| Items | Treatment (mg L−1) |
P value | ||||
|---|---|---|---|---|---|---|
| CTR 0.0 | CH-ZnONPs |
BIO-ZnONPs |
||||
| 0.5 | 1 | 0.5 | 1 | |||
| Feeding time (sec) | 122.0 ± 2.35bc | 138.0 ± 4.25ab | 71.0 ± 3.25c | 177.0 ± 2.15ab | 240.0 ± 2.45a | 0.015 |
| Feeding frequency | 6.0 ± 0.36c | 7.6 ± 0.68b | 6.0 ± 0.56c | 8.2 ± 0.85b | 11.3 ± 0.35a | 0.009 |
| Foraging time (Sec) | 148.0 ± 0.89 | 225.8 ± 1.35 | 76.0 ± 1.63 | 171.0 ± 0.69 | 115.0 ± 0.89 | 0.942 |
| Foraging frequency | 4.2 ± 0.42c | 7.4 ± 0.43ab | 5.0 ± 0.39bc | 8.2 ± 0.41a | 5.5 ± 0.52abc | 0.002 |
| Surfacing time (Sec) | 292.0 ± 1.26ab | 410.0 ± 1.36a | 198.0 ± 1.53b | 210.0 ± 2.35b | 222.5 ± 3.51ab | 0.025 |
| Surfacing frequency | 9.6 ± 0.51 | 8.8 ± 0.35 | 8.8 ± 0.68 | 9.2 ± 1.25 | 8.0 ± 0.42 | 0.436 |
| Swimming time (sec) | 1147.0 ± 0.26abc | 1074.0 ± 0.31bc | 914.0 ± 0.35c | 1334.0 ± 0.42ab | 1400.0 ± 0.27a | < 0.001 |
| Swimming frequency | 25.8 ± 0.05a | 28.0 ± 0.25a | 16.8 ± 0.03bc | 12.6 ± 0.23c | 21.0 ± 0.01ab | 0.014 |
| Resting time (sec) | 393.0 ± 1.08b | 294.0 ± 1.78bc | 561.0 ± 1.56a | 191.0 ± 2.35c | 255.0 ± 1.26bc | 0.002 |
| Resting frequency | 8.6 ± 0.21b | 7.6 ± 0.27b | 12.4 ± 0.34a | 8.4 ± 0.39b | 9.3 ± 0.28ab | 0.005 |
| Arousal time (sec) | 907.0 ± 1.36bc | 880.0 ± 0.89c | 1292.0 ± 0.72a | 1152.0 ± 1.02ab | 1128.8 ± 1.25abc | < 0.001 |
| Arousal frequency | 23.2 ± 0.05bc | 28.8 ± 0.21b | 38.8 ± 0.01a | 14.0 ± 0.03d | 18.8 ± 0.02 cd | < 0.001 |
| Aggression time (sec) | 197.0 ± 0.32a | 145.0 ± 0.19ab | 83.0 ± 0.14bc | 28.4 ± 0.16c | 19.8 ± 0.12c | 0.006 |
| Aggression frequency | 21.0 ± 0.05a | 17.8 ± 0.41a | 6.2 ± 0.06b | 4.0 ± 0.39b | 2.8 ± 0.37b | 0.023 |
| Body shaking | 0.80 ± 0.29 | 0.80 ± 0.08 | 1.20 ± 0.46 | 0.80 ± 0.04 | 1.25 ± 0.35 | 0.127 |
| Scratching | 0.6 ± 0.38 | 1.0 ± 0.42 | 1.0 ± 0.23 | 1.0 ± 0.63 | 1.0 ± 0.20 | 0.134 |
| Huddling time (sec) | 230.0 ± 0.86 | 330.0 ± 1.08 | 398.0 ± 1.05 | 151.0 ± 0.99 | 127.5 ± 1.36 | 0.574 |
| Huddling frequency | 3.8 ± 0.25b | 6.2 ± 0.48b | 15.4 ± 1.03a | 6.0 ± 0.89b | 5.0 ± 0.75b | 0.019 |
| Sexual behaviour time (sec) | 36.0 ± 0.45 | 26.0 ± 0.75 | 17.0 ± 0.42 | 46.4 ± 0.75 | 37.5 ± 0.63 | 0.204 |
| Sexual behaviour frequency | 3.4 ± 0.23 | 2.2 ± 0.04 | 1.2 ± 0.42 | 3.4 ± 0.01 | 2.3 ± 0.02 | 0.276 |
Means having different superscript letters in the same column are significantly different at P < 0.05.
3.8. Fish hematology, immunity, and hormonal changes
Table 4 showed the serum biochemical parameters of Nile tilapia treated with different levels of CH-ZnONPs and BIO-ZnONPs for ten weeks (means ± SE). Blood GLU level and serum ALT and AST values significantly increased in the fish group treated with 1 mg L-1 CH-ZnONPs after ten weeks compared with other groups. Regarding LYZ activity and NBT values, the fish group treated with 0.5 mg L−1 BIO-ZnONPs for ten weeks exhibited the highest LYZ and NBT, which were significantly elevated concerning the control group (P < 0.05). The hormonal changes of Nile tilapia treated with different levels of CH-ZnONPs and BIO-ZnONPs for ten weeks (means ± SE) were illustrated in Table 5. The FSH levels, GH and testosterone, were significantly increased (P < 0.05) in the fish group exposed to 0.5 mg L-1 BIO-ZnONPs compared to other groups. Meanwhile, Cortisol levels were significantly reduced in groups treated with 1 mg L−1 BIO-ZnONPs for ten weeks compared with the other groups. While all ZnONPs treated groups significantly have lower levels of FSH and progesterone compared with the control group.
Table 4.
