Abstract
At present, the effect of ganglioside combined with Jiaji electroacupuncture (Jiaji EA) on SCI still remains unclear. This study explores the effect of ganglioside combined with electroacupuncture on Nogo/NgR signal pathway in spinal cord tissue of spinal cord injury (SCI) rats. Basso Beattie Bresnahan (BBB) score was used to evaluate spinal cord function after modeling and 14 days post ganglioside and electroacupuncture treatment. RT-qPCR and western blot were performed to evaluate the expression levels of targets in spinal cord tissue. After 14 days of treatment, the BBB scores of Jiaji EA group, ganglioside group and combination group were all improved. The expression levels of IL-1β, IL-6 and TNF-α in Jiaji EA group, ganglioside group and combination group were significantly lower than those in model group. Both of mRNA and protein expression levels of Nogo-A, NgR and LINGO-1 in the model group were significantly higher than those in the Jiaji EA group, ganglioside group and combination group. Ganglioside combined with Jiaji EA has a stronger effect on promoting the recovery of nerve function. Its mechanism of action may be related to its inhibition of the expression of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α and Nogo-NgR signal pathway to promote neuronal growth. Our results will provide fundamental information for further SCI studies.
Keywords: Spinal cord injury, Ganglioside ester, Jiaji electroacupuncture, Nogo NgR signal pathway
1. Introduction
Acute spinal cord injury (SCI) is caused by local hemorrhage and edema after violent SCI, leading to apoptosis and necrosis of nerve cells in damaged spinal cord tissue and dysfunction of nervous system function (Ziegler et al., 2018, Hills, 2020, Gong et al., 2020). SCI is a serious trauma to the central nervous system, causing serious disability (Andrade et al., 2021, Jogia et al., 2021, Bilchak et al., 2021). There is no effective clinical treatment at present (Yates et al., 2021, Yoshizaki et al., 2021).
In the traditional Chinese medicine, electroacupuncture has been (EA) extensively used in clinical treatment because of its beneficial therapeutic effects. Jiaji refers to acupoints located on either side of the vertebral column and 15 mm below the spinal processes from T1 to L5 and totaling 17 pairs. Jiaji acupuncture entails the use of 4 needles: 2 above the level of damage and 2 below. Jiaji EA involves the application of an electric current to the needles. A pulsed electric field reportedly promotes vascularization and regeneration of nervous tissue, and electroacupuncture has been shown to promote functional recovery after spinal cord injury. Jiaji EA improves blood circulation and relieves edema after SCI, and protects and promotes axonal regeneration (Yarkony et al., 1995, Vaidyanathan et al., 2001, Killorin et al., 1992 Jun, Wang et al., 2009). Therefore, we predict the Jiaji EA may exhibit potential to improve locomotor function after SCI.
Ganglioside is an important component of nerve cell membrane and an important substance in the process of nerve cell growth and proliferation with the largest content in nerve cells (Suzuki et al., 2018, Acquotti et al., 2018). Ganglioside can protect damaged nerve spinal cord, antagonize oxygen free radicals and neurotoxicity, inhibit intracellular calcium overload and nitric oxide synthesis, inhibit cell apoptosis, reduce cerebral edema, promote nerve development, especially have regeneration and repair effects on damaged nerve, and protect secondary functional degeneration of damaged nerve (Baptiste and Fehlings, 2006, Hadjiconstantinou et al., 1992).
At present, the effect of ganglioside ester combined with Jiaji electro-acupuncture (Jiaji EA) on SCI is unclear. After SCI, local ischemia and oxidative stress will inhibit axonal eye contact and regeneration, consequently affecting the recovery of nerve function. Axon growth inhibitor protein involved in central nerve regeneration disorder. The inflammation has been recognized as an important cause of parallels disease severity (Ferretti and Cuello, 2011, Serpente et al., 2014). Previous studies demonstrated that NgR is expressed on microglia and the binding of Nogo-A with NgR could inhibit microglia adhesion and migration (Yan et al., 2012). After binding with NgR, Nogo-A transmits information to nerve cells and generates NgR/p75NTR/LINGO-1 complex, which further mediates axon growth inhibition (Kan et al., 2017, Trifunovski et al., 2004). The inflammation regulated by Nogo/NgR pathway was involved in SCI recovery.
