Abstract
Introduction: We evaluated the immunoglobulin (Ig) G antibody response against the nucleocapsid protein (NP) and the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 in a cohort of 86 individuals in Venezuela, before and after receiving the Sputnik V vaccine.
Methods: Antibody responses against NP and RBD were determined with an enzyme-linked immunosorbent assay just before, 3 weeks after the first, and 6 weeks after the second dose of the vaccine.
Results: Before vaccination, 59 individuals were seronegative, and 27 seropositive for NP and/or RBD. Of the seronegative cohort, 42% did not develop an IgG immune response against RBD after the first vaccine dose, but 100% had a strong IgG response after 2 doses. All seropositive individuals developed a strong IgG antibody response against RBD after the first vaccine dose, with antibody levels ∼40% higher than seronegative individuals who had received 2 doses. Previously seropositive subjects showed no significant increase in IgG antibody response against RBD after the second vaccine dose.
Conclusions: We demonstrate that 2 doses of the Sputnik V vaccine triggered antibody response in all study individuals. The second Sputnik V dose had no impact on IgG response for those seropositive for SARS-CoV-2 antigens before vaccination.
Keywords: Sputnik V vaccine, IgG antibodies, receptor-binding domain (RBD), nucleocapsid protein (NP), S/P ratio, vaccine booster dose
Introduction
Venezuela has authorized the use of the adenovirus-based vaccine Gam-COVID-Vac (Sputnik V) against COVID-19. Vaccination with Sputnik V relies on a heterologous prime-boost approach using 2 different adenovirus vectors (Logunov et al., 2021); people should get 2 doses, 3 weeks apart (Jones et al., 2021). Most other COVID-19 vaccines also require 2 doses, and current guidelines for COVID-19 vaccines assume that 2 doses are required to give full protection. However, in the light of a shortage in vaccine supplies and the high cost of vaccines, single-dose vaccination is an alternative strategy and a way to extend vaccine supply (Matrajt et al., 2021).
Several studies have shown that people with previous exposure to SARS-CoV-2 tend to mount powerful immune responses to a single vaccine dose and gain little added benefit from the second dose. In Sweden, a single dose of the viral vector vaccine Oxford-AstraZeneca vaccine provided protection in previously infected recipients (Havervall et al., 2021). In the USA, based on serology testing, health care workers with previous COVID-19 infections had higher antibody titer responses after a single dose of the mRNA vaccine of Pfizer-BioNTech or the viral vector vaccine Moderna than those who had not been previously infected (Saadat et al., 2021, Krammer et al., 2021, Bradley et al., 2021. Ebinger et al., 2021). In Israel, it was found that individuals infected with SARS-CoV-2 pre-vaccination had a strong and persisting IgG response after one dose of the Pfizer–BioNTech vaccine (BNT162b2). Dose 2 in those individuals had no impact on IgG responses (Jabal et al., 2021). In Argentina, individuals with anti-SARS-CoV-2 IgG responses at baseline showed significantly higher responses to the first dose of Sputnik V than people with no prior history of the disease (Chahla et al., 2021). In Italy, vaccination with BNT162b2 mRNA COVID-19 vaccine showed that in previously infected individuals, neutralizing antibody responses 7 days after the first vaccine dose were not significantly different from those observed in naïve subjects 7 days after the second vaccine dose (Gobbi et al., 2021). In the UK, among participants who had received their first dose of the BNT162b2 mRNA COVID-19 vaccine, the vaccination increased anti-Spike responses at least one order of magnitude greater than what was reported following a conventional prime-boost vaccine strategy in previously uninfected individuals (Manisty et al., 2021). Furthermore, in India, a single dose of the COVISHIELD vaccine elicited the highest neutralizing antibody response and protective immunity among individuals who had been previously infected (Sasikala et al., 2021).
