Significance
Social transmission of threat information by observation is effective in humans and other animals. However, it is unknown if such observation of others’ reacting to threats can retrieve memories that have been previously learned through direct, firsthand aversive experiences. Here, we show concordantly in humans and rats that observing a conspecific’s reactions to a threat is sufficient to recover associative memories of direct, firsthand aversive experiences, measured as conditioned threat responses (physiological responses and defensive behavior) in the observer. The reinstatement of threat responses by observation of others is specific to the context that is observed as being dangerous. Our findings provide cross-species evidence that observation of others’ threat reactions can recover associative memories of direct, firsthand aversive experiences.
Keywords: reinstatement, vicarious learning, social learning, threat conditioning
Abstract
Information about dangers can spread effectively by observation of others’ threat responses. Yet, it is unclear if such observational threat information interacts with associative memories that are shaped by the individual’s direct, firsthand experiences. Here, we show in humans and rats that the mere observation of a conspecific’s threat reactions reinstates previously learned and extinguished threat responses in the observer. In two experiments, human participants displayed elevated physiological responses to threat-conditioned cues after observational reinstatement in a context-specific manner. The elevation of physiological responses (arousal) was further specific to the context that was observed as dangerous. An analogous experiment in rats provided converging results by demonstrating reinstatement of defensive behavior after observing another rat’s threat reactions. Taken together, our findings provide cross-species evidence that observation of others’ threat reactions can recover associations previously shaped by direct, firsthand aversive experiences. Our study offers a perspective on how retrieval of threat memories draws from associative mechanisms that might underlie both observations of others’ and firsthand experiences.
Social transmission of information about dangers in the environment is central for the adaption of defensive behaviors (1–3). As such, observing a conspecific’s threat responses to a stimulus or context commonly results in aversive learning and avoidance, saving the observer from costly learning from firsthand experienced trial and error (4–6). Yet, it is still unanswered if such social threat learning interacts with previous memories resulting from firsthand experiences. In other words, can social information recover responses that have been learned by firsthand experiences?
To address this open question, we tested in rats and humans if watching another individual’s threat reactions leads to recovery (or reinstatement) of conditioned responses to a threat cue that was previously acquired through firsthand aversive experience (i.e., Pavlovian threat conditioning). Our observational reinstatement procedure was inspired by established paradigms using firsthand aversive experience to reinstate conditioned defensive responses (CRs) in humans and other animals (7–11).
In the reinstatement paradigm, individuals first acquire an association between a cue (conditioned stimulus [CS]) that is predictive of a firsthand aversive experience (unconditioned stimulus [US]), which results in CRs toward the CS. When the CS is no longer followed by the US (and hence, no longer predictive of the US), CRs attenuate or extinguish slowly. However, the initial CS–US association is not thought to be erased but rather, inhibited. This inhibition can be abolished by presentation of the US alone (reinstatement), which reinstates the CR to the CS. Importantly, the reinstatement of the initial CS–US association is critically dependent on the context in which the reinstatement USs were presented (12). While such reinstatement of threat responses after firsthand aversive (US) experiences is a central mechanism for adaptive and maladaptive behavior toward threats (13, 14), it is unclear if information about others’ aversive experiences can disinhibit threat responses and reinstate firsthand acquired threat memories. This knowledge would reveal how social information interacts with firsthand learning of threats and could thereby provide a mechanistic perspective for overlapping processes that underlie observational and firsthand learning.
We examined whether observation of others’ reactions to threats recovers directly, firsthand acquired and extinguished threat responses (using Pavlovian threat conditioning) in rats and humans and thereby reinstates conditioned threat memories in both species. We hypothesized that observation of others’ behavior toward a US reinstates CRs in humans and rats.
Results
Observation of Others’ Reactions to the US Reinstates Conditioned Threat Responses in Humans.
The first experiment was designed to establish that CRs return after extinction by mere observation of another individual who reacts to a US (i.e., observational reinstatement of CRs). We included two groups that underwent identical acquisition and extinction training that differed only with respect to the demonstrator receiving USs or no USs, respectively (the design is in Fig. 1A).
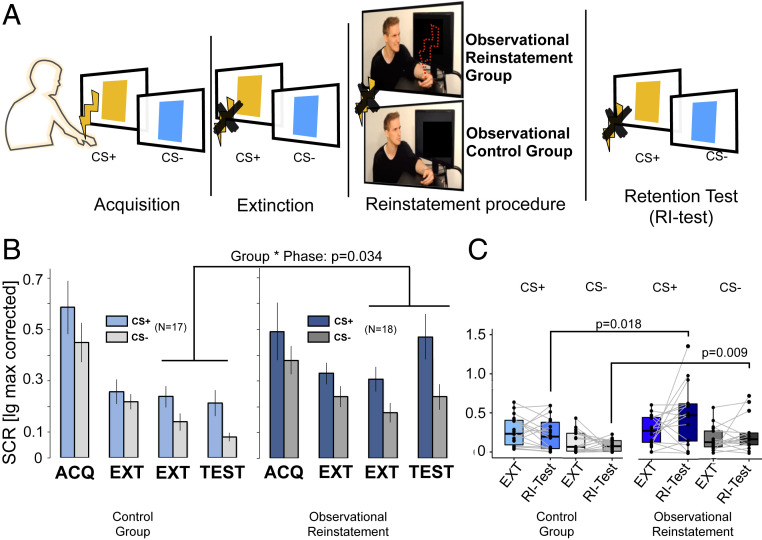
Fig. 1.
Observational reinstatement of firsthand acquired CRs. (A) Overview of the study design in experiment 1. Yellow lightning bolts denote firsthand aversive electrical stimulation to the observing participant. The red dotted lightning bolt denotes aversive electrical stimulation to the demonstrator, serving as an observational US for the observing participant. (B) Bar graphs representing the SCRs during acquisition, early (first block) and late extinction (second block), and the reinstatement test (first block) in the “no-reinstatement control” group (Left) and the observational reinstatement group (Right). SI Appendix, Fig. S1 shows blockwise SCRs during acquisition and the reinstatement test. Error bars represent the SEM. (C) Box plots of individual SCRs during late extinction (second block) and the reinstatement test (first block) for each of the CS+ in the control group (left four bars) and the observational reinstatement group (right four bars). P values indicate interaction in ANOVA (rmANOVA in B) or post hoc t test (one sided in C, corrected for multiple comparisons using Bonferroni–Holm). ACQ = acquisition training; EXT = extinction training.
