Abstract
Background
Since December 2019, the newly emerged SARS-CoV-2 virus continues to infect humans and many people died from severe Covid-19 during the last 2 years worldwide. Different approaches are being used for treatment of this infection and its consequences, but limited results have been achieved and new therapeutics are still needed. One of the most interesting biotherapeutics in this era are Nanobodies which have shown very promising results in recent researches.
Scope of review
Here, we have reviewed the potentials of Nanobodies in Covid-19 treatment. We have also discussed the properties of these biotherapeutics that make them very suitable for pulmonary drug delivery, which seems to be very important route of administration in this disease.
Major conclusion
Nanobodies with their special biological and biophysical characteristics and their resistance against harsh manufacturing condition, can be considered as promising, targeted biotherapeutics which can be administered by pulmonary delivery pharmaceutical systems against Covid-19.
General significance
Covid-19 has become a global problem during the last two years and with emerging mutant strains, prophylactic and therapeutic approaches are still highly needed. Nanobodies with their specific properties can be considered as valuable and promising candidates in Covid-19 therapy.
Keywords: SARS-CoV-2, Covid-19, Nanobody, Pulmonary drug delivery
1. Introduction
Coronavirus disease (Covid-19) is an infectious disease caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2, an enveloped, positive-sense single-stranded RNA virus [(+)ssRNA]) which was first appeared in Wuhan, China in December 2019 [1]. Most people infected with the SARS-CoV-2 virus will experience mild to moderate respiratory illness but older people, and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness [1]. In fact, Covid-19 rapid spread and pandemic, was a severe challenge for health care system and society [2].
The virus main transmission route is through mucosal tissues: nose, mouth and upper respiratory tract [3], and gains entry to the target cells via the Angiotensin Converting Enzyme 2 (ACE2) receptor by the interaction of the Receptor Binding Domain (RBD) of the Spike protein (as the key target for eliciting persistent neutralizing antibodies [4]) on the viral surface [5]. ACE2 receptors can be found in different tissues specially lung, small intestine, heart and digestive system, which reflect the diversity of symptoms and pathologies associated with Covid-19 [6].
2. Strategies for Covid-19 treatment
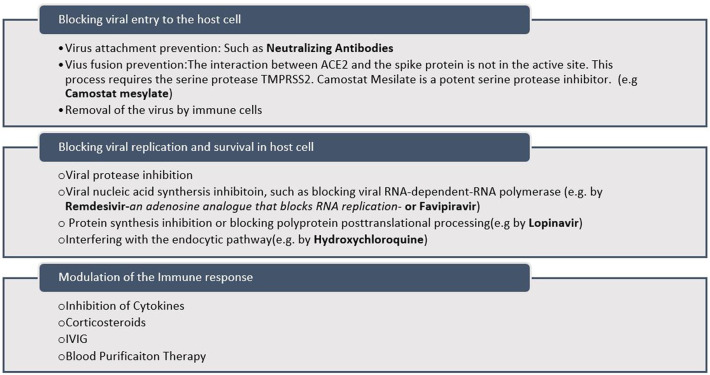
Based on the SARS-CoV-2 pathogenesis, different strategies for Covid-19 treatment are designed [[6], [7], [8]]. These approaches mainly include preventing the virus entrance to the target cell particularly by neutralizing antibodies [9,10] or virus fusion prevention [11], viral replication inhibition specially by targeting the virus proteases [[12], [13], [14]] and reducing the severity of immune response [15] which are summarized in Fig. 1 .
Fig. 1.
Summary of strategies for Covid-19 treatment.
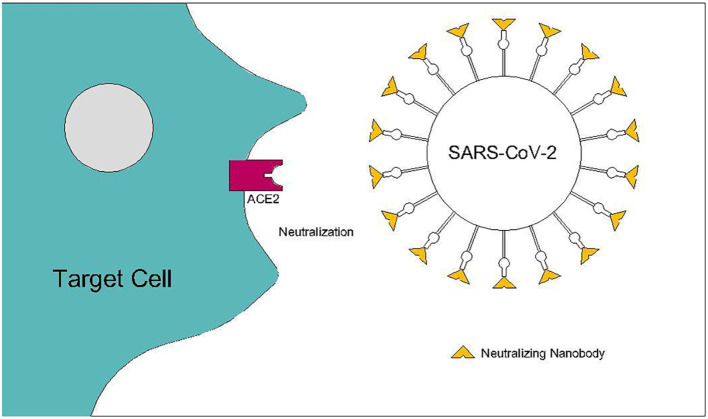
It is clear that despite all efforts, still therapeutic and preventive interventions against Covid-19 are an urgent need [16]. A potent target for drug discovery for Covid-19 is RBD-ACE2 interaction that offers a very safe and strong therapeutic target for binding and prevention of infection by antibodies and Nanobodies (Nbs) for researchers who are working on Covid-19(Fig. 2 ) [17].
Fig. 2.
SARS-CoV-2 neutralization by Nanobodies prevents viral entrance to the target cell. Spike RBD-ACE2 interaction is considered as a therapeutic target for binding and prevention of infection.
