Abstract
Better understanding of antibody responses against SARS-CoV-2 after natural infection might provide valuable insights into the future implementation of vaccination policies. Longitudinal analysis of IgG antibody titers was carried out in 32 recovered COVID-19 patients based in the Umbria region of Italy for 14 months after Mild and Moderately-Severe infection.Two FDA-approved immunoassays against SARS-CoV-2 Nucleocapsid protein (NCP) and anti-spike-receptor binding domain (S-RBD) were used for sequential serological tests at different time points. The demographics,clinical history and symptom profile associated with the magnitude and longevity of antibody responses were also analyzed. Anti-S-RBD IgG persisted in 96.8% (31 of 32) subjects at 14 months. Patients reporting loss of smell and taste during the clinical course of the disease developed significantly higher antibody titers. Anti-NCP IgG seronegative patients(n=7) at 10 months, tested positive for anti-S-RBD IgG at 12,13 and 14 months emphasizing on a higher false-negative rate for NCP protein-based antibody assays. This study also highlights the importance of adopting specific immunoassays for routine estimation of antibody titers and the decreased rate of re-infections in recovered patients.
Keywords: SARS-CoV-2, Covid19, Coronavirus, Humoral immunity, neutralizing antibodies
Graphical abstract
1. Introduction
As the worldwide vaccination implementation programs against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection causing Coronavirus disease 2019 (COVID-19) are progressing in full swing, information regarding the kinetics and longevity of acquired immunity post-natural infection necessitates analysis as well as documentation. The SARS-CoV-2 shares approximately 79.5% genomic homology with SARS-CoV-1 with a similar receptor-binding domain (RBD) structure. [1] Therefore, much understanding of the immunity offered post-SARS-CoV-2 infection is derived from real-time emerging data and previous experiences with SARS-CoV-1, where protective antibodies were found to persist for at least 2 years. [[2], [3], [4]] A “robust adaptive immune response” with positive S-specific neutralizing antibodies (nAbs), memory B cells, and circulating follicular helper T cells have been demonstrated in recovered patients after SARS-CoV-2 infection. [[5], [6], [7]]
In this study, we aimed to assess the dynamics of IgG antibody titers against SARS-CoV-2 in recovered COVID-19 patients over 14 months after Mild and Moderately-Severe infection. The demographics and clinical profile, that might be associated with the magnitude and longevity of antibody response were also analyzed. To our knowledge, the current study provides the longest follow-up (14 months) reported in the literature to date.
2. Materials and methods
2.1. Patient cohort
A monocentric pilot observational study, that longitudinally analyzed the presence of antibodies against SARS-CoV-2 was conducted in patients based in the Umbria region, Italy who had tested positive for SARS-CoV-2 in March 2020 by Reverse Transcriptase-Quantitative Polymerase Chain Reaction (RT-qPCR). The RT-qPCR tests were performed by the Local health regulatory authorities according to the national guidelines and standard operating procedures (SOPs). The patients were managed as per the set protocols by the treating doctor, prescribing home isolation for mild and moderate cases, hospitalization for cases with increased severity. On recovery, all subjects were informed about the seroprevalence study and were invited for voluntary participation. After written informed consent, serological samples were collected and antibody titers were analyzed using the MAGLUMI® 2019-nCoV lgM/lgG chemiluminescent analytical system (CLIA) assay and the MAGLUMI® SARS-CoV-2 S-RBD IgG CLIA. (New Industries Biomedical Engineering Co., Ltd. [Snibe], Shenzhen, China). Both these immunoassays; anti-nucleocapsid (anti-NCP) and the anti-Spike-RBD (anti-S-RBD) were granted Emergency Use Authorization by the US Food and Drug Administration. [8] At the first serum sample collection, the participants were asked to provide information about their COVID-19 clinical history along with symptoms and treatment undertaken using a standardized questionnaire. They were then invited for voluntary follow-up, periodically for sequential serum sample antibody assessment. The study participants did not receive any compensation or any other benefit but were informed individually about their antibody status.
2.2. Patient selection
From May 2020 to January 2021, anti-NCP antibodies developed against SARS-CoV-2 were analyzed using the MAGLUMI® 2019-nCoV lgM/lgG CLIA assay through sequential serum samples. We treated time as a factor and defined six different time points (TPs); (T0-T5). The first blood sample was collected in the month of May 2020, 2 months after the month of infection (March 2020), and was defined as T0. Consecutive serological samples were analyzed at different TPs; three months (T1), five months (T2), seven months (T3), eight months (T4), and ten months (T5) post-infection in June, August, October, November of 2020 and January 2021 respectively. At this point, a more specific immunoassay; MAGLUMI® SARS-CoV-2 S-RBD IgG CLIA was adopted for future assessments.
From late February 2021, an additional n = 12 patients (8 female and 4 male), who met the eligibility criteria for participation, were enrolled in the study and added to the original cohort (n = 30). These patients (n = 12), similar to the original cohort, had a history of testing positive for SARS-CoV-2 by RT-qPCR in March 2020, updating the sample size to n = 42.
