Abstract
Avian spermatozoa are highly susceptible to reactive oxygen species (ROS) produced during the cryopreservation. The aim of the current study was to investigate the antioxidant effects of resveratrol (RSV) during rooster semen cryopreservation. Changes in expression of AMP-activated protein kinase as a possible mechanism behind the beneficial effects of resveratrol were also evaluated. Semen samples were collected from ten Ross broiler breeders (52-wk) using abdominal massage, then divided into 4 equal aliquots and cryopreserved in Beltsville extender that contained different concentrations (0 µM, 0.01µM, 0.1µM, and 1µM) of RSV. higher percentage (P < 0.05) of total motility and membrane integrity was observed in RSV-0.1 compared to the other frozen groups. Moreover, higher percentage of sperm mitochondrial activity was observed in the RSV-0.01 and RSV-0.1 compared to the frozen control (P < 0.05). The lowest percentage of apoptotic like changes was found in the RSV-0.1 in comparison to the other groups (P < 0.05). RSV-0.01 and RSV-1 groups produced the lowest levels of H2O2 and O2− compared to the other frozen groups, respectively. Malondialdehyde (MDA) concentration, velocity average path (VAP), and linearity (LIN) were not affected by different concentrations of RSV (P > 0.05). We observed a dose-dependent increase in AMP-activated protein kinase expression in groups exposed to RSV. Thus, RSV-1 increased AMP-activated protein kinase phosphorylation but had no positive effects on post thaw sperm parameters. Our findings suggest that RSV-0.1 improve thawed sperm functions, and these effects might be mediated through activation of AMP-activated protein kinase.
Key words: cryopreservation, resveratrol, AMPK protein, rooster semen
INTRODUCTION
Avian sperm is highly susceptible to the various stresses in cryopreservation due to its unique biological and physiological features (Nabi et al., 2016). Avian sperm plasma membrane is rich in polyunsaturated fatty acids (PUFA), which make them vulnerable to lipid peroxidation. Using exogenous antioxidants could be an appropriate strategy against all the cryo-damages occurring during cryopreservation (Taylor et al., 2009; Zhu et al., 2015; Amidi et al., 2016) because they play an important role in eliminating ROS during cryopreservation (Eslami et al., 2018), thus prevent the injures to the sperm cells. Resveratrol (RSV) is one of the most important nonflavonoid polyphenols in red grapes, red wine and peanuts (Guerrero et al., 2009; Gambini et al., 2015), which have various biological activities, including anti-inflammation and antiapoptotic properties (Saiko et al., 2008; Longobardi et al., 2017). In addition, RSV has an effective role in eliminating a variety of ROS such as hydroxyl and superoxide radicals (Leonard et al., 2003). It has been shown that addition of RSV to freezing extenders can decrease DNA damage and protect plasma membrane integrity in human sperm during cryopreservation (Garcez et al., 2010). Moreover, RSV improves motility, mitochondrial activity, and DNA integrity in bull sperm after freeze-thaw process (Bucak et al., 2015). RSV as a natural phytoalexin acts as an AMP-activated protein kinase (AMPK) activator in multiple cell types (Baur and Sinclair, 2006; Hawley et al., 2010; Price et al., 2012). AMPK is an energy sensor of cellular metabolism (Hardie, 2011) which is sensitive to high levels of AMP, and AMP/ATP ratio (Suter et al., 2006). It is a heterotrimeric protein with 3 subunits namely α, β and γ; α is a catalytic subunit and β and γ subunits are regulatory subunits (Hardie, 2004; Hardie et al., 2006). The presence of AMPK protein has been confirmed in mature sperm of boar (De Llera et al., 2012), stallion (Córdova et al., 2014), human (Shabani Nashtaei et al., 2017), goat (Zhu et al., 2018), and chicken (Nguyen et al., 2014). In chicken sperm, the active form of AMPK (phospho-Thr172-AMPKα) is mainly localized in the flagellum and acrosome (Nguyen et al., 2014). Active form of AMPK protein can switch cells from an anabolic to a catabolic state, shut down the ATP-consuming synthetic pathways and restore energy balance (Rubin et al., 2005; Towler and Hardie, 2007). Since cryopreservation has deleterious effects on the sperm energetic metabolism like ATP content, we hypothesized that restoring ATP level through using RSV can improve the quality of cryopreserved sperm. Accordingly, here, we investigated protective effects of RSV as an activator of AMPK on functional parameters of post–thaw rooster sperm.
MATERIALS AND METHODS
Chemicals
Chemicals used for making different solutions were obtained from Sigma company (St. Louis, MO) and Merck company (Darmstadt, Germany), except where noted. Primary antibodies including anti-phospho-Thr172-AMPK and anti-AMPK as well as secondary antibodies (anti-Rabbit IgG) were prepared from Cell Signaling company. Approval for the study was obtained from the Research Ethics Committees of Tarbiat Modares University, Tehran, Iran.
Farm Management and Semen Collection
Ten 52-week-old broiler breeder roosters were kept individually in cages (70 × 60 × 75 cm) at 21 to 23°C, with 15 Light: 9 Dark schedule, and standard diet and water were available. Semen samples were routinely collected twice a week using dorsa-abdominal message method and transferred to separate microtubes. After that, samples were placed in a water bath (37°C) and transferred to laboratory for primary evaluations. Selected semen samples with the following criteria: volume 0.2 to 0.6 mL; sperm concentration ≥3 × 109 spermatozoa/mL; motility ≥80%, and abnormal morphology ≤10%, were pooled to eliminate individual effects and then divided into 4 equal aliquots.
