Abstract
Allelochemicals are secondary metabolites which are not edible and can be used as growth regulators and bio-herbicides. The goal of current study was to assess allelopathic ability of Lantana camara (Sage-plant) flowers against weeds viz. Avena fatua (Wild oat), Euphorbia helioscopia (Sun-spurge), Chenopodium album (Goosefoot), Phalaris minor (Canary-grass), and Rumex dentatus (Knotweed). Bioassay analysis of three methanolic fractions of the Combiflash from L. camara was performed at 50%, 75% and 100% concentration using germination percentage parameters, inhibition of plumule and radicle size. The fraction II of Combiflash strongly suppressed all weeds with negligible effect on T. aestivum. Gas chromatography-mass spectroscopy was conducted for the fraction, and isolated compounds were used to perform bioassays. From fraction II GC–MS detected four methyl esters of allelopathic fatty acid viz. Methyl oleate, methyl palmitate, methyl stearate and methyl linoleate. The evaluation of physiological effects of the bioassay revealed substantial suppression of chlorophyll, antioxidant enzymes (superoxide, dismutase peroxidase) and protein material in all weeds by methyl palmitate. Bioassay activity and study of physiological parameters revealed that the effective bio-herbicidal compound in Lantana camara flowers is methyl palmitate. This is the first time that methyl palmitate (a fatty acid methyl ester) has been related to herbicidal activity in L. camara flowers. It is proposed that field studies based on hormesis research and the mechanism of action of this compound be carried out.
Keywords: Combiflash, Phytotoxicity, Weed management, Physiological parameters, Methyl palmitate
1. Introduction
Triticum aestivum (Wheat) is one of the important cereal crops. Many factors including delayed sowing, water scarcity, less fertilizers, pests, lack of healthier seed supply, and dry periods decrease the yield of wheat. Weeds interfere with crops by competing for light, nutrients and moisture which cause low quality crops and less crop production. An estimate shows that crop production can be improved by 37 per cent by proper weed management (Anwar et al., 2019a). Mechanical and cultural approaches for controlling weeds are inefficient, weather hinged, and laborious. Only the latest methods for weed control (use of herbicides, synthetic chemicals) were not up to the mark (Arafat et al., 2015). If improved weed control techniques are not adequately formulated, there could be greater wheat production losses (Khan et al., 2016). The wheat crop usually includes about 30 different weed species. 12–16 weed species are widespread in this distribution and cause losses of up to a remarkable economic threshold (Anwar et al., 2019b). Because of the limited resources and tracts available to farmers it seems very difficult to eradicate weeds completely in the region. Allelopathy is based on the fact that plants contain many chemicals that are harmful to the neighbouring species known as allelochemicals. Such chemical compounds are released into ecosystems by numerous processes including processes of exudation, leaching, and decomposition. Allelochemical substances may also be perfect agrochemicals.
Lantana camara (Verbenaceae) is an allelopathic plant. Allelochemicals are present in all parts of the shrub. When released in surrounding, these chemicals restrict germination of other species e.g. Lemna paucicostata, Morrenia odorata, Eichhornia crassipes, Commmelina benghalensis, Digitaria sanguinalis, Echinocloa colonum, Panicum psilopodium, Microcystis aeruginosa, Abutilon theophrasti, Lepidium virginicum, Cyclosorus dentatus, Amaranthus hybridus, Parthenium hysterophorus (Bais et al., 2006, Ambika et al., 2003, Kumar et al., 2011). The leaf, root and stem possess allelochemicals that suppress neighbouring plant germination and growth (Rusdy and Ako, 2017). Allelochemicals belong to a range of species, including phenolic, aromatic and alkaloid species, monoterpenes, triterpenes, sesquiterpenes, flavonoids, phenyl and iridoid ethanoid glycosides (Ved et al., 2018). Such compounds display growth-inhibiting effects on germination and growth of adjacent plants through fluctuating microenvironments (Mishra, 2015, Murugesan et al., 2016, Saha et al., 2018).
L. camara is a dreadful weed that has a major negative impact on biodiversity (Choyal and Sharma, 2011). The toxins lantadene A and B found in its leaves and flowers make it unfit for ruminant herbivory. Because of the allelopathic effect of its root leachate, this weed stunts neighboring plants' growth. When favorable conditions prevail, the seeds germinate. Pruning increases the density of the thicket. So far, almost every attempt to eradicate this plant has failed (Patel, 2011). So, management of this weed by utilization is required (Lakshmi and Sekhar, 2018). Recent studies have reported that L. camara improves soil quality by enriching it with nitrogen, exhibits termiticidal effect, acts as lignocellulosic substrate for cultivation of edible mushrooms, acts as potential insecticide and fumigant for grains storage against weevils, antifungal agent, and herbicide against water hyacinths. L. camara has bioactive ingredients exhibiting anticancer, anti-ulcerogenic, hypolipidemic, larvicidal and anti-inflammatory activity (Sharma et al., 2003).
Essential oils (EOs) can be used to successfully suppress weeds, according to recent research. These chemicals provide a viable commercial alternative for organic weed control. Fatty acid methyl esters (FAMEs) are used as adjuvants in a number of consumer products based on the herbicidal action of EOs (Wang et al. 2015). Since FAMEs have a strong affinity for fatty compounds, they can easily pass through the plant cuticle. When FAMEs are added to a tank mixture, the herbicidal effect of commercial herbicides is greatly increased (Synowiec et al., 2017). Jones has proposed a composition and method for destroying unwanted plants using fatty acids and fatty acid esters as herbicides and carriers for herbicides (2008). Such compositions may be used in areas of both desirable and undesirable plants to destroy the undesirable plants without damaging the desirable ones. FAMEs have also been shown to have antibacterial and antifungal properties (Sati et al., 2017).
