Abstract
Lewisite is a strong vesicating and chemical warfare agent. Due to rapid transdermal absorption, cutaneous exposure to Lewisite can also elicit severe systemic injury. Lewisite (2.5, 5.0 and 7.5 mg/kg) was applied to skin of Ptch1+/−/SKH-1 mice and acute lung injury (ALI) was assessed after 24h. Arterial blood-gas measurements showed hypercapnia and hypoxemia in the Lewisite-exposed group. Histological evaluation of lung tissue revealed increased levels of proinflammatory neutrophils and a dose-dependent increase in structural changes indicative of injury. Increased inflammation was also confirmed by the altered cytokines expression, including increased IL-33 and a dose-dependent elevation of CXCL1, CXCL5 and GCSF was observed in the lung tissue. In the bronchoalveolar lavage fluid of Lewisite-exposed animals, there was significant increase in HMGB1, a damage associated molecular pattern molecule, as well as elevated CXCL1 and CXCL5, which coincided with neutrophils influx to the lung.. Complete blood cell analysis revealed eosinophilia and altered neutrophil/lymphocyte ratios, as a consequence of Lewisite exposure. Mean platelet volume and RBC distribution width, predictors of lung injury, were also increased in the Lewisite group. These data demonstrate that cutaneous Lewisite exposure causes ALI and may contribute to mortality in exposed populations.
Keywords: Lewisite, Acute lung Injury, Inflammation, Cutaneous, ARDS
Introduction
Lewisite (dichloro (2-chlorovinyl) arsine [C2H2AsCl3]), an organo-arsenic compound, was first introduced as an extremely poisonous chemical with toxicity significantly higher than Sulfur Mustard (HD)1. It is a colorless oily liquid with a smell resembling that of geranium. Owing to its lipophilic nature, Lewisite is rapidly absorbed by the skin, reacts faster and has higher solubility than HD2. Topical exposure to 2.6 g Lewisite can cause significant systemic damage, attributed to severe fluid loss due to capillary leakage (termed as Lewisite shock), and subsequent death over time3. Lewisite is also a potent chemical warfare agent with strong vesicating, erythematous and pain causing properties. There are no confirmed reports of its usage in warfare. However, due to possession of large quantities of this chemical by several nations, threat is posed by its accidental or intentional exposures to mass populations2, 4, 5. Investigations into Lewisite toxicity have focused primarily on its vesicating properties, but eyes and lungs in direct contact with external environment are also susceptible to injury. These assumptions are corroborated by a study carried out in 1970 by Nishitoma et al wherein they surveyed thousands of workers at Lewisite production sites (established before 1945) in the United States (Chicago) and Japan, and reported symptoms of respiratory injury, including chronic bronchitis and cough6, 7. Reduced FEV1/FVC ratio was a more common occurrence in highly exposed workers, as compared to the non-exposed or less exposed ones6. Similarly, contact of Lewisite with eyes causes instant edema and blepharospasm and ultimately superficial opacity8. In addition to effects caused by direct exposure, Lewisite has also been shown to cause acute kidney injury (AKI) upon exposure to skin indicating systemic injury4. Nonetheless, effects of cutaneous Lewisite exposure on the lungs have not been systematically evaluated.
Acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) can occur due to direct or indirect insult caused by toxicants and often results in acute hypoxemia and, under severe conditions, may lead to death9–11. To recognize and characterize ALI/ARDS in experimental animals, a standard criterion requires evaluation of histological evidence of injury, altered alveolar-capillary barrier, inflammatory response and impaired gas exchange12. ALI/ARDS is often experienced as a result of direct exposure to toxic chemicals9, 13. The effects of indirect insult, particularly those causing ALI/ARDS due to cutaneous lesions, is however, not well understood.
Lewisite, due to its rapid absorption through skin could become readily available to all organs through circulation. Lung being vital and the only organ to receive 100% right cardiac output is extremely susceptible to cutaneous Lewisite-induced toxic damage. This could make lungs more vulnerable to secondary microbial infections too. Importantly, due to the lack of any clinical data, management of acute toxic effect of Lewisite would be a major challenge for toxicologists or pulmonologists. Therefore, in the current study, we sought to characterize ALI/ARDS in mice exposed to cutaneous Lewisite.
2. Methods
2.1. Chemicals
Lewisite was prepared and used by personnel at MRIGlobal (Kansas City, MO). All chemicals and reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless mentioned otherwise.
2.2. Laboratory animal procedures
All animal procedures were conducted after approval from the Institutional Animal Care and Use Committees of MRIGlobal and the University of Alabama at Birmingham (UAB). In the current study, adult male and female Ptch1+/−/SKH-1 hairless mice (5–6 weeks) were purchased from Jackson Laboratory (Bar Harbor, ME) and bred at UAB. Animals were provided food and water ad libitum and maintained at 25°C in a 12-hour light/dark cycle room.
