Significance
Despite improved understanding of cellular and molecular events mediating type 2 immune responses in the gut, how these changes facilitate worm expulsion remains largely unknown. We identified pyroptosis-effector gasdermin C genes (Gsdmcs) as type 2 cytokine-regulated target genes in intestinal epithelial cells. Furthermore, we showed that overexpression of Gsdmc2 triggered pyroptosis. Organoid cultures and in vivo mouse models were used to show that activating type 2 immunity leads to lytic cell death in gut epithelium and cleavage of Gsdmcs. We thus revealed a role of gasdermin-mediated lytic cell death in worm-induced type 2 immunity. Parasitic infection often leads to malnutrition and retarded growth; since this may involve villus atrophy, gasdermin activity may provide a pharmaceutical target for treatment.
Keywords: gasdermin, pyroptosis, IL-4/IL-13, type 2 immunity, helminth
Abstract
“Taste-like” tuft cells in the intestine trigger type 2 immunity in response to worm infection. The secretion of interleukin-13 (IL-13) from type 2 innate lymphoid cells (ILC2) represents a key step in the tuft cell–ILC2 cell–intestinal epithelial cell circuit that drives the clearance of worms from the gut via type 2 immune responses. Hallmark features of type 2 responses include tissue remodeling, such as tuft and goblet cell expansion, and villus atrophy, yet it remains unclear if additional molecular changes in the gut epithelium facilitate the clearance of worms from the gut. Using gut organoids, we demonstrated that IL-4 and IL-13, two type 2 cytokines with similar functions, not only induced the classical type 2 responses (e.g., tuft cell expansion) but also drastically up-regulated the expression of gasdermin C genes (Gsdmcs). Using an in vivo worm-induced type 2 immunity model, we confirmed the up-regulation of Gsdmcs in Nippostrongylus brasiliensis–infected wild-type C57BL/6 mice. Consistent with gasdermin family members being principal effectors of pyroptosis, overexpression of Gsdmc2 in human embryonic kidney 293 (HEK293) cells triggered pyroptosis and lytic cell death. Moreover, in intestinal organoids treated with IL-4 or IL-13, or in wild-type mice infected with N. brasiliensis, lytic cell death increased, which may account for villus atrophy observed in worm-infected mice. Thus, we propose that the up-regulated Gsdmc family may be major effectors for type 2 responses in the gut and that Gsdmc-mediated pyroptosis may provide a conduit for the release of antiparasitic factors from enterocytes to facilitate the clearance of worms.
Helminth (worm) infection remains prevalent in developing countries and represents a significant health burden that negatively affects the development and health of infected children (1). Type 2 immunity is the primary host defense mechanism for helminth infection, mediating physiological changes that facilitate worm expulsion (2).
The interaction of tuft cells with type 2 innate lymphoid cells (ILC2) and the subsequent interaction of ILC2 cells with intestinal epithelial cells represent a primary circuit that initiates and sustains type 2 responses in the intestines (3–5). Tuft cells detect parasites, protozoans, and other infectious microbes via tuft cell–expressed Sucnr1 or other poorly characterized receptors to initiate type 2 responses (6–8). The activation of taste signaling elements expressed in tuft cells, such as gustducin and Trpm5, leads to the secretion of interleukin-25 (IL-25), leukotrienes, and other mediators (3–9). Subsequently, IL-25 activates mucosal ILC2 cells to secrete IL-13 (10–14), which contributes to gut tissue remodeling, including tuft and goblet cell expansion, and villus atrophy (3–5, 10, 15, 16). Despite the fact that worm-induced cellular remodeling of the gut epithelium is well established, how the gut gets rid of worms remains unclear, especially at the molecular level. We hypothesized that molecular changes in gut epithelium may occur upon worm infection, which may prepare intestinal epithelial cells to contribute to an inflammatory environment and facilitate the expulsion of worms from the gut. Because the type 2 cytokines IL-13 and IL-4 act as master cytokines that regulate type 2 immunity (15), the identification of target genes for these type 2 cytokines may fill the gap in our understanding of the molecular changes responsive to type 2 cytokines or infectious parasites.
Gasdermin proteins are key effectors of pyroptosis, a highly inflammatory form of programmed cell death triggered by intracellular and extracellular pathogens, which represents a prominent antimicrobial response (17–19). Rodents have four gasdermin C proteins (Gsdmcs) compared to a single GSDMC protein in humans. The functions of Gsdmcs within the family of gasdermin proteins are poorly explored. However, a recent publication showed that human GSDMC can facilitate the production of tumor necrosis by inducing pyroptosis (20). Here, we demonstrated that the up-regulation of Gsdmc genes is a key feature of mucosal type 2 immunity. Like other gasdermin family members, Gsdmc2 is a pyroptotic effector, and overexpression of Gsdmc2 can lead to pyroptosis in cell culture systems. Up-regulation of Gsdmcs and the cleavage of Gsdmcs correlate with lytic cell death in intestinal epithelial cells in mice infected with the parasitic worm Nippostrongylus brasiliensis, a strong inducer of type 2 immunity, and in cultured intestinal organoids treated with type 2 cytokines. Lytic cell death may be a part of the host defense mechanism to facilitate worm clearance through the release of antiparasitic factors into the gut lumen.
Results
Gsdmcs Are Target Genes of Type 2 Cytokines.
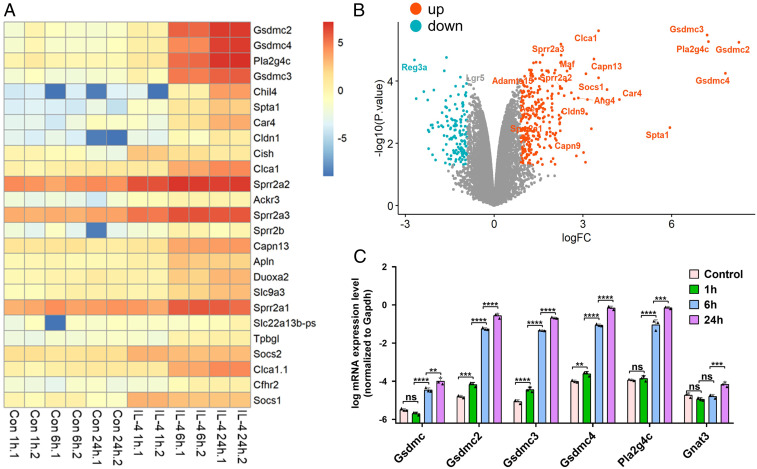
One of the characteristic type 2 immune responses is intestinal epithelial tissue remodeling, which includes tuft and goblet cell expansion, and villus atrophy (3–5). Despite these changes at the cellular level, the gene targets of type 2 cytokines that alter intestinal epithelial cell functionality to facilitate worm expulsion are largely unknown. We used intestinal organoids as an in vitro model system to study how the gut epithelial cells respond to type 2 cytokines (21). Intestinal organoids were treated with IL-4, and the transcriptome was analyzed 1, 6, and 24 h later and compared to control organoids without IL-4 treatment (Dataset S1). Three gasdermin Cs (Gsdmc2, Gsdmc3, and Gsdmc4) and Pla2g4c were prominent among the top 25 genes that showed a significant and time-dependent increase in gene expression (Fig. 1 A and B). Gsdmc was not found among the top 25 genes (Fig. 1 A and B), even though it increased markedly (Dataset S1), because the cutoff value to generate the graphs (Fig. 1 A and B) excluded low-expression genes like Gsdmc. Up-regulated expression of Pla2g4c in response to worm infection was reported previously (22). The increased expression of Gsdmcs and Pla2g4c in IL-4– or IL-13–treated organoids was further confirmed by qPCR analysis (Fig. 1 C and SI Appendix, Fig. S1). In contrast, Gnat3, which encodes the tuft cell marker gustducin, showed only a modest increase in expression 24 h after IL-4 treatment. As negative controls, the treatment of organoids with IL-25 or IL-33 (two alarmins secreted from tuft cells or epithelial cells to initiate type 2 immunity by activating ILC2 cells to release IL-13) (4) did not lead to the up-regulation of Gsdmcs, Pla2g4c, Dclk1 (tuft cell marker), or Gnat3 because of the lack of ILC2 cells in cultured intestinal epithelial organoids (SI Appendix, Fig. S1).