Participants (N = 35) in both groups acquired higher threat responses toward a CS+ that was followed by an aversive US (aversive electrical stimulation) as compared with a control stimulus (CS–; not followed by a US) measured as skin conductance responses [SCRs; main effect of CS type, F(1,33) = 5.71, P = 0.023; = 0.148, CS+ > CS–: t(33) = 2.39, = 0.022; Cohen’s d = 0.405; mean difference = 0.130 ± 0.054 (SD), 95% CI: 0.020/0.249] with no main effect or interaction with the factor group (P > 0.3) (SI Appendix, Fig. S1 shows details and blockwise SCRs). Subsequently, during extinction training, the CS+ was no longer followed by a US, leading to attenuated SCRs, albeit remaining elevated to the CS+ as compared with the CS– [main effect of CS type: F(1,33) = 9.48, P = 0.004; = 0.223; CS+ > CS−: t(33) = 3.06, = 0.004; Cohen’s d = 0.517; mean difference = 0.089 ± 0.029 (SD), 95% CI: 0.030/0.149], again without a main effect or interaction with the factor group (P > 0.16) (SI Appendix, Table S2 has details).
Importantly, participants who observed a demonstrator model reacting to the US after extinction (experimental group) showed enhanced SCRs as compared with participants in the control group who observed a calm demonstrator [late extinction (second block) to the reinstatement test (first block), group by phase interaction: F(1,33) = 4.90, P = 0.034; = 0.129 and main effect of group: F(1,33) = 9.136, P = 0.005; = 0.22] (Fig. 1B and SI Appendix, Table S3). This interaction effect was indicative of the hypothesized increase in SCRs from late extinction to reinstatement test in the experimental group as compared with the control group [one-sided t test: t(33) = −2.213, = 0.017, Cohen’s d = –0.748, mean difference = –0.156 ± 0.07 (SE), upper 95% CI: –0.037] (Fig. 1C and SI Appendix, Table S4). Hence, these analyses supported a reinstatement (or increase of CRs after extinction) as a function of observing the demonstrator reacting to the US. A post hoc test further confirmed our hypothesis that SCRs in the experimental group during the reinstatement test were enhanced to the CS+ and the CS– when compared with the control group [one-sided t test CS+: t(33) = −2.513, = 0.018, Cohen’s d = –0.850, mean difference = –0.259 ± 0.10 (SE), upper 95% CI: –0.085; CS–: t(33) = −2.90, = 0.009, Cohen’s d = –0.982, mean difference = –0.157 ± 0.05 (SE), upper 95% CI: –0.065] (Fig. 1C and SI Appendix, Table S4; SI Appendix, Table S5 has enhanced SCRs after observational reinstatement when considering the whole reinstatement test phase). This effect was concordant with an enhanced expectancy for the US after observational reinstatement as compared with the no-reinstatement control group [one-sided t test CS+: t(32) = 2.141, = 0.040; CS–: t(32) = 0.272, > 0.7], assessed by a postexperimental questionnaire.
In sum, the results of our first experiment suggest that observing another individual receiving a US reinstates conditioned threat responses that have been previously acquired by direct US experiences.
Observational Reinstatement Is Contingent on Contextual Information.
In the second experiment (N = 21), we tested if observational reinstatement displayed a key characteristic of direct reinstatement, namely contextual dependence. Previous research has established that enhancement of CRs is specific to the context in which the participants received firsthand reinstatement USs [humans (15); animals (7)]. Indeed, reinstatement USs are thought to render a context dangerous, which gates reinstatement of CRs at a later test. Accordingly, we examined if observation of reinstatement within a specific context (here, red illuminated room) leads to enhancement of CRs within the (red) context in which the demonstrator reacted defensively toward the US. Thereby, we would provide insights into how social information can exert contextual gating of CRs.
To address the context dependence of observational reinstatement, another sample of participants underwent firsthand acquisition and extinction training in context A followed by observation of the demonstrators’ reactions to the US in context B (reinstatement context) (Fig. 2A and Materials and Methods). Participants were then tested in contexts A and B for reinstatement of conditioned responses. Thus, context specificity of reinstatement was tested by within-subject comparisons of responses when participants were placed in context A and context B, respectively (cross-over, counterbalanced design). We expected context-specific reinstatement of CRs during the test, evident by enhanced CRs in context B (the context of the observational reinstatement) as compared with control context A. During acquisition training, participants acquired higher responses toward the CS+ as compared with the CS–, measured as SCR [CS type: F(1,20) = 7.987, P = 0.010; = 0.285; CS+ > CS–: t(20) = 2.826, = 0.010, Cohen’s d = 0.617, mean difference = 0.191 ± 0.07 (SE), 95% CI: 0.050/0.331] (SI Appendix, Fig. S4 shows blockwise SCRs, and SI Appendix, Table S9 has details). Subsequent extinction training led to decreasing responses [main effect of block: F(1,20) = 5.315, P = 0.032; = 0.210; Block 1 > Block 2: t(20) = 2.305, = 0.032, Cohen’s d = 0.503, mean difference = 0.081 ± 0.04 (SE), 95% CI: 0.008/0.115] (SI Appendix, Table S10), with no support for a difference between CSs (main effect of CS type: P > 0.19). Importantly, when comparing the SCRs during late extinction in context A with responses during the reinstatement test in control context A and in reinstatement context B, analyses revealed a main effect of context [main effect of context: F(2,28.7) = 5.554, P = 0.016; = 0.217; the factor context includes three levels: extinction, context A, context B] (Fig. 2B and SI Appendix, Table S11). In accordance with our hypothesis, participants exhibited enhancement of CRs from late extinction (second block) to the reinstatement test in the reinstatement context B [one-sided comparison: late extinction vs. context B: t(20) = −2.621, = 0.025, Cohen’s d = –0.572, mean difference = –0.330 ± 0.13 (SE), upper 95% CI: –0.001; late extinction vs. context A: t(20) = −1.702, = 0.052, Cohen’s d = –0.371, mean difference = –0.152 ± 0.089 (SE), upper 95% CI: 0.081; context A vs. context B: t(20) = −2.371, = 0.028, Cohen’s d = –0.517, mean difference = –0.178 ± 0.075 (SE), upper 95% CI: 0.018] (Fig. 2C and SI Appendix, Table S11 have details). Hence, reinstatement of responses was evident in the reinstatement context (i.e., the context in which the demonstrator was present during observational reinstatement). We found weak evidence for a stimulus-specific effect of observational reinstatement in the rmANOVA [CS type × context interaction: F(2,40) = 2.953, P = 0.082; = 0.129] that was characterized as enhancement of responses for both the CS+ and the CS– in the reinstatement context B [one-sided comparison: late extinction vs. context B CS+: t(20) = −2.398, = 0.039, Cohen’s d = –0.523, mean difference = –0.228 ± 0.10 (SE), upper 95% CI: –0.064; CS–: t(20) = 2.570, = 0.036, Cohen’s d = 0.561, mean difference = 0.432 ± 0.17 (SE), upper 95% CI: –0.142] (Fig. 2C and SI Appendix, Table S12). Such a generalized responding after reinstatement (i.e., to the CS+ and the CS–) has been frequently reported in humans (8) and might actually be a result of the strong contextual influence in this experiment that affects all stimuli that are presented within the dangerous context (8, 16). Control analyses, including the order of the contexts in reinstatement testing as well as accounting for individual differences in both dispositional and current anxiety, still revealed a contextual effect on observational reinstatement (SI Appendix, Tables S13–S15). In accordance with experiment 1, the postexperimental ratings revealed a differential reinstatement effect for the CS+ in retrospectively assessed expectations of the US (P < 0.001).
