Abstract
The impact of trace elements, especially zinc, selenium, copper, and magnesium, on male fertility has gained great interest and significance. Increased oxidative stress and altered trace element levels are probable etiological factors underlying male reproductive dysfunction and infertility. The present study focused on the evaluation of seminal oxidative markers, such as reactive oxygen species (ROS), malondialdehyde (MDA), and total antioxidant capacity (TAC), and trace element levels in the normozoospermic fertile control group (n = 40) and asthenozoospermic infertile group (n = 30). Semen from infertile men exhibited significantly higher ROS and MDA levels accompanied with significant decline in TAC and trace element (zinc and magnesium) levels. Furthermore, a significant correlation was observed between trace elements and oxidative markers with sperm motility. The current study revealed increased lipid peroxidation and oxidant-reductant imbalance that leads to deterioration of semen quality and male infertility. Thus, oxidative stress and trace elements can be considered important biomarkers of male infertility. Measurement of seminal oxidative stress with conventional seminological parameters must be integrated in fertility assessment from early stages to ensure healthy semen characteristics and fertility in men.
Keywords: Male infertility, Trace elements, Oxidative stress
1. Introduction
Globally, 8%–12% of the world’s population have infertility issues, and 40%–50% of infertility cases are cause by a male factor (Alahmar, 2017, Kasperczyk et al., 2015, Macanovic et al., 2009, Showell et al., 2014). Although the field of reproductive medicine has witnessed rapid development, causes of male infertility remains not fully addressed. Age, environment, occupational exposure, socioeconomic and nutritional status are few predisposing factors for decreased sperm quality (Kasperczyk et al., 2015). Moreover hypogonadism, varicocele, cryptorchidism, genetic factors etc also lead to infertility. About 25% of the couples are idiopathic with no reason/ cause identified underlying the problem (Wang et al., 2017). Growing evidences indicates oxidative stress (OS) and reactive oxygen species (ROS) as probable etiological factors underlying idiopathic infertility. A significant part of male factor infertility cases is believed to be due to the damaging effects of OS. Oxidative stress results from imbalance between ROS production and the semen’s natural antioxidant defenses. Many factors like high temperatures, electromagnetic radiation, pesticides, pollutants, and lifestyle factors (advanced age, alcohol consumption, smoking, stress, obesity, and poor diet) accounts for increased levels of ROS (Seshadri et al., 2009).
Under normal physiological conditions, highly reactive oxidizing agents termed ROS are generated in cells (Alahmar., 2019). Increased ROS levels have been hypothesized to result in sperm dysfunction by enhancing lipid peroxidation and reduced oxidant levels (Agarwal et al., 2006). Spermatozoa are susceptible to OS owing to the presence of abundant polyunsaturated fatty acids (PUFAs) in its membrane and subsequently to enhanced ROS production (Hesham et al., 2008). Malondialdehyde (MDA) is one of the prominent indicators of lipid peroxidation (Agarwal et al., 2003). Another important factor that is suggested to affect fertility is the level of trace elements (TE) that play a crucial role in the male reproductive development process. The possible influence of TE, especially copper (Cu), selenium (Se), and zinc (Zn), on male fertility is of great interest. Some studies have investigated the relationship between seminal plasma TE and male infertility but have shown inconsistent results. Zn and Se are considered essential in human testicular development and spermatogenesis (Akinloye et al., 2005, Colagar et al., 2009, Ebisch et al., 2007). The prostate gland is known to contain higher Zn and Mg levels. Vital processes, such as DNA transcription and protein synthesis, which are crucial for sperm formation, are dependent on the Zn levels (Chia et al., 2000). Zn and Cu acts as antioxidants forming a cofactor of antioxidant enzymes, such as copper or Zn-SOD. Zn mediates its function by binding to the sulfhydryl groups in proteins and interferes in oxidation reactions by blocking copper binding sites on DNA and lipids (Cologar et al., 2009). Recently, impact of Zn deficiency on loss of antioxidant status have been suggested to exacerbate OS leading to spermatozoal dysfunction (Cologar et al., 2009). Like Zn, Se is vital for sperm formation, motility and functioning (Agarwal and Sekhon, 2011, Colagar et al., 2009). It has anti-oxidizing properties which protects sperm DNA from oxidative attack. The antioxidative mechanism of Se and its role in improving sperm quality and fertility is still contentious.
