Abstract
Objective
The objectives of this pilot study were (1) to assess the feasibility of a larger evaluation of Smart About Meds (SAM), a patient-centered medication management mobile application, and (2) to evaluate SAM’s potential to improve outcomes of interest, including adherence to medication changes made at hospital discharge and the occurrence of adverse events.
Materials and Methods
We conducted a pilot randomized controlled trial among patients discharged from internal medicine units of an academic health center between June 2019 and March 2020. Block randomization was used to randomize patients to intervention (received access to SAM at discharge) or control (received usual care). Patients were followed for 30 days post-discharge, during which app use was recorded. Pharmacy claims data were used to measure adherence to medication changes made at discharge, and physician billing data were used to identify emergency department visits and hospital readmissions during follow-up.
Results
Forty-nine patients were eligible for inclusion in the study at hospital discharge (23 intervention, 26 control). In the 30 days of post-discharge, 15 (65.2%) intervention patients used the SAM app. During this period, intervention patients adhered to a larger proportion of medication changes (83.7%) than control patients (77.8%), including newly prescribed medications (72.7% vs 61.7%) and dose changes (90.9% vs 81.8%). A smaller proportion of intervention patients (8.7%) were readmitted to hospital during follow-up than control patients (15.4%).
Conclusion
The high uptake of SAM among intervention patients supports the feasibility of a larger trial. Results also suggest that SAM has the potential to enhance adherence to medication changes and reduce the risk of downstream adverse events. This hypothesis needs to be tested in a larger trial.
Trial registration
Clinicaltrials.gov, registration number NCT04676165.
Keywords: mobile application, medication adherence, adverse events, pilot randomized controlled trial
INTRODUCTION
Prescription medications play an important role in preventing and managing chronic diseases, which impose a significant burden on individuals and health-care systems. To maximize therapeutic benefit, adherence to prescribed medications is necessary. Unfortunately, medication nonadherence is common, with up to 30% of patients failing to fill a new prescription in some cases1–5 and 45% failing to take their filled medication as prescribed.6–10 Nonadherence is also a problem following discharge from hospital,11–13 when several changes are often made to patients’ medication regimens.14–16 In a recent study, we found that patients had an average of 4.4 changes made to their medication regimen at discharge, a significant proportion of which were not adhered to: 27% of newly prescribed medications were not filled, 12% of discontinued medications were refilled, and 30% of dose changes were filled at the wrong dose.11
The impact of medication nonadherence on health outcomes has been widely documented.17–19 Failure to take medications as prescribed increases the risk of adverse events such as emergency department (ED) visits, hospital admissions, and death,3,9,19–23 as well as associated health-care costs.24,25 Nonadherence to medication changes made at hospital discharge also increases the risk of adverse events,26 including the risk of death among patients discharged after a myocardial infarction.3 In our recent study, patients who did not adhere to any of the changes made to their medications had a 35% increased risk of adverse events post-discharge compared to patients who adhered to all changes.26
Numerous interventions targeting medication nonadherence have been evaluated.27 However, only moderate improvements in adherence have been achieved, at best.28,29 Fortunately, digital technologies have emerged in recent years as increasingly popular and potentially powerful tools to provide individualized support to change health behaviors. Various technologies, from telehealth to web-based technologies, have been used to provide a wide range of adherence tools, such as adherence tracking and feedback, patient education and counseling, and medication and refill reminders.30,31 However, many of these interventions involve a limited number of adherence-targeting components. This is despite research that suggests that multicomponent interventions are the most effective,27,28,32 likely due to the complex, multifactorial nature of nonadherence.25,33
Mobile applications have, by virtue of their versatile nature, the potential to bring several components targeting nonadherence together into one tool. They also have the advantage of being readily available to smartphone owners, who represent 81% of adults in the United States.34 Mobile apps have demonstrated success in effecting health behavior change35 and their potential to improve medication adherence has also been recognized.36 One only has to open the app store on a smartphone to access the dozens of medication-related apps available to users.37,38 However, the majority require tedious manual entry of medications,38 few include advanced features beyond pill reminders and adherence tracking,37–39 and even fewer have been evaluated.36,37,40,41 There is thus a clear need to design mobile apps that integrate multiple, advanced features aimed at improving medication adherence, and to conduct scientific evaluations of their impact on adherence and downstream adverse outcomes such as ED visits and hospital admissions.
We designed and developed Smart About Meds (SAM), a patient-centered mobile application that aims to enhance medication adherence and empowers patients to better manage their medications in accordance with their needs and values. We conducted a pilot randomized controlled trial (RCT) of SAM among patients discharged from 2 internal medicine units of an academic health center. Our objectives were to assess the feasibility of a larger trial and to evaluate the potential of an effect of SAM on outcomes of interest, including adherence to medication changes and adverse event risk.
MATERIALS AND METHODS
Study design and context
We conducted a pilot RCT among patients discharged from the 2 internal medicine units of the Royal Victoria and Montreal General sites of the McGill University Health Centre (MUHC). The MUHC is a consortium of 5 tertiary hospitals in Montreal, Quebec, where prescription drug insurance is mandatory and provided by the provincial health insurer (Régie de l’assurance maladie du Québec—RAMQ) to those who are over the age of 65, are not covered by their employer, or are on welfare.
Enrolled patients were randomized using permuted block randomization with varying block sizes of 2 and 4, with equivalent numbers of patients randomized to intervention and control in each block. Patients in the control arm received usual care at discharge, whereas those in the intervention arm received, in addition to usual care, access to the SAM mobile application. Given the nature of the intervention, study participants and recruiting research assistants were not blinded to group allocation, but data analysts were.
Following discharge from the hospital, patients were followed for 30 days, during which app use was recorded in our databases and used to determine utilization rates. Pharmacy claims data were obtained for the 30-day follow-up period to measure primary adherence to medication changes made at hospital discharge, and physician billing data were obtained to identify the occurrence of adverse events (ED visits and readmissions).
Study population
Patients were eligible for this study if they were 18 years of age or older at the time of hospital admission, were covered by the RAMQ prescription drug insurance program, owned a smartphone or tablet device with an internet connection, were fluent in English or French, and were discharged home. Patients who were not prescribed any medications at discharge, had a prognosis of survival of less than 3 months, were transferred to a non-study unit, or were discharged to a rehabilitation center were excluded from the study.
All eligible patients provided written informed consent prior to being enrolled in the study. Patients who were cognitively impaired or otherwise unable to provide informed consent were enrolled if consent was obtained from a legally authorized representative and if the caregiver responsible for acquiring and administering the patient’s medications agreed to use the SAM app on the patient’s behalf. The study was granted ethics approval by the MUHC Research Ethics Board.
Data sources
Patients’ discharge prescriptions were obtained from hospital charts. Medications prescribed at discharge, along with their reconciliation status (new, continued, dose change, discontinued), dosage, and special directives, were entered by a trained research assistant into structured data fields in a computerized study administration tool developed by our team.
Pharmacy claims data were obtained from the RAMQ for all study participants for the 3 months prior to hospital admission and the 30 days following hospital discharge. These data included information on medications dispensed to study participants, including the drug identification number (DIN), duration of the prescription, and quantity of pills dispensed. These data were linked using the DIN to data tables from Vigilance Santé, a drug database vendor in Quebec, to obtain further information on the strength of the dispensed medication, format, and typical route of administration.
