Significance
Endothermic animals must survive periods of seasonally lowered temperature, coincident with lowered food supply. While we know much about hibernation and migration as survival strategies, the responses of nonhibernating, nonmigrating species are more opaque, yet how these animals survive such periods is important to understand their potential susceptibility to climate change. Here, we report on a 13-y study of a species of lagomorph (plateau pika) from the Qinghai-Tibetan Plateau. We show pikas suppress their metabolism in winter and exploit a novel food source (feces of its supposed competitor, the domestic yak), which may contribute to their survival. Deposition of a fat store in the fall and its progressive utilization was not part of the overwinter strategy.
Keywords: winter survival, metabolic suppression, thyroid axis
Abstract
The Qinghai-Tibetan Plateau, with low precipitation, low oxygen partial pressure, and temperatures routinely dropping below −30 °C in winter, presents several physiological challenges to its fauna. Yet it is home to many endemic mammalian species, including the plateau pika (Ochotona curzoniae). How these small animals that are incapable of hibernation survive the winter is an enigma. Measurements of daily energy expenditure (DEE) using the doubly labeled water method show that pikas suppress their DEE during winter. At the same body weight, pikas in winter expend 29.7% less than in summer, despite ambient temperatures being approximately 25 °C lower. Combined with resting metabolic rates (RMRs), this gives them an exceptionally low metabolic scope in winter (DEE/RMRt = 1.60 ± 0.30; RMRt is resting metabolic rate at thermoneutrality). Using implanted body temperature loggers and filming in the wild, we show that this is achieved by reducing body temperature and physical activity. Thyroid hormone (T3 and T4) measurements indicate this metabolic suppression is probably mediated via the thyroid axis. Winter activity was lower at sites where domestic yak (Bos grunniens) densities were higher. Pikas supplement their food intake at these sites by eating yak feces, demonstrated by direct observation, identification of yak DNA in pika stomach contents, and greater convergence in the yak/pika microbiotas in winter. This interspecific coprophagy allows pikas to thrive where yak are abundant and partially explains why pika densities are higher where domestic yak, their supposed direct competitors for food, are more abundant.
Endothermic animals sustain stable high body temperatures in the face of environmental variation. As it gets colder the energy demands of sustaining endothermy increase (1, 2), particularly in small animals that have a less favorable surface-to-volume ratio (3). Since cold conditions during the arctic and temperate winter often also coincide with reductions in food supply, this creates a problem for small endothermic animals that do not migrate or hibernate. Understanding how such animals survive these conditions increases our understanding of the sorts of threats endotherms may face as climates change (4, 5). Although the integrated eco-physiological responses that underpin hibernation and migration have received extensive attention, we are less aware of the detailed survival strategies of most nonhibernating endotherms. For example, despite the theoretical impact of temperature on metabolic rates, a review of 16 species of small mammals showed that on average there was no difference in daily energy expenditure (DEE) between seasons (6), pointing to substantial acclimatization to seasonal cold conditions.
The mechanisms that underpin this acclimatization have been studied in many animals but in a largely piecemeal fashion, exploring individual components of the responses in isolation. Relatively few studies have integrated information across different approaches to further our understanding of the relative contribution of different strategies to overwinter survival. Examples of such integrated work include studies of North American red squirrels (Tamiasciurus hudsonicus) and snowshoe hares (Lepus americanus) living in the boreal forest of northern Canada. These species differ substantially in how they react to winter conditions. Red squirrels hoard food prior to winter and minimize physical activity (7–10), spending most of their time in well-insulated nests, and allowing some limited flexibility in their body temperature (11). In contrast, hares do not store food, do not exploit nests or burrows, and remain exposed to the winter environment. They survive primarily by adopting physiological mechanisms that increase thermogenesis and sustain high invariant body temperatures (8). Nevertheless, at temperatures below 0 °C energy demands decline as it gets colder, pointing to adoption of energy conservation mechanisms (12), the exact nature of which remain unknown.
The Qinghai-Tibetan Plateau is a high-altitude desert, averaging 4,500 m above sea level (ASL) in elevation, which covers an area roughly the size of Western Europe (2.5 million km2). The plant growth season is short, being around 134 to 152 d at low altitudes (3,200 to 3,300 m) and 90 to 120 d at 3,800 to 4,500 m. Consequently, the maximal summer biomass is about 420 g/m2 at 3,300 m but only 75 to 110 g/m2 at 4,000 m (13, 14). Winter on the plateau is cold, with nighttime temperatures averaging around −20 °C and routinely falling below −30 °C (13). High winds and solar radiation combined with low precipitation make the plant life in winter exceedingly dry (water content circa 20% compared with circa 70% in the summer), with reduced dry-matter levels of protein, sugars, and fat, but elevated fiber (15–17), and hence likely much less palatable than in summer. Moreover, the low partial pressure of oxygen at high altitude potentially constrains maximal aerobic metabolism and thermogenesis. Despite these environmental challenges, the plateau is home to several endemic mammalian species. Among the most ecologically important of these is the plateau pika (18–20) because its burrowing activity impacts soil physics and chemistry (21, 22), has positive effects on plant succession/biodiversity and budding of alpine meadow plants (23–25), provides nesting sites for lizards and burrow nesting birds (19), and it is an important prey item for many predators (26). Yet, pikas have been poisoned as an agricultural pest for decades because they are believed to compete with domestic yak for food (27, 28) and cause habitat degradation (29), although their role in this is disputed (30, 31).
The plateau pika is believed to be incapable of hibernation (32) because past research has never recorded a lagomorph entering torpor or hibernation (33). However, pikas flourish in the high-altitude meadows. A related species, the American pika (Ochotona princeps), lives in the Rocky Mountain range in North America, where it survives winter by caching hay underground (34). In summer, American pikas harvest and dry grasses in conspicuous “haypiles” (35, 36). At this time up to 55% of their above-surface activity is spent collecting plants for the haypile or transporting it into the rocky talus (35). Plateau pikas, however, have never been observed to build haypiles, never been observed carrying food into their burrows, and burrow excavations have never located underground food stores (37, 38). This is potentially because the plateau grasslands provide little shelter from prevailing winds and, hence, haypiles would be blown away. Moreover, in the open habitat, haypiles would be more visible and potentially vulnerable to kleptoparasitism (39). Furthermore, indirect evidence supporting the suggestion that underground food stores do not exist is that winter snowfall, which covers the food of plateau pika on the surface, often leads to high mortality (40, 41). This would not be expected if pikas had substantive underground stores on which to rely. Paradoxically, despite being suggested to be competitors, the pika reaches its highest densities in areas where yak are also most abundant (42, 43). One aspect of the plateau pika that separates it from the North American red squirrel, snowshoe hare, and American pika is that unlike these other species, the plateau pika lives in small gregarious social groups throughout the year (44). This difference in social behavior may affect their overwintering physiological strategies.
Previous work on the ecophysiology of the plateau pika has emphasized their relatively high metabolic rates, absence of diurnal variation in body temperature, seasonal variation in thermogenic capacity, maximal metabolic rate, and levels of the mitochondrial uncoupling protein (UCP-1) and how these features are regulated by temperature and photoperiod (45–48). How these physiological capabilities are integrated into an overall strategy to survive the Tibetan winter, however, remains unknown. We report here on the energy balance strategies of pikas that enable them to survive the Tibetan winter. This included studies of their resting and daily energy expenditure, fur insulation, body temperature and hormonal responses, food sources, and fat utilization.
Results
Weather Characteristics and General Topology of the Study Sites.
For photographs of the two study areas in summer and winter, see SI Appendix, Fig. S5. These images show that the lush green vegetation in summer is transformed to dry brown in winter. The general weather characteristics during midwinter based on average measures over 4 d at each site and then pooled across the two sites in each area are summarized in SI Appendix, Fig. S6. These images show the similarity between the two areas, despite them being 250-km apart. In both areas it was relatively calm at night and windy in the daytime. Solar radiation levels peaked at midday at an average level of 550 W/m2 at the high-density yak area and 650 W/m2 at the low-density area. The temperature of the operative temperature model was very similar to the recorded surface temperatures. Temperature fell slowly through the night to minima just prior to dawn. These minima averaged around −20 °C at the high-density yak area and −18 °C at the low-density yak area. Air temperatures were slightly warmer at around −17 to −18 °C. As soon as the sun rose the surface, air and operative temperatures began to rise. For the surface and operative temperature model temperatures, these peaked around noon at 15 to 20 °C, when the solar radiation was most intense. Air temperatures rose more slowly and peaked in midafternoon at −5 to −2 °C. We only recorded burrow temperatures in the high-density yak area, and these showed a dampened cycle with a peak around −10 °C recorded after nightfall until around 2200 hours, and a nadir around noon at −15 °C.
Doubly Labeled Water Measurements of Energy Expenditure.
