Abstract
Santolina chamaecyparissus is an important medicinal plant growing in the Mediterranean region and has been reported as a potent anti-inflammatory, antibacterial, antioxidant, and antifungal agent. The purpose of the current research is to identify the chemical constituents in ethyl acetate extract (EAE) from the leaves of S. chamaecyparissus, and to evaluate antidiabetic, and anticancer activity. Chemical constituents of EAE were identified by GC-MS, and the antidiabetic activity was evaluated by α-glucosidase inhibition assay. The anticancer activity was assessed by Epidermal Growth Factor Receptor (EGFR) expression in human breast cancer cell line (MCF7) by using quantitative RT-PCR method. GC-MS analysis of EAE of S. chamaecyparissus yielded 44 compounds. Tetrapentacontane (27.15%), eicosyl acetate (8.40%), 2-methylhexacosane (6.87%), and n-pentadecanol (5.44%) were found as major chemical constituents. The EAE of S. chamaecyparissus showed concentration dependant inhibition of α-glucosidase enzyme and the IC50 value (IC50 110 ± 4.25 µg/mL) was found comparable with standard acarbose (IC50 105 ± 3.74 µg/mL). The real-time qRT-PCR results showed that the EGFR protein (bcl-2) in human breast cancer cell line (MCF7) was negatively expressed with a value of −0.69297105 after treatment with EAE (100 µg/mL). The study results are suggesting the possible use of S. chamaecyparissus in the management of diabetes, and human breast cancer.
Keywords: Santolina chamaecyparissus, Diabetes, α-glucosidase, EGFR, Human breast cancer, GC-MS
1. Introduction
Medicinal plants are the major source of remedies for the treatment of chronic human ailments including diabetes, cancer, cardiovascular complications, etc. In recent years, natural products play a very important role in drug discovery for life-threatening diseases (Verpoorte, 2000). Natural drugs derived from medicinal plants are considered safe and effective compared to synthetic modern drugs, based on long history of use by humans as food and medicine (Newman and Cragg, 2016). Medicinal plants showed great promise as sources of novel anticancer drugs such as vincristine, vinblastine, topotecan, irinotecan, docetaxel, paclitaxel, etc. (Farnsworth et al., 1985). Even the discovery of approved and widely used antidiabetic drug, metformin came from the traditional approach of using Galega officinalis (Grover et al., 2002). Current therapy to alleviate cancer, and metabolic disorders such as diabetes mellitus, cardiovascular complications are not optimal and thus efforts have been made to develop effective and better drugs from natural sources (Newman et al., 2003).
Santolina (Asteraceae) species are found throughout the Mediterranean and European region. The most common species of the Santolina genus are S. viridis Wild (South of France, and North of Spain), S. africana Lag. (Iberian Peninsula) and S. chamaecyparissus L. (Synonym: Ormenis fricana). Santolina chamaecyparissus has been reported as anti-inflammatory (Cuellar et al., 1998, Sala et al., 2000); antioxidant and antimicrobial (Nouasri et al., 2015, Djeddi et al., 2012); anticandidal (Suresh et al., 1997); antibacterial, and antifungal (Salah-Fatnassi et al., 2017); CNS depressant, and anti‐cholinergic (Giner et al., 1988). Phytochemical investigations of essential oil of S. chamaecyparissus by GC-MS identified several phytochemicals, the most abundant ones belong to mono and sesqui-terpenes, the major constituents include camphor, cubenol, p-cymene, sabinene, 1,8-cineole, α-phellendrene, β-eudesmol and terpinene-4-ol (Garg et al., 2001, Perez-Alonso and Velasco-Negueruela, 1992, Derbesy et al., 1989). In the present research work, the chemical composition of ethyl acetate extract from leaves of S. chamaecyparissus was determined by GC-MS. The present study was also designed to evaluate S. chamaecyparissus extract efficacy in the treatment of diabetes mellitus via α-glucosidase enzyme inhibition. Further, the anticancer activity was assessed by evaluation of EGFR protein expression using quantitative RT-PCR technique.
2. Materials and methods
2.1. Plant materials and chemicals
The fresh S. chamaecyparissus L. leaves (500 g) were collected from the Northern region of Saudi Arabia. The authenticity of S. chamaecyparissus leaves (Voucher number: PL/2020-21/008) was ascertained by Pharmacognocist Dr. Abuzer Ali, College of Pharmacy, Taif University. Anticancer cell line study was carried out by Trichy Research Institute of Biotechnology Pvt. Ltd., Trichy, Tamil Nadu. The MCF-7 (human breast cancer) cell line was purchased from NCCS Pune, India. SUPER- SCRIPT™II RNase H- Reverse Transcriptase was received from Gibco (USA). Human EGFR and β-actin forward and reverse primers were purchased from Xcelris Pvt. Ltd. India (Table 1). The α-glucosidase enzyme was brought from Subra Scientific Company Chennai, India.
