Abstract
The incidence of autoimmunity is growing rapidly worldwide. Many epidemiological studies have found environmental factors, such as toxic chemicals (persistent organic pollutants, toxic metals, solvents, endocrine disruptors), to be a key factor in this rapid progression. Numerous mechanisms have been identified that can cause immune dysregulation and autoimmune reactivity from toxic chemical exposure to subsets of individuals who have genetic susceptibility in immune regulatory genes. In susceptible genotypes, toxic chemicals can induce epigenetic expressions, bind to immune and endocrine receptors throughout the body and promote immune dysregulation, bind to nucleic acids and promote anti-nuclear autoimmunity, deplete antioxidant reserves, promote immune barrier degradation, induce lymphocyte dysregulation, and alter normal antigen-presenting responses. This paper provides a detailed review of the specific immunological pathways involved with exposure to environmental toxins and autoimmunity.
Introduction
A recent epidemiological systematic review identified a 19.1% net % increased /year incidence and prevalence of autoimmune disease year in the past 30 years. It also reported a stronger influence of environmental factors, as opposed to genetic variables, on the development of autoimmune disease.1 The increase in autoimmune disease prevalence and incidence rates also have corresponding data with environmental toxic load exposure in individuals living in industrialized countries.2 Studies with twin subjects have found that genetics can only account for increased susceptibility to autoimmune diseases and that an environmental trigger is necessary to turn on the genetic expression of these diseases. These current findings suggest that environmental chemicals may be a significant factor in the development of autoimmunity in subsets of susceptible individuals.3
Persistent organic pollutants, toxic metals, solvents, and endocrine disruptors are now ubiquitous in our food, drinking water, household products, and even in the air we breathe. The human population has daily exposures to many toxic chemicals (Table). Substances such as mercury, aluminum, dioxin, pesticides, asbestos, trichlorethylene, and many other industrial and environmental toxins have been associated with autoimmunity in both animal and human models.4 These chemicals can induce oxidative stress, T cell dysregulation, and alterations of immune cell messenger systems.5,6
Table.
Common Chemicals of Exposure With Exposure Sources
| Chemical Category | Chemicals | Common Exposure Sources |
|---|---|---|
| Persistent Organic Pollutants | Dichloro-diphenyl-trichloroethane (DDT), aldrin, chlordane, tetrabromobisphenol A, dieldrin, endrin, heptachlor, mirex, polychlorinated biphenyls, hexachlorobenzene, dioxins and furans | Food, water, air, soil, flame retardants, breast milk |
| Toxic Solvents | Acetone, butanol, toluene, methanol, benzene, carbon tetrachloride, trichloroethylene, xylene | Paint, lacquer, glue, cleaning supplies, industrial soap, degreaser, paint stripper, ink, synthetic rubber |
| Toxic Heavy Metals | Mercury, lead, cadmium, arsenic | Food, water, air, dental amalgams, cosmetics |
| Endocrine Disruptors | Bisphenols, dioxins, phthalates, perchlorates, polychlorinated biphenyls, polybrominated diphenyl ethers | Plastic products, herbicides, pesticides, water, food, flame retardants, lining of cans, coating of paper plates |
Genetic susceptibility is a key factor in the development of autoimmune diseases. Genome-wide association studies have identified numerous polymorphisms for autoimmune disease development involving the regulatory regions of genes involved with immune responses.7 The strongest associations found with autoimmune susceptibility to environmental chemicals involve the human leukocyte antigen (HLA) alleles and cytokine receptor IL-23R cytokine receptor polymorphisms.8,9 However, the contribution of a single gene polymorphism leading to the development of an autoimmune disease is very small. There appears to be a combination of polymorphisms involved with autoimmune susceptibility in combination with environmental triggers, such as a toxic chemical to either initiate the development of autoimmune disease or to propagate inflammatory autoimmune responses.10
This paper provides a review of the key immunological mechanisms involved with exposure to toxic chemicals (persistent organic pollutants, toxic metals, solvents, etc.) and autoimmune disease development in genetically susceptible individuals. These autoimmune-mediated mechanisms that develop from toxic chemical exposure include chemical binding with aryl hydrocarbons, alteration of epigenetic mechanisms, depletion of glutathione levels, breakdown of immune barrier systems, chemical binding to nucleic acids, immune dysregulation, and neoantigen formation from chemicals binding to proteins and post-translation modifications of proteins (Figure). A multi-variate combination of these factors, in combination with a mix of susceptible polymorphisms, are theorized to play a role in the development of autoimmunity due to toxic chemical exposure.