Extender Preparation and Cryopreservation
Beltsville used as the base medium was composed of dipotassium phosphate (12.7 g /L), sodium glutamate (8.61 g /L), fructose (5 g /L), sodium acetate (4.3 g /L), TES (1.95 g /L), potassium citrate (0.64 g /L), monopotassium phosphate (0.06 g /L), and magnesium chloride (0.34 g /L) (Nabi et al., 2016). Soybean lecithin (1% w/v) and glycerol (3% v/v) were added to the basic medium (pH 7.5 and osmotic pressure was 340 mosmol/kg). Beltsville extender containing different concentrations of RSV (Cat. No.: R5010, Sigma Aldrich, St. Louis, MO) was used in the following groups: Beltsville without RSV (RSV-0, control), Beltsville plus 0.01 µM RSV (RSV-0.01), 0.1 µM RSV (RSV-0.1), and 1 µM RSV (RSV-1). The diluted semen sample was cooled at 5°C for 2 h, then vacated into 0.25 mL straws and stored in the liquid nitrogen for one week. The frozen straws were thawed individually (37°C) for 30 s in a water bath, and then evaluated.
Evaluation of Semen After Freezing-Thawing
Motility Parameters
Thawed sperm motility parameters were evaluated by sperm class analysis software (SCA; Version 5.1; Mi-croptic, Barcelona, Spain). Sperm cells were diluted with Phosphate-buffered saline (PBS), then, 10 µL of diluted semen was placed on a prewarmed chamber slide (38°C, Leja 4; 20 mm height; Leja Products, Luzernestraat B.V., Holland). At least 6 fields that contained a minimum of 400 sperm cells, were evaluated for motion characteristics including total motility (TM %), average path velocity (VAP, µm/s), straight line velocity (VSL, µm/s), curvilinear velocity (VCL, µm/s), amplitude of lateral head displacement (ALH, µm/s), straightness (STR %), and linearity (LIN %) (Nguyen et al., 2015).
Plasma Membrane Functionality
Sperm plasma membrane functionality was assessed by hypo-osmotic swelling test (HOST) (Revell and Mrode, 1994). The test is based on the resistance of sperm membrane in stress conditions in a hypo-osmotic medium. Briefly, 5 µL sperm suspension was added to 50 µL hypo-osmotic solution (100 mOsm/L, 57.6 mM fructose, and 19.2 mM sodium citrate), then incubated at 37°C for 20 min. At least, 300 sperm cells were assessed to determine the percentage of swollen tails, under a phase-contrast microscope, as sperm cells with intact membrane (CKX41, Olympus, Tokyo, Japan).
Lipid Peroxidation
Malondialdehyde (MDA) concentration as an index of lipid peroxidation level, is measured using the thiobarbituric acid reaction. At first, 1 mL of diluted semen sample (250 × 106) was mixed with 1 mL of cold 20% (w/v) trichloroacetic acid (TCA) to precipitate proteins. The precipitate was plated by centrifuging (960 × g) for 15 min, and 1 mL of the supernatant was incubated with 1 mL of 0.67% (w/v) thiobarbituric acid (TBA) in a boiling water-bath at 95°C for 10 min. After cooling, the absorbance was determined using a spectrophotometer (Shimadzu/UV-2100, Japan) at 532 nm; MDA concentrations is reported as nmol/mL (Fujihara and Koga, 1984).
Mitochondrial Activity
Mitochondrial activity in semen samples was evaluated using JC-1 (T4069, Sigma-Aldrich, St. Louis, MO). Briefly, 5 μL of the JC-1 solution was added to 300 μL of the PBS-diluted semen sample containing 2 × 106 spermatozoa and incubated for 15 min at 37°C in the dark. Mitochondrial activity of JC-1-stained spermatozoa was evaluated by flow cytometry. In this method, FL1 for green fluorescence (low or no active mitochondria) and FL2 for red/orange fluorescence (active mitochondria) were considered (Zhu et al., 2019).
Apoptosis
Phosphatidylserine translocation is as a sign of apoptosis and Annexin-V (IQP-116f, IQ Products) staining was performed to determine phosphatidylserine translocation in rooster sperm. First, sperm cells were washed with calcium buffer for adjusting the concentration of sperm to 1 × 106 sperm/mL and then, 10 µL of Annexin-V FITC (0.01 mg/mL) was added to 100 µL of the sperm suspension and incubated for 20 min on ice. Then, 10 µL of propidium iodide (PI) was added to the sperm suspension, incubated for at least 10 min on ice, and finally analyzed by a flow cytometer. The sperm subpopulations were classified as follows (1) live sperm (A−/PI−); (2) apoptotic sperm (A+/PI−); (3) dead sperm (A+/PI+); and (4) necrotic sperm (A−/PI+) (Hezavehei et al., 2019a).
Intracellular Reactive Oxygen Species (ROS) Concentration
We used dihydroethidium (DHE) and dichlorofluorescein diacetate (DCFH-DA) to determine levels of O2− and H2O2 in thawed sperm, respectively. Thawed sperm was washed with PBS to the final concentration of 3-5 × 106. Then, DHE (1.25 µM) and DCFH-DA (25 µM) were added separately to 1 mL of the sperm suspensions and incubated at 25°C for 20 min for DHE or 40 min for DCFH-DA, in the dark; samples were then analyzed using flow cytometry. The red fluorescence (DHE) was detected in the FL2 channel and green fluorescence (DCFH) was detected in the FL1 channel (Ghaleno et al., 2014).
The Level of Phosphorylation of AMPK Protein by Western Blotting
The total protein was extracted from thawed sperm by lysis buffer (pH 6.8) that consisted of 8 M urea, 1% SDS, 2% CHAPS, and 50 mL Tris-HCL. Total concentration of protein was determined by using Bradford Assay Kit (Thermo Scientific, Rockford, IL). Next, 10 µg protein from each sample was separated on 10% SDS-polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (PVDF Western Blotting Membranes, Roche). After that, the membranes were blocked by 1% bovine serum albumin (BSA) (Sigma Aldrich, St. Louis, MO) and nonfat milk, at room temperature for 1 h. Then, the membranes were washed 3 times with TBST (Tris-buffered saline [TBS: 100 Mm Tris-HCL, 150 Mm NaCl] supplemented with 0.05 Tween 20) and incubated overnight at 4°C with the primary antibody anti-phospho-Thr172-AMPKα (1:500 in TBST, Cat. No. Ph-Thr172-AMPKα (40HA) Rabbit mAb 2531, Cell Signaling). After 3 washes with TBST, bands were detected after 1.5 h incubation with the secondary antibody, antirabbit IgG (1:50000 in TBST, Sigma Aldrich, St. Louis, MO), at room temperature. The bonds were quantified using Gel Doc (UVITEC Cambridge, Cambridge, UK, Alliance Q9 Advanced). The loading control AMPKα (1:500 in TBST, Cat. No. AMPKα (D5A2) Rabbit mAb 5831, Cell Signaling) was used for data normalization. The results were quantified using image J software, version 1.50i (National Institutes of Health, Bethesda, MD).