Serum biochemical parameters and immune responses of Nile tilapia (Oreochromis niloticus) treated with different levels of chemically synthesized (CH-ZnONPs) and bio-fabricated zinc oxide nanoparticles (BIO-ZnONPs) for 10 weeks (means ± SE).
| Parameters | Treatment (mg L−1) |
P value | ||||
|---|---|---|---|---|---|---|
| CTR |
CH-ZnONPs |
BIO-ZnONPs |
||||
| 0.00 | 0.5 | 1 | 0.5 | 1 | ||
| GLU (mg/dl) | 105.83 ± 1.03b | 104.74 ± 1.93b | 120.70 ± 1.70a | 103.82 ± 1.80b | 105.81 ± 1.13b | 0.031 |
| ALT (U/l) | 56.04 ± 1.09b | 63.60 ± 1.76b | 70.03 ± 2.01a | 55.9 ± 1.48b | 50.60 ± 1.94b | 0.020 |
| AST (U/1) | 127.8 ± 1.12c | 145.16 ± 1.82b | 148.43 ± 1.32a | 127.57 ± 1.87c | 128.9 ± 1.28c | 0.006 |
| NBT (OD 630) | 0.34 ± 0.72d | 0.44 ± 0.80c | 0.12 ± 0.59e | 0.78 ± 0.92a | 0.56 ± 0.88b | 0.002 |
| LYZ (μg/mL) | 1.22 ± 0.67c | 1.57 ± 0.78b | 1.01 ± 0.47d | 1.89 ± 0.97a | 1.68 ± 0.87ab | <0.001 |
AST: Aspartate aminotransferase, ALT: Alanine transaminase, NBT: Nitro blue tetrazolium, LYZ: Lysozyme activity; Means having different superscript letters in the same row are significantly different at P < 0.05.
Table 5.
Serum hormonal changes of Nile tilapia (Oreochromis niloticus) treated with different levels of chemically synthesized (CH-ZnONPs) and bio-fabricated zinc oxide nanoparticles (BIO-ZnONPs) for 10 weeks (means ± SE).
| Parameters | Treatment (mg L−1) |
P value | |||||
|---|---|---|---|---|---|---|---|
| CTR |
CH-ZnONPs |
BIO-ZnONPs |
|||||
| 0.00 | 0.5 | 1 | 0.5 | 1 | |||
| FSH (mIU/ml) |
Males | 0.22 ± 0.02d | 0.29 ± 0.02c | 0.26 ± 0.01c | 0.48 ± 0.2a | 0.35 ± 0.02b | < 0.001 |
| Females | 0.96 ± 0.02a | 0.68 ± 0.03c | 0.76 ± 0.03b | 0.59 ± 0.01d | 0.35 ± 0.01e | < 0.001 | |
| Testosterone (ng/ml) | 2.15 ± 0.06c | 2.46 ± 0.08b | 1.54 ± 0.05b | 2.85 ± 0.04a | 2.73 ± 0.07a | < 0.001 | |
| Progesterone (ng/ml) | 0.81 ± 0.02a | 0.48 ± 0.01e | 0.55 ± 0.02d | 0.63 ± 0.02c | 0.69 ± 0.02b | 0.002 | |
| GH (ng/ml) | 0.57 ± 0.03d | 0.77 ± 0.03c | 0.88 ± 0.05b | 0.91 ± 0.02a | 0.50 ± 0.01e | 0.011 | |
| Cortisol (ng/ml) | 56.3 ± 0.91e | 70.5 ± 1.3b | 79.2 ± 2.1a | 66.9 ± 1.2c | 59.2 ± 1.1d | < 0.001 | |
FSH: Follicle stimulating hormone, GH: Growth hormone.; Means having different superscript letters in the same row are significantly different at P < 0.05.
3.9. Microbiological counts
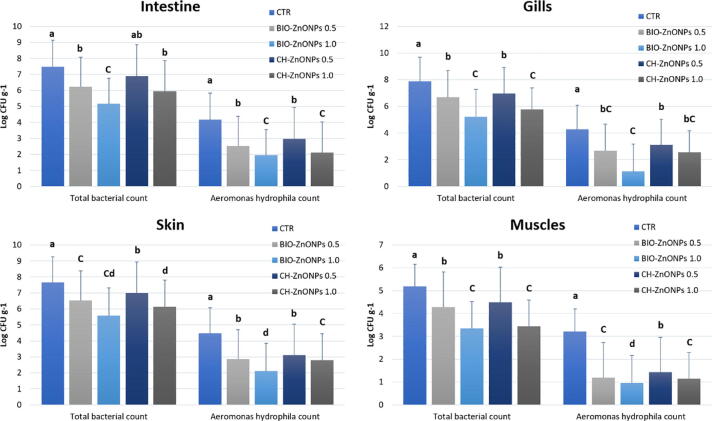
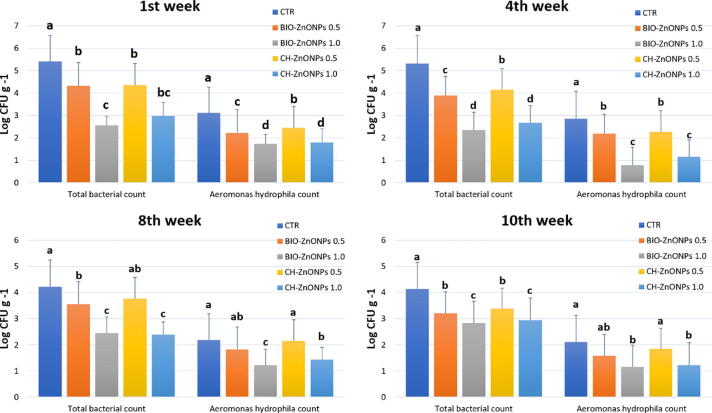
The changes occurred in the total bacterial counts and the total count of Aeromonas spp., in different fish tissues (intestines, gills, skin, and muscles) (Fig. 4), and water of different treatments 1st, 4th, 8th and 10th week of the experiment. Significant decrease in the total bacterial count and total Aeromonas spp., (P < 0.05) in BIO-ZnONPs groups (Fig. 5), the highest total bacterial counts and Aeromonas spp., counts were recorded from intestines, gills, skin, and fish muscles treated with CH-ZnONPs and in the control group. From these results, it can be suggested that BIO-ZnONPs are more efficient than CH-ZnONPs in lowering the total bacterial counts and Aeromonas spp., counts in water and fish organs.
Fig. 4.
Total bacterial count and the total count of Aeromonas hydrophila in different tissues of Nile tilapia (intestine, gills, skin, and muscles) treated with several levels of chemical and biological ZnONPs for 10 weeks.
Fig. 5.