Neutralizing antibodies that prevent the virus particles from entering the target cells by inhibiting the RBD-ACE2 interaction, are very interesting anti-viral agents for the treatment of Covid-19 [[18], [19], [20], [21]]. Recently, Zhiqiang Ku et al. have described strategies for the discovery and development of SARS-CoV-2 neutralizing antibodies [22]. However, despite all the potentials, antibodies also show some limitations such as size, stability and possible immunogenicity. While Nanobodies, which are the variable domains of camelid heavy chain-only antibodies, are a promising class of therapeutics and with their impressive characteristics, seems to be very effective molecules in inhibiting the RBD-ACE2 interaction and virus entry into the cells [2,23,24].
3. Nanobodies structure, characteristics and applications
Conventional immunoglobulin-γ (IgG) antibodies assembled from two identical heavy (H)-chains and two identical light (L)-chains. While sera of camelids contain a unique functional heavy (H)-chain antibody (HCAbs) in addition to conventional antibodies. The H chain of these homodimeric antibodies consists of one antigen-binding domain, the VHH, and two constant domains. The smallest intact functional antigen-binding fragment of HCAbs is the single-domain VHH, also known as a Nanobody (Fig. 4C) [25]. Nanobodies can be used in different formats such as bivalent monospecific, bivalent bispecific, bivalent bispecific, albumin-conjugated, trivalent bispecific and bispecific chimeric antigen receptor (CAR) T cell [23]. Nanobodies (including multi-specific, multivalent and bi-paratopic constructs) are encoded by single genes and are efficiently produced in various prokaryotic and eukaryotic hosts, including bacteria, yeast, and mammalian cells. They can be formulated at high concentrations and maintain low viscosity, enabling multiple routes of administration, including low volume injectables. (https://www.ablynx.com/technology-innovation/Nanobodies-competitive-features/).
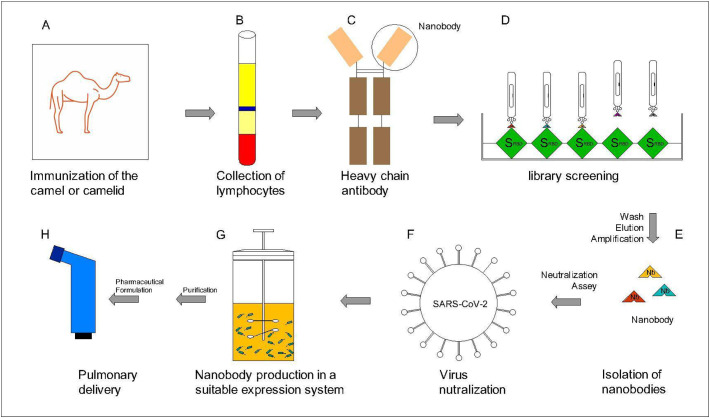
Fig. 4.
Schematic process and steps of Nanobody production against SARS-CoV-2 and pharmaceutical formulation in pulmonary dosage form. A. First a Nanobody library should be prepared for example by immunizing the camel or camelid by SARS-CoV-2 spike protein. B. After the defined duration, blood will be collected and lymphocytes are separated. C. The camel blood contains VHH antibody, the smallest functional part of the VHH antibody is called Nanobody. D. The library is screened for Nanobodies against spike SARS-CoV-2 protein (specially the RBD). E. Washing non-specific phages and elution and amplification of the specific-Nanobodies. F. Virus neutralization assay is performed to select the Nanobodies with the most affinity to the SARS-CoV-2 Spike. G. Expression (in E. coli or yeast or any other suitable expression systems) and Large-scale production of the selected Nanobody with the highest affinity for the SARS-CoV-2 virus. H. After purification and downstream processes, produced Nanobodies will be formulated in a suitable dosage form for pulmonary delivery.
Nanobodies show many interesting characteristics in comparison to conventional antibodies; such as [26]:
-
1.
Easily selection by Phage Display
-
2.
Reaching and recognizing unique epitopes due to small size
-
3.
Ease of manipulation
-
4.
High stability in harsh condition (such as chaotropic agents and pH extremes, so the route of administration can be intravenous, oral, intraperitoneal or intratumor)
-
5.
Low Immunogenicity
-
6.
High specificity
-
7.
Easy production and suitable cost
All these properties, plus combining the beneficial properties of small molecules and monoclonal antibodies, make Nanobodies attractive molecules in drug discovery in various fields including Covid-19.
Recently, a list of selected domain antibodies in development and in clinical trials are published [23,27], among them Cablivi™ (Caplacizumab, From Ablynx, a Sanofi company) is approved by FDA and EMA as the first Nanobody for treatment of aTTP (acquired thrombotic thrombocytopenic purpura), a rare blood-clotting disorder [23].
Nanobodies applications (alone or in conjugation with other molecules) can be classified as [26,27]:
-
1.
Therapy
-
2.
Diagnosis
-
3.
Intracellular targeting
-
4.