2.2. Lewisite exposure
All exposures to Lewisite were performed at the MRIGlobal facility under an approved animal protocol. Lewisite was applied once topically on dorsal skin of mice on a 2×4cm2 rectangular area, as explained elsewhere2. Briefly, prior to lewisite exposure, mice were anesthetized with 100 mg/kg of ketamine and 5–7 mg/kg of xylazine intraperitoneally (i.p.) and buprenorphine as analgesic 0.05 to 0.1 mg/kg. Lewisite was dissolved in 30 μl of ethanol and applied at 2.5 mg/kg, 5 mg/kg and 7.5 mg/kg doses. After 24 hours of exposure, buprenorphine (0.05–0.1mg/kg) was administered i.p., followed by ketamine (150 mg/kg) before further processing. (n=20/group).
2.3. Arterial blood gas (ABG) measurement
To assess pulmonary gas exchange and blood acid-base status, arterial blood gas (ABG) analysis was performed at necropsy, by collecting blood from the descending aorta. Blood was injected into a calibrated test card (EPOC-BGEM) and measurements of ABG parameters, such as pH, partial pressure of carbon dioxide (paCO2), sodium bicarbonate (HCO3−), partial pressure of oxygen (paO2), calculated total carbon dioxide (TCO2) and calculated oxygen saturation (cSO2) was performed using the EPOC-Blood gas and electrolyte analyzer (Loveland, CO).
2.4. Histology and immunohistochemistry
For fixation, lungs were inflated with intratracheal instillation of 4% buffered formalin and immersed in the same solution for 24 h and later placed in 70% ethanol until paraffin embedding. Separate lungs were used for fixation and were not lavaged or perfused. The fixed lungs embedded in paraffin were cut into 5 μm sections using microtome (Lieca biosystems, IL) and stained with Hematoxylin and Eosin (H&E) for histological examination and lung injury scoring, which was performed as described before12. For immunohistochemistry, deparaffinized and rehydrated lung tissue sections (5 μm) were incubated with citrate (10 mM; pH 6.0; 0.05% Tween-20) for 20 minutes for antigen retrieval followed by endogenous peroxidase quenching with 3% hydrogen peroxide for 15 minutes. Non-specific binding sites were blocked by 5% normal goat serum (NGS) followed by overnight incubation (4°C) with anti-MPO antibody (ab208760; Abcam, Cambridge, MA) at 1:400 dilution. Next day, tissue sections were incubated with HRP-conjugated anti-rabbit antibody (Bio-Rad Laboratories, Hercules, CA) at 1:1000 dilution for 45 minutes (room temperature). DAB (DAKO/Agilent, Santa Clara, CA) was used for color development and rabbit IgG control (DAKO/Agilent, Santa Clara, CA) was used at the same specifications as negative control. Tissues were counterstained with hematoxylin and images were acquired using a bright field microscope (VWR, Radnor, PA).
2.5. Bronchoalveolar lavage fluid (BALF) collection and differential staining
Bronchoalveolar lavage (BAL) was performed on whole lungs with 2×1ml of Ca2+ and Mg2+ free Dulbecco’s phosphate buffer saline (DPBS). Lavage fluid was centrifuged at 800 × g for 10 minutes at 4°C and supernatant was collected and stored at −80 °C until further use, whereas pellet was washed and resuspended in 500 μl of PBS. Further, 100 μl of BALF was centrifuged in Shandon Cytospin 3 (Shandon Inc. San Marcos, CA) and approximately 200 cells were used for differential staining to reveal neutrophils and macrophages (Protocol Hema 3; Fisher Diagnostics, Middletown, VA).
2.6. Western blotting
BALF supernatant (40 μl) was separated on a precast gradient gel (Bio-Rad laboratories, CA) and transferred to nitrocellulose membrane as described previously14. Polyclonal anti-HMGB1 antibody (NB100–2322; Novus Biologicals, Littleton, CO) was used at a dilution of 1:1000. Membranes were then incubated with HRP-conjugated anti-rabbit antibody (Bio-Rad laboratories, Hercules, CA) for 1hr at room temperature (1:3000 dilution) and detection was done by using Super Signal® West Femto maximum sensitivity substrate (Thermo Fisher, Waltham, MA) on a Gel Doc imaging system (Bio-Rad laboratories, Hercules, CA).
2.7. Cytokine profiling in lung lysates
Frozen lung tissues were homogenized using mortar-pestle and total protein was extracted with lysis buffer (0.1 M Tris-HCl pH 7.4, 1% Triton X-100, 1mM EGTA, 1 mM EDTA and 0.5% sodium deoxycholate) containing PMSF and protease inhibitor cocktail. Protein concentrations in the supernatants were measured by BCA method on Varioskan™ LUX multimode microplate reader (Thermo Fisher, Waltham, MA). A panel of cytokines were quantitated in the tissue lysates, using a bead-based multiplex immunoassay system (ProcartaPlex; eBioscience, San Diego, CA), as per the manufacturer’s protocol.
2.8. Pathway Analysis
IPA (Ingenuity Pathway Analysis) was used to evaluate the physiological pathways and cellular functions affected by the differentially expressed cytokines from the multiplex assay. Fold-changes in the cytokines were used to list the pathways that were predicted to be affected by the differentially expressed cytokines.
