Abstract
Brassinosteroids (BRs) have emerged as pleiotropic phytohormone owing to their wide function in crop growth and metabolism. Homobrassinolide (HBR) being an analogue of BRs is known to improve the growth, yield and quality parameters in many crop plants. Thus, an evaluation study was conducted for two years (2018 and 2019) to elucidate the performance of tomato plants (Solanum lycopersicum L.) to a novel group of phytohormone,HBR. The field experiment comprised of seven treatments with homobrassinolide 0.04% (Emulsifiable Concentrate) EC at four different concentrations (0.06, 0.08, 0.10 and 0.12 g active ingredient (a.i.) ha−1) and two well-known growth promoters viz., Gibberellic acid (GA), Naphthalene Acetic Acid (NAA) along with the untreated control. Plant height and chlorophyll concentration were found significantly different in both years of experiment as well as among the different treatments. HBR at 0.12 g a.i. ha−1 was found better with maximum number of fruits (77.36 plant−1), fruit length (6.72 cm), fruit breadth (6.45 cm) and fruit weight (80.52 g) over other concentrations and treatments. Fruit yield was more pronounced in the plots treated with plant growth regulators compared to untreated control. However, significantly higher fruit yield of 91.07 t ha−1 (62.58 t ha−1 with untreated control) along with improved quality traits viz., fruit firmness (4.11 kg cm−2), ascorbic acid content (24.09 mg 100 g−1), total soluble solids (4.43°Brix) and keeping quality (12.50 days) was recorded in 0.12 g a.i. ha−1 HBR treated plots. Thus, it can be inferred that HBRapplication would be a better option to enhance growth, yield as well as quality traits in tomato.
Keywords: Homobrassinolide, Tomato, Fruit yield, Total soluble solids, Keeping quality, Biomaterial
1. Introduction
Tomato is known as poor man’s orange and has a significant importance in human diet due to its antioxidant property as it cures ailment related to cardio-vascular system, lungs and stomach. It is one of the most widely grown vegetable throughout the world. As it is highly perishable, retaining the quality fruits is of prime concern. Several tomato hybrids have been developed with high yielding potential. Genotypes with both yield and the fruit quality are primary concern in crop improvement programs. Many crop growth promoters have indeed been established in this regard to improve the quality and productivity of crops (Aiman et al., 2014). Among them, BRs group of plant hormones arouse as one of the most effective and eco-friendly phytohormone by regulating multiple plant physiological process viz., cell division, cell elongation, preventing loss of photosynthetic pigments, pollen tube growth, vascular differentiation, retarding the abscission, ethylene biosynthesis and so on, thereby enhances crop growth and development even at stressed circumstances (Hasan et al., 2011, Hayat et al., 2012, Nolan et al., 2020). Because of their wide variety of applications in crop growth metabolism, brassinosteroids are popularly known as ‘pleiotropic hormone’ (Clouse, 2011, Ali, 2017). Thus, it becomes necessary to understand the mode of action of BRs in improving yield and quality in different crop species.
BRs are present in almost all plant species naturally (Sasse, 1997). They were first extracted from the pollen of rapeseed (Brassica napus) plants (Grove et al., 1979) and classified under sixth class of endogenous plant hormones by Mandava (1988). In India, BRs were first isolated from the leaves and seeds of tea (Camellia sinensis) (Kaur et al., 2002, Gupta et al., 2004). Many scientists have explored the ability of BRs in increasing growth and yield of crops such as rice (Krishnan et al., 1999, Castorina and Consonni, 2020), maize (Hu et al., 2017, Tian et al., 2019), broad bean (Helmy et al., 1997) and groundnut (Vardhini and Rao, 1998).
However, an analogue of BRs i.e., HBRs is gaining much attention in recent years because of their specific advantages like promotion of seed germination, early seedlings vigor, accelerating photosynthesis and assimilates translocation from source to economic parts. Increases the enzymes level which are responsible for the synthesis of proteins, nucleic acids, sugars. It imparts stress resistance by increased proline production under adverse environmental conditions, inducing flowering and increasing fruit set and fruit growth and so on, over other commonly available BRs (Bajguz, 2000). Aiman et al., 2014, Wang et al., 2019 already showed improvement in crop growth and fruit yield of tomato due to exogenous 28-homobrassinolide application. However, extensive studies with respect to different concentrations and time of application were not properly documented which play prime role in reducing the cost and quantity of their requirement. Thus, literature with respect to these aspects of HBR are meagre. In this juncture, the prime concern of our present study was to evaluate the potentiality of foliar applied BRs analogue homo HBR on crop growth, fruit yield and fruit quality aspects of tomato in comparison with plant growth promoters (PGRs) such as GA and NAA.The performance of GA and NAA on growth and yield was reviewed by Tomar et al. (2020) revealing them as the most promising growth regulators as they considerably increase fruits number per plant, fruit setting, fruit weight and thereby enhancing the final fruit yield per hectare.