Measurements of DEE, using the doubly labeled water (DLW) method, showed that in winter, pikas had lower energy expenditure than in summer (Fig. 1A) (ANCOVA; body mass effect: F1, 152 = 9.57 P = 0.002; season effect: F1, 152 = 1.25, P = 0.26; body mass × season: F1, 152 = 9.38, P = 0.003, n = 156). For the smaller sample, where we had information on sex, there was no significant sex effect (F1, 77 = 0.3, P = 0.588), and no sex-by-body mass interaction (F1, 77 = 0.18, P = 0.673). All measured animals were nonbreeding. The summer data were distributed into two groups comprising those weighing less than 120 g, which were young of the year, and those weighing >140 g, which were adults that were born in previous years. There was a strong effect of body mass in summer (r2 = 0.34, P < 0.001) but no effect in winter (r2 = <0.001, P > 0.05). There was a significant mass-by-season interaction (ANCOVA F = 9.37, P = 0.003). If we removed the data for the young of the year, there was no relationship between body mass and DEE in summer for the adults (r2 = 0.003, P > 0.05). Using the fitted regression curves including all the data, summer expenditure was higher than in winter, and the difference between seasons at a body weight of 150 g averaged 50.3 kJ/d (29.7% of the winter DEE). If data for the young of the year are removed, the difference between summer and winter DEE for adults was much greater. The average DEE in summer adults was 280.3 kJ/d (SD = 72) and in winter was 169.2 kJ/d (SD = 19.1). This average difference of 111.1 kJ/d was 65.7% of the winter DEE. This substantially lower winter DEE was despite the fact that the average daily air temperature in winter in both areas was around 25 °C lower than in summer. Even in their subterranean burrows, the average winter temperature was around −12 °C (SI Appendix, Fig. S6). DEE in winter did not differ between the areas with high and low yak densities (ANCOVA area effect F1, 83 = 1.50, P = 0.22) (Fig. 1B: model including area and body mass as factors). The lower DEE in winter than in summer could indicate a reduction in energy expenditure either when the animals were at rest, during activity, or both.
Fig. 1.
(A and B) DEE of the plateau pikas living wild on the Qinghai-Tibetan Plateau using the DLW method. (A) Measurements in white are from summer (July to September) and those in black are from winter (November to March). Lines refer to fitted linear regressions and equations for these are shown. (B) The winter data were split into those collected in areas where yak were abundant (white) and those where yak were scarce (black). Lines are fitted linear regressions for each group. (C) Resting energy expenditure of pikas measured in animals captured during summer and winter at various ambient (incubator) temperatures using indirect calorimetry. The summer values are in white and the winter values in black. There was a strong seasonal difference. (D) Resting energy expenditure at thermoneutral (RMRt 25 °C) in relation to body mass in summer and winter. Levels of (E) Tri-iodothyrionine (T3) and (F) levels of thyroxine (T4) in the blood from pikas captured in the wild in winter (W) and summer (S) and for experimental animals held in the field in winter and given ad libitum food (WF) or starved (Wst) Post hoc differences by Tukey test (P < 0.05) are indicated by letters above the bars.
Resting Metabolic Rate.
We measured the resting metabolic rate (RMR) of pikas from the two study areas in winter (n = 13) and in summer (n = 14). In winter, the mass of pikas was 167 ± 30.7 g (SD), while in summer it was 142 ± 22.2 g. None of the animals were breeding. Across all individuals and measurements there was a significant negative relationship between RMR and ambient (= incubator) temperature (Fig. 1C) (t = −9.5, P < 0.0001), with the RMR in winter significantly lower than that in summer by on average 28.7 kJ/d (t = −6.8, P < 0.0001). There was no effect of the site of origin (t = −0.09, P = 0.99 not significant, ns). There was a significant interaction between season and ambient temperature (t = 3.05, P = 0.0028) with the slope of the effect of ambient temperature in summer being steeper than that in winter, indicating increased pelage insulation in winter. During winter, RMR did not differ between the sexes (t = 1.3, P = 0.23, sex data not available for summer). RMR at thermoneutrality (RMRt) was positively related to body mass both during winter (RMRt = 0.59 × mass + 11.68; F1, 11 = 18.15; P = 0.001; r2 = 0.62) and summer (RMRt = 0.64 × mass + 24.42; F1, 14 = 11.84; P = 0.005; r2 = 0.45) (Fig. 1D). Examining both seasons together, the effect of season was significant (ANCOVA: F1, 24 = 10.88, P = 0.003), with the RMRt being on average 20.5 ± 6.2 kJ/d lower in winter than in summer (in this model, the mass coefficient was 0.62 ± 0.11; F1, 26 = 12.4, P = 0.002).
We used the pooled relationship between body mass and RMRt in the different seasons to estimate sustained metabolic scopes (SusMS = DEE/RMRt). For each individual for which we measured DEE in the field using DLW, we predicted its RMRt using the derived equations above and then estimated SusMS using the measured DEE and predicted RMRt. The estimated SusMS in summer was 1.99 (SD = 0.48, n = 71) and in winter was significantly lower (t155 = 7.65, P = 0.0013) at 1.60 (SD = 0.31, n = 85). This demonstrates that the energy conservation in winter was a combination of reduced energy expended on both RMRs and non-RMRs. Another mechanism animals may use to conserve energy is to huddle (49, 50). To see if pikas attained significant benefits from huddling, we performed five respirometry trials during winter involving two animals in the respirometry chamber. In these cases, combined metabolic rate (metabolic rate at chamber temperature = 0 °C was 285.2 ± 14.6 kJ/d; average mass per animal = 181.6 ± 2 g) was 5.4% lower than the predicted summed metabolic rates of two animals of the same mass (predicted metabolic rate in winter at 0 °C in a linear regression on mass averaged 150.8 kJ/d × 2 = 301.6 kJ/d), but this difference was not significant (t = 1.02, P > 0.05).
Thyroid Hormones.
Given the known importance of thyroid hormones as mediators of energy metabolism in free-living animals (e.g., ref. 51), we compared the levels of circulating thyroid hormones (T3 and T4) in four groups of pikas (Fig. 1 E and F). The level of thyroid hormones differed between the experimental groups (F3, 47 = 7.04, P < 0.001 and F3, 48 = 10.033, P < 0.001 for T3 and T4, respectively). For T4 the summer level was significantly higher than in winter (Tukey post hoc P < 0.05). We also found a similar, but not marginally significant, trend for T3 (Tukey post hoc P = 0.058). In winter we also kept pikas in cages at the field site (low-density yak area) for 24 h and experimentally manipulated these animals by feeding one group with ad libitum supplies of rabbit chow, while the others were starved. We then collected blood 24 h later. T3 and T4 levels in the fed animals matched the summer levels, while the levels in the starved animals remained at the levels for those sampled immediately in the field (not significantly different, Tukey post hoc P > 0.05) (Fig. 1 E and F). These data suggest reduced T3 and T4 levels may be an important contributor to the observed suppression of metabolism, and this system may be extremely flexible relative to changes in winter food supply.
Animals may also mitigate the impact of seasonally reduced temperatures by improving the insulation of their pelage, as indicated by the lower slope of the RMR vs. incubator temperature relationship in summer (Fig. 1C). To explore this further we measured the thermal characteristics of pelages removed from summer and winter animals using the cooled bottle technique (52). The thermal insulation of the pelage in winter was significantly greater than in summer (SI Appendix, Fig. S7).
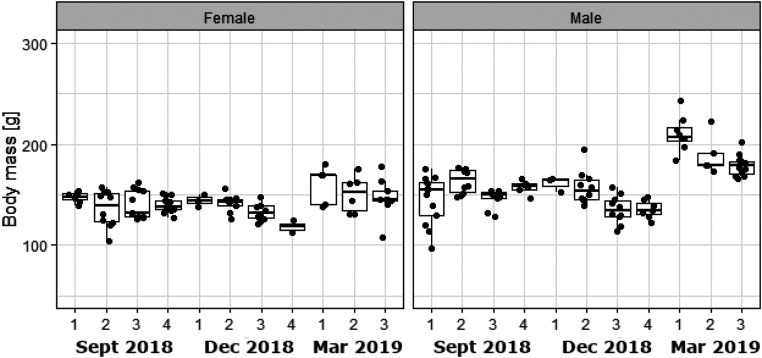
Body Composition.
The body weights of pikas trapped at two sites in a high yak and two sites in a low yak area in September, December, and March are shown in Fig. 2. There was a significant effect of site (F3, 171= 6.95, P < 0.001), measurement month (F2, 171 = 42.29, P < 0.001), and sex (F1, 171 = 68.31, P < 0.001). There was also a significant sex-by-month interaction (F2, 171 = 16.39, P < 0.001). There was no difference in the weights between the sexes in September and December (Tukey post hoc, P > 0.05), but males were significantly heavier than females in March by on average 40 g (Tukey post hoc, P < 0.001). Contrasting the expectation that weights might peak in September, reflecting deposition of a fat store, and then decline steadily through the winter, reflecting utilization of that store, the weights were not significantly different between September and December (Tukey post hoc, P > 0.05) and increased between December and March (Tukey post hoc, P < 0.01), particularly in males.
Fig. 2.
Body weights of pikas trapped at four sites (1 and 2 low yak density, 3 and 4 high yak density) in three different time periods: September 2018, December 2018, March 2019. It was not possible to access site 4 in March 2019. Data are stratified into males and females. Sample size at each time/site varied between 10 and 20. Total sample 180.
Body Temperature.