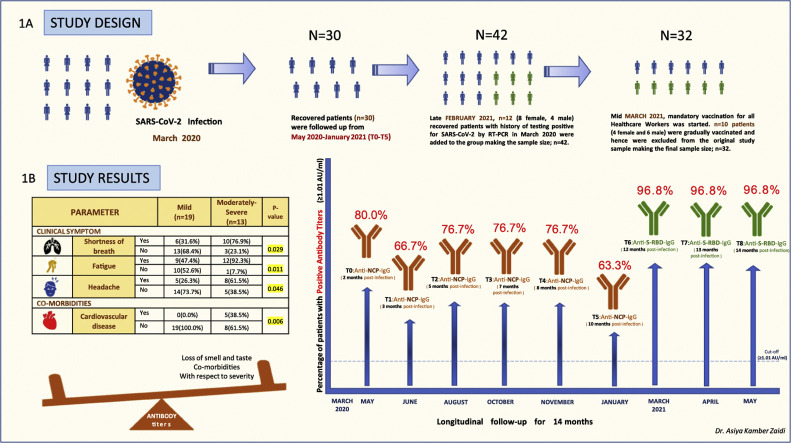
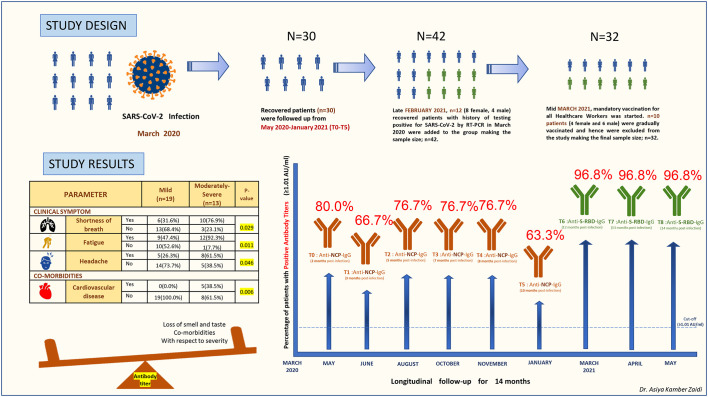
Since the legal provisions adopted by the Italian Ministry of Health advised mandatory vaccination for all Healthcare Workers, irrespective of previous disease status, n = 10 patients (4 female and 6 male) were gradually vaccinated from mid-March 2021 and hence excluded from the original cohort, making the revised final sample size to be n = 32. The study design, study findings, and the temporal distribution of sequential serological sampling time points are described in Fig. 1 . The study group was divided into two groups at each time point based on disease severity; Mild and Moderately-Severe and the antibody assessments were done accordingly. [9]
Fig. 1.
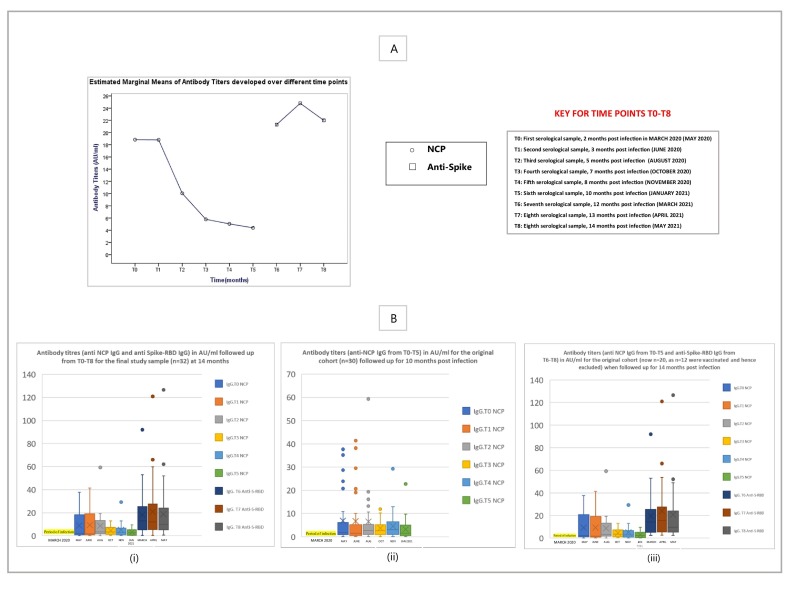
The study design in 1A, describing the recruitment of SARS-CoV-2-recovered individuals in the study, the timeline and the number of individuals analyzed at each time point. The study results in 1B, showing an increased severity of disease in patients with clinical symptoms such as shortness of breath, Fatigue, Headache and history of cardiovascular disease. Patients who experienced loss of smell and taste and had comorbidities with respect to severity developed higher antibody titers at 14 months. P values <0.05 were significant and have been highlighted in yellow. The graph represents the percentage of individuals with positive antibody titres (≥1.01 Au/ml) against Nucleocapsid (NCP) from March 2020 to January 2021 (T0-T5), and against the Spike-RBD from March 2021 to May 2021 (T6-T8), throughout the follow up period of 14 months. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Antibodies against NCP were analyzed from T0-T5 (for 10 months post-infection; March 2020–January 2021) in n = 30 patients followed by analysis of antibodies against Spike-RBD from T6-T8 (for 12, 13 and 14 months post-infection; March 2021–May 2021) in n = 32 patients.
The blood samples were collected after informed consent by the patients and with the approval of the ethics committee of the Associazione Naso Sano (Document number ANS-2020/001) at an accredited lab (Laboratory of Nuclear Lipid BioPathology, CRABION, Perugia, Italy). Data collection and analysis were masked from the main principal investigator, who was also a part of the study sample to avoid observer bias. [10,11] The study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. The STROBE statement checklist can be found in Supplementary Table S.
2.3. Analytical systems used in our study
The MAGLUMI® 2019-nCoV IgM/IgG CLIA is a capture chemiluminescence immunoassay for IgM and an indirect chemiluminescence immunoassay for IgG using the MAGLUMI 2000 series fully-automated chemiluminescence immunoassay analyzer. The cut-off is set at 1.0 arbitrary units per milliliter (AU/mL). According to the manufacturer, the assay has a diagnostic sensitivity and specificity of 91.2% and 97.3%, respectively. [12]
The MAGLUMI® SARS-CoV-2 S-RBD IgG CLIA is an in vitro chemiluminescence immunoassay for quantitative determination of S-RBD IgG antibodies to SARS-CoV-2 in human serum and plasma using MAGLUMI-series fully-automated chemiluminescence immunoassay analyzer. As per product specifications, it has a sensitivity of 100% with CI [99.9%–100.0%] at ≥15 days post symptom onset and specificity of 99.6%; CI [98.7%–100.0%].