Molecular Imaging
4. Nanobodies application against viral infections including SARS-CoV-2
Advantageous properties of Nanobodies, as mentioned above, and ease of their engineering and manipulation, make these single domain antibodies an interesting research tool and biotechnological medication [25]. So, researches have used these promising tools to fight many pathologic conditions including different viral infections [28,29], as well as HIV [[30], [31], [32]], Influenza A virus [33,34], Chikungunya virus [35] and HCV [36] and also in viral respiratory infections such as Respiratory Syncytial Virus (RSV) [37,38] and Middle East Respiratory Syndrome Coronavirus(MERS-CoV) [39,40].
According to the brilliant experience in previous viral infections, researches incline to study Nanobodies potential in prevention and treatment of Covid-19 caused by SARS-CoV-2. In addition, Nanobodies with their unique biophysical properties (including small size and thermostability) have this potential to be used as the pharmaceutical form of inhalation which can be directly deliver the therapeutic agent to the target organ, lung, conferring high pulmonary drug concentrations with minimal systemic side effects [41].
Notably, trimeric spike protein is conformationally flexible and allows each of its RBDs to have two different configurations: a “down” conformation that is thought to be less accessible and an “up” conformation that is receptor (ACE2) accessible conformation [2,42]. One of the best strategies to neutralize SARS-CoV-2 entry to the cells is to develop biotherapeutics with the potential of inducing conformational changes in a way that RBD cannot binds to its receptor anymore. Such as bivalent Nanobodies that induce post-fusion conformation of the SARS-CoV-2 spike to neutralize the virus [2,43]. Also, researchers have introduced some conserved epitopes for binding to their designed Nanobodies which were inaccessible to antibodies [44]. During the Covid-19 pandemic, several other studies have done to evaluate Nanobodies potentials for treatment of this syndrome, most of them are designed against SARS-CoV-2 spike RBD [45,46]. Binding kinetics between the SARS-CoV-2 RBD (including association(Ka) and dissociation(Kd) rates) are very important factors that affect the therapeutic outcome of the Nanobody and should be determined accurately [25] which are evaluated in some valuable studies [17,19,41,47]. Some important studies with the aim of development of anti-SARS-CoV-2 nanobodies are summarized in Table 1 .
Table 1.
Nanobodies developed against SARS-CoV-2 during the Covid-19 pandemic.
| Nanobody format (name) | Source | Expression System | SARS-Cov-2 target protein | Neutralization or binding ability | Claims | References | |
|---|---|---|---|---|---|---|---|
| 1 |
|
Immunized Camel with RBD | Pichia pastoris | RBD |
|
Good stability profile which was not impacted by nebulization. | [48] |
| 2 |
|
Synthetic | E. coli | RBD |
|
Binding small antiviral molecules to the Sybody can increase their antiviral potency due to recognizing secondary epitopes of the virus. | [49] |
| 3 |
|
Naïve library from llamas and alpacas | E. coli | RBD |
|
|
[50] |
| 4 |
|
Immunized llama with RBD | E. coli | RBD |
|
|
[41,44,51] |
| 5 |
|
Yeast surface-displayed library of synthetic nanobody sequences | E. coli | RBD |
|
|
[52] |
| 6 |
|
Immunized Alpaca with spike protein and RBD |
Fu2: E. coli Fu2-Fc: Mammalian cell |
RBD |
|
[53] | |
| 7 |
|
Immunized Llama and Transgenic mice (nanomice) with spike protein and RBD |
Unknown | RBD |
|
|
[54] |
| 8 |
|
Immunized Alpaca and Llama with RBD and formalin-inactivated SARS-CoV-2 | E. coli | RBD |
|
|
[2] |
| 9 |
|
Immunized Alpaca with spike protein | E. coli | RBD |
|
|
[55] |
| 10 |
|
Immunized Alpaca with RBD | Mammalian expression system | RBD |
|
|
[47] |
| 11 |
|
Naïve llama library | E. coli | RBD |
|
|
[19] |
| 12 |
|
Naïve llama library and humanized synthetic library | Mammalian cells |
RBD |
|
|
[56] |
| 13 |
|
Immunized Alpaca with spike protein | Mammalian cells | RBD |
|
|
[57] |
| 14 |
|
Synthetic sybody libraries | E. coli | RBD |
|
|
[58] |
| 15 |
|
Immunized Llama with spike protein | E. coli, Pichia pastoris | RBD |
|
|
[17] |
5. Nanobodies production and delivery, pharmaceutical point of view
Local pulmonary delivery of therapeutics may offer benefits for the treatment of lung diseases such as Covid-19. Advantages of pulmonary drug delivery includes the rapid onset of action, reduced systemic side effects, increased therapeutic window and the need for a lower dose to reach the desired therapeutic response, as well as non-invasive administration [59]. Although it is pharmaceutically possible to make different drug delivery systems and drug dosage forms for Nanobodies [26,60], if the target tissue of the drug is lung (for example, in diseases such as the Covid-19, which involves the respiratory system), the pulmonary method seems to be the best route of administration. Previous studies have shown that if the drug is administered systemically, only about 0.2% of the drug reaches the lungs, which means that in order to achieve a therapeutic dose in the lungs, much more concentration of the drug (and more side effects and more manufacturing costs as result) is needed in comparison with local pulmonary route. The same result was achieved in another study where it was shown that the inhalation route potentially offers rapid RSV neutralization, while simultaneously, t1/2 of about 20 h allows for once daily dosing [37]. Nevertheless, formulation of biotherapeutics for pulmonary delivery is challenging and requires proteins with favourable biophysical and biochemical properties suitable for inhaled formulation, delivery, and inhalation devices. In other words, since during pharmaceutical development of pulmonary dosage forms, typically multiple buffer systems with different ionic strengths and high heat is used, biomolecules to be formulated into the pulmonary form must have certain properties and specifications. Among all biotherapeutics and in comparison, with conventional antibodies, Nanobodies, are particularly suited for delivery by inhalation due to their small size, simple and robust structure, high thermal stability and solubility, short half-life in the systemic circulation, biological and physico-chemical stability (such as resistance against aggregation and shear forces during manufacturing) (Fig. 3 ). Furthermore, being only one-tenth the size of a normal immunoglobulin, a single dose of Nanobody packs in ten times more active molecules compared to the same dose of a classic immunoglobulin [59].