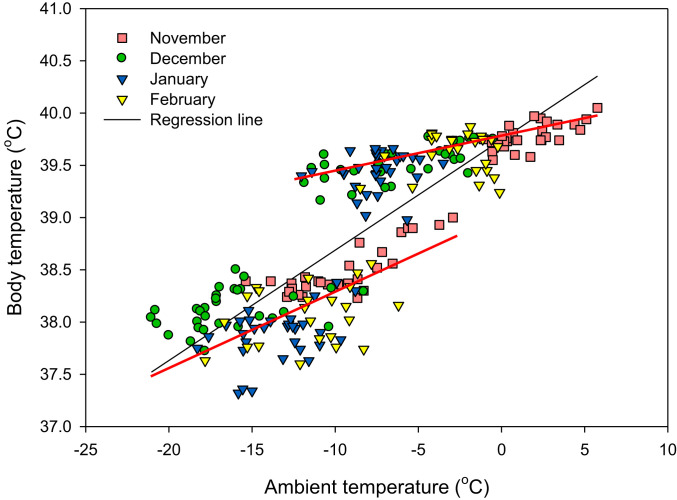
Some nonhibernating mammals may use daily torpor to minimize their energy expenditure (33). We implanted a total of 27 free-ranging pikas with temperature data loggers (ibuttons: Maxim Integrated products) at both areas at the start of winter, aiming to recapture them and remove the loggers in late February or early March. Only 6 (4 males and 2 females) of the 27 implanted pikas were recaptured, all from the area where yak were not abundant (see Materials and Methods for full details). Pikas were not recaptured at sites where yak were abundant because of poisoning events at these sites. Typical daily patterns of body temperature in November, December, January, and February are shown in SI Appendix, Fig. S8 A–D. All the implanted animals showed a profound diurnal cycle of body temperature, with temperature at night averaging 1.6 °C lower than in the daytime (ANOVA F2, 57,341 = 3,280.4, P < 0.001; day = 39.6 °C, night = 37.9 °C; mean for all 4 mo; mean difference = 1.62, SD = 0.31) (SI Appendix, Table S1). The temporal responses of the six animals were strongly correlated with each other (SI Appendix, Fig. S8 and Table S1B). Peak body temperature during the day coincided with the peak ambient temperature on the surface (a typical 2-d period illustrating this association is shown in SI Appendix, Fig. S8 E and F). Although in January body temperature dropped as low as 34 °C, we did not record any incidence of torpor, which for small mammals like pikas is generally defined as having a Tb less than 30 °C. We averaged the daytime (dawn to dusk) and nighttime (dusk to dawn) body and ambient temperatures (ground surface) for each animal and then took the average across all six animals. Average body temperature in both the daytime and nighttime was positively correlated with the ambient temperature (for day r = 0.681, P < 0.001; for night r = 0.527, P < 0.001) (Fig. 3).
Fig. 3.
The average night- and daytime body temperatures averaged across diurnal and nocturnal hours across all six implanted individuals plotted against the simultaneous ambient temperature. Lines show the fitted linear regressions. Red lines are linear regressions for daytime and nighttime temperatures, and black line is overall regression through all the data. Pikas lowered their body temperatures as it got colder and did this more so at night than in the day.
We measured the cooling rate at 1900 hours and warming rate at 0700 hours each day for five of the six animals between November 4th and February 8th (n = 97 d) by calculating the slope of the temperature vs. time line over the previous 80 min. For one animal, the resolution of the ibutton was only 0.5 °C and this precluded accurate calculations of the slopes. Cooling rates were transformed to positive values to make comparisons to warming rates (SI Appendix, Table S2). Across all individuals and measurements, warming rates averaged 0.83 °C/h (SD = 0.49, n = 485). Individuals differed significantly in their warming rates (F4, 480 = 3.0, P = 0.018), with individual 5 having a significantly slower warming rate (mean 0.70) than individual 4 (mean = 0.93), but otherwise there were no significant differences. There was only a weak correlation between the warming rates on different days across the individuals (SI Appendix, Fig. S9 and Table S2), with only 2 of the 10 correlations significant at P = 0.05 (adjusted for multiple testing using the Bonferroni correction). Nevertheless, in four of the five individuals there was a significant relationship between the rate of warming and the ambient temperature on the surface (SI Appendix, Fig. S9 and Table S2). Across all individuals the cooling rates averaged 2.38 °C/h (SD 0.93, n = 485). Individuals differed significantly in their cooling rates (F4, 480 = 16.2, P < 0.001), with individual 5 cooling significantly faster than individuals 2 and 3. For all individuals, the cooling rate in the afternoon was significantly greater than the warming rate in the morning on the same day (paired test, n = 97, for all individuals P < 0.001). Across all individuals, the average cooling rate was 3.9× faster than the warming rate on the same day (n = 457, SD = 3.8). Cooling was also more coordinated across the individuals (SI Appendix, Fig. S9), with 5 of 10 of the interindividual relationships significant when adjusted for multiple testing (SI Appendix, Table S2) and 4 of the 5 nonsignificant relationships being because 1 individual (ID1) was out of synchrony with the other 4. However, the cooling rate was generally not significantly related to the ambient temperature on the surface at the same time (SI Appendix, Fig. S9 and Table S2). In only one of the five individuals were cooling rates significantly related to ambient temperature. For all five individuals, there were no significant relationships (P > 0.05) between the rate of warming in the morning and the rate of cooling in the afternoon on the same day.
Although we only recaptured six long-term implanted animals at the sites where yak were scarce, we have data from a short-term pilot study (Materials and Methods) conducted at a site where yak were abundant. This involved five implanted animals that were released into the wild (September 2009) and then n = 4 were recaptured 3 d later, whereupon they spent 5 d in the laboratory before the transmitters were removed. These data, averaged across the four recaptured animals (SI Appendix, Fig. S10), show that when they were free-living in the field they also had a circadian difference between night and daytime measures with lower body temperatures by about 1.5 °C during the night. This shows that the data from the area where yak were rare was not unique to those populations. Daytime body temperatures in laboratory and field were similar, but in the laboratory the circadian cycle disappeared, and they remained at high body temperature throughout the night (SI Appendix, Fig. S10). The number of cycles available was insufficient to perform a formal periodogram analysis.
Physical Activity Patterns.
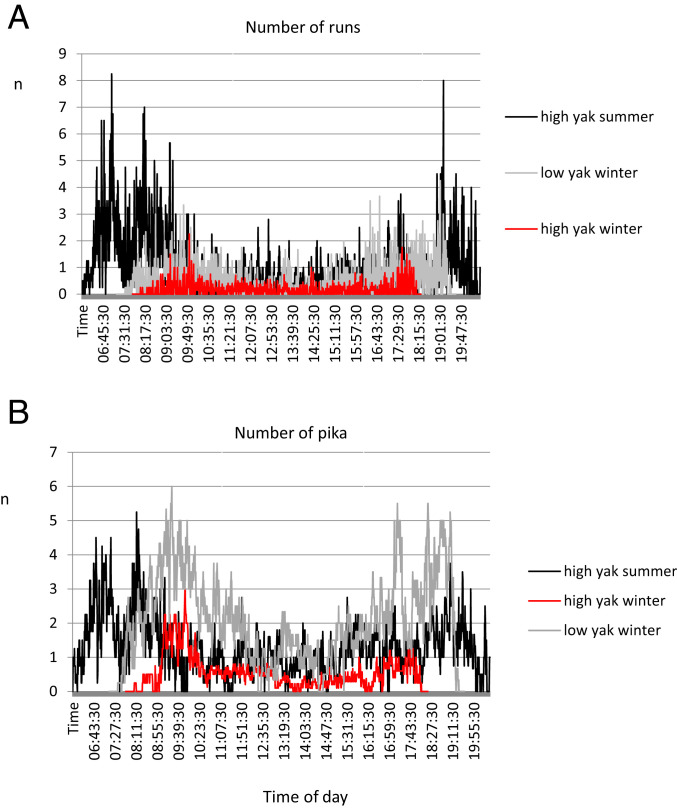
There was a strong diurnal cycle in ambient temperature, wind speed, and solar radiation at both high- and low-density yak areas, leading to profound changes in the operative temperature (SI Appendix, Fig. S5). This suggested that pikas might additionally reduce their energy expenditure by timing their feeding behavior above ground to coincide with the period of the day when it was warmest (1100 to 1500 hours). We filmed their activity in both summer and winter at the sites where yak (and pika) were abundant, and in winter at the sites where yak (and pika) were rare. At the high-density yak site there was considerably more above-surface activity in the summer compared to the winter (Wilcoxon test, W = 376,401, P < 0.001; W = 325,006, P < 0.001, for runs and numbers, respectively). Contrary to our predictions, pikas had their highest surface activity shortly after dawn and just before dusk (Fig. 4) in both summer and winter. The lowest levels of above-surface activity corresponded to the middle of the day, when operative temperatures were highest (SI Appendix, Fig. S5). Above-surface winter activity at the sites where yak were abundant was lower than where yak were scarce (Wilcoxon test, W = 554,800, P < 0.001; W = 138,837, P < 0.001, for runs and numbers, respectively).
Fig. 4.
Above-surface physical activity patterns of pikas at the high-density yak site in summer and winter and the low-density yak site in winter only. (A) The numbers of “runs” over each minute, and (B) the highest number of individuals simultaneously in frame during any particular minute. Data are pooled across the sites within each area and are based on films taken on at least four separate days in each minute. Independent of season and site, pikas were more active at dawn and dusk than in the middle of the day. Above-surface activity in winter was lower than in summer, and was lower at the sites where yak were abundant than the sites where yak were scarce, despite the pika population density being greater at the former.
Exploitation of Yak Feces.
Although pikas were more abundant at the area where yak were abundant, based on historical mark recapture analyses (44, 53, 54), the recorded above-ground activity in winter at the high-density yak area was much lower than at the low-density yak area (Fig. 4). We hypothesized that a potential reason for this lower activity was that pikas cohabiting with yak might be able to exploit yak feces as food. This latter behavior would potentially lower their surface activity as recorded on the cameras. During February 2010 at one of the high-density yak sites, we had two animals die during trapping, and when dissected the stomachs of these pikas we found them full of feces. The density of yak feces available to pikas was evaluated at the four sites by photographing the surface and counting the number of yak feces present in January to February. This confirmed that yak feces were significantly more abundant at the sites where yak were themselves more abundant (χ2 = 93.79, df = 1, P < 0.001) (SI Appendix, Table S4) by about sevenfold. This is not a foregone conclusion given that local Tibetan people harvest the yak feces and dry them to use as fuel. The fact that the density of feces was sevenfold higher, when the density of yaks themselves was only twofold higher, suggests such harvesting may have a strong impact on feces densities.