Statistical Analysis
All data were analyzed using general linear model procedure using Proc GLM of SAS 9.1 (SAS Institute, version 9.1, 2002, Cary, NC). Six replicates of semen were used for evaluation. Statistical differences among various groups were determined by Tukey's test and a P < 0.05 was considered statistically significant. Results are shown as mean ± SEM.
RESULTS
Motion Parameters
Effects of different concentrations of RSV on the motility parameters of post-thawed sperm are presented in Table 1. The percentage of total motility increased in the RSV-0.1 (60.9 ± 2.6) compared to the other groups (P < 0.05). The lowest percentages of total motility (30.8 ± 2.6), VAP (9.5 ± 1.2), VSL (8.3 ± 1.3), and LIN (25.4 ± 1.5) were observed in the RSV-1 compared to the other groups (P < 0.05). In addition, no significant difference was found in VCL, STR, or ALH among different groups (Table 1).
Table 1.
Effect of different concentrations of RSV on motility parameters of rooster sperm after freezing thawing.
| Parameter (unit) | RSV-0 | RSV-0.01 | RSV-0.1 | RSV-1 | SEM |
|---|---|---|---|---|---|
| TM (%) | 51.6b | 54.3b | 60.9a | 30.8c | 2.6 |
| VAP (µm/s) | 20.7a | 19.7a | 18.6a | 9.5b | 1.2 |
| VSL (µm/s) | 14.7a | 17.6a | 15.8a | 8.3b | 1.3 |
| VCL (µm/s) | 33.9 | 35.5 | 33.2 | 34.5 | 1.6 |
| ALH (µm/s) | 1.98 | 2 | 2.03 | 1.94 | 0.21 |
| STR (%) | 55.1 | 57.9 | 58.4 | 53.6 | 1.65 |
| LIN (%) | 38.2a | 39.1a | 40.7a | 25.4b | 1.5 |
Abbreviations: TM, total motility; VAP, average path velocity; VSL, straight-line velocity; VCL, curvilinear velocity; ALH, mean amplitude of the lateral head displacement; STR, straightness; LIN, linearity; and RSV, resveratrol.
a-cValues with different letters are significantly different (P < 0.05). Data are expressed mean ± SEM (n = 6).
Membrane Integrity, Mitochondrial Activity, and Lipid Peroxidation
Table 2 shows the mean percentage of membrane integrity, mitochondrial activity and MDA concentration of thawed rooster sperm exposed to different concentrations of RSV. RSV- 0.1 increased membrane integrity (60.7 ± 2.3) and mitochondrial activity (49.5 ± 2.2) compared to other frozen groups (P < 0.05). However, membrane integrity (40.4 ± 2.3) and mitochondrial activity (32.4 ± 2.2) reduced in the RSV-1 compared to the other groups (P < 0.05). The highest level of MDA was observed in the frozen control group (2.17 ± 0.19) in comparison to the other groups (P < 0.05). The level of MDA was not affected by different concentrations of RSV in thawed sperm (P > 0.05).
Table 2.
Effect of different concentrations of RSV on viability, membrane integrity, mitochondrial activity, and MDA concentration of rooster sperm after freezing thawing.
| Parameter (unit) | RSV-0 | RSV-0.01 | RSV-0.1 | RSV-1 | SEM |
|---|---|---|---|---|---|
| Membrane integrity (%) | 49.2b | 51.4b | 60.7a | 40.4c | 2.3 |
| Mitochondrial activity (%) | 40.3b | 47.1a | 49.5a | 32.4c | 2.2 |
| seminal MDA (nmol/mL) | 2.17a | 1.52b | 1.06b | 1.1b | 0.19 |
a-cValues with different letters in the same row are significantly different (P < 0.05).
Abbreviations: RSV, resveratrol. Data are expressed as mean ± SEM (n = 6).
Apoptotic-Like Changes
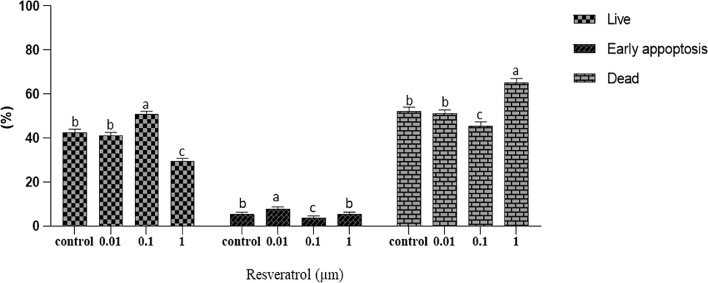
As shown in Figure 2, RSV-0.1 increased the presentage of live sperm (50.7 ± 1.5) compared to the frozen control (42.5 ± 1.5), RSV-0.01 (41.1 ± 1.5) and RSV-1 (29.4 ± 1.5; P < 0.05) groups. However, the lowest precentage of appoptotic cells was observed in the RSV-0.1 (2.7 ± 1) compared to the other groups (P < 0.05). Furthermore, RSV-0.1 decreased (45.6 ± 1.8) dead sperm percentage compared to the RSV-0 (52.2 ± 1.8), RSV-0.01 (51.1 ± 1.8), and RSV-1 (65.2 ± 1.8, P < 0.05) groups.
Figure 1.