2. Material and methods
2.1. Details of experimental site
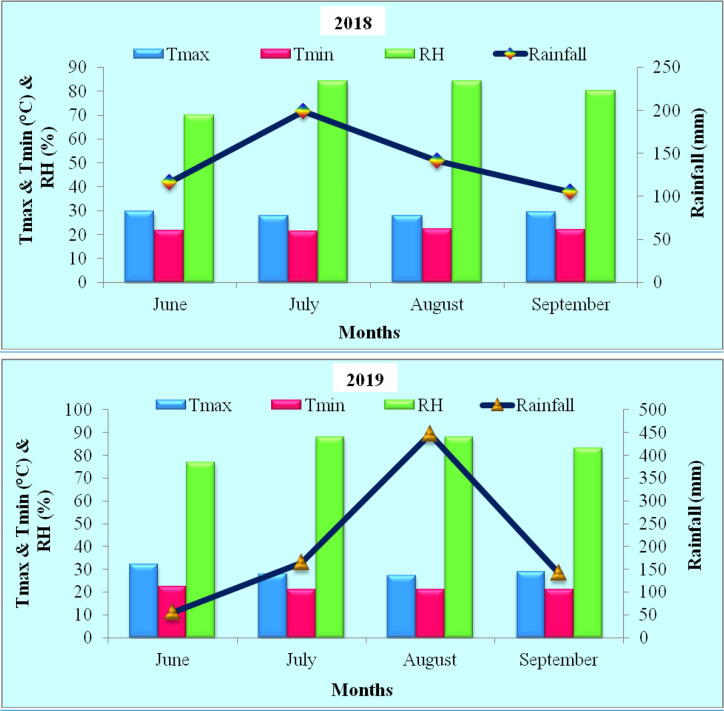
The present study was carried out in field at Center for Climate Resilient Agriculture operating at University of Agricultural and Horticultural Sciences, Navile, Shivamogga, Karnataka residing at 13.92°N longitude, 75.56°E latitude at an elevation of 569 m above mean sea level. The experimental soil was found e sandy loam soil, having organic carbon content of 7.5 g kg−1 and nutrient profile of 241 kg nitrogen (N) ha−1, 77 kg phosphorus as P2O5 ha−1 and 331.72 kg potassium as K2O ha−1. Experiment was conducted during kharif (June to September) season of two consecutive years, 2018 and 2019. The amount of rainfall recorded during the experimental period was 560.4 mm during 2018 and it was around 808 mm during 2019. During both the years of study, maximum temperature (Tmax) was recorded in June (29.5 and 32.2 °C) while, minimum temperature was recorded in July (21.2 °C) and August (21 °C), respectively during 2018 and 2019. The maximum relative humidity (RH) of 84 and 88 per cent (%) in both July and August was recorded during 2018 and 2019, respectively while lower was recorded during June (70 and 77%, respectively) (Fig. 1).
Fig. 1.
Weather conditions prevailed during first (2018) and second (2019) year study of tomato. Note: Tmax–Maximum temperature; Tmin–Minimum temperature; RH-Relative Humidity.
2.2. Treatment details
The experiment was planted in Randomized Complete Block Design (RCBD) with seven treatments in a plot size of 7.2 m × 5.4 m and replicated thrice. A high yielding F1 tomato hybrid Arka Rakshak having triple disease resistance to tomato leaf curl virus, bacterial and early blight was taken as a test crop. Transplanting of 25 days old tomato seedlings was done manually at a spacing of 120 cm × 90 cm with a population of 36 plants per plot. The recommended fertilizers of 125 kg N ha−1 as urea, 75 kg P2O5 ha−1 as single super phosphate and 60 kg K2O ha−1, and as muriate of potash was applied to the crop. In total, recommended dose of fertilizers was applied in three splits with 50% N and 25% P, K as basal dose at 4 days after transplanting and remaing fertilizers were applied at 30 days (25% N and 50% P, K) and 50 days (25% N, P, K) after transplanting. Commercial product of HBR 0.04% EC was tested at four different concentrations viz., 0.06, 0.08, 0.010 and 0.12 g (a.i.) ha−1 in comparison to two well known plant regulators, GA 0.001% L at 0.018 g a.i. ha−1 (Market product -Prime Gold) and NAA at 25 ppm (Market product – Bayer Planofix) along with an untreated control. As per treatments, first foliar application of crop growth promoters was made at flower initiation and subsequent second application was given fifteen days after first spray.
2.3. Growth and quality attributes
Growth parameter like height of the tomato plants length as influenced by the application of HBR 0.04% EC recorded during both the experimental seasons was measured from base of the plant to tip of the main stem and was expressed in centimeters. Total chlorophyll was measured by adopting Hiscox and Israelstam (1979) method using Dimethyl sulfoxide. Quality attributes such as keeping quality of the fruits (shelf life) in days as well as fruit firmness (kg cm−2) was measured as per the guidelines outlined by Nagoni (2015) and Total Soluble Solids (TSS) were estimated as per the Association of Official Analytical Chemists (AOAC, 2000) protocol. Ascorbic acid (mg 100 g−1) content was assessed as per the standard procedure described by Almajidi and Alqubury (2016).
2.4. Yield attributes
At each harvest, five randomly selected plants from each treatment were selected and recorded fruits per plant, fruit length and fruit breadth (cm). The average fruit weight per plant was computed by recording the weight of the fruits at each and every harvest from selected plants in each treatment.
2.5. Fruit yield
Fruit yield from each plot was determined by harvesting fruits after attaining the marketable size and edible maturity. In total from each plot, five pickings were taken at six days gap between the pickings and cumulative yield of all the pickings were taken as total yield of the plot. Later the yield was converted on hectare basis and expressed as tonnes per hectare.