Methyl palmitate is a fatty acid methyl ester with a nonpolar aliphatic carbon chain and a polar carboxyl group. It's a natural botanical compound found in a variety of plants (Qin et al., 2000, Goswami and Fernandes, 2003, Lin et al., 2005). A number of insects have been shown to use methyl palmitate as a semiochemical. Insect repellents containing methyl palmitate have been proposed. Methyl palmitate, on the other hand, appears to be healthy for vertebrates, as shown by its widespread use in food, medicinal, cosmetic, and industrial products (Wang et al., 2009). The amount of methyl palmitate that is safe and effective for humans has been confirmed to be in the range of 0.1–10 mg/kg body weight (Usha and Nazarine, 2003), Various phytophagous mites are poisoned by this selection. Human skin was only slightly affected by methyl palmitate, and fatty acids could be used as a complement to animal feeds. In conclusion, methyl palmitate is a promising botanical miticide that appears to be a good candidate for commercialization and agricultural use (Wang et al., 2009).
Keeping the facts in view, the current analysis was planned to test L. camara allelopathic ability against weeds viz. Rumex dentatus, Euphorbia helioscopia, Phalaris minor, Chenopodium album and Avena fatua.
2. Methods
The research was planned to assess the allelopathic ability of L. camara against Rumex dentatus, Euphorbia helioscopia, Chenopodium album, Phalaris minor and Avena fatua weeds. L. camara flowers were collected from the Rawalpindi district (latitude 33°36′N and longitude 73°02′E), Pakistan. The collected sample was dried at 30 °C in shade and pulverized with a heavy-duty blender (2 mm mesh size). Ground sample (300 g) was macerated in 2000 ml methanol. An aliquot of rotary evaporated methanol extract (15 g) on the Combiflash column (Combiflash® Rf + by Teledyne Isco) gave us three fractions. The allelopathic ability of three Combiflash fractions was tested against weeds (Rumex dentatus, Euphorbia helioscopia, Chenopodium album, Phalaris minor and Avena fatua) using germination rate, radicle, and plumule length parameters at 100%, 75%, 50% concentration. An aliquot (15 ml) was poured on 25 g of soil per petri plate using methanol as control for each concentration of the Combiflash fractions. Ten seeds of each research weed species were added to each Petri dish. The petri plates were set at 25 °C for 15 days in growth chamber (NTS, MI-25S). With each plant, the percentage of germination, length of the plumule and radicle was determined. Five replicates of the experiments were carried out. Completely randomized design was applied in STATISTIX v. 9 and ANOVA along Fisher's LSD test was applied for means separation.
Purification of active Combiflash fraction II by C-18. An aliquot (0.2 g) of fraction II was chromatographed on a column of 2x60 cm silica gel (Silica gel mesh 70–230, Merck, Germany) eluted with (n)-hexane that had collective ethyl acetate amounts [20 percent per step, 300 ml per step]. On thin layer chromatography (Silica gel 60 GFz54; Merck) with a mixture of chloroform and acetic acid (90:10 v/v), the active fraction was eluted from the silica gel column (Ezhilan and Neelamegam, 2012). Residue dissolved in 20 per cent aqueous methanol (5 ml v/v) was loaded on C18 Sep-Pak cartridges in reverse phase (Waters Co., Milford, USA). High performance liquid chromatography was used for residue purification using 20, 40, 60 and 80 percent aqueous methanol.
Gas Chromatography-Mass Spectrometry (GC–MS). On Thermo GC-TRACE ultra ver. 2.2 (film thickness: 0.25 μm; DB 5-MS capillary standard non-polar column 30 Mts, ID: 0.25 mm) GC–MS was performed (Thermo Scientific Co.). The results were evaluated using National Institute of Standards and Technology (NIST) database mass-spectra.
2.1. Bio-Assay effect of isolated FAMEs on seed germination and seedling growth
In mother solvent (methanol), the isolated compounds (methyl oleate, methyl stearate, methyl palmitate, and methyl linoleate) were dissolved. Ten seeds of each test species were placed on filter paper (Whatman No. 1) in petri dishes (85 mm diameter). Assays for isolated compound were performed at 10 μM, 20 μM, 30 μM, 40 μM, and 50 μM. Wrapped with aluminium foil, the petri dishes were incubated in the dark at 28 °C. After five days, germination, root and shoot-lengths were collected. All of the parameters were displayed as percentages. Non treated seeds were used as control. The student's t-test analysed statistical differences between the treatments to determine the statistical significance of disparity between two sample means.
Determination of chlorophyll contents. One gram of the leaf material was ground in liquid nitrogen for test weed species followed by addition and centrifugation of 15 ml of 80 per cent acetone (extraction solution) (8,000 rpm). Readings were reported at 645 nm and 662 nm using a spectrophotometer (UV- 3802, UNIC, China) using 80 per cent blank acetone. The chlorophyll a, chlorophyll b and complete chlorophyll concentrations have been determined (Hanh et al., 2016):
Quantification of peroxidase and superoxide dismutase contents. For each test weed species, 0.2 g of leaf material was homogenized for 5 min at 4 °C with 1 ml of 0.2 M perchloric acid, and then centrifuged (10,000 rpm) for 7 min. 0.2 M sodium hydroxide was used to change the supernatant's pH to 7.5. An aliquot (100 μl) moved through a column of 0.4 ml at Dowex AG. The eluate was used for H2O2 assay. In 0.1 M ethylene diamine tetra acetic acid, 0.02 M sodium phosphate buffer (7.8 pH), 4% polyvinyl pyrrolidone and 0.2% Triton X-100, an aliquot (0.2 g) of test plant leaves was homogenised. Solutions have been filtered and centrifuged for 20 min (10,000 rpm). Superoxide dismutase was measured using standard method for separating isoenzymes from superoxide dismutases. On 7.5 per cent acrylamide gel, non-denaturing polyacrylamide gel electrophoresis was performed. Superoxide dismutase activity reduction protocol for Nitroblue Tetrazolium was introduced (Zuo et al., 2012).
Determination of protein contents. 1 g of leaf material was combined with 10 ml of 2 per cent anhydrous sodium carbonate dissolved in 0.1 M sodium hydroxide for each test weed. Protein suspension (0.5 ml) was combined with 0.5 ml reagent (1 ml 0.5% Copper sulphate, 1 ml 1% sodium potassium tartate, and 48 ml 2% anhydrous sodium carbonate dissolved in 0.1 M sodium hydrooxide). The mixture had been permitted to stand 15 min at room temperature. Folin-Ciocalteau (0.5 ml) reagent was combined with solution and left to stand at room temperature for 30 min. Protein solution absorbance was measured on spectrophotometer at 700 nm (UV-3802, UNIC, China) (Javed, 2011).