Figure.
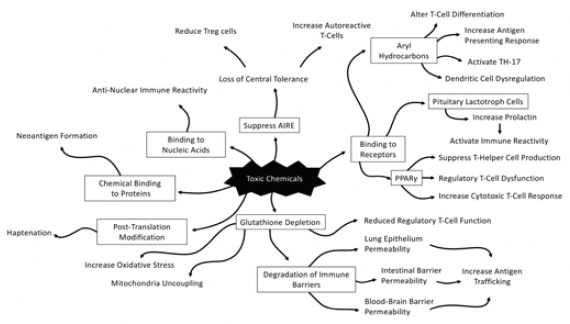
Physiological Pathways from Exposure to Environmental Toxins and Autoimmune Conditions
Chemicals and Epigenetic Expression of DNA Methylation In Autoimmunity
Environmental chemicals have the ability to impact epigenetic mechanisms of histone modifications, microribonucleic acid (RNA) gene expression, and deoxyribonucleic acid (DNA) methylation. The role of epigenetic expression has been best identified in monozygotic twins in which identical DNA sequences express discordance rates in various autoimmune diseases.11,12 Environmentally triggered epigenetic expressions impact various immunological pathways, including the modification of T cell differentiation involved with phenotypic autoimmune expression.13 Environmental chemicals also interfere with methyl group transfers, resulting in anomalous DNA expression.14 These expressions are thought to be attributed to epigenetic drifts that occur due to small differences or defects in the expression of information that occur through cell divisions from exposure to environmental factors.15 Polymorphisms involving the genes that regulate immunological pathways in combination with environmental triggers can result in the phenotypic expression of autoimmunity. Therefore, toxic chemicals have the ability to alter epigenetic expressions, leading to immune dysregulation and development of autoimmunity.
Chemicals Binding to Aryl Hydrocarbon Receptors (AHR)
Autoimmune diseases are modulated by aryl hydrocarbon receptors (AHR) that occur both endogenously and exogenously from environmental chemicals. Ligand binding to AHRs modulates T cell differentiation and regulatory T cell function; it also contributes to antigen-presenting cell responses, and is the primary pathway for activation of the T-helper (TH-17) response.16 Chemicals such as dioxin, polychlorinated bisphenol, and particulate matter mixtures can directly act as ligands for AHR and activate the expression of these receptors.17 These reactions can trigger autoimmune reactivity due to its impact on T cell differentiation and activation of the TH-17 pathway.18 AHR activation by toxic chemicals is a fundamental mechanism in autoimmune development.
Chemical Depletion of Glutathione and Antioxidants
Increased oxidative stress and depletion of antioxidant activity is a key metabolic feature of autoimmune diseases such as systemic lupus erythematosus (SLE).19 Glutathione is a tripeptide antioxidant occurring within most body cells and plays an essential role in quenching oxidative stress, supporting hepatic biotransformation, and serving as a critical immune modulator.20 Mild depletion of intracellular glutathione has a profound impact on lymphocyte function and immune modulation.21 Glutathione activity is essential for proper regulatory T cell function and has a direct role in autoimmune inflammatory T cell responses.22 Therapeutic strategies to prevent glutathione depletion have been effective in treating autoimmune rheumatic diseases.23 Low-dose exposures to persistent organic pollutants have been linked with glutathione depletion, mitochondrial dysfunction, and immune activation.24 Depletion of glutathione is a key variable in how toxic chemical exposures impact autoimmunity.
Chemicals and Autoimmune Regulator Protein (AIRE)
Autoimmune regulator (AIRE) protein is an essential transcriptional regulator of thymocytes. It is involved with modulation of central immune tolerance by eliminating autoreactive T cells and by inducing production of regulatory T cells.25 Dysfunction in AIRE expression leads to phenotypic expression of autoimmune diseases.26 Animals exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) have been found to lose immune tolerance to self-tissue and have reduced levels of AIRE in thymocytes.27,28 Environmental chemical disruption of AIRE is an important mechanism in the way toxic chemicals disrupt central immune tolerance.