2.6. Statistical analysis
The data collected on various parameters during the investigation was statistically analyzed by using Fisher’s method of analysis of variance as elaborated by Gomez and Gomez (1984). The least significant difference used in F test was at α = 0.05. Critical difference was calculated at five percent level of probability. All the statistical analysis was made by using SPSS V 25.0 statistical software in collaboration with colleagues from King Saud and Princess Nourah bint Abdulrahman Universities.
3. Results
3.1. Growth parameters and chlorophyll content of tomato
Growth attributing parameter such as plant height of tomato was statistically influenced by the HBR 0.04% EC spray during the different years of study and it was significantly higher during second year (124.34 cm). Similarly, the plant height was also influenced by different concentrations of HBR 0.04% EC application. Among the different concentrations, significantly higher plant height (135.30 cm) was noticed with the application of HBR 0.04% EC at 0.12 g a.i. ha−1 but which was on par with the HBR 0.04% EC at 0.10 g a.i. ha−1 application (129.87 cm) when compared to reference growth regulators application such as GA 0.001% L at 0.018 g a.i. ha−1 (120.61 cm) and NAA at 25 ppm (114.13 cm). However, significantly lower plant height was observed with the untreated plants (104.70 cm) (Table 1).
Table 1.
Plant height and chlorophyll content of tomato as influenced by HBR 0.04% EC application.
| Treatments | Plant height (cm) | Chlorophyll content (mg g−1 fresh weight) |
|---|---|---|
| Year | ||
| First year (2018) | 119.13b* | 2.82a |
| Second year (2019) | 124.34 a | 2.77b |
| LSD (p = 0.05) | 3.67 | 0.04 |
| Treatment (B) | ||
| HBR 0.04% EC at 0.06 g a.i. ha−1 | 121.47cb | 2.77d |
| HBR 0.04% EC at 0.08 g a.i. ha−1 | 126.03b | 2.86c |
| HBR 0.04% EC at 0.10 g a.i. ha−1 | 129.87ab | 3.09b |
| HBR 0.04% EC at 0.12 g a.i. ha−1 | 135.30a | 3.19a |
| GA 0.001% L at 0.018 g a.i. ha−1 | 120.61db | 2.65e |
| NAA at 25 ppm | 114.13de | 2.63e |
| Untreated control | 104.70f | 2.36f |
| LSD (p = 0.05) | 6.87 | 0.08 |
| A × B | NS | NS |
Note: HBR – Homobrassinolide; GA – Gibberllicacid; NAA – Napthalene Acetic Acid; NS – Non significant; *Values followed by similar alphabets are non-significant.
Application of HBR 0.04% EC significantly influenced the chlorophyll content of tomato foliage across the years as well as with different concentrations and significantly maximum chlorophyll content was extracted from the first year tomato leaves (2.82 mg g−1 fresh weight). Among the various growth regulators, tomato plants treated with HBR 0.04% EC at 0.12 g a.i. ha−1 registered significantly higher chlorophyll content (3.19 mg g−1 fresh weight) followed by HBR 0.04% EC at 0.10 g a.i.ha−1 (3.09 mg g−1 fresh weight) as against 2.65 and 2.63 mg g−1 fresh weight with GA 0.001% L at 0.018 g a.i. ha−1 and NAA at 25 ppm, respectively. Significantly lower chlorophyll concentration of 2.36 mg g−1 fresh weight was noticed with the untreated control (Table 1).
3.2. Yield and yield attributing traits of tomato
The number of fruits per plant in response to HBR 0.04% EC and reference plant growth regulators was varied significantly during the different years of study and a maximum number of fruits were noticed in second year (68.57 plant−1) compared to the first year (57.32). The other yield attributing characters viz., fruit length, fruit breadth, fruit weight and fruit yield were not statistically significant across the years. However, slight increase these fruit traits were noticed in first-year tomato crop.
Statistically significant difference in the yield and yield contributing parameters was noticed with the HBR 0.04% EC and reference plant growth regulators application to the tomato crop. Significantly higher fruits (77.36 plant−1), fruit length (6.72 cm), fruit breadth (6.45 cm), fruit weight (80.52 g) as well as the fruit yield (91.07 t ha−1) was registered with HBR 0.04% EC at 0.12 g a.i. ha−1 application which was closely followed by the HBR 0.04% EC at 0.10 g a.i. ha−1 (72.70, 6.39 cm, 6.19 cm, 76.08 g and 88.42 t ha−1, respectively) and these treatments were out performed against the popular plant growth regulators like GA 0.001% L at 0.018 g a.i. ha−1 (57.90, 5.52 cm, 5.01 cm, 70.50 g and 80.47 t ha−1, respectively) and NAA at 25 ppm (59.16, 5.64 cm, 4.56 cm, 68.14 g and 80.51 t ha−1, respectively). However, significantly lower fruits (49.90 plant−1), fruit length (4.01 cm), fruit breadth (3.74 cm), fruit weight (55.92 g) and fruit yield (62.58 t ha−1) was observed for untreated crop (Table 2).
Table 2.