3. Results
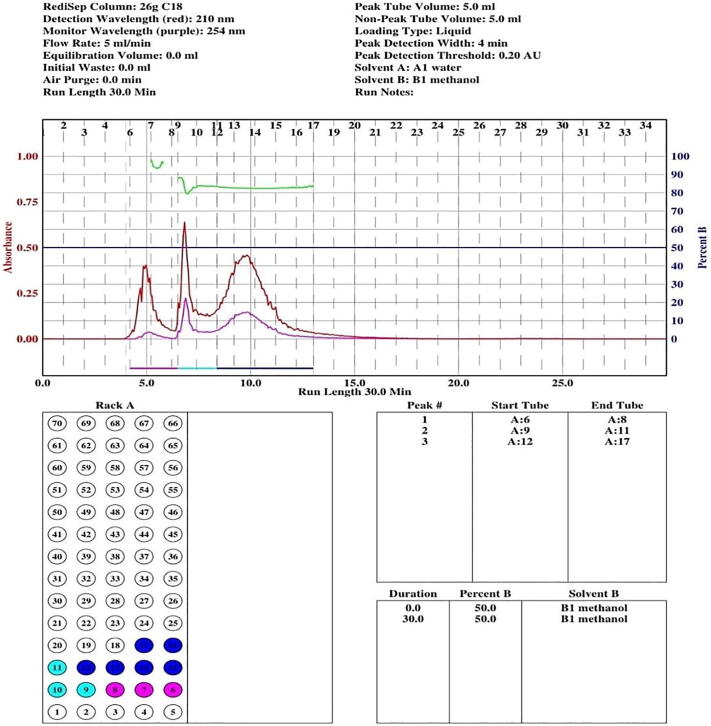
Three fractions were constructed from L. camara flowers methanol extract via Combiflash ® Rf + from Teledyne Isco (the chromatogram is shown in Fig. 1). Fraction I consisted of test tubes6-8, fraction II consisting of test tubes 9–11 and fraction III consisted of test tubes12-17. The fractions were collected using the rotary vacuum evaporator Buchi-Rotavapor R-300 to remove solvent and subsequent aqueous extract was lyophilized in vacuum (Stellar ® Tray Type Freeze Dryer). Finally, an aliquot was obtained of 301 mg (fraction I), 245 mg (fraction II) and 154 mg (fraction III). These fractions were tested against selected weeds for allelopathic bioassays. The three fractions of Combiflash impacted germination and seedling growth parameters of weeds with no noticeable effect on T. aestivum as shown in Fig. 2.
Fig. 1.
Three methanolic Combiflash fractions of L. camara flowers.
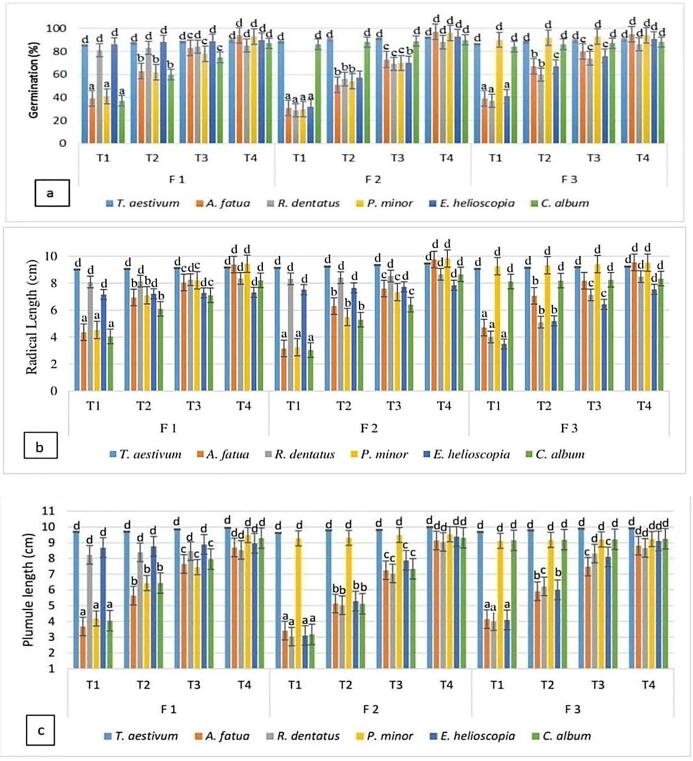
Fig. 2.
Phytotoxicity of Combiflash fractions (T1 = 100%, T2 = 75%, T3 = 50% and T4 = control).