Physician fee-for-service billing data were obtained from the RAMQ for all services provided to study participants in the 3 months prior to hospital admission and the 30 days post-discharge. These data included the date of the provided service and the service location (eg ED, inpatient ward).
SAM utilization data were retrieved from our databases, which store records of every action conducted by patient and caregiver users in the SAM app. Each record is timestamped, linked to a user, and categorized by the type of feature accessed (eg “drug information leaflet”, “side-effect checker”, “message pharmacist”, “resolve adherence alert”).
Intervention
Control arm
Patients in the control arm received usual care at discharge. Following medication reconciliation, they were provided with a written discharge prescription to be filled at their community pharmacy. Patients may or may not have received written or verbal instructions about their discharge prescription or about changes made to their medications.
Intervention arm
Patients in the intervention arm received, in addition to usual care, training in and access to the SAM mobile app at discharge. Patients’ own medications were used during training, which consisted of showing patient and caregiver users how to log into SAM and how to access its various features.
SAM was designed based on the Information-Motivation-Behavioral Skills (IMB) theoretical framework, which integrates key concepts from classic health behavior models (Theory of Planned Behavior, Self-Determination Theory, and Health Belief Model) to advance interventions targeting behavior change.42 In brief, IMB posits that successful behavior change requires individualized interventions to address the informational, motivational, and behavioral skills needs of patients. For medication adherence, individuals must first understand their condition and how prescribed medications help manage symptoms (information), must be committed to adhering to recommended therapy (motivation), and have the skills and support that facilitate long-term adherence (behavioral skills) (Table 1).
Table 1.
SAM features
Information
|
|---|
Motivation
|
|
|
Behavioral skills and support
|
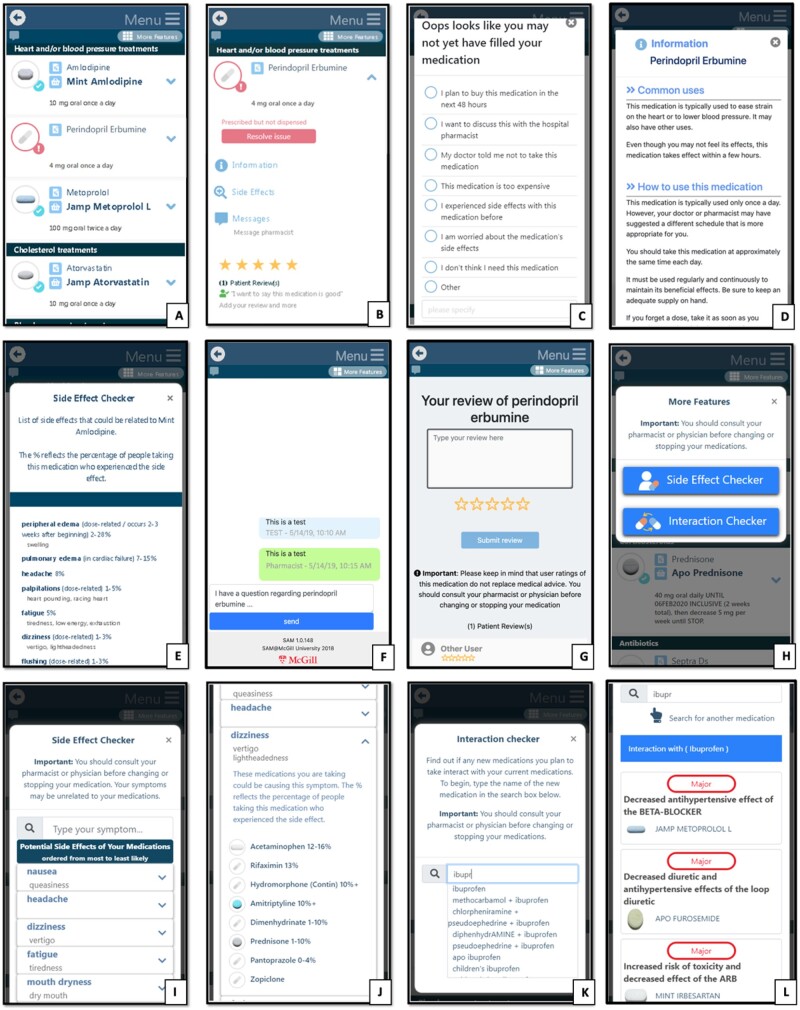
SAM was also designed with input from patients and caregivers through a user-centered design development process. The app retrieves the patient’s prescribed medications from the study administration tool and dispensed medications from RAMQ pharmacy claims data. SAM matches dispensed medications to prescribed medications based on ingredient, and generates a patient-friendly list of prescribed and dispensed medications in which medications are grouped by therapeutic class. The app also offers several features aimed not only at addressing barriers to adherence, but also empowering patients to be more informed about their medications and better able to manage them. These include pill images, drug information leaflets, adherence alerts, side effect and interaction checkers, pharmacist and caregiver connect features, and a rate-my-med feature (Table 1, Figures 1 and 2). These features empower patients to actively access information about their medications in a manner consistent with their needs and values, rather than having to depend on a health professional to convey this information.
Figure 1.
SAM main features. (A) List of prescribed and dispensed medications grouped by therapeutic class, with pill images and prescribed dosage. (B) Dropdown menu of one medication, showing buttons for an adherence alert, drug information leaflet, side effects, messaging feature, and Rate My Med feature. (C) Options to resolve an adherence alert. (D) Patient-friendly drug information leaflet. (E) List of side effects of one medication, with frequency of occurrence. (F) Pharmacist connect feature to send message to pharmacists. (G) Rate My Med feature. (H) Menu to access additional features. (I) Side effect profile of all of the user’s medications. (J) Medications associated with one of the side effects in the user’s side effect profile. (K) Search box for the interaction checker—user searches for medication they consider purchasing. (L) Interaction checker—displays interactions between medication selected in K and the user’s medication profile.
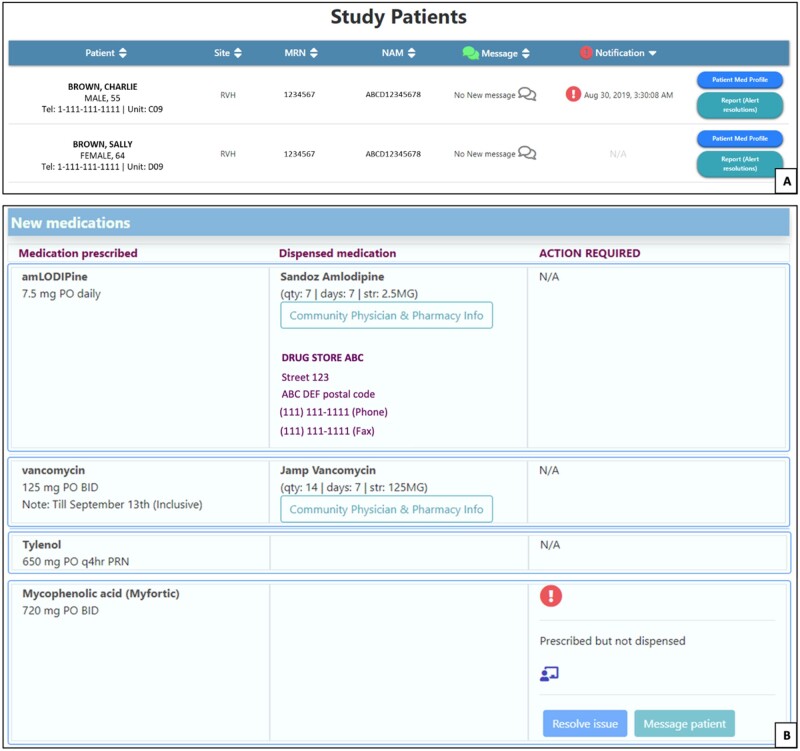
Figure 2.