Yield traits of tomato as influenced by HBR 0.04% EC application.
| Treatments | Number of fruits (plant−1) | Fruit length (cm) | Fruit Breadth (cm) | Fruit weight (g) | Fruit yield (t ha−1) |
|---|---|---|---|---|---|
| Year (A) | |||||
| First year (2018) | 57.32b* | 5.68a | 5.32a | 70.44a | 80.46a |
| Second year (2019) | 68.57a | 5.71a | 5.20a | 67.56a | 79.30a |
| LSD (p = 0.05) | 2.18 | NS | NS | NS | NS |
| Treatment (B) | |||||
| HBR 0.04% EC at 0.06 g a.i. ha−1 | 58.78d | 5.64d | 5.21c | 64.58c | 75.43c |
| HBR 0.04% EC at 0.08 g a.i. ha−1 | 64.81c | 5.93c | 5.67b | 67.22bc | 80.68b |
| HBR 0.04% EC at 0.10 g a.i. ha−1 | 72.70b | 6.39b | 6.19a | 76.08ab | 88.42a |
| HBR 0.04% EC at 0.12 g a.i. ha−1 | 77.36a | 6.72a | 6.45a | 80.52a | 91.07a |
| GA 0.001% L at 0.018 g a.i. ha−1 | 57.90d | 5.52d | 5.01c | 70.50b | 80.47b |
| NAA at 25 ppm | 59.16d | 5.64d | 4.56d | 68.14bc | 80.51b |
| Untreated control | 49.90e | 4.01e | 3.74e | 55.92d | 62.58d |
| LSD (p = 0.05) | 4.08 | 0.26 | 0.33 | 5.86 | 4.63 |
| A × B | NS | NS | NS | NS | NS |
Note: HBR – Homobrassinolide; GA – Gibberllicacid; NAA – Napthalene Acetic Acid; NS – Non significant; *Values followed by similar alphabets are non-significant.
3.3. Quality traits of tomato
The qualitative attributes of tomato fruit like fruit firmness and ascorbic acid content was not statistically differed across the years but they were statistically affected by the application of different plant growth regulators at varied level of concentrations. Significantly higher values of fruit firmness (4.11 kg cm−2) and ascorbic acid content (24.09 mg 100 g−1) were noticed with the application of HBR 0.04% EC at 0.12 g a.i. ha−1 to the tomato crop over the quality of fruits under untreated control crop (2.16 kg cm−2 and 14.50 mg 100 g−1, respectively). The other fruit quality attributes like total soluble solids and shelf life were also significantly influenced both across the years and with different plant growth regulators treatment. Across the years, significantly higher total soluble solids (4.85°Brix) were noticed in the first-year tomato fruits while maximum keeping quality was registered with the fruits grown during the second year (9.87 days). Among the different growth regulators, HBR 0.04% EC at 0.12 g a.i. ha−1 applied to tomato crop recorded significantly higher total soluble solids (4.43°Brix) with a shelf life of about 12.50 days which was closely followed by HBR0.04% EC at 0.10 g a.i. ha−1 application (4.28°Brix and 11.57 days, respectively). The tune of increase in keeping quality of fruits treated with HBR 0.04% EC at 0.12 g a.i. ha−1 was 49.88, 66.67 and 158.79 per cent over GA, NAA and untreated tomato fruits. However, significantly lower total soluble solids (3.34°Brix) and minimum shelf life of 4.83 days were noticed with tomato fruits harvested under untreated control crop (Table 3).
Table 3.
Quality attributes of tomato as influenced by HBR 0.04% EC application.
| Treatments | Fruit firmness (kg cm−2) | Ascorbic acid (mg 100 g−1) | TotalSolubleSugars(°Brix) | Shelf life (Days) |
|---|---|---|---|---|
| Year (A) | ||||
| First year (2018) | 3.21a* | 20.87a | 4.85a | 7.48b |
| Second year (2019) | 3.23a | 20.58a | 3.09b | 9.87a |
| LSD (p = 0.05) | NS | NS | 0.10 | 0.45 |
| Treatment (B) | ||||
| HBR 0.04% EC at 0.06 g a.i. ha−1 | 2.86de | 19.94c | 3.95cd | 6.67e |
| HBR 0.04% EC at 0.08 g a.i. ha−1 | 3.04 cd | 21.83b | 4.05c | 9.30c |
| HBR 0.04% EC at 0.10 g a.i. ha−1 | 3.66b | 23.86a | 4.28b | 11.57b |
| HBR 0.04% EC at 0.12 g a.i. ha−1 | 4.11a | 24.09a | 4.43a | 12.50a |
| GA 0.001% L at 0.018 g a.i. ha−1 | 3.53b | 21.59b | 3.94cd | 8.34d |
| NAA at 25 ppm | 3.19c | 19.26c | 3.81de | 7.50e |
| Untreated control | 2.16f | 14.50d | 3.34f | 4.83f |
| LSD (p = 0.05) | 0.31 | 1.27 | 0.18 | 0.83 |
| A × B | NS | NS | 0.25 | 1.17 |
Note: HBR – Homobrassinolide; GA – Gibberllicacid; NAA – Napthalene Acetic Acid; TSS – Total Soluble Solids; NS – Non significant; *Values followed by similar alphabets are non-significant.