Chemicals Binding to Human Serum Albumin
Environmental chemicals can bind to circulating proteins and thereby change the allosteric structure of proteins by inducing protein misfolding and configuring these proteins into neoantigens. A humoral response is generated against these new proteins in the formation of circulating antibodies.29 The most common target protein for chemical binding is albumin found in human serum, because it is the most abundant protein in serum. Chemical binding to albumin has been found to show high correlations with myelin basic protein and myelin oligodendrocyte glycoprotein autoimmunity.30 Chemical binding to albumin has also been found to significantly increase risk for alphasynuclein autoantibody formations.31
Chemicals Causing Protein Modification
Once proteins enter the body, they undergo the process of post-translational modification through chemical processes such as glycosylation, phosphorylation, and lipidation. Proteins also undergo modification by phase I and phase II hepatic biotransformation through processes such as glucuronidation, sulfation, acetylation, etc. These modifications occur in 50% to 90% of proteins in the human body. Environmental chemicals have the ability to alter post-translational modifications and promote immunogenicity of self-proteins.32 These protein modifications play a role in autoimmune reactivity and diseases such as multiple sclerosis and rheumatoid arthritis.33
Chemicals Binding to Nucleic Acids
Nucleic acids are responsible for conveying information from DNA to RNA in eukaryotic cells. Chemicals and drugs possess the potential to bind to DNA, small nuclear ribonucleoproteins, and chromatin and lead to the production of autoantibodies, called anti-nuclear antibodies, as found with drug-induced lupus or SLE.34,35 Chemicals have not only been found to flare up autoimmunity, but they also have the potential to lead to the development of SLE in animal models.36 Various environmental triggers have also been found to skew lupus CD4+ T cells toward autoreactivity from altered genetic expressions. Triggers such as mercury, silica, and cigarette smoke appear to bind and disrupt nucleic acid communication and play a role in autoimmune reactivity.37
Chemicals Acting As Endocrine Disruptors and Autoimmunity
Some chemicals possess the ability to bind to endocrine receptors, thereby competing with or altering the post-translational expression of hormones in the body. These chemicals are classified as endocrine-disrupting chemicals and include polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), bisphenol A (BPA), and dichlorodiphenyltrichloroethane (DDT). Endocrine-disrupting chemicals have the ability to alter several immunological pathways involved with autoimmune reactivity.38 These chemicals can also exert powerful effects on pituitary lactotroph cells and promote increased prolactin release.39 Increased prolactin levels play an immune-activating role with many autoimmune diseases, including Hashimoto’s, SLE, and type 1 diabetes.40 Endocrine-disrupting chemicals also have the ability to bind to estrogen receptors and promote upregulation of dendritic cells, induce B-cell dysfunction, activate T cells, and promote abnormal immune signaling on AHR and peroxisome proliferator-activated nuclear receptors.41-44
Chemicals Causing Regulatory T Cell Dysfunction
Regulatory T cells (Tregs) are essential to maintain immunological tolerance and avoid the development of autoimmune disease.45 There are diverse lists of environmental chemicals that have been shown to activate the peroxisome proliferator-activated receptor gamma (PPARγ) and promote Treg dysregulation.46 Disruption in Treg function by environmental chemicals can lead to suppressive T cell production, exacerbated cytotoxic T cell responses, and mechanisms involving exaggerated antigen presentation responses. These mechanisms may promote autoimmune disease development and propagation of autoimmune pathophysiology.47
Chemicals Causing Dendritic Cell Dysfunction
Intestinal dendritic cells are essentially gatekeepers that are involved in connecting the adaptive and innate immune system in the modulation of immune tolerance.48 Dysregulation of intestinal dendritic cells have been found to play a role in the pathogenesis of autoimmunity.49,50 Exposure to environmental contaminants can alter hydrocarbon receptors on dendritic cells, leading to disruption of immune tolerance and failure to induce the Foxp3 pathway for regulatory T cells.51 Dysfunction in this immunological pathway can lead to aggressive auto-responses and the promotion of autoimmunity.52,53
Chemicals Inducing Permeability of Immune Barriers
Disruption of the intestinal barrier has been found to be a key factor in the development of autoimmune diseases in animal models. The breakdown leads to trafficking intercellular tight junctions of macromolecules capable of inducing an exaggerated immune response in the development of inflammatory and autoimmune reactions.54,55 The interplay between the gut-associated lymphoid tissue in the intestinal epithelial barrier has been found to play a significant role in controlling the equilibrium between immune tolerance and immunity to antigens and haptens.56
Chemical exposure to the gastrointestinal microbiome induces dysbiosis and a reduction in occludin tight junction proteins with a resultant pattern of intestinal permeability, endotoxemia, and the upregulation of tolllike receptor 4 (TLR4), leading to systemic inflammatory responses in both the small intestine and brain of animals.57 In addition to the intestinal barrier, chemicals have been shown to cause disruption in the blood-brain barrier and pulmonary endothelial barrier. Animals exposed to cadmium in drinking water for 90 days were found to develop a reduction in antioxidant enzyme activity of superoxide dismutase, glutathione peroxidase, glutathione reductase, and catalase within microvessel preparations that led to disruption of the blood-brain barrier.58 Cigarette smoke has been found to disrupt the pulmonary epithelial barrier and cause alterations of alveolar micro-circulatory cytoskeletal arrangements.59 The key mechanism involved with barrier disruption with chemicals involves depletion of antioxidant reserves within the barrier systems, leading to oxidative stress and permeability.60 Chemical-induced barrier permeability may be a key factor in the promotion of autoimmune disease development.