The purpose of this study is to explore the effect of ganglioside combined with chiropractic acupuncture on NogoNgR signal pathway in spinal cord tissue of rats with SCI, analyze its therapeutic effect and mechanism, and provide new insights for clinical treatment of SCI.
2. Materials and methods
2.1. Grouping, modeling and delivery
Fifty healthy female Sprague Dawley (SD) rats, aged 8 weeks and weighing 280 ± 20 g were purchased from Experimental Animal Center of Gansu Provincial Hospital and used to ensure high reproducibility of the results. After 3 days adaptation, rats were randomly divided into five groups: Sham group, Model group, Jiaji EA treatment group, Ganglioside injection group and Jiaji EA combined with Ganglioside injection group. Each group contains 10 rats. All procedures involving animals and their care in this study were approved by the Ethics Committee of Gansu Provincial Hospital (GPH-2020-012).
Allen's method was used to establish a rat SCI model (Hu et al., 2013). Briefly, rats were anesthetized with chloral hydrate (10%) and fixed on the operating table prone. T9~T11 spines were exposed through longitudinal incision with T10 as the center. Stainless steel rods were used to impact T10 with an impact energy of 25 mm × 10 g and an injury diameter of 3 mm. After modeling, the incision was sutured, penicillin was injected intramuscularly to prevent infection and excretion function was promoted by massaging the lower abdomen. Rats in sham group were subjected to remove the lamina and spinous process without causing SCI.
The ganglioside group was injected intraperitoneally with ganglioside (Qilu corporation, China; 10 mg/kg) once a day, lasting for 14 days. The rats in the Jiaji EA group were restrained on a board. The 6 mm Hua Tuo filiform needle (0.30 mm) was vertically inserted into a depth of around 4–5 mm at the acupoint, a distance of 3–4 mm from the midline beside the spinous process space at the upper and lower ends of the injury area. Subsequently, the needles were connected to KWD-808II pulse electroacupuncture apparatus at a frequency of 2 Hz for 1.5 ms and rest for 1.5 ms. The output intensity is measured by slight twitch of back muscle. The treatment was performed for 30 min per day and lasted for 14 days. The dosage of ganglioside in combination group was the same as that in ganglioside group, and EA conditions were same as that in Jiaji EA group. The sham group and the model group were treated with saline.
2.2. Neurological function evaluation
Basso Beattie Bresnahan (BBB) score and somato-sensory evoked potential (SEP) were used to evaluate spinal cord function before modeling, after modeling and 14 days after intervention. The BBB score of rats was evaluated by two personnel respectively through double blind method, and the BBB score of rats was evaluated through the Rat Hindlimb Movement Evaluation Scale proposed by Basso et al. The sensory nerve conduction pathway of rats was detected by SEP.
2.3. Extraction of spinal cord tissue specimens
After 14 days of treatment, rats in each group were sacrificed with decapitation on an ice tray, and cardiac perfusion was performed with normal saline. The fresh T10-centered injured spinal cord tissue was isolated and placed in liquid nitrogen for subsequent experiments.
2.4. Western blot
The cellular proteins were extracted by RIPA buffer containing Halt protease inhibitor cocktail (ThermoFisher, USA) and the protein concentrations were measured by BCA method. The proteins were subjected to 10% SDS-PAGE and transferred onto a PVDF membrane followed by blocking with 5% fat-free milk at room temperature for 2 h. Subsequently, membrane was incubated with anti-IL-1β (ab216995, 1:1000; Abcam, USA), anti-IL-6 (ab233706, 1:1000; Abcam, USA), anti-TNF-α (ab255275, 1:1000; Abcam, USA), anti-Nogo-A (ab62024, 1:1000; Abcam, USA), anti-NgR (ab174323, 1:1000; Abcam, USA) and anti-LINGO-1 (ab23631, 1:1000; Abcam, USA) at 4 °C overnight, and incubated with goat anti-mouse IgG antibody (HRP conjugate, 1:3000, #91196; CST, USA) at room temperature for 2 h. ECL reagent was used for chemiluminescence detection. The β-actin (1:1000, #3700; CST, USA) was set as internal reference.