Fig. 2.
Observational reinstatement (SCR) is context specific. (A) Overview of the study design in experiment 2. Yellow lightning bolts denote firsthand aversive electrical stimulation to the observing participant. The red dotted lightning bolt denotes aversive electrical stimulation to the demonstrator, serving as an observational US for the observing participant. (B) Bar graphs representing the SCRs during acquisition, early (first block) and late extinction (second block), and the reinstatement test in context A (white lamp) and context B (red lamp). P values (corrected for multiple comparisons by Bonferroni–Holm) indicate comparisons between late extinction and both contexts. SI Appendix, Fig. S4 shows blockwise SCRs during acquisition. Error bars represent the SEM. (C) Box plots of individual SCRs to both CSs during late extinction (second block) and the reinstatement test (first block) in the reinstatement context. ACQ = acquisition training; EXT = extinction training.
In sum, our results from two studies in humans provide evidence for contextual-dependent reinstatement of threat responses after observation of a demonstrator reacting to a US, indicated by both psychophysiological measures (SCR) and US expectancy. Interestingly, responses are returning in a context that is potentially dangerous for the demonstrator, but was never predictive for a US to the participants.
Next, we wanted to examine observational reinstatement in an experimental model where the synaptic substrates of firsthand reinstatement have been established (17, 18). To this end, we tested for defensive responses measured as freezing behavior (which is commonly examined as a CR in rodents) after firsthand and observational reinstatement procedures in rats (experiment 3). We adapted the observational reinstatement protocol to meet species-specific demands, which allowed us to examine if observational reinstatement is robust to such changes. Our hypothesis was that rats, as humans, show observational reinstatement of defensive responses that are comparable with firsthand reinstatement.
Observational Reinstatement of Conditioned Responses Is Evident in Rats.
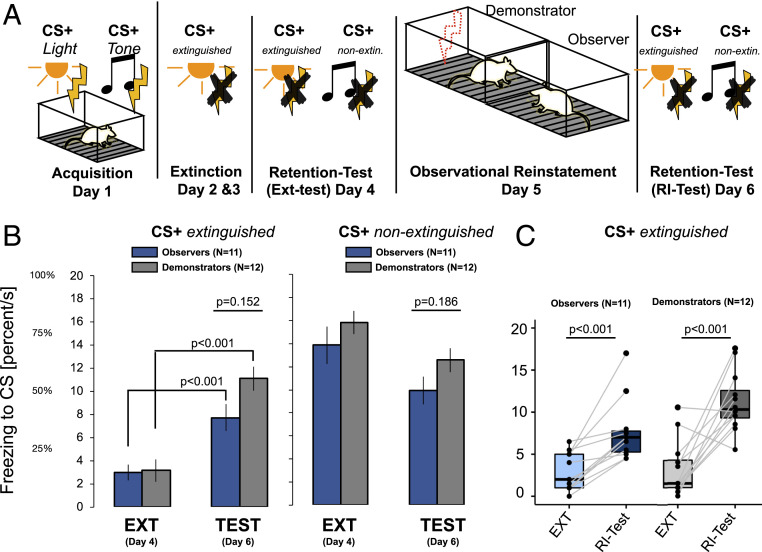
In experiment 3, 23 (11 pairs) of male cage-mate rats individually underwent acquisition training, in which a flashing light and a tone were both predictive of a foot shock US (day 1). Twenty-four hours later (day 2), the rats underwent extinction training to one of the CS+ (CS+extinguished) on 2 consecutive days (Fig. 3A). Behavioral analyses of the retention test (day 4) revealed successful acquisition and extinction training, indicated by longer duration of freezing behavior to the nonextinguished CS+nonextinguished when compared with freezing to the extinguished CS+extinguished, respectively [CS type: F(1,21) = 112.196, P < 0.001; = 0.842; CS+extinguished > CS+nonextinguished: t(20) = 10.548, < 0.001, Cohen’s d = −2.199, mean difference = −11.788 ± 1.12 (SE), 95% CI: −14.112/−9.464] (SI Appendix, Table S20 has details). There was no difference in freezing behavior between demonstrators and observers in the extinction retention test (no interaction or main effect, P > 0.62) (SI Appendix, Table S22). For the observational reinstatement procedure on the next day (day 5), rats (previous cage-mate pairs) were placed in two adjacent chambers, which were separated by plexiglass with holes that allowed for olfactory and auditory exchange (rat pairs were habituated to this chamber without a US before acquisition training). One of the rats (demonstrator rat) received four unsignaled foot shocks, whereas the rat (observer rat) in the other chamber did not receive any foot shocks. Strikingly, we found that freezing behavior to the extinguished CS+extinguished increased in both the demonstrator rats that received firsthand US reinstatement and the observer rats that observed the reinstatement procedure [main effect of reinstatement: F(1,21) = 4.802, P = 0.040; = 0.186] (Fig. 3B and SI Appendix, Table S21). Specifically, freezing behavior increased from the extinction test (day 4) to the reinstatement test (day 6) to the CS+extinguished and reached the same level of the CS+nonextinguished [CS type × reinstatement: F(1,21) = 38.186, P < 0.001; = 0.642; CS+extinguished increase: t(21) = −6.110, = 1.294e-6, mean difference = −6.366 1.042, 95% CI: −9.257/−3.474; CS+nonextinguished decrease: t(21) = 3.580, = 0.003, mean difference = 3.580 1.042, 95% CI: 0.688/6.471; CS+extinguished vs. CS+nonextinguished during reinstatement test: t(21) = −1.649, = 0.107, mean difference = −1.843 1.117, 95% CI: −4.937/1.251] (Fig. 3B and SI Appendix, Tables S21 and S22; SI Appendix, Tables S23 and S24 have separate post hoc tests in demonstrators and observers). These results show that observational reinstatement, which we found in humans in experiments 1 and 2, is also evident in rats. Interestingly, we found no statistical support for a difference in reinstatement of freezing behavior between observers and demonstrators (all interactions: P > 0.15) (SI Appendix, Table S21), although observers displayed less freezing when compared with demonstrators across all phases [main effect of being observer or demonstrator: F(1,21) = 6.774, P = 0.017; = 0.244; demonstrators vs. observers: t(21) = 2.603, = 0.017, Cohen’s d = 0.543, mean difference = 2.052 0.79, 95% CI: 0.412/3.692]. In other words, our results do not reveal any difference in CRs between rats that received a firsthand US vs. observed a cage-mate receiving the US. SI Appendix has an additional experiment to explore the expression of immediate early genes (c-fos protein) in rats within a similar protocol as in experiment 3.