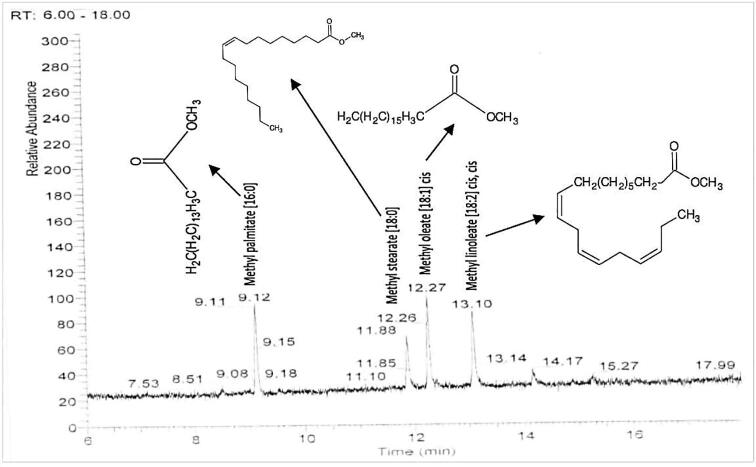
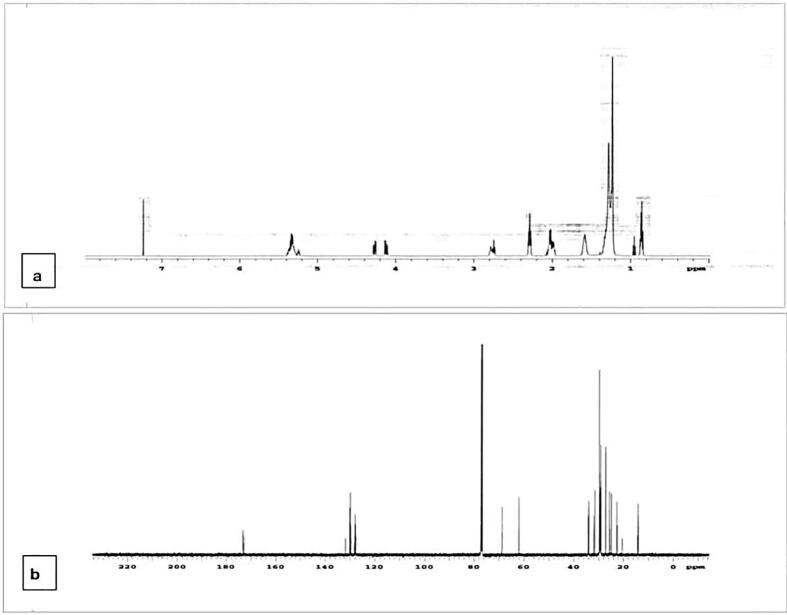
For fraction I, P. minor, C. album and A. fatua exhibited 55%, 57% and 58% germination inhibition respectively while germination inhibition of R. dentatus and E. helioscopia remained unchanged. Maximum germination was noted in E. helioscopia and R. dentatus (98 percent), while A. fatua displayed modest germination, i.e. 42 percent. P. minor, C. album and A. fatua demonstrated a radicle reduction of 51 percent, 52 percent and 54 respectively. For E. helioscopia and R. dentatus the maximum radicle length (98 per cent) was observed. Likewise, for A. fatua, radicle length of 46 per cent was found. C. album, P. minor and A. fatua reported a reduction of 56%, 56.5% and 57%, while E. helioscopia and R. dentatus remained unchanged. It was observed that E. helioscopia and R. dentatus showed the maximum (98 per cent) plumule growth. For A. fatua, the least plumule length (49 per cent) was observed. Seed germination of E. helioscopia, R. dentatus, A. fatua and P. minor was affected as 66 percent, 67 percent, 68 percent, and 69 percent, respectively, while C. album remained unchanged. The highest germination was noted for C. album (96 percent), while P. minor was the lowest, i.e. 31 percent. P. minor, A. fatua and C. album showed substantial suppression of the radicle length by 68%, 67% and 55% while E. helioscopia and R. dentatus remained unaffected. E. helioscopia and R. dentatus showed the maximum radicle length (97 per cent). The experiments for A. fatua reported slightly lower radicle length (32 per cent). Fraction II significantly repressed the plumule length of A. fatua, C. album, E. helioscopia and R. dentatus by 66%, 66%, 67%, and 69% respectively, while P. minor remained unaffected. P. minor was found to have the maximum plumule length (95 percent). R. dentatus was most prone in terms of total plumule length (31 per cent). Fraction III substantially suppressed E. helioscopia, R. dentatus and A. fatua germination by 55 percent, 57 percent and 59 percent compared to control while P. minor and C. album remained unchanged. For C. album and P. minor the highest germination (96 percent) was noted while for A. fatua it was lowest i.e. 41 percent. The bioassays for A. fatua reported slightly lower radicle length (32 per cent). Fraction II significantly repressed the plumule length of A. fatua, C. album, E. helioscopia and R. dentatus by 66%, 66%, 67%, and 69% respectively, while P. minor remained unaffected. P. minor was found to have the maximum plumule length (95 percent). R. dentatus was most prone with plumule length of 31 per cent. Fraction III substantially repressed E. helioscopia, R. dentatus and A. fatua germination by 55 percent, 57 percent and 59 percent compared to control while P. minor and C. album remained unchanged. For C. album and P. minor the highest germination (96 percent) was noted while for A. fatua, it was lowest i.e. 41 percent. For fraction II, highest suppression effects were noted, therefore, its characterisation was done. The GC–MS chromatogram and NMR analysis of fraction II showed four peaks with different retention periods (Fig. 3, Fig. 4). Four allelopathic fatty acid methyl esters (FAMEs) were found, i.e., methyl palmitate (R.T: 9.13), methyl stearate (R.T: 11.87), methyl oleate (R.T: 12.27), and methyl linoleate (R.T: 13.08).
Fig. 3.
The GC–MS spectrum of Combiflash fraction II.
Fig. 4.
(a): 1H NMR (b): 13C NMR of Combiflash fraction II.
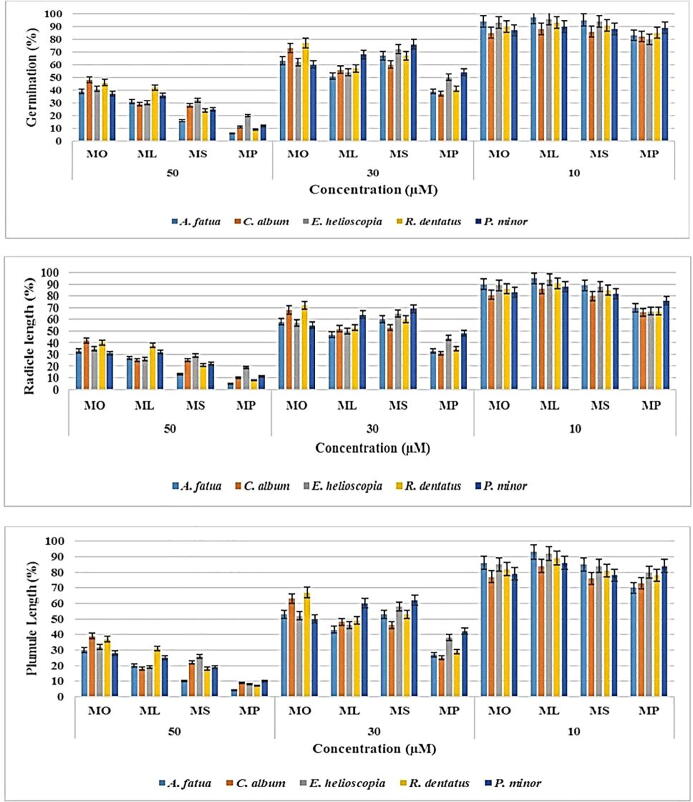
Pre-emergence bioassay with allelopathic fatty acid methyl esters (FAMEs): The seed germination of Avena fatua, Chenopodium album, Euphorbia helioscopia, Rumex dentatus and Phalaris minor was inhibited by methyl oleate, methyl stearate, methyl palmitate and methyl linoleate in a dose-dependent manner.