Fig. 3.
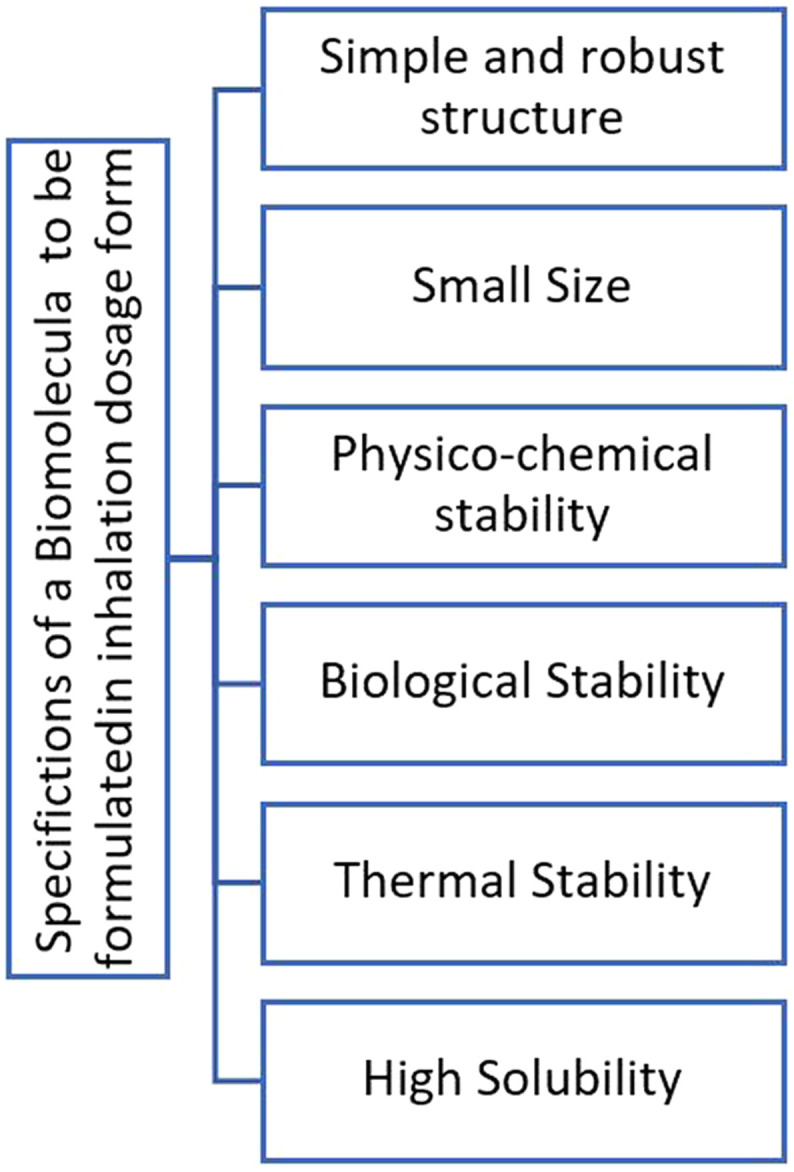
Key specifications of a biomolecule to be formulated in a pulmonary dosage form (which all can be found in Nanobodies).
To make a suitable inhaled formulation for a biological medicine such as Nanobody, the formulation method and the delivery device should provide not only the proper droplet size (0.5–5 μm aerodynamic diameter for good drug deposition in the lung [59]) but also preserve the structure and activity of the medicine [61]. Depending on the formulation, there are different devices currently used for pulmonary delivery: Nebulizers, pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs), each of them has its own characteristics and mechanism of action explained elsewhere [62,63]. pMDIs formulations need propellants and as long as most biologics are water-soluble, they do not easily solubilize in propellants and this results in a dose limitation in addition to changes in protein structure. DPIs are not easily applicable in critically ill patients, low consciousness or children; and also drying stage which is necessary in DPIs formulations may affect the protein or Nanobody construct which need extensive studies. Most biological products in the market are generally formulated in either solution or suspension or lyophilized powder. Generally, 75% of inhaled proteins in researches are prepared in the form of liquids for nebulization. Pulmonary delivery by nebulizer does not need a drying step as it may affect the drug properties and it can be used easily by all patients. There are different kinds of nebulizer: air-jet, ultrasonic and vibrating mesh. According to previous researches, It seems that the best method for nebulizing Nanobodies is the vibrating mesh method due to minimizing construct changes and multimerization of the molecules (2% vs 40% in air-jet method) [59,61]. The main advantages of a nebulizer compared to a pMDI or DPI is that oxygen can be administered in combination with the aerosol treatment and higher doses can be administered over a prolonged time. Furthermore, nebulizers can be used without the cooperation of the patient [59], which is a very important factor in hospitalized patients.