High concentration samples were diluted automatically by analyzers and the recommended dilution was 1:9 with the diluent in the kit. The sample, buffer, and magnetic microbeads coated with S-RBD recombinant antigen were mixed thoroughly and incubated, forming immune complexes. After precipitation, decanting of supernatant, and performing a wash cycle, ABEI labeled with anti-human IgG antibody was added, and incubated to form complexes. Again after precipitation in a magnetic field, decanting of supernatant, and performing another wash cycle, the Starter 1 + 2 were added to initiate a chemiluminescent reaction. The light signal was measured by a photomultiplier as relative light units (RLUs), which is proportional to the concentration of SARS-CoV-2 S-RBD IgG presented in the sample. The measurements and interpretation of results were made according to the manufacturer's instructions. The analyzer automatically calculates the concentration in each sample using a calibration curve which is generated by a 2-point calibration master curve procedure. The results were expressed in AU/mL. A result less than 1.00 AU/mL (<1.00 AU/mL) was considered to be non-reactive while a result greater than or equal to 1.00 AU/mL (≥1.00 AU/mL) was considered to be reactive. [13]
2.4. Statistical analysis
The descriptive statistics for the main characteristics of the study group were expressed as Median, [1st -3rd] quartile for continuous variables, and as absolute frequency (column percentage) for the categorical variables. The normal distribution of data was tested by the One-sample Kolmogorov-Smirnov test. The p-values resulted from the Mann Whitney U test, Friedman Test, Pearson's Chi-squared test (for cell frequency n ≥ 5), and Fisher's exact test (for cell frequency n < 5). Statistical significance was defined for p < 0.05. All analyses and data plotting were performed using SPSS Version 22.
3. Results
3.1. Baseline demographic, clinical features and disease characteristics of the study group
Out of the n = 32 subjects, n = 21 (66%) were females and n = 11 (34%) were males. The disease severity was rated as Mild in n = 19 (59.3%) and Moderately-Severe in n = 13 (40.7%). The median age for the group with Moderately-Severe disease was greater (56 years) as compared to the group with Mild disease (31 years).
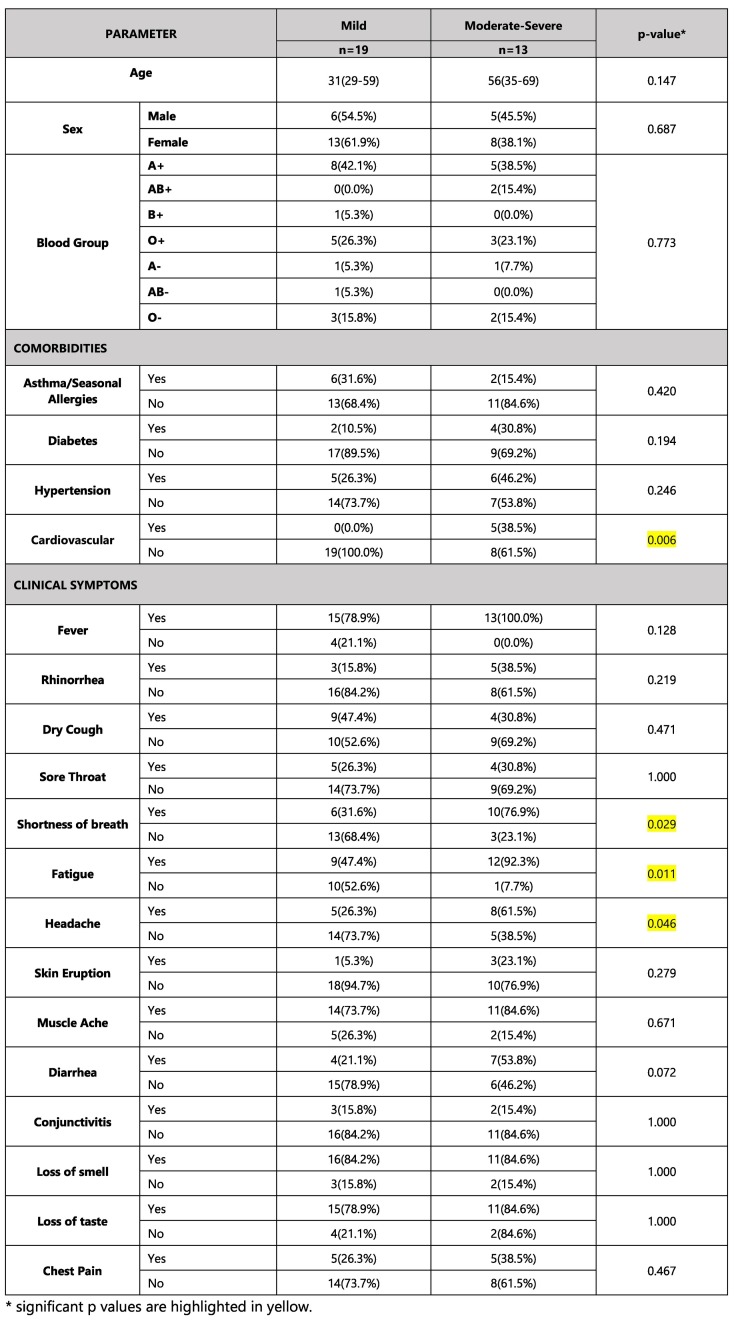
It was noted that n = 18 (56.2%) declared one or more comorbidities such as asthma/seasonal allergies, diabetes, hypertension, or cardiovascular diseases. Of these, n = 5 (38.5%) subjects of the Moderately-Severe category, had some form of cardiovascular disease which was a significant finding (p. = 0.006). In terms of clinical symptoms experienced, the subjects in the Moderately-Severe group (n = 13) had significant shortness of breath (n = 10, p = 0.029), fatigue (n = 12, p = 0.011) and headache (n = 8, p = 0.046). The baseline demographic, clinical features, and disease characteristics of the study subjects at the time of infection (March 2020) are reported in Table 1 . The main characteristics were expressed as Median (q2) with First and Third quartiles i.e., (q1-q3) for continuous variables and as absolute frequency and column percentage for binary variables.