Fig. 3.
Observational reinstatement in rats. (A) Overview of the study design in experiment 3 in rats that included acquisition of two CS+–US associations (using a light CS and a tone CS), one of which was extinguished (CS+extinguished), whereas the other was not (CS+nonextinguished). Yellow lightning bolts denote firsthand aversive electrical stimulation to all rats during acquisition. The red dotted lightning bolt denotes aversive electrical stimulation to the demonstrator rats, indicating an observational US to the observer rats. (B) Bar graphs illustrating an increase in freezing behavior to the CS+extinguished from the retention test before (day 4 extinction test; second block) to after observational reinstatement (day 6 RI test; first block). This increase was evident in both demonstrators (gray bars) and observers (blue bars) to the extinguished CSextinguished but not to the CSnonextinguished. There was no support for differences between demonstrators and observers with respect to freezing behavior in the RI test on day 6. (C) Box plots of individual freezing duration during the extinction test (second block) and the reinstatement test (first block) for each the CSextinguished in the observers (left two bars) and the demonstrators (right two bars). EXT = extinction retrieval test; RI-Test = reinstatement test.
Discussion
Our findings provide cross-species evidence that observation of others’ threat reactions can recover associations previously shaped by direct, firsthand aversive experiences. Specifically, in three experiments studying both humans and rats, we show that the observation of a conspecific exposed to a US can recover—“reinstate”—previously extinguished conditioned responses (SCR in humans and freezing behavior in rats). Importantly, the recovery of threat responses indicates that observing a conspecific’s US reaction overrides the previously learned inhibition of threat responses by firsthand experiences of the absence of the US (i.e., extinction training). Social transmission of threat information might therefore not only recover associations from firsthand aversive experiences but also diminish safety information that we learned from our own firsthand experience. Moreover, our results indicate that the recovery of threat responses through observation is context specific so that responses are displayed only in a situation that is potentially dangerous for the observed individual. We furthermore provide concordant results across humans and rats using species-specific adaptions of observational protocols and complementary measurements of conditioned responses (i.e., psychophysiological responses and defensive behavior, respectively) (19). Our findings indicate that observational reinstatement of conditioned responses is robust to changes in the protocol (e.g., live interaction vs. video clips, differential conditioning vs. nonextinguished cues, immediate vs. delayed extinction). Note that the terms “firsthand experience” and “observational experience” do not necessitate conscious experiences, in particular when examining peripheral arousal in humans and freezing behavior in animals.
Our results provide experimental evidence for the return of threat associations solely through social information. Thereby, our results extend current conditions for the return of threat responses (8, 11, 12, 14, 20, 21) that include shifts of the context (i.e., renewal), passage of time (i.e., spontaneous recovery), CS–US pairings (rapid reacquisition), and direct experience of the US (i.e., direct reinstatement). Moreover, we demonstrate that observational reinstatement shares a defining characteristic with direct reinstatement, namely contextual dependency. Indeed, the context where the observational reinstatement takes place (i.e., the context that surrounds the demonstrator) modulates reinstatement of conditioned responses, similar to contextual specificity of direct reinstatement (7, 15) and other forms of threat recovery (12, 14, 21). Such strong contextual influence might have been one reason for the increase in SCRs to the CS– during the reinstatement test in experiment 2 (i.e., generalized reinstatement), which is a common reinstatement phenomenon in human research (8). We further found that postexperimental US expectancy was increased for the CS+ (and not the CS–) after reinstatement, which might be due to the different sampling time points and the divergence in processes that underlie rating of US expectancy and psychophysiological arousal.
Interestingly, we found return of threat responses in the context in which the demonstrators’ threat reaction was observed, which was, however, never predictive for a US to the participants. Hence, the observational return of cued threat responses was transferred to a context, which has merely been observed as dangerous to another individual. Such a transfer of previously shaped firsthand association by contextual information that is derived from observation of others might be particularly adaptive to generalize threat responses to novel dangerous situations.
Previous research in humans showed that reinstatement of CRs is possible with exposure to a US that had not initially been paired with the CS, so-called “cross-US reinstatement” (22). However, there exist several differences between studies of such cross-US reinstatement and our results. First, cross-US reinstatement leads to expectancy for the US that was presented during reinstatement (22). In contrast, participants in our study still rated (in a postexperimental interview) high expectancy for the firsthand US, which was used during acquisition. Second, whereas cross-US reinstatement using different US types has not been found in rats (17, 18), our results provide evidence for observational reinstatement in rats. Instead, we suggest that observation of others’ responses to a US interacts with previously made associations of firsthand US experiences, which in turn, jointly gate recovery of threat responses. In support of such a mechanism underlying observational reinstatement are previous findings of reinstated threat responses through retrieval of firsthand US associations (instead of a firsthand US experience). In particular, reinstatement in rodents has been found after presentation of a conditioned CS (23) or a conditioned context (24, 25). Similarly, the observation of US responses in our experiments might also have signaled (or fostered retrieval of) association of the firsthand US experience. In fact, responses to others’ expression of distress are often explained by associative learning mechanisms and thought to underlie association with experiences of firsthand aversive events (26, 27). Our experimental evidence that the observational reinstatement procedure leads to recovery of what is learned from firsthand experience would speak in favor of a common associative learning mechanism in observational and firsthand learning. While we have employed extinction learning protocols with similar numbers of trials (28, 29) and there is no support for a relationship between the amount of extinction trials and the strength of reinstatement (8), future studies need to determine if prolonged (firsthand or observational) extinction might influence observational reinstatement.
