Abstract
Background
There are concerns about a link between the ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines against COVID-19 and cerebral venous thrombosis (CVT) and other thrombotic events. One key missing component of the risk-benefit analysis of using such vaccines is the risk of these severe thrombotic events following COVID-19.
Methods
Using a retrospective cohort study based on electronic health records primarily in the USA, the absolute risks of CVT and portal vein thrombosis (PVT) in the two weeks following a diagnosis of COVID-19 (made between January 20, 2020 and March 25, 2021) were calculated. The risks were compared to cohorts of patients with influenza (diagnosed within the same period) and people receiving an mRNA vaccine (i.e. not the ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines) against COVID-19 (matched for demographics and the main risk factors for CVT and PVT).
Findings
A total of 537,913 patients with a COVID-19 diagnosis were included. The incidence of CVT in the two weeks after a COVID-19 diagnosis was 42.8 per million people (95% CI 28.5–64.2). This was significantly higher than in a matched cohort of people who received an mRNA vaccine (RR = 6.33, 95% CI 1.87–21.40, P = 0.00014) and patients with influenza (RR = 2.67, 95% CI 1.04–6.81, P = 0.031). The incidence of PVT after COVID-19 diagnosis was 392.3 per million people (95% CI 342.8–448.9). This was significantly higher than in a matched cohort of people who received an mRNA vaccine (RR=4.46, 95% CI 3.12–6.37, P < 0.0001) and patients with influenza (RR=1.43, 95% CI 1.10–1.88, P = 0.0094).
Keywords: COVID-19, Cerebral venous sinus thrombosis, Portal vein thrombosis, Electronic health records, SARS-CoV-2
Research in context.
Evidence before this study
We searched PubMed with the terms ("COVID" OR "SARS-CoV-2″) AND ("cerebral venous thrombosis" OR "cerebral venous sinus thrombosis" OR "cerebral vein thrombosis" OR "portal vein thrombosis" OR "portal venous thrombosis") until June 6, 2021 included. A systematic review of cohort studies suggested an incidence of CVT among patients hospitalized with COVID-19 to be about 800 per million patients but it revealed evidence of selection, ascertainment, and reporting bias in all included studies. A few case reports and case series reported PVT associated with COVID-19. The incidence of CVT and PVT among both hospitalised and non-hospitalised patients with COVID-19 is unknown and the lack of control studies means that it is also unknown if COVID-19 increases the risk of CVT and PVT.
Added value of this study
Our study estimates that the absolute risk of CVT and PVT are respectively 42.8 and 392.3 per million patients (both hospitalised and non-hospitalised) in the 2 weeks after a diagnosis of COVID-19. COVID-19 increases the risk of CVT and PVT compared to patients diagnosed with influenza, and to people who have received a COVID-19 mRNA vaccine.
Implications of all the available evidence
The data highlight the risk of serious thrombotic events after COVID-19. These help contextualize the risks and benefits of vaccination.
Alt-text: Unlabelled box
Introduction
There are concerns about a possible association between vaccines against SARS-CoV-2 and cerebral venous thrombosis (CVT, also called cerebral venous sinus thrombosis [1]). The concern has focused primarily on ChAdOx1 nCoV-19 (“Oxford-AstraZeneca”) vaccine, [2,3] and more recently the Ad26.COV2.S (“Janssen”) vaccine [4], with much fewer events being reported after the BNT162b2 (‘Pfizer-BioNTech’) vaccine or the mRNA-1273 (‘Moderna’) vaccines [5]. Emerging data for the ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines suggest that the association reflects a ‘vaccine-induced thrombotic thrombocytopenia’ (VITT), also known as Thrombosis with Thrombocytopenia Syndrome (TTS), which also involves thrombotic events in other body systems, including portal vein thrombosis (PVT) [2,3]. Governments and regulatory authorities have reacted by restricting the use of the two vaccines in different subgroups of the population, based on a risk-benefit analysis [3,4]. Yet one key component of the risk-benefit calculation is currently unknown: the absolute risk of CVT following a diagnosis of COVID-19. To date there are only a few case series of CVT post-COVID-19, and a few cohort studies limited to hospitalized patients–a systematic review of which revealed evidence of selection, ascertainment, and reporting bias [6]. The evidence for an association between COVID-19 and PVT is limited to a few case reports and case series [7], [8], [9], [10].