Altogether, it seems that nebulizers are the most appropriate pulmonary dosage form for targeted delivery of Nanobodies to the lung. An example of a Nanobody against a respiratory virus that is formulated in inhalation dosage form is ALX-0171 (manufactured by Ablynx company), a potent trivalent Nanobody with antiviral properties against RSV [37] with good safety results in phase 1 clinical trials [59,64]. These successful experiences encouraged researches to use their knowledge for the treatment of new emerging corona virus too.
Physical properties of a nebulizer solution may impact drug product stability and device performance. To resist the shear stress during nebulization and to avoid physico-chemical degradation, high solubility, low viscosity, and physical stability in a physiologic buffer is needed (a solubility of at least 100 mg/ml and a viscosity below 2 Centipoise for a nebulizer solution) [59]. As shown in some valuable studies, Nanobodies production should be in a way that pre-and post-nebulization, the stability and function of the Nanobody molecule does not changed. For example Esparza et al. demonstrated in their study that their Nanobody (named NIH-CoVnb-112) stability and potent inhibition of SARS-CoV-2 pseudovirus following nebulization did not changed significantly before and after nebulization [17].
The method of design and development of Nanobodies are explained in different review articles [65]. In general, to develop a nanobody, different steps are considered including Nanobody generation, identification, characterization, activity assay and finally formulation and manufacturing. Accordingly, to manufacture a Nanobody against SARS-CoV-2 in pulmonary dosage form (or any other kind of Nanobody and dosage form), it is necessary to first generate a Nanobody library (Immune, synthetic or naive libraries). In immune Nanobody libraries which are very routine, it is necessary to immunize a camel or camelids (Llama or Alpaca) by spike protein of the virus which has strong immunomodulatory properties. After a defined duration (typically 4 to 6 weeks), the blood is collected and lymphocytes are extracted. A phage display library is made and then the Nanobodies with the most affinity to the RBD of the SARS-CoV-2 spike protein will be selected. After assaying Nanobodies for neutralization of SARS-CoV-2 pseudovirus (by Plaque reduction neutralization test (PRNT) which is considered as the “gold standard” for detecting and measuring antibodies that can neutralize the viruses [66]), the next step would be large-scale production in a suitable expression system (bacteria, yeast or mammalian expression system) and downstream process including purification and pharmaceutical formulation as a pulmonary drug dosage form (Fig. 4 ).
6. Conclusion and suggestions
During the last 2 years after appearance of SARS-CoV-2 in China and Covid-19 pandemic, researches and pharmaceutical companies strongly tried to develop therapeutic and prophylactic candidates against this disease. Among different approaches it seems that VHH, Nanobodies with their promising and outstanding properties truly can be a “magic bullet” against SARS-CoV-2 and Covid-19. It can be suggested that Nanobodies not only can be used as neutralizing agents, but also Nanobodies with anti-inflammatory effects such as anti-IL-6R Nanobody® ALX-0061 which is developed by Ablynx, (Gent, Belgium) primarily for rheumatoid arthritis, probably can be used in Covid-19 with the aim of reducing pulmonary inflammation like Tocilizumab (Actemera®) which is an anti-IL-6R monoclonal antibody with extensive use in Covid-19. According to pathogenesis of Covid-19 and its effects on different body tissues like heart and gastrointestinal system, different Nanobodies with different route of administrations can be designed and produced to help people against this society annoying virus. It should be noted that computational design can also complement experimental Nanobody development to identify new epitopes according to the Nanobodies structural-conformational information [67].
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
Authors are grateful to Mrs. Dina Bahrololoumi for her support in graphics. This work was financially supported by Tehran University of Medical Sciences.