Our findings and the suggested interaction between learned association from firsthand experiences and processing of signals from conspecifics are consistent with a previous finding in rodents that revealed enhanced acquisition of CR after interacting with conspecifics that underwent acquisition training (30). Our finding that observation of others’ responses to threats modulates the expression of defensive behaviors also aligns with research demonstrating enhanced recovery (here, contextual renewal) of CRs in an observer rat tested in the presence of a conspecific that exhibited defensive responses (31). Moreover, our results are consistent with reports in humans that previous firsthand experiences modulate responses toward aversive experience in others (32) and that observation of others’ distress biases choices that depend on firsthand experiences (33) since direct and observational processes interact during decision making (34).
It should be stressed that our results cannot be explained by imitation or social facilitation (i.e., when responses of an individual are modulated by the passive presence of a conspecific) because the demonstrator model is absent during the test. Neither can our results be explained by stimulus enhancement of responses to the CS (i.e., when observation of responses to an object facilitates acquisition of the observed response) because no CS was present during reinstatement (5). The experiments in humans used a relatively low number of extinction trials [as in previous experiments (28, 29)] that might have facilitated reinstatement, even though the number of trials during extinction has not been found to impact the strength of the reinstatement (8, 35). It is theoretically possible that our relatively low number of extinction trials in the human experiments might have facilitated reinstatement. Arguing against this possibility, however, is the finding in a recent review of the human reinstatement literature that a higher number of extinction, but not conditioning, trials is overrepresented in studies reporting differential reinstatement (and not a reduced reinstatement effect). Moreover, a study that statistically compared different numbers of extinction trials during extinction found no impact of the amount of extinction on the degree of the reinstatement (measured as startle) (35). Furthermore, we used a more extensive extinction protocol in the experiment in rats (2 d, each with 15 CS presentations), which renders it rather unlikely that the effect of extinction trials had a strong influence on the observational reinstatement effect. Our group has previously used six CS presentations as a standard extinction protocol (e.g., two earlier experiments that investigated the effect of direct [firsthand] vs. observational extinction in our group) (28, 29).
Importantly, observational reinstatement does not contradict that other sensory modalities, especially olfactory or auditory cues of conspecifics in the animal experiment, could have contributed to the observational effect. We want to highlight that other threat recovery processes, in particular renewal (recovery threat responses within novel or dangerous context), might have contributed to the enhancement of threat responses by observation of others’ US reactions. While our study was not designed to detect potential differences between male and female participants (when observing a US that is administered to a male demonstrator), we want to emphasize that differences in perceiving responses of males and females in pain (36) and identification with the demonstrator model (37) might influence observational reinstatement.
It is tempting to speculate that similar neural substrates of firsthand acquisition training were active after direct and observational reinstatement. This, however, remains to be tested in future research. We only present an exploratory investigation of c-fos expression in demonstrators and observers of reinstatement in SI Appendix in order to provide an initial readout of general, unspecific neural activity. The univariate analyses of our preliminary dataset did not reveal any differences between demonstrators and observers of reinstatement in key regions that process threats. However, finer-grained analyses might be more suitable to pick up differences in neural processes that code firsthand vs. observed reaction to reinstatement USs. In support of this idea, previous experiments in both rodents and humans have revealed a partial overlap in neural activations during observational and firsthand aversive experiences (38–45). It should, however, be highlighted that such an overlap in neural activation does not necessarily imply identical underlying processes (38, 42, 46, 47). Such divergent processes within overlapping central nervous pathways would further be in line with findings of spinal cord responses when observing others being exposed to aversive events that, however, are different from spinal responses to firsthand aversive experiences (48).
In sum, our results provide experimental evidence that observation of reactions to aversive events interacts with learned association that results from firsthand aversive experiences. Specifically, we found in rats and humans that observing another conspecific reacting to an aversive stimulus (US) reinstates conditioned threat responses that underlie associative memories of a firsthand aversive US experience. We furthermore found that recovery of threat responses by observation of others was specific to the context that was observed as dangerous. Our three experiments provide a unique cross-species perspective, suggesting that observation of others’ distress might underlie domain general associative learning mechanisms that gate threat responses in social contexts.
Materials and Methods
Human Experiments.
Participants.
Participants (no intake of prescription medication, no current or prior psychiatric disorders or neurological disorders) were recruited via advertisements at local universities in Stockholm as well as via a website. Thirty-nine participants were recruited in experiment 1, and 24 participants were recruited in experiment 2. Participants were reimbursed with two cinema tickets. Subjects were excluded for analyses if they could not report the stimulus contingencies (Procedure). In experiment 1, this resulted in exclusion of four subjects (control group = 1, experimental group = 3), resulting in a sample of 35 participants (19 females, age between 19 and 30 y, mean = 23.8, SD = 2.9) in total. In experiment 2, this resulted in exclusion of three subjects, resulting in a sample of 21 participants (16 female, age between 18 and 28 y, mean = 22.2, SD = 2.9) in total. Exclusion of participants did not conceptually change the results (SI Appendix, Tables S6 and S16).
Both experiments were approved by the Regional Ethical Review Board in Stockholm (https://etikprovningsmyndigheten.se/). Participants gave written informed consent after a short interview with the experimenter in which all questions were addressed.
Apparatus and Stimuli.
CSs.
In both experiments, two colored rectangles (562 × 762 pixels, bright yellow and blue) were presented in the middle of the screen on a black background, serving as CSs (counterbalanced as CS+ and CS–). The CSs were presented for 6 s, in a pseudorandomized order with the constraint that each CS was not presented more than two times in a row. The duration of the intertrial interval (ITI) between CSs was jittered between 11 and 15 s.
USs.
The US consisted of a 100-ms DC-pulse electric stimulation, which was administered using a constant voltage stimulator (STM200; BIOPAC Systems) applied to the participant’s right forearm through a surface electrode. A conductive gel (Sigma Gel) was applied between the electrode and the skin. Before the start of each experiment, the level of the US was individually adjusted to be “unpleasant but not painful” (experiment 1: range 24 to 56 mA, mean = 36.7 mA, SD = 9.12; experiment 2: range 15.7 to 41.1 mA, mean = 29.39 mA, SD = 7.63), and participants were asked to rate the unpleasantness of the US on a scale reaching from between 1 (“not unpleasant at all”) to 10 (“extremely unpleasant”; experiment 1: range 2 to 9, mean = 5.70, SD = 1.43; experiment 2: range 1 to 7, mean = 3.64, SD = 1.45).