Here, using an electronic health records network primarily based in the USA, we estimated the incidence of CVT and PVT occurring in confirmed COVID-19 cases (both hospitalized and non-hospitalized) and compared this incidence to two other groups: people who received a COVID-19 mRNA vaccine (i.e. the BNT162b2 or mRNA-1273 vaccine), and a cohort of patients with influenza. A direct comparison with rates after the ChAdOx1 nCoV-19 (“Oxford-AstraZeneca”) vaccine could not be made because this has not been used in the USA.
Methods
Data
TriNetX Analytics is a federated network of linked electronic health records (EHRs) recording anonymized data from 59 healthcare organizations (HCO), primarily in the USA, totalling 81 million patients. MT and PJH had access to the data. The data are based directly on the electronic health records used in clinical practice and not on claims data and so do not suffer the typical limitations of claim databases (such as inaccurate billing and lack of representativeness of the population) [11]. Details of the network have been described elsewhere [12] and in Supplementary Methods 1. In short, the HCOs are a mixture of hospitals, primary care, and specialist providers and contribute data from insured and uninsured patients alike. Most HCOs have both inpatient and outpatient data. The data from a typical HCO generally go back around 7 years, with some going back 13 years. HCO update their data frequently, with the vast majority refreshing every 1, 2, or 4 weeks. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating HCOs and their individual contribution to each dataset cannot be disclosed.
The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule, so that ethical approval from an institutional review board is not needed.
Primary analysis
A cohort of all patients who had a confirmed diagnosis of COVID-19 (ICD-10 code U07.1) between January 20, 2020 and March 25, 2021 was defined for study. The absolute risk of a diagnosis of CVT (ICD-10 code I67.6) was calculated by identifying those patients in the cohort who had the diagnosis in the two weeks following their diagnosis of COVID-19. The absolute risk of PVT (ICD-10 code I81) was also calculated. A focus on PVT was motivated by reports of thromboses of the splanchnic venous system (and specifically PVT) as part of VITT. PVT is a subtype of splanchnic vein thrombosis which is common and has its own ICD-10 code (unlike other kinds of thromboses of the splanchnic venous system).
For the whole COVID-19 cohort, and for cases with CVT or PVT following COVID-19, baseline characteristics are reported (details in Supplementary Methods 2). We identified patients who had a reported high D-dimer (> 5 mg/L), low fibrinogen (< 200 mg/dL), or thrombocytopenia (ICD-10 codes D69.49, D69.59, or D69.6) within the 2 weeks after their COVID-19 diagnosis. We also assessed how many of them had died (and, if so, when) by the time of the analysis (April 21, 2021).
A causal link between COVID-19 and CVT/PVT cannot be established with a cohort study. However, a testable corollary of a causal association is that the rate of new CVT/PVT diagnoses decreases with time from the index event. We tested this corollary by comparing the absolute risk within two weeks of diagnosis (Week 1 and 2) with the absolute risk within the next two weeks (Week 3 and 4) and the two weeks thereafter (Week 5 and 6). For this part of the analysis, only patients diagnosed on or before February 28, 2021 were included to allow for sufficient follow-up.
Two control cohorts based on other index events were used for comparison: a diagnosis of influenza (ICD-10 codes J09-J11) between January 20, 2018 and March 25, 2021 (an earlier start date was used for this event to achieve a sufficiently large sample), and receipt of a first dose of the two vaccines administered to this predominantly US population: the BNT162b2 (‘Pfizer-BioNTech’) vaccine or the mRNA-1273 (‘Moderna’) vaccine before March 25, 2021. We excluded from these cohorts any patients who had a diagnosis of COVID-19 on or after January 20, 2020. A detailed description of cohorts is provided in Supplementary Methods 3.
These two cohorts were then matched (see ‘Statistical analysis’ for details) to the cohort of patients with COVID-19 for the following covariates:
-
-
Age
-
-
Sex
-
-
Race
-
-
Overweight and obesity
-
-
History of cancer (matching for 16 separate ICD-10 codes capturing the main categories of cancer).
-
-
Previous use of hormonal therapy or contraceptive medications (matching for 18 separate codes of the VA Formulary).
For the analysis of PVT, cohorts were also matched for previous liver diseases (matching for 8 separate ICD-10 codes capturing the main categories of liver diseases). Details on the definition of covariates are presented in Supplementary Methods 4.