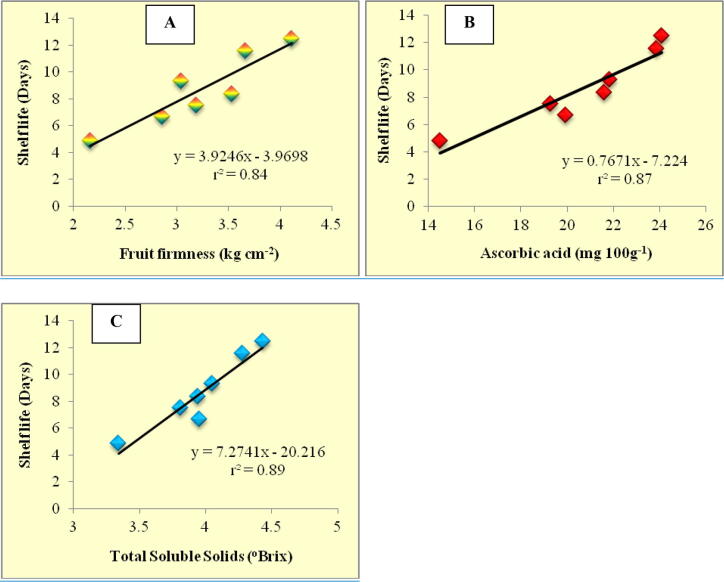
3.4. Correlation between quality parameters
Correlation coefficient values and regression equations for different quality parameters was evolved by using Spearman's correlation coefficient and simple linear regression analysis, respectively. The fruit quality parameters viz., firmness, ascorbic acid and TSS content have showed strong positive correlation towards the shelf life of tomato fruits with correlation values of 0.84, 0.87 and 0.89, respectively (Fig. 2). These values signifies that upto 84–89% variation in the keeping quality of the tomato fruits will be determined by the above mentioned fruit quality parameters. Thus, improvement in these fruit quality parameters ultimately improves the keeping quality of the tomato.
Fig. 2.
Relationship between shelf life of tomato fruits with fruit firmness (A), ascorbic acid content (B) and Total Soluble Solids (C).
4. Discussion
Application of HBR at different concentrations to the tomato crop resulted in significantly positive improvement in the growth attributing parameter like plant height. The increased cell division and cell elongation due to enhanced functional metabolism by the BRs might be the reason for enhancement in the growth of plants under treated condition (Clouse and Sasse, 1998). These steroidal hormones are also known to regulate gene expression and thus mediate growth activity (Felner, 2003). Vardhini and Rao, 2001, Ali et al., 2006, Hayat et al., 2011 have also reported the improved growth due to higher photosynthetic rate in tomato plants uponBRS application. To support this statement, BRs are known to activate the key enzymes of photosynthesis such as Rubisco (Yu et al., 2004) and catalase activity (Yusuf et al., 2011). Similarly, enhanced leaf area and biomass production in wheat under both irrigated and water stress conditions due to increased nitrate reductase activity, relative water content, chlorophyll content, water uptake, nitrogen assimilation rate and ultimately photosynthesis with brassinosteroids was also elucidated by the Altman, 1998, Sairam, 1994.
The significant improvement in the chlorophyll pigments content was observed with the homobrassinolide treated tomato leaves than the reference growth regulators like GA and NAA. BRs promoted transcription and translation process of the enzymes which are involved in chlorophyll synthesis in association with reduced level of catabolizing enzymes might be the prime cause for enhanced chlorophyll content (Bajguz, 2000, Sharma et al., 2017). Similarly, positive response of BRs induced chlorophyll biosynthesis in algae was also stated by Bajguz and Asami, 2005. In support of this statement, increase in chlorophyll content due to HBR spray was also revealed by Hayat et al., 2012, Ali et al., 2006 in Tomato; Bhatia and Kaur (1997) in mung bean, Fariduddin et al. (2004) in green gram and Ali et al. (2007) in chickpea.
Application of HBR also improved the yield contributing parameters and fruit yield of the tomato as BRs are known to delay the senescence process thereby a greater number of productive flowers will be retained in the plant for longer duration which ultimately enhances the fruit development in the crop (Iwahori et al., 1990, Kutschera and Wang, 2012). Higher assimilation of photosynthetic carbon along with better supply of nutrients and metabolites from source to sink by BRs contributes to higher fruit yield. Furthermore, they play a key role in the regulation of flower-to-fruit transformation and thus optimize fruit set by conferring resistance to biotic and abiotic stress (Tang et al., 2016). In tomato, Kamuro and Takatsuto, 1999, Ali et al., 2006 also reported improved fruit set by the regulation of source to sink ratio due to increased photosynthetic rate with brassinosteroids treatment. Increased accumulation of carotenoid in tomato fruits due to crop architecture regulation of by BRs were also revealed by Li et al. (2016). Increased yield of tomato due to higher plant height, leaf area, maximum number of fruits and fruit weight per cluster due to enhancement of autophosphorylation was also revealed by Wang et al. (2019) with BRs treatment. Similar kind of improvement in fruit yield with BRs application was also noticed in tomato by Vardhini and Rao, 2001, Ali et al., 2006, Hayat et al., 2012, Aiman et al., 2014, in mungbean by Ali et al. (2008), in guava by Lal et al. (2013), in onion by Doležalová et al. (2016) and in sugar apple by Mostafa and Kotb (2018).