Table 1.
Baseline clinical, demographic features and disease characteristics of the study group in March 2020. The main characteristics are expressed as Median (q2) with First and Third quartiles i.e., (q1-q3) for continuous variables and as absolute frequency and column percentage for binary variables.
3.2. Role of co-morbidities on antibody titers
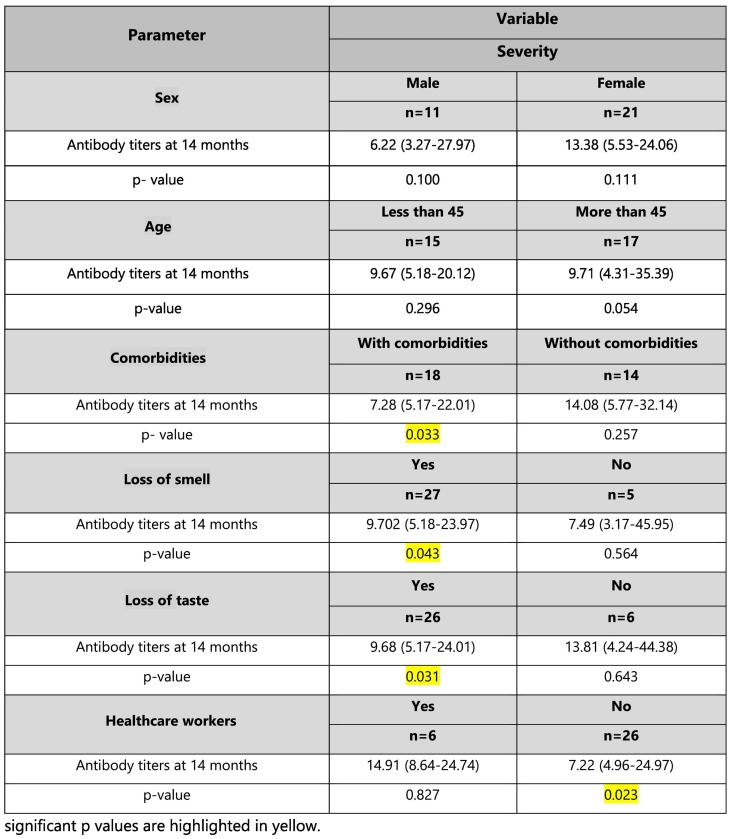
It was observed that the subjects with one or more comorbidities (n = 18) with respect to disease severity, developed a better antibody titer at 14 months as compared to the group (n = 14) without any comorbidity. The p-value was significant (p = 0.033). A study by Huang et al. also found that diabetes was associated with higher IgG levels. [14] This could also mean that subjects with more severe disease due to one or more comorbidities had a better antibody titer at 14 months. Similar findings were observed by studies by Gudbjartsson DF et al., Chirathaworn C et al., Huang M et al., Terpos E et al., and Zhao J et al. [[15], [16], [17], [18]] However, larger studies are needed to draw stronger conclusions regarding these associations.
3.3. Role of loss of smell and taste on antibody titers
It was observed that the subjects who experienced loss of smell and taste during infection (March 2020), with respect to disease severity, developed a better antibody titer at 14 months (p = 0.043 and p = 0.031 respectively).
3.4. Role of occupation on antibody titers
Although similar p values of significance were observed for lower antibody titers developed at 14 months in healthcare workers as compared to non-healthcare workers (p = 0.023), a generalized comment cannot be justified. A significant p-value in such a situation could be due to n = 6 subjects in the healthcare workers group and n = 26 for non-healthcare workers resulting in bias.
The antibody titers (Anti-S-RBD IgG) expressed as Median (q1-q3) in AU/ml at 14 months based on the demographics, comorbidities, loss of smell and taste, and healthcare as an occupation with respect to the disease severity are reported in Table 2 .
Table 2.
Antibody titers (Anti-S-RBD IgG) expressed as Median (q1-q3) in AU/ml developed in n = 32 recovered patients at 14 months based on the demographics, comorbidities, loss of smell and taste during the clinical course of disease, and healthcare as an occupation with respect to overall disease severity but irrespective of their categorization.
3.5. Serologic status at 14 months post-infection
At 14 months post-infection in May 2021, 96.8% (31 out of 32) patients were positive for anti-SARS-CoV-2-S-RBD IgG. This was also observed for the preceding 12 and 13 months (31/32 positive for anti-S-RBD antibodies) but the median neutralization titer (MNT) differed at each time point.
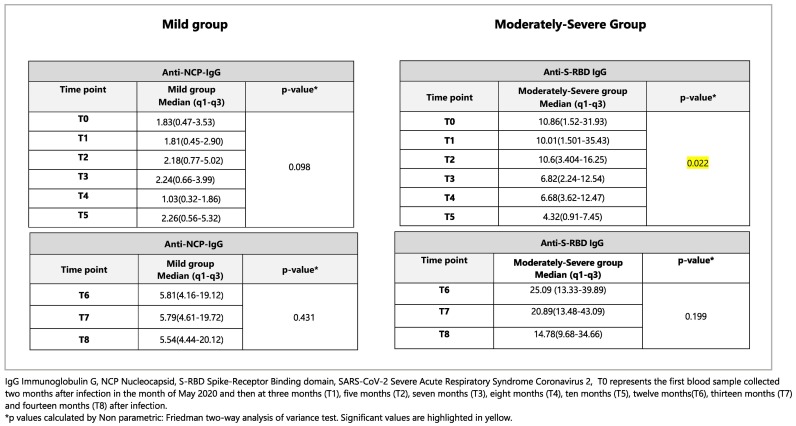
Related-Samples Friedman's Two-way analysis of Variance by Ranks was applied to analyze the antibody titers developed at 14 months for each disease severity group (Mild and Moderately-Severe). It was observed that, differences were observed in the values of Anti-NCP antibody titers (quantitative variable) at different time points from T0-T5 for only the Moderately-Severe group and this finding was statistically significant (p < 0.001). In terms of anti-S-RBD antibody titers from T6-T8, significant differences were not observed for both, Mild and Moderately Severe groups. This could be because only three tests were done for anti-S-RBD (T6-T8) as compared to six tests (T0-T5)for anti-NCP throughout the follow-up period of 14 months. The data analysis for anti-S-RBD IgG titers at 17 months (T9-T11) is ongoing, so that we will be able to compare the results of both tests (six each) in the near future. The results are described in Table 3 .