Insight into the literature on male infertility and its causes in Saudi Arabia, very few studies were reported from this region. Mukhtar and his group (2017) found that about half million couples require assisted conception (Mukhtar et al., 2017). Male factor infertility (72%) was accountable for increased rate of infertility among infertile couples (Alasmari et al., 2018, Alosaimi et al., 2017). In view of the above, the present study intends to validate the limited results reported in the literature and clarify the impact of seminal oxidative status. More importantly, this study aimed to evaluate the effect of some important TE levels on sperm quality of infertile Saudi men diagnosed with asthenozoospermia using automated sperm motility assessment to eliminate measurement subjectivity and increase reproducibility in which participants were grouped based on their reproductive pathologies
2. Material and method
2.1. Study setting and design
All semen samples used in this cross-sectional study were obtained from consenting patients attending the Assisted Conception Unit of King Khalid University Hospital, Riyadh. Prior to the study, ethical approval was obtained from the Institutional Review Board of King Khalid university Hospital Riyadh, Saudi Arabia and signed consent forms were collected. The study examined infertile asthenozoospermics semen samples (n = 30); low sperm motility, in relation to normal semen samples (n = 40) from healthy males with proven fertility confirmed by previous successful conception used as a control. A brief questionnaire was filled by the participants and those with prolong illness, vitamin intake or antioxidant supplement treatment, azoospermia, leukocytospermia were excluded from the study.
2.2. Semen collection and sample preparation
Semen samples were collected following the World Health Organization (WHO) guidelines [19] into a sterile polypropylene non-toxic container (Alpha Laboratories, Eastleigh, UK) after 2–3 days abstinence. Samples were left to liquefy at 37 °C for 30 min. Computer-assisted semen analysis (CASA) system was employed (Hamilton Thorne Biosciences, Beverly, MA USA) for analysis of semen following WHO grading system guidelines [WHO reports., 2010].
On the basis of the semen analysis, samples were enrolled into one of the following groups; normozoospermics fertile control group (n = 40) showing normal parameters with sperm concentration of ≥ 15 × 106 sperm/mL, total sperm motility of ≥ 40%, normal morphology of ≥ 4% of sperm, and a minimum volume of 1 mL. The second group included as thenozoospermics (AST) infertile group (n = 30) exhibiting normal sperm concentration of ≥ 15 × 106 sperm/mL and normal morphology of ≥ 4% of sperm but with lower than normal level of total sperm motility (<40%).
Samples were then processed for seminal plasma collection by centrifugation at 400g for 15 min. Seminal plasma was then preserved at −80 °C until the time of the detection of ROS, total antioxidant capacity (TAC), MDA and trace elements; Zinc (Zn), Selenium (Se), Copper (Cu), and Magnesium (Mg).
2.3. Measurement of seminal total antioxidant capacity (TAC)
Seminal TAC was measured using total antioxidant status colorimetric assay kit (Calbiochem, USA) following the manufacturer’s protocol. The kit is designed to measure antioxidants in the sample inhibiting oxidation of ABTS™ (2,2′-azino-di-[3-ethylbenz-thiazoline sulfonate]) substrate to ABTS™•+ by metmyoglobin, a peroxidase. The amount of ABTS™•+ was monitored by reading the absorbance at 600 nm. Under the reaction conditions used, antioxidants in the sample cause suppression of the absorbance at 600 nm to a degree that is proportional to their level in nM concentration.
2.4. Estimation of ROS levels in seminal plasma
The OxiSelect™ In Vitro ROS/RNS Assay Kit (Cell Biolabs Inc., San Diego, CA, USA) was used to determine the ROS level in seminal plasma. Fluorescence of the plate was read separately at 480 nm (excitation) and 530 nm (emission) to measure the rate of end product formed 2′, 7′-dichlorodihydrofluorescein (DCF) proportional to ROS level formed in the sample.
2.5. Determination of lipid peroxidation of sperm plasma membrane
Lipid peroxidation was estimated by measuring thiobarbituric-acid reactive substances and expressed in terms of MDA using Lipid Peroxidation Colorimetric Assay Kit (Abcam, USA). A blue color product that was generated was measured at 695 nm using absorbance microplate readers.