Table 1.
Primers used in EGFR expression study.
| Genes | Directions | Sequence (5́ – 3́) | TM | Product size |
|---|---|---|---|---|
| Human EGFR |
Forward | 5′-GGCACTTTTGAAGATCATTTTCTC-3′ | 7.06 | 163 |
| Reverse | 5′-CTGTGTTGAGGGCAATGAG-3′ | |||
| Human β-actin |
Forward | 5′-ATCGTGCGTGACATTAAGGAGAAG-3′ | 61.5 | 179 |
| Reverse | 5′-AGGAAGGAAGGCTGGAAGAGTG-3′ |
2.2. Preparation of ethyl acetate extract (EAE) of S. chamaecyparissus
The leaves of the S. chamaecyparissus were dried for one week in the shade. S. chamaecyparissus, leaves were pulverized into a coarse powder. Coarse powder (50 g) was extracted at 200 W ultrasonic power with ethyl acetate (250 mL) in an ultrasonic flask (Elma, Germany) at 35 °C for 20 min. The extract was filtered and concentrated using a rotary evaporator (Buchi, Switzerland). Further, EAE was freeze-dried and stored at 2–4 °C.
2.3. GC-MS analysis and identification of chemical constituents of S. chamaecyparissus
The chemical composition of S. chamaecyparissus EAE was determined by GC-MS. The sample was run on Agilent Bench Top GC-MS (Agilent Technologies, Wilmington, DE, USA) equipment and was fitted with a capillary column of DB-5 glass (30 m × 0.25 mm i.d.; film thickness of 0.25 μm). Helium was used at a flow rate of 1 mL/min as the carrier gas. The temperature of the oven was set to 50 °C for 1 min and then isothermally kept for 2 min at 320 °C, while the injector port was maintained at 250 °C. The EAE was injected (1 μL) with hexane (1:1), and the split ratio was 1:5. Data capture took place at 70 eV using scanning times of 1.5 sec in the mass range of 50–1000 amu. By comparison of retention time and comparison of the fragmentation pattern of mass spectra acquired by GC-MS analysis with those contained in the database of NIST, NBS 54 K.L, WILEY8 libraries, and published literature, the individual peaks/constituents were identified (Adams, 2007, Ali, 2001, Giner et al., 1988, Garg et al., 2001, Perez-Alonso and Velasco-Negueruela, 1992, Derbesy et al., 1989). The percent composition of each compound was calculated in the EAE from leaves of S. chamaecyparissus and is presented in Table 3.
Table 3.
Chemical composition of EAE from leaf of S. chamaecyparissus.
| No. | Name of chemical compound | RT | Concentration (%) |
|---|---|---|---|
| 2-Butanone | 4.071 | 1.22 | |
| trans-Cyclo-butyl-sesqui lavandulol | 6.445 | 0.28 | |
| 2,4,5,7-Tetramethyl-2,6-octadiene | 8.204 | 1.21 | |
| β-Camphor | 10.413 | 0.5 | |
| 1,1,3-Trimethylcyclopentane | 11.383 | 0.84 | |
| 1-Pentadecene | 15.966 | 1.6 | |
| α-Curcumene | 17.906 | 0.35 | |
| Dimethyldiazene | 18.362 | 2.14 | |
| t-Octylphenol 2- (1,1,3,3-Tetramethyl butyl) phenol | 18.488 | 1.08 | |
| Diethyl phthalate | 20.04 | 4.10 | |
| Tetramethyl-2-heptene | 22.067 | 1.59 | |
| Pentadecane | 22.145 | 0.47 | |
| t-Butylamine | 22.264 | 1.5 | |
| 5-Methylundecane | 23.968 | 0.5 | |
| N-Acetyl-tyrosine | 25.004 | 0.72 | |
| 3,3-Dimethylhexane | 25.69 | 0.51 | |
| Dibutyl phthalate | 26.6 | 2.95 | |
| 1-Octadecene | 27.23 | 4.40 | |
| 1-Bromo-8-chloronaphthalene | 27.34 | 2.23 | |
| 1,3-Propanediol, decyl ethyl ether | 28.127 | 0.34 | |
| n-Pentadecanol | 28.686 | 5.44 | |
| 3-Methylheptanol | 30.319 | 0.56 | |
| Dodecyl propan-2-yl sulfite | 30.39 | 0.66 | |
| Eicosyl acetate | 30.515 | 8.40 | |
| trans-4-(Dimethylamino)-3-buten-2-one | 30.786 | 0.31 | |
| Decyl propionate | 31.844 | 0.92 | |
| 3-Methylpiperidine | 31.943 | 0.79 | |
| 1-Oxaspiro[4.4]nonan-4-one, 2-isopropyl | 32.341 | 0.79 | |
| 2,3,4-Trimethyl Hexane | 33.228 | 0.89 | |
| Nonadecane | 34.557 | 1.27 | |
| 18-Methyl-nonadecane-1,2-dio, trimethylsilyl ether | 35.775 | 0.37 | |