Serology testing can help to determine whether vaccination results in the production of SARS-CoV-2 specific antibodies. Serology has been used to follow up on vaccination with several vaccines in various countries. Moreover, serology can provide us with data regarding the antibody response acquired from receiving a single versus double dose of the vaccine. Serological testing can also detect a previous COVID-19 infection, irrespective of whether the individual had a severe or mild illness or even asymptomatic infection. COVID-19 vaccines do not induce antibodies to the virus nucleocapsid protein (NP) but induce antibodies against the receptor-binding domain (RBD) of the spike protein. However, SARS-CoV-2 infection induces IgG antibodies against RBD and NP (Assis et al., 2021). Here, we evaluated the IgG antibody response against the NP and the RBD of the spike protein of SARS-CoV-2 in 86 individuals just before, after the first, and after the second dose of the Sputnik V vaccine. The study was carried out on a cohort of hospital and laboratory workers, and serology differentiated this population into individuals with previous SARS-CoV-2 seroconversion and, according to their medical records, previously infected and SARS-CoV-2 antigen seronegative individuals with no previous history of SARS-CoV-2 infection or disease. We determined if one dose of the Sputnik V vaccine could lead to seroconversion and established the benefits of a second vaccine dose.
Material and methods
Participants
We randomly enrolled individuals who presented for vaccination (n=149) at a public hospital in Caracas, Venezuela. Before receiving the first dose of the Sputnik V vaccine, the participants were interviewed, and personal data, underlying illnesses and prior signs/symptoms or proof (reverse transcriptase-polymerase chain reaction (RT-PCR) positive) of a previous SARS-CoV-2 infection were registered and a blood sample taken for serology studies. Only vaccine recipients who provided a baseline (pre-vaccine) sample, a sample at application of dose 2 and a sample 6 weeks after dose 2 were included in this study (n= 86). Of these, 74 were workers at Hospital Vargas, a public hospital in Caracas, and consisted of administrative personnel, doctors, and nurses. Twelve participants came from a research institute (IVIC) in Los Teques, Miranda State, Venezuela.
Vaccination
Individuals received 2 doses of the Sputnik V vaccine, 3 weeks apart. Blood samples were taken at 3 time points: on day 0 pre-vaccination; day 21 after the first dose; and day 42 after the second dose. All participants (86) of this study were vaccinated with the first vaccine dose during the first week of February 2021.
ELISA and determination of specific IgG antibodies
An in-house enzyme-linked immunosorbent assay (ELISA) was employed to quantify circulating levels of SARS-CoV-2 anti-NP IgG and RBD anti-spike IgG. The antigens utilized in the ELISA were provided by “My BIOSOURCE” (RBD and NP proteins cat. numbers MBS8574742 and MBS8574741, respectively) and used for the coating of the ELISA plates (Greiner HB) in a concentration of 1 ug/ml. After coating, the plates were blocked for 1 hour at 37 ○C with 300 ul of blocking buffer containing bovine serum albumin (Sigma A7906). Serum samples were used in the ELISA at a dilution of 1:100 in blocking buffer and then incubated for 60 minutes at room temperature (20–25°C). After washing, 100 μl of Goat Anti-Human IgG Fc labeled with HRP and diluted at 1:10.000 (ABCAM ab97225) in blocking buffer was added to the wells, and the samples were incubated for 60 minutes at room temperature (20–25°C). Following the second wash cycle, 100 μl TMB substrate (T0440 Sigma-Aldrich) was added to the wells, and the samples were incubated for 20 minutes at room temperature (20–25°C). Lastly, a stop solution was added (0.16 M sulfuric acid) to the wells to terminate the reaction. The optical density of each well was determined using a microplate reader set to 450 nm within 10 minutes.
Positive and negative ELISA controls
To allow the results to be compared between plates, 2 serum samples (one of a COVID-19 patient, RT-PCR confirmed, who tested positive for IgG antibodies against NP and RBD, and one of a pool of negative controls) were always loaded into 2 wells each on all ELISA plates. The positive control was selected out of 10 randomly chosen serum samples from convalescent COVID-19, RT-PCR confirmed patients with IgG antibodies for NP and RBD protein, and the serum sample that was closest to the mean color development (OD) of these 10 serum samples. This positive control serum tended to generate an ELISA absorbance value in the range of 0.800–1.00 for the NP and the RBD antigen. The negative control was a pool of 5 human serum samples taken before the COVID-19 pandemic in 2019 and randomly chosen from the serum collection of healthy subjects available in our institution.