Fig. 1.
Up-regulation of Gsdmcs in intestinal organoids treated with the type 2 cytokine IL-4. (A and B) RNA-seq analyses of organoids treated with IL-4. (A) Heat map showing the top 25 up-regulated genes 1, 6, and 24 h after IL-4 stimulation (100 ng/mL) versus unstimulated controls (Con). Two biological replicates for each time point. (B) Volcano plot showing the fold change and P value of up-regulated (red) and down-regulated (blue) genes 24 h after IL-4 stimulation (100 ng/mL) versus unstimulated controls. x-axis: log fold change (FC). Cutoff for logFC is 0.9. (C) qPCR confirmation of the up-regulation of Gsdmc, Gsdmc2, Gsdmc3, Gsdmc4, Pla2g4c, and Gnat3 1, 6, and 24 h after IL-4 stimulation (100 ng/mL). **P < 0.01; ***P < 0.001; ****P < 0.0001; NS, not significant. Data (mean ± SEM) are technical replicates. qPCR experiments were repeated two more times with similar results.
Up-Regulation of Gsdmcs in Mice Infected with N. brasiliensis.
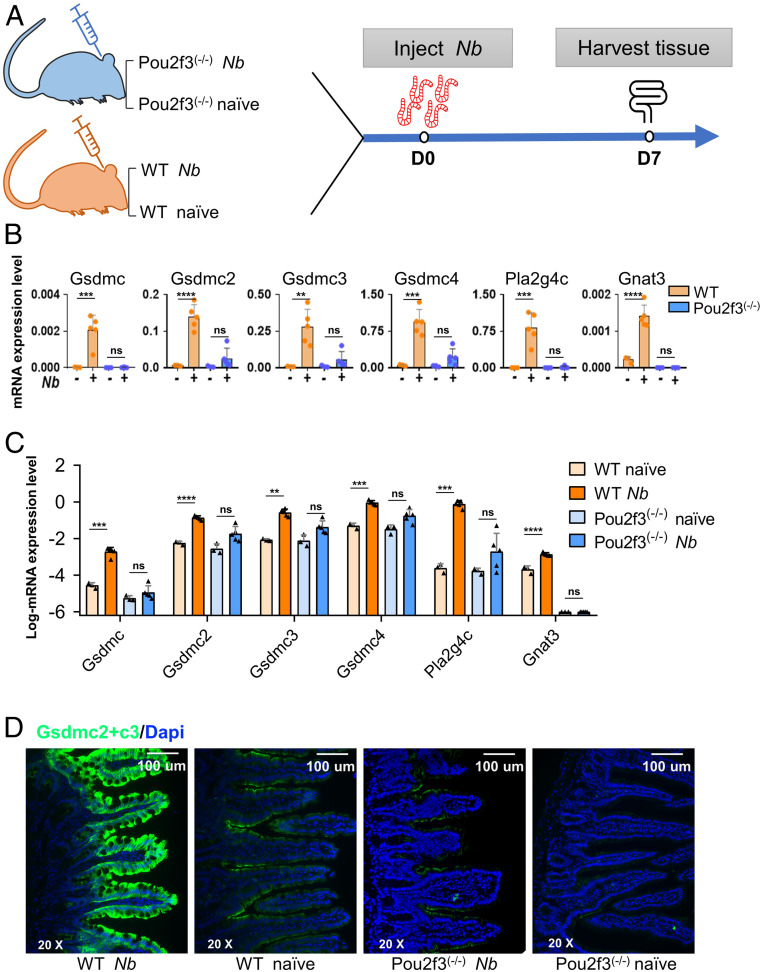
To determine if gasdermin C genes are up-regulated in the intestinal epithelium in type 2 immunity in vivo, N. brasiliensis, which induced strong type 2 immunity throughout the small intestine (e.g., tuft cell expansion; SI Appendix, Fig. S2), was used (4, 5, 23, 24). Both wild-type and Pou2f3−/− mice, which lack tuft cells (5), were inoculated with N. brasiliensis, and intestinal tissue was collected for qPCR analyses at 7 d postinoculation (dpi) (Fig. 2A). As reported previously, Pou2f3−/− mice showed delayed worm expulsion compared with wild-type mice because of a lack of tuft cells that initiate type 2 immunity (SI Appendix, Fig. S3) (5, 9). As anticipated, in wild-type mice, all four Gsdmcs showed up-regulated expression after N. brasiliensis infection: Gsdmc, Gsdmc2, Gsdmc3, and Gsdmc4 were up-regulated 72.8-, 23.9-, 33.0-, and 18.3-fold, respectively, compared to naïve mice (Fig. 2 B and C). Nevertheless, the fold increase was less than that in organoids treated with IL-4 (∼1,000 to 4,000 times in RNA sequencing [RNA-seq]), possibly due to a high amount of IL-4 (100 ng/mL) used to treat the organoids. Consistent with the organoid data, the expression of Pla2g4c was also increased around 3,000 times in the small intestine of mice infected with N. brasiliensis (Fig. 2 B and C). Tuft cell hyperplasia is a hallmark feature of type 2 immunity. As expected, expression of the tuft cell markers Gnat3 and IL-25 was also up-regulated in N. brasiliensis–infected wild-type mice (Fig. 2 B and C and SI Appendix, Fig. S4 A and B). Consistent with worm-induced activation and proliferation of ILC2 cells, IL-13 (ILC2 marker) was up-regulated as well. In contrast, IL-4, a type 2 cytokine not expressed in gut-residing ILC2 cells despite having functions similar to IL-13, showed no significant increase in expression, nor did we find changes in IL-33, an epithelial cell–derived cytokine that is neither expressed in tuft cells nor directly involved in sensing worm infection (SI Appendix, Fig. S4 A and B). In contrast to wild-type mice, the expression levels of Gsdmcs were markedly lower in naïve tuft cell–lacking Pou2f3−/− mice and slightly up-regulated after N. brasiliensis infection (Fig. 2 B and C). There was no significant increase in expression of Pla2g4c in N. brasiliensis–infected Pou2f3−/− mice (Fig. 2 B and C). As expected, Pou2f3−/− mice had little or no Gnat3 with or without N. brasiliensis infection (Fig. 2 B and C). Before entering the gut, N. brasiliensis resides in the lung tissue for a short period of time (∼2 d) (25); we therefore also examined if Gsdmcs are up-regulated in the lung. Unlike in the gut tissue, there was no significant change in the expression of Gsdmcs or in other genes examined at 7 dpi (SI Appendix, Fig. S5).