Summary of AMPK protein activation pathways by resveratrol in sperm. (A) Decreasing ATP level (adenosine triphosphate) and increasing AMP/ATP ratio cause AMPK protein phosphorylation. Resveratrol inhibits PDEs (phosphodiesterases), which lead to an increase in cAMP (cyclic AMP) levels and intracellular calcium, as well as AMPK phosphorylation and PKA (Protein kinase A) activation. PKA actives PKC (protein kinase C) and finally phosphorylates AMPK. Resveratrol activates SIRT1 and then LKβ1 that result in AMPK phosphorylation. Also, an increase in NAD+/NADH level activates the SIRT1 downstream of AMPK. (B) Presence of AMP-activated protein kinase and AMPK in rooster sperm by western blotting. Bands for phospho-Thr172-AMPKa were detected at 62 kDa (upper film). AMPKα was used as the loading control (62 kDa) (lower film). (C) The phosphorylated protein p-AMPKα/AMPKα ratio. Values represent means± SEM. Different superscripts (a, b, c, and d) indicate significant differences between control and other groups (P < 0.05). Abbreviations: AMP, adenosine monophosphate; AMPK, 5′AMP-activated protein kinase; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; LKB1, liver kinase B1; CaMKKα/β, Ca2+/calmodulin-dependent protein kinase kinase α/β; NAD+, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; PDE, phosphodiesterase; PKA, protein kinase A; PKC, protein kinase C; SIRT1, silent information regulator 1: regulation inhibition; and: regulation direction.
AMPK Phosphorylation
The effects of different concentrations of RSV (0, 0.01, 0.1 and 1 µM) on the level of AMPK phosphorylation in thawed sperm, were evaluated (Figure 1B). RSV increased phospho-Thr172-AMPK levels in a dose-dependent manner compared to untreated frozen–thawed semen (Figure 1C; P < 0.05). Therefore, we observed the highest level of AMPK phosphorylation in the RSV-1 group compared to other groups (Figure 1)
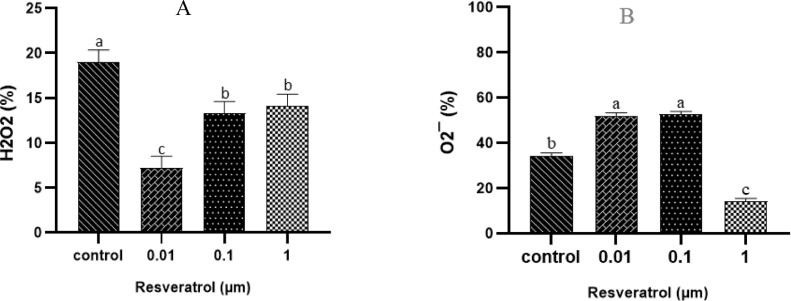
Figure 3.
Effect of different concentrations of RSV on H2O2 level (A) and O2- (B) in frozen-thawed rooster sperm (n=6). Values with different letters are significantly different (P < 0.05).
ROS Measurement
As depicted in Figure 3 The lowest level of H2O2 (7.2 ± 1.32) were observed in sperm of the RSV-0.01 group in comparison to the other groups (P < 0.05). Moreover, the highest percentage of H2O2 was observed in frozen control (19 ± 1.32) compared to the other groups (P < 0.05). The lowest percentages of O2− was in found in the RSV-1 (25.3 ± 2.56) in comparison to the other groups (P < 0.05).
Figure 2.
The effects of different concentrations of RSV on the percentage of viable, apoptotic, and dead thawed rooster sperm (n = 6), Values with different letters are significantly different (P < 0.05). Data are expressed as mean ± SEM.
DISCUSSION
Semen cryopreservation is a valuable technique for long-term storage of poultry species sperm (Shahverdi et al., 2015). This technique affects some cellular physiochemical processes which can cause lethal and sublethal damage to sperm (Hezavehei et al., 2019b). Increased ROS and oxidative stress result in reduction of the viability and fertility ability of sperm during cryopreservation. Resveratrol is a natural nonflavonoid polyphenolic compound that found largely in red fruits. This substance has antioxidant properties and has been introduced as free radicals scavenger against lipid peroxidation (LPO). This phenol has important pleiotropic effects including anticancer, antiaging, cardio- and neuroprotectant. The chemical structure of RSV enables the molecule to exert its antioxidant activity through different pathways. It can deactive many number of oxidants (such as superoxide anion, hydrogen peroxide) with a process of hydrogen atom transfer and sequential proton loss. Furthermore, RSV decreases ROS accumulation by enhancing mitochondrial biogenesis and reducing electron flow. Also, RSV indirectly able to upregulate intrinsic antioxidant enzymes including SOD and catalase (Falchi et al., 2020). previous studies reported that cryopreservation has deleterious effects on sperm metabolism (e.g., it influences ATP level) (Agarwal et al., 2006; Aitken and Baker, 2006). Therefore, sperm cells need energy to conserve their essential functions. It was suggested that AMPK activators could lead to phosphorylation of AMPK protein which has a protective role against cryo-injury. In the present study, we hypothesized that RSV as AMPK activator can improve functional parameters of rooster thawed sperm through activation of 5’ AMP-activated protein kinase. Our results indicated that 0.1 µM RSV could improve rooster sperm motility after freeze-thaw process. Zhu et al. (2019) confirmed the positive effect of RSV on boar sperm motility during cryopreservation (Zhu et al., 2019). AMPK might phosphorylate downstream substrates including proteins of the axoneme or structures that are indispensable for sperm flagellar motility (Martin-Hidalgo et al., 2018). Moreover, we observed a decrease in motility in the RSV-1. It seems that increasing and decreasing AMPK activity above physiological levels have negative effect on sperm motility in human (Calle‐Guisado et al., 2017) and boar (Hurtado de Llera et al., 2015). Therefore, a particular physiological level of AMPK activity is essential to accomplish optimal sperm motility. Our results confirmed negative correlation between phospho-AMPK levels above physiological levels (1 µM RSV) and sperm motility.
