Abstract
The premise of the pharmacology of natural product is to explore benefits of natural resources for the mankind. Medicines extracted from natural resources are considered as primary source for drug discovery. Thus, the current study was designed to evaluate the safety profile and explore the analgesic and anti-inflammatory activity of ethanol extract of Cucurbita maxima (C. maxima) and Cucumis sativus (C. sativus) seeds. These seeds are edible, good in taste and have been used for several therapeutic purposes. Acute toxicity of the seeds was evaluated by Lorke’s method while Eddy’s hot plate and tail immersion methods were used to assess analgesic activity in mice. Anti-inflammatory activity was evaluated by rat hind paw edema method. The seed extracts of C. maxima and C. sativus were found to be safe and showed significant analgesic and anti-inflammatory activity in comparison with the control group. The therapeutic effects of these extracts were almost comparable to aspirin and brufen. Therefore, the seeds can be used as effective analgesic and anti-inflammatory agents.
Keywords: C. maxima, C. sativus, Extract, Safety, Analgesic, Anti-inflammatory
1. Introduction
Pain and inflammation are two immune responses triggered in response to injury, irritants or pathogen causing the symptoms of pain, redness, immobility, edema and heat. Uncontrolled chronic inflammation is accountable for several disorders such as atherosclerosis, rheumatoid arthritis, ischemic heart diseases or many others. Infectious diseases also have common symptoms of pain and inflammation along with non-infectious diseases like leprosy, syphilis, tuberculosis, asthma, peritonitis, inflammatory bowel syndrome, vasculitis, nephritis, celiac diseases, auto-immune disorders etc. (Chen et al., 2017).
Steroidal and non-steroidal anti-inflammatory drugs (NSAIDs) are mainly used to control the above-mentioned symptoms but after continued usage, these drugs can cause harmful effects to kidney, liver, GIT, CVS, CNS and lungs (Bindu et al., 2020). Hence, despite large number of available analgesic and anti-inflammatory drugs, still there is a need of new analgesics and ant-inflammatory drugs with minimum side effects. Medicinal plants are among one of the best options to discover newer medicinal agent as these are rich in phytochemicals.
Inflammation with pain is related with different illnesses like rheumatism, pneumonia, fibrosis esophagitis, encephalitis, cancer and heart problems, Non-steroidal anti-inflammatory drugs are generally used for the treatment of pain associated with inflammation. However these drugs may cause several adverse effects. Therefore every year, hundreds of plants are evaluated for their potential anti-inflammatory and analgesic activity nonetheless only few of them are approved in health care system after laborious clinical research (Sen et al., 2010).
C. maxima (Pumpkin) and C. sativus (cucumber) are the members of Cucurbitaceae family. C. maxima is traditionally used as a source of food due to high content of polysaccharides, proteins, lipid, ash, sterols, Para amino-benzoic acids, mono- and polyunsaturated fatty acids and fixed oil. Its phytochemical evaluation has shown that it has carotenoids, γ-amino butyric acid in seeds and fruit (Matus et al., 1993, Murkovic et al., 2002), phenolic glycosides, 11E-octa decatrienoic acid in leaves and seeds (Glew et al., 2006), flavonoids, alkaloids, phenolic derivatives, proteins, tannins, carbohydrates, saponins and proteins in ethanol extract (Muchirah et al., 2018). Among all the most important class of triterpenoids, Cucurbitacin has gained importance due to their biological attributes (Salehi et al., 2019a, Salehi et al., 2019b).
C. maxima seeds have enough magnesium (Edward et al, 2013) which acts as NMDA receptor blocker hence is effective in reducing acute or chronic pain, especially nerve pain (Na et al 2011).
C. sativus is cultivated in large quantities in the Indo-Pak region and mainly famous for its elongated cylindrical green accessory fruit eaten as salad (Huang et al., 2009). Therapeutic value of different parts of the C. sativus i.e. leaf, stem, fruit and seeds have been explored and its fruits extract is mainly used in several skin formulations for management of aging (Maity et al., 2011, Mukherjee et al., 2013) (See Table 1, Table 2, Table 3).
Table 1.
Analgesic Activity of C. maxima and C. sativus by Hot plate method.
|
Groups & Doses mg/Kg |
Reaction time (Sec) |
||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| Control | 6.71 ± 0.42 | 7.86 ± 0.40 | 8.07 ± 0.46 | 7.11 ± 0.44 | 5.15 ± 0.42 |
| C. maxima 50 | 5.99 + 0.28 | 10.8 + 0.35* | 11.07 + 0.57* | 10.44 + 0.42** | 8.46 + 0.64 |
| C. maxima 100 | 7.21 + 0.52 | 10.65 + 0.67* | 10.10 + 0.36 | 9.74 + 0.30* | 8.40 + 0.34 |
| C. maxima 200 | 7.26 + 0.45 | 10.9 + 0.67* | 11.3 + 0.56** | 10.30 + 0.68* | 9.35 + 0.82 |
| C. sativus 50 | 7.10 + 0.46 | 9.36 + 0.62 | 12.14 + 0.49** | 11.86 + 0.29* | 9.53 + 0.53 |
| C. sativus 100 | 7.35 + 0.36 | 12.53 + 0.52** | 12.19 + 0.64** | 10.63 + 0.56* | 9.44 + 0.65 |
| C. sativus 200 | 6.94 + 0.33 | 13.40 + 0.65** | 13.40 + 0.78** | 10.09 + 0.37 | 9.78 + 0.58 |
| Aspirin 300 | 7.27 + 0.32 | 13.73 + 0.35** | 12.73 + 0.56** | 11.48 + 0.50** | 11.61 + 0.58** |
| Brufen 100 | 7.02 + 0.45 | 14.23 + 0.56** | 12.35 + 1.00** | 11.77 + 0.92** | 11.93 + 0.34** |
n = 10, Mean ± SEM; *P < 0.05 significant; ** P < 0.01 highly significant as compare to control.