Using these matched cohorts, we calculated the relative risk (RR) of a CVT diagnosis, a PVT diagnosis, and a diagnosis of thrombocytopenia in the two weeks after COVID-19 diagnosis compared to the other index events (i.e. influenza or vaccination).
Secondary analyses
The analyses were repeated in four different ways to test the robustness of the findings to the choice of outcome, cohort, covariates, and statistical model. First, analyses were repeated after broadening the diagnostic criteria for CVT to include I63.6 (cerebral infarction due to central thrombosis, non-pyogenic), G08 (intracranial and intraspinal phlebitis and thrombophlebitis), O22.5 (CVT in pregnancy) and O87.3 (CVT in the puerperium), in line with recent epidemiological studies that have used this definition of CVT [13,14].
Second, the analyses were repeated after excluding those patients who had a prior diagnosis of the event of interest (CVT or PVT).
Third, the analyses were repeated after additionally matching for previous use of anticoagulants, in addition to all other covariates already included in the primary analysis.
Finally, the analyses were repeated using a time-to-event analysis (details below) to account for the timing of outcomes within the 2-weeks’ follow-up and to account for patients who made no further contacts with an HCO within the network.
Statistical analyses
Fisher's exact tests were used to compare characteristics (baseline and laboratory) and death rates between patients with COVID-19 who had a CVT (or PVT) compared to patients with COVID-19 who did not. Fisher's exact tests were also used to test the null hypothesis that the relative risks of CVT and PVT in the two weeks after COVID-19 vs. influenza and vs. mRNA vaccine were equal to 1. Confidence intervals for absolute risks were based on Wilson score intervals. Confidence intervals for relative risks were based on Wald confidence limits, with Agresti-Coull adjustment, which provide better coverage than ‘exact’ intervals when the risks are small [15].
Propensity score matching was used to create cohorts with matched baseline characteristics (see above), and carried out within TriNetX. Propensity score 1:1 matching used a greedy nearest neighbor matching approach with a caliper distance of 0.1 pooled standard deviation of the logit of the propensity score. Any characteristic with a standardized mean difference (SMD) between cohorts lower than 0.1 is considered well matched [16].
Time-to-event analysis was conducted using the Kaplan-Meier estimator and the Cox proportional hazard model. The proportional hazard assumption was tested using the generalized Schoenfeld approach.
Statistical significance was set at a 2-sided P-value < 0.05. Analyses were performed using R version 3.6.3. This study follows the STROBE reporting guidelines (see Supplement for a checklist). Further details about the statistical analyses are provided in Supplementary Methods 5.
Role of the funding source
The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Results
Absolute risk of CVT and PVT after COVID-19
537,913 patients with a confirmed diagnosis of COVID-19 were included in this study (54.9% females, mean [SD] age 46.2 [21.4]; Table 1 and Supplementary Table 1). Of these, 23 were diagnosed with a CVT in the two weeks following their diagnosis (absolute risk: 42.8 per million people, 95% CI 28.5–64.2, equivalent to an incidence of 111.5 per 100k person-years). The risk was significantly higher among patients with a history of cardiovascular diseases, specifically arterial diseases (P = 0.00086), cerebral/precerebral artery stenosis/occlusion (P = 0.00019), and intracranial hemorrhage (P < 0.0001; Table 1).
Table 1.
Baseline characteristics of the whole COVID-19 cohort and the groups who received a diagnosis of CVT or PVT in the two weeks after COVID-19 diagnosis. The P-values from Fisher exact test (or t-test for age) for CVT and PVT groups compared to the rest of the COVID-19 cohort are shown.