S/P ratio of serum samples
The relative amounts of antibodies in serum samples were calculated by reference to the positive and the negative control. This relationship is expressed as the S/P ratio (Sample to Positive Ratio) and was calculated using the following formula: ((OD sample-OD negative control)/(OD positive control-OD negative control)) x 100%. An S/P value of 40% or more was considered positive and corresponded with the mean of the 5 negative sera and 2 SDs. The sensitivity and specificity of the ELISA were determined with 40 RT-PCR positive serum samples taken 4–6 weeks after the initial diagnosis and 30 negative control samples of healthy individuals collected before the COVID-19 pandemic. The sensitivity and specificity for the RD and NP antigens were, 97.5/100% and 96.7/100% respectively.
Survey
With simple and direct questions, participants were asked for the following information: sex, age, whether they had experienced any of the most common symptoms of COVID-19 (fever, a persistent dry cough, fatigue and a loss of smell and/or taste) over the last year, results of previous COVID tests, use of medicines (particularly the use of immunosuppressants), and underlying illnesses.
Statistical analysis
Statistical analysis was performed with the R software package
Ethical considerations
The study was approved by Hospital Vargas de Caracas’ ethics committee. Participants gave oral permission for an interview and signed informed consent document for the use of their blood samples for serological studies regarding previous SARS-CoV-19 infection serological responses to the Sputnik V vaccine. Participants were individually informed concerning the results of this study and the presence or absence of antibodies induced by the vaccine or natural infection.
Results
Participants of the study
Of the 146 individuals who initially collaborated, only 86 were eligible for this study as the others did not provide a sample after the first or second vaccine dose. The ages of the participants ranged from 21 to 76 with a mean age of 41±13.2, and 49 (57%) were women. At least one chronic condition was reported by 30% of individuals: hypertension (17 individuals), diabetes (4) and asthma (5).
Assessment of SARS-CoV-2 antibodies at baseline
Before the first vaccine dose, IgG antibody levels against RBD and NP were measured with the ELISA. No detectable IgG antibodies against the SARS-CoV-2 antigens NP and RBD were found in 59 individuals; they were therefore classified as seronegative in this study. The remaining 27 individuals had detectable IgG antibodies at baseline against NP or RBD and were classified as seropositive. All seropositive individuals had detectable IgG antibodies against NP (mean S/P 140, SD 109), and 15 of the 27 subjects had detectable IgG antibodies against RBD (mean S/P 108, SD 60). Six seropositive participants reported a positive RT-PCR, and 22, including the RT-PCR positives, reported COVID-19 specific symptoms and signs such as fever (22), a dry cough (20), headache (15) and tiredness (16) or a loss of smell (16) in the past. Five individuals with SARS-CoV-2 specific antibodies reported no COVID-19 signs or symptoms in the past.
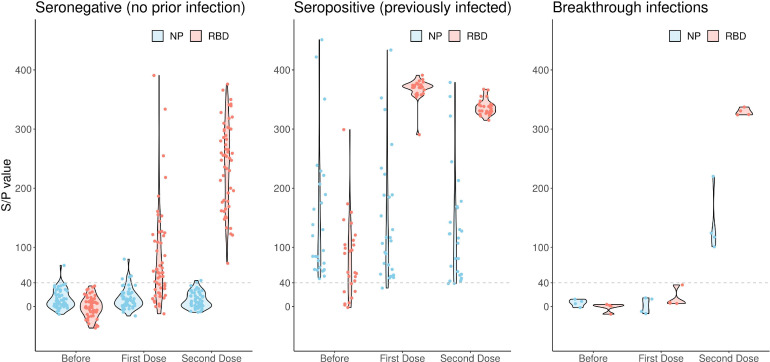
See Figure 1 for the distribution of the S/P values of the antibody responses against RBD and NP before vaccination.