SAM pharmacist dashboard. (A) Main dashboard listing patients using the app who the pharmacist is currently managing. Patient information, hospital discharge site, hospital identification number (MRN), and medicare number (NAM) are displayed. New incoming messages from patients or caregivers are displayed, as are any unresolved notifications (i.e. adherence alerts). Through this dashboard, the pharmacist can also access the patient’s medication profile and documentation services. (B) Patient’s medication profile, with prescribed medications and dosage in the far left column, dispensed medications (if any) in the middle column along with dispensed strength, duration, quantity, and dispensing pharmacy, and any adherence alerts that require attention in the far right column.
Outcome measures
As part of usual care, medication reconciliation was conducted for all patients at discharge. This process, which consists of comparing patients’ community medications to those prescribed at discharge, allows for the assignment of a reconciliation action to each medication prescribed at discharge: new, continue, discontinue, or dose change. New medications are those which patients were not taking prior to hospitalization and which were newly prescribed at discharge. Continued and discontinued medications are those which patients were taking prior to hospitalization and which were re-prescribed as is or discontinued at discharge, respectively. Dose changes are medications that patients were taking prior to hospitalization and that were re-prescribed at discharge, but at a different daily dose. For the purposes of this study, we defined medication changes as medications that had a reconciliation status of new, discontinue, or dose change at discharge.
Primary adherence to medication changes
Primary adherence was measured at the medication level as the proportion of medication changes adhered to during the 30-day follow-up period. Adherence was measured overall and by study group, and separately for each type of medication change.
Primary adherence to medication changes was assessed by comparing medications dispensed to patients in the 30 days post-discharge to their discharge prescriptions. Thirty days is a typical length of follow-up for measuring primary adherence, which is usually defined as a new prescription being filled within this timeframe.49 In this study, primary adherence to medication changes was defined as (1) filling newly prescribed medications within 30 days, (2) not refilling discontinued medications, or (3) filling dose changes at the correct daily dose. For new and discontinued medications, adherence was assessed only for medications reimbursed by the provincial drug formulary and, for new medications, those which were not prescribed on an “as needed” basis. For dose changes, adherence was assessed only for those medications that were actually filled post-discharge. This ensured that our measurement of adherence to dose changes did not include patients who used a leftover supply of medication to modify the daily dose.
The prescribed daily dose was measured by multiplying the prescribed dose per intake by the prescribed number of daily intakes. The dispensed daily dose was measured by multiplying the strength of the dispensed medication by the quantity dispensed and dividing by the duration of the prescription. If the dispensed daily dose of a medication differed from the prescribed daily dose by 25% or more, the medication was considered to have been dispensed at the wrong daily dose. Dose changes dispensed at the incorrect daily dose were considered nonadherence, even if the result of a dispensing error.
Adverse events: emergency department visits and hospital readmissions
Adverse event occurrence was measured, overall and by study group, as the proportion of patients who had an ED visit, hospital readmission, or either event in the 30 days post-discharge. Medical fee-for-service billing data were used to identify ED visits and hospital readmissions occurring during follow-up. Previous research has shown that emergency physicians accurately diagnose admissions as medication-related only 51% of the time and of those accurately diagnosed, only up to 28% are reported as medication-related in administrative health data.50,51 Given this low sensitivity of detecting medication-related ED visits and hospital readmissions using administrative health data, we included all such events in the outcome regardless of the recorded diagnosis.
SAM app utilization
Time-stamped records of each action conducted in SAM were retrieved from our databases. Overall SAM utilization was measured as the proportion of intervention patients who used SAM post-discharge. Among those users, utilization rates for various SAM features were measured as the median number of times each feature was accessed, per patient and caregiver user, over the 30-day follow-up period.
Records of pharmacist actions in the pharmacist dashboard were similarly obtained to determine the number of times each action was conducted.
Data analysis
Descriptive statistics were used to characterize the study population (age, sex, caregiver presence, prescribed medications, and prior ED visits and hospitalizations) and to assess adherence to medication changes, the occurrence of adverse events, and SAM app utilization. Medication adherence and adverse event rates in intervention and control groups were assessed using an intention-to-treat approach, that is, patients randomized to the intervention group who did not use the app post-discharge were not excluded. Utilization of SAM features, on the other hand, was assessed only among intervention patients who used the app post-discharge. All analyses were conducted using SAS version 9.4.
RESULTS
Study cohort
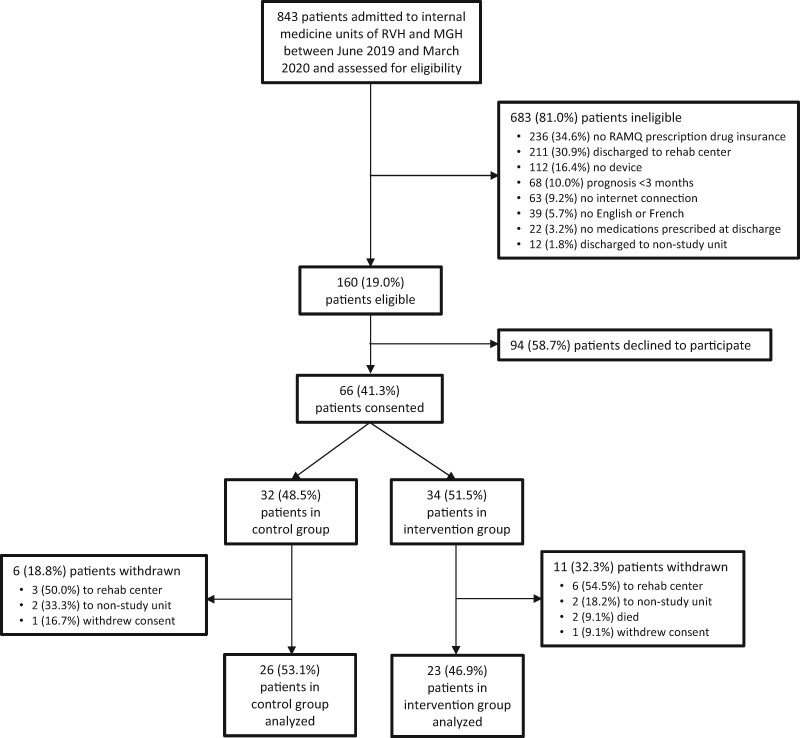
Between June 2019 and March 2020, 843 patients admitted to study sites were assessed for eligibility (Figure 3). Of these, 683 (81.0%) were ineligible for various reasons, chief among which were the lack of RAMQ prescription drug insurance (N = 236, 34.6%), expected discharge to a rehabilitation center (N = 211, 30.9%), and lack of a smartphone or tablet device (N = 112, 16.4%). The remaining 160 (19.0%) patients were eligible, among whom 66 (41.3%) consented to participate and were enrolled in the study. Following randomization, which allocated 32 (48.5%) patients to the control group and 34 (51.5%) to the intervention group, 15 (22.7%) patients became ineligible because of unexpected discharge to a rehabilitation center (N = 9, 60.0%), unexpected transfer to a non-study unit (N = 4, 26.7%), or death (N = 2, 13.3%). An additional 2 patients withdrew consent during follow-up (1 intervention, 1 control). This left 49 patients, 26 (53.1%) in the control group and 23 (46.9%) in the intervention group, who were analyzed. The pilot trial was unexpectedly terminated in March 2020 due to the Coronavirus pandemic.