Importantly, in the present study, the rate of apoptosis was reduced while the rate of viability, and membrane integrity were increased in sperm treated with 0.1 μM RSV. Our results are in agreement with those reported by Zhu et al. and Lv et al. who reported that RSV increased membrane integrity in boar and goat sperm after freeze-thaw process (Lv et al., 2019; Zhu et al., 2019). Our results also showed that 0.01 and 0.1 µM RSV could enhance the percentage of Mitochondrial Membrane Potential (MMP) in thawed sperm during cryopreservation. It agrees with the results of Najafi et al. (2019) who demonstrated the protective effect of RSV on rooster sperm mitochondrial activity after freeze-thawing (Najafi et al., 2019).
Mitochondria plays a critical role in ATP synthesis and any damage to mitochondria leads to extending apoptosis (Paoli et al., 2011; Piomboni et al., 2012). Cryodamage may have destructive effects on MMP and ATP generation. (Tchir and Acker, 2010). It is noteworthy that AMPK has a key role in energy balance and a specific level of AMPK activity in sperm is essential to maintain a suitable MMP. The involvement of AMPK in controlling MMP has been demonstrated in boar (De Llera et al., 2013; Hurtado de Llera et al., 2015), mice (Tartarin et al., 2012), and human sperm (Shabani Nashtaei et al., 2017). Also, it has been reported that AMPK α1 knockout mice had a lower number of mitochondria and a reduced MMP (Tartarin et al., 2012). Therefore, AMPK activity at physiologic levels can be necessary to maintain sperm MMP (Hurtado de Llera et al., 2015; de Llera et al., 2016). AMPK protein acts as an energy regulator by activating metabolic pathways and producing ATP and simultaneously inhibiting ATP-consuming anabolic pathways (Kahn et al., 2005; Hardie, 2011; De Llera et al., 2013). Therefore, activation of the AMPK protein is necessary to maintain ATP levels under ATP-limiting conditions (Hurtado de Llera et al., 2015). Accordingly, phosphorylation of AMPK protein by RSV has been performed through various mechanisms and clearly revealed in the previous studies (Yun et al., 2014; Nashtaei et al., 2018; Zhu et al., 2019). AMPK protein is extremely sensitive to AMP level and increasing the AMP / ATP ratio stimulates AMPK activity due to a decrease in cellular energy (Suter et al., 2006). Indeed, AMP can cause increased phosphorylation at Thr-172 located in the α subunit, finally leading to phosphorylation of AMPK protein (Suter et al., 2006). Resveratrol can cause AMPK phosphorylation via upstream serine/threonine kinases such as LKB1 (Dasgupta and Milbrandt, 2007; Biasutto et al., 2012). Furthermore, RSV through signaling pathways including calcium/calmodulin-dependent protein kinase β (CaMKKb), cyclic adenosine monophosphate (cAMP), silent information regulator 1 (SIRT1), protein kinase A (PKA) and protein kinase C (PKC), which lead to AMPK phosphorylation in sperm (Figure 1A) (Vingtdeux et al., 2010; Shabani Nashtaei et al., 2017; Martin-Hidalgo et al., 2018). In this study, we showed that 1 µM RSV directly or via intermediate elements, could increase expression level of AMPK phosphorylation in rooster sperm after freeze-thawing. Our results were in agreement with the findings of Zhu et al. (2019), who reported that 50 µM RSV could increase phosphorylation of AMPK and improve motility, membrane integrity and membrane mitochondrial potential in boar sperm after thawing (Zhu et al., 2019). In addition, 25 µM RSV increased AMP-activated protein kinase phosphorylation in human frozen-thawed spermatozoa (Shabani Nashtaei et al., 2017). In one study was reported that apoptosis-like changes increased during freezing process (Martin et al., 2004). It was reported that RSV as a free radical scavenger can reduce apoptosis-like changes in sperm cells (Najafi et al., 2019). Our findings were in agreement with those reported by Attia et al. (2012), who reported that transfer of phosphatidylserine to the cell surface and caspase-3 activity reduced following treatment with RSV (Attia, 2012). Jiang and colleagues similarly reported that RSV protected rat sperm against apoptotic damages (Jiang et al., 2008). Moreover, 50 µM RSV led to increased percentage of live sperm and decreased percentage of dead sperm in goat (Lv et al., 2019). It was reported that RSV can decrease ROS production and improve antioxidative defense system in terms of catalase, SOD, GPx, and GSH level in sperm (Zhu et al., 2019). Oxidative stress generates the lipid peroxidation in sperm plasma membrane and one of the end products of lipid peroxidation is malondialdehyde (MDA). An increase in free radicals can lead to overproduction of MDA which is commonly known as a marker of oxidative stress and the antioxidant deficiency (Gaweł et al., 2004). Also, MDA increases usage of various antioxidants to fight the free radicals, and therefore a reduction happens in total antioxidant capacity of sperm. Polyphenol compound especially flavonoid compounds such as RSV inhibits lipid peroxidation to capture free radicals (Aziz et al., 2021). Our results indicated that 0.01, 0.1 or 1 µM RSV decreased MDA level in sperm after thawing. A previous study showed that adding 40 µM RSV to freezing extender prevented MDA production in thawed rooster sperm (Najafi et al., 2019) potentially due to the antioxidant properties of phenolic groups in RSV (Burkitt and Duncan, 2000). It is also worth mentioning that lipophilic structure in RSV may inhibit lipid peroxidation induced by Fenton reaction products (Berrougui et al., 2009). Collodel et al. (2011) reported that RSV could decrease lipid peroxidation stimulated by tert-butyl hydroperoxide in human sperm (Collodel et al., 2011). In this study, higher concentration of MDA in control group compared to the other groups indicating that ROS not only can destroy sperm membrane but also can reduce sperm motility and viability as well (Salehi et al., 2020). However, using of RSV stopped this detrimental effects because, RSV inhibited ROS cumulation inside the sperm cells (Sun et al., 2020). Many studies indicated that RSV suppresses ROS production by activating AMPK phosphorylation and enhancing the antioxidative system performance including GSH level and activities of glutathione peroxidase (GPx), SOD, and catalase (Pasquariello et al., 2020). Our finding indicated that 0.01 and 1 µM RSV could reduce level of H2O2 and O2− in thawed rooster sperm, respectively. Mojica-Villegas et al. (2014), demonstrated that RSV significantly reduced the production of ROS in mouse sperm (Mojica-Villegas et al., 2014). Sato et al. reported that RSV via reducing ROS, had a positive effect on mitochondrial quality (Sato et al., 2014). In the present study, we observed that 0.1 µM RSV improved sperm functional parameters after thawing, while higher phosphorylation of AMPK protein induced by 1 µM RSV, in a dose-dependent manner, had destructive effects on sperm quality. It seems that level of AMPK phosphorylation induced by 0.1 µM RSV may be suitable for maintaining rooster sperm functionality after thawing. Therefore, further investigations are needed to explain the mechanism underlying RSV protective effects on sperm cryoinjury particularly its influence on AMP-activated protein kinase.