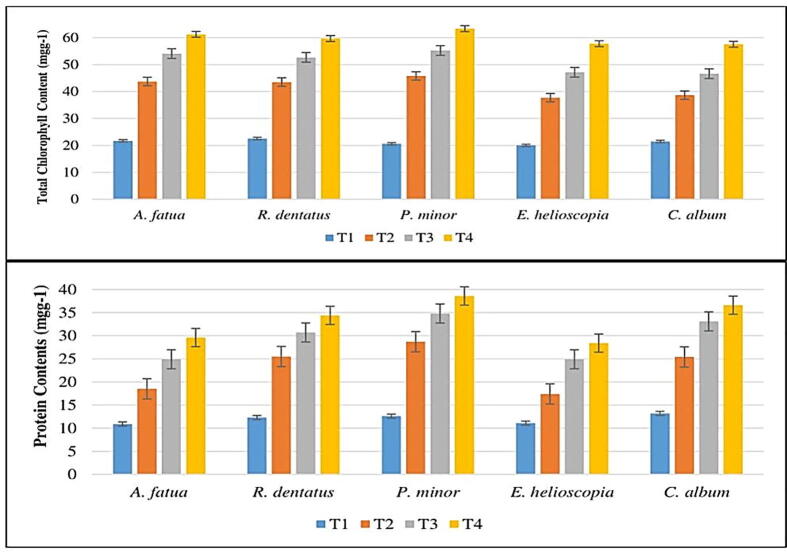
Methyl palmitate has demonstrated the highest inhibition effects; physiological parameters (chlorophyll a & b, peroxidase content, and protein content) have also been tested for this compound. The effect of methyl palmitate on the content of chlorophyll a & b and total chlorophyll content is shown in Fig. 5. The chlorophyll content of P. minor, A. fatua, E. helioscopia, C. album and R. dentatus was substantially suppressed by 66%, 64%, 63%, 62% and 60%. For C. album, R. dentatus, A. fatua, E. helioscopia, and P. minor chlorophyll b content was suppressed by 63%, 64%, 66%, 67%, and 68% respectively. For P. minor, E. helioscopia, A. fatua, C. album, and R. dentatus chlorophyll content was suppressed by 67%, 66%, 65%, 63%, and 62% respectively.
Fig. 5.
Phytotoxicity of FAMs (Methyl oleate, Methyl palmitate, Methyl stearate and Methyl linoleate).
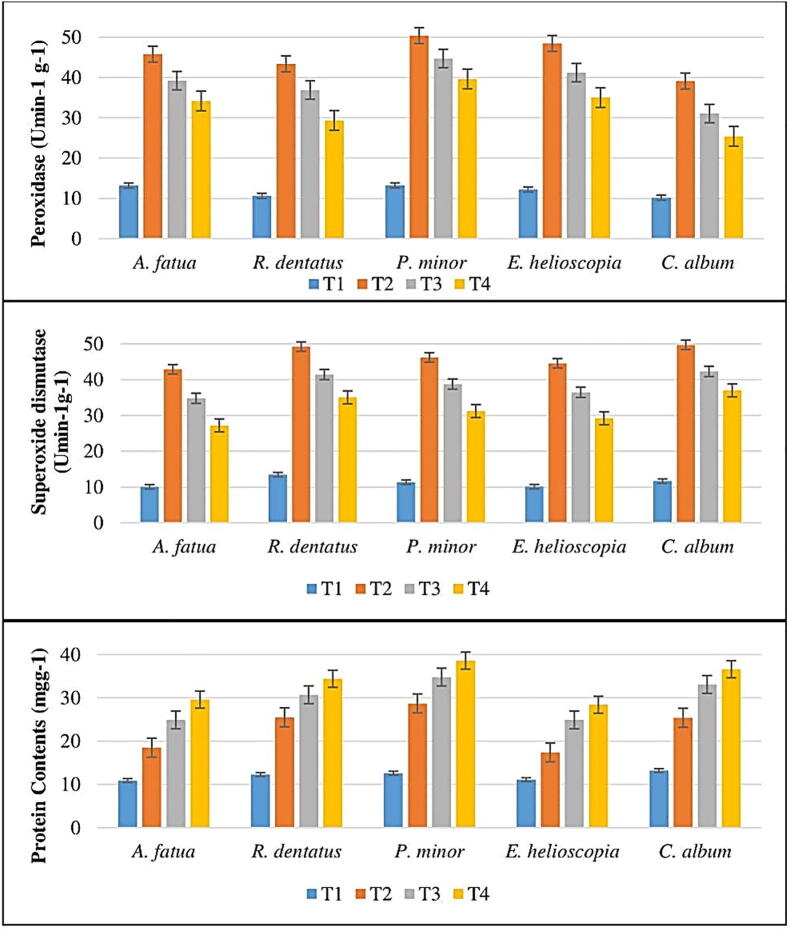
The role of antioxidant enzymes and the suppression of protein content by methyl palmitate is shown in Fig. 6. C. album, A. fatua, R. dentatus, E. helioscopia and P. minor peroxidase content were suppressed by 60 percent, 61 percent, 64 percent, 65 percent and 67 percent respectively at T1, while for C. album, R. dentatus, E. helioscopia, A. fatua and P. minor peroxidase content was suppressed by 46 percent, 52 percent, 62 percent, 66 percent and 72 percent at T2. At T1, 62 percent, 63 percent, 64 percent, 66 percent, and 68 percent suppression of superoxide dismutase content was noted for R. dentatus, A. fatua, P. minor, E. helioscopia, and C. album respectively. Superoxide dismutase was 42 percent, 47 percent, 52 percent, 60 percent and 66 percent respectively at T2 for A. fatua, E. helioscopia, P. minor, R. dentatus and C. album. Methyl palmitate substantially suppressed E. helioscopia, A. fatua, R. dentatus, C. album and P. minor protein content by 61%, 63%, 64%, 67% and 68% respectively (Fig. 7).