Figure 3.
CONSORT flow diagram.
Patient characteristics
The majority of study participants were male (N = 30, 61.2%) and the median age was 64.6 years (interquartile range [IQR] 45.3–75.0) (Table 2). Participants in the intervention group were younger than those in the control group (median age 54.6 vs 68.6 years, respectively) and less likely to be male (52.2% vs 69.2%, respectively). In the 3 months prior to the index hospitalization, 29 (59.2%) patients had at least one ED visit and 10 (20.4%) were hospitalized. A similar proportion of intervention and control patients had a hospital admission prior to the index hospitalization (21.7% and 19.2%, respectively), but a larger proportion of intervention patients (65.2%) had an ED visit compared to control patients (53.8%). During enrollment, 8 (16.3%) patients indicated that a caregiver aids them with medication management, 1 (4.4%) of whom was in the intervention group and 7 (26.9%) who were in the control group. The caregiver in the intervention group was granted access to the SAM app.
Table 2.
Patient characteristics
| Patient characteristic | Overall (N = 49) | Intervention group (N = 23) | Control group (N = 26) |
|---|---|---|---|
| Sex, male | 30 (61.2%) | 12 (52.2%) | 18 (69.2%) |
| Agea | 64.6 (45.3, 75.0) | 54.6 (38.2, 74.2) | 68.6 (62.1, 76.4) |
| Patient has caregiver | 8 (16.3%) | 1 (4.4%) | 7 (26.9%) |
| Medical services in 3 months prior to admission | |||
| ED visit | 29 (59.2%) | 15 (65.2%) | 14 (53.8%) |
| Hospitalization | 10 (20.4%) | 5 (21.7%) | 5 (19.2%) |
| Discharge prescription | |||
| # of prescribed medicationsa | 15.0 (11.0, 18.0) | 13.0 (8.0, 16.0) | 15.5 (11.0, 19.0) |
| # of continued medicationsa | 9.0 (5.0, 13.0) | 6.0 (4.0, 12.0) | 11.5 (6.0, 14.0) |
| # of medication changesa | 7.0 (4.0, 10.0) | 6.0 (4.0, 11.0) | 7.0 (3.0, 9.0) |
| # of new medicationsa | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) | 3.0 (1.0, 5.0) |
| # of dose changesa | 1.0 (1.0, 2.0) | 1.0 (0.0, 2.0) | 1.0 (1.0, 2.0) |
| # of discontinued medicationsa | 2.0 (1.0, 3.0) | 2.0 (0.0, 3.0) | 2.0 (1.0, 3.0) |
| # of medications dispensed in 30 days postdischargea,b | 11.0 (6.0, 16.0) | 12.0 (5.0, 15.0) | 11.0 (7.0, 17.0) |
Abbreviations: ED: Emergency Department.
Results presented as median (interquartile range).
If a medication was dispensed more than once in the 30-days post-discharge, it was counted only once.
Study participants were prescribed a median of 15.0 (IQR 11.0–18.0) medications at discharge, with a median of 7.0 (IQR 4.0–10.0) changes made to their medication regimen. Of medication changes, a median 3.0 (IQR 1.0–5.0) were new medications, 1.0 (IQR 1.0–2.0) were dose changes, and 2.0 (IQR 1.0–3.0) were discontinued medications. Intervention patients were prescribed a slightly lower median number of medications at discharge (13.0) compared to control patients (15.5). There was little difference between intervention and control patients in the median number of medication changes per patient (6.0 vs 7.0, respectively), including new medications (3.0 vs 3.0), dose changes (1.0 vs 1.0), and discontinued medications (2.0 vs 2.0).
In the 30 days following hospital discharge, intervention and control patients were dispensed a similar median number of medications (12.0 and 11.0, respectively).
Adherence to medication changes
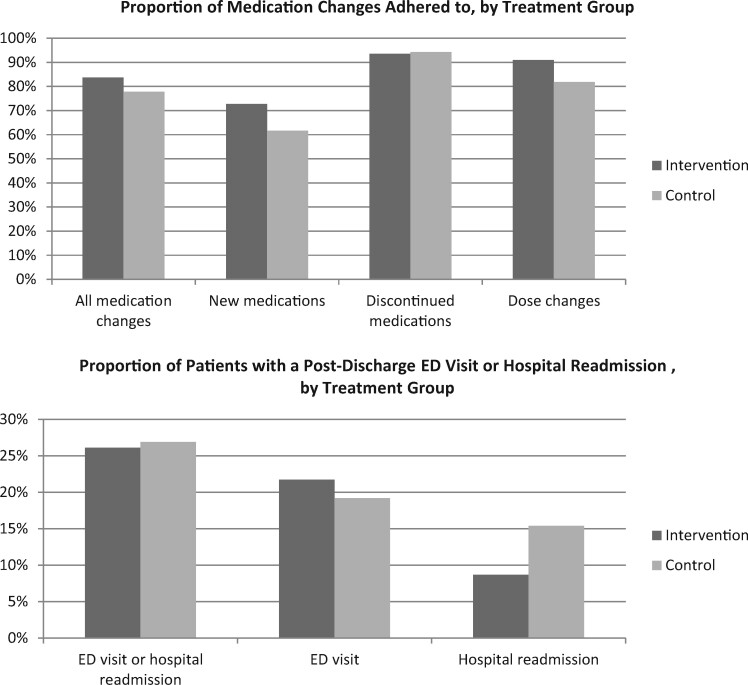
In the 30 days following hospital discharge, a larger proportion of medication changes were adhered to in the intervention group (83.7%) compared to the control group (77.8%) (Table 3, Figure 4). Similar findings were observed for newly prescribed medications (72.7% dispensed in the intervention group vs 61.7% dispensed in the control group) and dose changes (90.9% dispensed at correct daily dose in the intervention group vs 81.8% in the control group). There was little difference between intervention and control groups in the proportion of discontinued medications not refilled (93.5% vs 94.3%, respectively).
Table 3.
Adherence to medication changes and adverse events in 30 days post-discharge, by treatment group
| Proportion of medication changes adhered to, n/N (%) | |||
|---|---|---|---|
| Medication change | Overall | Intervention | Control |
| All changes | 208/258 (80.6%) | 103/123 (83.7%) | 105/135 (77.8%) |
| New medicationsa | 77/115 (67.0%) | 40/55 (72.7%) | 37/60 (61.7%) |
| Discontinued medicationsa | 93/99 (93.9%) | 43/46 (93.5%) | 50/53 (94.3%) |
| Dose changesb | 38/44 (86.4%) | 20/22 (90.9%) | 18/22 (81.8%) |
|
| |||
| Number of patients (%) experiencing adverse events | |||
|
| |||
| Adverse event | Overall (N = 49) | Intervention (N = 23) | Control (N = 26) |
|
| |||
| ED visit or hospital readmission | 13 (26.5%) | 6 (26.1%) | 7 (26.9%) |
| ED visit | 10 (20.4%) | 5 (21.7%) | 5 (19.2%) |
| Hospital readmission | 6 (12.2%) | 2 (8.7%) | 4 (15.4%) |
Abbreviations: ED: Emergency Department.
Adherence was assessed for new and discontinued medications that are covered by the RAMQ and, in the case of new medications, for those not prescribed “as needed”.