| All patients with COVID-19 | Patients with COVID-19 and CVT |
Patients with COVID-19 and PVT |
|||
|---|---|---|---|---|---|
| n (%) | mean (SD) | n (%) | mean (SD) | P | n (%) | mean (SD) | P | |
| Sample size, n | 537,913 (100.0) | 23 (100.0) | – | 211 (100.0) | – |
| Age, mean (SD), y | 46.2 (21.4) | 46.5 (21.5) | 0.95 | 57.2 (14.6) | <0.0001 |
| Sex, n (%) | |||||
| Female | 295,220 (54.9) | 16 (69.6) | 0.21 | 94 (44.5) | 0.0029 |
| Male | 242,439 (45.1) | 7 (30.4) | 0.21 | 117 (55.5) | 0.0028 |
| Race, n (%) | |||||
| White | 326,258 (60.7) | 18 (78.3) | 0.091 | 145 (68.7) | 0.017 |
| Black | 93,712 (17.4) | 4 (17.4) | 1 | 34 (16.1) | 0.72 |
| Asian | 14,498 (2.7) | 2 (8.7) | 0.13 | 3 (1.4) | 0.39 |
| Other | 3909 (0.7) | 0 (0.0) | 1 | 1 (0.5) | 1 |
| Unknown | 99,536 (18.5) | 2 (8.7) | 0.29 | 28 (13.3) | 0.051 |
| Comorbidities at baseline, n (%) | |||||
| Obesity | 92,903 (17.3) | 4 (17.4) | 1 | 57 (27.0) | 0.00048 |
| Hypertension | 153,305 (28.5) | 8 (34.8) | 0.49 | 115 (54.5) | <0.0001 |
| CKD | 35,582 (6.6) | 2 (8.7) | 0.66 | 51 (24.2) | <0.0001 |
| Ischemic heart diseases | 48,767 (9.1) | 5 (21.7) | 0.052 | 42 (19.9) | <0.0001 |
| Cardiac failure | 29,536 (5.5) | 2 (8.7) | 0.36 | 26 (12.3) | 0.00011 |
| Arterial diseases | 38,606 (7.2) | 7 (30.4) | 0.00086 | 44 (20.9) | <0.0001 |
| Venous diseases | 33,222 (6.2) | 4 (17.4) | 0.05 | 171 (81.0) | <0.0001 |
| Cerebral/Pre-cerebral artery stenosis | 20,817 (3.9) | 6 (26.1) | 0.00019 | 21 (10.0) | <0.0001 |
| Intracranial hemorrhage | 4056 (0.8) | 5 (21.7) | <0.0001 | 9 (4.3) | <0.001 |
| Dementia | 11,872 (2.2) | 1 (4.3) | 0.4 | 3 (1.4) | 0.64 |
| Chronic lower resp. disease | 90,617 (16.8) | 6 (26.1) | 0.26 | 69 (32.7) | <0.0001 |
| Connective tissue disorders | 9671 (1.8) | 1 (4.3) | 0.34 | 15 (7.1) | <0.0001 |
| Liver disease | 32,972 (6.1) | 2 (8.7) | 0.65 | 148 (70.1) | <0.0001 |
| Diabetes mellitus | 77,038 (14.3) | 6 (26.1) | 0.13 | 73 (34.6) | <0.0001 |
| Malignancy | 40,636 (7.6) | 3 (13.0) | 0.25 | 100 (47.4) | <0.0001 |
| Past CVT | 90 (0.02) | 4 (17.4) | <0.0001 | 0 (0.0) | 1 |
| Past PVT | 676 (0.1) | 0 (0.0) | 1 | 117 (55.5) | <0.0001 |
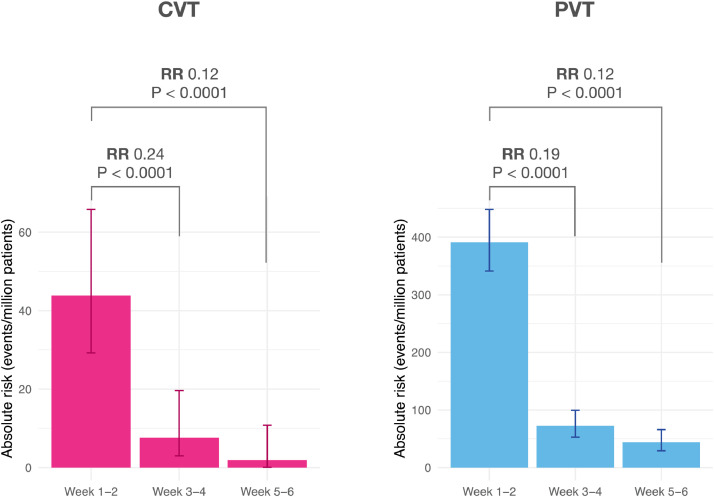
Among the 23 events, 7 were observed in patients under the age of 30, 4 between 30 and 39, 2 between 40 and 49, 3 between 50 and 59, 2 between 60 and 69, and 5 between 70 and 79. Four patients had also had a CVT diagnosed prior to their COVID-19 diagnosis, one between 4 and 8 weeks beforehand, and the other 3 > 8 weeks prior. The incidence of CVT following COVID-19 significantly decreased with time from the index event (RR in 3rd and 4th week vs. first 2 weeks 0.24, 95% CI 0.098–0.59, P < 0.0001; RR in 5th and 6th week vs. first 2 weeks 0.12, 95% CI 0.036–0.40, P < 0.0001; Fig. 1).