2.5. RT-qPCR
RT-qPCR was used to detect the expression levels of Nogo-A, NgR and LINGO-1mRNA in spinal cord tissue. Spinal cord tissue was ground and lysed under the protection of liquid nitrogen, and then total RNA was extracted by Trizol reagent (ThermoFisher, USA) and dissolved in DEPC water. Reverse transcription assay was performed on RNA samples using a reverse transcription kit (Invitrogen, USA) to synthesize cDNA. The reaction conditions of reverse transcriptase were 37 °C for 15 min, and the inactivation conditions of reverse transcriptase were 85 °C for 15 s. RT-qPCR was performed using SYBR Prellix Ex TaqTM real-time PCR kit (TaKaRa, Japan). PCR was performed by activating DNA polymerase at 95 °C for 5 min, then 40 cycles of two-step PCR (95 °C for 10 s and 60 °C for 30 s) were performed, and finally extended at 75 °C for 10 min and stored at 4 °C. The mRNA expression was analyzed by 2-△△CT. The primer sequences are shown in Table 1.
Table 1.
The primer sequences.
| Primer name | Sequence (5′-3′) |
Product size (bp) |
|---|---|---|
| Nogo-A-Forward | GGTGCCTTGTTCAATGGTC | |
| Nogo-A-Reverse | AATCTGCTTTGCGCTTCA | 108 |
| NgR-Forward | AGAAAGAACCGCACCCGTAG | |
| NgR-Reverse | GGCCCAAGCACTGTCCAA | 198 |
| LINGO-1-Forward | GCTGACGCTGGAGAAATG | |
| LINGO-1-Reverse | GAAGGAGTAGTCCCGTATGG | 224 |
| GAPDH-Forward | GCAACTTCAACGGCACAG | |
| GAPDH-Reverse | GCCAGTAGACTCCACGACAT | 204 |
2.6. Statistical treatment
All data were analyzed by SPSS 25.0. The data were analyzed by one-way ANOVA and LSD-t test. P < 0.05 was statistically significant.
3. Results
3.1. Comparison of BBB scores of rats in each group
As shown in Table 2, after modeling, the score of rats decreased significantly (P < 0.05). After 14 days of intervention, the BBB scores of Jiaji EA group, ganglioside group and combination group were all improved, and the BBB scores of combination group were significantly higher than those of Jiaji EA group and ganglioside group (P < 0.05).
Table 2.
Comparison of BBB scores in rats of each group(score).
| Group | n | Before modeling | After modeling | After 14 days of intervention |
|---|---|---|---|---|
| Sham group | 10 | 21.17 ± 0.53 | 20.95 ± 0.41 | 21.06 ± 0.33 |
| Model group | 10 | 21.34 ± 0.62 | 6.67 ± 0.75a | 7.71 ± 0.91*a |
| Jiaji EA group | 10 | 21.28 ± 0.49 | 6.54 ± 0.81a | 9.85 ± 0.62*b |
| Ganglioside group | 10 | 21.31 ± 0.67 | 6.59 ± 0.63a | 10.13 ± 0.58*b |
| Combination group | 10 | 21.45 ± 0.36 | 6.57 ± 0.54a | 11.36 ± 0.75*b,c,d |
Compared with that after modeling, *P < 0.05; Compared with sham group, aP < 0.05; Compared with the model group, bP < 0.05; Compared with Jiaji EA group, cP < 0.05; Compared with ganglioside group, dP < 0.05.
3.2. Comparison of SEP incubation period of rats in each group
The SEP incubation period increased significantly (P < 0.05), and the SEP incubation period of Jiaji EA group, ganglioside group and combination group decreased after intervention, and the SEP incubation period of combination group was significantly lower than that of Jiaji EA group and ganglioside group (P < 0.05, Table 3).