The animals in the videos we took from fixed locations to monitor activity were too distant to discern exactly what the pikas were eating. We did, however, manage to film several pikas eating yak feces. Four of these clips are shown in Movies S1–S4. These directly observed instances of pikas eating yak feces did not only occur in winter, suggesting that this behavior occurs year-round at sites where yak are abundant, but that it probably increases in frequency during the winter. As part of another project on pika morphology, we collected a total of 304 pikas from 9 separate sites (144 in September 2018, 79 in December 2018, and 81 in March 2019). We examined the stomach contents removed from these animals for evidence of yak DNA utilizing primers designed to amplify the mitochondrial DNA cytochrome oxidase 1 locus of yak but not of pika. The presence of yak DNA in the stomach samples is summarized in SI Appendix, Table S3. In total, we detected yak DNA in 22.7% of the samples (69 of 304), suggesting that eating yak feces is a common behavior. This is likely an underestimate because yak feces may sit on the surface for some time before being consumed, during which time they are exposed to the harsh UV light and the DNA may have already degraded. Indeed, when we tested yak feces collected from the field, we only detected yak DNA in 5 of 22 samples, probably for this reason (SI Appendix, Fig. S4). Moreover, the DNA may also be degraded in the stomach prior to us capturing the animals. The absence of yak DNA on extracts from plants collected in the field (SI Appendix, Fig. S4) suggests the positive results from the stomach contents are unlikely to reflect incidental environmental contamination on other food items. Surprisingly, evidence of yak feces consumption based on stomach DNA analysis was less common in December (11.4%) than in September (29.8%) or March (21.0%). However, this was mostly because yak feces were less abundant at these trapping sites in December (SI Appendix, Table S3). Feces density explained 35% of the variation in the probability of a pika stomach containing yak feces (F1, 19 = 10.38, P < 0.004) (SI Appendix, Fig. S11). Feces may be less abundant in December because local yak herders harvest them more for fuel at this time of year, or because they keep their herds closer to their dwellings in midwinter and away from the sites where we trapped.
As another test whether pikas were consuming yak feces, we compared the microbiota of pika and yak in the summer and winter at a high-density yak field site. When we plotted the operational taxonomic units (OTUs) found in pika feces against those found in yak feces, we found that the relationships of the microbiota between the species changed between summer and winter (SI Appendix, Fig. S12 A and B). In particular, during winter the most abundant OTUs in yak feces (loge abundance > 3) were on average 3.9× more abundant in the pika feces in the winter than they were in the summer (loge winter abundance in pika = 2.48; loge summer abundance = 1.11). This is consistent with pikas ingesting a greater amount of yak feces in winter and acquiring more aspects of the yak microbiota as a consequence. In addition, when we compared the microbiota of the pika and yak at the genus level, it was clear that in winter the correspondence between the microbiota was much greater than it was in summer (SI Appendix, Fig. S12 C and D). This convergence of microbiota in winter was consistent with greater utilization of yak feces by pikas in winter than in summer.
Discussion
Although it was on average about 25 °C colder in winter (13), the directly measured daily energy demands of plateau pikas were about 30% lower in winter (when corrected for body mass). In many species of arctic and temperate zone nonhibernating mammals, energy demands do not differ between summer and winter, despite the sometimes large seasonal ambient temperature difference (6). Although in most studies winter expenditure is either elevated or unchanged, similar reductions in daily energy demands during winter have been reported previously. For example, North American red squirrels have energy demands averaging 20% lower in midwinter compared with spring/summer (9, 55). This lowered metabolism is enabled by two features of squirrel behavior that are absent in plateau pikas. First, they cache food in large middens in the autumn, meaning they do not have to spend much time out foraging. Second, they build large well-insulated nests close to these middens. They then spend most of their time inside such nests, increasingly so the colder it gets, and foray out only during the warmest times of day, thereby minimizing their exposure to low temperatures (7, 9, 10). Although plateau pikas do not store food or build nests, their energy demands were still very low. At 1.6× RMRt, the pikas in winter had comparable metabolic scopes to that in squirrels, and among the lowest metabolic scopes yet recorded in wild animals (6, 56).
We anticipated that pikas might mitigate the energy demands of being out on the surface in the cold temperatures and wind by timing their activity to coincide with the hottest time of day, in the same way that squirrels do (7). In the open alpine meadow habitat, they might also take advantage of absorbed solar radiation to further reduce energy requirements. Operative temperatures between 1100 and 1500 hours were around 15 to 20 °C (SI Appendix, Fig. S5), while actual air temperatures at the same time were −5 to 0 °C. Being underground, pika burrows are buffered from the external temperatures and maintain a relatively stable −10 to −15 °C throughout the day (SI Appendix, Fig. S5). Surface and air temperatures show a more pronounced diurnal cycle. Hence, it is warmer in the burrow at night but warmer on the surface in the day. Pika diurnality may then reflect a strategy of minimizing exposure to cold temperatures. Pygmy rabbits (Brachylagus idahoensis) also use their burrows as thermal refugia to minimize exposure to cold temperatures and conserve energy expenditure in winter (57). We do not know how deep the pika burrows were at the respective sites. If pikas dig extremely deep burrows (>2 m) they may be able to access more stable and warmer temperatures at depth. However, burrow excavations suggest that the maximum depth of burrows is only 55 cm (38). We excavated a total of five burrows at one of the high-density sites and also found burrows were not deeper than 0.5 m. Hence, the burrow temperatures we measured about 1.5 m down the burrows likely reflected the underground environment encountered by the pikas.
Despite the potential thermal advantages of being active on the surface around noon and early afternoon, the pikas were least active on the surface in the middle of the day, and instead focused their activities at dawn and dusk (Fig. 4). Exposure to temperatures above 25.5 °C can cause hyperthermia in American pikas, leading them to avoid the hottest times of day (58–60), but that seems an unlikely explanation of the diurnal activity patterns in plateau pikas reported here, as the temperatures (and operative temperatures) were never so high. In fact, surface activity was much higher in the summer when it was considerably warmer. Moreover, there were large differences in surface activity between sites in relation to yak abundance (Fig. 4) while the microclimate of the sites was very similar (SI Appendix, Fig. S5). This suggests that above-surface activity patterns were not strongly driven by microclimate/thermal factors. The reduced surface activity around noon remains unexplained, but may be an antipredator behavior, since pika behavior is sensitive to predator population density (61) or perceived predation risk (62), and pikas are susceptible to predation by multiple diurnal predators that are common in both areas (63). Previous work in the American pika has suggested that activity may decline in relation to wind speed because wind affects the effectiveness of alarm calling to alert conspecifics about predators (64). The reduction in activity at midday may then be a response to the higher wind speed at this time of day (SI Appendix, Fig. S5). An early study of activity in plateau pikas in captivity also reported peaks in the morning and afternoon, with a lull around midday, but only in July. In other months the activity was more uniform throughout the day, particularly in January (65). This difference strongly suggests ecological factors, such as predation risk or windspeed generated the patterns observed here. Peaks in activity in the early morning and late afternoon have also been observed in free-living Royle’s pika (Ochotona roylei) in the Himalayas. Contrasting this extensive surface winter activity, American pikas remain completely under the snowpack (35), discontinuing surface feeding, and relying on their large haypiles to survive the winter. Estimates of the food available in excavated haypiles suggest they contain more than sufficient food to sustain the animals for the entire winter (36).
In addition to reduced winter activity, the biggest contributor to saving energy in winter was due to reduced RMR and the observed flexibility in body temperature. The reduction in RMR involved two components. The first was a reduction in RMR at thermoneutrality (25 °C) that may be linked to changes in thyroid status (see below). Other species have also been shown to exhibit acclimatization of resting metabolic rate in winter. For example, Arctic ground squirrels (Spermophilus parryii) show a gradual decline in the regulated core body temperature over a period of about 45 d as they approach the winter hibernation season. This body temperature reduction correlates with a decline in RMR (66, 67). European badgers (Meles meles) also show that reduced RMR in winter (68) correlated with a reduction in T4 levels in midwinter. Similarly, reduced RMR in African striped mice during the seasonal dry period was correlated with changes in T3 levels (69), as was reduced RMR in Maximowiczi’s vole (Microtus maximowiczii) (70).
We also observed a reduction in whole-body thermal conductance (slope of the RMR vs. ambient temperature line) in winter, linked to improvements in pelage thermal insulation properties (SI Appendix, Fig. S7). The thickening and/or lengthening of the pelage in winter to reduce thermal conductance is well established (1; reviewed in ref. 71) and has been shown previously in many small mammal species, including other lagomorphs Lepus californicus (72) and L. americanus (12). MacArthur and Wang (73) also observed lower thermal conductance of American pika in winter. Together, the reduction in RMR and elevated thermal insulation meant that at 5 °C (approximately the average operative temperature they experience) the plateau pika had lowered resting energy demands by around 30 kJ/d in winter (Fig. 1 C and D). Hence, these modifications in RMRt and thermal conductance accounted for about 60% of the 50 kJ/d difference between summer and winter DEE (Fig. 1A). These measurements of RMR in the laboratory do not capture the energy savings made by the pikas by reducing their body temperature at night, since they were made during daytime.