Adherence was assessed for dose changes that were dispensed in the 30 days following hospital discharge.
Figure 4.
Adherence to medication changes and adverse event rates, by treatment group.
ED visits and hospital readmissions
In the 30 days following hospital discharge, 13 (26.5%) patients had an ED visit or hospital readmission, 6 (26.1%) of whom were in the intervention group, and 7 (26.9%) who were in the control group (Table 3, Figure 4). While there was little difference between treatment groups in the proportion of patients who had an ED visit (21.7% of intervention patients, 19.2% of control patients), a smaller proportion of intervention patients (8.7%) were readmitted to hospital than control patients (15.4%).
SAM app and pharmacist dashboard utilization
Of the 23 intervention patients, 15 (65.2%) accessed and used the SAM app in the 30 days following hospital discharge (Table 4). Patients who used SAM conducted a median of 16.0 (IQR 8.0–31.0) actions in the app. The side effect checker was the most frequently used feature, having been accessed a median 9.0 times per user. Other frequently used features included the pharmacist connect feature (median 2.0 messages per user) and the drug information leaflets (median 3.0 times per user).
Table 4.
Utilization of SAM, overall and by feature, and of the pharmacist dashboard
| Overall utilization of SAM | |
|---|---|
| n/N (%) | |
| Intervention patients who used SAM | 15/23 (65.2%) |
|
| |
| Utilization, by feature, among SAM users (N = 15) | |
|
| |
| SAM feature | Median (IQR) # of times accessed per user |
|
| |
| Any feature | 16.0 (8.0, 31.0) |
| Side effect checker | 9.0 (2.0, 23.0) |
| Drug information leaflet | 3.0 (1.0, 4.0) |
| Message pharmacist | 2.0 (0.0, 6.0) |
| Review of medication | 1.0 (0.0, 1.0) |
| Interaction checker | 0.0 (0.0, 0.0) |
| Alert resolutiona | 0.0 (0.0, 1.0) |
|
| |
| Utilization of pharmacist dashboard | |
|
| |
| Pharmacist dashboard feature | Total # of times accessed |
|
| |
| Message patient | 86 |
| Alert resolutionb | 66 |
Alert resolution refers to the selection, from a dropdown menu, of an option explaining why the patient has not yet filled a prescribed medication, refilled a discontinued medication, or filled a medication at the wrong daily dose.
Alert resolution refers to the pharmacist’s documentation of the result of follow-up of an adherence alert with a patient.
Over the 30-day post-discharge period, hospital pharmacists conducted a total 152 actions in the pharmacist dashboard (Table 4); 86 of these consisted of sending patient and caregiver users a message through the app and 66 consisted of documenting the results of a follow-up the pharmacist initiated with a SAM user in response to an adherence alert.
DISCUSSION
In this pilot RCT, SAM was used by the majority (65%) of intervention patients following hospital discharge. An important overlap exists between the 8 intervention patients who never used the app and the 2 who, for logistical reasons, were not trained in the use of SAM. This could explain why these 2 intervention patients did not use SAM. It is also important to note that it is typical for a certain proportion of individuals who download an app to never use it. Although comparable utilization statistics are lacking, one survey of mobile health app use among older adults indicates that 24% of individuals who downloaded a health app never used it.52 This could be due to the large number of apps that smartphone users typically download and across which their attention is split. In the survey, 58.9% of individuals who had a health app had at least 11 other apps on their smartphone.52 These data support the notion that SAM uptake in this pilot was consistent with typical health app use, which supports the feasibility of future, larger evaluations of SAM. This is despite the relatively low recruitment rate, driven primarily by a low eligibility rate (19%). Our pilot was hindered by other ongoing trials at study sites, which for months restricted our access to patients over the age of 65, who are much more likely to meet the eligibility criterion of possessing public prescription drug insurance. We expect that in a larger trial unhindered by these restrictions, we will be able to recruit study participants at a reasonably faster rate.
Intervention patients who used SAM post-discharge conducted a median of 16 actions in the app, most frequently to check medication side effects, view drug information leaflets, and message hospital pharmacists. Given that SAM is one of very few apps that both display a comprehensive list of medications and have been evaluated, similar data on app use against which we can compare these utilization rates are lacking. However, we can note 2 things. First, our utilization rates do not consider the action of simply viewing the list of prescribed and dispensed medications, as this action was not recorded in our databases. The estimated median of 16 actions per user therefore likely underestimates the actual use of SAM. Second, when interpreting usage rates, it is sometimes more useful to do so in the context of the number of newly prescribed medications, rather than overall medications. This is because patients are more likely to seek information about new medications than those they have been taking for a while. For intervention patients, the median number of new medications at discharge was 3. In this context, a median of 9 actions per user in the side effect checker, for example, seems reasonable.
Our results also suggest that, compared to control patients, intervention patients adhered to a larger proportion of medication changes made at discharge, including newly prescribed medications and dose changes. In addition, although a similar proportion of intervention and control patients had an ED visit post-discharge, a smaller proportion of intervention patients were readmitted to hospital compared to control patients. It is important to note that due to the small sample size of this pilot, no conclusions can be drawn about the effect of SAM on adherence and adverse events. Indeed, this pilot had less than 10% power to detect clinically relevant differences of 5% in adherence rates between treatment groups. However, our results do suggest some potential for SAM to enhance medication adherence and reduce the risk of subsequent adverse events. This hypothesis needs to be tested in a larger RCT.
Larger evaluations may in fact reveal a larger impact of SAM on adherence to new medications and dose changes than what the results of this pilot suggest. This is owing to caregivers’ involvement in medication management, which is known to contribute to medication adherence.53,54 In this pilot, a much larger proportion of control patients (26.9%) had a caregiver compared to intervention patients (4.4%). This imbalance may have underestimated the difference in adherence between treatment groups, a bias that would be corrected in a larger RCT. Other potential biases which would also be corrected in a larger evaluation are those resulting from imbalances in age, sex, and the number of medications prescribed at discharge. Intervention patients were younger than control patients, more likely to be female, and had fewer medications prescribed at discharge. This is important to note, as older patients tend to adhere better to medications than younger patients, as do men compared to women, and those who have fewer prescribed medications.5,11,55
Interestingly, a similar proportion of discontinued medications was adhered to in intervention and control groups. One possible explanation for this is that, unlike new medications and dose changes, discontinued medications are not displayed in the app unless they are dispensed, triggering an adherence alert. SAM therefore has the potential to improve adherence to discontinued medications, but perhaps only after they have actually been dispensed. The primary adherence measures used in this study consider a patient who had a discontinued drug dispensed as nonadherent even if they did heed the SAM alert and refrained from taking the drug. This could explain why our results suggest no effect of SAM on discontinued medications. On the other hand, secondary adherence measures, which assess continued adherence, may be more likely to show an effect, if it exists. To enhance SAM’s potential for such an effect, we will develop a feature that shows patients the changes that were made to their medication regimen, including which medications were discontinued, which were modified, and which were newly prescribed.