2.6. Measurements of TE levels
Atomic absorption spectrophotometer (Shimadzu AA-6200, Japan) was used for estimation of levels of TEs of Zn, Se, Cu, and Mg in seminal plasma. All glassware used in the measurement of zinc, copper, and magnesium were boiled in 6 mol/L nitric acid for 60 min and then washed twice with deionized water. Digestion of semen was conducted using ultrapure nitric acid. To achieve mineralization, samples were mixed with concentrated nitric acid in 1:10 dilution in closed Teflon vessels and digested in a microwave oven system. At the end of the digestion process, seminal plasma samples were diluted to a final volume of 10 mL with demineralized Milli-Q water for copper measurements and 1% lanthanum chloride for magnesium measurement. Then, the samples were placed into the atomic absorption flame emission spectrophotometer for measurement of Zn, Se, Cu, and Mg at wavelengths of 213.9, 196.0, 324.7, and 285.2 nm, respectively.
2.7. Statistical analysis
Kolmogorov–Smirnov test was used to explore data normality, and normality histograms were conducted. Data were not normally distributed and were log10 (x + 2) transformed. The differences between the groups were assessed by one-way analysis of variance, and Tukey test was used for post hoc pairwise comparison for significance determination. Pearson’s correlation was conducted to examine relationships between seminal redox status, oxidative biomarkers, targeted TE levels, and semen quality. Quantitative variables were calculated, and descriptive data were expressed as mean ± standard deviation (SD). All analyses were performed using a computer statistical program (IBM SPSS version 19.0, Chicago, IL, USA) and testing was two sided, with a P-value < 0.05 considered statistically significant and P-value < 0.001 considered highly significant.
3. Result
3.1. Samples characteristics
Table 1 illustrates the seminal characteristics of the fertile and infertile subjects participated in this study. There was no change in mean age and semen volume between the normozoospermic control and the asthenozoospermics infertile groups. However, infertile group (AST) shows significantly lower (P < 0.001) sperm total motility compared with normozoospermic group as expected and significantly lower count and normal morphology; P < 0.01, P < 0.05, respectively.
Table 1.
Semen parameter analysis in normozoospermic (fertile) and asthenozoospermic (infertile) groups.
| Age (years) | Volume (ml) | Sperm motility (%) | Sperm concentration (106/ml) | Normal sperm morphology (%) | |
|---|---|---|---|---|---|
| Fertile (n = 40) | 36.80 ± 4.91 | 3.26 ± 1.91 | 59.12 ± 6.82 | 78.41 ± 6.83 | 46.34 ± 4.67 |
| AST (n = 30) | 37.74 ± 5.41 | 3.15 ± 1.22 | 29.73 ± 4.88*** | 48.83 ± 5.80** | 31.61 ± 4.56* |
Values are mean ± SD. ***p < 0.001, **p < 0.01 *p < 0.05 in comparison with fertile group.
3.2. Redox status of seminal plasma
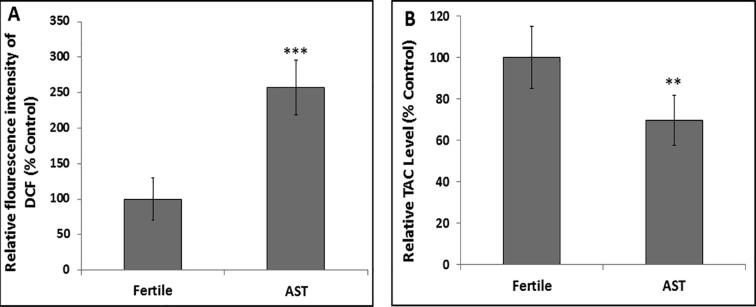
Fig. 1A shows fluorescent 2′, 7′-dichlorodihydrofluorescein (DCF) indicating ROS levels in seminal plasma. Compared to the control, levels of the ROS were highly significantly increased (P < 0.001) in the AST group. On the other hand, Fig. 1B illustrates significant decrease (P < 0.01) of TAC levels in seminal plasma of AST group in relation to control indicating compromised redox status of the infertile group.
Fig. 1.
Redox status of the seminal plasma in fertile and asthenozoospermic (AST) groups. A) Reactive Oxygen Species (ROS) levels and B) Total Antioxidant Capacity (TAC) in seminal plasma of the AST group compared to the normozoospermic (control fertile) group. Values represent means ± SD. *** P < 0.001, **P < 0.01 in comparison with fertile group.