Conclusion
Toxic environmental chemicals (persistent organic pollutants, toxic metals, solvents, and endocrine disruptors) induce several physiological mechanisms that lead to autoimmunity (Figure). These chemicals can bind to gamma aryl hydrocarbon and peroxisome proliferator-activated receptors, leading to abnormal antigen-presenting responses and lymphocyte dysregulation. Toxic chemicals that possess endocrine-disrupting properties can bind to estrogen and pituitary receptors, leading to immune dysfunction. In addition to directly binding to receptors throughout the body, toxic chemicals can bind directly to nucleic acids and promote the activation of anti-nuclear antibodies, or simply bind to circulating proteins found throughout the body and induce protein misfolding, thereby configuring proteins into neoantigens. These mechanisms all contribute to direct immune activation and dysregulation. Toxic chemicals can also induce epigenetic expressions of DNA, which leads to autoimmunity. Lastly, toxic chemicals deplete antioxidants such as glutathione and promote immune dysregulation and deterioration of immune barriers, including the blood-brain barrier, the intestinal barrier, and the pulmonary epithelial barrier. The combination of these physiological alterations, in combination with genotypes that have polymorphisms in immune regulatory regions, leads to autoimmune reactivity or autoimmune disease development
Footnotes
Conflicts Of Interest
The author declares no conflicts of interest involved with this manuscript.
References
- 1.Lerner A, Jeremias J, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2015;3(4):151-155. [Google Scholar]
- 2.Parks CG, Miller FW, Pollard KM, et al. Expert panel workshop consensus statement on the role of the environment in the development of autoimmune disease. Int J Mol Sci. 2014;15(8):14269-14297. PMID:25196523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crinnion WJ. Environmental medicine, part one: the human burden of environmental toxins and their common health effects. Altern Med Rev. 2000;5(1):52-63. PMID:10696119 [PubMed] [Google Scholar]
- 4.Pollard KM, Hultman P, Kono DH. Toxicology of autoimmune diseases. Chem Res Toxicol. 2010;23(3):455-466. PMID:20078109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floreani A, Leung PS, Gershwin ME. Environmental basis of autoimmunity. Clin Rev Allergy Immunol. 2016;50(3):287-300. PMID:25998909 [DOI] [PubMed] [Google Scholar]
- 6.Kreitinger JM, Beamer CA, Shepherd DM. Environmental immunology: lessons learned from exposure to a select panel of immunotoxicants. J Immunol. 2016;196(8):3217-3225. PMID:27044635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marson A, Housley WJ, Hafler DA. Genetic basis of autoimmunity. J Clin Invest. 2015;125(6):2234-2241. PMID:26030227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marson A, Housley WJ, Hafler DA. Genetic basis of autoimmunity. J Clin Invest. 2015;125(6):2234-2241. PMID:26030227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho JH, Feldman M. Heterogeneity of autoimmune diseases: pathophysiologic insights from genetics and implications for new therapies. Nat Med. 2015;21(7):730-738. PMID:26121193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. 2015;125(6):2228-2233. PMID:25893595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang Z, Yang Y, Chang C, Lu Q. The epigenetic mechanism for discordance of autoimmunity in monozygotic twins. J Autoimmun. 2017;83:43-50. PMID:28412046 [DOI] [PubMed] [Google Scholar]
- 12.Blossom SJ, Gilbert KM. Epigenetic underpinnings of developmental immunotoxicity and autoimmune disease. Curr Opin Toxicol. 2018;10:23-30. PMID:30613805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16(5):649-660. PMID:12049717 [DOI] [PubMed] [Google Scholar]
- 14.Hossain MB, Vahter M, Concha G, Broberg K. Low-level environmental cadmium exposure is associated with DNA hypomethylation in Argentinean women. Environ Health Perspect. 2012;120(6):879-884. PMID:22382075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacFarlane AJ, Strom A, Scott FW. Epigenetics: deciphering how environmental factors may modify autoimmune type 1 diabetes. Mamm Genome. 2009;20(9-10):624-632. PMID:19697079 [DOI] [PubMed] [Google Scholar]