Total bacterial counts and the total count of Aeromonas hydrophila of rearing water samples treated with several levels of chemical and biological ZnONPs sampled during the experimental period (10 weeks).
4. Discussion
Microbes are potent eco-friendly Nano factories and can control the size and shape of biological nanoparticles (Reda et al., 2021, El-Saadony et al., 2021a,b). The biological Synthesis of metallic nanoparticles, can be split into two categories, bioreduction and biosorption. In bioreduction, metal ions are biologically reduced into more stable forms which is coupled with oxidation of enzymes. Biosorption involves binding metal ions from an aqueous solution or soil sample onto the organism. It does not require energy (Pantidos and Horsfall, 2014). Nevertheless, only a few microbes are reported to have synthesized ZnO NPs. There is a need to investigate more potential microbes to synthesize ZnONPs (Yusof et al., 2019). The Zinc tolerant fungus Aspergillus niger TS16 was used in the present study was isolated from soil and used for biotransformation of ZnONPs. Aspergillus niger can produce ZnONPs by extracellular and intracellular routes. However, it has more potential for extracellular synthesis. Extracellular synthesis is enzyme-mediated such as a nitrate reductase enzyme secreted in the medium and reduces metal ions to metal nanoparticles. The ZnONPs are formed when electrons are transported from NADH by an enzyme NADH reductase to Zn+2 which are reduced into ZnONPs. In intracellular synthesis, metal ions are transported within the cell wall. These are reduced by enzymes present there and then the nanoparticles were formed in the periplasmic space and cytoplasm. The intracellular mechanism for nanoparticles bio-fabricated by verticillium sp., consisting of three steps trapping, bioreduction, and capping (Yusof et al., 2019). The optimization process is essential to achieve the better size of obtained ZnONPs. The increase of pH value led to an increase of NPs size since the pH of the reaction mixture changes the catalytic activity of the enzymes secreted by the fungus (Kathiresan et al., 2009). Also the size of NPs increased at high temperatures because of the accumulation of particles (Suvith and Philip, 2014). Besides, the increments in fungal supernatant ere decreased nanoparticles sizes and reaction time because of the extensive amount of fungal enzyme. Nevertheless, further supernatant increase led to the increment of nanoparticles size that may be the saturation of reaction mixture (enzyme + substrate) (Shahzad et al., 2019). MSM was the best media due to the presence of the necessary nutrient for optimum fungal growth to release reducing agents to produce NPs. Also, agitation allows more collision between salt and fungi supernatant, and thus produces more NPs. In this finding, the produced biological and chemical ZnONPs were characterized by several instruments. The obtained results were in accordance with Fakhari et al. (2019) found that the UV spectrum of ZnONPs was in the range of 320–390 nm, also, Sharmila et al. (2018) found that ZnONPs SPR peak was observed at 375 nm. On the other hand, Naveed Ul Haq et al. (2017) stated that ZnO nanoparticles with an average size of 57.72 nm. Lactobacillus sporogens was investigated to produce ZnONPs of diameter of 5–15 nm. Fungal supernatant is incubated with a zinc salt solution and kept in the dark for a particular time in the intracellular synthesis while in the extracellular synthesis fungal filtrate is treated with salt solution. Jain et al. (2013) demonstrated that Aspergillus aeneus isolate NJP12 exhibited the highest capacity for extracellular synthesis of ZnO nanoparticles. Jacob et al. (2014) found that synthesized ZnONPs by Aspergillus niger supernatant were spherical with an average diameter of 39.4–82.6 nm. Both BIO-ZnONPs and CHE-ZnONPs showed respectable antibacterial activities against all the tested bacterial strains. Their effects were more pronounced on Gram-positive bacteria than Gram-negative bacteria. Consistent with the findings, it can be summarized that BIO-ZnONPs are considered as effective antibacterial agents against both Gram-positive and Gram-negative bacteria (Meruvu et al., 2011). These findings were also similar to the study of Emami-Karvani and Chehrazi (2011), who illustrated that ZnONPs were effective against Gram-positive than Gram-negative bacteria. Similarly, Busi et al. (2016) reported that the potential antibacterial activities of ZnONPs at concentrations of 100, 200, 300, 400, and 500 µg mL−1. Soren et al. (2018) illustrated that the MIC of ZnONPs inhibited the growth of E. coli and Vibrio parahaemolyticus was observed at 100 and 200 µg/mL, respectively, meanwhile, the MBC of ZnONPs against E. coli and V. parahaemolyticus were reported at 200 and 400 µg mL−1. To the best of our knowledge, the structure of the outer layer of Gram-positive bacteria is composed of peptidoglycan; meanwhile, Gram-negative bacteria are composed of phospholipids, and both undergo different interactions when exposed to ZnONPs. Several studies suggested some possible antibacterial mechanisms of ZnONPs; they easily penetrated bacterial cells and released zinc ions (Zn2+) that inhibit active transport, bacterial enzymes required for metabolic activity and finally induced cell death because of the huge reactive surface of NPs. The other proposed that the formation of reactive oxygen species (ROS) generated from the surface of NPs leads to oxidative stress and subsequent cell damage (Agarwal et al., 2018).