In theory, the energy demands in the cold burrows could be mitigated by huddling. The impact of huddling on energy demands stems from two effects: reduced surface area exposed to cold temperatures and changes in the local microclimate due to locally trapped heat (49). Many previous studies have documented thermal benefits derived from huddling (e.g., refs. 49, 50, and 74). In contrast, we did not observe any metabolic benefits of huddling when pikas were housed together in the respirometer. This was potentially because they did not modulate the local temperature inside the chamber and derived low benefits from proximity because they were insulated from each other. The sample size involved was small but suggests that pikas may not benefit significantly from huddling to conserve energy, although we cannot discount the possibility that larger groups might derive benefits by huddling or through local microclimate heating (49). Indeed, Wei and Zhang (38), on the basis of burrow architecture, suggested family groups of pikas may huddle in “nesting chambers” during winter to conserve energy. There is no objective evidence to support or refute this. In other species there are profound changes in social behavior with season that include animals becoming more gregarious to permit huddling (75). Although there have been some previous studies of pika personality traits (76), these have not included evaluation of seasonal impacts in relation to gregariousness.
Pikas showed a strong diurnal pattern in their body temperatures, dropping by on average 1.5 °C at night. Such relatively small reductions in Tb, compared to the drops observed in torpor and hibernation, have been shown in other animals to produce significant energy savings (77). The fall in body temperature, corresponding to nightfall, was rapid, with pikas dropping their body temperature at on average 3.4 °C/h. But the rise in temperature, commencing at dawn, was much slower and averaged only 0.8 °C/h (SI Appendix, Table S2). Reheating the body can be energetically expensive (33, 78), and the slow rate of warming may reflect the possibility that the pikas were passively rewarming. Therefore, another reason pikas may time their activity at dawn is to passively reheat themselves, taking advantage of elevating ambient temperatures and basking in the morning solar radiation. Basking has been previously well established to reduce energy demands at ambient temperatures below thermoneutral (79–81), and is used as a strategy to rewarm from periods of torpor in several species (81, 82). In this way pikas may take advantage of reduced body temperature at night to conserve energy, without incurring the energy cost of reheating. In favor of this interpretation, the rates of warming were related to the ambient temperature in four of the five animals measured (SI Appendix, Fig. S10 and Table S2). Contrasting the situation with warming, cooling rates were poorly associated with the simultaneously recorded surface temperature (SI Appendix, Fig. S10 and Table S2). This could be because evening surface conditions do not closely reflect the down burrow environment where the animals were cooling down.
Although most small mammals show diurnal patterns of body temperature change, this pattern of body temperature change was unexpected because previous laboratory studies have emphasized that pikas lack such a diurnal cycle (83). When we kept some implanted pikas in the laboratory for a short period, we also noted there was no diurnal cycle in their body temperatures (SI Appendix, Fig. S10). Reduction of body temperature at night is clearly a facultative response to the ambient conditions. At high constant temperatures and constant food supply, which typify laboratory housing, pikas do not reduce their body temperatures at night because they have no need to conserve energy. Our data therefore suggest the absence of a diurnal body temperature cycle in pikas (as described in ref. 83) is probably an artifact of captivity. The cycle of temperature appeared to be regulated in relation to the ambient temperature (R2 of relation between body and ambient temperature for day and for night was 0.464 and 0.278, respectively) and, hence, putative energy demands for thermoregulation. Moreover, the slope for linear regression was steeper during nighttime than daytime (0.044 vs. 0.033, night vs. day, respectively, P < 0.001). Pikas dropped their body temperature more at night and were also colder in the day, when the ambient temperature was lower (Fig. 3). Previous laboratory work in plateau pikas suggests T3 and T4 may also be responsive to ambient temperature changes (84, 85) and, hence, the thyroid axis may be an important regulator of these effects. Similarly, in trumpet-tailed rats (Octodon degus), circulating thyroid levels are responsive to both chronic and acute changes in ambient temperature (86). Supporting this interpretation, when we captured pikas directly from the field they had lower T3 and T4 levels in winter than in summer, consistent with their lower metabolic rates in winter. Several other studies in mammals have also shown that DEE, RMR, or nonshivering thermogenesis in the field are related to levels of thyroid hormones (70, 87, 88), and in humans it has been long established that thyroid hormone levels correlate positively with basal metabolic rate (89). However, two studies in birds have shown links of thyroid hormone levels to resting but not daily energy demands (90, 91).
Several previous laboratory studies have demonstrated experimental links between levels of thyroid hormones, metabolic rate, and food intake (e.g., refs. 92–94). But the relationship of these effects to ambient temperature is complex. For example, experimental treatment of striped hamsters (Cricetulus barabensis) in the laboratory with levothyroxine elevated resting metabolic rate and food intake (95), paralleling the effects of cold exposure. However, treatment with levothyroxine in the cold had no significant impact. We suggest that the responsivity of pikas to cold ambient temperatures, mediated via the thyroid axis, probably includes sensitivity to food supply, since we experimentally demonstrated the T3 and T4 levels were acutely responsive to changes in starvation status of pikas held captive overnight. Exactly how energy balance drives changes in the thyroid axis leading to counter regulatory changes in body temperature and DEE remains unclear. However, in hibernating Djungarian hamsters (Phodopus sungorus), thyroid hormone status affects the expression of daily torpor (96), showing a direct link of the thyroid axis to body temperature regulation. This acute responsivity of body temperature to environmental conditions has also been shown in other species in the wild (51, 97), although whether it is linked to thyroid status is unknown. However, several studies also show high variability of thyroid status in relation to environmental conditions (98, 99), suggesting this may be a more general endocrine mechanism coupling environment to physiology.
Contrasting the strategy shown by most hibernating mammals to deposit a large fat store in the fall, that is progressively utilized through the winter, plateau pikas did not show a peak in body weight in September nor any reduction over the winter (Fig. 2). Indeed, body weight increased between September and March. These data match those collected previously for the same species at a single site at low altitude (Haibei) over spring, summer, autumn, and winter, showing no large body mass increase in the fall and maintained body weight (at fall levels) in midwinter (46). Wang et al. (32) showed similar stable body weights in Haibei between October and January (October 132.2 g, SD 2.7; January 129.6 g, SD 1.4) and also showed no significant decline in dissected body fat content (October 5.2 g, SD 0.83; January 3.75 g, SD 0.2). Hence, seasonal body fat deposition and utilization over winter is not part of their overwintering strategy. Similar patterns of change in body weight have also been reported for American pikas (73).
These strategies were combined with interspecific coprophagy on yak feces, as demonstrated by direct observation, the presence of yak DNA in pika stomach contents (SI Appendix, Figs. S4 and S11), and convergence of the pika and yak microbiota in winter (SI Appendix, Fig. S12). Although self-coprophagy and caecotrophy are extremely common in mammals, particularly among herbivores (100), including pikas, interspecific coprophagy is a rare feeding behavior. However, it is not unique and is probably underreported. For some other records of interspecific coprophagy, see SI Appendix, Table S5. Previous studies have noted that American pikas include feces in their haypiles and that these are a particularly nutritious component of the food cache (101). Feces of other species may also be included.
A primary benefit of eating yak feces may be that it reduces levels of physical activity. This could have two positive consequences. The first is reduction in exposure to above-ground predators; the second is reduction in DEE. Our measurements of DEE at the areas where yak were abundant or scarce were not significantly different (P = 0.22). However, given the large variation we had relatively low power to detect a significant effect. The average difference in DEE at a body weight of 160 g was 11 kJ (6.7%). To detect a significant difference of this magnitude at the given individual levels of variation and α = 0.05 would have required a sample of 118 animals per group, compared to the 40 per group that we managed to collect. Note, that our study already has one of the largest sample sizes of DLW measurements for a free-living mammal and was sufficiently powered to detect the main effect of season. Therefore, it is still possible that there was a biologically important impact of reduced activity on energy expenditure that we failed to detect due to power issues.
In addition to reducing required surface activity levels, there are several additional benefits pikas may derive from this interspecific coprophagy. First, having been processed by the yak digestive system, yak feces may have lower levels of plant toxicants. American pika put plants containing high levels of phenolics into their haypiles and do not consume them until the levels have declined (102). Second, because the food is partially digested by the yak, it may be more easily digested by the pika. Animal excreta generally are suggested to be rich in amino acids, vitamins, and minerals (101, 103). Coprophagy in chimpanzees (Pan troglodytes) has been speculated to improve digestion of Dialium seeds (Dialium spp. [Caesalpiniaceae]) (104). We were able to perform some limited measurements of digestibility of captive pika feeding on rabbit chow, grass, and yak feces. These showed that digestibility of grass (33% SD = 2.4, n = 3) was significantly lower than either commercial rabbit chow (64% SD = 3.8, n = 10) or yak feces (62%, SD = 18.0, n = 4; comparison across diets: F2, 14 = 14.0, P = 0.0004; Tukey post hoc P < 0.0025). In addition to harvesting more energy, eating feces may also provide access to otherwise scarce nutrients. For example, Egyptian vultures (Neophron percnopterus) are suggested to obtain carotenoids to enhance the intensity of color in their yellow face patches by eating ungulate feces (105). Third, given the suggested links between the microbiota and thermoregulatory abilities (106), an advantage may be that the yak microbiota create a more favorable metagenomic profile, favoring better thermal tolerance. However, previous metagenomics work on the plateau pika microbiome, contrasting summer to winter, suggested gene function related to energy and lipid metabolism was reduced in winter (107). Moreover, the link of the microbiota to thermoregulatory ability has been questioned (108). Fourth, yak feces may provide a source of water, given the available vegetation is so dry, and any surface water is frozen. Feeding on a highly digestible and readily available food source like yak feces may reduce the time they need to spend on the surface (Fig. 4), and such reduced surface activity may lead to benefits in reduced exposure to aerial predators, such as, peregrine falcon (Falco peregrinus) and buzzards (Buteo refectus) that are routinely seen at these sites, along with terrestrial predators like the Tibetan fox (Vulpes ferrilata), a specialist pika predator (72), none of which can access the pikas when they are in their burrows. American pika also show flexibility in their diet choice in situations where their main food sources are reduced, specializing, for example, on moss (101). There are also potential costs associated with the feces eating behavior. Eating yak feces may increase susceptibility to infection with gut parasites. The avoidance of feces-contaminated patches of grass by sheep (Ovis aeries) is believed to be an antiparasitic behavior (109). Although there has been considerable work on the gut parasites of American pika, very little is published on the gut parasites of the plateau pika (110) and nothing on their relation to gut parasites found in yak.