2.9. Statistical analysis
All statistical analyses were performed by using one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparisons test in Prism 8.0 (GraphPad, La Jolla, CA), unless otherwise indicated. Data has been presented as mean values with standard error of the mean (SEM). p<0.05 was considered significant.
3. Results
3.1. Cutaneous exposure to Lewisite induces acute lung injury and increases neutrophils in lung tissue and BALF
Lewisite was applied once to dorsal skin of mice at a single dose of 2.5, 5.0 or 7.5 mg/kg of body weight. Mice similarly exposed to the diluent ethanol served as control. After 24 h of exposure, the vesicating effects of Lewisite were evident across, all doses, as compared to control (Figure 1A). To identify lung injury in response to Lewisite, we assessed lung tissues of mice exposed to Lewisite for histology and lung injury score determination. A gross histological evaulation revealed minimum to mild lung injury, which was characterized primarily by global edema in alveolar interstitium, mild perivascular edema and increased number of intra-alveolar as well as interstitial neutrophils. (Figure 1B). Hyaline membrane formation was also evident in some animals of the Lewisite exposed group but was absent in control animals (Figure 1B). Moreover, few mice in Lewisite exposed groups demonstrated mild perivascular and intra-alveolar hemorrhages (Figure1B). Quantification revealed that animals exposed to Lewisite had an increased lung injury score (LIS) when compared to control, which was more pronounced at higher doses (Figure 1C). These results show that cutaneous exposure to Lewisite can potentially cause lung damage.
Figure 1. Cutaneous exposure to Lewisite causes acute lung injury and neutrophil infiltration in lung and BALF.
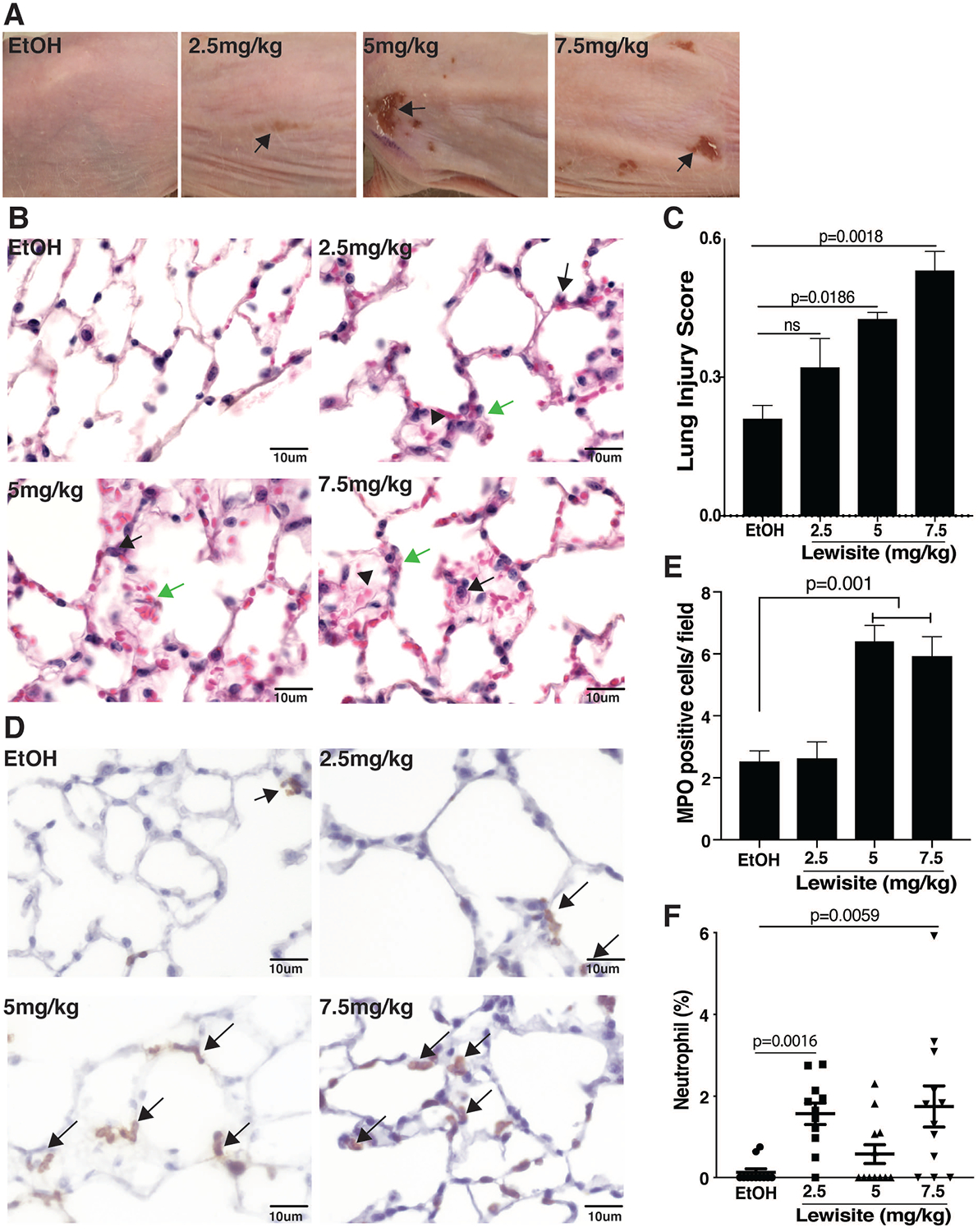
Lewisite at doses of 2.5, 5.0 and 7.5 mg/kg were applied to the skin of hairless Ptch+/−/SKH-1mice and (A) skin damage (arrows) was assessed after 24 hours. Representative images of dorsal skin of mice (ethanol; EtOH group or 2.5, 5.0 and 7.5 mg/kg Lewisite groups) are shown (n=20/group). Twenty-four hours post exposure, animals were sacrificed and lungs were fixed as described in the ‘Methods‘. (B) Representative images of H&E stained lung