Table 3.
SARS-CoV-2 specific antibody titers for Mild (n = 19) and Moderately-Severe (n = 13) groups in AU/ml expressed as Median (q1-q3) for the study group (n = 32) evaluated separately at each time point from T0-T5 for anti-NCP-IgG and from T6-T8 for anti-S-RBD IgG.
Since the data repeats over a period of time, multiple comparison tests; i.e. The Friedman's Two-way ANOVA test was applied and expressed as a graph representing the estimated marginal means of antibody titers (Anti-nucleocapsid; NCP and anti-Spike-Receptor Binding Domain; S-RBD) developed over different time points from T0-T8 for the study cohort in panel A of Fig. 2 . Box and Whisker plots represent the antibody titres (anti NCP and anti-S-RBD in AU/ml) for the final study sample, n = 32 at 14 months in (i), Antibody titres (only anti NCP) for n = 30, from T0-T5; 10 month follow-up post infection in (ii), and antibody titres (both anti-NCP and anti-S-RBD) for n = 20 of the original cohort (after exclusion of vaccinated individuals) when followed up for 14 months in (iii) of Panel B of Fig. 2.
Fig. 2.
Panel A represents the estimated marginal means of antibody titers (Anti-nucleocapsid; NCP and anti-Spike-Receptor Binding Domain; S-RBD) developed at different time points from T0-T8 for the study group. Panel B shows Box and Whisker plots representing the antibody titers (both anti NCP and anti-S-RBD) in AU/ml for the final study sample, n = 32 at 14 months in (i) only anti NCP IgG titers for n = 30, from T0-T5; 10 month follow-up post infection in (ii), and both anti-NCP IgG titers and anti-S-RBD IgG titers for n = 20 of the original cohort (after exclusion of vaccinated individuals) when followed up for 14 months in (iii).
3.6. Role of disease severity on antibody titers
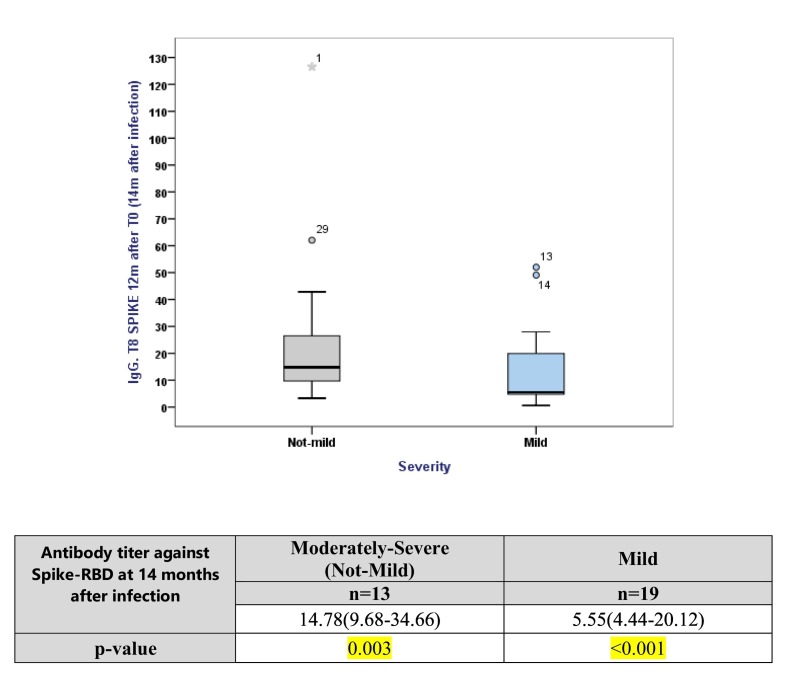
Median neutralization titer (MNT) was calculated for both disease severity groups. It was observed that the subjects of the Moderately-Severe group developed a higher median antibody tire (14.78 AU/ml) at 14 months when compared to the mild group (5.55 AU/ml) but the dispersion or variation was higher for the Moderately-Severe group, indicating a larger degree of variability in the development of antibodies. Fig. 3 shows a box plot with the Median line at the 25th percentile in both the cases indicating that the data is positively skewed i.e., some values are towards the higher end. The subjects in the mild group show less variability in terms of developing antibody titers and have a smaller median while subjects of the Moderately-Severe group exhibit larger variation and also have a higher median.
Fig. 3.
Box plot and table representing inferential statistics for which the data was analyzed for the Outcome variable i.e., Group had either Mild or Moderately-Severe symptoms individually to the exposure variable which was Continuous. The main characteristics are expressed as Median (q2) and First and Third quartiles i.e., (q1-q3) for continuous variables. The continuous variable was non-normal and so the p-values result from one sample Kolmogorov–Smirnov test.