Table 2.
Analgesic activity of C. maxima and C. sativus seeds extract by Tail flick method.
| Groups & Doses mg/Kg |
Reaction time (Sec) |
||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| Control | 1.79 ± 0.15 | 1.91 ± 0.20 | 2.76 ± 0.51 | 2.210 ± 0.26 | 1.58 ± 0.17 |
| C. maxima 50 | 1.67 ± 0.12 | 4.65 ± 0.17** | 4.13 ± 0.31** | 3.58 ± 0.26** | 3.46 ± 0.20** |
| C. maxima 100 | 1.76 ± 0.09 | 4.88 ± 0.25** | 3.42 ± 0.25* | 3.63 ± 0.35** | 3.56 ± 0.28** |
| C. maxima 200 | 1.60 ± 0.08 | 3.68 ± 0.34** | 4.10 ± 0.32** | 3.03 ± 0.13* | 2.93 ± 0.23* |
| C. sativus 50 | 1.21 ± 0.07 | 3.51 ± 0.3 | 3.27 ± 0.27 | 2.86 ± 0.40 | 2.71 ± 0.23 |
| C. sativus 100 | 1.70 ± 0.16 | 3.94 ± 0.19** | 3.79 ± 0.30* | 3.60 ± 0.33** | 2.63 ± 0.28 |
| C. sativus 200 | 1.71 ± 0.12 | 4.36 ± 0.27** | 3.52 ± 0.29* | 3.37 ± 0.29** | 2.34 ± 0.25 |
| Aspirin 300 | 1.60 + 0.14 | 5.23 + 0.57** | 4.16 + 0.43** | 3.91 + 0.38** | 3.75 + 0.35** |
| Brufen 100 | 1.46 + 0.13 | 4.97 + 0.25** | 4.89 + 0.57** | 5.35 + 0.59** | 3.70 + 0.21** |
n = 10, Mean ± SEM; *P < 0.05 significant; ** P < 0.01 highly significant as compare to control.
Table 3.
Anti-inflammatory Activity of C. maxima and C. sativus seed extracts.
| Groups & Doses mg/kg |
Paw Volume (mL) with % inhibition in edema |
|||||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | 5 h | 24 h | |
| Control | 0.74 ± 0.22 | 1.85 ± 0.22 | 2.27 ± 0.15 | 1.71 ± 0.09 | 1.52 ± 0.11 | 0.62 ± 0.16 |
| C. maxima 50 | 0.74 ± 0.15 (8.83%) |
1.69 ± 0.37 (16.12%) |
1.74 ± 0.29 (26.80%) |
1.17 ± 0.26 (31.29%) |
0.83 ± 0.33* (45.31%) |
0.36 ± 0.16* (41.61%) |
| C. maxima 100 | 0.56 ± 0.09 (31.01%) |
1.61 ± 0.37 (20.00%) |
1.31 ± 0.38* (44.68%) |
0.88 ± 0.31* (48.35%) |
0.42 ± 0.19** (72.59%) |
0.10 ± 0.3** (74.52%) |
| C. maxima 200 | 0.69 ± 0.11 (14.74%) |
1.25 ± 0.24 (37.38%) |
1.05 ± 0.22** (55.56%) |
0.65 ± 0.28** (62.00%) |
0.41 ± 0.15** (72.98%) |
0.14 ± 0.11* (77.74%) |
| C. sativus 50 | 0.70 ± 0.19 (14.25%) |
1.43 ± 0.21 (28.66%) |
1.79 ± 0.20 (24.44%) |
1.47 ± 0.31 (13.65%) |
1.18 ± 0.25 (22.89%) |
0.55 ± 0.20 (11.61%) |
| C. sativus 100 | 0.76 ± 0 + 0.10 (5.87%) |
1.12 ± 0.06* (44.38%) |
1.56 ± 0.21 (34.14%) |
1.17 ± 0.20 (31.38%) |
0.76 ± 0.16* (50.16%) |
0.21 ± 0.08* (65.81%) |
| C. sativus 200 | 0.56 ± 0.12 (30.76%) |
1.27 ± 0.23 (37.01%) |
1.42 ± 0.19 (40.04%) |
0.98 ± 0.04* (42.35%) |
0.52 ± 0.06** (66.16%) |
0.20 ± 0.03* (67.10%) |
| Aspirin 300 | 0.22 ± 0.02* (72.4%) |
0.4 ± 0.15** (79.3%) |
0.2 ± 0.05** (90.8%) |
0.09 ± 0.03** (94.8%) |
0.2 ± 0.14** (88.7%) |
0.05 ± 0.01** (92.6%) |
| Brufen 100 | 0.68 ± 0.03 (16.47%) |
0.65 ± 0.12* (67.76%) |
0.7 ± 0.2** (68.71%) |
0.6 ± 0.2** (63.65%) |
0.42 ± 0.2** (72.59%) |
0.14 ± 0.03**(92%) |
n = 10, Mean ± SEM; *P < 0.05 significant; ** P < 0.01 highly significant as compare to control.
C. sativus has high nutritional value since it contains carbohydrates, pectin, amino acids and some secondary metabolites like carotenoid, vitamins A, C, E and K, terpenoids, flavonoids, saponins, and Cucurbitacins A-D, orientin and isoorientin, tannins and some important minerals (Wang et al., 2007, Kumar et al., 2010, Uzuazokaro et al., 2018). C. sativus fruit is mainly used for its antioxidant, anti-wrinkle, anti-aging, anticancer, anti-diabetes, analgesic and hypolipidemic activities (Mukherjee et al., 2013).