CONCLUSIONS
Our findings indicated that cryosurvival of rooster semen can be improved by exposure to RSV. Application of 0.1 µM RSV as an AMP-activated protein kinase activator, before cryopreservation increased phospho-Thr172-AMPK levels and numerous functional parameters of thawed sperm.This study demonstrated that RSV may protecte thawed sperm quality by activation of AMPK. This study is the first report on the effects of RSV on the level of AMPK phosphorylation in rooster sperm during cryopreservation. Therefore, regulatory roles of intermediate signaling components in activation of AMP protein kinase in sperm cryobiology should be assessed in future.
Acknowledgments
ACKNOWLEDGMENTS
The authors appreciate the financial support provided by the Deputy of Research of Tarbiat Modares University and Royan Institute.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Agarwal A., Said T.M., Bedaiwy M.A., Banerjee J., Alvarez J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006;86:503–512. doi: 10.1016/j.fertnstert.2006.02.088. [DOI] [PubMed] [Google Scholar]
- Aitken R.J., Baker M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell. Endocrinol. 2006;250:66–69. doi: 10.1016/j.mce.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Amidi F., Pazhohan A., Nashtaei M.S., Khodarahmian M., Nekoonam S. The role of antioxidants in sperm freezing: a review. Cell Tissue Bank. 2016;17:745–756. doi: 10.1007/s10561-016-9566-5. [DOI] [PubMed] [Google Scholar]
- Attia S.M. Influence of resveratrol on oxidative damage in genomic DNA and apoptosis induced by cisplatin. Mutat. Res. Toxicol. Environ. Mutagen. 2012;741:22–31. doi: 10.1016/j.mrgentox.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Aziz A.M., Permatasari N., Soeharto S., Nugrahenny D. Effect of Physalis angulata L. leaf water extract on malondialdehyde (MDA) testis, calcium intracellular sperm and total motile sperm of male wistar rats (Rattus novergicus) model of hypertension. Med. Leg. Updat. 2021;21:69–75. [Google Scholar]
- Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Berrougui H., Grenier G., Loued S., Drouin G., Khalil A. A new insight into resveratrol as an atheroprotective compound: inhibition of lipid peroxidation and enhancement of cholesterol efflux. Atherosclerosis. 2009;207:420–427. doi: 10.1016/j.atherosclerosis.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Biasutto L., Mattarei A., Zoratti M. Resveratrol and health: the starting point. Chembiochem. 2012;13:1256–1259. doi: 10.1002/cbic.201200193. [DOI] [PubMed] [Google Scholar]
- Bucak M.N., Ataman M.B., Başpınar N., Uysal O., Taşpınar M., Bilgili A., Öztürk C., Güngör Ş., Inanc M.E., Akal E. Lycopene and resveratrol improve post-thaw bull sperm parameters: sperm motility, mitochondrial activity and DNA integrity. Andrologia. 2015;47:545–552. doi: 10.1111/and.12301. [DOI] [PubMed] [Google Scholar]
- Burkitt M.J., Duncan J. Effects of trans-resveratrol on copper-dependent hydroxyl-radical formation and DNA damage: evidence for hydroxyl-radical scavenging and a novel, glutathione-sparing mechanism of action. Arch. Biochem. Biophys. 2000;381:253–263. doi: 10.1006/abbi.2000.1973. [DOI] [PubMed] [Google Scholar]
- Calle-Guisado V., Hurtado de Llera A., González-Fernández L., Bragado M.J., Garcia-Marin L.J. Human sperm motility is downregulated by the AMPK activator A769662. Andrology. 2017;5:1131–1140. doi: 10.1111/andr.12423. [DOI] [PubMed] [Google Scholar]
- Collodel G., Federico M.G., Geminiani M., Martini S., Bonechi C., Rossi C., Figura N., Moretti E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011;31:239–246. doi: 10.1016/j.reprotox.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Córdova A., Strobel P., Vallejo A., Valenzuela P., Ulloa O., Burgos R.A., Menarim B., Rodríguez-Gil J.E., Ratto M., Ramírez-Reveco A. Use of hypometabolic TRIS extenders and high cooling rate refrigeration for cryopreservation of stallion sperm: presence and sensitivity of 5′ AMP-activated protein kinase (AMPK) Cryobiology. 2014;69:473–481. doi: 10.1016/j.cryobiol.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Dasgupta B., Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslami M., Hashem E.Z., Ghaniei A., Sayyah-Atashbeig H. Evaluation of linoleic acid on lipid peroxidative/antioxidative parameters, motility and viability of rooster spermatozoa during cold storage. Cell Tissue Bank. 2018;19:799–807. doi: 10.1007/s10561-018-9738-6. [DOI] [PubMed] [Google Scholar]
- Falchi L., Pau S., Pivato I., Bogliolo L., Zedda M.T. Resveratrol supplementation and cryopreservation of buck semen. Cryobiology. 2020;95:60–67. doi: 10.1016/j.cryobiol.2020.06.005. [DOI] [PubMed] [Google Scholar]
- Fujihara N., Koga O. Prevention of the production of lipid peroxide in rooster spermatozoa. Anim. Reprod. Sci. 1984;7:385–390. [Google Scholar]
- Gambini J., Inglés M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell. Longev. 2015;2015 doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez M.E., dos Santos Branco C., Lara L.V., Pasqualotto F.F., Salvador M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil. Steril. 2010;94:2118–2121. doi: 10.1016/j.fertnstert.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Gaweł S., Wardas M., Niedworok E., Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. (Warsaw, Pol. 1960) 2004;57:453–455. [PubMed] [Google Scholar]