Fig. 6.
(a) Chlorophyll content and (b) protein contents of weeds treated by methyl oleate [T1 = 100%, T2 = 75%, T3 = 50%, T4 = control]
Fig. 7.
(a) Peroxidase contents (b) superoxide dismutase contents (c) protein contents of weeds treated by methyl palmitate.
4. Discussion
Plant allelopathy can have positive effects, e.g., in agricultural management, such as weed control, crop restore or crop protection. Allelochemicals may possibly be used as regulators for production, insecticides, herbicides and crop protection products. Here we appraised L. camara methanol extract from flowers as a source of natural herbicide against selected weed species viz. A. fatua, Chenopodium album, Phalaris minor, Euphorbia helioscopia and Rumex dentatus. The degree of phytotoxicity was dependent upon concentration. Past studies have reported that extract of methanol from L. camara supress Raphanus sativus, Phaseolus mungo, Cicer arietinum, Cucumis sativa, Brassica juncea, Eichhornia crassipes, Phaseolus mungo and Microcystis aeruginosa (Mishra, 2015). In our case, Combiflash fraction II from L. camara flowers had the greater inhibitory ability than fractions 1 and 3 (see section MM for the fractionation procedure). Methanol extracts of L. camara flowers depressed growth parameters, protein content, chlorophyll content and antioxidant enzymes in test species. Reduction of chlorophyll by phytochemicals is possibly due to degradation of chlorophyll, retardation in chlorophyll production along with photosystem II malfunction. Allelochemicals reduce chlorophyll content by 52% −62% in young leaves and 72%-92% in mature leaves (Biljana and Kragujevac, 2015, Anwar et al., 2018). In addition, phytochemicals produce reactive oxygen species (ROS) which cause Ca2+ signalling cascade leading to gene expression manipulation and shoot death (Nekonam et al., 2014). The effects of cinnamic acid on the ability of the enzyme to scavenge ROS, the rate of generation of ROS and subsequent growth in cucumber were recorded. Allelochemical stress is linked to ROS production and oxidative stress. Phytotoxicity enhances the potential for SOD which causes H2O2 deposition and increased membrane peroxidation (Zhang et al., 2018). Lantana leaf extract's toxic potential is probably due to oxidative stress. L. camara leaf extract substantially suppressed POD activity. The POD is linked to biochemical and physiological activities such as cell formation, fruit production, ethylene biosynthesis and growth, and the response to environmental toxins and stresses (Zaytseva and Neumann, 2016). In Lycopersicon esculentum, Phaseolus vulgaris and Zea mays the substantial reduction in protein content is stated earlier by the leaf methanol extract of L. camara (Zuo et al., 2012). Allelochemicals inhibit cell division and cellular processes including enzyme activity, membrane permeability, respiratory and photosynthetic ETC retardation, ion uptake, protein damage, and cell death due to DNA (Li et al., 2018, Maiti et al., 2010). Allelochemicals change enzyme functionality. Reduction of the permeability of the cell membrane, protein formation, gibberellins and indole acetic acid caused by allelochemicals leads to reduced mitotic activity and growth rate (Alcântara et al., 2017).
In recent years, analytics technology has enabled the identification of minute amounts of allelochemicals. GC–MS study of L. camara flowers methanol extract (Combiflash fraction II) classified four methyl esters of fatty acids (FAMEs) as possible allelochemicals viz. Methyl linoleate, methyl stearate, methyl palmitate and methyl oleate. There is no earlier evidence of allelopathic ability of these compounds. From other plant species, including T. aestivum, Cucumis sativus and Echinochloa crusgalli, esters and long fatty acids with allelochemical properties are reported earlier (Cheng et al., 2016). At a concentration of 1000 μg/mL, methyl oleate and methyl linoleate are reported to inhibit radicle by 80% and plumule by 60%. Alpha-linolenic acid and linoleic acid (essential fatty acids) have been reported to be allelochemical compounds from Typha domingensis leachates (Nea et al., 2017). However, no phytotoxic activity of methyl palmitate from L.camara flowers has ever been identified. These compounds may have inhibitory effects on ATP generation and electron transport in chloroplasts and mitochondria, similar to other phytotoxins (Qureshi et al., 2021). Allelochemicals usually work through pathways that synthetic compounds don't, making natural compounds a potential source of new herbicide leads. It is proposed that the action mechanism of an isolated compound be investigated as only a few of the hundreds of allelochemicals known have had their mode of action determined.