Thus present study was designed to investigate the safety profile, analgesic and anti-inflammatory activity of the ethanol extract of C. maxima and C. sativus seeds. The objective of the study was to explore a potential analgesic and anti-inflammatory agent with minimal side effects.
2. Materials and methods
The study was performed under the approval of Board for Advance Studies and Research (reference No 03297/pharm), University of Karachi dated April 2017 and Departmental Research Committee, Pharmacology for the use of animals according to the guidelines of National institute of Health.
2.1. Collection of plant material
The seeds of C. maxima and C. sativus were purchased from the local market of Karachi and were identified at the Herbarium, Centre for Plant Conservation; University of Karachi. The botanist of the same institute issued specimen number GH# 94589 to C. sativus and GH # 9501 to C. maxima which were then placed in the herbarium as reference for authentication.
2.2. Extracts preparation
The seeds of these plants were precisely weighed, washed, dried, coarsely crushed and then macerated for 21 days in ethanol. Ethanol was used in portions for soaking 4 Kg of both seeds kept in tightly closed containers with intermittent shaking. Filtration was done after the maceration period using muslin cloth and Whatman filter paper #1. Solvent was removed by rotatory evaporator at 40 °C under reduced pressure followed by freeze drying of both extracts. The obtained extracts were stored in refrigerator at 4 °C. The yield of C. maxima and C. sativus seeds extracts was15.8% and 12.4%.
2.3. Drugs and chemicals
All chemical used in the experiment were highly purified. Carrageenan and Dimethyl sulfoxide (≥99.9%) were of Sigma- Aldrich purchased from Multi Chem Corporation, Karachi. Brufen suspension100 mg/5 mL of Abbot Laboratories, Karachi and Disprin (Aspirin 300 mg) of Reckitt Benckiser Pakistan, were used as standard drugs
2.4. Study design
Healthy adult mice and rats of either sex, bred in the animal house of Department of Pharmacology, University of Karachi, were used in the study. Five animals were kept per polycarbonate cage with free access to food and water ad libitum at controlled room temperature. Mice between 25 and 30 g while rats between 200 and 250 g were used all animals were divided in eight groups, each group having 10 animals. Control group received 5% DMSO by mouth, equivalent to the volume of doses as per weights. Aspirin was used as standard drug to compare anti-inflammatory effect in the dose of 300 mg/Kg (Rahman et al., 2015) and brufen was used as standard analgesic drug in the dose of 100 mg/Kg (Lalan et al., 2015). Test groups received ethanol extracts of C. maxima and C. sativus seed at doses of 50 mg/Kg, 100 mg/Kg and 200 mg/Kg.
2.5. Toxicity studies
2.5.1. Acute toxicity study
Mice were denied food overnight followed by the administration of extracts on next day. The animals were divided into three groups as per Lorke’s method (1983) at doses of 10 mg/Kg, 100 mg/Kg and 1000 mg/Kg. After dosing, mice were continuously monitored for the symptoms of toxicity such as convulsions, tonic extension, tremors, and loss of righting reflex, ataxia, muscle spasm, sedation or hypnosis, lacrimation, diarrhea, salivation, writhing up to 4 h. Meanwhile, mortality was observed for up to 48 h (Riaz et al., 2010).
2.5.2. Sub-acute toxicity
Sub chronic studies were carried out at selected doses of the extracts i.e. 50 mg/Kg, 100 mg/Kg, and 200 mg/Kg for 30 days. Moreover, gross behavioral changes as described in acute toxicity were also observed for up to 30 days and mortality of animals was also noted at selected doses.
2.6. Analgesic activity
Analgesic activity of these extracts was tested using hot plate and tail flick method.
2.6.1. Hot plate test
Anti-nociceptive effect is measured by increase in the latency by withdrawal or licking time of paw through electrically generated heat. Only those mice were included in the study which showed
reaction within 15 s. The cut-off time of 15 s was used to avoid paw damage due to heat. Extracts and standard drugs were administered 1 h before the test. The temperature maintained on hot plate was 55 ± 2 °C and mice were kept on hot plate to record withdrawal time in seconds or the start of licking or jumping (Turner and Ebborn, 1965). Mean increase in latency for reaction time was thought to exhibit analgesic effect (Eddy et al., 1950).
2.6.2. Tail flick test
Tail flick method is an alternative method to evaluate anti-nociceptive effects of drug by thermal stimuli for confirmation of analgesic activity (Luiz et al., 1988). The latency time in seconds was recorded by placing the tail of the mice in pre-heated water at 50 °C. Readings were noted at 1 h, 2 h, 3 h, and 4 h of administration of ethanol extracts. Mean increase in latency time of tail withdrawal from hot water after the administration of the extracts and reference standard were deemed indicators of the analgesic effects.
2.7. Anti-inflammatory activity
The water displacement method using digital plethysmometer as adopted by Winter et al. (1962) was used to determine anti-inflammatory activity with some minor alterations. Ethanol extracts of these seeds were administered 1 h before the induction of edema through orogastric tube. Edema was induced in normal rats by injecting 0.1 mL 1% carrageenan (normal saline) in sub-plantar tissue of left paw. Measurement of paw volume was carried out at intervals of 1, 2, 3, 4, 5 and 24 h of the extracts and standard drug administration. The difference in paw volume after administration of extracts was measured from baseline paw volume of each rat. Two parameters, reduction in paw edema and percentage inhibition were calculated in comparison to control and standard drugs aspirin and brufen (Sood et al., 2009, Jamil et al., 2017). The percentage inhibition in paw edema was determined by formula described by Suleyman et al., 1991.
where Vc and Vt are the difference of mean paw volumes of control and treated group animals respectively.