| Eicosane | 35.839 | 2.54 | |
| 2-Methylhexacosane | 37.069 | 6.87 | |
| 1,3:4,6-Di-O-benzylidene-D-mannitol | 38.335 | 0.59 | |
| 2-Methylmercaptoaniline | 38.375 | 0.65 | |
| 2-Methoxyformanilide | 38.408 | 1.5 | |
| cis-1-formylbicyclo[3.3.0]octane | 38.495 | 0.76 | |
| 1-(Piperidin-1-yl)dodecan-1-one | 38.749 | 1.83 | |
| Tetrapentacontane | 39.515 | 27.15 | |
| Hexatriacontane | 39.911 | 4.04 | |
| 1-Ethyl-1-methylindan | 39.945 | 0.85 | |
| Convicine | 39.985 | 1.18 | |
| Piperazine | 40.12 | 0.24 | |
| Phytol | 40.175 | 0.85 |
2.4. In-vitro α-glucosidase inhibitory activity
The α-glucosidase inhibitory activity was assessed by method described earlier (Ahamad et al., 2016). Briefly, 60 μL of S. chamaecyparissus EAE in dimethyl sulfoxide with varying concentrations (3.125 to 100 μg/mL), and 50 μL of 0.1 M phosphate buffer (pH 6.8) containing α-glucosidase solution (0.2 U/mL) were incubated at 37 °C for 20 min in 96 well plates. A 50 μL of 5 mM p-nitrophenyl-alpha-D-glucopyranoside (PNPG) solution in a 0.1 M phosphate buffer (pH 6.8) was applied to each well after pre-incubation and incubated for another 20 min at 37 °C. The reaction was then stopped by adding 160 mL of 0.2 M NaCO3 into each well, and absorbance were recorded at 405 nm and compared to a control which had 60 μL of buffer solution in place of the test sample. Acarbose was used as a positive control and evaluated same as test sample. The % inhibition of α-glucosidase enzyme was calculated by using the following formula:
where Abs is absorbance of the control, and absorbance of the sample.
2.5. Anticancer activity: EGFR expression by quantitative RT-PCR
2.5.1. Cell culture and cell treatment
The cell line of MCF-7 was cultured in liquid Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS), 100 u/mL penicillin, and 100 μg/mL streptomycin and maintained at 37 °C under a 5% CO2 atmosphere. The S. chamaecyparissus EAE sample was tested for EGFR and β-actin gene expression, using MCF-7 cells according to the method reported earlier (Eimani et al., 2014). In brief, trypsinization was used to harvest the MCF-7 cells, pooled in a 15 mL tube. The cells were then inserted into a 6-well tissue culture plate in a DMEM medium containing 10% FBS and 1% antibiotic solution for 24 h at 37 °C at a density of 1 to 106 cells/well (1 mL). The wells were washed with sterile PBS, and 100 μg/mL of S. chamaecyparissus EAE was treated in a serum free DMEM medium and incubated at 37 °C in a humidified 5% CO2 incubator for 24 h. The complete RNA from the MCF-7 cell line was isolated using the Trizol method after the incubation time (Neah and Ujjwala, 2011).
2.5.2. RNA isolation
Total RNA isolation was performed using the Trizol method (Chomczynski and Mackey, 1995). The samples were centrifuged, using diethylpyrocarbonate treated centrifuge tubes at 5000 rpm for 10 min to get the cell pellet. To cell pellet (1 × 107 cells), Trizol (700 µL) was added to cell lysis. The lysate was collected into 1.5 mL tubes and vigorously pipetted. Then 300 µL of chloroform was added and mixed vigorously for 5 min at room temperature. The aqueous layer was separated by centrifugation at 12000 rpm for 20 min at 4 °C. The aqueous layer was collected in a fresh 1.5 mL tube. RNA was precipitated by adding 700 µL of isopropanol. Precipitated RNA was pelleted by centrifugation at 12000 rpm for 20 min at 4 °C. The pellet was washed with 70% ethanol. Finally, air-dried RNA pellet was mixed into 30 µL double distilled autoclaved water and stored at −80 °C till the next use. The quantity and quality of the isolated RNA were estimated by Labman UV–visible spectrometer and resolved in 1.5% agarose gel, respectively.