Pika exploitation of yak feces may also contribute to our understanding of a long-standing conundrum, in that while pikas and yak are widely regarded as competitors for food, the abundance of pikas is generally greater in areas where yak are extensively grazed. Experimental manipulation of grazing alters pika populations, with more pikas reported from grazed than ungrazed plots (111). This effect has been previously attributed to yak reducing the height of vegetation, which provides pikas with more protection from predators, although experimental evidence does not support this interpretation (30). The alternative hypothesis posited here is that yak feces potentially provide a resource that pikas can utilize to enhance their overwinter survival.
Limitations.
A major limitation of this work is the small sample sizes for some of the measurements. The extended temperature recording was available for only 6 pikas and the number of animals measured for RMR was only 13 in summer and 14 in winter. These reflect in part the difficulties of working at these field sites. Apart from this probably, the biggest limitation of this work is the issue of whether pikas store food underground, which then enables them to reduce above-ground activity in midwinter. We inferred from a number of indirect pieces of information that they do not. However, proving a negative is fundamentally problematical. Although we collected one of the largest samples of field metabolic rate measures using the DLW method for any wild animal studied to date, and this sample was sufficient to distinguish our primary question of what energy demands are in winter compared to summer, we had insufficient power to detect the potentially subtle impact of feeding on yak feces on DEE. Moreover, our measurements of RMR were made during the day, and therefore did not capture the savings made by suppressing body temperature at night. However, even if we had made measurements at night, these would likely not reflect the actual savings because we showed that the animals sustain a more stable and elevated body temperature in the laboratory than they exhibit in the field (SI Appendix, Fig. S10). Measuring energy expenditure during the period of suppressed temperature at night would provide a clearer picture of the savings made by this physiological suppression. However, how to get animals to show this suppression in a situation where their RMR could be measured is challenging. Another issue in the quantification of energy demands relates to the impact of huddling. We made relatively few measurements of the impact of such behavior using respirometry, and we had no access to information whether they huddle underground or not.
Conclusions
Surviving extremely cold winters is a feature exhibited by many small nonhibernating, nonmigrating endotherms, and the integrated strategy they use to survive such conditions has been seldom documented, although individual components of such strategies have been extensively studied. Previous work shows that different species have reached individual solutions to this problem, including, for example: in North American squirrels building well-insulated nests as refuges from the cold and caching food, in Snowshoe hares’ metabolic suppression, and in American pika caching food. Our work showed that winter survival of the plateau pika on the Qinghai-Tibetan Plateau involves an integrated change in physical activity, resting metabolism, and body temperature regulation, probably mediated via the thyroid axis, which together mean that despite it being on average 25 °C colder than in summer, daily energy requirements in winter were reduced by about 30%. Some pikas also exploit yak feces as a novel food source. Deposition of a fat store in the fall, and its progressive utilization over winter, and caching food was not part of their overwinter survival strategy.
Materials and Methods
All procedures were reviewed and approved by the Ethical Review Board of the Institute of Zoology, Chinese Academy of Sciences, Beijing (approval number IOZ20150065-1). We primarily studied pikas in two study areas between 3,400 and 4,000 m ASL between 2007 and 2020. The data are based on a total of about 20 field trips plus extended periods of study of animals brought to the Chinese Academy of Science Northwest Plateau Biology Institute in Xining. The study sites included two sites at a study area with high yak densities (3,500 m ASL ∼3.6 km north of Qinghai lake, Gangcha county: 37.25 N, 100.29 E) and two sites in a study area with lower yak densities (3,900 m ASL; Guolou district Maqin county: 34.46 N, 100.36 E). We studied the energy expenditure in the field using the DLW technique and resting energy demands in the laboratory using indirect calorimetry. We studied physical activity patterns in the field by filming the animals. Long-term body temperature responses were recorded using implanted temperature monitors. We made measurements and experimental manipulations of their energy balance to explore the potential role of the thyroid axis in mediating environmental impacts on energy budgets. Finally, we measured the presence of yak DNA in the stomach contents of Pika using PCR and explored the convergence of the pika and yak microbiomes using 16S sequencing. Detailed materials and methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grants 31670394 and 31570410), the strategic program of the Chinese Academy of Sciences (Grant XDB13030100), The National Key Research and Development Program of China (Grant 2016YFC0501905-04), and a United Kingdom–China collaboration grant from the Royal Society and National Science Foundation of China (NSFC-RS 30711130224). J.R.S. was also supported by the President’s International Fellowship Initiative professorial fellowship program and a Wolfson merit award. Ł.O. was supported by a PIFI2017 (Presidents International Fellowship Initiative 2017).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2100707118/-/DCSupplemental.
Data Availability
The data have been deposited in the Open Science Framework (https://osf.io/vg3mc/).
Change History
July 22, 2021: The author line has been updated.
References
- 1.Scholander P. F., Walters V., Hock R., Irving L., Body insulation of some arctic and tropical mammals and birds. Biol. Bull. 99, 225–236 (1950). [DOI] [PubMed] [Google Scholar]
- 2.Anderson K. J., Jetz W., The broad-scale ecology of energy expenditure of endotherms. Ecol. Lett. 8, 310–318 (2005). [Google Scholar]
- 3.Tattersall G. J., et al., Coping with thermal challenges: Physiological adaptations to environmental temperatures. Compr. Physiol. 2, 2151–2202 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Tomanek L., The importance of physiological limits in determining biogeographical range shifts due to global climate change: The heat-shock response. Physiol. Biochem. Zool. 81, 709–717 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Valladares F., et al., The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351–1364 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Speakman J. R., The cost of living: Field metabolic rates of small mammals. Adv. Ecol. Res. 30, 177–297 (2000). [Google Scholar]
- 7.Pauls R. W., Behavioural strategies relevant to the energy economy of the red squirrel (Tamiasciurus hudsonicus). Can. J. Zool. 56, 1519–1525 (1978). [Google Scholar]
- 8.Menzies A. K., et al., Body temperature, heart rate and activity patterns of boreal homeotherms in winter: Homeostasis, allostasis and ecological coexistence. Funct. Ecol. 34, 2292–2301 (2020). [Google Scholar]
- 9.Humphries M. M., et al., Expenditure freeze: The metabolic response of small mammals to cold environments. Ecol. Lett. 8, 1326–1333 (2005). [Google Scholar]
- 10.Studd E. K., et al., Optimisation of energetic and reproductive gains explains behavioural responses to environmental variation across seasons and years. Ecol. Lett. 23, 841–850 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Brigham R. M., Geiser F., Do red squirrels (Tamiasciurus hudsonicus) use daily torpor during winter? Ecoscience 19, 127–132 (2012). [Google Scholar]
- 12.Sheriff M. J., Speakman J. R., Kuchel L., Boutin S., Humphries M. M., The cold shoulder: Free‐ranging snowshoe hares maintain a low cost of living in cold climates. Can. J. Zool. 87, 956–964 (2009). [Google Scholar]
- 13.Wang C. T., et al., Changes in plant species diversity and productivity along an elevational gradient in an alpine meadow. Acta Phytoecol. Sin. 28, 240–245 (2004). (in Chinese). [Google Scholar]
- 14.Li Y. N., Zhao X. Q., Cao Y. M., Analyses on climates and vegetation productivity background at Haibei alpine meadow ecosystem research station. Plateau Meteorol. 23, 558–567 (2004). [Google Scholar]
- 15.Geng W. C., Optimum study of sown pasture sheep systems. III. Introduction and cultivation of several winter survival soiling crops. Caoye Kexue Z1, 40–42 (2000). [Google Scholar]
- 16.Zhuoga L., Luobuciren, Dejiyangzong, Quda, Xiu-hai Y., Bianbadunzhu, Survey on the grassland conditions and biomass in Nagqu County of Tibetan Autonomous Region. Caoye Kexue 26, 11–17 (2009). [Google Scholar]
- 17.Fu H., et al., Environment and host species identity shape gut microbiota diversity in sympatric herbivorous mammals. Microb. Biotechnol. (2020), 10.1111/1751-7915.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith A. T., Foggin J. M., The plateau pika (Ochotona curzoniae) is a keystone species for biodiversity on the Tibetan Plateau. Anim. Conserv. 2, 235–240 (1999). [Google Scholar]