- 16.O’Driscoll CA, Mezrich JD. The aryl hydrocarbon receptor as an immune-modulator of atmospheric particulate matter-mediated autoimmunity. Front Immunol. 2018;9:2833. PMID:30574142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrysík Z, Vondráček J, Marvanová S, et al. Activation of the aryl hydrocarbon receptor is the major toxic mode of action of an organic extract of a reference urban dust particulate matter mixture: the role of polycyclic aromatic hydrocarbons. Mutat Res. 2011;714(1-2):53-62. PMID:21762708 [DOI] [PubMed] [Google Scholar]
- 18.O’Driscoll CA, Owens LA, Gallo ME, et al. Differential effects of diesel exhaust particles on T cell differentiation and autoimmune disease. Part Fibre Toxicol. 2018;15(1):35. PMID:30143013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perl A, Hanczko R, Lai ZW, et al. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: implications for activation of the mechanistic target of rapamycin. Metabolomics. 2015;11(5):1157-1174. PMID:26366134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pizzorno J. Glutathione! Integr Med (Encinitas). 2014;13(1):8-12. PMID:26770075 [PMC free article] [PubMed] [Google Scholar]
- 21.Dröge W, Breitkreutz R. Glutathione and immune function. Proc Nutr Soc. 2000;59(4):595-600. PMID:11115795 [DOI] [PubMed] [Google Scholar]
- 22.Mak TW, Grusdat M, Duncan GS, et al. Glutathione primes t cell metabolism for inflammation. Immunity. 2017;18;46(4):675-689. doi: 10.1016/j.immuni.2017.03.019. Erratum in: Immunity. 2017 Jun 20;46(6):1089-1090. PMID: 28423341 [DOI] [PubMed] [Google Scholar]
- 23.Perl A. Review: metabolic control of immune system activation in rheumatic diseases. Arthritis Rheumatol. 2017;69(12):2259-2270. PMID:28841779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DH, Jacobs DR, Jr. Hormesis and public health: can glutathione depletion and mitochondrial dysfunction due to very low-dose chronic exposure to persistent organic pollutants be mitigated? J Epidemiol Community Health. 2015;69(3):294-300 PMID:25271248 [DOI] [PubMed] [Google Scholar]
- 25.Zhao B, Chang L, Fu H, Sun G, Yang W. The role of autoimmune regulator (AIRE) in peripheral tolerance. J Immunol Res. 2018;2018:3930750. PMID:30255105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramson J, Husebye ES. Autoimmune regulator and self-tolerance - molecular and clinical aspects. Immunol Rev. 2016;271(1):127-140. PMID:27088911 [DOI] [PubMed] [Google Scholar]
- 27.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287-312. PMID:19302042 [DOI] [PubMed] [Google Scholar]
- 28.Ishimaru N, Takagi A, Kohashi M, et al. Neonatal exposure to low-dose 2,3,7,8-tetrachlorodibenzo-p-dioxin causes autoimmunity due to the disruption of T cell tolerance. J Immunol. 2009;182(10):6576-6586. PMID:19414813 [DOI] [PubMed] [Google Scholar]
- 29.Vojdani A, Kharrazian D, Mukherjee PS. Elevated levels of antibodies against xenobiotics in a subgroup of healthy subjects. J Appl Toxicol. 2015;35(4):383-397. PMID:25042713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kharrazian D, Vojdani A. Correlation between antibodies to bisphenol A, its target enzyme protein disulfide isomerase and antibodies to neuron-specific antigens. J Appl Toxicol. 2017;37(4):479-484. PMID:27610592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharrazian D, Herbert M, Vojdani A. The associations between immunological reactivity to the haptenation of unconjugated bisphenol a to albumin and protein disulfide isomerase with alpha-synuclein antibodies. Toxics. 2019;7(2):E26. PMID:31064082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci. 2005;1050:1-9. PMID:16014515 [DOI] [PubMed] [Google Scholar]
- 33.Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665-673. PMID:29925983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. PMID:9324032 [DOI] [PubMed] [Google Scholar]