2.5.3. DNase treatment
DNA contamination was removed by the DNase treatment. The reaction volume was set up to 20 µL containing 1U of DNase. It was incubated at 37 °C for 45 min, then 20 µM of 2 µL EGTA was added and further incubated at 66 °C for another 10 min. Sodium acetate (1/10 V) and absolute ethanol (2 V) were added and incubated at −20 °C for 60 min. Then the mixture was centrifuged at 12000 rpm for 20 min at 4 °C, the supernatant was discarded, and the pellet was washed with 500 µL of 75% ethanol. The sample was air-dried and dissolved in 20 µL of sterile water and stored till further process.
2.5.4. Gene level detection of micrometastases
Total RNA (1.5 µg) was converted to cDNA using a reaction mixture containing Reverse transcriptase (MMLV). The cDNA synthesis was carried out at 25 °C for 10 min followed by 37 °C for 2 h. Denaturation of cDNA and RNA hybrid along with inactivation of reverse transcriptase was carried out at 85 °C for 2 min. The prepared cDNA was used as a template to detect metastasis. The expression levels of the selected genes (Primer sequence, Table 1) were assessed by qRT-PCR in ABI StepOne Plus (Applied Biosystems, CA, USA) using the relative quantification (2^-ΔΔCT) method. Expression was normalized using the endogenous control (β-actin), and control cells were used as the calibrator.
2.5.5. RT-PCR condition
The initial melting temperature was set at 95 °C for 15 min, followed by 40 cycles of 95 °C for 10 sec. Annealing at 57 °C for 15 sec, and the extension temperature was at 72 °C for 20 sec (Table 2). The real-time data was captured at the end of each extension stage.
Table 2.
The working condition of QRT-PCR.
| Fragments | Initial denaturation | Number of cycles | Denaturation | Annealing | Extension |
|---|---|---|---|---|---|
| EGFR | 95 °C-15 min | 40 | 95 °C for 10 sec | 57 °C-15 s | 72 °C-20 s |
| β-actin | 95 °C-15 min | 40 | 95 °C for 10 sec | 61 °C-15 s | 72 °C-20 s |
3. Results
3.1. GC-MS analysis of EAE of S. chamaecyparissus
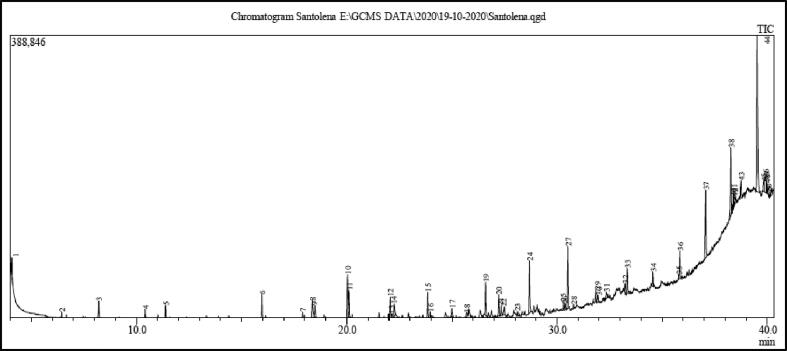
The chemical composition of EAE from S. chamaecyparissus leaves was determined by GC-MS method (Fig. 1) and results are presented in Table 3. A total of 44 components representing 97.62% were identified. The major constituents in EAE from S. chamaecyparissus included tetrapentacontane (27.15%), eicosyl acetate (8.40%), 2-methylhexacosane (6.87%), n-pentadecanol (5.44%), 1-octadecene (4.40%), diethyl phthalate (4.10%), hexatriacontane (4.04%), dibutyl phthalate (2.95%), eicosane (2.54%), and Dimethyldiazene (2.14%). The other components detected more than 1% includes 1-pentadecene (1.60%), tetramethyl-2-heptene (1.59%), t-butylamine (1.50%), 2-methoxyformanilide (1.5%), nonadecane (1.27%), 2-butanone (1.22%), 2,4,5,7-tetramethyl-2,6-octadiene (1.21%), and convicine (1.18%).
Fig. 1.
GC-MS chromatogram of EAE from leaves of S. chamaecyparissus.