tissues showing neutrophils (black arrows), edema in alveolar interstitium (green arrows) across the different doses of cutaneous Lewisite exposure. Alveolar hemorrhages (red arrows) are shown in the Lewisite exposed animals. Images were captured at 400x magnification and cropped; scale bar represents 50 μm. (C) Images (5 random images/animal) were then processed to perform quantification using Image J (NIH) and quantification of lung injury represented as lung injury score (LIS) (n=5/group), as described in ‘Methods‘ is shown. (D) Representative images of MPO staining of the lungs demonstrating increased levels in the Lewisite exposed mice (brown spots), as compared to control, which is also represented (E) graphically to demonstrate semi-quantitative statistical evaluation of MPO expression. (F) Bronchoalveolar lavage fluid (BALF) was collected for performing cytospins and lungs were fixed and immunohistochemistry performed on 5 μm sections as described in ‘Methods‘. Graphical representation of differential staining with Hema-3 was performed on BALF cytospin slides for counting neutrophils. Values are expressed as mean±SEM.
To further evaluate lung injury in Lewisite exposed animals, lung sections were analysed for myeloperoxidase (MPO) which is a heme-containing peroxidase, mainly expressed in neutrophils and used extensively as a neutrophil marker15. Lung sections of Lewisite exposed mice showed a marked increase in MPO staining in alveolar as well as interstitial spaces in comparision to control animals (Figure 1D). MPO positive cells were significantly increased, paticularly at the higher 5 mg/kg and 7.5 mg/kg doses, as compared to control (Figure 1E). In the BALF, we observed significantly increased neutrophils in the Lewisite groups, when compared to control (Figure 1F). These observations support increased neutrophils in lungs as well as BALF of Lewisite exposed animals. Here it is imperative to mention that separate animals were used for whole lung BALF collection and fixation for histology.
3.2. Cutaneous Lewisite exposure impaires gas exchange and decreases arterial blood pH