SAM is not the first mobile application to target medication management, but it is one of very few that have been evaluated. Despite hundreds of previously developed medication management mobile apps, a systematic review published in 2020 by Armitage et al56 shows only 9 such apps have been evaluated in RCTs for success in improving adherence. Although some demonstrated success, SAM has important advantages over this small number of previously evaluated apps. First, SAM is the first to benefit from real-time linkage to pharmacy claims data. This not only allows for the generation of real-time adherence alerts, which itself is novel, but also allows for an objective measurement of adherence. This is in contrast to self-reported measures used in most previous RCTs,56 which overestimated adherence.57,58 In addition, SAM appears to be one of few apps that integrates several advanced features targeting barriers to adherence. Some of the apps reviewed by Armitage et al appear to be basic medication reminder apps.59–61 Others offer additional features such as telephone support,62 adherence tracking,63,64 refill reminders,64 peer support,63 symptom tracking,65 or even advanced AI monitoring.66 However, medication nonadherence is a complex phenomenon arising from a multitude of factors and, unlike SAM, none of these apps appear to take full advantage of the versatility of this medium to target more than 2 or 3 barriers to adherence. Communication with a health-care professional is one example of a feature available in SAM that is a missed opportunity in many others, particularly as it plays an important role in supporting adherence67–69 and is valued by patients and caregivers. Finally, unlike most other apps, SAM generates medication lists based on digitized prescription and dispensation data, rather than relying on tedious manual entry of medications by users. This addresses a gap highlighted in a 2016 study that assessed the quality of adherence apps.70 The authors underlined the need for apps that interface with prescribing or dispensing data sources to generate a medication list, particularly as older users with complex medication regimens represent those most likely to use and benefit from adherence apps.70,71
Most RCTs reviewed in Armitage et al were characterized by small to moderate sample sizes that had limited statistical power to detect improvements in adherence, much less in the risk of downstream health outcomes. To address this gap in current knowledge, we will build on this pilot and conduct large RCTs of SAM among patients discharged from hospital and patients prescribed medications in primary care settings. The latter will be achieved by linking SAM to an electronic prescriber previously developed by our team,72 work which is already in progress. In addition, we will develop a more extensive version of SAM to be tested in these trials. Version 2.0 will include new features such as daily pill reminders and a weekly dosing schedule. We will also incorporate patient preferences into the app via a feature that shows patients alternatives to their medications based on personal preferences regarding medication benefits, adverse effects, and cost. Research has shown that patients differ in their preferences regarding these factors73–83 and that tailoring mobile apps to individual users enhances their success in improving medication adherence.56
Our pilot had some limitations that should be considered. First, secondary adherence was not measured. This could have overestimated adherence to newly prescribed medications, as patients may not have necessarily taken purchased medications as prescribed.6–10 However, adherence is likely to have been overestimated in both treatment groups. Furthermore, we plan to incorporate secondary adherence measures in future evaluations of SAM. This will be done using daily adherence tracking, a new feature that will be developed in SAM, and by obtaining pharmacy claims data over a 6-month follow-up period to measure the Medication Possession Ratio.49 Second, patients randomized to the intervention received training in the use of SAM using their own medications. This potential Hawthorne effect, resulting from the additional focus on medications this group received compared to control patients, could have increased primary adherence among intervention patients and led to an overestimated effect of SAM on adherence. However, we expect this bias to be minimal, particularly as control patients were aware of the purpose and outcomes of the study. A third limitation of this pilot is its potentially limited generalizability, as it was conducted in an older, hospitalized population. However, these individuals represent the patient population with the largest number of prescribed medications, on average, and therefore, at the highest risk of nonadherence and associated adverse outcomes.84–86 Targeting this population is likely to have significant impacts. That said, our team is working on accessing drug dispensation data for individuals who do not necessarily have public prescription drug insurance. This would allow us to assess the effectiveness of SAM across age groups. We also plan to conduct a trial of SAM among patients prescribed medications in primary care, which would also enhance the generalizability of future studies. A final limitation, which also impacts the generalizability of our pilot, is that eligibility was restricted to patients who possessed a smartphone or tablet device with an internet connection. However, this “tech-savvy” patient population represents those who are most likely to download and use SAM if it were made publicly available, and among whom the effect of SAM on adherence and adverse events is therefore most relevant.
CONCLUSION
In this pilot RCT of the SAM mobile application, a high uptake of SAM among intervention patients supports the feasibility of a future, larger trial. Results also suggest that SAM has potential to improve medication adherence and reduce the risk of downstream adverse events. This hypothesis needs to be tested in larger evaluations of SAM.
FUNDING
This work was supported by the Quebec Ministry of Economy, Science and Innovation (MESI) through the Fonds de soutien à l’innovation en santé et services sociaux (FSISSS)—grant number 2-53.
AUTHOR CONTRIBUTIONS
All authors contributed to the concept and design of the study and of the SAM mobile application. RT acquired the data necessary for the SAM app and outcome measures. MT and TT developed the mobile app. MB and BH coordinated the clinical trial. SM and MB recruited patients. BH conducted statistical analyses and drafted the manuscript. All authors provided feedback on the intellectual content of the manuscript and read and approved the final manuscript.
ETHICS APPROVAL
This study was authorized by the Research Ethics Board (REB) of the McGill University Health Centre (MUHC)—study number 2019-4597.
ACKNOWLEDGMENTS
The authors wish to thank the hospital pharmacists at the internal medicine units of the Royal Victoria Hospital and the Montreal General Hospital, whose collaboration was indispensable to the successful implementation of SAM.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared due to privacy and ethical reasons.
REFERENCES
- 1. Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J.. Prevalence of and factors associated with primary medication non-adherence in chronic disease: a systematic review and meta-analysis. Int J Clin Pract 2019; 73 (6): e13350. [DOI] [PubMed] [Google Scholar]
- 2. Lemstra M, Nwankwo C, Bird Y, Moraros J.. Primary nonadherence to chronic disease medications: a meta-analysis. Patient Prefer Adherence 2018; 12: 721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackevicius CA, Li P, Tu JV.. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation 2008; 117 (8): 1028–36. [DOI] [PubMed] [Google Scholar]
- 4. Storm A, Andersen SE, Benfeldt E, Serup J.. One in 3 prescriptions are never redeemed: primary nonadherence in an outpatient clinic. J Am Acad Dermatol 2008; 59 (1): 27–33. [DOI] [PubMed] [Google Scholar]
- 5. Tamblyn R, Eguale T, Huang A, Winslade N, Doran P.. The incidence and determinants of primary nonadherence with prescribed medication in primary care. Ann Intern Med 2014; 160 (7): 441–50. [DOI] [PubMed] [Google Scholar]