Table 3.
Comparison of SEP incubation period of rats in each group.
| Group | n | Before modeling | After modeling | After 14 days of intervention |
|---|---|---|---|---|
| Sham group | 10 | 6.85 ± 0.45 | 6.89 ± 0.32 | 6.83 ± 0.56 |
| Model group | 10 | 6.83 ± 0.51 | 21.05 ± 1.67a | 18.15 ± 1.89*a |
| Jiaji EA group | 10 | 6.82 ± 0.69 | 20.33 ± 1.41a | 14.46 ± 1.33*b |
| Ganglioside group | 10 | 6.84 ± 0.53 | 20.91 ± 1.35a | 13.68 ± 1.27*b |
| Combination group | 10 | 6.82 ± 0.72 | 21.01 ± 1.68a | 11.73 ± 0.91*b,c,d |
P < 0.05: Compared with sham group; bP < 0.05: Compared with the model group; cP < 0.05: Compared with Jiaji EA group; dP < 0.05: Compared with ganglioside group.
3.3. Comparison of expression levels of IL-1β, IL-6 and TNF-α protein in spinal cord tissue of each group
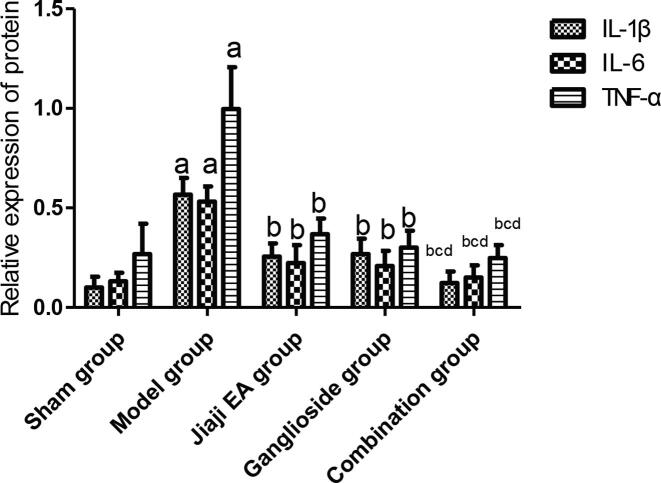
After 14 days of intervention, the expression levels of IL-1β, IL-6 and TNF-α protein in Jiaji EA group, ganglioside group and combination group were significantly lower than those in model group (P < 0.05). The expression levels of IL-1β, IL-6 and TNF-α protein in combination group after intervention were significantly lower than those in Jiaji EA group and ganglioside group (P < 0.05), as shown in Figs. 1 and 2.
Fig. 1.
The results of quantitative detection of IL-1β, IL-6 and TNF-α protein expression by Western blot (n = 10).
Fig. 2.
Semi-quantitative analysis of IL-1β, IL-6 and TNF-α protein expression levels (n = 10). aP < 0.05: Compared with sham group; bP < 0.05: Compared with the model group; cP < 0.05: Compared with Jiaji EA group; dP < 0.05: Compared with ganglioside group.
3.4. Comparison of expression levels of Nogo-A, NgR and LINGO-1 protein in spinal cord tissues of each group
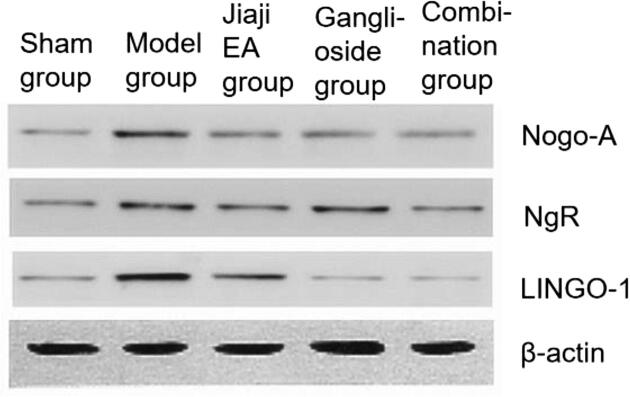
After 14 days of intervention, the expression levels of Nogo-A, NgR and LINGO-1 protein in the model group were significantly higher than those in the sham group (P < 0.05). The expression levels of Nogo-A, NgR and LINGO-1 protein in the Jiaji EA group, ganglioside group and combination group were significantly lower than those in the model group (P < 0.05), and the expression levels of Nogo-A, NgR and LINGO-1protein in the combination group were significantly lower than those in Jiaji EA group and ganglioside group (P < 0.05) after intervention, as shown in Fig. 3, Fig. 4.