3.2. α-Glucosidase inhibition activity of EAE from S. chamaecyparissus
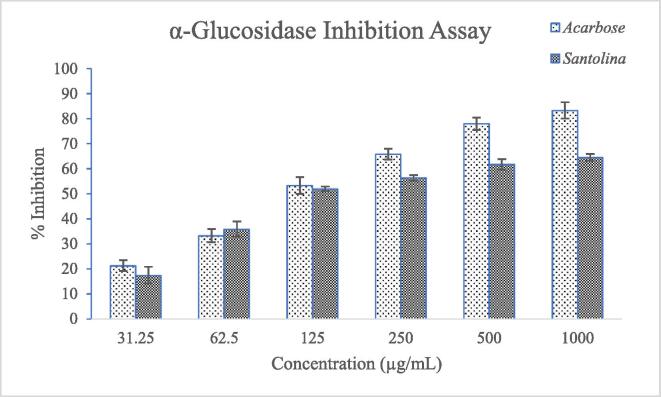
The EAE from leaves of S. chamaecyparissus showed concentration dependant α-glucosidase enzyme inhibition that varies from 17.36 ± 3.32 to 64.51 ± 1.37 µg/mL for concentration ranging from 31.25 to 1000 µg/mL (Table 4 and Fig. 2). Acarbose was used as a positive standard and it also showed concentration dependant inhibition of α-glucosidase enzyme ranging from 21.94 ± 2.45 to 81.26 ± 3.21 µg/mL for the same concentration as the test sample. The IC50 values for S. chamaecyparissus and acarbose were found as 110 ± 4.25 and 105 ± 3.74 µg/mL, respectively against the α-glucosidase enzyme.
Table 4.
α-Glucosidase enzyme inhibitory activity of EAE from S. chamaecyparissus.
| Conc. (µg/mL) | Acarbose (% inhibition) |
S. chamaecyparissus (% inhibition) |
|---|---|---|
| 31.25 | 21.94 ± 2.45 | 17.36 ± 3.32 |
| 62.5 | 35.44 ± 2.63 | 35.82 ± 3.01 |
| 125 | 51.23 ± 3.31 | 51.91 ± 0.94 |
| 250 | 63.82 ± 2.47 | 56.32 ± 1.13 |
| 500 | 76.81 ± 2.96 | 61.66 ± 2.13 |
| 1000 | 81.26 ± 3.21 | 64.51 ± 1.37 |
| IC50 values | 105 ± 3.74 | 110 ± 4.25 |
Data were presented as mean of triplicate determinations ± SD.
Fig. 2.
α-Glucosidase enzyme inhibitory activity of EAE from leaves of S. chamaecyparissus (Data were presented as mean of triplicate determinations ± SD).
3.3. Anticancer activity: Expression of EGFR in human breast cancer cell line (MCF-7)
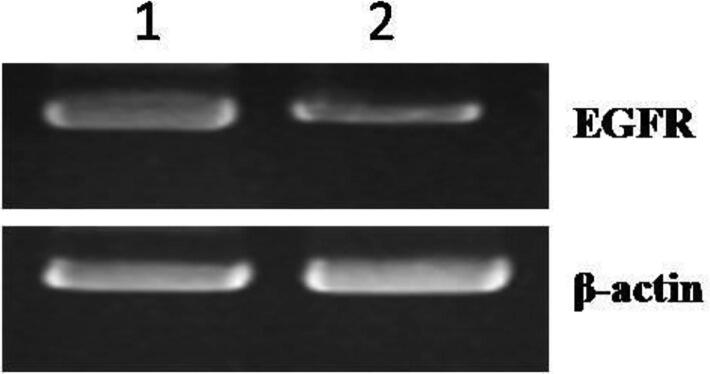
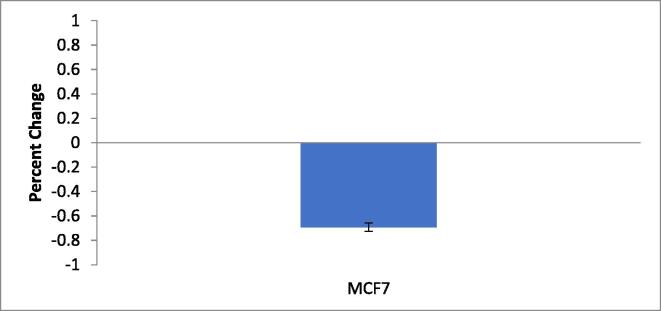
An investigation was performed to assess the EGFR expression using human breast cancer cell line (MCF7) and the results are presented in Table 5 and Fig. 3a, Fig. 3b. The results showed negative expression of EGFR protein compared to the control group. Β-Actin protein was used as an endogenous control for normalization of expression EGFR (Fig. 3a). Table 5 also represents the EGFR (bcl-2) protein levels from multiple quantitative real-time RT-PCR runs relative to normalized levels of β-actin. The results showed that the downregulation of EGFR protein with a value of −0.69297105 after treatment with EAE from leaves of S. chamaecyparissus (100 µg/mL).
Table 5.