Fig. 2.
Up-regulation of Gsdmcs in gut epithelial tissue of mice infected with N. brasiliensis. (A) Schematic illustration of the treatment regimen. Intestinal tissues were collected from uninfected control (naïve) or N. brasiliensis (Nb)-infected wild-type or Pou2f3−/− mice at 7 dpi (D7). RNA was extracted, cDNA was generated, and qPCR was performed using primer sets for the indicated genes. (A and B) Expression levels of Gsdmcs, Pla2g4c, and Gnat3. (B) Data normalized to Gapdh [n = 3 (naïve) or 5 (Nb-infected)]. Each point represents a single mouse. Experiments were repeated two more times with similar results. (C) Log expression of data presented in B. **P < 0.01; ***P < 0.001; ****P < 0.0001; NS, not significant. (D) Representative images of anti-Gsdmc2/3 antibody staining of jejunal tissue sections. Strong staining was detected only in N. brasiliensis–infected wild-type mice and not in infected Pou2f3−/− mice. Data (mean ± SEM) are biological replicates (n = 3 or 5). Experiments were repeated two more times with similar results.
We further confirmed the qPCR results using a validated anti-Gsdmc2/3 antibody (95.2% homology between Gsdmc2 and Gsdmc3 protein sequences). In N. brasiliensis–infected wild-type mice, there was strong staining in the apical surface of enterocytes and potentially other types of intestinal epithelial cells throughout the villus but not in the crypt cells (Fig. 2D). In contrast, in naïve mice, only weak staining was found in the apical surface of villus cells and slightly stronger staining in the villous cells near the villus/crypt junction (Fig. 2D). In N. brasiliensis–infected Pou2f3−/− mice, weak staining was detectable, whereas no or little signal was detected in naïve Pou2f3−/− mice (Fig. 2D).
Succinate activates tuft cell–expressed Sucnr1 to trigger type 2 immunity (6–8). Concomitantly, the up-regulation of Gsdmcs was also evident in a single-cell RNA-seq data set analyzing the succinate-treated ileum/colon (24) (SI Appendix, Fig. S6). This set of data further supports that up-regulation of Gsdmcs is a common feature of the intestinal type 2 immune response.
Overexpression of Gsdmc2 Induced Pyroptosis and Lytic Cell Death in HEK293 Cells.
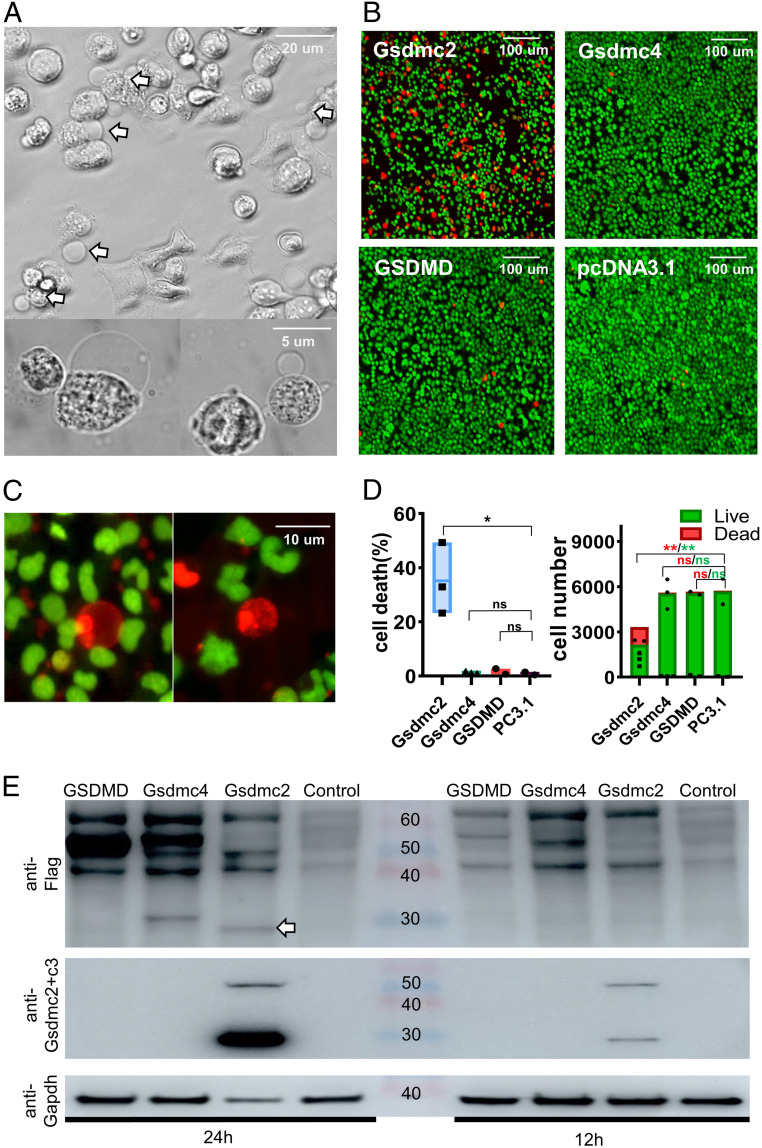
Gasdermin family members are principal mediators of pyroptosis and lytic cell death (26–28), but whether Gsdmcs can lead to pyroptosis remains unknown. To address this question, we used HEK293 cells, which have been utilized routinely for pyroptosis-related work (26–28), to determine if overexpression of Gsdmcs can lead to pyroptosis and if proteinases such as caspases are required to cleave Gsdmcs into functional fragments. HEK293 cells were transfected with C-terminal flag-tagged Gsdmc2, Gsdmc4 (the two most abundantly up-regulated Gsdmcs), GSDMD, or control vector pcDNA3.1 (two negative controls) and analyzed 24 h later. Strikingly, cells expressing Gsdmc2 without any other manipulation showed robust pyroptosis, such as cell swelling (bubbling) (Fig. 3A). However, such an effect was not observed with Gsdmc4 or GSDMD (the most studied member of the gasdermin family) (SI Appendix, Fig. S7). When tested with SYTOX orange (cell-impermeant nucleic acid stain for dead cells), we found abundant SYTOX orange–labeled cells in Gsdmc2-transfected wells but not in Gsdmc4-transfected, GSDMD-transfected, or sham-transfected (pcDNA3.1) wells (Fig. 3B). Fig. 3C shows single Gsdmc2-induced pyroptotic cells that were labeled with SYTOX orange. Overexpression of Gsdmc2 led to a significant increase in lytic cell death in about 35% of cells (Fig. 3D). The presence of live cells could be partly due to transfection efficiency. Fewer cells and more dead cells were present in Gsdmc2-transfected wells than in Gsdmc4-, GSDMD-, or mock-transfected wells (Fig. 3D). As a positive control, cotransfection of GSDMD and caspase-4 led to numerous SYTOX orange–labeled cells as reported previously (26, 29) (SI Appendix, Fig. S8).