References
- 1.Wang M.Y., Zhao R., Gao L.J., Gao X.F., Wang D.P., Cao J.M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front. Cell. Infect. Microbiol. 2020;10:1–17. doi: 10.3389/fcimb.2020.587269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koenig P.-A., Das H., Liu H., Kümmerer B.M., Gohr F.N., Jenster L.-M., et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science (80- ) 2021;371 doi: 10.1126/science.abe6230. eabe6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subbarao Kanta, Siddartha M. Respiratory virus infections: understanding COVID-19. Immunity. 2020;52(6):905–909. doi: 10.1016/j.immuni.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y., Du L. SARS-CoV-2 spike protein: a key target for eliciting persistent neutralizing antibodies. Signal Transduct. Target Ther. 2021;6:95. doi: 10.1038/s41392-021-00523-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nitulescu G.M., Paunescu H., Moschos S.A., Petrakis D., Nitulescu G., Ion G.N.D., et al. Comprehensive analysis of drugs to treat SARS-CoV-2 infection: mechanistic insights into current COVID-19 therapies (review) Int. J. Mol. Med. 2020;46:467–488. doi: 10.3892/ijmm.2020.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hossein-khannazer N., Shokoohian B., Shpichka A., Aghdaei H.A., Timashev P., Vosough M. An update to novel therapeutic approaches for treatment of COVID-19. J. Mol. Med. 2021;99:303–310. doi: 10.1007/s00109-020-02027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty Rohit, Ghosh Arkasubhra, Honavar Santosh G., Pooja Khamar S.S. Therapeutic opportunities to manage COVID-19/SARS-CoV-2 infection: Present. Indian J. Ophthalmol. 2017;17:1. doi: 10.4103/ijo.IJO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang S., Zhang X., Yang Y., Hotez P.J., Du L. Neutralizing antibodies for the treatment of COVID-19. Nat. Biomed. Eng. 2020;4:1134–1139. doi: 10.1038/s41551-020-00660-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renn A., Fu Y., Hu X., Hall M.D., Simeonov A. Fruitful neutralizing antibody pipeline brings hope to defeat SARS-Cov-2. Trends Pharmacol. Sci. 2020;41:815–829. doi: 10.1016/j.tips.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Xia S., Wang Q., Xu W., Li W., Lu L., et al. Broad-spectrum coronavirus fusion inhibitors to combat COVID-19 and other emerging coronavirus diseases. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seth S., Batra J., Srinivasan S. COVID-19: targeting proteases in viral invasion and host immune response. Front. Mol. Biosci. 2020;7:1–9. doi: 10.3389/fmolb.2020.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padmanabhan P., Desikan R., Dixit N.M. Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARSCoV- 2 infection. PLoS Comput. Biol. 2020;16:1–28. doi: 10.1371/journal.pcbi.1008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M., Hofmann-Winkler H., Smith J.C., Krüger N., Arora P., Sørensen L.K., et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021 doi: 10.1016/j.ebiom.2021.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang L., Yin Z., Hu Y., Mei H. Controlling cytokine storm is vital in COVID-19. Front. Immunol. 2020;11:1–14. doi: 10.3389/fimmu.2020.570993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020;20:401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esparza T.J., Martin N.P., Anderson G.P., Goldman E.R., Brody D.L. High affinity nanobodies block SARS-CoV-2 spike receptor binding domain interaction with human angiotensin converting enzyme. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-79036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the novel coronavirus sars-cov-2. Int. J. Biol. Sci. 2020;16:1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huo J., Le Bas A., Ruza R.R., Duyvesteyn H.M.E., Mikolajek H., Malinauskas T., et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020;27:846–854. doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- 20.Ho M. Perspectives on the development of neutralizing antibodies against SARS-CoV-2. Antib. Ther. 2020;3:109–114. doi: 10.1093/abt/tbaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuttuvan Valappil Sajna S.K. Antibodies at work in the time of severe acute respiratory syndrome coronavirus 2. Cytotherapy. 2020;23:101–110. doi: 10.1016/j.jcyt.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku Z., Ye X., To G., Zhang N., An Z. Antibody therapies for the treatment of COVID-19. Antib. Ther. 2020;3:101–108. doi: 10.1093/abt/tbaa007. [DOI] [Google Scholar]
- 23.Jovčevska I., Muyldermans S. The therapeutic potential of nanobodies. BioDrugs. 2020;34:11–26. doi: 10.1007/s40259-019-00392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ackaert C., Smiejkowska N., Xavier C., Sterckx Y.G.J., Lahoutte T., Muyldermans S., et al. Vol. 12. 2021. Immunogenicity Risk Profile of Nanobodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muyldermans S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 26.Hu Y., Liu C., Muyldermans S. Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison C. Nanobody approval gives domain antibodies a boost. Nat. Rev. Drug Discov. 2019;18:485–487. doi: 10.1038/d41573-019-00104-w. [DOI] [PubMed] [Google Scholar]