Observational reinstatement US.
Experiment 1.
The observational reinstatement US consisted of a video sequence depicting a male demonstrator model (age = 29 y) receiving three unannounced, unpleasant electrical shocks while sitting in front of a black screen. The demonstrator reacted to the shocks by slightly twitching the arm and showing a facial expression of pain (resulting from an electric stimulation of the shock electrode that was visibly attached to the demonstrator’s right wrist). The observational reinstatement USs control (no-reinstatement control group) in experiment 1 consisted of a video of the same male learning model calmly sitting in front of a black screen. Acquisition, extinction, the observational reinstatement procedure, and the reinstatement test took place in a sound-attenuated cabin, illuminated by white light.
Experiment 2.
The context-specific observational reinstatement US used the same video sequences as described for the observational reinstatement US in experiment 1 but toned the light surrounding the demonstrator to a red color (red context). In experiment 2, acquisition, extinction, and the observational reinstatement procedure took place within the white control context, as described for experiment 1. The control context during the reinstatement test in experiment 2 employed this white control context as well. The context-specific testing environment consisted of another identical sound-attenuated cabin with a red light (red testing context), which was adjacent to the white control context. Participants were placed in each context.
Procedure.
Each experiment consisted of five phases: habituation, acquisition, extinction, observational reinstatement procedure, and reinstatement test. Habituation consisted of two nonreinforced presentations of each CS. In the acquisition phase, the presentation of the CS+ coterminated with the presentation of the US on 4 of 6 presentations in experiment 1 and on 9 of 12 presentations in experiment 2. The CS–, which was never paired with the US, was presented the same number of times as the CS+ in each experiment. The immediately following extinction phase consisted of six unreinforced presentations of each CS. During the observational reinstatement procedure, participants were presented with the observational US (i.e., a video showing a male learning model receiving three electrical stimulations that were similar to the US that the participants experienced during acquisition; see above). During the reinstatement procedure, the participants themselves never received a shock. After the observational reinstatement procedure in experiment 1, a black screen was presented for 60 s. In experiment 2, participants waited outside the testing chamber after observational reinstatement for 60 s before they entered the chamber and the reinstatement test was conducted. The following reinstatement test consisted of 12 CSs (6 CS+ and 6 CS–) presentations, which were not reinforced by the US. CS presentations were in randomized order and counterbalanced for the starting of a CS+ or CS– presentation. In experiment 1, all 12 CSs were presented in the same context. In experiment 2, six CSs (three CS+ and three CS–) were presented in the white illuminated context A, and six CSs (three CS+ and three CS–) were presented in the red illuminated context B.
Finally, CS–US contingency awareness as well as retrospective expectancy of the US after the observational reinstatement procedure was assessed by visual analog scales (0 to 100).
Questionnaires.
State and trait anxiety levels were assessed using the State-Trait Anxiety Inventory [STAI (49)] prior to the experiment. Following the experiment, participants filled out a questionnaire assessing CS–US contingency awareness (e.g., if a colored square was followed by a US and which color was followed by the US). In this postexperimental questionnaire, participants further rated retrospectively their expectancy of the US after the observational reinstatement USs (visual analogue scale from 0 [no expectancy] to 10 [sure about a US] for each CS and the ITI). Additionally, participants were asked to fill out a short questionnaire on their health status (intake of medication, etc.).
Skin conductance acquisition and data reduction.
A pair of pregelled Ag/AgCl electrodes was attached to the palmar side of the distal phalanges of the index and middle fingers of the left hand. The physiological signals were amplified and wirelessly recorded (in order to allow us to switch contexts in experiment 2) using a BIOPAC 150 system at a rate of 200 samples per second. Data were analyzed using AcqKnowledge 4.1 software (BIOPAC Systems). The raw signal was filtered (low pass: 1 Hz, high pass: 0.01 Hz), and SCRs were manually scored for each CS trial as the base to peak amplitude for the first response (in micro-Siemens) with base of the amplitude in the latency window from 0.5 to 4.5 s after stimulus onset using AcqKnowledge 4.1 software [as in previous experiments (50, 51)]. Trials with obvious electrode artifacts and reactions with a rise time longer than 4.5 s (indicating artifacts from breathing) were scored as missing reactions. Amplitudes were range corrected for the maximal SCR during the extinction phase to account for interindividual variability in extinction training and logaritmized. Range correction to the maximum during acquisition training did not conceptually change the results (SI Appendix, Figs. S3 and S6 and Tables S8 and S18).
Data analysis.
Physiological and retrospective US expectancy data were analyzed using JASP (version 0.14). Our main analysis focused on the return of conditioned responses from the end of extinction toward the reinstatement test. To this aim, in both experiments in humans, SCRs were averaged across a block of three trails in all phases of the experiment. Thereby, we could focus on the second half of extinction training and the first half during the reinstatement test. Importantly, our decision to include three trials was based on a previous study that revealed temporal stability of reinstated conditioned responses across three trials in humans (52). Hence, the main analysis consisted of a 2 (CS type; CS+, CS–) × 2 (phase; last block of extinction, first block of the reinstatement test) repeated measures ANOVA. As defined previously (8), a main effect of phase would indicate a generalized reinstatement, and a stimulus × phase interaction would be indicative of a differential reinstatement effect. To this end, planned comparisons included contrasts from the end of extinction to the reinstatement test in general and for each CS. To explore individual differences in responding during the reinstatement test, correlation between individual STAI scores and differential SCRs (CS+ − CS– trials) for the reinstatement test was calculated. The significance level was set at <0.05 for all analyses. Greenhouse–Geisser degrees of freedom correction was used if assumptions of sphericity were violated. Post hoc comparisons that followed upon the ANOVA were corrected for multiple comparisons using the Bonferroni–Holm method, correcting for comparisons accordioning to our hypotheses (i.e., increases from extinction to reinstatement within one group/context and within the reinstatement test between groups/contexts).
Animal Experiments.
Animals.