4. Discussion
The SARS-CoV-2 is an enveloped virus with four structural proteins: spike (S) protein, membrane (M) protein, envelope (E) protein, and nucleocapsid (N) protein. Among these four structural proteins, the S and N proteins are the main immunogens. The S protein is a major protective antigen that elicits highly potent neutralizing antibodies (nAbs) and plays an essential role in viral attachment, fusion, entry, and transmission. The S protein comprises of an N-terminal Sl subunit responsible for virus-receptor binding and a C-terminal S2 subunit responsible for virus-cell membrane fusion. The S1 subunit is further divided into an N-terminal domain (NTD) and a receptor-binding domain (RBD). The RBD within Sl interacts directly with host receptors, human angiotensin-converting enzyme 2 (hACE2). [19] The immunity against any infectious disease is comprised of two arms: innate immunity and adaptive or acquired immunity. The adaptive arm contains humoral (B cells) and the cell-mediated (T cell) immune elements.
Antibodies are synthesized and secreted by plasma cells that are derived from the B cells of the immune system and can be used as a correlate of immunity. Antibody tests also known as serological tests, detect the presence of antibodies against a particular disease-causing agent in the blood, to evaluate the immune response against it. Antibodies can be of different varieties known as isotypes or classes which differ in their biological properties and ability to deal with different antigens and are called Immunoglobulins. Immunoglobulin (IgM) eliminates pathogens in the early stages of B Cell-mediated immunity and is a marker of active infection, while immunoglobulin G (IgG) provides long-lasting antibody-mediated immunity and is the only antibody capable of crossing the placenta providing passive immunity to the fetus.
In a recent study by Turner et al., it was observed that the SARS-CoV-2 infection induces a robust antigen-specific, long-lived humoral immune response in humans. For the patients who experienced mild infections (n = 77), serum anti-SARS-CoV-2 spike (S) antibodies declined rapidly in the first 4 months after infection and then more gradually over the following 7 months, remaining detectable at least 11 months after infection. The S-binding bone marrow plasma cells (BMPCs) are quiescent, indicating that they are part of a long-lived compartment. [20]
The neutralizing antibodies (nAbs) are capable of preventing an infectious agent from infecting a cell by neutralizing or inhibiting its biological effect. The most critical target for SARS-CoV-2 nAbs is the RBD within the Sl subunit of S protein. Such nAbs can interrupt the interaction of RBD and its receptor ACE2. Anti-S-RBD nAbs produced in COVID-19 recovered patients can block viral infection of human cells in vitro and counter viral replication in vivo. [[21], [22], [23]] Thus, SARS-CoV-2 S-RBD IgG antibody levels in human serum or plasma correlates with protective immune responses in individuals recovered from SARS-CoV-2 infection. Information regarding herd immunity at a population level would be helpful in planning of clinical management for patients with past or ongoing COVID-19 infection.
Neutralizing antibody titers (and total Spike antibody titers) have demonstrated a positive correlation with COVID-19 disease severity in large cohort studies. [7,11,24]. This was also observed in our study findings. The subjects in the Moderately-Severe group developed a higher Median Neutralization Titer (14.78 AU/ml, p = 0.003) at 14 months when compared to the Mild group (5.55 AU/ml, p < 0.001). [Fig. 3] This result was statistically significant. However, the relationship between the neutralizing antibodies, T follicular helper cells (Tfh cells), and COVID-19 disease severity appear to be complex. A higher neutralizing antibody titer is associated with severe disease and potentially “extrafollicular B cell responses” [24] whereas the SARS-CoV-2-specific Tfh cells are associated differently. Moreover, antibodies could act as a useful surrogate marker of CD4+ T cell responses in many infections, since antibody assays are much easier to perform and more sensitive in small blood volumes when compared to antigen-specific T cell assays. [25]
A recent study by Abu-Raddad LJ et al. in Qatar assessed the cumulative risk as well as the incidence rate of SARS-CoV-2 reinfection in a nationwide cohort of 43,044 antibody-positive individuals. This study with a follow period of up to 35 weeks, demonstrated and confirmed through viral genome sequencing that SARS-CoV-2 reinfection occurs, but “only rarely” with a cumulative risk of ~2 per 1000 persons and reinfection incidence rate of <1 per 10,000 person weeks as compared to the complement cohort of 149,923 antibody-negative persons with a much higher cumulative risk of re-infection (~31 per 1000 persons after 46 weeks of follow-up) and estimated incidence rate of infection (~14 per 10,000 person-weeks). The estimated efficacy of natural infection against reinfection was 95%. Moreover, this study showed no evidence of waning protective immunity against reinfection in this cohort for over 7 months. [26]
An important point that needs to be highlighted in our study is zero cases of re-infection despite the fact that the Umbria region has been experiencing multiple waves with mutant strains since late 2020. [11]
4.1. Adoption of anti-S-RBD immunoassay
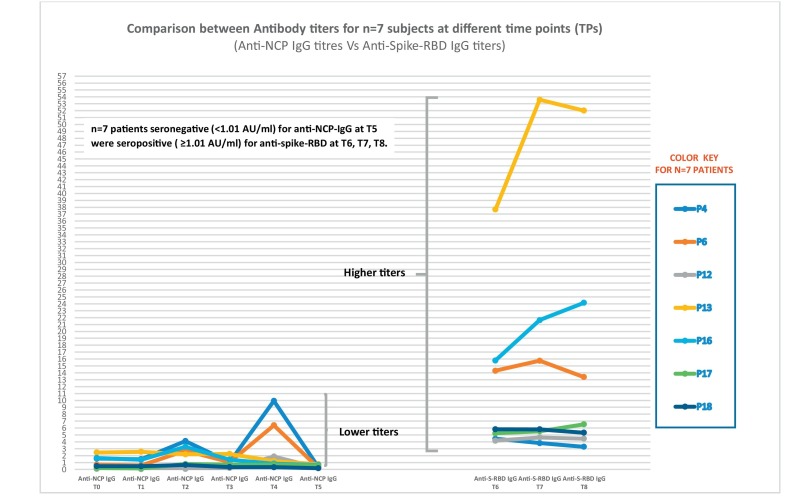
The S1 subunit has low evolutionary protein homologies within the coronavirus family suggesting less cross-reactivities among the endemic coronaviruses, but the N protein-based antibody assays exhibit a higher false-negative rate compared with the S1 subunit, making the anti-S-RBD assays more specific. Although the Nucleocapsid and Spike IgG titers are highly correlated [25], the spike is the target of SARS-CoV-2 neutralizing antibodies, and the RBD of Spike is the target of >90% of neutralizing antibodies in COVID-19 cases [24,[27], [28], [29]], with some neutralizing antibodies instead targeting the N-terminal domain (NTD). [30] When the study group was followed up for 10 months (T0-T5), a decreasing trend in anti-NCP antibodies was observed and therefore, a more specific assay was adopted to detect the presence of antibodies against the S-RBD. It was observed in our study the subjects, n = 7 (P4, P6, P12, P13, P16, P17, P18) who were seronegative for anti-NCP antibodies at T5, were found to be seropositive at T6, T7, and T8 for anti-S-RBD antibodies emphasizing on the fact that N protein-based antibody assays exhibit a higher false-negative rate when compared with the anti-S-RBD assays. Further prospective studies are needed to determine if antibodies against NCP and S-RBD develop at the same time or sequentially but since anti-S-RBD titers at T6-T8 were of a much higher range (upto 53.59 AU/ml) in terms of magnitude when compared with anti-NCP (upto 10 AU/ml), we can hypothesize that these anti-S-RBD IgG would have been also detectable in the preceding months. [Fig. 4 ].