5. Conclusion
Allelochemicals have wide potential for enhancing crop produce, defense and biocontrol. In the current study, allelopathic potential of methanol extract from flowers of Lantana camara was evaluated against selected weeds viz. Phalaris minor, Chenopodium album, Avena fatua and Rumex dentatus. Results provide evidence that methyl palmitate isolated from L. camara flowers has herbicidal potential. Studies are suggested on the degree and extent of the phytotoxicity of isolated compounds in agronomic conditions at different stages of development.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alcântara B.K., Rizzi V., Salete A., Gaziola A.RA. Soluble amino acid profile, mineral nutrient and carbohydrate content of maize kernels harvested from plants submitted to ascorbic acid seed priming. Anais da Academia Brasileira de Ciências. 2017;89:695–704. doi: 10.1590/0001-3765201720160399. [DOI] [PubMed] [Google Scholar]
- Ambika S.R., Poornima S., Palaniraj R., Sati S.C., Narwal S.S. Allelopathic plants: Lantana camara. Allelopathic J. 2003;12:147–162. [Google Scholar]
- Anwar T., Ilyas N., Qureshi R., Malik M.A. Allelopathic potential of Carica papaya against selected weeds of wheat crop. Pak J Bot. 2019;51:279–287. [Google Scholar]
- Anwar T., Ilyas N., Qureshi R., Munazir M., Rahim B.Z., Qureshi H., Kousar R., Maqsood M., Abbas Q., Bhatti M.I., Panni M.K. Allelopathic potential of Pinus roxburghii needles against selected weeds of wheat crop. Appl Ecol Env Res. 2019;7:1717–1739. [Google Scholar]
- Anwar T., Panni M.K., Khalid S., Qureshi H. Allelopathic management of noxious weeds in Helianthus annuus, Zea mays and Triticum aestivum by selected plants. Pak J Weed Sci Res. 2018;24:257–265. [Google Scholar]
- Arafat Y., Khalid S., Lin W., Fang C., Sadia S., Ali N., Azeem S.J. Allelopathic evaluation of selected plants extract against broad and narrow leaves weeds and their associated crops. Acad J Agric Res. 2015;3:226–234. [Google Scholar]
- Bais H.P., Weir T.L., Perry L.G., Gilorys V.JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Rev Plant Biol. 2006;57:233–266. doi: 10.1146/annurev.arplant.57.032905.105159. [DOI] [PubMed] [Google Scholar]
- Biljana M.B., Kragujevac D.Z.J. Allelopathic relations of selected cereal and vegetable species during seed germination and seedling growth. J Sci. 2015;37:135–142. [Google Scholar]
- Cheng F., Cheng Z., Meng H., Tang X. The garlic allelochemical diallyl disulfide affects tomato root growth by influencing cell division, phytohormone balance and expansin gene expression. Front Plant Sci. 2016;7:1199. doi: 10.3389/fpls.2016.01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choyal R., Sharma S.K. Evaluation of allelopathic effects of Lantana camara (Linn) on regeneration of Pogonatum aloides in culture media. Asian J Plant Sci Res. 2011;1:41–48. [Google Scholar]
- Ezhilan B.P., Neelamegam R. GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacognosy Res. 2012;4:11–14. doi: 10.4103/0974-8490.91028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U, Fernandes N (2003) Bioactivity of methyl palmitate obtained from a mangrove plant Salvadora persica L. Patents granted during 2003-2004. (http://www. nio.org/jsp/patent2.jsp).
- Hanh N.T., Hoang N.Y., Thao P.T.P. Change of chlorophyll and vitamin c in green peas (Pisum sativum) during thermal processing. Vietnam J Agri Sci. 2016;14:1068–1074. [Google Scholar]
- Javed K. Impact of allelopathy of sunflower (Helianthus annuus L.) roots extract on physiology of wheat (Triticum aestivum L.) Afr J Biotechnol. 2011;10:14465–14477. [Google Scholar]
- Jones AL (2008) Fatty acids and fatty acid esters as herbicidal agents and carriers. United States Patent Application Publication. Pub. No.: US 2008/0153708 A1.
- Khan R., Khan M.A., Uddin S., Ali S., Ilyas M. Bioherbicidal potential of plant extracts against weeds of wheat crop under agro-climatic conditions of Peshawar-Pakistan. Pak J Weed Sci Res. 2016;22:285–294. [Google Scholar]
- Kumar M., Singh S., Singh S. In vitro morphogenesis of a medicinal plant–Aloe vera L. Asian journal of plant science and Research. 2011;1:31–40. [Google Scholar]
- Lakshmi C.S., Sekhar C.C. Impact, management and uses of Lantana camara – A noxious weed. Bull Env Pharmacol Life Sci. 2018;7:170–180. [Google Scholar]
- Li X., Zhang X., Wu Y., Li B., Yang Y. Physiological and biochemical analysis of mechanisms underlyingcadmium tolerance and accumulation in turnip. Plant Diversity. 2018;40:19–27. doi: 10.1016/j.pld.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.R., Xu F., Li Z.H., Yang L.H., Li B., Deng G.G. Analysis of volatile composition in the perfume of Ficus carica Lam. produced by fermentation. Biotechnology. 2005;15:54–56. [Google Scholar]
- Maiti PP, Bhakat RK, Bhattacharjee (2010) An evaluation of allelopathic potential of an obnoxious weed using mung bean as a standard bioassay material. Int J Sci Nat 1: 236–241.