2.8. Statistical analysis
Data was analysed by using SPSS software version 20 by One-way ANOVA and general linear model using repeated measures followed by Dunnet Post hoc tests. Statistically results were considered significant when p value was <0.05 and highly significant when p value was <0.01. Values were expressed as mean ± S.E.M.
3. Results
3.1. Acute toxicity
The results of in-vivo oral acute toxicity of the ethanol seed extract of C. maxima and C. sativus reveals that these seeds are safe as there was no mortality, while no toxic symptoms were observed at selected doses of both extracts. During the experimental period, there were no symptoms of lacrimation, salivation, laboured breathing, diarrhoea, constipation, anorexia, hyperesthesia, weight loss, polyuria, polyphagia, polydipsia, skin damage, hyperesthesia, abnormal body tone, in coordinated movement, haemorrhage, sedation, nasal congestion/ rhinorrhoea, loss of autonomic reflexes, and collapse, hypothermia, or hyperthermia, twitching, spasticity, tremors, convulsion, fasciculation, writhing and respiratory depression.
3.2. Sub-acute toxicity
One month sub-acute dosing of ethanol extract of C. maxima produced no changes in gross behavioural parameters at 50 mg/Kg like convulsions, tremors, aggression, vocalization, fearfulness, body tone, righting reflex, staggering gait and grip strength, while spontaneous activity or passivity, pain response, touch response, corneal and light response were decreased. Sedation, piloerection and startle response were increased. The effects after 100 mg/kg and 200 mg/kg were almost same as compared to 50 mg/Kg dose of C. maxima, however, with the increment of dose grip strength, light and corneal reflex were decreased.
There was decrease in some gross behavioural parameter like awareness, alertness, fearfulness, vocalization, aggression, spontaneous activity and pain response, in mice during the one month sub-acute dosing of ethanol extract of C. sativus at 50 mg/Kg while no changes were observed in induction of, convulsions, seizures, tremors, corneal reflex, light reflex, body and limb tone, righting reflex, staggering gait. However there was increase in startle response, grip strength, alertness and balance beam at 100 mg/Kg and 200 mg/Kg. Pain response and touch response were decreased at each selected doses.
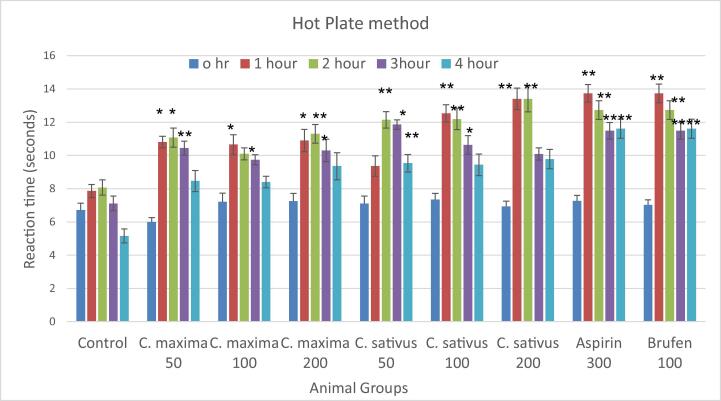
3.3. Hot plate analgesia
Fig. 1 shows results of C. maxima and C. sativus seeds extracts at 50 mg/Kg, 100 mg/Kg and 200 mg/Kg by hot plate method. There was significant increase in basal reaction time as compared to control in animals received C. maxima 50 mg/Kg at 1 h, and 2 h, while highly significant increase was observed at 3 h. There was significant increase in reaction time at 1 h and 3 h by C. maxima at100mg/Kg. C. maxima at 200 mg/Kg significantly increased reaction time at 1 h and 3 h, while there was highly significantly increase in reaction time at 2 h. However, these increments were less than the standards drugs brufen and aspirin.
Fig. 1.
Analgesic Activity of C. maxima and C. sativus by Hot plate method.
The onset of action in C. sativus was slightly delayed at 50 mg/Kg, showed highly significant increase in reaction time at 2 h followed by significant increase at 3 h as compared to control.
C. sativus at 100/Kg and 200 mg/Kg produced highly significant increase in reaction time at 1 h and 2 h as compared to control.
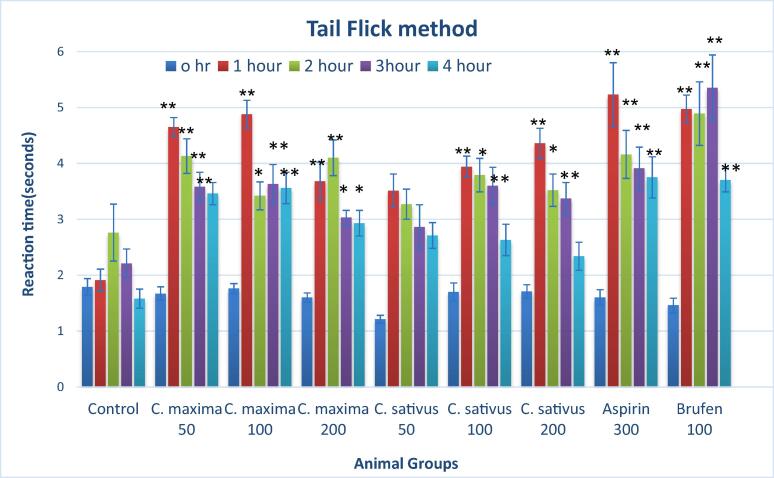
3.4. Tail flick analgesia
Fig. 2 shows results for analgesic effects of C. maxima and C. sativus seed extracts at 50 mg/Kg, 100 mg/Kg and 200 mg/Kg by tail flick method. C. maxima at 50 mg/Kg showed highly significant increase in mean reaction time at 1 h, 2 h, 3 h and 4 h. While C. maxima at 100 mg/Kg showed highly significant increase in mean reaction time at 1 h, 3 h and 4 h, however increase at 2 h was significant. C. maxima at 200 mg/Kg showed highly significant increase in mean reaction time at 1 h and 2 h and significant increase in reaction time at 3 h and 4 h as compare to control.