The quality attributes of tomato viz., fruit firmness, ascorbic acid content, total soluble solids and shelf life was significantly improved with the plant growth regulators application at different concentrations. This significant improvement was mainly attributed to regulation of ethylene production with the BRs application, as ethylene play prime role in fruit ripening and thereby determines the fruit firmness and shelf life. Similar kind of improvement in the quality of tomato fruits with BRs application was also revealed by the Vardhini and Rao, 2002, Zhu et al., 2015. Improved quality parameters viz., fruit firmness, ascorbic acid and TSS with HBR application was also showed positive impact on the shelf life of tomato fruits. This statement was further strongly evidenced by the strong positive correlation of these quality traits with the shelf life of fruits (r2 = 0.84, r2 = 0.87 and r2 = 0.89, respectively). Increase in ascorbic acid content observed in the present investigation due to foliar application of HBR is in accordance with studies of Roghabadi and Pakkish (2014). Total soluble solids are another best indicator of fruit quality as it mainly indicates the sugar levels in fruits. This kind of improved TSS with the application of HBR was also elucidated by Gomes et al. (2006) in passion fruit and Samira et al. (2012) in pepper. Similarly, improved quality traits such as total soluble solids, total sugars as well as lycopene content of watermelon sprayed with BRs @ 0.1 ppm was also observed by Susila et al. (2012). Fruit quality improvement with BRs spray was also reported by Li et al., 2010, Coll et al., 2015, Wei and Li, 2016 in different fruit crops. In addition, brassinosteroids also stimulates various physiological and biological processes such as cell division, hyperpolarization of membranes, ATPase activity, orientation of cortical microtubules, antioxidant enzyme activities, photosynthetic and chlorophyll contents that impart tolerance to many biotic and abiotic stresses (Anwar et al., 2018) which might be the plausible reason for improving fruit quality traits.
5. Conclusions
The present investigation revealed that application of HBR 0.04% EC at 0.12 g a.i. ha−1 as well as at 0.10 g a.i. ha−1 concentration showed significant improvement in the growth and yield contributing traits as well as fruit yield of the tomato. Compared to promising plant growth regulators such as GA and NAAA, HBR application significantly improved the fruit firmness, ascorbic acid content, total soluble solids and keeping quality of the tomato fruits. Thus, current study opens the option for improving the yield, quality and keeping quality of tomato fruits with HBR application as a potential source of BRs when compared to available plant growth regulators.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Shankarappa Sridhara, Email: sridharas1968@gmail.com.
Nissren Tamam, Email: nmtamam@pnu.edu.sa.
Hosam O. Elansary, Email: helansary@ksu.edu.sa.
Ahmed M. El-Sabrout, Email: elsabroutahmed@alexu.edu.eg.
References
- Aiman, H.S., Mohammad, I., Shamsul, H., 2014. Response of tomato cultivars on yield andquality attributes applied with two different modes of BR Analogues: A Comparative study. In: Int. Conference on Advances in Agricultural, Biological & Environmental Sciences (AABES-2014) Oct 15-16, Dubai (UAE).
- Ali B. Practical applications of brassinosteroids in horticulture some field perspectives. Sci. Hortic. 2017;225:15–21. doi: 10.1016/j.scienta.2017.06.051. [DOI] [Google Scholar]
- Ali B., Hasan S.A., Hayat S., Hayat Q., Yadav S., Fariduddin Q., Ahmad A. A role of brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiate L. Wilczek) Environ. Exp. Bot. 2008;62:153–159. doi: 10.1016/j.envexpbot.2007.07.014. [DOI] [Google Scholar]
- Ali B., Hayat S., Ahmad A. 28-Homobrassinolide ameliorates the saline stress in chickpea (Cicer arietinum L.) Environ. Exp. Bot. 2007;59:217–223. doi: 10.1016/j.envexpbot.2005.12.002. [DOI] [Google Scholar]
- Ali B., Hayat S., Hasan A., Ahmad A. Effect of root applied 28-homobrassinolide on the performance of Lycopersicon esculentum. Sci. Hortic. 2006;110(3):267–273. doi: 10.1016/j.scienta.2006.07.015. [DOI] [Google Scholar]
- Almajidi M.I.H., Alqubury H.Y. Determination of Vitamin C (ascorbic acid) contents in various fruit and vegetable by UV-spectrophotometry and titration methods. J. Chem. Pharm. Sci. 2016;9(4):2972–2974. [Google Scholar]
- Altman T.A. Tale of dwarfs and drugs: Brassinosteroids in the rescue. Trends Genet. 1998;14:490–495. doi: 10.1016/s0168-9525(98)01598-4. [DOI] [PubMed] [Google Scholar]
- AOAC, 2000. The Official Methods of Analysis. Association of Official Analytical Chemists, Washington, DC.