- 6. Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA.. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine (Baltimore) 2017; 96 (4): e5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al AlShaikh S, Quinn T, Dunn W, Walters M, Dawson J.. Predictive factors of non-adherence to secondary preventative medication after stroke or transient ischaemic attack: a systematic review and meta-analyses. Eur Stroke J 2016; 1 (2): 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKenzie SJ, McLaughlin D, Clark J, Doi SAR.. The burden of non-adherence to cardiovascular medications among the aging population in Australia: a meta-analysis. Drugs Aging 2015; 32 (3): 217–25. [DOI] [PubMed] [Google Scholar]
- 9. Tuppin P, Neumann A, Danchin N, et al. Evidence-based pharmacotherapy after myocardial infarction in France: adherence-associated factors and relationship with 30-month mortality and rehospitalization. Arch Cardiovasc Dis 2010; 103 (6–7): 363–75. [DOI] [PubMed] [Google Scholar]
- 10. Naderi SH, Bestwick JP, Wald DS.. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med 2012; 125 (9): 882–7.e1. [DOI] [PubMed] [Google Scholar]
- 11. Weir DL, Motulsky A, Abrahamowicz M, et al. Challenges at care transitions: failure to follow medication changes made at hospital discharge. Am J Med 2019; 132 (10): 1216–24.e5. [DOI] [PubMed] [Google Scholar]
- 12. Fallis BA, Dhalla IA, Klemensberg J, Bell CM.. Primary medication non-adherence after discharge from a general internal medicine service. PLoS One 2013; 8 (5): e61735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wooldridge K, Schnipper JL, Goggins K, Dittus RS, Kripalani S.. Refractory primary medication nonadherence: prevalence and predictors after pharmacist counseling at hospital discharge. J Hosp Med 2016; 11 (1): 48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris CM, Sridharan A, Landis R, Howell E, Wright S.. What happens to the medication regimens of older adults during and after an acute hospitalization? J Patient Saf 2013; 9 (3): 150–3. [DOI] [PubMed] [Google Scholar]
- 15. Viktil KK, Blix HS, Eek AK, Davies MN, Moger TA, Reikvam A.. How are drug regimen changes during hospitalisation handled after discharge: a cohort study. BMJ Open 2012; 2 (6): e001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cochrane RA, Mandal AR, Ledger-Scott M, Walker R.. Changes in drug treatment after discharge from hospital in geriatric patients. BMJ 1992; 305 (6855): 694–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes CM. Medication non-adherence in the elderly. Drugs Aging 2004; 21 (12): 793–811. [DOI] [PubMed] [Google Scholar]
- 18. Capoccia K, Odegard PS, Letassy N.. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ 2016; 42 (1): 34–71. [DOI] [PubMed] [Google Scholar]
- 19. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008; 155 (4): 772–9. [DOI] [PubMed] [Google Scholar]
- 20. Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006; 166 (17): 1836–41. [DOI] [PubMed] [Google Scholar]
- 21. Fitzgerald AA, Powers JD, Ho PM, et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J Card Fail 2011; 17 (8): 664–9. [DOI] [PubMed] [Google Scholar]
- 22. Vik SA, Hogan DB, Patten SB, Johnson JA, Romonko-Slack L, Maxwell CJ.. Medication nonadherence and subsequent risk of hospitalisation and mortality among older adults. Drugs Aging 2006; 23 (4): 345–56. [DOI] [PubMed] [Google Scholar]
- 23. Feldman CH, Yazdany J, Guan H, Solomon DH, Costenbader KH.. Medication nonadherence is associated with increased subsequent acute care utilization among medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res 2015; 67 (12): 1712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cutler RL, Fernandez-Llimos F, Frommer M, Benrimoj C, Garcia-Cardenas V.. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open 2018; 8 (1): e016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization (WHO; ); 2003. [Google Scholar]
- 26. Weir DL, Motulsky A, Abrahamowicz M, et al. Failure to follow medication changes made at hospital discharge is associated with adverse events in 30 days. Health Serv Res 2020; 55 (4): 512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kini V, Ho PM.. Interventions to improve medication adherence: a review. JAMA 2018; 320 (23): 2461–73. [DOI] [PubMed] [Google Scholar]
- 28. Conn VS, Ruppar TM.. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Prev Med 2017; 99: 269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014; 2014 (11): CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mistry N, Keepanasseril A, Wilczynski NL, et al. ; Patient Adherence Review Team. Technology-mediated interventions for enhancing medication adherence. J Am Med Inform Assoc 2015; 22 (e1): e177–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gandapur Y, Kianoush S, Kelli HM, et al. The role of mHealth for improving medication adherence in patients with cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes 2016; 2 (4): 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conn VS, Ruppar TM, Chase J-AD, Enriquez M, Cooper PS.. Interventions to improve medication adherence in hypertensive patients: systematic review and meta-analysis. Curr Hypertens Rep 2015; 17 (12): 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Náfrádi L, Galimberti E, Nakamoto K, Schulz PJ.. Intentional and unintentional medication non-adherence in hypertension: the role of health literacy, empowerment and medication beliefs. J Public Health Res 2016; 5 (3): 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson M. Mobile Technology and Home Broadband 2019. Washington, DC: Pew Research Center; 2019.
- 35. Rathbone AL, Prescott J.. The use of mobile apps and SMS messaging as physical and mental health interventions: systematic review. J Med Internet Res 2017; 19 (8): e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dayer L, Heldenbrand S, Anderson P, Gubbins PO, Martin BC.. Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc (2003) 2013; 53 (2): 172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed I, Ahmad NS, Ali S, et al. Medication adherence apps: review and content analysis. JMIR Mhealth Uhealth 2018; 6 (3): e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park JYE, Li J, Howren A, Tsao NW, De Vera M.. Mobile phone apps targeting medication adherence: quality assessment and content analysis of user reviews. JMIR mHealth Uhealth 2019; 7 (1): e11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Santo K, Richtering SS, Chalmers J, Thiagalingam A, Chow CK, Redfern J.. Mobile phone apps to improve medication adherence: a systematic stepwise process to identify high-quality apps. JMIR mHealth Uhealth 2016; 4 (4): e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haase J, Farris KB, Dorsch MP.. Mobile applications to improve medication adherence. Telemed J E Health 2017; 23 (2): 75–9. [DOI] [PubMed] [Google Scholar]
- 41. Ng R, Carter SR, El-Den S.. The impact of mobile applications on medication adherence: a systematic review. Transl Behav Med 2019; 10 (6): 1419–1435. [DOI] [PubMed] [Google Scholar]
- 42. DiMatteo MR, Haskard-Zolnierek KB, Martin LR.. Improving patient adherence: a three-factor model to guide practice. Health Psychology Review 2012; 6 (1): 74–91. [Google Scholar]
- 43. Gadkari AS, McHorney CA.. Medication nonfulfillment rates and reasons: narrative systematic review. Curr Med Res Opin 2010; 26 (3): 683–705. [DOI] [PubMed] [Google Scholar]
- 44. Kennedy J, Tuleu I, Mackay K.. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm 2008; 14 (6): 553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eibergen L, Janssen MJA, Blom L, Karapinar-Çarkit F.. Informational needs and recall of in-hospital medication changes of recently discharged patients. Res Social Adm Pharm 2018; 14 (2): 146–52. [DOI] [PubMed] [Google Scholar]
- 46. Birkelund R, Larsen LS.. Patient–patient interaction—caring and sharing. Scand J Caring Sci 2013; 27 (3): 608–15. [DOI] [PubMed] [Google Scholar]
- 47. Larsen LS, Larsen BH, Birkelund R.. A companionship between strangers—the hospital environment as a challenge in patient–patient interaction in oncology wards. J Adv Nurs 2014; 70 (2): 395–404. [DOI] [PubMed] [Google Scholar]
- 48. Sarasohn-Kahn J. The Wisdom of Patients: Health Care Meets Online Social Media. Oakland, CA: California HealthCare Foundation; 2008. [Google Scholar]