Cutaneous exposure to Lewisite resulted in modest decreases in arterial blood pH in all groups which, however, did not reach statistical significance (Figure 2A). Partial pressure of carbon-dioxide (PaCO2) was also increased in all groups and the increase was particularly significant in 5.0 mg/kg group (p<0.05) (Figure 2B), whereas total carbon dioxide (TCO2) and bicarbonate (HCO3−) levels were significantly increased in all Lewisite exposed groups (p<0.05), in comparison to control (Figure 2C, 2D).
Figure 2. Effect of cutaneous exposure to Lewisite on blood-gas parameters in mice.
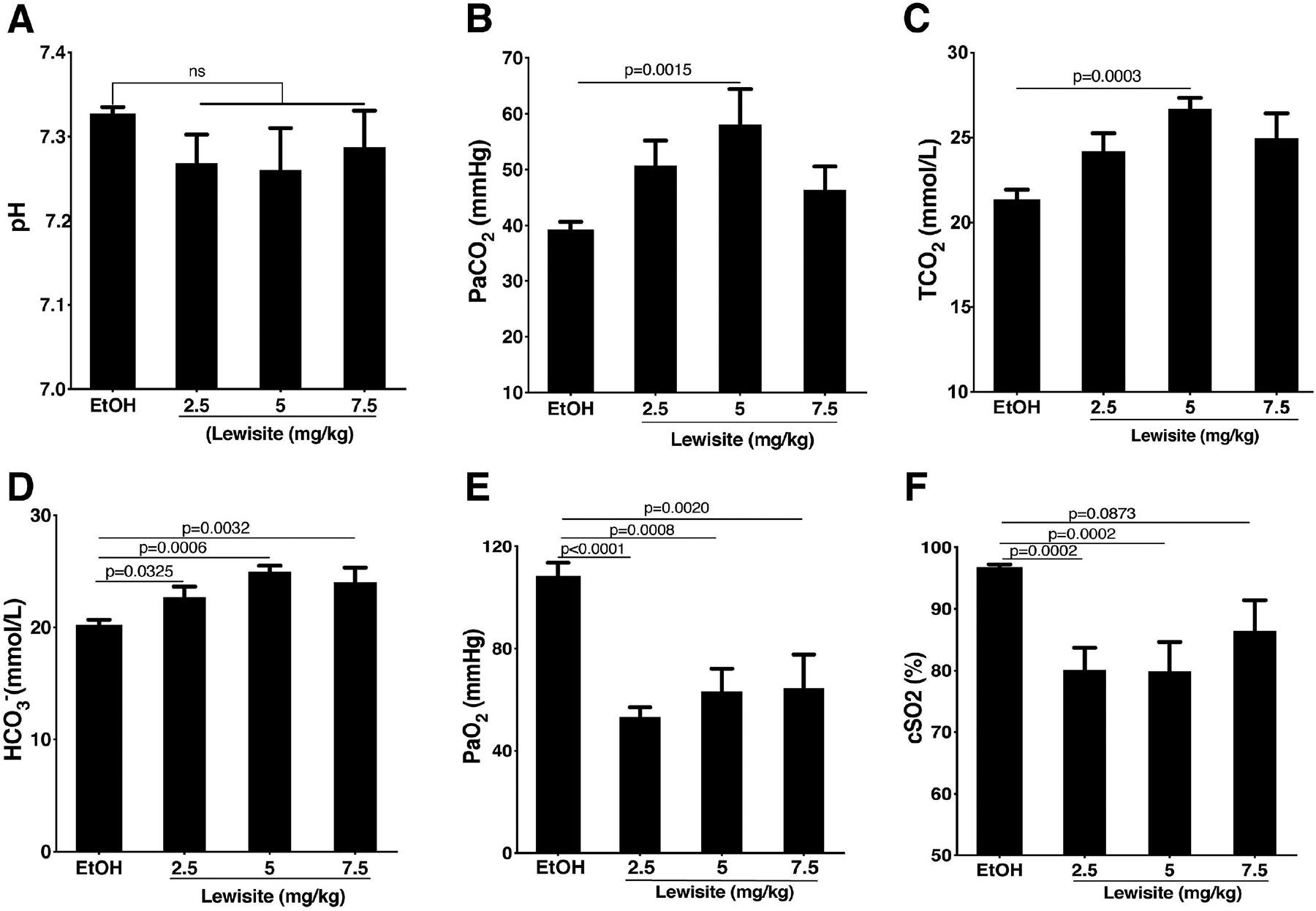
Blood gas measurements were taken 24 hours after cutaneous Lewisite exposure. Effect of increasing doses of Lewisite on (A) pH of arterial blood, (B) partial pressure of arterial carbon dioxide (PaCO2), (C) calculated total carbon dioxide (TCO2), (D) bicarbonate levels (HCO3-), (E) partial pressure of arterial oxygen (PaO2) and (F) calculated oxygen saturation (cSO2) is shown, compared to the control (Ethanol: EtOH treated) group. Values are expressed as mean±SEM (n=8/group).
Exposure to Lewisite also resulted in a significant decrease in partial pressure of arterial oxygen (PaO2) (p<0.01) in all Lewisite exposed groups when compared to controls (Figure 2E). Calculated oxygen saturation (cSO2) in arterial blood was also decreased in all groups (Figure 2F). Collectively, these results suggest impaired gas exchange acutely following cutaneous Lewisite exposure in mice.
3.3. Exposure to Lewisite alters the expression of cytokines in lung lysates
To further characterise lung injury caused by acute cutaneous exposure to Lewisite in mice, we evaluated the expression of a panel of cytokines using multiplex assay. As shown in Figure 3A, we observed an increased expression of a number of cytokines. This data was then subjected to pathway analysis, using IPA and alteration of cellular processes was evaluated. A number of altered cytokines were found to be involved in adhesion of granulocytes as well as recruitment of mononuclear leukocytes (Figure 3B). The pathway analysis also revealed a number of putative cellular processes affected which included amonst others, cell to cell signaling, immune cell trafficking, inflammatory response and respiratory disease (Figure 3C).
Figure 3. Effect of cutaneous Lewisite exposure on cytokines and IPA analysis for altered disease and cellular pathways.