- 19.Lai C. H., Smith A. T., Keystone status of plateau pika (Ochotona curzoniae): Effect of control on biodiversity of native birds. Biodivers. Conserv. 12, 1901–1912 (2003). [Google Scholar]
- 20.Smith A. T., Badingqiuying, Wilson M. C., Hogan B. W., Functional-trait ecology of the plateau pika Ochotona curzoniae in the Qinghai-Tibetan Plateau ecosystem. Integr. Zool. 14, 87–103 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Li W. J., Zhang Y. M., Impacts of plateau pika on soil organic matter and moisture content in alpine meadow. Shou Lei Xue Bao 26, 331–337 (2006). [Google Scholar]
- 22.Pang X. P., Guo Z. G., Plateau pika disturbances alter plant productivity and soil nutrients in alpine meadows of the Qinghai-Tibetan Plateau, China. Rangeland J. 39, 133–144 (2017). [Google Scholar]
- 23.Wang Q., et al., The disturbance and disturbance intensity of small and semi-fossorial herbivores alter belowground bud density of graminoids in alpine meadows. Ecol. Eng. 113, 35–42 (2018). [Google Scholar]
- 24.Liu Y. S., Fan J. W., Shi Z., Yang X., Harris W., Relationships between plateau pika (Ochotona curzoniae) densities and biomass and biodiversity indices of alpine meadow steppe on the Qinghai-Tibet Plateau China. Ecol. Eng. 102, 509–518 (2017). [Google Scholar]
- 25.Guo Z. G., et al., Response of alpine meadow communities to burrow density changes of plateau pika (Ochotona curzoniae) in the Qinghai-Tibet Plateau. Acta Ecol. Sin. 32, 44–49 (2012). [Google Scholar]
- 26.Zhao X. Q., Zhao L., Xu T. W., Xu S. X., The plateau pika has multiple benefits for alpine grassland ecosystem in Qinghai-Tibet Plateau. Ecosyst. Health Sustain. 6, 1750973 (2020). [Google Scholar]
- 27.Harris R. B., Rangeland degradation on the Qinghai-Tibetan Plateau: A review of the evidence of its magnitude and causes. J. Arid Environ. 74, 1–12 (2010). [Google Scholar]
- 28.Wu L., Wang H., Poisoning the pika: Must protection of grasslands be at the expense of biodiversity? Sci. China Life Sci. 60, 545–547 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Dong Q. M., Zhao X. Q., Wu G. L., Shi J. J., Ren G. H., A review of formation mechanism and restoration measures of “black-soil-type” degraded grassland in the Qinghai-Tibetan Plateau. Environ. Earth Sci. 70, 2359–2370 (2013). [Google Scholar]
- 30.Wangdwei M., Steele B., Harris R. B., Demographic responses of plateau pikas to vegetation cover and land use in the Tibet Autonomous Region, China. J. Mammal. 94, 1077–1086 (2013). [Google Scholar]
- 31.Sun F., Chen W. Y., Liu L., Cai Y. M., Smith P., Effects of plateau pika activities on seasonal plant biomass and soil properties in the alpine meadow ecosystems of the Tibetan Plateau. Grassl. Sci. 61, 195–203 (2015). [Google Scholar]
- 32.Wang J. M., Zhang Y. M., Wang D. H., Seasonal thermogenesis and body mass regulation in plateau pikas (Ochotona curzoniae). Oecologia 149, 373–382 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Ruf T., Geiser F., Daily torpor and hibernation in birds and mammals. Biol. Rev. Camb. Philos. Soc. 90, 891–926 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakopak R. P., Hall L. E., Chalfoun A. D., Organising the pantry: Cache management improves quality of overwinter food stores in a montane mammal. J. Mammal. 98, 1674–1681 (2017). [Google Scholar]
- 35.Conner D. A., Seasonal changes in activity patterns and the adapative value of haying in pikas (Ochotona princeps). Can. J. Zool. 61, 411–416 (1983). [Google Scholar]
- 36.Dearing M. D., The function of haypiles of pikas (Ochotona princeps). J. Mammal. 78, 1156–1163 (1997a). [Google Scholar]
- 37.Liu W., et al., Food selection by plateau pikas in different habitats during plant growing season. Shou Lei Xue Bao 28, 358–366 (2008). [Google Scholar]
- 38.Wei W. R., Zhang W. G., Architecture characteristics of burrow system of plateau pika, Ochotona curzoniae. Pak. J. Zool. 50, 311–316 (2018). [Google Scholar]
- 39.McKechnie A. M., Smith A. T., Peacock M. M., Kleptoparasitism in pikas (Ochotona princeps)—Theft of hay. J. Mammal. 75, 488–491 (1994). [Google Scholar]
- 40.Zong H., Xia W. P., Sun D., The influence of a heavy snow on the population density of small mammals. Acta Biol. Plateau Sin. 5, 85–90 (1986). [Google Scholar]
- 41.Qu J. P., Li W. J., Yang M., Ji W. H., Zhang Y. M., Life history of the plateau pika (Ochotona curzoniae) in alpine meadows of the Tibetan Plateau. Mamm. Biol. 78, 68–72 (2013). [Google Scholar]
- 42.Pech R. P., Jiebu, Arthur A. D., Zhang Y. M., Lin H., Population dynamics and responses to management of plateau pikas (Ochotona curzoniae) in the Naqu District of Tibet. J. Appl. Ecol. 44, 615–625 (2007). [Google Scholar]
- 43.Arthur A. D., Pech R. P., Davey C., Zhang Y. M., Lin H., Livestock grazing, plateau pikas and the conservation of avian biodiversity on the Tibetan Plateau. Biol. Conserv. 141, 1972–1981 (2008). [Google Scholar]
- 44.Qu J. P., et al., Effects of climate change on the reproduction and offspring sex ratio of plateau pika (Ochotona curzoniae) on the Tibetan Plateau. J. Arid Environ. 134, 66–72 (2016). [Google Scholar]
- 45.Wang D. H., Wang Z. W., Strategies for survival of small mammals in a cold alpine environment: II. Seasonal changes in the capacity of nonshivering thermogenesis in Ochotona curzoniae and Microtus oeconomus. Shou Lei Xue Bao 10, 40–53 (1990). [Google Scholar]
- 46.Wang D. H., Wang Z. W., Seasonal variations in thermogenesis and energy requirements of plateau pikas (Ochotona curzoniae) and root voles (Microtus oeconomus). Acta Theriol. (Warsz.) 41, 225–236 (1996). [Google Scholar]
- 47.Wang D., Sun R., Wang Z., Liu J., Effects of temperature and photoperiod on thermogenesis in plateau pikas (Ochotona curzoniae) and root voles (Microtus oeconomus). J. Comp. Physiol. B 169, 77–83 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Li J., et al., Chronic cold exposure results in subcutaneous adipose tissue browning and altered global metabolism in Qinghai-Tibetan Plateau pika (Ochotona curzoniae). Biochem. Biophys. Res. Commun. 500, 117–123 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Hayes J. P., Speakman J. R., Racey P. A., The contributions of local heating and reducing exposed surface area to the energetic benefits of huddling by short-tailed field voles (Microtus agrestis). Physiol. Zool. 65, 742–762 (1991). [Google Scholar]
- 50.Scantlebury M., Bennett N. C., Speakman J. R., Pillay N., Schradin C., Huddling in groups leads to daily energy savings in free-living African four-striped grass mice, Rhabdomys pumilio. Funct. Ecol. 20, 166–173 (2006). [Google Scholar]
- 51.Riek A., et al., Energy expenditure and body temperature variations in llamas living in the High Andes of Peru. Sci. Rep. 9, 4037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Z. J., Król E., Moille S., Gamo Y., Speakman J. R., Limits to sustained energy intake. XV. Effects of wheel running on the energy budget during lactation. J. Exp. Biol. 216, 2316–2327 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Wang X. G., Dai K. H., Studies on the population reproduction ecology of plateau pika. Zool. Res. 12, 155–161 (1991). [Google Scholar]
- 54.Qu J. P., et al., Five-year population dynamics of plateau pikas (Ochotona curzoniae) on the east of Tibetan Plateau. Eur. J. Wildl. Res. 63, e51 (2017). [Google Scholar]
- 55.Fletcher Q. E., et al., Seasonal stage differences overwhelm environmental and individual factors as determinants of energy expenditure in free-ranging red squirrels. Funct. Ecol. 26, 677–687 (2012). [Google Scholar]
- 56.Speakman J. R., Król E., Maximal heat dissipation capacity and hyperthermia risk: Neglected key factors in the ecology of endotherms. J. Anim. Ecol. 79, 726–746 (2010). [DOI] [PubMed] [Google Scholar]
- 57.Milling C. R., et al., Seasonal temperature acclimatization in a semi-fossorial mammal and the role of burrows as thermal refuges. PeerJ 6, e4511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacArthur R. A., Wang L. C. H., Behavioral thermoregulation in the pika Ochotona princeps: A field study using radiotelemetry. Can. J. Zool. 52, 353–358 (1974). [DOI] [PubMed] [Google Scholar]
- 59.Smith A. T., Distribution and dispersal of pikas—Influences of behavior and climate. Ecology 55, 1368–1376 (1974). [Google Scholar]
- 60.Camp M. J., Shipley L. A., Varner J., Waterhouse B. D., Activity patterns and foraging behavior of American pikas (Ochotona princeps) differ between craters of the moon and alpine talus in Idaho. West. N. Am. Nat. 80, 46–69 (2020). [Google Scholar]
- 61.Yin B. F., Yang S. M., Shang G. Z., Wei W. H., Effects of predation risk on behavior, hormone levels and reproductive success of plateau pikas. Ecosphere 8, e01643 (2017). [Google Scholar]
- 62.Holmes W. G., Predator risk affects foraging behavior of pikas—Observational and experimental evidence. Anim. Behav. 42, 111–119 (1991). [Google Scholar]
- 63.Badingqiuying P., Smith A., Senko J., Siladan M. U., Plateau pika Ochotona curzoniae poisoning campaign reduces carnivore abundance in southern Qinghai, China. Mammal Study 41, 1–8 (2016). [Google Scholar]
- 64.Hayes A. R., Huntly N. J., Effects of wind on the behavior and call transmission of pikas (Ochotona princeps). J. Mammal. 86, 974–981 (2005). [Google Scholar]
- 65.Zong H., Xia W. P., Studies of the diurnal cycle of pika (Ochotona curzoniae) in the plateau of China. Shou Lei Xue Bao 7, 211–223 (1987). [Google Scholar]