- 49. Raebel MA, Schmittdiel J, Karter AJ, Konieczny JL, Steiner JF.. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care 2013; 51 (8 Suppl 3): S11–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hohl CM, Kuramoto L, Yu E, Rogula B, Stausberg J, Sobolev B.. Evaluating adverse drug event reporting in administrative data from emergency departments: a validation study. BMC Health Serv Res 2013; 13 (1): 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hohl CM, Robitaille C, Lord V, et al. Emergency physician recognition of adverse drug-related events in elder patients presenting to an emergency department. Acad Emerg Med 2005; 12 (3): 197–205. [DOI] [PubMed] [Google Scholar]
- 52. Rasche P, Wille M, Bröhl C, et al. Prevalence of health app use among older adults in Germany: National Survey. JMIR mHealth Uhealth 2018; 6 (1): e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Trivedi RB, Bryson CL, Udris E, Au DH.. The influence of informal caregivers on adherence in COPD patients. Ann Behav Med 2012; 44 (1): 66–72. [DOI] [PubMed] [Google Scholar]
- 54. Aggarwal B, Liao M, Mosca L.. Medication adherence is associated with having a caregiver among cardiac patients. Ann Behav Med 2013; 46 (2): 237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ.. Patient characteristics associated with medication adherence. Clin Med Res 2013; 11 (2): 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Armitage LC, Kassavou A, Sutton S.. Do mobile device apps designed to support medication adherence demonstrate efficacy? A systematic review of randomised controlled trials, with meta-analysis. BMJ Open 2020; 10 (1): e032045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M.. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. PharmacoEconomics 2010; 28 (12): 1097–107. [DOI] [PubMed] [Google Scholar]
- 58. Gallagher BD, Muntner P, Moise N, Lin JJ, Kronish IM.. Are two commonly used self-report questionnaires useful for identifying antihypertensive medication nonadherence? J Hypertens 2015; 33 (5): 1108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hammonds T, Rickert K, Goldstein C, et al. Adherence to antidepressant medications: a randomized controlled trial of medication reminding in college students. J Am Coll Health 2015; 63 (3): 204–8. [DOI] [PubMed] [Google Scholar]
- 60. Mertens A, Brandl C, Miron-Shatz T, et al. A mobile application improves therapy-adherence rates in elderly patients undergoing rehabilitation: A crossover design study comparing documentation via iPad with paper-based control. Medicine (Baltimore) 2016; 95 (36): e4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mira JJ, Navarro I, Botella F, et al. A Spanish Pillbox App for elderly patients taking multiple medications: randomized controlled trial. J Med Internet Res 2014; 16 (4): e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Svendsen MT, Andersen F, Andersen KH, et al. A smartphone application supporting patients with psoriasis improves adherence to topical treatment: a randomized controlled trial. Br J Dermatol 2018; 179 (5): 1062–71. [DOI] [PubMed] [Google Scholar]
- 63. Morawski K, Ghazinouri R, Krumme A, et al. Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP Randomized Clinical Trial. JAMA Intern Med 2018; 178 (6): 802–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santo K, Singleton A, Rogers K, et al. Medication reminder applications to improve adherence in coronary heart disease: a randomised clinical trial. Heart 2019; 105 (4): 323–9. [DOI] [PubMed] [Google Scholar]
- 65. Lakshminarayana R, Wang D, Burn D, et al. Using a smartphone-based self-management platform to support medication adherence and clinical consultation in Parkinson's disease. NPJ Parkinsons Dis 2017; 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A.. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke 2017; 48 (5): 1416–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Young HN, Len-Rios ME, Brown R, Moreno MM, Cox E.. How does patient-provider communication influence adherence to asthma medications? Patient Educ Couns 2017; 100 (4): 696–702. [DOI] [PubMed] [Google Scholar]
- 68. Chiang N, Guo M, Amico KR, Atkins L, Lester RT.. Interactive two-way mHealth interventions for improving medication adherence: an evaluation using the behaviour change wheel framework. JMIR mHealth Uhealth 2018; 6 (4): e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Finitsis DJ, Pellowski JA, Johnson BT.. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One 2014; 9 (2): e88166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Heldenbrand S, Martin BC, Gubbins PO, et al. Assessment of medication adherence app features, functionality, and health literacy level and the creation of a searchable Web-based adherence app resource for health care professionals and patients. J Am Pharm Assoc (2003) 2016; 56 (3): 293–302. [DOI] [PubMed] [Google Scholar]
- 71. Becker S, Brandl C, Meister S, et al. Demographic and health related data of users of a mobile application to support drug adherence is associated with usage duration and intensity. PLoS One 2015; 10 (1): e0116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tamblyn R, Huang A, Kawasumi Y, et al. The development and evaluation of an integrated electronic prescribing and drug management system for primary care. J Am Med Inform Assoc 2006; 13 (2): 148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abraham NS, Naik AD, Street RL Jr, et al. Complex antithrombotic therapy: determinants of patient preference and impact on medication adherence. Patient Prefer Adherence 2015; 9: 1657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hauber AB, Mohamed AF, Johnson FR, Falvey H.. Treatment preferences and medication adherence of people with Type 2 diabetes using oral glucose-lowering agents. Diabet Med 2009; 26 (4): 416–24. [DOI] [PubMed] [Google Scholar]
- 75. Unson CG, Siccion E, Gaztambide J, Gaztambide S, Mahoney Trella P, Prestwood K.. Nonadherence and osteoporosis treatment preferences of older women: a qualitative study. J Womens Health (Larchmt) 2003; 12 (10): 1037–45. [DOI] [PubMed] [Google Scholar]
- 76. Florek AG, Wang CJ, Armstrong AW.. Treatment preferences and treatment satisfaction among psoriasis patients: a systematic review. Arch Dermatol Res 2018; 310 (4): 271–319. [DOI] [PubMed] [Google Scholar]
- 77. Moia M, Mantovani LG, Carpenedo M, et al. Patient preferences and willingness to pay for different options of anticoagulant therapy. Intern Emerg Med 2013; 8 (3): 237–43. [DOI] [PubMed] [Google Scholar]
- 78. Laba TL, Essue B, Kimman M, Jan S.. Understanding patient preferences in medication nonadherence: a review of stated preference data. Patient 2015; 8 (5): 385–95. [DOI] [PubMed] [Google Scholar]
- 79. Bulcun E, Ekici M, Ekici A.. Assessment of patients' preferences regarding the characteristics associated with the treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014; 9: 363–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B.. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence 2019; 13: 1253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Svedsater H, Leather D, Robinson T, Doll H, Nafees B, Bradshaw L.. Evaluation and quantification of treatment preferences for patients with asthma or COPD using discrete choice experiment surveys. Respir Med 2017; 132: 76–83. [DOI] [PubMed] [Google Scholar]
- 82. Andersen KM, Kelly A, Lyddiatt A, et al. Patient perspectives on DMARD safety concerns in rheumatology trials: results from inflammatory arthritis patient focus groups and OMERACT Attendees Discussion. J Rheumatol 2019; 46 (9): 1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Smith MY, Hammad TA, Metcalf M, et al. Patient engagement at a tipping point-the need for cultural change across patient, sponsor, and regulator stakeholders: insights from the DIA Conference, “Patient Engagement in Benefit Risk Assessment Throughout the Life Cycle of Medical Products”. Ther Innov Regul Sci 2016; 50 (5): 546–53. [DOI] [PubMed] [Google Scholar]
- 84. Morin L, Johnell K, Laroche M-L, Fastbom J, Wastesson JW.. The epidemiology of polypharmacy in older adults: register-based prospective cohort study. CLEP 2018; 10: 289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Stoehr GP, Lu SY, Lavery L, et al. Factors associated with adherence to medication regimens in older primary care patients: the Steel Valley Seniors Survey. Am J Geriatr Pharmacother 2008; 6 (5): 255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pasina L, Brucato AL, Falcone C, et al. Medication non-adherence among elderly patients newly discharged and receiving polypharmacy. Drugs Aging 2014; 31 (4): 283–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article cannot be shared due to privacy and ethical reasons.