3.3. Seminal MDA level
To determine the impact of impaired redox status, the level of MDA, an indicator of lipid peroxidation, was estimated in the seminal plasma of both fertile and infertile samples. The mean MDA level was found to be significantly increased (P < 0.01) in the seminal plasma of the AST group compared to the normozoospermic control group (Fig. 2).
Fig. 2.
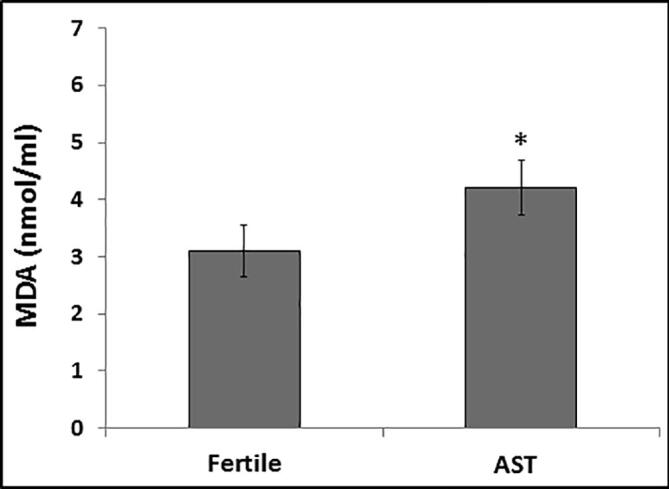
Comparison of malondialdehyde (MDA) levels in seminal plasma of asthenozoospermic (AST) samples in relation to the fertile normozoospermic (control) samples. The mean MDA level in the infertile group was significantly higher than that in the control group. Values represent means ± SD. *P < 0.05 in comparison with fertile group.
3.4. Seminal TEs
Seminal Zn and Mg levels significantly decreased (P < 0.01) in the AST infertile group compared to normozoospermic fertile group (Table 2). Although no significant difference was detected among the Se and Cu levels in seminal plasma, a slight increase was observed in the fertile group.
Table 2.
Seminal trace element concentrations in the normozoospermic (fertile) control group and the asthenozoospermic (infertile) group.
| Selenium (µg/L) | Copper (mg/L) | Zinc (mg/L) | Mg (mg/L) | |
|---|---|---|---|---|
| Fertile (n = 40) | 60.73 ± 18.12 | 165.56 ± 40.13 | 141.7 ± 30.23 | 13.14 ± 3.65 |
| AST (n = 30) | 43.12 ± 19.65 | 159.88 ± 36.01 | 119.8 ± 30.75** | 7.11 ± 3.88** |
Values are mean ± SD. **p < 0.01 in comparison with fertile group.
3.5. Pearson’s correlation of seminal TEs with sperm parameters and antioxidant status
Seminal Zn and Se levels exhibited strong significant correlation with sperm motility (r = 0.381, P < 0.01; r = 0.33, P = 0.01 respectively) and normal morphology (r = 0.41, P < 0.001; r = 0.36, P < 0.01 respectively). Mg also showed significant positive correlations with sperm motility (r = 0.18, P < 0.05) and morphology (r = 0.114, P < 0.05). However, seminal plasma copper levels correlated negatively but not significantly with normal sperm morphology (r = − 0.036, P = 0.17) and motility (r = − 0.028, P = 0.11) in the control and AST groups, respectively (Table 3). While Se (r = 0.025, P = 0.18), Zn (r = 0.019, P = 0.21), and Mg (r = 0.022, P = 0.11) showed positive and Cu (r = − 0.031, P = 0.13) showed negative but insignificant correlation with sperm concentration in the fertile and infertile groups (Table 3).
Table 3.
The value correlation coefficients calculated between the oxidative stress markers trace elements and sperm parameters.
| Total motility | Sperm concentration | Normal sperm morphology | |
|---|---|---|---|
| ROS | r = − 0.48**, P = 0.001 | r = − 0.28*, P = 0.02 | r = − 0.51**, P < 0.001 |
| TCA | r = 0.31**, P = 0.008 | r = 0.12*, P = 0.05 | r = 0.41**, P < 0.01 |
| MDA | r = −0.39**, P < 0.01 | r = − 0.31**, P = 0.007 | r = − 0.43**, P < 0.001 |
| Zn | r = 0.381**, P < 0.01 |
r = 0.025, P = 0.18 NS |
r = 0.41**, P < 0.001 |
| Se | r = 0.33**, P = 0.01 |
r = 0.019, P = 0.21 NS |
r = 0.36**, P < 0.01 |
| Mg | r = 0.18*, P < 0.05 |
r = 0.022, P = 0.11 NS |
r = 0.114*, P < 0.05 |
| Cu |
r = −0.028, P = 0.11 NS |
r = −0.031, P = 0.13 NS |
r = −0.036, P = 0.17 NS |
*p ≤ 0.05, **p ≤ 0.01. NS-non-significant.