Fig. 2.
Analgesic activity of C. maxima and C. sativus seeds extract by Tail flick method. n = 10, Mean ± SEM; *P < 0.05 significant; ** P < 0.01 highly significant as compare to control.
C. sativus at 100 mg/Kg and 200 mg/Kg showed highly significant increase the reaction time at 1 h and 3 h and significantly increase in reaction time at 2 h as compare to control.
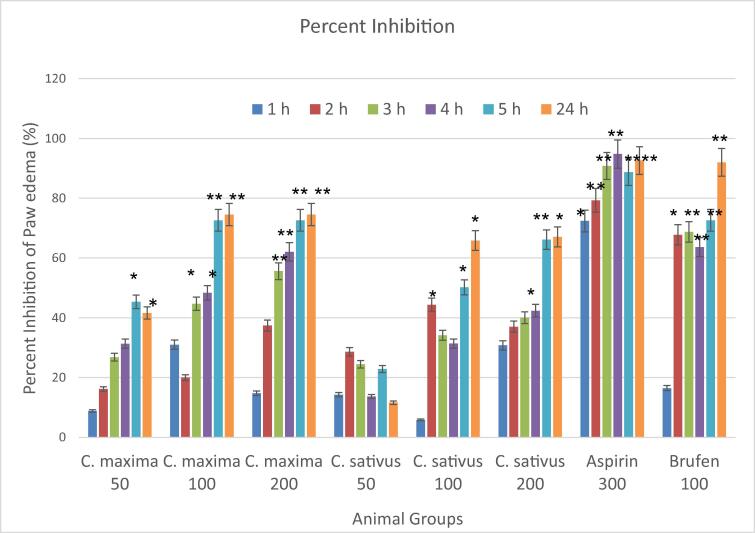
3.5. Anti-inflammatory activity
Fig. 3 shows results for anti-inflammatory effects of C. maxima and C. sativus seed extracts at 50 mg/Kg, 100 mg/Kg and 200 mg/Kg. C. maxima at 50 mg/Kg showed significant reduction in paw edema at 5 h and 24 h with 45.3% and 41.6% inhibition in paw edema as compared to control. C. maxima at 100 mg/Kg demonstrated significant reduction in paw edema at 3 h and 4 h with 44.7% and 48.3% inhibition in paw edema as compared to control. However, at 5 h and 24 h a highly significant reduction in paw edema was observed with 72.6% and 74.5% inhibition in paw edema as compared to control. C. maxima at 200 mg/Kg demonstrated highly significant reduction in paw edema at 3 h, 4 h and 5 h, while at 24 h a significant reduction in paw edema was observed with 77.7% inhibition in edema as compared to control.
Fig. 3.
Anti-inflammatory Activity of C. maxima and C. sativus seed extracts. n = 10, Mean ± SEM; *P < 0.05 significant; ** P < 0.01 highly significant as compare to control.
C. sativus at 100 mg/Kg showed significant reduction in paw edema at 5 h and 24 h with 50.2% and 65.8% inhibition in paw edema as compared to control. C. sativus at 200 mg/Kg demonstrated significant reduction in paw edema at 4 h and 24 h, however at 5 h reduction in paw edema was highly significant with 67.1% inhibition in edema as compared to control. C. maxima was found to be more effective anti-inflammatory then C. sativus.
4. Discussions
The main objective in the management of chronic pain and inflammatory disorders is elimination of underlying cause rather than transient suppression of symptoms. Currently available NSAIDS are basically prostaglandin synthesis inhibitors which mainly suppress the body immunity response in all conditions.
The results of both acute and sub chronic toxicity testing revealed that these seeds are safe for oral use even up to 1000 mg/Kg. This finding is in line with the previous studies that indicate safety of these seeds up to 5000 mg/Kg. All tested seed extracts were non-toxic and can be classified according to the global harmonization system as category 5 having LD50 value greater than 2000–5000 mg/Kg. According to Cruz et al. (2008), LD50 of C. maxima seeds is more than 5000 mg/Kg. Similarly, toxicity of C. sativus seed oil, leave and fruit extracts also reveals its safety up to 5000 mg/kg. Hence, the present study confirms safety of these seeds following oral use.
The analgesic and anti-inflammatory properties of ethanol seed extracts of C. maxima and C. sativus were investigated in mice and rats respectively. Hot plate and tail-flick tests were used to assess thermal and neuronal induced algesia. Although, the stimuli were thermal in both tests, spinal reflexes were mainly involved in paw licking and tail-flick responses (Suh et al., 1992, Schmauss and Yaksh, 1984). The antinociceptive effect in the tail flick and hot plate tests indicates that these extracts have effect on CNS as demonstrated through inhibition of the spinal reflex and supraspinal centres (Dewey et al., 1970).