Fig. 3.
The results of quantitative detection of Nogo-A, NgR and LINGO-1 protein expression by Western blot (n = 10).
Fig. 4.
Semi-quantitative analysis of Nogo-A, NgR and LINGO-1 protein expression levels (n = 10). aP < 0.05: Compared with sham group; bP < 0.05: Compared with the model group; cP < 0.05: Compared with Jiaji EA group; dP < 0.05: Compared with ganglioside group.
3.5. Comparison of mRNA expression levels of Nogo-A, NgR and LINGO-1 mRNA in spinal cord tissues of each group
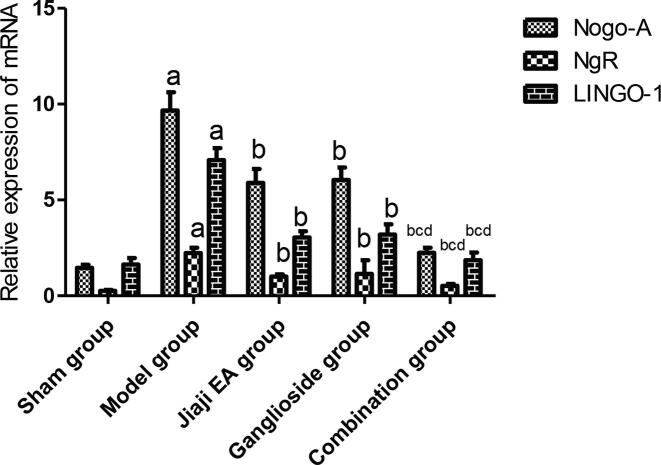
The mRNA expression levels of Nogo-A, NgR and LINGO-1 in the model group were significantly higher than those in the sham group (P < 0.05), and the mRNA expression levels of Nogo-A, NgR and LINGO-1 in the Jiaji EA group, ganglioside group and combination group were significantly lower than those in the model group (P < 0.05), and the mRNA expression levels of Nogo-A, NgR and LINGO-1 in the combination group were significantly lower than those in the Jiaji EA group and ganglioside group (P < 0.05) after the intervention See Fig. 5.
Fig. 5.
Semi-quantitative analysis of Nogo-A, NgR and LINGO-1 mRNA expression levels (n = 10). aP < 0.05: Compared with sham group; bP < 0.05: Compared with the model group; cP < 0.05: Compared with Jiaji EA group; dP < 0.05: Compared with ganglioside group.
4. Discussion
After SCI occurs, nerve cells in the damaged spinal cord tissue will be lost, leading to neurological dysfunction and limited recovery ability. In this study, the Allen's method was used to establish a SCI model. After modeling, the dural mater of the rats was not injured, but the injury site was swollen and both hind limbs showed flaccid paralysis, indicating the modeling was successful. BBB can evaluate the recovery of hind limb function in rats and is considered as an important indicator reflecting the recovery of spinal cord injury. SEP incubation period can reflect spinal cord nerve function in rats. Our results demonstrated that the BBB score of rats after modeling was significantly reduced, and the BBB score of combination group was significantly higher than that of Jiaji EA group and ganglioside group after 14 days of intervention (P < 0.05). The SEP incubation period of combination group was significantly lower than that of Jiaji EA group and ganglioside group (P < 0.05), indicating that Jiaji EA group and ganglioside ester treatment can better promote the regeneration and repair of injured nerves. Previous studies have shown that Jiaji EA has certain therapeutic effect on SCI in rats (Sun, 1996, Xu et al., 2006, Juarez Becerril et al., 2015)and ganglioside can promote the recovery of neurological function after SCI (Constantini and Young, 1994, Geisler et al., 1993, Constantini and Young, 1994, Donaldson et al., 2000). Our results further suggested that the combination of Jiaji EA and ganglioside ester effectively promoted the recovery of spinal cord nerve function in spinal cord rats.