Also, Seil and Webster (2012) suggested that positively charged ZnONPs electrostatically bind the bacterial cell membrane and damage the bacterial cell integrity, intracellular contents released and cell demolished. It can be concluded that the obtained results showed that BIO-ZnONPs are more efficient in inactivating pathogenic bacteria than in CHE-ZnONPs and zinc nitrate. There were significant differences in FBW, TFI, WG, SGR, and FCR (P < 0.05) between different groups in the current study. The maximum values of FBW, TFI, WG, SGR, and FCR were recorded in both groups of BIO-ZnONPs compared with CH-ZnONPs and control groups. The differences that exist in WG and SGR are linked to the response of fish to different sources and concentrations of Zn, along with the increased bioavailability of Zn from the used ZnONPs (Tan and Mai, 2001). The data indicated that ZnONPs promoted the growth more than the control groups. Similar results were recorded by Taheri et al. (2017). Attractively, ZnONPs with small particle size facilitates the absorption of Zn through gills, digestive tract, and skin directly from water and feed (Al-Taee et al., 2015). Also, the enhancement of feed efficiency and utilization in biological ZnO NPs treated groups could be due to the effects of small-sized of Zn on promoting a varied physiological activity, including sensing, digestion, absorption, and storage produced energy (Jiang et al., 2018). We assume that this effect is closely linked to their beneficial effect in enhancing the TFI, thereby stimulate the fish to take more feed. Moreover, its small size has facilitated the passage of particles across tissues and cell membranes, leading to improved gastrointestinal tract absorption and bioavailability (Onuegbu et al., 2018). Also, BIO-ZnONPs may stimulate both RNA and DNA synthesis and cell division for somatic growth (Şıklar et al., 2003). Zinc (Zn) is an essential microelement for physiological functions in fish. The application of ZnNPs in fish diet enhanced the bioavailability and absorbance of Zn in fish (Mohammedy et al., 2021). These findings indicated that Zn is essential for the growth and development of fish at lower concentrations, but higher Zn levels are harmful (Murugan et al., 2008). The higher survival rate percentage was recorded in fish groups treated with low doses of BIO-ZnONPs, and this suggests that this form is non-toxic for exposed fish (Abdelazim et al., 2018). Knowledge of feeding behavior of tilapia adult fish reared under ZnONPs treated water is fundamental to understanding the role of these critical elements on fish health and reproduction in the ecosystem (Williamson, 1986). According to our study, the feeding behavior (time and frequency) of fish treated was significantly improved (P < 0.05) with the addition of 1 mg L−1 BIO-ZnONPs. On the other trend, the treatment of fish water with 1 mg L−1 CH-ZnONPs induced a significant adverse effect (P < 0.05). Our results were supported by Rajendran (2013), who reported that feed additives in nanoforms enhanced the growth and improved the immunity via enhancement of the ration criteria. Moreover, the dietary supplementation of ZnONPs can increase the feeding rate, and subsequently, growth rates and growth hormone (Zhang et al., 2017). The freshwater fish increasingly uptake Zn from the diet. It is the predominant route (Kaya et al., 2015). The behavioral effects might be due to the small size of biological ZnONPs form and different physicochemical properties compared with other synthetic procedures. As a result, BIO-ZnONPs with higher mobility and uptake throughout the membrane has improved interaction between the tissues biological processes. Consequently, an increment of the nanoparticle surface area could increase reactive groups more than their conventional form and improve their reactivity. It can also lead to a high biological response in living cells, otherwise not observable in other chemical forms (Yusof et al., 2019). On the contrary, chemical metals in the aquatic environment could suppress motivation for feeding and induce disturbance of the proper feeding behavior, food searching, and disturbances in the foraging behavior (Kasumyan, 2001). Thus, it was described that high levels of elemental Zn could be considered neurotoxic, inducing the central nervous system (Sarasamma et al., 2018). Our findings illustrate that BIO-ZnONPs with low concentration is believed to be more eco-friendly, economical, and non-toxic than chemically and physically synthesized forms (Jiang et al., 2018). Our results illustrated that the fish took a long-time swimming with a high level of BIO-ZnONPs in treated water (P < 0.05). However, the resting and arousal behavior (time and frequency) was significantly increased with a high CH-ZnONPs treatment level (P < 0.05). Alkaladi et al. (2015) revealed that the 96 h LC50 value of CH-ZnONPs in Nile tilapia was 3.1 ± 0.4 mg L−1. According to Taherian et al. (2019), the 96 h LC50 value of BIO-ZnO-NP was 25.50 mg L−1 implied the low risk of biologically produced nanoparticles compared to chemically prepared ones. The safety properties of biological methods for the synthesis of nanoparticles can be referred to using nontoxic and environmentally materials combined with green technology and the final products are more acceptable and safer for the aquatic environment than conventional methods (Shah et al., 2015).
Considering the foraging behavior and sensitivity of fish to food stimuli, the fish group treated with 0.5 mg L−1 BIO-ZnONPs rise to the water surface. Concerning the aggression (time and frequency), the aggression was increased with 0.5 mg L−1 CH-ZnONPs treatment if compared with the BIO-ZnONPs treatment; meanwhile, it was suppressed if compared with the control group and these results differed from that obtained by Ellgaard et al. (1995). Fish growth hormone (GH) was related to improved fish behavior via improving appetite, aggression, swimming activities, and reducing the anti-predator behavior of fish, thus undesirable fluctuations in fish behaviors and feed consumption when exposed to CH-ZnONPs that reduced GH (JÖnsson and BjÖrnsson, 2002). The biological ZnO-NPs play an important role in enhancing serum biochemical response, oxidative stress, liver function, hormones, immunity antioxidant enzymes capacity, growth-promoting, modulating the digestive enzymes, and related gene expression due to the high Zn availability (Mohammedy et al., 2021). The present study showed hyperglycemic Nile tilapia after rearing water with 1 mg L−1 of CH-ZnONPs. There was a significant increase in ALT and AST levels in the same treated group with hyperglycemia. Parallel to our study, Taheri et al. (2017) reported that the activities of liver enzymes of common carp (Cyprinus carpio) were significantly elevated in the 15 mg ZnONPs kg−1 diet supplemented group. It might be linked to a block of glucose transfer inside the cells, which may subsequently lead to elevation of blood glucose levels and reduced glycogenesis in both liver and muscles (Sharafeldin et al., 2015). Besides, Jyothi and Narayan (1999) reported that hyperglycemia might be attributed to the partial or fully blocking process of glucose transport from the bloodstream to the liver by stress and impaired insulin secretion due to toxicity. Also, Sharafeldin et al. (2015) revealed that such enhancement of glycogen breakdown in the liver of tilapia after carbaryl exposure is probably due to the toxicity effect, which caused hyperglycemia and increase of catecholamine secretion and corticosteroid hormones. This attribution was in cooperation with our study finding that increasing the CH-ZnONPs levels followed by increasing serum glucose and cortisol levels.
Regarding LYZ activity and NBT values in our study, although there were significant differences among different groups, the 0.5 mg L−1 BIO-ZnONPs group showed significantly higher levels than the other groups. Our results were in concordance with the findings of Kaya et al. (2016). They illuminated that LYZ levels of Nile tilapia were non-significantly decreased during exposure to 1 mg L−1 of small form ZnONPs and exposure to10 mg L−1 of large form ZnONPs versus the control group. Meanwhile, the group exposed to 1 mg L−1 large size ZnONPs exhibited a significant reduction in LYZ levels versus the control group.