Fig. 1.
Incidence of CVT and PVT per million patients as a function of the time since diagnosis of COVID-19, from the first 2 weeks post diagnosis to the 5th and 6th week post diagnosis. The height of the bars represent absolute risks while the error bars represent 95% confidence intervals.
The absolute risk of PVT in the two weeks following COVID-19 was 392.3 per million people (95% CI 342.8–448.9), equivalent to 1022.7 per 100k person-years. Among the 211 affected patients, 117 also had a PVT prior to their COVID-19 diagnosis. The incidence of PVT following COVID-19 significantly decreased with time from the index event (RR in 3rd and 4th week vs. first 2 weeks 0.19, 95% CI 0.14–0.27, P < 0.0001; RR in 5th and 6th week vs. first 2 weeks 0.12, 95% CI 0.080–0.18, P < 0.0001; Fig. 1).
Laboratory data and death rate
Laboratory data were available for a subset of the COVID-19 patients (Table 2). Although the data do not cover most patients with a diagnosis of CVT, they suggest that patients with CVT after COVID-19 were significantly more likely to have elevated D-dimer level than patients with COVID-19 who did not have CVT (P = 0.012), whereas patients with PVT after COVID-19 were significantly more likely to have low fibrinogen level (P < 0.0001) and thrombocytopenia (P < 0.0001). The death rate among patients with CVT in the two weeks after COVID-19 was 17.4% (4 out of 23 patients, 95% CI 6.98–37.1%; Supplementary Figure 1) and that among patients with PVT after COVID-19 was 19.9% (42 out of 211 patients, 95% CI 15.1–25.8%; Supplementary Figure 1) and were significantly higher than among patients with COVID-19 who did not have those events (P = 0.0050 for CVT and P < 0.0001 for PVT).
Table 2.
Laboratory characteristics of the patients in each group. P values are from Fisher's exact test, comparing the CVT and PVT groups to the rest of the COVID-19 cohort.
| All patients with COVID-19 | Patients with COVID-19 and CVT |
Patients with COVID-19 and PVT |
|||
|---|---|---|---|---|---|
| n (%) | n (%) | P | n (%) | P | |
|
D-dimer > 5 mg/L n/n with measurement (%) |
2047/68,313 (3.0) | 2/6 (33.3) | 0.012 | 4/66 (6.1) | 0.14 |
|
Fibrinogen < 200 mg/dL n/n with measurement (%) |
1167/19,602 (6.0) | 1/6 (16.7) | 0.31 | 22/46 (47.8) | <0.0001 |
|
Thrombocytopenia (ICD-10 codes D69.49, D69.59, D69.6) |
8840 (1.8) | 0 (0.0) | 1 | 59 (28.0) | <0.0001 |
| Death | 16,619 (3.1) | 4 (17.4) | 0.0050 | 42 (19.9) | <0.0001 |
Relative risks of CVT and PVT compared to matched cohorts
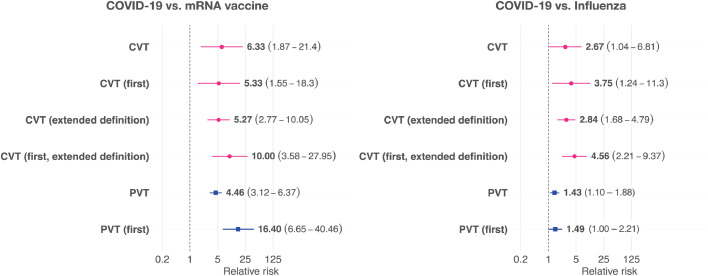
The two-week risk of being diagnosed with a CVT was significantly higher in the cohort diagnosed with COVID-19 compared to a matched cohort receiving an mRNA vaccine (N = 389,034 in each cohort; RR=6.33, 95% CI 1.87–21.40, P = 0.00014; Fig. 2 and Supplementary Table 2) and compared to a matched cohort diagnosed with influenza (N = 393,972 in each cohort; RR = 2.67, 95% CI 1.04–6.81, P = 0.031; Fig. 2 and Supplementary Table 3). Similarly, the two-week risk of being diagnosed with a PVT was significantly higher in the cohort diagnosed with COVID-19 compared to a matched cohort receiving an mRNA vaccine (N = 388,298 in each cohort, RR = 4.46, 95% CI 3.12–6.37, P < 0.0001; Fig. 2 and Supplementary Table 4) or compared to a matched cohort diagnosed with influenza (N = 393,848 in each cohort, RR=1.43, 95% CI 1.10–1.88, P = 0.0094; Fig. 2 and Supplementary Table 5). A diagnosis of thrombocytopenia was also significantly more likely in the two weeks after COVID-19 than following an mRNA vaccine (RR = 23.96, 95% CI 21.49–26.73, P < 0.0001) or a diagnosis of influenza (RR = 1.23, 95% CI 1.18–1.28, P < 0.0001).