In order to further explore the effect and mechanism of Jiaji EA and ganglioside on SCI rats, we detected the expression levels of IL-1β, IL-6, TNF-α and Nog-oNgR signal pathways in spinal cord tissue respectively. The results show that the combination of Jiaji EA and ganglioside ester can inhibit the expression of proinflammatory cytokines IL-1β, IL-6, TNF-α, thus reducing the secondary injury caused by inflammatory reaction to spinal cord and achieving the effect of protecting spinal cord.
LINGO-1 is a nervous system-specific transmembrane protein that binds NgR1 and p75 and that is an additional functional component of the NgR1/p75 signaling complex (Mi et al., 2004). Previous study indicated that blocking Nogo/NgR promoted axon regeneration and functional recovery in the spinal cord-injured rat (Buchli and Schwab, 2005). In this study, we demonstrated that the protein and mRNA expression levels of Nogo-A, NgR and LINGO-1 in the model group were significantly higher than those in the sham group. Treatment with Jiaji EA combined with ganglioside ester significantly reduced Nogo-A, NgR and LINGO-1 expression compared to Jiaji EA or ganglioside ester single treatment group. Since Nogo protein and NgR are the key structures that block or antagonize the growth of nerve axons after injury, LINGO-1 is a specific protein located on CNS neurons and oligodendrocytes and also an essential negative regulatory factor that inhibits nerve growth. NEP1-40 promotes axon growth and extension after injury through competitive antagonism or blocking NgR binding, and plays a leading role in regeneration and repair process after central nerve injury (Yu et al., 2008). It can be inferred that the combined use of Jiaji EA and ganglioside ester may effectively reduce the inhibitory effect on nerve axon growth and further play a protective role on SCI by reducing the signal regulation of Nogo-A, NgR and LINGO-1 gene expression, especially the change of LINGO-1 expression. Considering that there is no effective clinical treatment currently (Yates et al., 2021, Yoshizaki et al., 2021), our study provided alternative therapeutic strategy for spinal cord injury.
5. Conclusion
Ganglioside ester combined with Jiaji EA has a stronger effect on promoting the recovery of nerve function. Its mechanism of action may be related to its inhibition of the expression of proinflammatory cytokines IL-1β, IL-6 and TNF-α, and inhibition of Nogo-NgR signal pathway to promote neuronal growth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Acquotti D., Mauri L., Sonnino S. Nuclear magnetic resonance of gangliosides. Methods Mole. Biol. 2018;1804:241–284. doi: 10.1007/978-1-4939-8552-4_12. [DOI] [PubMed] [Google Scholar]
- Andrade M.J., Quintas F.L., Silva A.M., Cruz P. Is autonomic dysreflexia a cause of respiratory dysfunction after spinal cord injury? Spinal Cord Ser Cases. 2021;7(1):4. doi: 10.1038/s41394-020-00372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste D.C., Fehlings M.G. Pharmacological approaches to repair the injured spinal cord. J. Neurotrauma. 2006;23:318–334. doi: 10.1089/neu.2006.23.318. [DOI] [PubMed] [Google Scholar]