Figure 1.
Violin Plots showing the distribution of the IgG responses in the sub-groups before, during and after the vaccination with the Sputnik V vaccine. IgG responses against the NP and RBD protein are expressed as S/P ratios on the vertical line of the graph and calculated as described in the Material and Methods section. The participants were divided in 3 sub-groups: seronegative (most likely never infected), seropositive (most likely previously infected) and infected during the study period (breakthrough infections). A positive antibody response or seroconversion, was defined as a titer with an S/P ratio of at least 40%. S/P ratios of < 40% were considered negative.
IgG antibodies after the first vaccine dose
Three weeks after the first vaccine dose, the participants received the second vaccine dose and blood samples were taken for serological studies. Of the seronegative cohort, 34/59 (58%) showed detectable IgG antibody responses against the RBD protein (mean S/P 119, SD 80) without seroconversion for the NP protein. All (100%) of the seropositive individuals who had received one dose had much higher detectable IgG antibody responses against RBD (mean S/P 368, SD 17) than the seronegatives and maintained the NP response (see Figure 1 and Table 1 ). No statistically significant difference was observed in the seronegative cohort between antibody responses elicited in males and females or individuals with an underlying disease. However, although not significantly different, a lower IgG response was associated with older age. The mean for the IgG response in the older age group (60 years or more, n=8) was 36, SD 42 compared with 87, SD 98 for the the age bracket 21–59.
Table 1.
Anti-SARS-CoV-2 NP and RBD IgG antibody responses among 86 healthcare workers who received the Sputnik V vaccine in February (first dose) and March 2021 (second dose) in Caracas, Venezuela. Shown are the mean S/P values with the standard deviation (SD) at the baseline and every stage of the vaccination and the percentage of seropositive individuals of 3 sub-groups; seronegative or seropositive before the first vaccine dose and NP protein seroconverters (breakthrough infection after the first vaccine dose). S/P values of 40 or more are considered a positive antibody response or seroconversion. See also figure 1 for the distribution of the S/P values for each vaccination stage.
| Sub-group | N | IgG at the baseline |
IgG 3 weeks after the first dose |
IgG 6 weeks after the second dose |
|||
|---|---|---|---|---|---|---|---|
| NP (SD) | RBD (SD) | NP (SD) | RBD (SD) | NP (SD) | RBD (SD) | ||
| A.-Seronegative | 55 | 13 (15) | 1 (19) | 13 (18) | 119 (80) | 15 (35) | 244 (73) |
| % seropositive | 0% | 0% | 0% | 55% | 0% | 100% | |
| B.-Seropositive | 27 | 148 (112) | 80 (67) | 153 (133) | 368 (18) | 131 (96) | 337 (12) |
| % seropositive | 100% | 70% | 100% | 100% | 100% | 100% | |
| C.- Seronegative | |||||||
| Breakthrough infection | 4 | 6 (6) | 2 (7) | 2 (14) | 14 (15) | 141 (54) | 329 (6) |
| % seropositive | 0% | 0% | 0% | 0% | 100% | 100% | |
IgG antibodies after the second vaccine dose
Six weeks after receiving the second dose, all participants were invited back to be interviewed and for blood samples to be taken. By then all participants of the seronegative cohort had measurable IgG antibody responses (mean S/P 244, SD 73) that were approximately 2 times higher than after the first vaccine dose. The IgG antibody responses of the seropositive cohort remained high, without a significant change after the second vaccine dose (S/P 337, SD 12) and were on average 40% higher than the seronegative cohort. Equally, the second dose did not increase the IgG responses in the seropositive cohort (See Figure 1 and Table 1). Again, no statistically significant difference was observed between antibody responses elicited in males and females or individuals with an underlying condition. Similarly, when the participants of the seronegative cohort were grouped according to age (age ranges of 21–49 (n=20); 50–59 (n=25); and 60–76 (n=8)), no statistical difference was found between mean antibody responses elicited by each age group. However, the IgG response in the older age group was likewise lower (30%) in comparison with the other age groups (S/P 188, SD 70 vs S/P 255, SD 70).