- 66.Sheriff M. J., Williams C. T., Kenagy G. J., Buck C. L., Barnes B. M., Thermoregulatory changes anticipate hibernation onset by 45 days: Data from free-living arctic ground squirrels. J. Comp. Physiol. B 182, 841–847 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Sheriff M. J., Richter M. M., Buck C. L., Barnes B. M., Changing seasonality and phenological responses of free-living male arctic ground squirrels: The importance of sex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120480 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McClune D. W., et al., Winter is coming: Seasonal variation in resting metabolic rate of the European badger (Meles meles). PLoS One 10, e0135920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rimbach R., Pillay N., Schradin C., Both thyroid hormone levels and resting metabolic rate decrease in African striped mice when food availability decreases. J. Exp. Biol. 220, 837–843 (2017). [DOI] [PubMed] [Google Scholar]
- 70.Chen J. F., Zhong W. Q., Wang D. H., Seasonal changes in body mass, energy intake and thermogenesis in Maximowiczi’s voles (Microtus maximowiczii) from the inner Mongolian grassland. J. Comp. Physiol. B 182, 275–285 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Lovegrove B. G., Seasonal thermoregulatory responses in mammals. J. Comp. Physiol. B 175, 231–247 (2005). [DOI] [PubMed] [Google Scholar]
- 72.Harris R. B., et al., Evidence that the Tibetan fox is an obligate predator of the plateau pika: Conservation implications. J. Mammal. 95, 1207–1221 (2014). [Google Scholar]
- 73.MacArthur R. A., Wang L. C. H., Physiology of thermoregulation in the pika, Ochotona princeps. Can. J. Zool. 51, 11–16 (1973). [DOI] [PubMed] [Google Scholar]
- 74.Canals M., Rosenmann M., Bozinovic F., Geometrical aspects of the energetic effectiveness of huddling in small mammals. Acta Theriol. (Warsz.) 42, 321–328 (1997). [Google Scholar]
- 75.Karasov W. H., Wintertime energy conservation by huddling in antelope ground squirrels, (Ammospermophilus lecurus). J. Mammal. 64, 341–345 (1983). [Google Scholar]
- 76.Qu J., Réale D., Fletcher Q. E., Zhang Y., Among-population divergence in personality is linked to altitude in plateau pikas (Ochotona curzoniae). Front. Zool. 16, 26 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Glanville E. J., Seebacher F., Advantage to lower body temperatures for a small mammal (Rattus fiscipes) experiencing chronic cold. J. Mammal. 91, 1197–1204 (2010). [Google Scholar]
- 78.Lyman C. P., The oxygen consumption and temperature regulation of hibernating hamsters. J. Exp. Zool. 109, 55–78 (1948). [DOI] [PubMed] [Google Scholar]
- 79.Bartholomew G. A., Rainy M., Regulation of body temperature in the rock hyrax, Heterohyrax brucei. J. Mammal. 52, 81–95 (1971). [PubMed] [Google Scholar]
- 80.Geiser F., Drury R. L., Radiant heat affects thermoregulation and energy expenditure during rewarming from torpor. J. Comp. Physiol. B 173, 55–60 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Geiser F., et al., Basking hamsters reduce resting metabolism, body temperature and energy costs during rewarming from torpor. J. Exp. Biol. 219, 2166–2172 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Warnecke L., Geiser F., Basking behavior and torpor use in free-ranging Planigale gilesi. Aust. J. Zool. 57, 373–375 (2009). [Google Scholar]
- 83.Luo Z. W., et al., Anatomical and neurochemical peculiarities of the pika retina: Basis for lack of circadian rhythm of core temperature. Neurosci. Lett. 259, 13–16 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Liu J. S., Li Q. F., Seasonal variations of thermogenesis in brown adipose tissue and liver in plateau pika (Ochotona curzoniae). Shou Lei Xue Bao 16, 155–157 (1995). (in Chinese). [Google Scholar]
- 85.Li Q. F., Lu S., The effects of thyroid hormone on adaptive thermogenesis in Ochotona curzoniae [in Chinese]. Alpine Meadow Ecosystem 4, 117–126 (1995). [Google Scholar]
- 86.Vaughan, M. K., Little, J. C., Buzzell, G. R., Menendezpelaz, A.Reiter, R. J.. Natural decreasing temperature and photoperiod conditions of acute cold exposure affect circulating thyroid hormones, serum cholesterol and type-II 5'-deiodinase in brown adipose tissue in the trumpet tailed rat, Octodon degus. Biomedical Research–Tokyo 10, 469–474 (1989). [Google Scholar]
- 87.Brinkmann L., Gerken M., Hambly C., Speakman J. R., Riek A., Thyroid hormones correlate with field metabolic rate in ponies, Equus ferus caballus. J. Exp. Biol. 219, 2559–2566 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Rawson R. E., Concannon P. W., Roberts P. J., Tennant B. C., Seasonal differences in resting oxygen consumption, respiratory quotient, and free thyroxine in woodchucks. Am. J. Physiol. 274, R963–R969 (1998). [DOI] [PubMed] [Google Scholar]
- 89.Danforth E. Jr, Burger A., The role of thyroid hormones in the control of energy expenditure. Clin. Endocrinol. Metab. 13, 581–595 (1984). [DOI] [PubMed] [Google Scholar]
- 90.Elliott K. H., et al., Thyroid hormones correlate with resting metabolic rate, not daily energy expenditure, in two charadriiform seabirds. Biol. Open 2, 580–586 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welcker J., et al., Thyroid hormones correlate with basal metabolic rate but not field metabolic rate in a wild bird species. PLoS One 8, e56229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banta M. R., Holcombe D. W., The effects of thyroxine on metabolism and water balance in a desert-dwelling rodent, Merriam’s kangaroo rat (Dipodomys merriami). J. Comp. Physiol. B 172, 17–25 (2002). [DOI] [PubMed] [Google Scholar]
- 93.Dittner C., Lindsund E., Cannon B., Nedergaard J., At thermoneutrality, acute thyroxine-induced thermogenesis and pyrexia are independent of UCP1. Mol. Metab. 25, 20–34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khakisahneh S., et al., Thyroid hormones mediate metabolic rate and oxidative, anti-oxidative balance at different temperatures in Mongolian gerbils (Meriones unguiculatus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 216, 101–109 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Wen J., et al., Effects of thyroid hormones and cold acclimation on the energy metabolism of the striped hamster (Cricetulus barabensis). J. Comp. Physiol. B 189, 153–165 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Bank J. H. H., Kemmling J., Rijntjes E., Wirth E. K., Herwig A., Thyroid hormone status affects expression of daily torpor and gene transcription in Djungarian hamsters (Phodopus sungorus). Horm. Behav. 75, 120–129 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Hume T., Geiser F., Currie S. E., Körtner G., Stawski C., Responding to the weather: Energy budgeting by a small mammal in the wild. Curr. Zool. 66, 15–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Williams C. T., et al., Environmental heterogeneity affects seasonal variation in thyroid hormone physiology in free-living arctic ground squirrels (Urocitellus parryii). Can. J. Zool. 97, 783–790 (2019). [Google Scholar]
- 99.Wilsterman K., Buck C. L., Barnes B. M., Williams C. T., Energy regulation in context: Free-living female arctic ground squirrels modulate the relationship between thyroid hormones and activity among life history stages. Horm. Behav. 75, 111–119 (2015). [DOI] [PubMed] [Google Scholar]
- 100.Hirakawa H., Coprophagy in lepordids and other mammalian herbivores. Mammal Rev. 31, 61–80 (2001). [Google Scholar]
- 101.Varner J., Dearing M. D., Dietary plasticity in pikas as a strategy for atypical resource landscapes. J. Mammal. 95, 72–81 (2014). [Google Scholar]
- 102.Dearing M. D., The manipulation of plant toxins by a food-hoarding herbivore, Ochotona princeps. Ecology 78, 774–781 (1997b). [Google Scholar]
- 103.Flachowsky G., Animal excreta as feedstuff for ruminants—A review. J. Appl. Anim. Res. 12, 1–40 (1997). [Google Scholar]
- 104.Krief S., Jamart A., Hladik C. M., On the possible adaptive value of coprophagy in free-ranging chimpanzees. Primates 45, 141–145 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Negro J. J., et al., Coprophagy: An unusual source of essential carotenoids. Nature 416, 807–808 (2002). [DOI] [PubMed] [Google Scholar]
- 106.Li B. G., et al., Microbiota depletion impairs thermogenesis of brown adipose tissue and browning of white adipose tissue. Cell Rep. 26, 2720–2737 (2019). [DOI] [PubMed] [Google Scholar]
- 107.Wang Y., Zhou R., Yu Q., Feng T., Li H., Gut microbiome adaptation to extreme cold winter in wild plateau pika (Ochotona curzoniae) on the Qinghai-Tibet Plateau. FEMS Microbiol. Lett. 367, fnaa134 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Krisko T. I., et al., Dissociation of adaptive thermogenesis from glucose homeostasis in microbiome-deficient mice. Cell Metab. 31, 592–604.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hutchings M. R., Gordon I. J., Kyriazakis I., Jackson F., Sheep avoidance of faeces contaminated patches leads to a trade-off between intake rate of forage and parasitism in subsequent foraging decisions. Anim. Behav. 62, 955–964 (2001). [Google Scholar]
- 110.Wang C., et al., Parasite species associated with wild plateau pika (Ochotona curzoniae) in southeastern Qinghai Province, China. J. Wildl. Dis. 45, 288–294 (2009). [DOI] [PubMed] [Google Scholar]
- 111.Badingqiuying,Smith A.T., Harris R. B., Summer habitat use of plateau pikas (Ochotona curzoniae) in response to winter livestock grazing in the alpine steppe Qinghai-Tibetan Plateau. Arct. Antarct. Alp. Res. 50, e1447190 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data have been deposited in the Open Science Framework (https://osf.io/vg3mc/).