Fig. 4.
Graph showing antibody responses in n = 7 patients (P4, P6, P12, P13, P16, P17 and P18) that were seronegative (<1.01 AU/ml) for anti-NCP-IgG at T5 were seropositive (≥1.01 AU/ml) for anti-spike-RBD at T6, T7, T8.
4.2. Antibody seropositivity in the cohort
In this study, at T0, 2 months after the initial infection, 24 out of 30 (80%) subjects were positive for SARS-CoV-2 NCP IgG antibodies, followed by a slight dip with 20 out of 30 (66.7%) subjects with antibody seropositivity at T1, 3 months after infection. This antibody seropositivity trend remained stable in 23 out of 30 (76.7%) at T2, T3, T4 and a second dip was observed at T5. However, after the adoption of anti-S-RBD immunoassay, 31 out of 32 (96.8%) subjects showed antibody persistency of much higher magnitude at all the three-time points; T6, T7, and T8, at 12, 13 and 14 months post-infection respectively.
Our results are in line with previous studies showing similar longevity and pattern of anti-SARS-CoV-2 Ab responses, with Ab levels reaching a peak at 23 days following symptom onset and being maintained for at least 4 months, [11,15,[31], [32], [33], [34], [35], [36], [37]] yet contradictory to others, in which a low prevalence and rapid decay (within 3 months) of anti-SARS-CoV-2 Abs in COVID-19 patients with either mild or severe disease were observed. [38,39]
4.3. Patient persistently seronegative in the cohort
Interestingly, the patient (P31: 55 yrs./Female) that tested negative on all three occasions (T6-T8) for anti-S-RBD IgG was a known case of multiple myeloma. A study by E. Terpos et al. demonstrated production of lower levels of Nabs against SARS-CoV-2 among patients with multiple myeloma after the first dose of BNT162b2 compared with non-MM controls of similar age and sex and without malignant disease. This may be due to the effect of myeloma cells suppressing normal B-cell expansion and immunoglobulin production [40] or could be due to myeloma cells producing abnormal antibodies that the body cannot use.
Our study had some limitations. First, a small sample size (n = 32). Second, ideally, simultaneous antibody titer detection of each patient at each time point using both NCP and S-RBD assay would have given best results for comparative analysis but S-RBD assay received emergency approval only later in 2020. Moreover, retesting of old samples for anti-S-RBD titers during the pandemic had its own logistic and compliance issues.
In Conclusion, our study findings are consistent with recent studies reporting antibody persistency suggesting that induced SARS-CoV-2 immunity through natural infection, might be very efficacious against re-infection (>90%) and could persist for more than six months. Our study followed up patients up to 14 months demonstrating the presence of anti-S-RBD IgG in 96.8% of recovered COVID-19 subjects.
This study also provides valuable information for future implementation of health policies including vaccine distribution. Further studies need to be conducted to determine the differences between the antibody responses developed in patients infected by the original wild type virus versus antibody responses developed in patients infected by the mutant strains.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2021.108814.
Appendix A. Supplementary data
Supplementary materail 1
Supplementary materail 2
References
- 1.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020 Feb 22;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. Epub 2020 Jan 30. PMID: 32007145; PMCID: PMC7159086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L.P., Wang N.C., Chang Y.H., et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg. Infect. Dis. 2007 Oct;13(10):1562–1564. doi: 10.3201/eid1310.070576. PMID: 18258008; PMCID: PMC2851497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall V.J., Foulkes S., Charlett A., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021 Apr 17;397(10283):1459–1469. doi: 10.1016/S0140-6736(21)00675-9. Epub 2021 Apr 9. PMID: 33844963; PMCID: PMC8040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matusali G., Colavita F., Lapa D., Meschi S., Bordi L., Piselli P., Gagliardini R., Corpolongo A., Nicastri E., Antinori A., Ippolito G., Capobianchi M.R., Castilletti C., Inmi Covid-Laboratory Team SARS-CoV-2 serum neutralization assay: a traditional tool for a brand-new virus. Viruses. 2021 Apr 10;13(4):655. doi: 10.3390/v13040655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.015. 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juno J.A., Tan H.-X., Lee W.S., Reynaldi A., Kelly H.G., Wragg K., Esterbauer R., Kent H.E., Batten C.J., Mordant F.L., et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID19. Nat. Med. 2020;26:1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 7.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MAGLUMI nCoV IgM/IgG - Letter of Authorization. 2019. fda.gov
- 9.World Health Organization International guidelines for certification and classification (coding) of COVID-19 as cause of death. https://www.who.int/classifications/icd/Guidelines_Cause_of_Death_COVID-19-20200420-EN.pdf?ua=1 Available from: Document Number: WHO/HQ/DDI/DNA/CAT. Accessed on June 1, 2020.