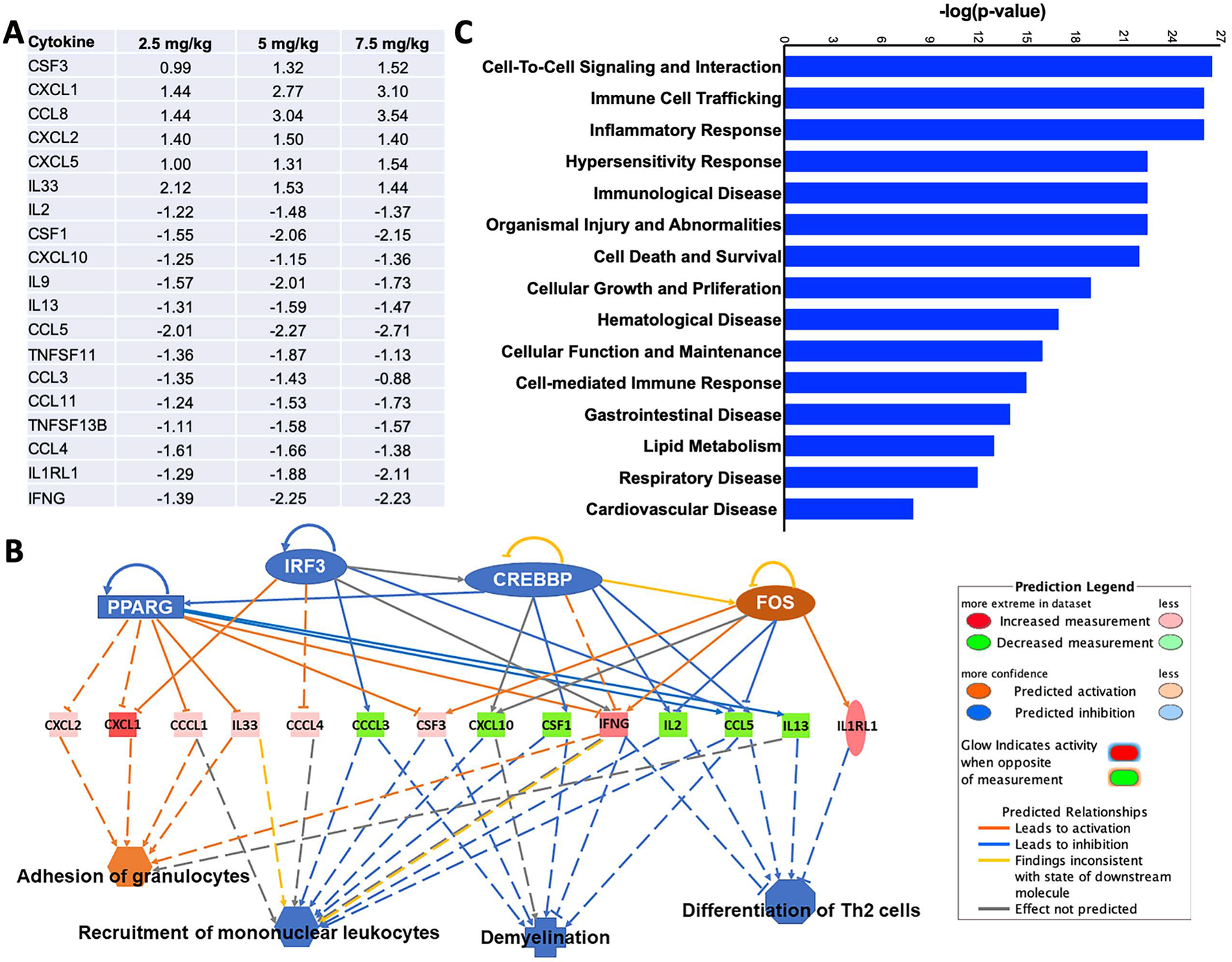
Animals were exposed to indicated doses of Lewisite. (A) A panel of cytokines was evaluated in the lung tissue lysates using a bead-based Luminex multiplex immunoassay system and only the significantly altered cytokines are shown. This data was further analyzed by Ingenuity Pathway Analysis (IPA) and the predicted effects of altered cytokines on (B) immunological/inflammatory and (C) cellular and disease processes are shown.
Adhesion of granuloctes is considered a hallmark of inflammatory process and, in addition, inflammatory response was one of the highly affected cellular process revealed by IPA, therefore we next evaluated, as a proof of concept, a set of cytokines that are known to be involved in inflammatory response. A dose-dependent increase in levels of proinflammatory CXCL1 (Figure 4A) and CXCL5 (Figure 4B) was observed in Lewisite treated animals. We also observed a significant increase in IL-33 levels (Figure 4C). GCSF (CSF3), a key regulator for maturation and differentiation of neutrophils from neutrophil progenitor cells16 was also significantly increased in lung tissues of Lewisite exposed animals, relative to control group (Figure 4D).
Figure 4. Cutaneous Lewisite exposure increased pro-inflammatory cytokines in lung lysates.