- Mishra A. Allelopathic properties of Lantana camara. Int Res J Basic Clin Stud. 2015;3:13–28. [Google Scholar]
- Murugesan S., Senthilkumar N., Suresh B.D., Rajasugunasekar D. Chemical constituents and toxicity assessment of the leaf oil of Lantana camara Linn from Tamilnadu regions. Asian J Plant Sci Res. 2016;6:32–42. [Google Scholar]
- Nea F., Tanoh E.A., Yapi T.A., Garcia G., Tomi F., Tonzibo Z.F. Chemical investigation on leaf, flower and fruit oils of Lantana camara from côte d’ivoire. Nat Prod Commun. 2017;12:607–610. [PubMed] [Google Scholar]
- Nekonam M.S., Kraimmojeni H., Sharifnabi B., Razmjoo J., Amini H., Bahrami F. Assessment of some medicinal plants for their allelopathic potential against redroot pigweed (Amaranthus retroflexus) J Plant Prot Res. 2014;54:90–95. [Google Scholar]
- Patel S.A. Weed with multiple utility: Lantana camara. Rev Environ Sci Biotechnol. 2011;10:341–351. [Google Scholar]
- Qin B., Gao H.X., Wang H.Q., Lu R.H., Wang M. Chemical constituent essential oil from Delavaya yunnanensis Franch J Instrum Anal. 2000;19:1–4. [Google Scholar]
- Qureshi H., Anwar A., Ali Q., Haider M.Z., Habib N., Fatima S., Waseem M., Bibi Y., Arshad M., Adkins S.W. Isolation of natural herbicidal compound from Lantana camara. Int J Environ Anal Chem. 2021;101:631–638. [Google Scholar]
- Rusdy M., Ako A. Allelopathic effect of Lantana camara and Chromolaena odorata on germination and seedling growth of Centroma pubescens. Int J Appl Environ Sci. 2017;12:1769–1776. [Google Scholar]
- Saha D., Marble S.C., Pearson B.J. Allelopathic effects of common landscape and nursery mulch materials on weed control. Front Plant Sci. 2018;9:733. doi: 10.3389/fpls.2018.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sati A., Sati S.C., Sati N., Sati O.P. Chemical composition and antimicrobial activity of fatty acid methyl ester of Quercus leucotrichophora fruits. Nat Prod Res. 2017;31:713–717. doi: 10.1080/14786419.2016.1217202. [DOI] [PubMed] [Google Scholar]
- Sharma P.K., Ladha J.K., Verma T.S., Bhagat R.M., Padre A.T. Rice-wheat productivity and nutrient status in a lantana- (Lantana spp.) amended soil. Biol Fertil Soils. 2003;37:108–114. [Google Scholar]
- Synowiec A., Halecki W., Wielgusz K., Byczyńska M., Czaplicki S. Effect of fatty acid methyl esters on the herbicidal effect of essential oils on corn and weeds. Weed Technol. 2017;31:301–309. [Google Scholar]
- Usha G, Nazarine F (2003) Bioactivity of methyl palmitate obtained from a mangrove plant Salvadora persica L. (http://www.freepatentsonline.com/6638546.html).
- Ved A., Arsi T., Prakash O., Gupta A. A review on phytochemistry and pharmacological activity of Lantana camara Linn. Int J Pharm Sci Res. 2018;9:37–43. [Google Scholar]
- Wang Y.N., Wang H.X., Shen Z.J., Zhao L.L., Clarke S.R., Sun J.H., Du Y.Y., Shi G.L. Methyl Palmitate, an acaricidal compound occurring in green walnut husks. J Econ Entomol. 2009;102(1):196–202. doi: 10.1603/029.102.0128. [DOI] [PubMed] [Google Scholar]
- Wang W., Wei H., Du Z., Tai X., Wang G. Formation and characterization of fully dilutable microemulsion with fatty acid methyl esters as oil phase. ACS Sust Chem Engin. 2015;3:443–450. [Google Scholar]
- Zaytseva O., Neumann G. Carbon nanomaterials: production, impact on plant development, agricultural and environmental applications. Chem Boil Technol Agric. 2016;3:17. [Google Scholar]
- Zhang W., Lu L.W., Hu L.Y., Cao W., Sun K., Sun Q.B., Siddikee A., Shi R.H., Dai C.C. Evidence for the involvement of auxin, ethylene and ROS signaling during primary root inhibition of Arabidopsis by the allelochemical benzoic acid. Plant and Cell Physiol. 2018;59:1889–1904. doi: 10.1093/pcp/pcy107. [DOI] [PubMed] [Google Scholar]
- Zuo S.P., Ma Y.Q., Ye L.T. Invitro assessment of allelopathic effects of wheat on potato. Allelopathy J. 2012;30:1–10. [Google Scholar]
Further Reading
- Anwar T., Khalid S., Mazhar R., Qureshi H., Rashid M. Herbicidal potential of selected species to overcome weed infestation in Triticum aestivum, Zea mays and Helianthus annuus. Pak J Weed Sci Res. 2017;23:49–63. [Google Scholar]
- Anwar T., Khalid S., Saeed M., Mazhar R., Qureshi H., Rashid M. Allelopathic interference of leaf powder and aqueous extracts of hostile weed: Parthenium hysterophorus (Asteraceae) Sci Int. 2016;4:86–93. [Google Scholar]
- Gallardo-Williams M.T., Geiger C.L., Pidala J.A., Martin D.F. Essential fatty acids and phenolic acids from extracts and leachates of southern cattail (Typha domingensis P.) Phytochem. 2002;59:305–308. doi: 10.1016/s0031-9422(01)00449-6. [DOI] [PubMed] [Google Scholar]
- Hossain M.K., Alam M.N. Allelopathic effects of Lantana camara leaf extract on germination and growth behavior of some agricultural and forest crops in Bangladesh. Pak J Weed Sci Res. 2010;16:217–226. [Google Scholar]
- Landon J., Rohowetz K.JG., Koulen P. Reactive oxygen species-mediated damage of retinal neurons: drug development targets for therapies of chronic neurodegeneration of the retina. Int J Mol Sci. 2018;19:3362. doi: 10.3390/ijms19113362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler K., Buschmann C. Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad E.R., Acree T.E., editors. Handbook of Food Analytical Chemistry. John Wiley and Sons; New Jersey: 2001. pp. 171–179. [Google Scholar]
- Mumtaz M.M., Al-Zuaidy M.H., Hamid A.A., Danish M., Akhtar M.T., Mukhtar H. Metabolite profiling and inhibitory properties of leaf extracts of Ficus benjamina towards α-glucosidase and α-amylase. Int J Food Prop. 2018;21:1560–1574. [Google Scholar]
- Phung T.T., Xuan T.D., Anh T.T.T., Van T.M., Ahmad A., Elzaawely A.A., Khanh T.D. Weed suppressing potential and isolation of potent plant growth inhibitors from Castanea crenata Sieb. et Zucc. Molecules. 2018;23:345. doi: 10.3390/molecules23020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M.E.A., Silva N.L., Siqueira D.E.P., Santos D.F.J.L., Lima L.A.R.S. Allelopathic effect of fatty acid methyl esters from corn and sunflower oils. Biochem Biotech Rep. 2013;2:44–48. [Google Scholar]
- Zheng H.H.C.Q., Xu Q.Y., Yang J.N., Zhan Y.W., Lei Y.R. Inference of allelopathy about Spartina alterniflora to Scirpus mariqueter by effects of activated carbon on soil. Procedia Environmental Sciences. 2011;10:1835–1840. [Google Scholar]