Additionally, seminal ROS levels exhibited strong negative correlation with total sperm motility (r = − 0.48, P = 0.001), concentration (r = − 0.28, P = 0.02), and normal morphology (r = − 0.51, P = 0.001). TAC level measured in seminal plasma showed positive correlation with sperm motility (r = 0.31, P = 0.008) and normal morphology (r = 0.41, P < 0.01). Seminal lipid peroxidation measured as MDA level was negatively associated with sperm motility (r = r = − 0.39, P < 0.01) and normal sperm morphology (r = − 0.43, p = 0.001) (Table 3).
4. Discussion
Through a decade, there has been growing awareness of the impact of OS in etiology underlying infertility in men, yet the underlying mechanism is still unresolved. Probing into the investigations on the impact of OS on male infertility, seminal altered redox status and diminished TE level as the paramount cause of reduced sperm motility among AST men were thus validated in the current study. A significant increase in seminal ROS and MDA levels and diminished levels (P < 0.01) of TEs, such as Cu, Se, Zn, and Mg, were significantly correlated with reduced sperm motility (P < 0.01) in the AST group compared to fertile samples Nevertheless, ROS at minimal ranges are required for maturation of spermatozoa, acrosome reaction, capacitation, hyperactivation, and sperm-oocyte fusion (Fatima, 2018, Huang et al., 2018) yet, excessive ROS production has devastating effect on the neutralizing capability of antioxidants in the seminal plasma. Spermatozoa are highly vulnerable to attack by ROS due to the presence of large amounts of unsaturated fatty acid (UFA) in the cytoplasm and plasma membrane (Agarwal et al., 2014). ROS are known to mediate sperm dysfunction by triggering a cascade of peroxidation cycles resulting in oxidation of the membrane lipids and fragmentation of nucleic acid (Agarwal et al., 2006). Enhanced lipid peroxidation (MDA) is a reflective of considerable lipid peroxidation of the spermatozoa membrane that subsequently may have lowered sperm motility in AST group. In addition, correlation between oxidative stress markers and sperm motility was assessed to further evaluate the link between them. This confirmed the findings evident by the strong negative correlation between MDA and ROS and a positive correlation of TAC with sperm motility, indicating the negative impact of oxidative stress on motility, which is supported by recent studies (Dutta et al., 2019).
Semen is known to contain various nonenzymatic antioxidants like minerals such as Zn, Cu, Se, and Mg in addition to vitamins, GSH, coenzyme Q10,etc (Agarwal et al., 2003). TEs such as Zn, Cu, Se, and Mg were suggested to have important effect on sperm quality and normal function due to their involvement in key enzymatic reactions that are crucial for maintaining sperm normal physiology. The imbalance of TEs may impair sperm function by inducing OS, thus increasing the risk of male infertility (Tahmasbpour et al., 2019). Current results showed marked decrease (P < 0.01) in the levels of Zn, Se, and Mg in AST group compared to fertile group. Further, the correlation assessment confirms significant positive association of Zn, Se, and Mg with sperm motility, suggesting the fact that these elements play a vital role in maintaining redox balance in seminal ejaculate (Atig et al., 2012, Nenkova et al., 2017). A review of the literature shows significant inconsistency on the effect of levels of TEs found in semen on sperm function. Contradictory to our findings, recent studies reported no significant changes in the levels of these elements in the AST group (Hashemi et al., 2018, Palani and Alshatteri, 2017).
Seminal Zn and Mg levels are thought to be reflective of prostate secretory function as these elements originate primarily from the prostate gland. Level of these elements tend to alter in prostate gland infection could be indicated by a decrease in Zn and Mg levels in semen can be the cause of premature ejaculate and infertility (Omu and Fernandes., 2001). Low level of Mg could be due to presence of chelating agents working as Mg diminishing factors in the semen or hypomagnesemia due to low Mg consumption (Wong et al., 2001). Zn, which is known to exhibit antioxidative property, might play an important role in sperm motility since it is involved in disulfide bridge formation during sperm maturation, a critical step in motility generation in sperm (Khan et al., 2011). Furthermore, the antioxidant properties of Zn might be important in maintaining sperm viability via inhibiting DNase activity (Ebisch et al., 2007). The impact of low seminal Zn and Mg levels on sperm motility and morphology observed in the AST group in the present study is in accordance with recent findings (Abdul-Rasheed, 2010, Colagar et al., 2009).