The enhancement of non-specific immune status may be due to the nanoscale of the Zn macromolecule, which altered its properties and improved its efficiency (Rather et al., 2011). Hence, the ZnONPs can stimulate the immune response by activating toll-like receptors and down-regulating pro-inflammatory cytokines (Tawfik et al., 2017). Also, Zn has a high positive antioxidant property, reducing the interaction between antioxidants and immune systems (Rather et al., 2011). The fish hormonal changes are closely linked with environmental pollution, mainly induced by metal toxicity (Bakshi and Panigrahi, 2018). Furthermore, Scott and Sloman (2004) elucidated that pollutants and environmentally toxic substances caused hormonal disruption, neurological alterations, and metabolic disorders in many fish. In our study, higher GH levels and testosterone were detected in tilapia males treated with 0.5 mg L−1 of BIO-ZnONPs, with a significant decrease in cortisol level. At the same trend, Alkaladi et al. (2020) reported a significant in serum GH, FSH, testosterone with a substantial elevation of cortisol levels of Nile tilapia exposed to a higher dose (1.23 and 2.05 µ L−1) of CH-ZnONPs. There no available studies on reducing microbial load in fish organs by BIO-ZnONPs.
5. Conclusion
In this study, ZnONPs were successfully synthesized by the biological method that was simple, cheap, safer and eco-friendly than the chemical and physical methods. In the biological approach, natural sources act as stabilizing and reducing agents for forming nanoparticles with controlled size and shape. ZnONPs can easily be synthesized using fungi as the biological system. Aspergillus niger TS16 can be manipulated under controlled conditions and has great potential for extracellular synthesis of ZnONPs. The proteins stabilized the biosynthesized ZnONPs in fungal supernatant. The application of biological ZnONPs to water enhanced the growth and behavior of fish. Also, immunity, liver function, and growth hormones were enhanced. In another way, their potential in vitro antibacterial effects against most common bacterial pathogens infection Nile tilapia directs our vision for possible in vivo application to control the invading and emergent pathogens. Plans are directed towards testing the biological ZnONPs form on other freshwater fish species and expand our research for in vivo treatment trials of some virulent bacterial pathogens.
Funding
The current work was funded by Taif University, Saudi Arabia, for financial support through its Researchers Supporting Project (TURSP-2020-105).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors appreciate and thank Taif University, Saudi Arabia, for financial support through its Researchers Supporting Project (TURSP-2020-105).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdelazim A.M., Saadeldin I.M., Swelum A.A.A., Afifi M.M., Alkaladi A. Oxidative stress in the muscles of the fish Nile tilapia caused by zinc oxide nanoparticles and its modulation by vitamins C and E. Oxid. Med. Cell. Longev. 2018;1–9 doi: 10.1155/2018/6926712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Khalek A.A., Morsy K., Shati A. Comparative assessment of genotoxic impacts induced by zinc bulk-and nano-particles in nile tilapia, Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2020;104(3):366–372. doi: 10.1007/s00128-020-02799-9. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19(1):1046–1056. doi: 10.1080/1828051X.2020.1815598. [DOI] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A., Abd El-Hack M.E., Al-Shargi O.Y., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 doi: 10.1016/j.livsci.2020.104220. [DOI] [Google Scholar]
- Agarwal H., Menon S., Kumar S.V., Rajeshkumar S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018;286:60–70. doi: 10.1016/j.cbi.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Aguilar-Méndez M.A., San Martín-Martínez E., Ortega-Arroyo L., Cobián-Portillo G., Sánchez-Espíndola E. Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides. J. Nanoparticle Res. 2011;13(6):2525–2532. doi: 10.1007/s11051-010-0145-6. [DOI] [Google Scholar]
- Aizen J., Kasuto H., Golan M., Zakay H., Levavi-Sivan B. Tilapia Follicle-Stimulating Hormone (FSH): immunochemistry, stimulation by gonadotropin-releasing hormone, and effect of biologically active recombinant FSH on steroid secretion. Biol. Reprod. 2007;76(4):692–700. doi: 10.1095/biolreprod.106.055822. [DOI] [PubMed] [Google Scholar]
- Akintobi A., Okonko I., Agunbiade S., Akano O., Onianwa O. Isolation and identification of fungi associated with the spoilage of some selected fruits in Ibadan, South Western Nigeria. Acad. Arena. 2011;3(11):1–10. [Google Scholar]
- Akl B., Nader M.M., El-Saadony M.T. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020;11(1):1–8. doi: 10.21608/jacb.2020.76656. [DOI] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276(2) doi: 10.1016/j.anifeedsci.2021.114920. [DOI] [Google Scholar]
- Alkaladi A., Afifi M., Ali H., Saddick S. Hormonal and molecular alterations induced by sub-lethal toxicity of zinc oxide nanoparticles on Oreochromis niloticus. Saudi J. Biol. Sci. 2020;27(5):1296–1301. doi: 10.1016/j.sjbs.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkaladi A., El-Deen N.A.N., Afifi M., Zinadah O.A.A. Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J. Biol. Sci. 2015;22(5):556–563. doi: 10.1016/j.sjbs.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Taee S.K.E., Al-Hamdani A.H.A. Effect of CuSO4 on toxicity of nano zinc oxide (nZnO) in carp fish (Cyprinus carpio L.) J. Limnol. Freshw. Fisheries Res. 2015;1(3):99–102. doi: 10.17216/LimnoFish-5000114439. [DOI] [Google Scholar]
- Anras, M., L.,B., Lagardère, J.P., 2004. Measuring cultured fish swimming behaviour: first results on rainbow trout using acoustic telemetry in tanks. Aquaculture. 240(1-4):175-86. DOI: 10.1016/j.aquaculture.2004.02.019
- Ashour E.A., El-Hack M.E.A., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10(10):457. doi: 10.3390/agriculture10100457. [DOI] [Google Scholar]
- Bakshi A., Panigrahi A. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol. Rep. 2018;5:440–447. doi: 10.1016/j.toxrep.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becheri A., Dürr M., Nostro P.L., Baglioni P. Synthesis and characterization of zinc oxide nanoparticles: application to textiles as UV-absorbers. J. Nanoparticle Res. 2008;10(4):679–689. doi: 10.1007/s11051-007-9318-3. [DOI] [Google Scholar]
- Bhattacharyya A., Reddy S., Hasan M., Adeyemi M., Marye R. Nanotechnology-a unique future technology in aquaculture for the food security. Int. J. Bioassays. 2015;4(7):4115–4126. doi: 10.21746/IJBIO.2015.07.0017. [DOI] [Google Scholar]
- Busi S., Rajkumari J., Pattnaik S., Parasuraman P., Hnamte S. Extracellular synthesis of zinc oxide nanoparticles using Acinetobacter schindleri SIZ7 and its antimicrobial property against foodborne pathogens. J. Microbiol. Biotechnol. Food Sci. 2016;5(5):407. doi: 10.15414/jmbfs.2016.5.5.407-411. [DOI] [Google Scholar]
- Chattopadhyay S., Dash S.K., Ghosh T., Das D., Pramanik P., Roy S. Surface modification of cobalt oxide nanoparticles using phosphonomethyl iminodiacetic acid followed by folic acid: a biocompatible vehicle for targeted anticancer drug delivery. Cancer Nanotechnol. 2013;4(4):103–116. doi: 10.1007/s12645-013-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan, P., 1986. The motivational basis of fish behaviour. The behaviour of teleost fishes: Springer; 23-46.