- 28.De Vlieger D., Ballegeer M., Rossey I., Schepens B., Saelens X. Single-domain antibodies and their formatting to combat viral infections. Antibodies. 2018;8:1. doi: 10.3390/antib8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahbarizadeh F., Ahmadvand D., Sharifzadeh Z. Nanobody; An old concept and new vehicle for immunotargeting. Immunol. Investig. 2011;40:299–338. doi: 10.3109/08820139.2010.542228. [DOI] [PubMed] [Google Scholar]
- 30.Forsman A., Beirnaert E., Aasa-Chapman M.M.I., Hoorelbeke B., Hijazi K., Koh W., et al. Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J. Virol. 2008;82:12069–12081. doi: 10.1128/jvi.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boons E., Li G., Vanstreels E., Vercruysse T., Pannecouque C., Vandamme A.M., et al. A stably expressed llama single-domain intrabody targeting rev displays broad-spectrum anti-HIV activity. Antivir. Res. 2014;112:91–102. doi: 10.1016/j.antiviral.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Weiss R.A., Verrips C.T. Nanobodies that neutralize HIV. Vaccines. 2019;7:1–14. doi: 10.3390/vaccines7030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashour J., Schmidt F.I., Hanke L., Cragnolini J., Cavallari M., Altenburg A., et al. Intracellular expression of camelid single-domain antibodies specific for influenza virus nucleoprotein uncovers distinct features of its nuclear localization. J. Virol. 2015;89:2792–2800. doi: 10.1128/jvi.02693-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei G., Meng W., Guo H., Pan W., Liu J., Peng T., et al. Potent neutralization of influenza a virus by a single-domain antibody blocking M2 ion channel protein. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J.L., Shriver-Lake L.C., Zabetakis D., Anderson G.P., Goldman E.R. Selection and characterization of protective anti-chikungunya virus single domain antibodies. Mol. Immunol. 2019;105:190–197. doi: 10.1016/j.molimm.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Thueng-in K., Thanongsaksrikul J., Srimanote P., Bangphoomi K., Poungpair O., Maneewatch S., et al. Cell penetrable humanized-VH/VHH that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Detalle L., Stohr T., Palomo C., Piedra P.A., Gilbert B.E., Mas V., et al. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob. Agents Chemother. 2016;60:6–13. doi: 10.1128/AAC.01802-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schepens B., Ibañez L.I., De Baets S., Hultberg A., Bogaert P., De Bleser P., et al. Nanobodies® specific for respiratory syncytial virus fusion protein protect against infection by inhibition of fusion. J. Infect. Dis. 2011;204:1692–1701. doi: 10.1093/infdis/jir622. [DOI] [PubMed] [Google Scholar]
- 39.He L., Tai W., Li J., Chen Y., Gao Y., Li J., et al. Enhanced ability of oligomeric nanobodies targeting MERS coronavirus receptor-binding domain. Viruses. 2019;11 doi: 10.3390/v11020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Guangyu, He Lei, Sun Shihui, Qiu Hongjie, Tai Wanbo, Chen Jiawei, Li Jiangfan, Chen Yuehong, Guo Yan, Wang Yufei, Shang Jian, Ji Kaiyuan, Fan Ruiwen, Du Enqi, Jiang Shibo, Li Fang, Du Lanying, Zhoua Yusen. A novel nanobody targeting Middle East respiratory syndrome coronavirus (MERS-CoV) receptor-binding domain has potent cross-neutralizing activity and protective efficacy against MERS-CoV. J. Virol. 2018;92:1–15. doi: 10.1128/JVI.00837-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang Y., Nambulli S., Xiao Z., Liu H., Sang Z., Duprex W.P., et al. Versatile, multivalent nanobody cocktails efficiently neutralize SARS-CoV-2. Science (80- ) 2020;370(652):1479–1484. doi: 10.1101/2020.08.24.264333. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (80- ) 2020;367:1260–1263. doi: 10.1126/science.aax0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sasisekharan R., Ph D. Preparing for the future — Nanobodies for Covid-19? New Engl. J. Med. 2021:1568–1571. doi: 10.1056/NEJMcibr2101205. [DOI] [PubMed] [Google Scholar]
- 44.Sun D., Sang Z., Kim Y.J., Xiang Y., Cohen T., Belford A.K., et al. Potent neutralizing nanobodies resist convergent circulating variants of SARS-CoV-2 by targeting novel and conserved epitopes. BioRxiv. 2021:1–44. doi: 10.1038/s41467-021-24963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konwarh R. Nanobodies: prospects of expanding the gamut of neutralizing antibodies against the novel coronavirus, SARS-CoV-2. Front. Immunol. 2020;11:1–6. doi: 10.3389/fimmu.2020.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zare H., Aghamollaei H., Hosseindokht M., Heiat M., Razei A. Nanobodies, the potent agents to detect and treat the Coronavirus infections: A systematic review. Mol. Cell. Probes. 2021;55 doi: 10.1016/j.mcp.2020.101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma Huan, Zeng Weihong, Meng Xiangzhi, Huang Xiaoxue, Yang Yunru, Zhao Dan, Zhou Peigen, Wang Xiaofang, Zhao Changcheng, Sun Yong, Wang Peihui, Ou Huichao, Hu Xiaowen, Yan Xiang T.J. Potent neutralization of SARS-CoV-2 by hetero-bivalent alpaca nanobodies targeting the spike receptor-binding domain. J. Virol. 2021 doi: 10.1128/JVI.02438-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gai J., Ma L., Li G., Zhu M., Qiao P., Li X., et al. A potent neutralizing nanobody against SARS-CoV-2 with inhaled delivery potential. MedComm. 2021;2:101–113. doi: 10.1002/mco2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter J.D., Hutter C.A.J., Zimmermann I., Earp J., Egloff P., Hürlimann L.M., et al. Synthetic nanobodies targeting the SARS-CoV-2 receptor- binding domain. BioRxiv. 2020 doi: 10.1101/2020.04.16.045419. [DOI] [Google Scholar]