- Bilchak J.N., Yeakle K., Caron G., Malloy D., Côté M.P. Enhancing KCC2 activity decreases hyperreflexia and spasticity after chronic spinal cord injury. Exp. Neurol. 2021;13(338):113605. doi: 10.1016/j.expneurol.2021.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchli A., Schwab M. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann. Med. 2005;37(8):556–567. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- Constantini Shlomo, Young Wise. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 1994;80(1):97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Constantini S., Young W. The effects of methylprednisolone and the ganglioside GM1 on acute spinal cord injury in rats. J. Neurosurg. 1994;80(1):97–111. doi: 10.3171/jns.1994.80.1.0097. [DOI] [PubMed] [Google Scholar]
- Donaldson N., Perkins T.A., Fitzwater R. FES cycling may promote recovery of leg function after incomplete spinal cord injury. Spinal Cord. 2000;38(11):680–682. doi: 10.1038/sj.sc.3101072. [DOI] [PubMed] [Google Scholar]
- Ferretti M.T., Cuello A.C. Does a pro-inflammatory process precede Alzheimer’s disease and mild cognitive impairment? Curr. Alzheimer Res. 2011;8(3):164–174. doi: 10.2174/156720511795255982. [DOI] [PubMed] [Google Scholar]
- Geisler F.H., Dorsey F.C., Coleman W.P. Past and current clinical studies with GM-1 ganglioside in acute spinal cord injury. Ann. Emerg. Med. 1993;22(6):1041–1047. doi: 10.1016/s0196-0644(05)82748-9. [DOI] [PubMed] [Google Scholar]
- Gong L., Lv Y., Li S., Feng T., Zhou Y., Sun Y., Mi D. Changes in transcriptome profiling during the acute/subacute phases of contusional spinal cord injury in rats. Ann Transl Med. 2020;8(24):1682. doi: 10.21037/atm-20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinou M., Karadsheh N.S., Rattan A.K., Tejwani G.A., Fitkin J.G. Neff NH.GM1 ganglioside enhances cholinergic parameters in the brain of senescent rats. Neuroscience. 1992;46:681–686. doi: 10.1016/0306-4522(92)90154-t. [DOI] [PubMed] [Google Scholar]
- Hills T.E. Caring for patients with a traumatic spinal cord injury. Nursing. 2020;50(12):30–40. doi: 10.1097/01.NURSE.0000721724.96678.5a. [DOI] [PubMed] [Google Scholar]
- Hu J.Z., Huang J.H., Xiao Z.M., Li J.H., Li X.M., Lu H.B. Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. J. Neurol. Sci. 2013;324(1–2):94–99. doi: 10.1016/j.jns.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Jogia T., Lübstorf T., Jacobson E., Scriven E., Atresh S., Nguyen Q.H., Liebscher T., Schwab J.M., Kopp M.A., Walsham J., Campbell K.E., Ruitenberg M.J. Prognostic value of early leukocyte fluctuations for recovery from traumatic spinal cord injury. Clin. Transl. Med. 2021;11(1):e272. doi: 10.1002/ctm2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez Becerril O., Salgado Ceballos H., Anguiano Solis C., Alvarado Sanchez B., Lopez Hernandez M.E., Diaz Ruiz A., Torres Castillo S. Electro-acupuncture at GV.4 improves functional recovery in paralyzed rats after a traumatic spinal cord injury. Acupuncture Electro-Therapeutics Res. 2015;40(4):355–369. [PubMed] [Google Scholar]
- Kan Q.C., Zhang H.J., Zhang Y., Li X., Xu Y.M., Thome R., Zhang M.L., Liu N., Chu Y.J., Zhang G.X., Zhu L. Matrine treatment blocks NogoA-induced neural inhibitory signaling pathway in ongoing experimental autoimmune encephalomyelitis. Mole. Neurobiol. 2017;54:8404–8418. doi: 10.1007/s12035-016-0333-1. [DOI] [PubMed] [Google Scholar]
- Killorin W., Gray M., Bennett J.K., Green B.G. The value of urodynamics and bladder management in predicting upper urinary tract complications in male spinal cord injury patients. Paraplegia. 1992 Jun;30(6):437–441. doi: 10.1038/sc.1992.95. [DOI] [PubMed] [Google Scholar]