- Anwar, A.,, Liu, Y, Dong, R, Bai, L, Yu, X, Li, Y The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol. Res. 2018;51(46) doi: 10.1186/s40659-018-0195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajguz A. Effect of brassinosteroids on nucleic acid and protein content in cultured cell of Chlorella vulgaris. Plant Physiol. Biochem. 2000;38:209–215. [Google Scholar]
- Bajguz A., Asami T. Suppression of Wolffia arrhiza growth by brassinazole, an inhibitor of brassinosteroid biosynthesis and its restoration by endogenous 24-epibrassinolide. Phytochemistry. 2005;66:1787–1796. doi: 10.1016/j.phytochem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bhatia D.S., Kaur J. Effect of homobrassinolide and humicil on chlorophyll content, hill activity and yield components in mung bean [Vigna radiata (L.) Wilczek] Phytomorphology. 1997;47:421–426. [Google Scholar]
- Castorina G., Consonni G. The role of brassinosteroids in controlling plant height in poaceae: A genetic perspective. Int. J. Mol. Sci. 2020;21(4):1191. doi: 10.3390/ijms21041191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse, S.D., 2011. Brassinosteroids-Arabidopsis Book. 9: e0151. doi: 10.1199/tab.0151. [DOI] [PMC free article] [PubMed]
- Clouse S.D., Sasse J.M. Brassinosteroids: essential regulators of plant growth and development. Annu. Rev. Pl. Physiol. Pl. Mol. Biol. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Coll Y., Coll F., Amorós A., Pujol M. Brassinosteroids roles and applications: an up-date. Biologia. 2015;70(6):726–732. doi: 10.1515/biolog-2015-0085. [DOI] [Google Scholar]
- Doležalová J., Koudela M., Sus J., Ptácek V. Effects of synthetic brassinolide on the yield of onion grown at two irrigation levels. Scientia Horticulturae. 2016;202:125–132. doi: 10.1016/j.scienta.2016.02.023. [DOI] [Google Scholar]
- Fariduddin Q., Ahmad A., Hayat S. Response of Vigna radiata to foliar application of 28-homobrassinolide and kinetin. Biol. Plant. 2004;48(3):465–468. doi: 10.1023/b:biop.0000041106.77930.d6. [DOI] [Google Scholar]
- Felner, M., 2003. Brassinosteroids: Bioactivity and Crop Productivity. Kluwer Academic Publishers, Dordrecht.
- Gomes, M.D.M.A., Campostrini E., Leal N.R., Viana, A.P., Ferraz, T.M., do- Nascimento Siqueira L., Rosa, R.C.C., Netto, A.T., Nunez-Vázquez, M., Zullo, M.A.T., 2006. Brassinosteroid analogue effects on the yield of yellow passion fruit plants (Passiflora edulis f. flavicarpa). Sci. Hortic. 110, 235-240.
- Gomez, K.A., Gomez, A.A., 1984. Statistical Procedure for Agriculture Research, second ed., John Willey and Sons, New York, p. 68.
- Grove M.D., Spencer F.G., Rohwedder W.K., Mandava N.B., Worley J.F., Warthen J.D., Jr., Steffens G.L., Flippen-Anderson J.L., Cook J.C., Jr. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979;281:216–217. [Google Scholar]
- Gupta D., Bhardwaj R., Nagar P.K., Kaur S. Isolation and characterization of brassinosteroids from Camellia sinensis (L.) O. Kuntze. Plant Growth Regul. 2004;43(2):97–100. doi: 10.1023/B:GROW.0000040118.68011.6a. [DOI] [Google Scholar]
- Hasan S.A., Hayat S., Ahmad A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere. 2011;84(10):1446–1451. doi: 10.1016/j.chemosphere.2011.04.047. [DOI] [PubMed] [Google Scholar]
- Hayat S., Alyemeni M.S., Hasan S.A. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J. Biol. Sci. 2012;19(3):325–335. doi: 10.1016/j.sjbs.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayat S., Yadav S., Wani A.S., Irfan M., Ahmad A. Comparative effect of 28-homobrassinolide and 24-epibrassinolide on the growth, carbonic anhydrase activity and photosynthetic efficiency of Lycopersicon esculentum. Phtosynthetica. 2011;49(3):397–404. [Google Scholar]
- Helmy Y.T., Sawan O.M.N., Abdel-Halim S. Growth, yield and endogenous hormones of broad bean plants as affected by brassinosteroids. Egypt. J. Hort. 1997;24:109–115. [Google Scholar]
- Hiscox J.D., Israelstam G.F. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57(12):1332–1334. [Google Scholar]
- Hu S., Sanchez D.L., Wang C., Lipka A.E., Yin Y., Gardner C.A. Brassinosteroid and gibberellin control of seedling traits in maize (Zea mays L.) Plant Sci. 2017;263:132–141. doi: 10.1016/j.plantsci.2017.07.011. [DOI] [PubMed] [Google Scholar]
- Iwahori S., Tominaga S., Higuchi S. Retardation of abscission in citrus leaf and fruitlet explants by brassinolide. Plant Growth Regul. 1990;9:119–125. [Google Scholar]
- Kamuro, Y., Takatsuto, S., 1999. Brassinosteroids: Steroidal Plant Hormones. Springer-Verlag, Tokyo, pp. 223–241.
- Kaur, S., Bhardwaj, R., Nagar, P.K., 2002. Isolation and characterization of brassinosteroids from immature seeds of Camelia sinensis (L.) O. In: 13th Congress of the Federation of European Societies of Plant Physiology. Hersonissos, Heraklion, Crete. Abstract 206.