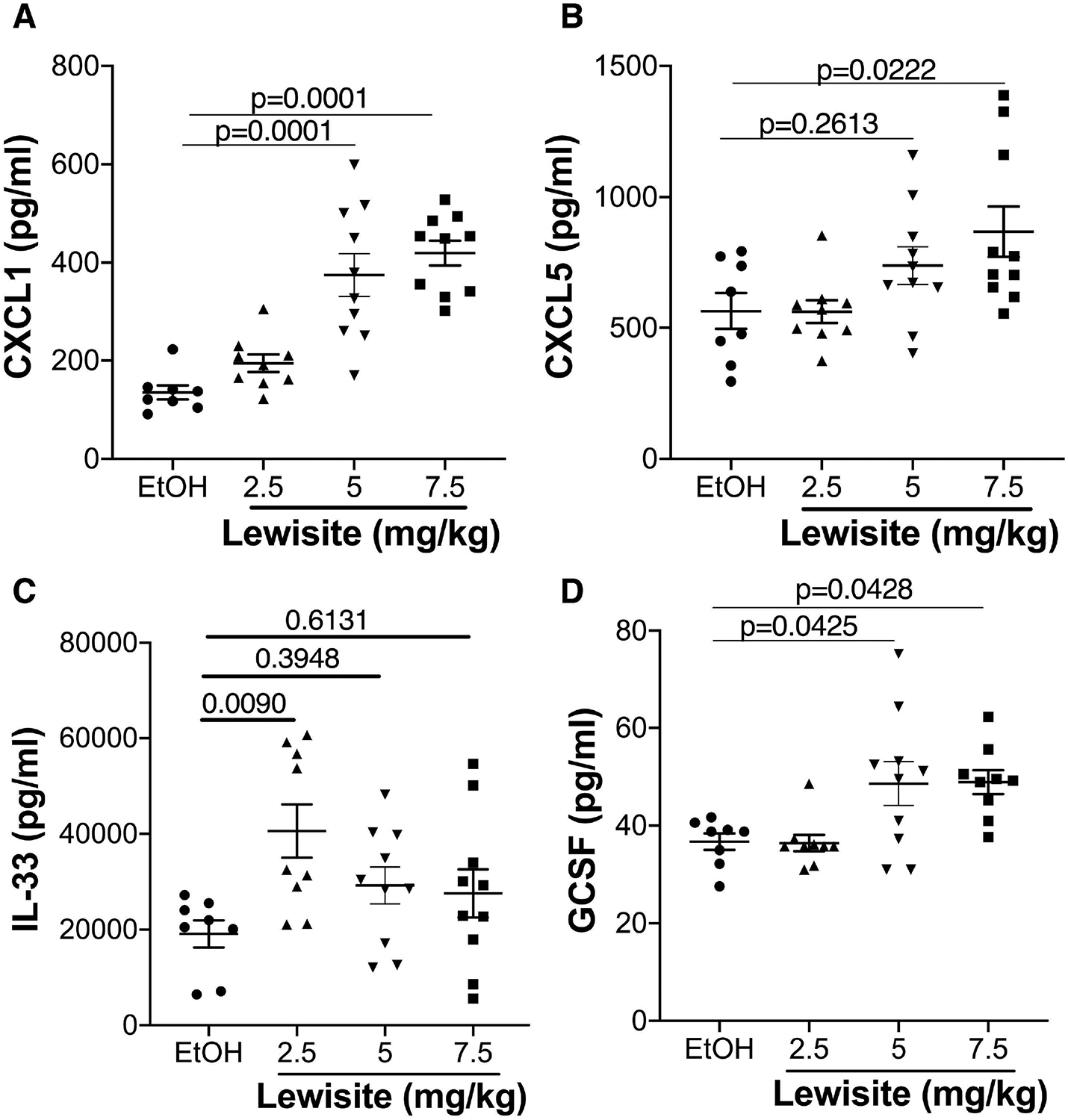
Lung tissue homogenates from all groups of mice were prepared as described in ‘Methods‘. Graphical representation of selected cytokines (A) CXCL1, (B) CXCL5, (C) IL-33 and (D) GCSF in lung tissue is shown. Values are expressed as mean±SEM.
3.4. Lewisite exposure increases CXCL1, CXCL5 and HMGB1 in BALF
Since we observed increased inflammatory cytokines in the lungs of animals exposed to Lewisite, we further analysed BALF from mice in 2.5, 5.0, 7.5 mg/kg and control groups for expression of select cytokines. We observed that animals exposed to Lewisite had significantly increased levels of CXCL1 protein in the BALF, which was more pronounced in the 7.5 mg/kg group (p<0.05) (Figure 5A). Similarly, BALF CXCL5 was also increased the most in the 7.5 mg/kg group (p<0.05) (Figure 5B). HMGB1, a DAMP (Damage Associated Molecular Pattern) molecule and an indicator of inflammation17 was also significant increased in BALF of mice exposed to Lewisite as compared to the control group (p<0.05) (Figure 5C). Such release of HMGB1 in BALF resulted in its decreased expression in the lung lysates (Figure 5D). Collectively, these observations suggest that pro-inflammatory responses evident in lung tissues could also be corroborated in the BALF of Lewisite exposed animals.
Figure 5. Cutaneous Lewisite exposure increases CXCL1, CXCL5 and HMGB1 in BALF.