- Coppedge, B.R., Shaw, J.H., 1997. Effects of horning and rubbing behavior by bison (Bison bison) on woody vegetation in a tallgrass prairie landscape. Am. Midl. Nat. 138:189-96. https://doi.org/10.2307/2426665
- Cuisset B., Pradelles P., Kime D.E., Kühn E.R., Babin P., Davail S., Le Menn F. Enzyme immunoassay for 11-ketotestosterone using acetylcholinesterase as laberl: application to the measurement of 11-ketotestosterone in plasma of Siberian sturgeon. Comp. Biochem. Physio. Part C: Pharmacology, Toxicology and Endocrinology. 1994;108(2):229–241. doi: 10.1016/1367-8280(94)90035-3. [DOI] [Google Scholar]
- Ellgaard E., Ashley S., Langford A. Kinetic analysis of the swimming behavior of the goldfish, Carassius auratus, exposed to nickel: hypoactivity induced by sublethal concentrations. Bull. Environ. Contam. Toxicol. 1995;55(6):929–936. doi: 10.1007/BF00209475. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69(1) [Google Scholar]
- El-Saadony M.T., Desoky E.S.M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony, M.T., El-Hack, A., Mohamed, E., Taha, A.E., Fouda, M.M., Ajarem, J.S., N. Maodaa, S., Allam, A.A., Elshaer, N., 2020a. Ecofriendly Synthesis and Insecticidal Application of Copper Nanoparticles against the Storage Pest Tribolium castaneum. Nanomaterials. 10(3):587. https://doi.org/10.3390/nano10030587 [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9(5):639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N., El-Fattah H., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7(4):238–249. doi: 10.17582/journal.aavs/2019/7.4.238.249. [DOI] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah A., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles biosynthesized by Bacillus subtilis ssp spizizenii MT5 isolated from heavy metals polluted soil. Zagazig J. Agric. Res. 2018;45(6):2439–2454. doi: 10.21608/zjar.2018.47889. [DOI] [Google Scholar]
- El-Sayed, A. F. M. Tilapia culture in salt water: environmental requirements, nutritional implications and economic potentials. Avances en Nutricion Acuicola.. 2006:96–106. [Google Scholar]
- Emami-Karvani Z., Chehrazi P. Antibacterial activity of ZnO nanoparticle on gram-positive and gram-negative bacteria. Afr. J. Microbiol. Res. 2011;5(12):1368–1373. doi: 10.5897/AJMR10.159. [DOI] [Google Scholar]
- Fakhari, S., Jamzad, M., Kabiri, Fard, H., 2019. Green synthesis of zinc oxide nanoparticles: a comparison. Green. Chem. Lett. Rev. 12(1):19-24. https://doi.org/10.1080/17518253.2018.1547925
- FAO, 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. https://doi.org/10.4060/ca9229en
- Forough M., Fahadi K. Biological and green synthesis of silver nanoparticles. Turkish J. Eng. Env. Sci. 2011;34(4):281–287. doi: 10.3906/muh-1005-30. [DOI] [Google Scholar]
- Glass G.V. Testing homogeneity of variances. Am. Educ. Res. J. 1966;3(3):187–190. doi: 10.2307/1161802. [DOI] [Google Scholar]
- Hao, L., Chen, L., 2012. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 80:103-10. doi:https://doi.org/10.1016/j.ecoenv.2012.02.017 [DOI] [PubMed]
- He, X., Hwang, H.M., Aker, W.G., Wang, P., Lin, Y., Jiang, X., He, X., 2014. Synergistic combination of marine oligosaccharides and azithromycin against Pseudomonas aeruginosa. Microbiol. Res. 169 (9-10): 759-767. DOI: 10.1016/j.micres.2014.01.001 [DOI] [PubMed]
- Hunt J., Boddy L., Randerson P.F., Rogers H.J. An evaluation of 18S rDNA approaches for the study of fungal diversity in grassland soils. Microb. Ecol. 2004;47(4):385–395. doi: 10.1007/s00248-003-2018-3. [DOI] [PubMed] [Google Scholar]
- Jacob S., Bharathkumar R., Ashwathram G. Aspergillus niger mediated synthesis of ZnO nanoparticles and their antimicrobial and in vitro anticancerous activity. World J. Pharm. Res. 2014;3(2):3044–3054. [Google Scholar]
- Jain N., Bhargava A., Tarafdar J.C., Singh S.K., Panwar J. A biomimetic approach towards synthesis of zinc oxide nanoparticles. Appl. Microbiol. Biotechnol. 2013;97(2):859–869. doi: 10.1007/s00253-012-3934-2. [DOI] [PubMed] [Google Scholar]
- Jiang J., Pi J., Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018;3:1–18. doi: 10.1155/2018/1062562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JÖnsson, E., BjÖrnsson, B.T., 2002. Physiological functions of growth hormone in fish with special reference to its influence on behaviour. Fish Sci. 68(sup1):742-8. https://doi.org/10.2331/fishsci.68.sup1_742
- Jyothi B., Narayan G. Certain pesticide-induced carbohydrate metabolic disorders in the serum of freshwater fish Clarias batrachus (Linn.) Food Chem. Toxicol. 1999;37(4):417–421. doi: 10.1016/s0278-6915(99)00020-4. [DOI] [PubMed] [Google Scholar]
- Kalpana V.N., Kataru B.A.S., Sravani N., Vigneshwari T., Panneerselvam A., Devi Rajeswari V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano. 2018;3:48–55. doi: 10.1016/j.onano.2018.06.001. [DOI] [Google Scholar]