Fig. 2.
Relative risk of CVT and PVT after a diagnosis of COVID-19 compared to matched cohorts of people receiving an mRNA vaccine (left) or with a diagnosis of influenza (right). Horizontal lines and numbers in brackets represent the 95% confidence intervals.
Robustness analyses
When the definition of CVT in terms of ICD-10 codes was broadened, the incidence of CVT in the two weeks after COVID-19 was 148.7 per million people (95% CI 119.5–185.1), which was significantly higher than in the matched cohort of people receiving an mRNA vaccine (RR = 5.27, 95% CI 2.77–10.04, P < 0.0001; Fig. 2) and the matched cohort of patients with influenza (RR=2.84, 95% CI 1.68–4.79; P < 0.0001).
When excluding patients who had also had a CVT or PVT prior to COVID-19, the incidence of a first CVT or PVT post-COVID-19 diagnosis were reduced accordingly (CVT: absolute risk 35.3 per million, 95% CI 22.6–55.2; PVT: absolute risk 175.0 per million, 95% CI 143.0–214.1), but all RRs were similar to those in the primary analysis (Fig. 2).
All results were very similar when cohorts were matched for previous use of anticoagulants in addition to all other covariates (Supplementary Figure 2).
The results using a Kaplan-Meier estimator (mean/median follow-up times: 11.3/14 days for the comparison with mRNA vaccines, and 10.7/14 days for the comparison with influenza) were consistent with the results based on relative risks: hazard ratios were significantly greater than 1 for the comparison between COVID-19 and matched cohorts in terms of incidence of CVT (HR 17.87, 95% CI 2.38 – 134.32, P < 0.001 compared to people receiving an mRNA vaccine, and HR 3.64, 95% CI 1.20–11.08, P = 0.012 compared to patients diagnosed with influenza), PVT (HR 4.90, 95% CI 3.40–7.07, P < 0.001 compared to mRNA vaccines, and HR 1.52, 95% CI 1.15 – 1.99, P = 0.0025 compared to influenza), and thrombocytopenia (HR 25.10, 95% CI 22.50–28.00, P < 0.001 compared to mRNA vaccines, and HR 1.27, 95% CI 1.22–1.32, P < 0.001 compared to influenza). The proportional hazard assumption was respected for most comparisons (Supplementary Table 6 and Supplementary Fig. 3).
Discussion
In a large electronic health records network, the absolute incidence of CVT and PVT in the 14 days after COVID-19 diagnosis was 42.8 and 392.3 per million patients respectively. The incidence rapidly decreased in the following weeks, compatible with a causal link between COVID-19 and those thrombotic events. However, causation cannot be demonstrated with the current study and residual confounding (e.g. increased medical monitoring directly after COVID-19 vs. a few weeks later, or time-limited disease processes increasing both the risk of COVID-19 and thromboses) might contribute to this observation.
The incidence of CVT and PVT after COVID-19 is substantially greater than in the matched control cohorts. The incidence of CVT after a diagnosis of COVID-19 is also substantially greater than the expected incidence in the general population in the USA, estimated to be between 0.53 and 0.77 per million people in any 2-week period [14] and the rate is significantly higher than the highest of these estimates (binomial test: P < 0.001).