Subjects were 23 male Sprague–Dawley rats (Hilltop Laboratory Animals) weighing 225 to 400 g at the beginning of the experiments. The animals were housed in pairs during at least 2 wk before the beginning of the experiments. After conditioning, rats were single housed. The environment was temperature and humidity controlled and maintained on a 12/12 light/dark cycle. Rats had ad libitum access to food and water. All conditions and procedures followed the National Research Council’s Guide for the Care and Use of Laboratory Animals (53), and all procedures were approved by the New York University Animal Care and Use Committee.
Apparatus and stimuli.
All experiments were conducted using a Habitest Linc system controlled by Graphic State 2 software. Conditioning boxes (Model H10-11R-TC; Coulbourn Instruments; 30-cm width, 25-cm depth, 30-cm height) were placed in sound-isolating cubicles (Model H10-24A; Coulbourn Instruments). For the habituation session and for reinstatement, modified shuttle boxes with a transparent plexiglass dividing them were also used (Model H10-11R-SC; Coulbourn Instruments; 50-cm width, 25-cm depth, 30-cm height). Every box had rod flooring connected to shock generators (Model H13-15; Coulbourn Instruments), a house light, a speaker connected to a tone generator (Model A12-33; Coulbourn Instruments), and infrared cue lights.
CS and US.
The CS was a continuous tone (20-s duration; 5 kHz; 80 dB; CStone) or the house light on for a duration of 20 s (CSlight). The US consisted of an electrical foot shock (0.5 s, 1 mA).
Observational reinstatement US.
For reinstatement, we used unsignaled foot shocks (0.5 s, 1 mA) delivered to the side of the shuttle box where the demonstrator was placed. During conditioning, the US coterminated with the CS. The observational reinstatement procedures consisted of four unsignaled US foot shocks applied to the demonstrator chamber. The demonstrator rat reacted to the shocks by jumping and vocalization.
Procedure.
After being housed in pairs for at least 2 wk, on day 0, rats were habituated to the reinstatement context by placing them in the modified shuttle boxes for 30 min. In these boxes, each pair of rats was able to see and smell each other’s odor, but they could not establish physical contact. Twenty-four hours later (day 1), the rats were conditioned individually in the conditioning boxes by the presentation of three pairings of CSlight-US and the presentation of three pairings of CStone-US. On days 2 and 3, all rats underwent extinction training to the CSlight by presentations of 15 unreinforced CSlight on each day. Extinction training took place in a modified context with a variable ITI ranging from 90 to 300 s (mean ITI was 180 s). The first CS was preceded by a 5-min acclimation period. This was in the same box as the conditioning session but with peppermint odor and a smooth black plastic floor covering the rod flooring. All sessions of all individual procedures were run for six rats at a time. On day 4, memory for both CSs was tested (extinction test) by presenting five unreinforced CSlight and five unreinforced CStone in the modified conditioning boxes after 4 min of acclimation period with a variable ITI (90 to 300 s; mean: 180 s); 24 h later, each pair of rats was placed in the modified shuttle boxes, and after 7 min of acclimation, four unsignaled foot shocks with variable ITI (60 to 270 s; mean: 140 s) were delivered only to the demonstrators (randomly selected) while the observer was present on the other side of the box separated by a transparent plexiglass. Rats were returned immediately to their home cages, and the animals used for immunohistochemistry were euthanized 90 min after reinstatement. Post-reinstatement memory was tested individually 24 h later by placing them on the modified conditioning boxes and presenting five unreinforced CSlight and five unreinforced CStone after an acclimation period of 5 min with variable ITI (90 to 300 s; mean: 180 s).
Assessment of freezing.
Videos recorded during the extinction test and during the post-reinstatement test sessions were analyzed for freezing by at least one rater who was blind to rat condition (demonstrators or observers). Freezing was defined as cessation of all movement other than respiration. Freezing during each CS presentation was timed with a digital stopwatch and presented as duration of freezing in seconds. Analogue to the human experiments, we averaged freezing behavior across the last block (two trials) of the extinction retention test and the first block (two trials) of the post-reinstatement test.
Statistical analysis.
The analysis of the animal data was analogous to the analyses of the human experiments and consisted of a repeated measures ANOVA with two within-subject factors: CS type (CS+extinguished, CS+nonextinguished) and reinstatement (extinction test, post-reinstatement) and type of learning (observer, demonstrator) as a between-subject variable. Post hoc comparisons were corrected for multiple comparisons using the Bonferroni–Holms method. Additional analyses (suggested by reviewing experts) that excluded outliers in the reinstatement test in each experiment did not conceptually change the results (SI Appendix, Figs. S2, S5, and S7 and Tables S7, S17, and S25).
Supplementary Material
Acknowledgments
This project was supported by German Research Foundation Research Grant HA 7470/1-1 (to J.H.) and German–Israeli Foundation for Scientific Research and Development Young Investigator Grant 2528 (to J.H.). G.G.-B. was supported by Ministry of Education of Spain Grant PRX17/00284 and Ministry of Economy, Industry and Competitiveness of Spain Grant PSI2017-84290-R. A.O. was supported by Knut and Alice Wallenberg Foundation Grant KAW 2014.0237, European Research Council Starting Grant 284366 (Emotional Learning in Social Interaction Project), and Swedish Research Foundation Consolidator Grant 2018-00877 (Vetenskapsrådet).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101290118/-/DCSupplemental.
Data Availability
Individual datasets to reproduce the figures and analyses and the analyses files are available at Open Science Framework (54).