- Kasumyan A. Effects of chemical pollutants on foraging behavior and sensitivity of fish to food stimuli. J. Ichthyol. 2001;41(1):76–87. [Google Scholar]
- Kathiresan K., Manivannan S., Nabeel M., Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf. B Biointerface. 2009;71(1):133–137. doi: 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Kaya H., Aydın F., Gürkan M., Yılmaz S., Ates M., Demir V., Arslan Z. Effects of zinc oxide nanoparticles on bioaccumulation and oxidative stress in different organs of tilapia (Oreochromis niloticus) Environ. Toxicol. Pharmacol. 2015;40(3):936–947. doi: 10.1016/j.etap.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Kaya H., Aydın F., Gürkan M., Yılmaz S., Ates M., Demir V., Arslan Z. A comparative toxicity study between small and large size zinc oxide nanoparticles in tilapia (Oreochromis niloticus): Organ pathologies, osmoregulatory responses and immunological parameters. Chemosphere. 2016;144:571–582. doi: 10.1016/j.chemosphere.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Kramer B., Bauer R. Agonistic behaviour and electric signalling in a mormyrid fish. Gnathonemus petersii. Behav. Ecol. Sociobiol. 1976;1(1):45–61. doi: 10.1007/BF00299952. [DOI] [Google Scholar]
- Langfield R.D., Scarano F.J., Heitzman M.E., Kondo M., Hammond G.B., Neto C.C. Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J. Ethnopharmacol. 2004;94(2–3):279–281. doi: 10.1016/j.jep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Mailafia, S., 2017. God’spower Richard Okoh HO, Olabode K, Osanupin R. Isolation and identification of fungi associated with spoilt fruits vended in Gwagwalada market, Abuja, Nigeria. Vet. World. 10(4):393. DOI: 10.14202/vetworld.2017.393-397 [DOI] [PMC free article] [PubMed]
- Majeed S., Danish M., Zahrudin A.H., Dash G.K. Biosynthesis and characterization of silver nanoparticles from fungal species and its antibacterial and anticancer effect. Karbala Int. J. Mod. Sci. 2018;4(1):86–92. doi: 10.1016/j.kijoms.2017.11.002. [DOI] [Google Scholar]
- Meruvu H., Vangalapati M., Chippada S.C., Bammidi S.R. Synthesis and characterization of zinc oxide nanoparticles and its antimicrobial activity against Bacillus subtilis and Escherichia coli. J. Rasayan. Chem. 2011;4(1):217–222. [Google Scholar]
- Mohammady E.Y., Soaudy M.R., Abdel-Rahman A., Abdel-Tawwab M., Hassaan M.S. Comparative effects of dietary zinc forms on performance, immunity, and oxidative stress-related gene expression in Nile tilapia. Oreochromis niloticus. Aquaculture. 2021;532 [Google Scholar]
- Murugan S.S., Karuppasamy R., Poongodi K., Puvaneswari S. Bioaccumulation pattern of zinc in freshwater fish Channa punctatus (Bloch.) after chronic exposure. Turkish J. Fish Aquat. Sci. 2008;8(1):55–59. [Google Scholar]
- Nash J.P., Cuisset B.D., Bhattacharyya S., Suter H.C., Le Menn F., Kime D.E. An enzyme linked immunosorbant assay (ELISA) for testosterone, estradiol, and 17,20β-dihydroxy-4-pregenen-3-one using acetylcholinesterase as tracer: application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 2000;22(4):355–363. doi: 10.1023/A:1007850014021. [DOI] [Google Scholar]
- Naveed Ul Haq, A., Nadhman, A., Ullah, I., Mustafa, G., Yasinzai, M., Khan, I., 2017. Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity. J. Nanomater. 2017. https://doi.org/10.1155/2017/8510342
- Ngugi C.C., Bowman J.R., Omolo B. Oregon State University; 2007. A new guide to fish farming in Kenya. Oregon: Aquaculture Collaborative Research Support Program (ACRSP) [Google Scholar]
- Noga E.J. John Wiley & Sons; 2010. Fish disease: diagnosis and treatment. [Google Scholar]
- Onuegbu C., Aggarwal A., Singh N. ZnO nanoparticles as feed supplement on growth performance of cultured African catfish fingerlings. J. Sci. Ind. Res. 2018;77(4):213–218. [Google Scholar]
- Padmavathy N., Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles—an antimicrobial study. Sci. Technol. Adv. Mater. 2008;9(3) doi: 10.1088/1468-6996/9/3/035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantidos N., Horsfall L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014;5(5):1. doi: 10.4172/2157-7439.1000233. [DOI] [Google Scholar]
- Rajendran D. Application of nano minerals in animal production system. Res. J. Biotechnol. 2013;8(3):1–3. [Google Scholar]
- Rather M.A., Sharma R., Aklakur M., Ahmad S., Kumar N., Khan M., Ramya V.L. Nanotechnology: a novel tool for aquaculture and fisheries development. A prospective mini-review. Fish Aquacult. J. 2011;16:1–15. doi: 10.4172/2150-3508.1000016. [DOI] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20(1):324–335. doi: 10.1080/1828051X.2021.1886001. [DOI] [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10(5):754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Sarah, C., Watkinson, L.B., Nicholas, 2017. Money The Fungi 3d edition.