- Krishnan S., Azhakanandam K., Abenezer G.A.I., Samson N.P., Dayanandan P. Brassinosteroids and benzylaminopurine increases yield in IR50 indica rice. Curr. Sci. 1999;76:145–147. [Google Scholar]
- Kutschera U., Wang Z.Y. Brassinosteroid action in flowering plants: a Darwinian perspective. J. Exp. Bot. 2012;63:3511–3522. doi: 10.1093/jxb/ers065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal N., Das R.P., Verma L.R. Effect of plant growth regulators on flowering and fruit growth of guava (Psidium guajava L.) cv. Allahabad safeda. Asian J. Agric. Horti. Res. 2013;8(1):54–56. [Google Scholar]
- Li J., Li Y., Chen S., An L. Involvement of brassinosteroid signals in the floral-induction network of Arabidopsis. J. Exp. Bot. 2010;61:4221–4230. doi: 10.1093/jxb/erq241. [DOI] [PubMed] [Google Scholar]
- Li X.J., Chen X.J., Guo X., Yin L.L., Ahammed G.J., Xu C.J., Chen K.S., Liu C.C., Xia J.X., Shi K., Zhou Y.H., Yu J.Q. Dwarf over expression induces alteration in phytohormone homeostasis, development, architecture and carotenoid accumulation in tomato. Plt. Biotechnol. J. 2016;14:1021–1033. doi: 10.1111/pbi.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandava N.B. Plant growth promoting brassinosteroids. Annu. Rev. Pl. Physiol. Pl. Mol. Biol. 1988;39:23–52. [Google Scholar]
- Mostafa L.Y., Kotb H.R.M. Effect of Brassinosteroids and Gibberellic acid on parthenocarpic fruit formation and fruit quality of Sugar Apple (Annona squamosa L.) Middle East. J. Agric. Res. 2018;7(4):1341–1351. [Google Scholar]
- Nagoni, S.P., 2015. Effect of integrated nutrient management on growth, yield and quality of tomato (Solanum lycopersicum L.) var. Arka Rakshak. M.Sc. Thesis (Unpub.), Univ. Agric. Sci., Bangalore.
- Nolan, T.M., Vukašinović, Liu, D., Russinova, E., Yanhai Yin, 2020. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. The Plant Cell 32, 295–318. [DOI] [PMC free article] [PubMed]
- Roghabadi M.A., Pakkish Z. Role of brassinosteroid on yield, fruit quality and postharvest storage of ‘TakDanehe Mashhad’ sweet cherry (Prunus avium L.) Agric. Commun. 2014;2:49–56. [Google Scholar]
- Sairam R.K. Effect of homo brassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Pl. Growth Regul. 1994;14:173–181. [Google Scholar]
- Samira I.M.H., Mansour-Gueddes S.B., Dridi-Mouhandes B., Denden M. 24-epibrassinolide enhances flower and fruit production of pepper (Capsicum annuum L.) under salt stress. J. Stress Physiol. Biochem. 2012;8(3):224–233. [Google Scholar]
- Sasse J.M. Recent progress in brassinosteroid research. Physiol. Pl. 1997;100:696–701. [Google Scholar]
- Sharma I., Kaur N., Pati P.K. Brassinosteroids: A Promising option in deciphering remedial strategies for abiotic stress tolerance in Rice. Front. Plant Sci. 2017;8:1–17. doi: 10.3389/fpls.2017.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susila T., Reddy S.A., Rajkumar M., Padmaja G., Rao P. Effects of sowing date and spraying of brassinosteroid on yield and fruit quality characters of watermelon. World J. Agric. Sci. 2012;8(3):223–228. [Google Scholar]
- Tang J., Han Z., Chai J. Q&A: what are brassinosteroids and howdo they act in plants? BMC Biol. 2016;14:113–118. doi: 10.1186/s12915-016-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J., Wang C., Xia J., Wu L., Xu G., Wu W. Teosinte ligule allele narrows plant architecture and enhances high-density maize yields. Science. 2019;365:658–664. doi: 10.1126/science.aax5482. [DOI] [PubMed] [Google Scholar]
- Tomar S., Rajiv, Singh D.P., Kumari M. Effect of GA and NAA on growth and yield of tomato 3 (Lycopersicon Esculentum Mill.) – A Review. Plant Archiv. 2020;20:71–72. [Google Scholar]
- Vardhini B.V., Rao S.S.R. Effect of brassinosteroids on growth, metabolic content and yield of Arachis hypogea. Phytochemistry. 1998;48:927–930. [Google Scholar]
- Vardhini B.V., Rao S.S.R. Effect of brassinosteroids on growth and yield of tomato (Lycopersicon esculentum Mill.) under field conditions. Indian J. Pl. Physiol. 2001;6(3):326–328. [Google Scholar]
- Vardhini B.V., Rao S.S.R. Acceleration of ripening of tomato pericarp disc by brassinosteroids. Phytochemistry. 2002;16:843–847. doi: 10.1016/s0031-9422(02)00223-6. [DOI] [PubMed] [Google Scholar]
- Wang S., Liu J., Zhao T., Du C., Nie S., Zhang Y. Modification of Threonine-1050 of SlBRI1 regulates BR Signalling and increases fruit yield of tomato. BMC Plant Biol. 2019;19(1):256. doi: 10.1186/s12870-019-1869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Li J. Brassinosteroids regulate root growth, development and symbiosis. Mol. Plant. 2016;9:86–100. doi: 10.1016/j.molp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Yu J.Q., Huang L.F., Hu W.H., Zhou Y.H., Mao W.H., Ye S.F., Nogues S. A role of brassinosteroids in the regulation of photosynthesis in Cucumis sativus. J. Exp. Bot. 2004;55:1135–1143. doi: 10.1093/jxb/erh124. [DOI] [PubMed] [Google Scholar]
- Yusuf M., Fariduddin Q., Hayat S., Hasan S.A., Ahmad A. Protective responses of 28-homobrssinolide in cultivars of Triticum aestivum with different levels of nickel. Arch. Environ. Contam Toxicol. 2011;60:68–76. doi: 10.1007/s00244-010-9535-0. [DOI] [PubMed] [Google Scholar]
- Zhu T., Tan W.R., Deng X.G., Zheng T., Zhang Da.-W., Lin H.H. Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol. Technol. 2015;100:196–204. [Google Scholar]