- 35.Crowl JT, Gray EE, Pestal K, Volkman HE, Stetson DB. Intracellular nucleic acid detection in autoimmunity. Annu Rev Immunol. 2017;35:313-336. PMID:28142323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leiss H, Niederreiter B, Bandur T, et al. Pristane-induced lupus as a model of human lupus arthritis: evolvement of autoantibodies, internal organ and joint inflammation. Lupus. 2013;22(8):778-792. PMID:23817510 [DOI] [PubMed] [Google Scholar]
- 37.Mak A, Tay SH. Environmental factors, toxicants and systemic lupus erythematosus. Int J Mol Sci. 2014;15(9):16043-16056. PMID:25216337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharrazian D. The potential roles of bisphenol a (BPA) pathogenesis in autoimmunity. Autoimmune Dis. 2014;2014:743616. PMID:24804084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138(5):1780-1786. PMID:9112368 [DOI] [PubMed] [Google Scholar]
- 40.Borba VV, Zandman-Goddard G, Shoenfeld Y. Prolactin and Autoimmunity. Front Immunol. 2018. February 12;9:73. doi: 10.3389/fimmu.2018.00073. PMID: 29483903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40(1):66-73. PMID:20352526 [DOI] [PubMed] [Google Scholar]
- 42.Schooling CM, Zhao J. Estrogenic endocrine disruptors and autoimmune disease. Int J Epidemiol. 2015;44(1):363-364. PMID:24997209 [DOI] [PubMed] [Google Scholar]
- 43.Csaba G. Aromatic hydrocarbon receptors in the immune system: review and hypotheses. Acta Microbiol Immunol Hung. 2019;66(3):273-287. PMID:30803253 [DOI] [PubMed] [Google Scholar]
- 44.Rogers JA, Metz L, Yong VW. Review: Endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol Immunol. 2013;53(4):421-430. PMID:23123408 [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8-27. PMID:16903903 [DOI] [PubMed] [Google Scholar]
- 46.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor γ is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178(5):2940-2949. PMID:17312139 [DOI] [PubMed] [Google Scholar]
- 47.Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665-673. PMID:29925983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waisman A, Lukas D, Clausen BE, Yogev N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol. 2017;39(2):153-163. PMID:27456849 [DOI] [PubMed] [Google Scholar]
- 49.Lebre MC, Tak PP. Dendritic cells in rheumatoid arthritis: which subset should be used as a tool to induce tolerance? Hum Immunol. 2009;70(5):321-324. PMID:19236901 [DOI] [PubMed] [Google Scholar]
- 50.Panda SK, Kolbeck R, Sanjuan MA. Plasmacytoid dendritic cells in autoimmunity. Curr Opin Immunol. 2017;44:20-25. PMID:27855321 [DOI] [PubMed] [Google Scholar]
- 51.Kreitinger JM, Beamer CA, Shepherd DM. Environmental immunology: lessons learned from exposure to a select panel of immunotoxicants. J Immunol. 2016;196(8):3217-3225. PMID:27044635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor γ is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178(5):2940-2949. PMID:17312139 [DOI] [PubMed] [Google Scholar]
- 53.Doyle HA, Mamula MJ. Posttranslational protein modifications: new flavors in the menu of autoantigens. Curr Opin Rheumatol. 2002;14(3):244-249. PMID:11981321 [DOI] [PubMed] [Google Scholar]
- 54.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91(1):151-175. PMID:21248165 [DOI] [PubMed] [Google Scholar]
- 55.Vojdani A, Vojdani E, Kharrazian D. Fluctuation of zonulin levels in blood vs stability of antibodies. World J Gastroenterol. 2017;23(31):5669-5679. PMID:28883692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42(1):71-78. PMID:22109896 [DOI] [PubMed] [Google Scholar]
- 57.Alhasson F, Das S, Seth R, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. PMID:28328972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shukla A, Shukla GS, Srimal RC. Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol. 1996;15(5):400-405. PMID:8735464 [DOI] [PubMed] [Google Scholar]
- 59.Schweitzer KS, Hatoum H, Brown MB, et al. Mechanisms of lung endothelial barrier disruption induced by cigarette smoke: role of oxidative stress and ceramides. Am J Physiol Lung Cell Mol Physiol. 2011;301(6):L836-L846. PMID:21873444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front Biosci. 2008. May 1;13:7210-26. doi: 10.2741/3223. PMID: 18508729 [DOI] [PMC free article] [PubMed] [Google Scholar]