Se, another important element examined in here, is known to be essential for testosterone biosynthesis, formation and normal sperm development (Akinloye et al., 2005). However, few studies have investigated the role of selenium in semen and the direct impact of its levels on male oxidative stress and fertility (Villaverde et al., 2014). Se mediate its functions as one of two selenoproteins forms; phospholipid hydroperoxide glutathione peroxidase (PHGPx) and selenoprotein P. PHGPx is the major selenoprotein expressed by germ cells in the testis, holding multiple functions and thus representing an important link between Se and sperm quality thus male fertility (Alahmar and Sengupta, 2020, Ursini et al., 1999). Se deficiency is thought to potentially affect chromatin condensation and reorganization processes via imposing oxidative stress, which in turn affects sperm quality and thus cause reduced fertilization capacity (Sánchez-Gutiérrez et al., 2008). Furthermore, Se deficiency might adversely affect antioxidant status in the male reproductive organs, and therefore it is reasonable to infer that Se deficiency potentially decreases the biosynthesis of selenoproteins with essential role in redox regulation, resulting in oxidative stress and consequent reduced male fertility. Negative correlation of Cu with sperm parameters indicates that excess Cu in seminal plasma can be harmful for the sperm parameters and thus for the male reproductive capacity (Kasperczyk et al., 2016). Although current study showed no significant difference in the seminal Cu levels in fertile and infertile groups, Cu is recognized as a crucial element for male gametes production (Ogorek et al., 2017).
Seminal plasma includes donations from accessory glands and testis in different amounts, resulting in variations between individuals. Semen viscosity and its ingredients, including lipids, sugars, and proteins, can certainly cause variation among individuals in terms of levels of free radicals or scavenging elements (Aljaser et al., 2017). Applying sperm washing techniques or density gradient separation may potentially cause relative alterations that perhaps contribute into the final data inconsistency, which represents protocol limitation. Thus, future work may include random and large sample size while involving experimentation of the optimal protocol prior to conducting the trial.
5. Conclusion
Data obtained are reflective of a diminished antioxidant status in infertile men. Decreased levels of seminal plasma TE were in synergy with increased production of ROS and MDA, which was associated with low sperm motility and male infertility. Thus, it can be concluded that decreased seminal plasma TE levels have considerable adverse impact on male fertility by generating imbalance or loss of oxidant-reductant equilibrium. Findings confirm the essential need of uniformed randomized large-sample-sized studies, evaluating the efficiency of TE and antioxidant supplementation on sperm quality.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education, Riyadh, Saudi Arabia for funding this research work through the project number IFKSURG-1442-012.
Authors contribution
All authors have contributed in this work. Aljaser F and Fatima S have conceptualized and performed the research, analyzed the data, and wrote the manuscript. Tabassum H has performed OS and TE lab work and revised tables. AbuDawood M and Aljaser F have reviewed and directed the data analysis and figures. Banu N has revised and contributed to the final approval of the version to be published. Both Aljaser F and Fatima S contributed in the scientific responsibility in addition to directing the research.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdul-Rasheed O.F. Association between seminal plasma copper and magnesium levels with oxidative stress in iraqi infertile men. Oman Med. J. 2010;25(3):168–172. doi: 10.5001/omj.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Saleh R.A., Bedaiwy M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003;79(4):829–843. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Sekhon L.H. Oxidative stress and antioxidants for idiopathic oligoasthenoteratospermia: Is it justified? Indian J. Urol. 2011;27:74–85. doi: 10.4103/0970-1591.78437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A., Sharma R., Nalella K.P., Thomas A.J., Alvarez J.G., Sikka S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006;86:878–885. doi: 10.1016/j.fertnstert.2006.02.111. [DOI] [PubMed] [Google Scholar]