Seroconversion for antibodies against the NP antigen (breakthrough infections)
Four participants of the seronegative cohort showed seroconversion for the NP antigen after the second vaccine dose. All 4 reported COVID-19 symptoms and signs, including a loss of smell for 3, a few days after receiving the second vaccine dose. None had any detectable IgG responses against the RBD or the NP protein when they received the second vaccine dose. However, 42 days after the second dose, these 4 participants showed an IgG response against the NP protein and a sharp rise in IgG responses against RBD, comparable with individuals of the seropositive cohort and significantly higher than the seronegative cohort. See Figure 1 for the distribution of the ELISA S/P values for these 4 individuals. The mean S/P values, with their respective SDs, for seronegative, seropositive and seroconverted individuals as well as the serum sample results before, during and after vaccination are summarized in Table 1.
Discussion and Conclusions
This study aimed to evaluate IgG responses to the Sputnik V vaccine among vaccinated individuals in Venezuela with reference to serostatus for SARS-CoV-2 antigens before vaccination.
Assessment of SARS-CoV-2 antibodies at baseline. The 27 seropositive individuals had detectable IgG antibodies against NP or RBD at baseline. The prevalence of individuals in our cohort with specific antibodies against SAR-CoV-2 antigens RBD and NP before vaccination was relatively high because the study was primarily performed with hospital workers, who are highly prone to contracting a SARS-CoV-2 infection. Therefore we assume that all seropositive individuals in this study can be considered to have been previously infected. For 6 of the seropositive individuals, previous infection was confirmed with a positive PCR. Of the other 21, who were never PCR tested, 16 reported clinical specific COVID-19 symptoms and signs accompanied by a loss of smell and were treated at home as COVD-19 patients. Five individuals in the seropositive cohort reported no signs/symptoms of a SARS-CoV-2 infection. For these 5 we have little proof of infection before vaccination; however, all had antibodies against NP and 4 had RBD antibodies as well. In addition, these 5 displayed a booster or secondary immune response after the first vaccine dose and thus can be considered asymptomatic SARS-CoV-2 infections. Elsewhere in Spain, at least a third of infections determined by serology were asymptomatic (Pollán et al. 2020). Seven of the seropositive individuals had no detectable RBD antibodies, likely indicating that the immune response had wained over time; 2 were RT-PCR COVID-19 symptomatic cases diagnosed in August 2020, approximately 7 months before vaccination, and 4 had disease-specific symptoms/signs, including a loss of smell in April and May of 2020. Regarding the sensitivity of a "loss of smell" for COVID-19 diagnosis, see the discussion below. A waning of the antibody response over time has been assessed in several serological studies; however, the findings are not uniform, with some studies claiming rapid waning and others showing that antibody persistence can only be accurately determined at the individual level (Chia et al. 2021). Our study suggests longevity of the IgG response against NP over time compared with the RBD antibody response.
IgG response after vaccination in the seronegative cohort. Our study demonstrates that 42% (25 out of 59) of our seronegative cohort did not develop a detectable IgG response against RBD after the first vaccine dose and seroconverted only after receiving the second dose, highlighting the importance of receiving a second dose of the vaccine. Furthermore, we showed that a single dose did not always protect against a symptomatic SARS-CoV-2 infection. Of the 25 individuals without a detectable IgG response against RBD after the first vaccine dose, 4 developed symptomatic COVID-19 just before or shortly after the second dose. The infection was not confirmed with an RT-PCR; however, the 4 individuals reported fever (4), dry cough (3), headache (2) and tiredness/fatigue (4) as the most common symptoms and 3 out of 4 lost their smell. A loss of smell is a good predictor for COVID-19, with studies reporting high specificity (97%) and sensitivity of 65% (Gerkin et al., 2021, Haehner et al., 2020, Said et al., 2021). A rapid olfactory test as a potential screening tool for COVID-19 has been reported (Said et al., 2021). These 4 individuals also developed a strong IgG response against the NP antigen after the second vaccine dose; only a natural infection and not the vaccine induces an antibody response against the NP antigen (Assis et al., 2021) Additionally, these 4 people showed a high IgG booster antibody response against the RBD antigen after their second dose (see Figure 1 and Table 1). We consequently consider these 4 cases as breakthrough infections and it therefore vital not to assume that individuals are fully protected after their first dose alone. No breakthrough infections were registered in the seropositive cohort.