Fig. 3.
Overexpression of Gsdmc2 in HEK293 cells led to pyroptosis and lytic cell death. HEK293 cells were transfected with flag-tagged Gsdmc2, Gsdmc4, GSDMD, or control vector pcDNA3.1. Images were taken 24 h after transfection. (A) Representative brightfield images showing pyroptosis in Gsdmc2-transfected cells (arrows). (B) Representative fluorescent images of Gsdmc2-, Gsdmc4-, or GSDMD-expressing cells stained with SYTO green (membrane-permeable dye to stain all cells) or SYTOX orange (membrane-impermeable dye to stain dead cells) at low magnification. Lytic cell death occurred in Gsdmc2-transfected cells but not in others. (C) Representative high-resolution images showing pyroptosis in Gsdmc2-transfected cells stained with SYTO green and SYTOX orange. (D) Statistical analysis of the percentage of dead cells (Left) and count of cell numbers (Right) in an 11 mm2 area. Data are mean ± SEM; *P < 0.05; **P < 0.01; NS, not significant. (E) Western blot analyses of flag-tagged Gsdmc2, Gsdmc4, and GSDMD protein using an anti-flag antibody (Top) or an anti-Gsdmc2/3 antibody (Middle). A 30-kDa band (truncated, arrow) was detected only in Gsdmc2-transfected samples. Gapdh (Bottom) was used as a control. Experiments were repeated five or more times, except Western blotting using anti-Gsdmc2/3 antibody, which was repeated three more times, all with similar results.
To further corroborate Gsdmc2-induced lytic cell death, the lactate dehydrogenase (LDH) assay, another indicator of lytic cell death, was performed. Increased release of LDH was noted in HEK293 cells expressing Gsdmc2 but not in cells expressing Gsdmc4 or GSDMD (SI Appendix, Fig. S9).
To determine if Gsdmc2 undergoes cleavage into functional fragments, we performed Western blotting analysis by staining for the C-terminal flag tag. As shown in Fig. 3 E, Top, several bands were detected 24 or 12 h after transfection with Gsdmc2. Cells transfected with Gsdmc4 or GSDMD showed a similar pattern, except for a band around 30 kDa, which was detected only in the sample prepared from Gsdmc2-transfected cells. Using the anti-Gsdmc2/3 antibody, we confirmed the presence of the prominent 30 kDa band, corresponding to the truncated C-terminal fragment of Gsdmc2, and the 55 kDa band, corresponding to the full-length Gsdmc2 (Fig. 3 E, Middle). Therefore, this set of data suggested that, like other gasdermin family members, Gsdmc2 may be subject to cleavage into functional fragments, and the cleaved N-terminal fragment may mediate pyroptosis. Unlike other members of the gasdermin family, no exogenous proteinases are required for the cleavage of Gsdmc2 into functional fragments in HEK293 cells.
Increased Lytic Cell Death in Organoids Treated with IL-4 or IL-13.
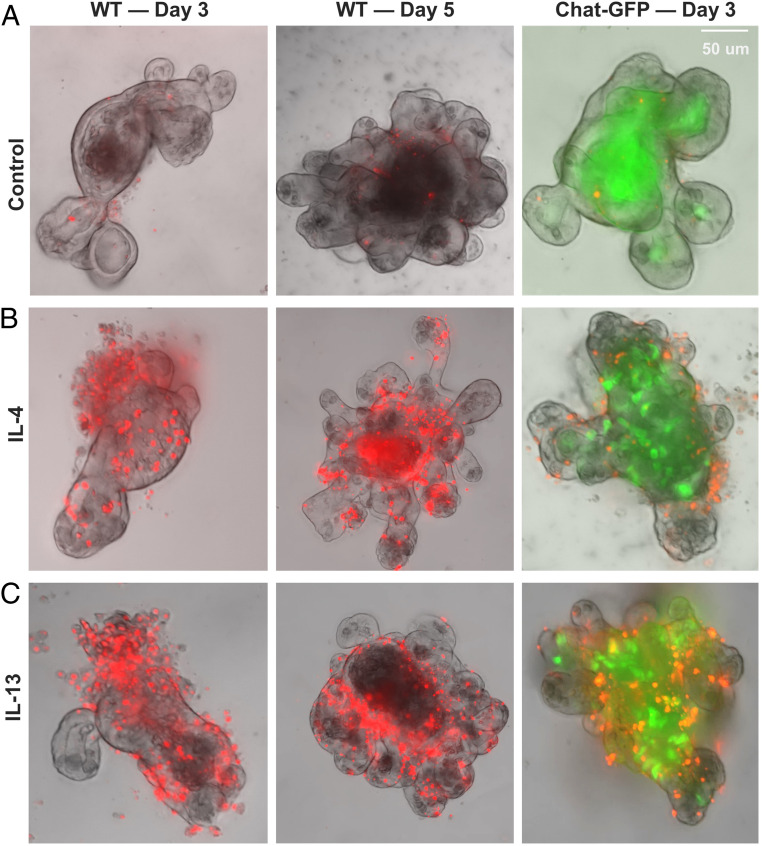
Given that overexpression of Gsdmcs can lead to pyroptosis and lytic cell death in HEK cells, we next asked whether up-regulated Gsdmcs in intestinal organoids correlated with increased lytic cell death. We treated organoids with IL-4 or IL-13 for 3 or 5 d and examined if cells in organoids take up SYTOX orange dye. In control-untreated organoids, only a few cells in the epithelial layer of the organoid were stained with SYTOX orange (Fig. 4A). In contrast, in IL-4– or IL-13–treated organoids, many cells in the epithelial layer were stained with SYTOX orange (Fig. 4 B and C). In line with the previous observation that IL-4 or IL-13 induced tuft cell hyperplasia (3–5), multiple cells positive for ChAT-GFP (a marker or tuft cells) (30) were present in IL-4/IL-13–treated organoids generated from ChAT-GFP transgenic mice (31), whereas no or few ChAT-GFP+ tuft cells were observed in control-untreated organoids generated from ChAT-GFP transgenic mice due to a low frequency of such cells in the intestinal epithelium (Fig. 4 A–C, Right). Again using the LDH assay as another way to assess lytic cell death, we detected the increased release of LDH in organoids treated with IL-4 or IL-13 for 5 d but not with IL-25 or IL-33 (SI Appendix, Fig. S10). Together, it appeared that increased lytic cell death occurred in IL-4/IL-13–treated organoids, consistent with the idea that up-regulated Gsdmcs may be responsible for lytic cell death.