The incidence is also many-fold higher than the latest reported incidence of CVT following administration of the first dose of the ChAdOx1 nCoV-19 (‘Oxford-AstraZeneca’) vaccine (reported by the European Medicines Agency to be around 5 per million vaccinated people [17] and by a predominantly female population-based cohort study in Denmark and Norway to be about 24.9 per million [18]) and the latest reported incidence of CVT following administration of the Ad26.COV2.S (“Janssen”) vaccine (reported to be about 1.7 per million vaccinated people [4]).
The increased rate of CVT in COVID-19 is notable, with relative risks higher than those for commoner forms of stroke and cerebral hemorrhage [12]. The PVT data highlight that COVID-19 is associated with thrombotic events that are not limited to the cerebral vasculature, likely reflecting the key pathogenic role of endothelial dysfunction [19].
Importantly, the present study cannot be used to draw conclusions on the relative risk of developing a CVT or PVT after receiving an mRNA vaccine compared to the baseline incidence or compared to other vaccines. Far larger samples are needed (such as those used by the EMA and the FDA pharmacovigilance studies) because the events have so far been found to be extremely rare. The observed incidence of CVT in the matched cohort of people who received an mRNA vaccine is compatible with even the lowest estimate of the baseline rate in the USA of 0.53 per million people in any 2-week period [14] (binomial test: P = 0.18). Thus, the results of this study are consistent with the hypothesis that these vaccines are not associated with an increased rate of CVT.
Achieving accurate estimates of the incidence of CVT after COVID-19 is challenging due to the rarity of events. Retrospective cohort studies conducted following the observation of one case are bound to have selection bias (retrospective cohort studies rarely report zero cases) [6]. Using a large EHR network can provide more accurate estimates because HCOs contribute data whether or not a case of CVT post-COVID-19 has been observed (indeed the number of HCOs in TriNetX exceeds the number of cases observed in the whole cohort). In quantitative terms, even if all CVTs occurred among the patients in our cohort who had been hospitalized at the time of their COVID-19 diagnosis (n = 117,528), the incidence of CVT post-COVID-19 would be 196 per million; this is substantially lower than the incidence reported in a recent systematic review (800 per million hospitalized patients) [6] suggesting that a substantial proportion of the selection bias has been addressed in this study. In addition, the use of matched cohorts provides evidence that COVID-19 is associated with an increased rate of CVT, above and beyond what is observed in a similar cohort not infected with COVID-19.
Our study also has several limitations and results should be interpreted with caution. First, ascertainment of CVT and PVT might differ between cohorts. Addressing ascertainment bias is extremely challenging for rare outcomes. Second, there are limitations inherent to studies based on EHR data, described in Supplementary Methods 1. In short, no information on diagnostic accuracy or completeness is available; undiagnosed cases of COVID-19 would not appear in the cohort and so our findings cannot be generalized to those patients; and lack of linkage might occur for patients who receive part of their care outside the HCO network (e.g. the relative risk estimates may not apply to patients receiving their vaccine elsewhere). Third, while cohorts are matched for age, sex, race, and for the main shared risk factors of COVID-19 and CVT/PVT, there might be residual confounding. Fourth, the absence of key laboratory data (and in particular anti-PF4 antibodies) from many patients limits our ability to comment on the mechanism of CVT after COVID-19 (and there is no reason to believe a priori that it is similar to VITT [2,3]). Given that data are strictly anonymized, there is no mechanism by which a particular patient's health record can be retrieved for further examination, nor opportunities to recruit a specific group of patients for follow-up investigations. Fifth, while we have information about hospitalizations for patients, the cause of hospitalization cannot be inferred (e.g. if a patient with a new diagnosis of COVID-19 is hospitalized 3 days later and has a diagnosis of CVT recorded 1 day later, it is unclear whether they were hospitalized for COVID-19 or due to their CVT).
In summary, COVID-19 is associated with a markedly increased incidence of CVT compared to patients with influenza, people who have received BNT162b2 or mRNA-1273 vaccines and compared to the best estimates of the general population incidence. The risk with COVID-19 also appears greater than with ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines, although as noted this conclusion is indirect and tentative. The rarity of CVT in all populations means that larger sample sizes are required to confirm the results, and complementary study designs are needed to aid interpretation. Nevertheless, the current data highlight the risk of serious thrombotic events in COVID-19 and can help contextualize and inform debate about the risk-benefit ratio for COVID-19 vaccines.