- Agarwal A., Virk G., Ong C., du Plessis S.S. Effect of oxidative stress on male reproduction. World J. Mens. Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinloye O., Arowojolu O., Shittu B., Adejuwon C.A., Osotimehin B. Selenium status of idiopathic infertile Nigerian males. Biol. Trace Elem. Res. 2005;104:9–18. doi: 10.1385/BTER:104:1:009. [DOI] [PubMed] [Google Scholar]
- Alahmar A.T., Sengupta P. Impact of coenzyme Q10 and selenium on seminal fluid parameters and antioxidant status in men with idiopathic infertility. Biol. Trace Elem. Res. 2020:1–7. doi: 10.1007/s12011-020-02251-3. [DOI] [PubMed] [Google Scholar]
- Alahmar A. Effect of Vitamin C, Vitamin E, zinc, selenium, and coenzyme Q10 in infertile men with idiopathic oligoasthenozoospermia. Int. J. Infertil. Fetal. Med. 2017;8:45–49. [Google Scholar]
- Alahmar A.T. Role of oxidative stress in male infertility: an updated review. J. Hum. Reprod. Sci. 2019;12(1):4–18. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari W., Edris F., Albar Z., Gari A., Eskandar M., Al Fageah M., Zawawi S. High proportion of abnormal semen characteristics among Saudi infertile couples. Clin. Med. Diag. 2018;8:14–20. [Google Scholar]
- Aljaser F., Campbell B.K., Almajed F.S., Tomlinson M.J. Efficacy of ultra-rapid freezing by direct immersion into liquid nitrogen for sperm cryopreservation and its potential for male fertility preservation. Int. J. Innovative Res. Med. Sci. 2017;2:1401–1413. [Google Scholar]
- Alosaimi F.D., Bukhari M., Altuwirqi M., Habous M., Madbouly K., Abotalib Z., Binsaleh S. Gender differences in perception of psychosocial distress and coping mechanisms among infertile men and women in Saudi Arabia. Human Fertility. 2017;20:55–63. doi: 10.1080/14647273.2016.1245448. [DOI] [PubMed] [Google Scholar]
- Atig F., Raffa M., Habib B.A., Kerkeni A., Saad A., Ajina M. Impact of seminal trace element and glutathione levels on semen quality of Tunisian infertile men. BMC Urol. 2012;12:6. doi: 10.1186/1471-2490-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S.E., Ong C.N., Chua L.H., Ho L.M., Tay S.K. Comparison of zinc concentrations in blood and seminal plasma and the various sperm parameters between fertile and infertile men. J. Androl. 2000;21:53–57. [PubMed] [Google Scholar]
- Colagar A.H., Marzony E.T., Chaichi M.J. Zinc levels in seminal plasma are associated with sperm quality in fertile and infertile men. Nutr. Res. 2009;29(2):82–88. doi: 10.1016/j.nutres.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Dutta S., Majzoub A., Agarwal A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019;17(2):87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch I.M., Thomas C.M., Peters W.H., Braat D.D., Steegers-Theunissen R.P. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum. Reprod. Update. 2007;13(2):163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- Examination and processing of human semen, WHO 2010 https://www.who.int
- Fatima S. Novel Prospects Based on Oxidative/Nitrosative Stress in Health and Disease. INTECH publisher; Croatia: 2018. Role of Reactive Oxygen Species in Male Reproduction. [Google Scholar]
- Hashemi M.M., Behnampour N., Nejabat M., Tabandeh A., Ghazi-Moghaddam B., Joshaghani H.R. Impact of seminal plasma trace elements on human sperm motility parameters. Rom. J. Intern. Med. 2018;56(1):15–20. doi: 10.1515/rjim-2017-0034. [DOI] [PubMed] [Google Scholar]
- Hesham, N., Moemen, L.A., and Abu Elela, M,H. 2008. Studying the levels of malondialdehyde and antioxidant parameters in normal and abnormal human seminal plasma. Aust. J. Basic Appl. Sci. 2:773-778