Fig. 4.
Lytic cell death in intestinal organoids treated with IL-4 or IL-13. Intestinal organoids generated from wild-type mice (Left and Middle) or ChAT-GFP mice (Right) were treated with IL-4 or IL-13 for 3 or 5 d and then assayed for the uptake of SYTOX orange. (A) Representative images of control organoids with no or few cells in the epithelial wall. Some cells in the organoid cores were dead cells that were shed and were labeled with SYTOX orange. (B) Representative images of organoids treated with IL-4. Multiple cells in the epithelial wall of organoids are labeled with SYTOX orange. (C) Representative images of organoids treated with IL-13. Multiple cells in the epithelial wall of organoids are labeled with SYTOX orange. The patterns of SYTOX orange staining in IL-13– and IL-4–treated organoids are similar. Note an increased number of tuft cells (ChAT-GFP, green) in IL-4– and IL-13–treated organoids that were generated from crypts of the small intestine of ChAT-GFP transgenic mice (B and C, Right). Experiments were repeated five or more times with similar results.
Increased Lytic Cell Death in Mice Infected with N. brasiliensis.
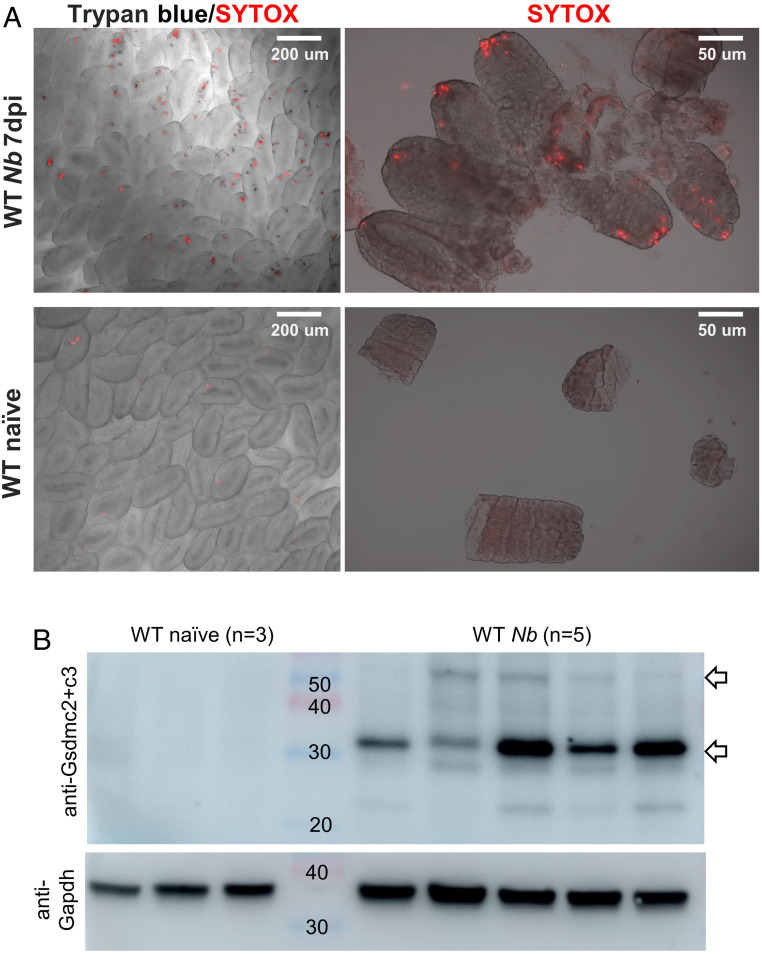
Given the up-regulation of Gsdmcs in N. brasiliensis–infected mice, we next asked if lytic cell death occurred in the intestinal epithelium in N. brasiliensis–infected mice. At 7 dpi, the jejunal tissue was harvested from these mice and stained with Trypan blue and SYTOX orange. Consistent with data obtained from IL-4/IL-13–treated organoids, quite a few cells at the tips of intestinal villi and upper villi were stained positive with SYTOX orange and/or Trypan blue (Fig. 5A). In naïve mice, no or few cells in the tip of the villi were stained with SYTOX orange and/or Trypan blue (Fig. 5A). Western blot analyses of jejunal tissue lysates showed a prominent Gsdmc2/3-immunoreactive band (Fig. 5B) only in N. brasiliensis–infected wild-type mice, corresponding to the 30 kDa band observed in HEK293 cells (Fig. 3E), consistent with lytic cell death observed in the worm-infected gut. Thus, Gsdmc2 (or Gsdmc3) is cleaved to a functional fragment in vivo upon worm infection. As another control, Pou2f3−/− mice were also inoculated with N. brasiliensis; no or few cells in the villi were stained with SYTOX orange (SI Appendix, Fig. S11), most likely due to reduced type 2 immunity in the absence of tuft cells in Pou2f3−/− mice. This set of data indicate that the up-regulation of Gsdmcs may trigger lytic cell death in worm-infected mice (illustrated in SI Appendix, Fig. S12).
Fig. 5.
Lytic cell death in intestinal epithelium of wild-type mice infected with N. brasiliensis. (A) Representative images of intestinal villi, stained with SYTOX orange and/or Trypan blue, from N. brasiliensis–infected (n = 5) or naïve (n = 3) wild-type mice. Jejunal tissues were freshly collected at 7 dpi or from control (naïve) mice and stained with SYTOX orange and/or Trypan blue. Cells at the tips of villi or upper villi are frequently labeled with SYTOX orange and/or Trypan blue in wild-type mice infected with N. brasiliensis but virtually none in naïve mice. (B) Western blot analyses of Gsdmc2/3 in jejunal lysates from naïve (n = 3) and N. brasiliensis–infected (n = 5) wild-type mice. Experiments were repeated one more time with similar results.
Discussion
In the present work, by performing transcriptome and PCR analysis of IL-4–treated organoids, we identified several type 2 cytokine target genes, with the Gsdmc family being the primary targets. In parallel, we demonstrated that the up-regulation of the Gsdmc family is a key feature of worm-induced type 2 immunity. We further revealed that Gsdmc2 is a pyroptotic effector and that overexpression of Gsdmc2 in HEK293 cells led to extensive pyroptosis and lytic cell death. Moreover, worm infection led to increased lytic cell death and cleavage of Gsdmcs in mice. Similarly, IL-4/IL-13 treatment of gut organoids led to lytic cell death. Together, these sets of data reveal that the involvement of lytic cell death in type 2 immunity is potentially mediated by Gsdmcs. Lytic cell death may help release antiparasitic factors from gut cells to fight these and other infectious agents.
Target Genes of Type 2 Cytokines.