IgG response after vaccination in the seropositive cohort
We showed that all individuals seropositive for SARS-CoV-2 antibodies displayed RBD- IgG responses that were approximately 40% higher after receiving one dose of Sputnik than their fully vaccinated (2 doses) seronegative counterparts. Equally, the second dose did not increase IgG responses in the seropositive group. These findings suggest that the second dose of Sputnik V had no direct benefits for seropositive individuals. Our findings agree with other vaccination studies, reported in the introduction, with viral vector and/or mRNA COVID-19 vaccines in different countries. All these studies conclude that vaccinating previously infected individuals with only a single dose of the aforementioned vaccines generated a boost-type response in IgG antibodies and elicited a higher antibody response in subjects with prior COVID-19 infections than 2 doses in uninfected individuals. Therefore, some countries, including France and Israel, are starting to endorse a single dose for infected individuals, since the benefit of the second dose in this group of individuals remains unclear. A single-dose regime for people with previous SARS-CoV-2 infection could improve the use of available doses, stretch the limited supply, and accelerate the vaccine rollout. However, the Sputnik vaccine has 2 different vector components: rAd26-S (primary dose) and rAd5-S (booster dose). It has not been tested whether the booster rAd5-S dose, potentially no longer needed in previously infected individuals, can be used as the primary dose in previously infected people and will induce the same strong booster effect as the rAd26-S vector. Otherwise, the booster vaccine dose with the rAd5-S vaccine will become redundant so as to make better use of available vaccine doses. Until sufficient data concerning protection are available, the 2-dose regimen should continue to be implemented in previously infected persons.
Limitations of the study
Our study measured circulating antibody levels. Interpreting the significance of differences in IgG levels for protection is difficult. No levels of neutralizing antibodies or virus neutralization assay were performed; thus, we would not presume to interpret our data with respect to vaccine efficacy. Further studies are needed to assess how antibody levels relate to vaccine protection against COVID-19.
Our study demonstrates no benefits for the vaccine-induced IgG response from receiving a second dose among individuals who were seropositive before vaccination. However, the numbers in our study (and in most other studies mentioned in the introduction) are small and the results need to be confirmed in larger and more diverse populations, with more power regarding sample size and across demographic and clinical sub-groups that are known to exhibit variations in antibody response following vaccination. Underlying illness and sex had no significant impact on the IgG response in our study; however, the older age group, although not statistically significant, showed a lower IgG response compared with younger age brackets.
RT-PCR testing was scarcely practiced in our study population; we, therefore, had to rely on signs, symptoms (particularly a loss of smell), and elevated antibody levels against RBD or NP to classify our patients into 2 groups: seronegative or possibly never infected and seropositive or possibly previously infected individuals. Yet, we cannot exclude that some high IgG responders after one vaccine dose in the seronegative cohort had been previously infected but lost their antibody response over time and that the first vaccine dose acted as a booster.
We tested for vaccine-induced antibodies 6 weeks after the second vaccine dose. It remains to be seen how long vaccine-induced antibody responses among seropositive, naturally infected individuals will last compared with non-infected seronegative individuals; this requires long-term follow-up.
Authors’ contribution
FECA, DS and JHdW designed the study, developed the ELISA assay and, with the participation of RR, recruited the participants for this study. FC, DS and MX performed the laboratory experiments. The authors had full access to all data, searched for relevant literature, participated in writing the first draft, and read and approved the final manuscript. Both FECA and DS can be considered as primary authors and contributed equally. All authors declare no competing interests.