Expression of EGFR in breast cancer cells (MCF-7) following treatment with S. chamaecyparissus.
| Sample | Reference Gene | Target gene |
Delta Ct |
Delta-Delta Ct EGFR | Expression EGFR | Ln10 expression (fold variation) EGFR | |
|---|---|---|---|---|---|---|---|
| β-actin | EGFR | β-actin | EGFR | ||||
| Control | 12.248 | 17.401 | 3.32 | 5.622 | 2.302 | 0.202781789 | −0.69297105 |
| S. chamaecyparissus | 15.568 | 23.023 | |||||
Fig. 3a.
1: Control group; 2: treated with S. chamaecyparissus (100 μg/mL).
Fig. 3b.
Real-time quantitative measurement of EGFR in breast cancer cells (MCF-7) cell lines in response to 48 h treatment with S. chamaecyparissus (130.4 μg/ml).
4. Discussion
Essential oils obtained from plants emerged as the major source of fragrance and are used as medicines; an important component in aromatherapy, and used as flavouring agents in foods, medicinal products, and cosmetics (Ali, 2001). S. chamaecyparissus is an important medicinal plant of the Mediterranean region and has been reported as anti-inflammatory, antioxidant, antimicrobial, anticandidal, antibacterial, antifungal, CNS depressant, and anti‐cholinergic (Tundis and Loizzo, 2018). GC-MS analysis of S. chamaecyparissus yielded 44 chemical constituents in EAE and the major constituents were tetrapentacontane (27.15%), eicosyl acetate (8.40%), and 2-methylhexacosane (6.87%). However, phytol (0.85%), N-acetyl-tyrosine (0.72%), β-camphor (0.5%), and α-curcumene (0.35%) were detected in trace. This is the first GC-MS study on EAE of S. chamaecyparissus. However, the previous studies on essential oil composition of S. chamaecyparissus had shown the presence of camphor, artemisia ketone, β-phellandrene, aromadendrene, cubenol, p-cymene, ɑ-terpinol, caryophyllene oxide, and 1,8-cineole as major chemical constituents (El-sharkawy, 2014; Giner et al., 1988, Garg et al., 2001, Perez-Alonso and Velasco-Negueruela, 1992, Derbesy et al., 1989). In the current study, Phytol detected in EAE, is an aromatic diterpene alcohol, reported to have immunostimulant, antioxidant, antiallergic, anti-inflammatory, antinociceptive, and antimicrobial potentials (Moraes et al., 2014). N-acetyl-L-tyrosine found in EAE improves cognitive function, as it acts as a precursor for dopamine. Oral N-acetyl-L-tyrosine is also reported increasing L-tyrosine levels in the brain (Jongkees et al., 2015, Topall and Laborit, 1989). α-Curcumene identified in EAE is reported as antibacterial and antifungal against selected microorganisms (Silva et al., 2015). Camphor detected in EAE, is used as a skin penetration enhancer, and reported to possess counterirritant, rubefacient, mild analgesic, antimicrobial, insecticidal, anticancer, and antitussive activities (Zuccarini and Soldani, 2009, Chen et al., 2013).
Inhibition of carbohydrate metabolizing enzymes such as α-amylase and α-glucosidase is an important strategy to control postprandial hyperglycaemia in diabetic patients (Subramanian et al., 2008). The results of the current enzyme inhibition study indicate that the S. chamaecyparissus EAE moderately inhibit α-glucosidase enzyme with IC50 value of 110 ± 4.25 µg/mL. α-Amylase and α-glucosidase enzymes are present in brush border of the gastrointestinal tract and responsible for the breakdown of polysaccharides and disaccharides, respectively. Inhibition of these enzymes leads reduction of monosaccharides available for absorption in blood and ultimately it controls the sudden rise of blood glucose level after meal. The increased blood sugar level after meal is known as postprandial hyperglycaemia and controlling it by inhibiting such enzymes is a remarkable strategy in the management of type-2 diabetes mellitus. The inhibitors of α-amylase and α-glucosidase enzymes such as acarbose and miglitol are non-specific in their action, the strong inhibition of both enzymes leads to decreased metabolism of polysaccharides which causes flatulence and distension as side effects due to bacterial fermentation from undigested carbohydrates (Ahamad et al., 2020). This is the first report on inhibition of α-glucosidase enzyme by EAE of S. chamaecyparissus. Hence, the present study provides preliminary evidence that S. chamaecyparissus may be a potential candidate for the development of antidiabetic drugs through the inhibition of carbohydrate metabolizing enzyme i.e. α-glucosidase.