- 10.Xydakis M.S., Dehgani-Mobaraki P., Holbrook E.H., et al. Smell and taste dysfunction in patients with COVID-19. Lancet Infect. Dis. 2020 Sep;20(9):1015–1016. doi: 10.1016/S1473-3099(20)30293-0. Epub 2020 Apr 15. PMID: 32304629; PMCID: PMC7159875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dehgani-Mobaraki P, Kamber Zaidi A, Porreca A et. al. Antibody persistency and trend post-SARS-CoV-2 infection at eight months. Ann. Ig. 2021 May. doi: 10.7416/ai.2021.2455. Online ahead of print. [DOI] [PubMed]
- 12.https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas
- 13.https://www.snibe.com/zh_en/en_newsView.aspx?id=647
- 14.Huang M., Lu Q.B., Zhao H., et al. Temporal antibody responses to SARS-CoV-2 in patients of coronavirus disease 2019. Cell Discovery. 2020;6:64. doi: 10.1038/s41421-020-00209-2. 32983570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gudbjartsson D.F., Norddahl G.L., Melsted P., et al. Humoral immune response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020 Sep 1 doi: 10.1056/NEJMoa2026116. 32871063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chirathaworn C., Sripramote M., Chalongviriyalert P., et al. SARS-CoV-2 RNA shedding in recovered COVID-19 cases and the presence of antibodies against SARS-CoV-2 in recovered COVID-19 cases and close contacts, Thailand, April-June 2020. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0236905. 33119712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terpos E., Politou M., Sergentanis T.N., et al. Anti– SARS-CoV-2 antibody responses in convalescent plasma donors are increased in hospitalized patients;subanalyses of a phase 2 clinical study. Microorganisms. 2020;8(12):1885. doi: 10.1080/22221751.2020.17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J., Yuan Q., Wang H., et al. Antibody Responses to SARS-CoV-2 in Patients With Novel Coronavirus Disease 2019. Clin. Infect. Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. PMID: 32221519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020 doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner J.S., Kim W., Kalaidina E., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021 doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 21.Zohar T., et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell. 2020;183(1508–1519) doi: 10.1016/j.cell.2020.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu F., et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai. China JAMA Intern. Med. 2020;180:1356–1362. doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piccoli L., Park Y.J., Tortorici M.A., et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell. 2020 Nov 12;183(4) doi: 10.1016/j.cell.2020.09.037. 1024–1042.e21. Epub 2020 Sep 16. PMID: 32991844; PMCID: PMC7494283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021 Feb 18;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. Epub 2021 Jan 12. PMID: 33497610; PMCID: PMC7803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Raddad L.J., Chemaitelly H., Coyle P., et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClin.Med. 2021 May;35:100861. doi: 10.1016/j.eclinm.2021.100861. Epub 2021 Apr 28. PMID: 33937733; PMCID: PMC8079668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brouwer P.J.M., Caniels T.G., van der Straten K., et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020 Aug 7;369(6504):643–650. doi: 10.1126/science.abc5902. Epub 2020 Jun 15. PMID: 32540902; PMCID: PMC7299281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers T.F., Zhao F., Huang D., et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 Aug 21;369(6506):956–963. doi: 10.1126/science.abc7520. Epub 2020 Jun 15. PMID: 32540903; PMCID: PMC7299280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suthar M.S., Zimmerman M.G., Kauffman R.C., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Rep Med. 2020 Jun 23;1(3):100040. doi: 10.1016/j.xcrm.2020.100040. Epub 2020 Jun 8. PMID: 32835303; PMCID: PMC7276302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Wang P., Nair M.S., et al. Potent neutralizing antibodies directed to multiple epitopes on SARS-CoV-2 spike. bioRxiv. 2020 doi: 10.1101/2020.06.17.153486. [DOI] [PubMed] [Google Scholar]
- 31.Bolke E., Matuschek C., Fischer J.C. Loss of anti-SARS-CoV-2 antibodies in mild covid-19. N. Engl. J. Med. 2020;383:1694–1695. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 32.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., Mills R., Teng E., Kamruzzaman M., Garcia-Beltran W.F., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., Li Z., Chao G., Rojas O.L., Bang Y.M., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., et al. Robust neutralizing antibodies to SARSCoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartley G.E., Edwards E.S.J., Aui P.M., Varese N., Stojanovic S., McMahon J., Peleg A.Y., Boo I., Drummer H.E., Hogarth P.M., et al. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roltgen K., Powell A.E., Wirz O.F., Stevens B.A., Hogan C.A., Najeeb J., Hunter M., Wang H., Sahoo M.K., Huang C., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dan J.M., Mateus J., Kato Y., Hastie K.M., Yu E.D., Faliti C.E., Grifoni A., Ramirez S.I., Haupt S., Frazier A., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., Ferbas K.G., Tobin N.H., Aldrovandi G.M., Yang O.O. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kutsuna S., Asai Y., Matsunaga A. Loss of anti–SARS-CoV-2 antibodies in mild covid-19. N. Engl. J. Med. 2020;383:1695–1696. doi: 10.1056/NEJMc2027051. [DOI] [PubMed] [Google Scholar]
- 40.Terpos Evangelos, Trougakos Ioannis P., Gavriatopoulou Maria, Papassotiriou Ioannis, Sklirou Aimilia D., Ntanasis-Stathopoulos Ioannis, Papanagnou Eleni-Dimitra, Fotiou Despina, Kastritis Efstathios, Meletios A. Dimopoulos; low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137(26):3674–3676. doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materail 1
Supplementary materail 2