Type 2 cytokines or activated type 2 immunity up-regulates the expression of Gsdmcs and several other genes in gut organoids and in the intestinal epithelium of N. brasiliensis–infected mice. Mechanistically, it seems that IL4ra and IL13ra1, the receptors for IL-4 and IL-13 (32, 33), are responsible for mediating the effects of IL-4/IL-13. Transcriptome profiling of gut epithelial cells showed that both IL4ra and IL13ra1 are expressed in the gut epithelium (34). Specifically, these two receptor subunits appear to be expressed in stem cells, transient amplifying cells, and enterocytes. The acute up-regulation of Gsdmcs (within an hour) suggested that IL-4 or IL-13 may act directly on differentiated cells such as enterocytes without the requirement of the differentiation of stem cells into enterocytes. Stat6 is the master transcription factor that mediates the effect of IL-4/IL-13 (15). Thus, it is likely that the increased expression of Gsdmcs by IL-4/IL-13 may be regulated by Stat6.
Previously, IL-4 and IL-13 were shown to promote tuft cell hyperplasia in intestinal organoids (3–5); we have confirmed that observation by using organoids generated from ChAT-GFP transgenic mice. ChAT (choline acetyltransferase) is the enzyme responsible for generating acetylcholine and is selectively expressed in tuft cells but not in other types of epithelial cells; thus, ChAT-GFP marks tuft cells (28). The ability of IL-4/IL-13 to promote tuft cell hyperplasia is likely mediated by IL4ra- and Il13ra1-expressing intestinal stem cells. This is supported by the observation that expanded tuft cells were generated from Lgr5+ intestinal stem cells (4, 35).
Thus, in the gut, the activity of IL-4 and IL-13 is bifurcated. On the one hand, they promote expression of Gsdmcs presumably in enterocytes. On the other hand, they activate a different set of genes to alter stem cell fate determination, that is, tuft and goblet cell hyperplasia. Thus, we speculate that both activities may cooperatively create a gut environment unfavorable for worm dwelling and facilitate worm clearance.
Slight up-regulation of Gsdmcs was also detected in Pou2f3−/− mice. This agrees with the residual type 2 immunity observed in the absence of tuft cells, as N. brasiliensis induced mild goblet cell hyperplasia (5).
Gsdmcs Are Pyroptosis Effectors.
Gasdermin family members are pyroptosis effectors involved in inflammatory responses. For the most studied member of the gasdermin family, GSDMD, the cleavage of GSDMD into N-terminal and C-terminal fragments by caspase-11/4/5 or caspase-1 is necessary to generate the pore-forming N-terminal fragment (26–28). The pore form is essential for the release of IL-1β and other antimicrobial products from cells undergoing pyroptosis (36). The activity of other members of the gasdermin family also depends on cleavage of the proteins by different proteinases (19). However, in the case of Gsdmc2, simply transfecting HEK293 cells with Gsdmc2 led to extensive pyroptosis and lytic cell death, suggesting that Gsdmc2 may undergo self-cleavage to functional fragments or that HEK293 cells may express some endogenous enzymes that are constitutively active and can cleave Gsdmc2 into functional fragments. Indeed, a band around 30 kDa was visible only in the sample prepared from Gsdmc2-transfected HEK293 cells and not in those from Gsdmc4- or GSDMD-transfected HEK293 cells, favoring the self-cleavage scenario, leading to a functional N-terminal fragment necessary for the pyroptotic activity of Gsdmc2 in HEK293 cells. This is further supported by the observation of presumably the same 30-kDa fragment in the gut tissue in N. brasiliensis–infected mice. Nevertheless, it is still possible that Gsdmc2 and other Gsdmcs in intestinal tissue may be subject to proteinase cleavage to generate functional fragments; this is worthy of future investigation.
We noted that quite a few cells at the tips of intestinal villi and upper villi of N. brasiliensis–infected mice were stained positive with SYTOX orange and/or Trypan blue. However, strong Gsdmc2/3 immunoreactivity was detected in the entire villus. Why don’t all these Gsdmc2/3+ cells undergo lytic cell death and take up the dyes? Although the exact reason is unknown, we speculate that the normal fast turnover of the intestinal epithelial cells (sloughing off at the tip of the villus) or an epithelial escalator-based mechanism (37) in combination with the worm-induced expression of Gsdmcs may lead to our observation of concentrated lytic cell death at the tips of intestinal villi or upper villi of N. brasiliensis–infected mice.
Lytic Cell Death May Lead to the Secretion of Antiparasitic Factors for Host Defenses.
How the lytic cell death of gut epithelial cells contributes to host defense is unclear. Gasdermin-mediated lytic cell death is known to open up cells by forming pores in the cell surface that allow the secretion of antimicrobial substances such as IL-1β and other mediators (36). By analogy, it is possible that lytic cell death, potentially induced by up-regulated and cleaved Gsdmcs in response to worm infection, may release antiparasitic factors. Consistent with this idea, genes encoding small proline-rich proteins, such as Sprr2a1, Sprr2a2, and Sprr2a3, are also up-regulated in gut organoids treated with type 2 cytokines. It is plausible that Sprr2a1-3, along with others, may be released into the intestinal lumen and facilitate worm expulsion (38–40). Furthermore, Pla2g4c is significantly up-regulated in intestinal epithelial cells. Pla2g4c encodes a phospholipase that has a significant role in membrane lipid remodeling and the generation of lipid mediators of the inflammatory response (22). Thus, it may also contribute to the inflammatory response to worm infection. Indeed, Entwistle et al. (41) demonstrated that epithelial cell–derived Pla2g1b, another member of the phospholipase A2 class of enzymes, is anthelmintic via directly reducing phospholipid abundance in infective larvae. It is plausible that up-regulated Pla2g4c may be secreted via Gsdmcs-formed pores to exert its effect directly on worms as well.
Our data support a major role of Gsdmcs in worm-induced type 2 immunity. Our work demonstrated a correlation among the up-regulation of the pyroptotic effects of Gsdmcs, the cleavage of Gsdmc2/3, and lytic cell death in the worm-infected gut. A cause–effect relationship between Gsdmcs and lytic cell death remains to be firmly established via ablating all four Gsdmcs in mice. Furthermore, we speculate that, in the absence of Gsdmcs, worm clearance may be delayed, but tuft and goblet cell hyperplasia may not be affected because tuft and goblet cell hyperplasia is initiated by the activity of IL-13 on intestinal stem cells, whereas worm clearance requires coordinated action of different types of intestinal cells, including secretion of antiparasitic factors released from pyroptotic enterocytes. This hypothesis remains to be tested using mice lacking Gsdmcs and the identification of antiparasitic factors released from pyroptotic enterocytes. Additionally, all four Gsdmcs are up-regulated in the gut epithelium; whether they have similar functions remains unexplored. It is plausible that these four Gsdmcs may form heteromers in native tissue to coordinately participate in lytic cell death and the release of antiparasitic factors into the lumen where worms reside.
Materials and Methods
Mouse Models.
Wild-type C57BL/6 mice were obtained from the Jackson Laboratory and maintained at the Monell Center. Pou2f3-deficient mice were maintained on the C57BL/6 genetic background. ChAT-GFP mice were obtained from Sukumar Vijayaraghavan (University of Colorado). Adult male and female mice (10 wk old) were used. Littermate mice were housed in single-sex groups unless otherwise specified. All experiment procedures were performed under NIH guidelines for the care and use of animals in research and approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Organoid Cultures.