- Ghaleno L.R., Valojerdi M.R., Janzamin E., Chehrazi M., Sharbatoghli M., Yazdi R.S. Evaluation of conventional semen parameters, intracellular reactive oxygen species, DNA fragmentation and dysfunction of mitochondrial membrane potential after semen preparation techniques: a flow cytometric study. Arch. Gynecol. Obstet. 2014;289:173–180. doi: 10.1007/s00404-013-2946-1. [DOI] [PubMed] [Google Scholar]
- Guerrero R.F., Garcia-Parrilla M.C., Puertas B., Cantos-Villar E. Wine, resveratrol and health: a review. Nat. Prod. Commun. 2009;4:635–658. 1934578X0900400503. [PubMed] [Google Scholar]
- Hardie D.G. AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D.G. The AMP-activated protein kinase pathway–new players upstream and downstream. J. Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Hawley S.A., Scott J.W. AMP‐activated protein kinase–development of the energy sensor concept. J. Physiol. Paris. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S.A., Ross F.A., Chevtzoff C., Green K.A., Evans A., Fogarty S., Towler M.C., Brown L.J., Ogunbayo O.A., Evans A.M. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezavehei M., Kouchesfahani H.M., Shahverdi A., Sharafi M., Salekdeh G.H., Eftekhari-Yazdi P. Preconditioning of sperm with sublethal nitrosative stress: a novel approach to improve frozen–thawed sperm function. Reprod. Biomed. Online. 2019;38:413–425. doi: 10.1016/j.rbmo.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Hezavehei M., Kouchesfahani H.M., Shahverdi A., Sharafi M., Salekdeh G.H., Eftekhari-Yazdi P. Induction of sublethal oxidative stress on human sperm before cryopreservation: a time-dependent response in post-thawed sperm parameters. Cell J. 2019;20:537. doi: 10.22074/cellj.2019.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado de Llera A., Martin-Hidalgo D., Gil M.C., Garcia-Marin L.J., Bragado M.J. AMPK up-activation reduces motility and regulates other functions of boar spermatozoa. Mol. Hum. Reprod. 2015;21:31–45. doi: 10.1093/molehr/gau091. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Tao P., Yong L.U.O., Li M., Lin Y. Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2, 5-hexanedione. Chin. Med. J. (Engl). 2008;121:1204–1209. [PubMed] [Google Scholar]
- Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Leonard S.S., Xia C., Jiang B.-H., Stinefelt B., Klandorf H., Harris G.K., Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- de Llera A.H., Martin-Hidalgo D., Gil M.C., Garcia-Marin L.J., Bragado M.J. New insights into transduction pathways that regulate boar sperm function. Theriogenology. 2016;85:12–20. doi: 10.1016/j.theriogenology.2015.05.008. [DOI] [PubMed] [Google Scholar]
- De Llera A.H., Martin-Hidalgo D., Gil M.C., Garcia-Marin L.J., Bragado M.J. AMP-activated kinase AMPK is expressed in boar spermatozoa and regulates motility. PLoS One. 2012;7:e38840. doi: 10.1371/journal.pone.0038840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Llera A.H., Martin-Hidalgo D., Rodriguez-Gil J.E., Gil M.C., Garcia-Marin L.J., Bragado M.J. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim. Biophys. Acta (BBA)-Biomembranes. 2013;1828:2143–2151. doi: 10.1016/j.bbamem.2013.05.026. [DOI] [PubMed] [Google Scholar]
- Longobardi V., Zullo G., Salzano A., De Canditiis C., Cammarano A., De Luise L., Puzio M.V., Neglia G., Gasparrini B. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology. 2017;88:1–8. doi: 10.1016/j.theriogenology.2016.09.046. [DOI] [PubMed] [Google Scholar]
- Lv C., Larbi A., Wu G., Hong Q., Quan G. Improving the quality of cryopreserved goat semen with a commercial bull extender supplemented with resveratrol. Anim. Reprod. Sci. 2019;208 doi: 10.1016/j.anireprosci.2019.106127. [DOI] [PubMed] [Google Scholar]
- Martin-Hidalgo D., Hurtado de Llera A., Calle-Guisado V., Gonzalez-Fernandez L., Garcia-Marin L., Bragado M.J. AMPK function in mammalian spermatozoa. Int. J. Mol. Sci. 2018;19:3293. doi: 10.3390/ijms19113293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G., Sabido O., Durand P., Levy R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004;71:28–37. doi: 10.1095/biolreprod.103.024281. [DOI] [PubMed] [Google Scholar]
- Mojica-Villegas M.A., Izquierdo-Vega J.A., Chamorro-Cevallos G., Sánchez-Gutiérrez M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients. 2014;6:489–503. doi: 10.3390/nu6020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi M.M., Kohram H., Zhandi M., Mehrabani-Yeganeh H., Sharideh H., Zare-Shahaneh A., Esmaili V. Comparative evaluation of Nabi and Beltsville extenders for cryopreservation of rooster semen. Cryobiology. 2016;72:47–52. doi: 10.1016/j.cryobiol.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Najafi A., Kia H.D., Hamishehkar H., Moghaddam G., Alijani S. Effect of resveratrol-loaded nanostructured lipid carriers supplementation in cryopreservation medium on post-thawed sperm quality and fertility of roosters. Anim. Reprod. Sci. 2019;201:32–40. doi: 10.1016/j.anireprosci.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Nashtaei M.S., Nekoonam S., Naji M., Bakhshalizadeh S., Amidi F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 5′ AMP-activated protein kinase activation. Cell Tissue Bank. 2018;19:87–95. doi: 10.1007/s10561-017-9642-5. [DOI] [PubMed] [Google Scholar]
- Nguyen T.M.D., Alves S., Grasseau I., Métayer-Coustard S., Praud C., Froment P., Blesbois E. Central role of 5′-AMP-activated protein kinase in chicken sperm functions. Biol. Reprod. 2014;91:121. doi: 10.1095/biolreprod.114.121855. [DOI] [PubMed] [Google Scholar]