- Huang C., Cao X., Pang D., Li C., Luo Q., Zou Y., Feng B., Li L., Cheng A., Chen Z. Is male infertility associated with increased oxidative stress in seminal plasma? A-meta analysis. Oncotarget. 2018;9:24494–24513. doi: 10.18632/oncotarget.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperczyk A., Dobrakowski M., Czuba Z.P., Kapka-Skrzypczak L., Kasperczyk S. Environmental exposure to zinc and copper influences sperm quality in fertile males. Ann. Agric. Environ. Med. 2016;23(1):138–143. doi: 10.5604/12321966.1196869. [DOI] [PubMed] [Google Scholar]
- Kasperczyk A., Dobrakowski M., Horak S., Zalejska-Fiolka J., Birkner E. The influence of macro and trace elements on sperm quality. J. Trace Elem. Med Biol. 2015;30:153–159. doi: 10.1016/j.jtemb.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Khan M.S., Zaman S., Sajjad M., Shoaib M., Gilani G. Assessment of the level of trace element zinc in seminal plasma of males and evaluation of its role in male infertility. Int. J. Appl. Basic Med. Res. 2011;1(2):93–96. doi: 10.4103/2229-516X.91152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macanovic B., Vucetic M., Jankovic A. Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: a pilot study. Dis. Markers. 2009;2015 doi: 10.1155/2015/436236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar H.B., Shaman A., Mirghani H.O., Almasalmah A.A. The outcome of assisted reproductive techniques among couples with male factors at Prince Khalid Bin Sultan Fertility Centre, Kingdom of Saudi Arabia. Macedonian J. Med. Sci. 2017;5:603–607. doi: 10.3889/oamjms.2017.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenkova G., Petrov L., Alexandrova A. Role of trace elements for oxidative status and quality of human sperm. Balkan Med. J. 2017;34:343. doi: 10.4274/balkanmedj.2016.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogórek M., Gąsior Ł., Pierzchała O., Daszkiewicz R., Lenartowicz M. Role of copper in the process of spermatogenesis. Postepy Hig. Med. Dosw. 2017;71:663–683. doi: 10.5604/01.3001.0010.3846. [DOI] [PubMed] [Google Scholar]
- Omu A.E., Fernandes S. The relationship between zinc/cadmium ratio in human semen: Effect on immune response. Kuwait Med. J. 2001;33:38–43. [Google Scholar]
- Palani A.F., Alshatteri A.H.A. Impact of trace elements in the seminal plasma on sperm quality in infertile men. ZJPAS. 2017;29:150–156. [Google Scholar]
- Sánchez-Gutiérrez M., García-Montalvo E., Izquierdo-Vega J., Del Razo L. Effect of dietary selenium deficiency on the in vitro fertilizing ability of mice spermatozoa. Cell Biol. Toxicol. 2008;24:321–329. doi: 10.1007/s10565-007-9044-8. [DOI] [PubMed] [Google Scholar]
- Seshadri S., Bates M., Vince G., Jones D.I.L. The role of cytokine expression in different subgroups of subfertile men. Am. J. Rep. Immunol. 2009;62:275–282. doi: 10.1111/j.1600-0897.2009.00736.x. [DOI] [PubMed] [Google Scholar]
- Showell M.J., Mackenzie-Proctor R., Brown J., Yazdani A., Stankiewicz M.T., Hart R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014;12 doi: 10.1002/14651858.CD007411.pub3. [DOI] [PubMed] [Google Scholar]
- Tahmasbpour E., Ebrahimi P., Beigi Harchegani A. An overview on role of some Mirnamniha M, Faroughi trace elements in human reproductive health, sperm function and fertilization process. Rev. Environ. Health. 2019;18;34(4):339–348 doi: 10.1515/reveh-2019-0008. [DOI] [PubMed] [Google Scholar]
- Ursini F., Heim S., Kiess M., Maiorino M., Roveri A. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:225–228. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- Villaverde A.I.S., Fioratti E.G., Ramos R.S., Neves R.C., Ferreira J.C.P., Cardoso G.S., Padilha P.M., Lopes M.D. Blood and seminal plasma concentrations of selenium, zinc and testosterone and their relationship to sperm quality and testicular biometry in domestic cats. Anim. Reprod. Sci. 2014;150:50–55. doi: 10.1016/j.anireprosci.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Wang Y.X., Wang P., Feng W. Relationships between seminal plasma metals/metalloids and semen quality, sperm apoptosis and DNA integrity. Environ. Pol. 2017;224:224–234. doi: 10.1016/j.envpol.2017.01.083. [DOI] [PubMed] [Google Scholar]
- Wong W.Y., Flik G., Groenen P.M., Swinkels D.W., Thomas C.M., Copius-Peereboom J.H. The impact of calcium, magnesium, zinc, and copper in blood and seminal plasma on semen parameters in men. Reprod. Toxicol. 2001;15(2):131–136. doi: 10.1016/s0890-6238(01)00113-7. [DOI] [PubMed] [Google Scholar]