After euthanizing the mice, jejunal (middle part of the small intestine) tissue was collected and washed with cold phosphate-buffered saline (PBS). Then, after removing villi, the tissue was digested with 2 mM ethylenediaminetetraacetic acid for 30 min on ice. Intestine crypts were harvested by filtering the suspensions with 70-µm nylon mesh. After suspension with Matrigel, intestine crypts were cultured in Dulbecco’s Modified Eagle Medium/F12 medium supplemented with R-spondin conditional medium (20%), Noggin conditional medium (10%), N-acetylcysteine (1 mM, Sigma), epidermal growth factor (50 ng/mL, Life Technologies), N2 (1%, Life Technologies), and B27 (2%, Life Technologies).
Organoids were passaged every 5 to 7 d, and 5 to 10 generations were used for this study. Recombinant mouse IL-4, IL-13, IL-25, and IL-33 protein (R&D Systems) was dissolved in PBS and then added to organoid culture medium at 100 ng/mL at indicated times.
RNA Extraction and RT-qPCR.
Organoids, intestine tissue (a 1-cm long segment of the jejunum from the middle of the small intestine for each sample), and lung tissue (half left lung for each sample) were kept on ice or stored in RNAlater before RNA extraction. RNA was extracted with the RNA Mini Kit (Invitrogen). Complementary DNAs (cDNAs) were made using the PrimeScript RT Reagent Kit (Takara). qPCR was conducted with the SYBR Green PCR Master Mix (Applied Biosystems) and primers (listed in SI Appendix, Table S1). Statistical analysis was performed for Figs. 1C and 2 B and C and SI Appendix, Figs. S1, S4A, and S5 using ANOVA with post hoc Tukey tests.
RNA-Seq and RNA-Seq Analysis.
Cultured organoids were treated with or without IL-4 (100 ng/mL) for 1, 6, and 24 h. RNA was extracted from culture organoids using a commercial kit (Nucleospin RNA XS kit; Clontech no. 740902.50). cDNA preparation and sequencing were performed as described previously. Briefly, cDNA was generated using a SMART-Seq v4 Ultra Low Input RNA Kit for Sequencing (Clontech no. 634888). Then, libraries were generated by KAPA Hyper Prep Kits for Illumina (Kapa Biosystems) and sequenced using an Illumina HiSEeq 2500 to generate 100–base pair reads. GRCm38 (mm10) was used for mapping sequence reads after quality control with filtering, using an algorithm implemented in CLC Genomics Workbench 8.0.3 (CLC Bio). A negative binomial generalized linear model, similar to that of edgeR, implemented in CLC Genomics Workbench 9.5.3 with the Advanced RNA-Seq plugin, was used for statistical tests. Likelihood ratio tests (ANOVA-like tests) were used to calculate differential gene expression across different time points while controlling for differences between replications. The read data have been deposited to the DNA Data Bank of the Japan Sequence Read Archive (accession no. DRA007998).
Further RNA-seq data analyses and visualization were conducted in R.4.0.2. Briefly, all transcripts per kilobase million (TPKM) data were normalized by package preprocessCore. Genes whose expression level in over half of the samples was zero were excluded. Expression change was conducted by the package Limma, and figures were made by the package ggPlot.
Worm Infection and Worm Counting.
N. brasiliensis eggs were collected and cultured to infective third-stage larvae (L3). A total of 200 L3 worms were purified and subcutaneously injected into mice. For worm counting, the whole intestine was cut open and incubated in saline (0.9% NaCl) at 37 °C for 30 min until all the worms sunk to the bottom. Statistical analysis was performed for SI Appendix, Fig. S3 using ANOVA.
Transfection.
pcDNA3.1-based expression constructs for mouse Gsdmc2 (NM_177912.4), Gsdmc4 (NM_028992.1), GSDMD (NM_026960.4), and caspase 4 (NM_007609.3) with a flag tag at the C-terminal were purchased from GenScript. HEK293 cells (peakRapid, ATCC) were cultured in Opti-minimal essential medium (Opti-MEM, ThermoFisher Scientific) supplemented with 4% fetal bovine serum (FBS) before transfection. We used Lipofectamine 2000 to transfect the abovementioned constructs (0.1 µg DNA + 0.5 µl Lipofectamine per well for 96-well plates or 4 µg DNA + 10 µl Lipofectamine per well for 6-well plates). After 4 h, the medium was replaced with fresh Opti-MEM supplemented with 4% FBS.
Live/Dead Cell Staining.
SYTO green and SYTOX orange nucleic acid stain (Invitrogen) were used in live/dead cell staining. For cultured organoids and HEK293 cells, dyes (5 nM) were added directly into culture medium 30 min before imaging. For mice infected with N. brasiliensis, jejunal tissue was collected and washed three times in cold PBS and then incubated in 0.4% Trypan blue and/or SYTOX orange (5 nM in PBS) for 2 min on ice. After staining, intestine tissue was washed three times in cold PBS and then fixed with 4% paraformaldehyde before imaging. Villi were collected with a clean coverslip and kept in PBS before imaging. Images were acquired using an Olympus-BX63 microscope. Live/dead cell counting was conducted by ImageJ. Statistical analysis was performed for Fig. 3D using ANOVA with post hoc Tukey tests.
Western Blotting.
HEK293 cells in each well of a 6-well plate were homogenized in 100 µl CelLytic M (Sigma) containing a protease inhibitor mixture (1:100, Sigma) and phenylmethylsulfonyl fluoride (10 mM, Thermo Scientific) and then centrifuged at 18,000 g for 5 min. The supernatants (10 µl) were mixed with the same amount of loading buffer (Bio-Rad) and loaded onto Mini-PROTEAN TCX gel (Bio-Rad). For Western blot analysis, jejunal intestinal tissue (5-mm long) was homogenized in 500 µl CelLytic M as mentioned above; 100 µg protein from each sample was loaded onto Mini-PROTEAN TCX gel (Bio-Rad).
Anti-flag (GenScript no. A00187, 1:1,000), anti-Gsdmc2/3 (Abcam no. ab229896, 1:1,000), anti-Gapdh (Cell Signaling Technology no. 2118, 1:1,000), and secondary antibodies anti-mouse IgG, human ads–horseradish peroxidase (HRP) (SouthernBiotech no. 1030–05, 1:3,000), and anti-rabbit IgG HRP-linked antibody (Cell Signaling no. 7074, 1:3,000) were used in this experiment. Exposure time was 10 s. Images were taken by an Amersham Imager 680 blot and gel imager.
Immunostaining.
A 2-cm long segment of the jejunum from the middle of the small intestine was harvested, fixed in 4% paraformaldehyde overnight, cryoprotected in 30% sucrose, and then embedded in optimal cutting temperature compound. Anti-Gsdmc2/3 antibody (Abcam, ab229896, 1:200), anti-Dclk1 antibody (Abcam, ab31704, 1:1,000), anti-beta-actin antibody (Invitrogen, AM4302, 1:500), and secondary antibodies purchased from Abcam were used in this study. Images were acquired using an Olympus-BX63 microscope with the identical setting and identical exposure time.