Carrageenan sub plantar injection acts as a local irritant that increases membrane phospholipase activity triggering synthesis of several pain and inflammatory cytokines like histamines, 5HT, bradykinins, leukotrienes, eicosanoids and prostaglandins which increases the paw size. First phase of inflammation starts within one hour of carrageenan injection by the release of histamine, serotonin and activation of cytoplasmic enzyme followed by Plateau phase maintained by bradykinin like substances. Both C. maxima and C. sativus initially showed little effect in reducing paw size however, during the second phase of inflammation which is due to the release of prostaglandin showed significant reduction in paw size at 3 hrs that last up to 24 hr thus indicating the presence of some COX enzyme inhibitors in these extracts.
C. sativus and C. maxima are the rich sources for various phytochemicals especially tetracyclic terpenoids type cucurbitacin. These are hepatoprotective, highly oxygenated phytochemicals having a basic structure of cucurbitane skeleton (Dhiman et al., 2012, Torkova et al., 2018). Seed of the C. maxima have been found to contain high quantities of cucurbitacin B and small quantities of Cucurbitacin D and E (Salehi et al., 2019a, Salehi et al., 2019b) while Cucurbitacins A, B, C, D, E and I were also recognized in seeds of different verities of C. sativus (Rice et al., 1981, Mukherjee et al., 2013). These terpenes possess good antineoplastic, antibacterial, COX 2 inhibition and anti-fungal characteristics (Rajasree et al., 2016). Furthermore, these compounds have been also investigated for their anti-inflammatory activities, cardiovascular and anticoagulant characteristics that is mainly due to the inhibition of the cyclooxygenase (COX) enzymes (Miro, 1995, Peters et al., 1997, Yesilada et al., 1998). The cucurbitacin are classified into twelve categories- cucurbitacins A-T differ with respect to oxygen functional group at various positions (Chen et al., 2005). Some cardiac glycosides are also present in these seeds having anti-inflammatory activity besides cardiac activity. Tannins are also found in the ethanol extract of C. maxima and C. sativa that have astringent, metal ion chelating, proton precipitating and antioxidant properties that accelerate wound healing and decrease inflammation of membrane. Phytosterols like carotenoids have also been found in these extracts. Furthermore, presence of high content of magnesium i.e., 348 ppm in C. maxima (Habib et al., 2015) and (8.5%) in seeds of C. sativus (Mariod et al., 2017) may also be responsible for its synergistic analgesic effects through central nervous system. Magnesium has a role in the inhibition of central sensitization and attenuation of pain especially in case of hypersensitivity of different pains such as migraine and neuropathic pain associated with diabetes. The mechanism of action of magnesium seems to involve antagonism of voltage-gated N-methyl-D-aspartate (NMDA) receptors so it provides putative function in pain transduction in various clinical conditions related to acute or chronic pain (Na et al 2011). This supports the idea as magnesium supplements in various salt form is used clinically as adjuvant in different pre-operative and post-operative surgical procedures in combination with propofol and lidocaine and in chemotherapy induced neuropathy (Na et al 2011).
Pumpkin-based foodstuff is reported as a source of anti-inflammatory herbal remedy for arthritis treatment due to the presence of carotenoid (Seo et al., 2005) β-Carotene is the major carotenoid with concentrations more than 70 μg/g (Provesi and Amante, 2015). Pumpkin seed oil used as an adjuvant in induced arthritis rats model similar to indomethacin, a well-known anti-inflammatory substance. Its clinical applicability as an antioxidant was also assessed in rheumatoid arthritis compared with indomethacin (Van-Vugt et al., 2008, Dixon, 2015).
The analgesic activity of these extracts may probably be mediated either by the inhibition of COX enzymes or NMDA receptors in CNS since both seed extracts have high magnesium contents. As far as anti-inflammatory effects are concerned, these may be due to cucurbitacins or carotenoids. The findings of present study suggest isolation of active components having analgesic and anti-inflammatory effects.
5. Conclusion
According to the results obtained, one can conclude that ethanol extracts of C. maxima and C. sativa seeds are safe and efficacious analgesic and anti-inflammatory agents with effects comparable to standard drugs brufen and aspirin. These seeds extracts were safe up to tested period of 30 days. Nonetheless the study has some limitation since does not include clinical studies which are necessary for safe use of any drug before general use in humans. However clinical studies, may be carried out in future to assure the safe use of C. maxima and C. sativa seeds extract.
Authors contributions
All authors were involved in the interpretation of the studies. S.W: carried out the experimental work, statistical analysis, and wrote the initial draft. R. A. K: developed concept of the study and edited the initial draft, A. A: reviewed the manuscript.
7. Impact statement
The findings of the present study lead us to an alternate of NSAIDs, which are likely to cause serious side effects when used for the management of chronic illnesses. Thus, outcomes of this study provide a relatively safer method to deal states of acute or chronic distress allowing the consumer to maintain well-being at low cost. The results also endorse the ill-informed OTC use of natural products by providing physicians scientific grounds for their use instead. Therefore these results may help altering arena of pharmacology to treat ailments by the use of natural medicines with negligent adverse effects.