Contributions
MT and PJH were granted unrestricted access to TriNetX Analytics for the purposes of research, and with no restrictions as to the analyses done or the decision to publish; they designed the study and directly accessed the TriNetX Analytics web interface to do it. MT and PJH have verified the data. MT, JRG, MH, and PJH defined cohort inclusion and exclusion criteria, and the outcome criteria and analytical approaches. MT did the data analyses, assisted by SL and PJH. MT, JRG, MH, and PJH contributed to data interpretation. MT wrote the paper with input from PJH, JRG, MH, and SL. PJH and MT had full access to all the data in the study. The corresponding author had full access to all the data in the study, conducted the analysis, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
NIHR Oxford Health Biomedical Research center (BRC-1215–20,005). MT is an NIHR Academic Clinical Fellow and NIHR Oxford Health Biomedical Research Centre Senior Research Fellow. MH is a Wellcome Principal Research Fellow and supported by the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or the Department of Health.
Data sharing statement
The TriNetX system returned the results of the analyses as .csv files, which were downloaded and archived. Data presented will be freely accessible to anyone at: https://osf.io/h2mt7. In addition, TriNetX will grant access to researchers if they have a specific concern (through a third-party agreement option).
Declaration of Competing Interest
SL is an employee of TriNetX. MH reports a Wellcome Trust Principal Research Fellowship. All other authors declare: no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Footnotes
Interpretation: The incidence of CVT and PVT is significantly increased after COVID-19. The data highlight the risk of serious thrombotic events in COVID-19 and can help contextualize the risks and benefits of vaccination in this regard.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101061.
Appendix. Supplementary materials
References
- 1.Silvis S.M., de Sousa D.A., Ferro J.M., Coutinho J.M. Cerebral venous thrombosis. Nat Rev Neurol. 2017;13:555–565. doi: 10.1038/nrneurol.2017.104. [DOI] [PubMed] [Google Scholar]
- 2.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104840. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schultz N.H., Sørvoll I.H., Michelsen A.E. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021 doi: 10.1056/NEJMoa2104882. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.See I., Su J.R., Lale A. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021 doi: 10.1001/jama.2021.7517. published online April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cines D.B., Bussel J.B. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;384:2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldini T., Asioli G.M., Romoli M. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Neurol. 2021 doi: 10.1111/ene.14727. published online Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Mura V., Artoni A., Martinelli I. Acute portal vein thrombosis in SARS-CoV-2 infection: a case report. Am J Gastroenterol. 2020;115:1140–1142. doi: 10.14309/ajg.0000000000000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borazjani R., Seraj S.R., Fallahi M.J., Rahmanian Z. Acute portal vein thrombosis secondary to COVID-19: a case report. BMC Gastroenterol. 2020;20:386. doi: 10.1186/s12876-020-01518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franco-Moreno A., Piniella-Ruiz E., Montoya-Adarraga J. Portal vein thrombosis in a patient with COVID-19. Thromb. Res. 2020;194:150–152. doi: 10.1016/j.thromres.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Low S.-.W., Swanson K.L., McCain J.D., Sen A., Kawashima A., Pasha S.F. Gastric ischemia and portal vein thrombosis in a COVID-19-infected patient. Endoscopy. 2020;52:E465–E466. doi: 10.1055/a-1230-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klabunde C.N., Warren J.L., Legler J.M. Assessing comorbidity using claims data: an overview. Med Care. 2002;40(IV):26–35. doi: 10.1097/00005650-200208001-00004. [DOI] [PubMed] [Google Scholar]
- 12.Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handley J.D., Emsley H.C. Validation of ICD-10 codes shows intracranial venous thrombosis incidence to be higher than previously reported. Health Inf Manag. 2020;49:58–61. doi: 10.1177/1833358318819105. [DOI] [PubMed] [Google Scholar]
- 14.Otite F.O., Patel S., Sharma R. Trends in incidence and epidemiologic characteristics of cerebral venous thrombosis in the United States. Neurology. 2020;95:e2200–e2213. doi: 10.1212/WNL.0000000000010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agresti A., Coull B.A. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 16.Haukoos J.S., Lewis R.J. The propensity score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Medicines Agency. AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. EMA Website. 2021 https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood [online] Available from. [Accessed June 8, 2021] [Google Scholar]
- 18.Pottegård A., Lund L.C., Karlstad Ø. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonaventura A., Vecchié A., Dagna L. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.