The EGFR protein is a member of the transmembrane receptor tyrosine kinases family of human epidermal growth factor (HER) that is widely expressed in different tissues. The EGFR protein is important for cellular processes such as proliferation, differentiation, and production of cells and it is responsible for the development and growth of cancer when overexpressed. Low expression or downregulation of EGFR is normally correlated with good cancer prognosis, and several studies have clearly shown a negative association between EGFR status and breast cancer relapse-free and overall survival (Kumaraswamy et al., 2015). The anticancer activity of S. chamaecyparissus growing in Saudi Arabia was investigated by El-sharkawy (2014), against A549, HCT116, HepG2, and MCF-7 cell lines; the study results showed that the S. chamaecyparissus was effective against HepG2 cell lines. S. chamaecyparissus also reported by other researchers as a potential plant with anticancer activity (Elsharkawy and Aljohar, 2016, Al-Zahrani, 2018). The current study findings indicated the possible role of S. chamaecyparissus in negative regulation of EGFR protein, which can be helpful in the management of human breast cancer.
5. Conclusion
The ethyl acetate extract (EAE) from leaves of S. chamaecyparissus was determined by the GC-MS method, and tetrapentacontane, eicosyl acetate, 2-methylhexacosane, n-pentadecanol were found as major chemical constituents. The in-vitro α-glucosidase enzyme inhibition activity of EAE from leaves of S. chamaecyparissus showed concentration dependant enzyme inhibition, and IC50 value was found comparable with standard acarbose. The study findings also indicate the possible anticancer effects of S. chamaecyparissus, as it showed the negative expression of EGFR protein in human breast cancer cell line (MCF7). The current findings support the possible use of S. chamaecyparissus in the management of diabetes and in human breast cancer. Further research studies are required to isolate the chemical compounds from S. chamaecyparissus and to evaluate their possible antidiabetic and anticancer effects.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgement
Dr Abuzer Ali is thankful to Taif University Researchers Supporting Project Number (TURSP-2020/124), Taif University, Taif, Saudi Arabia. Authors are thankful to Trichy Research Institute of Biotechnology Pvt. Ltd., Trichy, Tamil Nadu, India for conducting anticancer cell line study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Zahrani A.A. Saudi anti-human cancer plants database (SACPD): a collection of plants with anti-human cancer activities. Oncol. Rev. 2018;12(1):349. doi: 10.4081/oncol.2018.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouasri A., Dob T., Krimats S., Dahmane D., Toumi M., Lynda L., Chelgoume C., Racheme F. Chemical composition, antioxidant and antimicrobial activities of the essential oil of Santolina chamaecyparissus L. of Algeria. J. Coastal Life Med. 2015;3(3):220–227. [Google Scholar]
- Sala A., Recio M.C., Giner R.M., Manez S., Rios J.L. Antiphospholipase A2 and anti-inflammatory activity of Santolina chamaecyparissus. Life Sci. 2000;66:35–40. doi: 10.1016/s0024-3205(99)00578-0. [DOI] [PubMed] [Google Scholar]
- Eimani B.G., Sanati M.H., Houshmand M., Ataei M., Akbarian F., Shakhssalim N. Expression and prognostic significance of Bcl-2 and Bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 2014;15(4):362–1356. [PMC free article] [PubMed] [Google Scholar]
- Jongkees B.J., Hommel B., Kuhn S., Colzato L.S. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands – a review. J. Psychiatr. Res. 2015;70:50–57. doi: 10.1016/j.jpsychires.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Suresh B., Sriram S., Dhanaraj S.A., Elango K., Chinnaswamy K. Anticandidal activity of Santolina chamaecyparissus volatile oil. J. Ethnopharmacol. 1997;55:151–159. doi: 10.1016/s0378-8741(96)01490-0. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M., Snader K.M. Natural products in therapeutic. J. Nat. Prod. 2003;66:1022. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- Elsharkawy E., Aljohar H. Anticancer screening of medicinal plants growing in the Northern region of Saudi Arabia. Natl. J. Physiol. Pharm. Pharmacol. 2016;6(3):241–246. [Google Scholar]
- Kumaraswamy E., Wendt K.L., Augustine L.A., Stecklein S.R., Sibala E.C. BRCA1 regulation of Epidermal growth factor receptor (EGFR) expression in human breast cancer cells involves MicroRNA-146a and is Critical for its tumor suppressor function. Oncogene. 2015;34(33):4333–4346. doi: 10.1038/onc.2014.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-sharkawy E.R. Anticancer effect and seasonal variation in oil constituents of Santolina chamaecyparissus. Chem. Mater. Res. 2014;6(3):85–91. [Google Scholar]