LDH Assay.
LDH assays were conducted following the protocol of the Cytotoxicity Assay Lit (Promega no. G1780). For HEK293 cells, 50 µl culture medium was collected for each well (96-well plate) 24 h after transfection. For organoids, 10 µl culture medium was collected for each well (24-well plate) and diluted to 50 µl with blank organoid culture medium. Then, the medium was mixed with 50 µl LDH reagent in a fresh 96-well plate. After 30 min of incubation at room temperature, 50 µl stop solution was added into each well, and the optic density at 490 nm (OD490) was obtained by a spectrophotometer (Molecular Devices, FlexStation 3). Statistical analysis was performed for SI Appendix, Figs. S9 and S10 using ANOVA with post hoc Tukey tests.
Schematic Illustration.
The schematic illustration in SI Appendix, Fig. S12 was created with https://BioRender.com.
Supplementary Material
Acknowledgments
We thank members of the P.J. laboratory for discussion. We thank Dr. Jeffrey Whitsett (University of Cincinnati) for the Rspo2 stable cell line to produce R-spondin–conditioned medium and Binqing Zhang (Children's Hospital of Philadelphia) and Jianming Zeng (University of Macau) for their R reference codes. We also thank Dr. Chider Chen (University of Pennsylvania) for providing the blot imager and Dr. Sukumar Vijayaraghavan (University of Colorado) for providing ChAT-GFP mice to M.T. The work was supported by Monell institutional funds (P.J.) and the Doctoral Graduate Student’s Academic Visit Fund of Sichuan University (R.X.). We thank the Monell Genotyping, Phenotyping, and Histology and Cellular Localization Cores, supported in part by funding from NIH Core Grant P30DC011735 (to R.F.M.), for animal facilities (G20 OD020296 to Danielle R. Reed, Monell Chemical Senses Center).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026307118/-/DCSupplemental.
Data Availability
Organoid RNA-seq data have been deposited in the National Center for Biotechnology Information Sequence Read Archive (BioProject PRJDB7964) (42). Previous published single-cell RNA-seq data were available from the National Center for Biotechnology Information Sequence Read Archive (BioProject PRJNA608457) (43).
References
- 1.de Silva N. R., et al., Soil-transmitted helminth infections: Updating the global picture. Trends Parasitol. 19, 547–551 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Pulendran B., Artis D., New paradigms in type 2 immunity. Science 337, 431–435 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howitt M. R., et al., Tuft cells, taste-chemosensory cells, orchestrate parasite type 2 immunity in the gut. Science 351, 1329–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Moltke J., Ji M., Liang H. E., Locksley R. M., Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 529, 221–225 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerbe F., et al., Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei W., et al., Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. U.S.A. 115, 5552–5557 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadjsombati M. S., et al., Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49, 33–41.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider C., et al., A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGinty J. W., et al., Tuft-cell-derived leukotrienes drive rapid anti-helminth immunity in the small intestine but are dispensable for anti-protist immunity. Immunity 52, 528–541.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fort M. M., et al., IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Neill D. R., et al., Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price A. E., et al., Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 11489–11494 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saenz S. A., et al., IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature 464, 1362–1366 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moro K., et al., Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Urban J. F. Jr, et al., IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 (1998). [DOI] [PubMed] [Google Scholar]
- 16.Hyoh Y., et al., Enhancement of apoptosis with loss of cellular adherence in the villus epithelium of the small intestine after infection with the nematode Nippostrongylus brasiliensis in rats. Parasitology 119, 199–207 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Shi J., Gao W., Shao F., Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Xia S., Hollingsworth L. R. IV, Wu H., Mechanism and regulation of gasdermin-mediated cell death. Cold Spring Harb. Perspect. Biol. 12, a036400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broz P., Pelegrín P., Shao F., The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Hou J., et al., PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1264–1275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato T., et al., Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Brown J. K., et al., Trichinella spiralis induces de novo expression of group IVC phospholipase A2 in the intestinal epithelium. Int. J. Parasitol. 38, 143–147 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Miller H. R., Nawa Y., Immune regulation of intestinal goblet cell differentiation. Specific induction of nonspecific protection against helminths? Nouv. Rev. Fr. Hematol. 21, 31–45 (1979). [PubMed] [Google Scholar]
- 24.Banerjee A., et al., Succinate produced by intestinal microbes promotes specification of tuft cells to suppress ileal inflammation. Gastroenterology 159, 2101–2115.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camberis M., Le Gros G., Urban J. Jr, Animal model of Nippostrongylus brasiliensis and Heligmosomoides polygyrus. Curr. Protoc. Immunol. Chapter 19:Unit 19.12(2003). [DOI] [PubMed] [Google Scholar]
- 26.Shi J., et al., Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Ding J., et al., Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Kayagaki N., et al., Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Hu J. J., et al., FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schütz B., et al., Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front. Physiol. 6, 87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grybko M. J., et al., A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur. J. Neurosci. 33, 1786–1798 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilton D. J., et al., Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc. Natl. Acad. Sci. U.S.A. 93, 497–501 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly-Welch A. E., Hanson E. M., Boothby M. R., Keegan A. D., Interleukin-4 and interleukin-13 signaling connections maps. Science 300, 1527–1528 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Haber A. L., et al., A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker N., et al., Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). [DOI] [PubMed] [Google Scholar]
- 36.He W. T., et al., Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cliffe L. J., et al., Accelerated intestinal epithelial cell turnover: A new mechanism of parasite expulsion. Science 308, 1463–1465 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Hooper L. V., et al., Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Sun F. J., et al., Decreased gastric bacterial killing and up-regulation of protective genes in small intestine in gastrin-deficient mouse. Dig. Dis. Sci. 48, 976–985 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Nozaki I., et al., Small proline-rich proteins 2 are noncoordinately upregulated by IL-6/STAT3 signaling after bile duct ligation. Lab. Invest. 85, 109–123 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Entwistle L. J., et al., Epithelial-cell-derived phospholipase A2 group 1B is an endogenous anthelmintic. Cell Host Microbe 22, 484–493.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka K., Iwatsuki K., Gene expression analysis of mouse intestinal organoids treated with IL-4. NCBI Sequence Read Archive (SRA). https://www.ncbi.nlm.nih.gov/bioproject/PRJDB7964. Deposited 24 May 2021.
- 43.Lau K., Ileal single-cell analysis in the context of inflammation focused on tuft cells. NCBI Sequence Read Archive (SRA). https://www.ncbi.nlm.nih.gov/bioproject/PRJNA608457. Deposited 24 August 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Organoid RNA-seq data have been deposited in the National Center for Biotechnology Information Sequence Read Archive (BioProject PRJDB7964) (42). Previous published single-cell RNA-seq data were available from the National Center for Biotechnology Information Sequence Read Archive (BioProject PRJNA608457) (43).