References
- 1.LeDoux J., Rethinking the emotional brain. Neuron 73, 653–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mineka S., Ohman A., Phobias and preparedness: The selective, automatic, and encapsulated nature of fear. Biol. Psychiat. 52, 927 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Olsson A., Phelps E. A., Social learning of fear. Nat. Neurosci. 10, 1095–1102 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Heyes C. M., Social learning IN animals: Categories and mechanisms. Biol. Rev. 69, 207–231 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Hoppitt W., Laland K. N., Social Learning: An Introduction to Mechanisms, Methods, and Models (Princeton University Press, 2013). [Google Scholar]
- 6.Olsson A., Knapska E., Lindström B., The neural and computational systems of social learning. Nat. Rev. Neurosci. 21, 197–212 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Bouton M. E., Bolles R. C., Role of conditioned contextual stimuli in reinstatement of extinguished fear. J. Exp. Psychol. Anim. Behav. Process. 5, 368–378 (1979). [DOI] [PubMed] [Google Scholar]
- 8.Haaker J., Golkar A., Hermans D., Lonsdorf T. B., A review on human reinstatement studies: An overview and methodological challenges. Learn. Mem. 21, 424–440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hermans D., et al. , Reinstatement of fear responses in human aversive conditioning. Behav. Res. Ther. 43, 533–551 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Pavlov I. P., Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex (Oxford UP, 1927). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rescorla R. A., Heth C. D., Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1, 88–96 (1975). [PubMed] [Google Scholar]
- 12.Bouton M. E., Context and behavioral processes in extinction. Learn. Mem. 11, 485–494 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Craske M. G., et al. , Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther. 46, 5–27 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Vervliet B., Baeyens F., Van Den Bergh O., Hermans D., Extinction, generalization, and return of fear: A critical review of renewal research in humans. Biol. Psychol. 92, 51–58 (2013). [DOI] [PubMed] [Google Scholar]
- 15.LaBar K. S., Phelps E. A., Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav. Neurosci. 119, 677–686 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Sjouwerman R., Lonsdorf T. B., Experimental boundary conditions of reinstatement-induced return of fear in humans: Is reinstatement in humans what we think it is? Psychophysiology 57, e13549 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Debiec J., Díaz-Mataix L., Bush D. E. A., Doyère V., Ledoux J. E., The amygdala encodes specific sensory features of an aversive reinforcer. Nat. Neurosci. 13, 536–537 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz-Mataix L., Debiec J., LeDoux J. E., Doyere V., Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J. Neurosci. 31, 9538–9543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haaker J., et al. , Making translation work: Harmonizing cross-species methodology in the behavioural neuroscience of Pavlovian fear conditioning. Neurosci. Biobehav. Rev. 107, 329–345 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goode T. D., Maren S., Animal models of fear relapse. ILAR J. 55, 246–258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maren S., K. L. P., Liberzon I., The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokol N., Lovibond P. F., Cross-US reinstatement of human conditioned fear: Return of old fears or emergence of new ones? Behav. Res. Ther. 50, 313–322 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Halladay L. R., Zelikowsky M., Blair H. T., Fanselow M. S., Reinstatement of extinguished fear by an unextinguished conditional stimulus. Front. Behav. Neurosci. 6, 18 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goode T. D., Kim J. J., Maren S., Relapse of extinguished fear after exposure to a dangerous context is mitigated by testing in a safe context. Learn. Mem. 22, 170–178 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris R. W., Furlong T. M., Westbrook R. F., Recent exposure to a dangerous context impairs extinction and reinstates lost fear reactions. J. Exp. Psychol. Anim. Behav. Process. 31, 40–55 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Heyes C., Empathy is not in our genes. Neurosci. Biobehav. Rev. 95, 499–507 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Heyes C., Pearce J. M., Not-so-social learning strategies. Proc. Biol. Sci. 282, 20141709 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golkar A., Selbing I., Flygare O., Öhman A., Olsson A., Other people as means to a safe end vicarious extinction blocks the return of learned fear. Psychol. Sci. 24, 2182–2190 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Golkar A., Haaker J., Ida S., Olsson A., Neural signals of vicarious extinction learning. Soc. Cog. Affect. Neurosci. 11, 1541–1549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knapska E., Mikosz M., Werka T., Maren S., Social modulation of learning in rats. Learn. Mem. 17, 35–42 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak A., Werka T., Knapska E., Social modulation in extinction of aversive memories. Behav. Brain Res. 238, 200–205 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y., et al. , Expertise modulates the perception of pain in others. Curr. Biol. 17, 1708–1713 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Lindström B., Golkar A., Jangard S., Tobler P. N., Olsson A., Social threat learning transfers to decision making in humans. Proc. Natl. Acad. Sci. U.S.A. 116, 4732–4737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L., Gläscher J., A brain network supporting social influences in human decision-making. Sci. Adv. 6, eabb4159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Golkar A., Bellander M., Öhman A., Temporal properties of fear extinction–does time matter? Behav. Neurosci. 127, 59–69 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Zhang L., Losin E. A. R., Ashar Y. K., Koban L., Wager T. D., Gender biases in estimation of others’ pain. J. Pain 5900, 00035 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golkar A., Olsson A., The interplay of social group biases in social threat learning. Sci. Rep. 7, 7685 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allsop S. A., et al. , Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallo S., et al. , The causal role of the somatosensory cortex in prosocial behaviour. eLife Sciences 7, e32740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeon D., et al. , Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamm C., Decety J., Singer T., Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Lindström B., Haaker J., Olsson A., A common neural network differentially mediates direct and social fear learning. Neuroimage 167, 121–129 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Meffert H., Brislin S. J., White S. F., Blair J. R., Prediction errors to emotional expressions: The roles of the amygdala in social referencing. Soc. Cognit. Affect. Neurosci. 10, 537–544 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsson A., Nearing K. I., Phelps E. A., Learning fears by observing others: The neural systems of social fear transmission. Soc. Cognit. Affect. Neurosci. 2, 3–11 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singer T., et al. , Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Krishnan A., et al. , Somatic and vicarious pain are represented by dissociable multivariate brain patterns. eLife Sci. 5, e15166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockwood P. L., Apps M. A. J., Chang S. W. C., Is there a ‘social’ brain? Implementations and algorithms. Trends Cognit. Sci. 24, 802–813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tinnermann A., Büchel C., Haaker J., Observation of others’ painful heat stimulation involves responses in the spinal cord. Sci. Adv. 7, eabe8444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spielberger C. D., Gorsuch R. L., Lushene R. E., Manual for the State-Trait Anxiety Inventory (Consulting Psychologists Press, Palo Alto, CA, 1970). [Google Scholar]
- 50.Pan Y., Olsson A., Golkar A., Social safety learning: Shared safety abolishes the recovery of learned threat. Behav. Res. Ther. 135, 103733 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Haaker J., Molapour T., Olsson A., Conditioned social dominance threat: Observation of others’ social dominance biases threat learning. Soc. Cognit. Affect. Neurosci. 11, 1625–1637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharfenort R., Menz M., Lonsdorf T. B., Adversity-induced relapse of fear: Neural mechanisms and implications for relapse prevention from a study on experimentally induced return-of-fear following fear conditioning and extinction. Transl. Psychiatry 6, e858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 54.Haaker J., et al. , Observational_reinstatement. Open Science Framework. https://osf.io/j6sc5/view_only=90e63c921e8746508f28f5268a1cbed7. Deposited 11 January 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual datasets to reproduce the figures and analyses and the analyses files are available at Open Science Framework (54).