Funding
This study was funded by the “Fondo Nacional de Ciencia y Tecnología” (FONACIT). JhdW is supported by the Universidad de Las Americas, in Quito, Ecuador. The funders had no role in study design, data collection, or the decision to publish this article.
Ethical Approval
The study was approved by the Institutional Ethics Committee of Hospital Vargas de Caracas, Caracas, Venezuela
Acknowledgments
We especially thank: Dr. Oscar Noya from Instituto Medicina Tropical at the Universidad Central; Dr. Tirso Silva, director of Hospital Vargas de Caracas and Dr. Flor Pujol, Instituto Venezolano de Investigacion Cientifico (IVIC) for their comments and collaboration during the study period; and Jesús Perez for the statistical analysis.
References
- Assis R, Jain A, Nakajima R, Jasinskas A, Kahn S et al. Distinct SARS-CoV-2 Antibody Responses Elicited by Natural Infection and mRNA Vaccination. bioRxiv 2021.04.15.440089; doi: https://doi.org/10.1101/2021.04.15.440089 [DOI] [PMC free article] [PubMed]
- Bradley T, Grundberg E, Selvarangan R. Antibody Responses after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(20):1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahla RE, Tomas-Grau RH, Cazorla SI, et al. Past SARS-CoV-2 infection elicits a strong immune response after a single vaccine dose MedRxiv doi: 2021 https://doi.org/10.1101/2021.03.14.21253039
- Chia WN, Zhu F, Ong SWX, Young BE, Fong SW. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2(6):e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebinger JE, Fert-Bober J, Printsev I, Wu M. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021 doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem. Senses. 2021;46 doi: 10.1093/chemse/bjaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi F, Buonfrate D, Moro L, Rodari P. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses. 2021;13(3):422. doi: 10.3390/v13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Draf J, Dräger S, de With K, Hummel T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL J Otorhinolaryngol Relat Spec. 2020;82(4):175–180. doi: 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havervall, S., Marking U., Greilert-Norin N., Ng H., Salomonsson AC. et al. Antibody Responses after a Single Dose of ChAdOx1 nCoV-19 Vaccine in Healthcare Workers Previously Infected with SARS-CoV-2. medRxiv preprint. 2021. Doi: https://www.medrxiv.org/content/10.1101/2021.05.08.21256866v1 [DOI] [PMC free article] [PubMed]
- Jabal KA, Ben-Amram H, Beiruti K, Brimat I, Saada AA, et al. SARS-CoV-2 Immunogenicity in individuals infected before and after COVID-19 vaccination: Israel, January-March 2021: Implications for vaccination policy. MedRxiv 2021.04.11.21255273; doi: https://doi.org/10.1101/2021.04.11.21255273
- Jones I, Roy P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet. 2021;397(10275):642–643. doi: 10.1016/S0140-6736(21)00191-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C, Otter AD, Treibel TA, McKnight Á. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrajt L, Eaton J, Leung T, Dimitrov D, Schiffer JT, Swan DA, Janes H. Optimizing vaccine allocation for COVID-19 vaccines: critical role of single-dose vaccination. medRxiv preprint 2021:2020.12.31.20249099. doi: 10.1101/2020.12.31.20249099. [DOI] [PMC free article] [PubMed]
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S, Rikhtegaran Tehrani Z, Logue J. Binding and Neutralization Antibody Responses After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021;325(14):1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said M, Davis P, Davis S, Smart K, Davis S, Yan CH. A Rapid Olfactory Test as a Potential Screening Tool for COVID-19. JAMA Otolaryngol Head Neck Surg. 2021 doi: 10.1001/jamaoto.2021.1456. Published online July 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikala M, Shashidhar J, Deepika G. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int J Infect Dis. 2021;108:183-186. doi: 10.1016/j.ijid.2021.05.034. Int J Infect Dis. 2021;S1201-9712(21) doi: 10.1016/j.ijid.2021.05.034. 00436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]