- 50.Ye Gang, Gallant Joseph P., Massey Christopher, Ke Shi W.T., Zheng Jian, Odle Abby E., Vickers Molly A., Jian Shang Y.W., Drelich Aleksandra, Kempaiah Kempaiah R., Vivian Tat S.P., Du Lanying, Tseng Chien-Te, Aihara Hideki, LeBeau FL Aaron M. The development of a novel nanobody therapeutic for SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.11.17.386532. [DOI] [Google Scholar]
- 51.Nambulli S., Xiang Y., Tilston-Lunel N.L., Rennick L.J., Sang Z., Klimstra W.B., et al. Inhalable Nanobody (PiN-21) prevents and treats SARS-CoV-2 infections in Syrian hamsters at ultra-low doses. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abh0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoof M., Faust B., Saunders R.A., Sangwan S., Rezelj V., Hoppe N., Boone M., Billesbølle C.B., Puchades C., Azumaya C.M., Kratochvil H.T., Zimanyi M., Deshpande I., Liang J., Dickinson S., Nguyen H.C., Chio C.M., Merz G.E., Thompson M.C., Diwanji D., Schaefer K., Anand A.A., Dobzinsk M.A. An ultra-high affinity synthetic nanobody blocks SARS-CoV-2 infection by locking Spike into an inactive conformation. Sci. 2020;370:1473–1479. doi: 10.1126/science.abe3255. Doi 101101/20200808238469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanke L., Das H., Sheward D.J., Vidakovics L.P., Urgard E. A bispecific monomeric nanobody induces SARS-COV-2 spike trimer dimers. BioRxiv. 2021:1–37. doi: 10.1038/s41467-021-27610-z. Doi:101101/20210320436243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J., Xu K., Jung S., Conte A., Lieberman J., Chuang G., et al. Multimeric nanobodies from camelid engineered mice and llamas potently neutralize SARS-CoV-2 variants. BioRxiv. 2021 doi: 10.1101/2021.03.04.433768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valenzuela Nieto G., Jara R., Watterson D., Modhiran N., Amarilla A.A., Himelreichs J., et al. Potent neutralization of clinical isolates of SARS-CoV-2 D614 and G614 variants by a monomeric, sub-nanomolar affinity nanobody. Sci. Rep. 2021;11:1–14. doi: 10.1038/s41598-021-82833-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong J., Huang B., Wang B., Titong A., Gallolu Kankanamalage S., Jia Z., et al. Development of humanized tri-specific nanobodies with potent neutralization for SARS-CoV-2. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-74761-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu X., Cheng L., Fu M., Huang B., Zhu L., Xu S. A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration. BioRxiv. 2021 doi: 10.1016/j.celrep.2021.109869. (February 09, 2021, doi:101101/20210208429275 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Custódio T.F., Das H., Sheward D.J., Hanke L., Pazicky S., Pieprzyk J., et al. Selection, biophysical and structural analysis of synthetic nanobodies that effectively neutralize SARS-CoV-2. Nat. Commun. 2020 doi: 10.1038/s41467-020-19204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Heeke G., Allosery K., De Brabandere V., De Smedt T., Detalle L., De Fougerolles A. Nanobodies as inhaled biotherapeutics for lung diseases Gino. Pharmacol. Ther. 2016 doi: 10.1016/j.pharmthera.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 60.Ablynx . 2015. Nanobodies as a Versatile Approach and Clinically Validated Drug Platform. [Google Scholar]
- 61.Liang W., Pan H.W., Vllasaliu D., Lam J.K.W. Pulmonary delivery of biological drugs. Pharmaceutics. 2020;12:1–28. doi: 10.3390/pharmaceutics12111025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreno-Sastre M., Pastor M., Salomon C.J., Esquisabel A., Pedraz J.L. Pulmonary drug delivery: a review on nanocarriers for antibacterial chemotherapy. J. Antimicrob. Chemother. 2015;70:2945–2955. doi: 10.1093/jac/dkv192. [DOI] [PubMed] [Google Scholar]
- 63.Timsina M.P., Martin G.P., Marriott C., Ganderton D., Yianneskis M. Drug delivery to the respiratory tract using dry powder inhalers. Int. J. Pharm. 1994;101:1–13. doi: 10.1016/0378-5173(94)90070-1. [DOI] [Google Scholar]
- 64.De Bruyn S., De Smedt T., Allosery K., Crabbe P., De Brabandere V., Detalle L., Mortier K., Schoolmeester A., Wouters H., De Stohr T. ALX-0171: safety and therapeutic potential of an inhaled anti-RSV nanobody. Respir. Drug Deliv. Eur. 2015;1:37–48. [Google Scholar]
- 65.Muyldermans S. A guide to: generation and design of nanobodies. FEBS J. 2021 doi: 10.1111/febs.15515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lv J., Yang L., Qu S., Meng R., Li Q., Liu H., et al. Detection of neutralizing antibodies to Tembusu virus: implications for infection and immunity. Front. Vet. Sci. 2019;6:1–10. doi: 10.3389/fvets.2019.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serapian S.A., Marchetti F., Triveri A., Morra G., Meli M., Moroni E., et al. The answer lies in the energy: how simple atomistic molecular dynamics simulations may hold the key to epitope prediction on the fully glycosylated SARS-CoV-2 spike protein. J. Phys. Chem. Lett. 2020;11:8084–8093. doi: 10.1021/acs.jpclett.0c02341. [DOI] [PMC free article] [PubMed] [Google Scholar]