- Sarasamma S., Audira G., Juniardi S., Sampurna B.P., Liang S.T., Hao E., Lai Y.H., Hsiao C.D. Zinc chloride exposure inhibits brain acetylcholine levels, produces neurotoxic signatures, and diminishes memory and motor activities in adult zebrafish. Int. J. Mol. Sci. 2018;19(10):3195. doi: 10.3390/ijms19103195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G.R., Sloman K.A. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004;68(4):369–392. doi: 10.1016/j.aquatox.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Secombes C.J., Olivier G. Elsevier; Furunculosis: 1997. Host—Pathogen Interactions in Salmonids; pp. 269–296. [Google Scholar]
- Seil J.T., Webster T.J. Antimicrobial applications of nanotechnology: methods and literature. Int. J. Nanomedicine. 2012;7:2767. doi: 10.2147/IJN.S24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi Y., Kohshima S. Resting behaviors of captive bottlenose dolphins (Tursiops truncatus) Physiol. Behav. 2003;79(4–5):643–653. doi: 10.1016/s0031-9384(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Shah M., Fawcett D., Sharma S., Tripathy S.K., Poinern G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8(11):7278–7308. doi: 10.3390/ma8115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahzad A., Saeed H., Iqtedar M., Hussain S.Z., Kaleem A., Abdullah R., Sharif S., Naz S., Saleem F., Aihetasham A., Chaudhary A. Size-controlled production of silver nanoparticles by Aspergillus fumigatus BTCB10: likely antibacterial and cytotoxic effects. J. Nanomater. 2019;2019 doi: 10.1155/2019/5168698. [DOI] [Google Scholar]
- Sharafeldin K., Abdel-Gawad H., Ramzy E., Sweilum M., Nagy M. Harmful impact of profenofos on the physiological parameters in Nile tilapia, Oreochromis niloticus. Int. J. Basic. Appl. Sci. 2015;4:19–26. doi: 10.14419/ijbas.v4i1.3832. [DOI] [Google Scholar]
- Sharmila G., Muthukumaran C., Sandiya K., Santhiya S., Pradeep R.S., Kumar N.M., Thirumarimurugan M. Biosynthesis, characterization, and antibacterial activity of zinc oxide nanoparticles derived from Bauhinia tomentosa leaf extract. J. Nanostructure Chem. 2018;8(3):293–299. doi: 10.1007/s40097-018-0271-8. [DOI] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., El-Hack Abd, Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10(3):430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şıklar Z., Tuna C., Dallar Y., Tanyer G. Zinc deficiency: a contributing factor of short stature in growth hormone deficient children. J. Trop. Pediatr. 2003;49(3):187–188. doi: 10.1093/tropej/49.3.187. [DOI] [PubMed] [Google Scholar]
- Soltani M., Pourgholam R. Lysozyme activity of grass carp (Ctenopharingodon idella) following exposure to sublethal concentrations of organophosphate, diazinon. J. Vet. Res. 2007;62(2):50–52. [Google Scholar]
- Soren S., Kumar S., Mishra S., Jena P.K., Verma S.K., Parhi P. Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method. Microb. Pathog. 2018;119:145–151. doi: 10.1016/j.micpath.2018.03.048. [DOI] [PubMed] [Google Scholar]
- Suvith V., Philip D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014;118:526–532. doi: 10.1016/j.saa.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Taheri S., Banaee M., Haghi B.N., Mohiseni M. Effects of dietary supplementation of zinc oxide nanoparticles on some biochemical biomarkers in common carp (Cyprinus carpio) Int. J. Aquat. Biol. 2017;5(5):286–294. doi: 10.22034/ijab.v5i5.329. [DOI] [Google Scholar]
- Taherian S.M.R., Hosseini S.A., Jafari A., Etminan A., Birjandi M. Acute toxicity of zinc oxide nanoparticles from Satureja hortensis on rainbow trout (Oncorhynchus mykiss) Turkish J. Fish. Aquat. Sci. 2019;20(6):481–489. doi: 10.4194/1303-2712-v20_6_06. [DOI] [Google Scholar]
- Tan B., Mai K. Zinc methionine and zinc sulfate as sources of dietary zinc for juvenile abalone. Haliotis discus hannai Ino. Aquaculture. 2001;192(1):67–84. doi: 10.1016/S0044-8486(00)00435-X. [DOI] [Google Scholar]
- Tawfik, M., Moustafa, M., Abumourad, I., El-Meliegy, E., Refai, M., editors. 2017. Evaluation of Nano Zinc Oxide feed additive on tilapia Growth and Immunity. 15th International Conference on Environmental Science and Technology, Rhodes, Greece; 1342(1):1-9.
- Thakkar K.N., Mhatre S.S., Parikh R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine: NBM. 2010;6(2):257–262. doi: 10.1016/j.nano.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Tintos A., Míguez J.M., Mancera J.M., Soengas J.L. Development of a microtitre plate indirect ELISA for measuring cortisol in teleosts, and evaluation of stress responses in rainbow trout and gilthead sea bream. J. Fish Biol. 2006;68(1):251–263. doi: 10.1111/j.0022-1112.2006.00898.x. [DOI] [Google Scholar]
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969;6(1):24–27. doi: 10.1177/000456326900600108. [DOI] [Google Scholar]
- Williamson C.E. The swimming and feeding behavior of Mesocyclops. Hydrobiologia. 1986;134(1):11–19. [Google Scholar]
- Yehia R.S., Ahmed O.F. In vitro study of the antifungal efficacy of zinc oxide nanoparticles against Fusarium oxysporum and Penicilium expansum. Afr. J. Microbiol. Res. 2013;7(19):1917–1923. doi: 10.5897/AJMR2013.5668. [DOI] [Google Scholar]
- Yusof H.M., Mohamad R., Zaidan U.H. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 2019;10(1):57. doi: 10.1186/s40104-019-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.N., Zhang H.J., Wang J., Yue H.Y., Qi X.L., Wu S.G., Qi G.H. Effect of dietary supplementation of organic or inorganic zinc on carbonic anhydrase activity in eggshell formation and quality of aged laying hens. Poult. Sci. 2017;96(7):2176–2183. doi: 10.3382/ps/pew490. [DOI] [PubMed] [Google Scholar]