- Silva G.N.S.D., Pozzatti P., Rigatti F., Horner R., Alves S.H., Mallmann C.A., Heinzmann B.M. Antimicrobial evaluation of sesquiterpene α-curcumene and its synergism with imipenem. J. Microbiol. Biotech. Food Sci. 2015;4(5):434–436. [Google Scholar]
- Topall G., Laborit H. Brain tyrosine increases after treating with prodrugs: comparison with tyrosine. J. Pharm. Pharmacol. 1989;41(11):789–791. doi: 10.1111/j.2042-7158.1989.tb06368.x. [DOI] [PubMed] [Google Scholar]
- Ahamad J., Hasan N., Amin S., Mir S.R. Swertiamarin contributes to glucose homeostasis via inhibition of carbohydrate metabolizing enzymes. J. Nat. Remed. 2016;16(4):125–130. [Google Scholar]
- Ahamad J., Hasan N., Amin S., Mir S.R. Standardized extract from Enicostemma littorale ameliorates post-prandial hyperglycaemia in normal and diabetic rats. J Biol. Active Products Nat. 2020;10(1):34–43. [Google Scholar]
- Moraes J.D., Oliveira R.N.D., Costa J.P., Junior A.L.G., Sousa D.P.D., Freitas R.M., Allegretti S.M., Pinto P.L.S. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014;8(1) doi: 10.1371/journal.pntd.0002617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover J.K., Yadav S., Vats V. Medicinal plants of India with antidiabetic potential. J. Ethnopharmacol. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Salah-Fatnassi K.B.H., Hassayoun F., Cheraif I., Khan S., Jannet H.B., Hammami M. Chemical composition, antibacterial and antifungal activities of flower head and root essential oils of Santolina chamaecyparissus L., growing wild in Tunisia. Saudi J. Biol. Sci. 2017;24:875–882. doi: 10.1016/j.sjbs.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neah L., Ujjwala M.W. A unique method for isolation and solubilization of proteins after extraction of RNA from tumor tissue using trizol. J. Biomol. Tech. 2011;22(1):37–44. [PMC free article] [PubMed] [Google Scholar]
- Ali M. Birla Publication; Delhi, India: 2001. Techniques in terpenoid identification; pp. 4–51. [Google Scholar]
- Derbesy M., Touche J., Zola A. The essential oil of Santolina chamaecyparissus L. J. Essential Oil Res. 1989;1:269–275. [Google Scholar]
- Cuellar M.J., Giner R.M., Recio M.C., Just M.J., Máñez S., Cerda S., Ríos J.L. Screening of anti-inflammatory medicinal plants used in traditional medicine against skin diseases. Phytother. Res. 1998;12:18–23. [Google Scholar]
- Perez-Alonso M.J., Velasco-Negueruela A. Essential oil components of Santolina chamaecyparissus L. Flavour and Fragrance J. 1992;7:37–41. [Google Scholar]
- Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull WHO. 1985;63:965–981. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Mackey K. Short technical report. modification of the TRIZOL reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques. 1995;19(6):942–945. [PubMed] [Google Scholar]
- Zuccarini P., Soldani G. Camphor: benefits and risks of a widely used natural product. Acta Biol. Szeged. 2009;53(2):77–82. [Google Scholar]
- Giner R.M., Rios J.L., Villar A. CNS depressant effects, anti-inflammatory activity and anti-cholinergic actions of Santolina chamaecyparissus extracts. Phytotherapy Research. 1988;2(1):37–41. [Google Scholar]
- Adams R.P. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. [Google Scholar]
- Subramanian R., Asmawi M.Z., Sadikun A. In-vitro α-glucosidase and α-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim. Polon. 2008;55(2):391–398. [PubMed] [Google Scholar]
- Tundis R., Loizzo M.R. A review of the traditional uses, phytochemistry and biological activities of the genus Santolina. Planta Med. 2018;84(09/10):627–637. doi: 10.1055/a-0585-6153. [DOI] [PubMed] [Google Scholar]
- Verpoorte R. Pharmacognosy in the new millennium: lead finding and biotechnology. J. Pharm. Pharmacol. 2000;52:253–262. doi: 10.1211/0022357001773931. [DOI] [PubMed] [Google Scholar]
- Djeddi S., Djebilec K., Hadjbourega G., Achourd Z., Argyropoulou C., Skaltsa H. In-vitro antimicrobial properties and chemical composition of Santolina chamaecyparissus essential oil from Algeria. Nat. Prod. Comm. 2012;7(7):937–940. [PubMed] [Google Scholar]
- Garg S.N., Gupta D., Mehta V.K., Kumar S. Volatile constituents of the essential oil of Santolina chamaecyparissus Linn. from the southern hills of India. J. Essent. Oil Res. 2001;13:234–235. [Google Scholar]
- Chen W., Vermaak I., Viljoen A. Camphor-A fumigant during the black death and a coveted fragrant wood in ancient Egypt and Babylon – A Rev. Molecules. 2013;18(5):5434–5454. doi: 10.3390/molecules18055434. [DOI] [PMC free article] [PubMed] [Google Scholar]