- Nguyen T.M.D., Seigneurin F., Froment P., Combarnous Y., Blesbois E. The 5’-AMP-activated protein kinase (AMPK) is involved in the augmentation of antioxidant defenses in cryopreserved chicken sperm. PLoS One. 2015;10 doi: 10.1371/journal.pone.0134420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoli D., Gallo M., Rizzo F., Baldi E., Francavilla S., Lenzi A., Lombardo F., Gandini L. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil. Steril. 2011;95:2315–2319. doi: 10.1016/j.fertnstert.2011.03.059. [DOI] [PubMed] [Google Scholar]
- Pasquariello R., Verdile N., Brevini T.A.L., Gandolfi F., Boiti C., Zerani M., Maranesi M. The controversial roles of resveratrol in mammalian reproduction. Molecules. 2020;25:4554. doi: 10.3390/molecules25194554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomboni P., Focarelli R., Stendardi A., Ferramosca A., Zara V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012;35:109–124. doi: 10.1111/j.1365-2605.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- Price N.L., Gomes A.P., Ling A.J.Y., Duarte F.V, Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell S.G., Mrode R.A. An osmotic resistance test for bovine semen. Anim. Reprod. Sci. 1994;36:77–86. [Google Scholar]
- Rubin L.J., Magliola L., Feng X., Jones A.W., Hale C.C. Metabolic activation of AMP kinase in vascular smooth muscle. J. Appl. Physiol. 2005;98:296–306. doi: 10.1152/japplphysiol.00075.2004. [DOI] [PubMed] [Google Scholar]
- Saiko P., Szakmary A., Jaeger W., Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat. Res. Mutat. Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Salehi M., Mahdavi A.H., Sharafi M., Shahverdi A. Cryopreservation of rooster semen: evidence for the epigenetic modifications of thawed sperm. Theriogenology. 2020;142:15–25. doi: 10.1016/j.theriogenology.2019.09.030. [DOI] [PubMed] [Google Scholar]
- Sato D., Itami N., Tasaki H., Takeo S., Kuwayama T., Iwata H. Relationship between mitochondrial DNA copy number and SIRT1 expression in porcine oocytes. PLoS One. 2014;9:e94488. doi: 10.1371/journal.pone.0094488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani Nashtaei M., Amidi F., Sedighi Gilani M.A., Aleyasin A., Bakhshalizadeh S., Naji M., Nekoonam S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5’AMP-activated protein kinase activation. Andrology. 2017;5:313–326. doi: 10.1111/andr.12306. [DOI] [PubMed] [Google Scholar]
- Shahverdi A., Sharafi M., Gourabi H., Yekta A.A., Esmaeili V., Sharbatoghli M., Janzamin E., Hajnasrollahi M., Mostafayi F. Fertility and flow cytometric evaluations of frozen-thawed rooster semen in cryopreservation medium containing low-density lipoprotein. Theriogenology. 2015;83:78–85. doi: 10.1016/j.theriogenology.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Sun L., Fan X., Zeng Y., Wang L., Zhu Z., Li R., Tian X., Wang Y., Lin Y., Wu D. Resveratrol protects boar sperm in vitro via its antioxidant capacity. Zygote. 2020;28:417–424. doi: 10.1017/S0967199420000271. [DOI] [PubMed] [Google Scholar]
- Suter M., Riek U., Tuerk R., Schlattner U., Wallimann T., Neumann D. Dissecting the role of 5′-AMP for allosteric stimulation, activation, and deactivation of AMP-activated protein kinase. J. Biol. Chem. 2006;281:32207–32216. doi: 10.1074/jbc.M606357200. [DOI] [PubMed] [Google Scholar]
- Tartarin P., Guibert E., Touré A., Ouiste C., Leclerc J., Sanz N., Brière S., Dacheux J.-L., Delaleu B., McNeilly J.R. Inactivation of AMPKα1 induces asthenozoospermia and alters spermatozoa morphology. Endocrinology. 2012;153:3468–3481. doi: 10.1210/en.2011-1911. [DOI] [PubMed] [Google Scholar]
- Taylor K., Roberts P., Sanders K., Burton P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online. 2009;18:184–189. doi: 10.1016/s1472-6483(10)60254-4. [DOI] [PubMed] [Google Scholar]
- Tchir J., Acker J.P. Mitochondria and membrane cryoinjury in micropatterned cells: effects of cell–cell interactions. Cryobiology. 2010;61:100–107. doi: 10.1016/j.cryobiol.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Towler M.C., Hardie D.G. AMP-activated protein kinase in metabolic control and insulin signaling. Circ. Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H., Park S., Kim M., Yang W.K., Im D.U., Yang K.R., Hong J., Choe W., Kang I., Kim S.S. AMP-activated protein kinase mediates the antioxidant effects of resveratrol through regulation of the transcription factor FoxO1. FEBS J. 2014;281:4421–4438. doi: 10.1111/febs.12949. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Fan X., Lv Y., Zhang N., Fan C., Zhang P., Zeng W. Vitamin E analogue improves rabbit sperm quality during the process of cryopreservation through its antioxidative action. PLoS One. 2015;10 doi: 10.1371/journal.pone.0145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Li R., Fan X., Lv Y., Zheng Y., Hoque S.A., Wu D., Zeng W. Resveratrol improves boar sperm quality via 5AMP-activated protein kinase activation during cryopreservation. Oxid. Med. Cell. Longev. 2019;2019:5921503. doi: 10.1155/2019/5921503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Li R., Ma G., Bai W., Fan X., Lv Y., Luo J., Zeng W. 5’-AMP-Activated protein kinase regulates goat sperm functions via energy metabolism in vitro. Cell. Physiol. Biochem. 2018;47:2420–2431. doi: 10.1159/000491616. [DOI] [PubMed] [Google Scholar]