Acknowledgement
Authors are grateful to the Department of Pharmacology, University of Karachi for the support delivered to complete this piece of work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bindu, S., Mazumder, S., Bandyopadhyay, U., 2020. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem. Pharmacol. 180, 114147. Adv. Online Publication. Doi: 10.1016/j.bcp.2020.114147. [DOI] [PMC free article] [PubMed]
- Chen J.C., Chiu M.H., Nie R.L., Cordell G.A., Qiu S.X. Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat. Prod. Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- Edward M., Muntean N., Duda M.M. Cucurbita maxima duch. as a medicinal plant. Hop Med. Plants. 2013;1–2:75–80. [Google Scholar]
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W.G. Rheumatoid arthritis: Biological drugs and risk of infection. Lancet. 2015;386:224–225. doi: 10.1016/S0140-6736(14)61907-3. [DOI] [PubMed] [Google Scholar]
- Cruz R.C.B., Meurer C.D., Silva E.J., Schaefer C., Santos A.R.S., Bella-Cruz A., Filho V.C. Toxicity Evaluation of Cucurbita maxima. Seed Extract in Mice. Pharm. Biol. 2008;44:301–303. [Google Scholar]
- Dewey W.I., Harris L.S., Howes J.F., Nuite J.A. The effect of various neurohumoral modulators on the activity of morphine and the narcotic antagonists in the tail-flick and the phenylquinone tests. J. Pharmacol. Exp. Ther. 1970;175:435–442. [PubMed] [Google Scholar]
- Dhiman K., Gupta A., Sharma D.K., Gill N.S., Goyal A. A review on the medicinally important plants of the family cucurbitaceae. Asian J. Clin. Nutr. 2012;4:16–26. [Google Scholar]
- Eddy N.B., Touchberry C.F., Lieberman I.E. Synthetic analgesics: a methadone isomer and derivatives. J. Pharmacol. Exp. Ther. 1950;98:121–137. [PubMed] [Google Scholar]
- Glew R.H., Glew R.S., Chuang L.T., Huang Y.S., Millson M., Constans D., Vanderiaqt D.J. Amino acid, mineral and fatty acid content of C. maxima seeds (Cucurbita spp) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods Hum. Nutr. 2006;61:51–56. doi: 10.1007/s11130-006-0010-z. [DOI] [PubMed] [Google Scholar]
- Habib A., Biswas M.S., Siddique M.A.H., Manirujjaman M., Belal U., Hasan M.S., Khan M.M.H., Meftah U., Islam M.M., Rahman M. Nutritional and lipid composition analysis of pumpkin seed (Cucurbita maxima Linn) J. Nutr. Food. 2015;5(4) doi: 10.4172/2155-9600.1000374. [DOI] [Google Scholar]
- Huang S., Li R., Zhang Z. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 2009;41:1275–1281. doi: 10.1038/ng.475. [DOI] [PubMed] [Google Scholar]
- Jamil S., Khan R.A., Ahmed S. Evaluation of anti-inflammatory and antioxidant potential of seed extracts of Vernonia anthelmintica. Pak. J. Pharma. Sci. 2017;30:755–760. [PubMed] [Google Scholar]
- Kumar D., Kumar S., Singh J., Rashmi N., Vashistha B.D., Singh N. Free radical scavenging and analgesic activities of Cucumis sativus L. fruit extract. J. Young Pharm. 2010;2:365–368. doi: 10.4103/0975-1483.71627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalan, B.K., Hiray, R.S., Ghongane, B.B., 2015. Evaluation of Analgesic and Anti-Inflammatory Activity of Extract of Holoptelea Integrifolia and Argyreia Speciosa in Animal Models. J. Clin. Diag. Res. 9, FF01–FF04. doi: 10.7860/JCDR/2015/ 12059. 6200. [DOI] [PMC free article] [PubMed]
- Luiz C.D., Costa M., Medacolli S.L., Kirizawa M., Gomes C., Trolin G. Screening in mice of some medicinal plants used for analgesic purposes in the state of Sao Paulo. J. Ethnol. Pharmacol. 1988;24:205–211. doi: 10.1016/0378-8741(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Maity N., Mukherjee P.K., Nema N.K., Sarkar B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19:64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Mariod A.A., Mirghani M.E.S., Hussein I. Cucumis sativus cucumber. Unconvent. Oilseeds Oil Sources. 2017;89–94:Elsevier. doi: 10.1016/b978-0-12-809435-8.00016-0. [DOI] [Google Scholar]
- Matus Z., Molnar P., Szabo L.G. Main carotenoids in pressed seeds (Cucurbitae semen) of oil C. maxima (Cucurbita pepo convar. pepo var. styriaca) Acta Pharm. Hung. 1993;63:247–256. [PubMed] [Google Scholar]
- Miro M. Cucurbitacins and their pharmacological effects. Phytother. Res. 1995;9:159–168. [Google Scholar]
- Muchirah P.N., Waihenya R., Muya S., Abubakar L., Ozwara H., Makokha A. Characterization and anti-oxidant activity of Cucurbita maxima Duchesne pulp and seed extracts. J. Phytopharmacol. 2018;7:134–140. [Google Scholar]
- Mukherjee P.K., Nema N.K., Maity N., Sarkar B.K. Phytochemical and therapeutic potential of C. sativus. Fitoterapia. 2013;84:227–236. doi: 10.1016/j.fitote.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Murkovic M., Mulleder U., Neunteufl H. Carotenoid content in different varieties of Pumpkins. J. Food Compos. Anal. 2002;15:633–638. [Google Scholar]
- Na, H.S., Ryu, J.H., DO, S.H., 2011. The role of magnesium in pain. In: Vink R, Nechifor M, editors. Magnesium in the Central nervous System. Adelaide, University of Adelaide Press; 2011. PMID: 29920000. [PubMed]
- Peters R.R., Farias M.R., Ribeiro-do-Valle R.M. Anti-inflammatory and analgesic effects of cucurbitacins from Wilbrandia ebracteata. Planta Medica. 1997;63:525–528. doi: 10.1055/s-2006-957755. [DOI] [PubMed] [Google Scholar]
- Provesi, J.G., Amante, E.R., 2015. Carotenoids in Pumpkin and Impact of Processing Treatments and Storage. Processing and Impact on Active Components in Food. Academic Press. Doi: 10.1016/B978-0-12-404699-3.00009-3.