- Mi S., Lee X., Shao Z., Thill G., Ji B., Relton J. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nature Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Serpente M., Bonsi R., Scarpini E., Galimberti D. Innate immune system and inflammation in Alzheimer’s disease: from pathogenesis to treatment. NeuroImmunoModulation. 2014;21(2–3):79–87. doi: 10.1159/000356529. [DOI] [PubMed] [Google Scholar]
- Sun W. Electroacupuncture at “huatuo jiaji point” inhibits the expression of Fos protein in rat spinal cord induced by traumatic pain. Zhen Ci Yan Jiu. 1996;21(1):60–64. [PubMed] [Google Scholar]
- Suzuki A., Suzuki M., Ito E., Nitta T., Inokuchi J.I. Mass spectrometry of gangliosides. Methods Mole. Biol. 2018;1804:207–221. doi: 10.1007/978-1-4939-8552-4_9. [DOI] [PubMed] [Google Scholar]
- Trifunovski A., Josephson A., Ringman A., Brené S., Spenger C., Olson L. Neuronal activity-induced regulation of Lingo-1. NeuroReport. 2004;15:2397–2400. doi: 10.1097/00001756-200410250-00019. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S., Hughes P., Soni B.M. Occlusion of left common iliac vein by a distended urinary bladder in a male with paraplegia due to spinal cord injury. Spinal Cord. 2001;39:394–398. doi: 10.1038/sj.sc.3101165. [DOI] [PubMed] [Google Scholar]
- Wang J.H., Chen B.G., Yin J. Effect of electroacupuncture of different acupoints on the excitability of detrusor muscle and the expression of BDNF and TrkB in the spinal cord of rats with urinary retention due to spinal cord injury. Zhen Ci Yan Jiu. 2009;34(6):387–392. [PubMed] [Google Scholar]
- Xu J.-N., Xiao D., Ju J.-H. Effect of electro-acupuncture on treatment of spinal cord injuries in rats. Chin. J. Clin. Rehabilit. 2006;10(23):180–182. [Google Scholar]
- Yan J., Zhou X., Guo J.J., Mao L., Wang Y.J., Sun J. Nogo-66 inhibits adhesion and migration of microglia via GTPase Rho pathway in vitro. J. Neurochem. 2012;120(5):721–731. doi: 10.1111/j.1471-4159.2011.07619.x. [DOI] [PubMed] [Google Scholar]
- Yarkony G.M., Chen D., Palmer J., Roth E.J., Rayner S., Lovell L. Management of impotence due to spinal cord injury using low dose papaverine. Paraplegia. 1995;33:77–79. doi: 10.1038/sc.1995.19. [DOI] [PubMed] [Google Scholar]
- Yates A.G., Jogia T., Gillespie E.R., Couch Y., Ruitenberg M.J., Anthony D.C. Acute IL-1RA treatment suppresses the peripheral and central inflammatory response to spinal cord injury. J. Neuroinflammat. 2021;18(1):15. doi: 10.1186/s12974-020-02050-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki S., Tamaru T., Hara M., Kijima K., Tanaka M., Konno D.J., Matsumoto Y., Nakashima Y., Okada S. Microglial inflammation after chronic spinal cord injury is enhanced by reactive astrocytes via the fibronectin/beta1 integrin pathway. J. Neuroinflammat. 2021;18(1):12. doi: 10.1186/s12974-020-02059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Huang L., Zou J., Yu Z., Wang Y., Wang X., Xu L., Liu X., Xu X.M., Lu P.H. Immunization with recombinant Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of function after spinal cord injury in rats. Neurobiol. Dis. 2008;32:535–542. doi: 10.1016/j.nbd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Ziegler G., Grabher P., Thompson A., Altmann D., Hupp M., Ashburner J., Friston K., Weiskopf N., Curt A., Freund P. Progressive neurodegeneration following spinal cord injury: Implications for clinical trials. Neurology. 2018;90:e1257–e1266. doi: 10.1212/WNL.0000000000005258. [DOI] [PMC free article] [PubMed] [Google Scholar]