- Rahman N.U., Riaz M., Khan A., Haq M.Z., Dima L. Mechanism of anti-inflammatory and anti-nociceptive actions of Acacia modesta in animal models. Pak. J. Zool. 2015;47:1723–1730. [Google Scholar]
- Rajasree R.S., Sibi P., Francis F., William H. Phytochemicals of cucurbitaceae family. Int. J. Pharmacog. Phytochem. Res. 2016;8:113–123. [Google Scholar]
- Riaz A., Khan R.A., Ahmed S., Afroz S. Assessment of acute toxicity and reproductive capability of a herbal combination. Pak. J. Pharm. Sci. 2010;23:291–294. [PubMed] [Google Scholar]
- Rice C.A., Rymal K.S., Chambliss O.L., Johnson F.A. Chromatographic and mass spectral analysis of cucurbitacins of three Cucumis sativus cultivars. J. Agric. Food Chem. 1981;29:194–196. [Google Scholar]
- Salehi, B., Sharifi-Rad, J., Capanoglu, E., Adrar, N., Catalkaya, G., Shaheen, S., Jaffer, M., Giri, L., Suyal, R., Jugran, A.K., Calina, D., Docea, A.O., Kamiloglu, S., Kregiel, D., Antolak, H., Pawilkowska, E., Sen, S., Acharya, K., Cho. 2019. Cucurbita Plants: From Farm to Industry. App. Sci. 9, 3387. doi:10.3390/app9163387.
- Salehi B., Capanoglu E., Adrar N., Catalkaya G., Shaheen S., Jaffer M., Giri L., Suyal R., Jugran A.K., Calina D., Docea A.O., Kamiloglu S., Kregiel D., Antolak H., Pawlikowska E., Sen S., Acharya K., Selamoglu Z., Sharifi-rad J., Martorell M., Capasso R. Cucurbits plants: a key emphasis to its pharmacological potential. Molecules (Basel, Switzerland) 2019;24(10):1854. doi: 10.3390/molecules24101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmauss C., Yaksh T.L. In vivo studies on spinal opiate receptor systems mediating antinociception. II. Pharmacological profiles suggesting a differential association of mu, delta and kappa receptors with visceral, chemical and cutaneous thermal stimuli in the rat. J. Pharmacol. Exper. Therap. 1984;228:1–12. [PubMed] [Google Scholar]
- Sen S., Chakraborty R., Biplab De, Ganesh T., Raghavendra H.G., Debnath S. Analgesic and anti-inflammatory herbs: a potential source of modern medicine. IJPSR. 2010;11:32–44. [Google Scholar]
- Seo J.S., Burri B.J., Quan Z., Neidlinger T.R. Extraction and chromatography of carotenoids from pumpkin. J. Chroma. A. 2005;1073:371–375. doi: 10.1016/j.chroma.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Sood S., Bansal S., Muthuraman S., Gill N.S., Bali M. Therapeutic potential of Citrus medica L. peel extract in carrageenan induced inflammatory pain in rat. Res. J. Medi. Plant. 2009;3:123–133. [Google Scholar]
- Suh H.H., Fujimoto J.M., Tseng L.F. Different radiant heat intensities differentiate intracerebro ventricular morphine from b-endorphin-induced inhibition of the tail-lick response in the mouse. Euro. J. Pharmacol. 1992;213:337–341. doi: 10.1016/0014-2999(92)90622-b. [DOI] [PubMed] [Google Scholar]
- Suleyman H., Demirezer L.O., Kuruuzum A., Banoglu Z.N., Gocer F., Ozbakir G., Gepdiremen A. Anti-inflammatory effect of the aqueous extract from Rumex patientia L. roots. J. Ethno Pharmacol. 1991;65:141–148. doi: 10.1016/s0378-8741(98)00175-5. [DOI] [PubMed] [Google Scholar]
- Torkova A.A., Lisitskaya K.V., Filimonov I.S., Glazunova O.A., Kachalova G.S., Golubev V.N., Fedorova T.V. Physicochemical and functional properties of Cucurbita maxima Pumpkin pectin and commercial citrus and apple pectins: a comparative evaluation. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0204261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R.A., Ebborn P. Academic Press; New York: 1965. In: Analgesics: Screening Methods in Pharmacology. [Google Scholar]
- Uzuazokaro M.M.A., Okwesili F.C.N., Chioma A.A. Phytochemical and proximate composition of C. sativus (Cucumis sativus) fruit from Nsukka, Nigeria . Afr. J. Biotechnol. 2018;17:1215–1219. [Google Scholar]
- Van-Vugt R.M., Rijken P.J., Rietveld A.G., Van-Vugt A.C., Dijkmans B.A.C. Antioxidant intervention in rheumatoid arthritis: results of an open pilot study. Clin. Rheumatol. 2008;27:771–775. doi: 10.1007/s10067-008-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.H., Joobeur T., Dean R.A., Staub J.E. Cucurbits-genome mapping and molecular breeding in plants. Vegetables. 2007;5:375. [Google Scholar]
- Winter C.A., Risley E.A., Nuss G.W. Carrageenan-induced edema in hind paws of the rat as an assay for anti-inflammatory drugs. Exper. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Yesilada E., Tanaka S., Sezik E., Tabata M. Isolation of anti-inflammatory principles from the fruit juice of Ecballium elaterium. J. Nat. Prod. 1998;51:504–508. doi: 10.1021/np50057a008. [DOI] [PubMed] [Google Scholar]