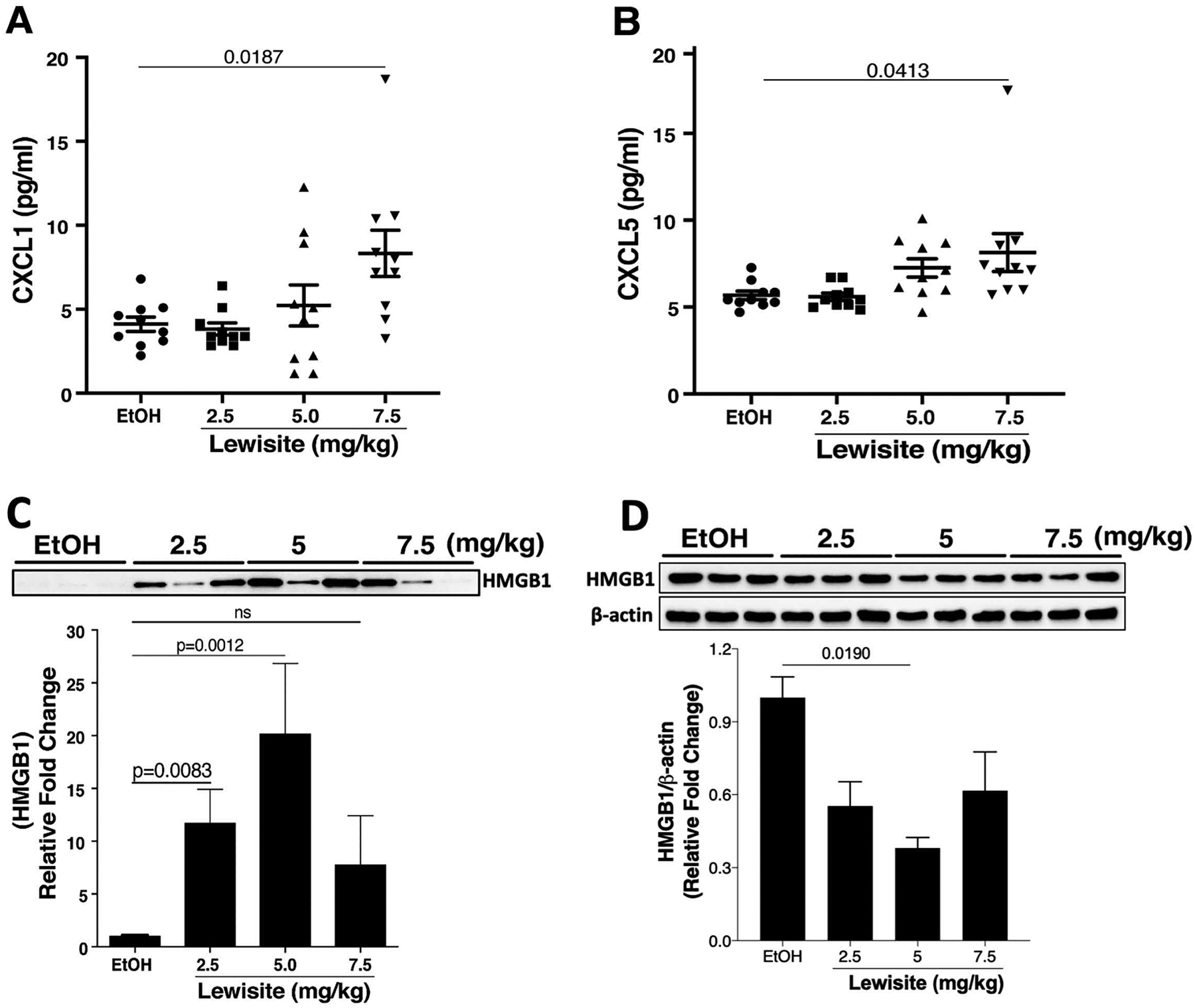
Cutaneous Lewisite exposures were performed at different doses as described above and mice were sacrificed 24 hours later. BALF was collected as described in the Methods. Concentrations of (A) CXCL1 and (B) CXCL5 in BALF of animals exposed to either EtOH (ethanol: control) or increasing doses of Lewisite are shown. Values are expressed as mean±SEM. Immunoblotting of HMGB1 was performed on (C) BALF and (D) lung tissue samples from control and Lewisite treated animals. The top panels demonstrate representative HMGB1 immunoblots. Densitometric analysis of blots are shown as a graph in the lower panel. Densitometric analysis of HMGB1 immunoblot for lung tissue lysates is plotted, relative to the expression of β-actin. Values are expressed as mean±SEM.
3.5. Effects of acute cutaneous Lewisite exposure on complete blood count
Our data clearly demonstrated an increase in neutrophils in lungs of mice exposed to Lewisite. To further understand the systemic effects of cutaneous Lewisite exposure we analyzed blood cells and observed a significant increase in circulating neutrophils in Lewisite exposed mice, as shown in Figure 6A. There was, however, no change in monocyte population in the blood (data not shown). Lymphocytes and Eosinophils were increased in Lewisite groups, as compared to control (Figure 6B, 6D). Neutrophil-lymphocyte ratio (NLR) has been recently recognised as an independent prognostic indicator of systemic inflammation18 and organ failure19. This ratio was found elevated upon Lewisite exposure (Figure 6C). Certain blood parameters like mean platelet volume (MPV) and red blood cell distribution width (RDW) percentage have been utilized in recent years as independent predictors of respiratory disease severity (like COPD, pulmonary hypertension)20, 21. We observed a significant increase in both MPV and RDW in Lewisite exposed mice, as compared to control (Figure 6E, 6F).
Figure 6. Effects of cutaneous Lewisite exposure on complete blood counts.
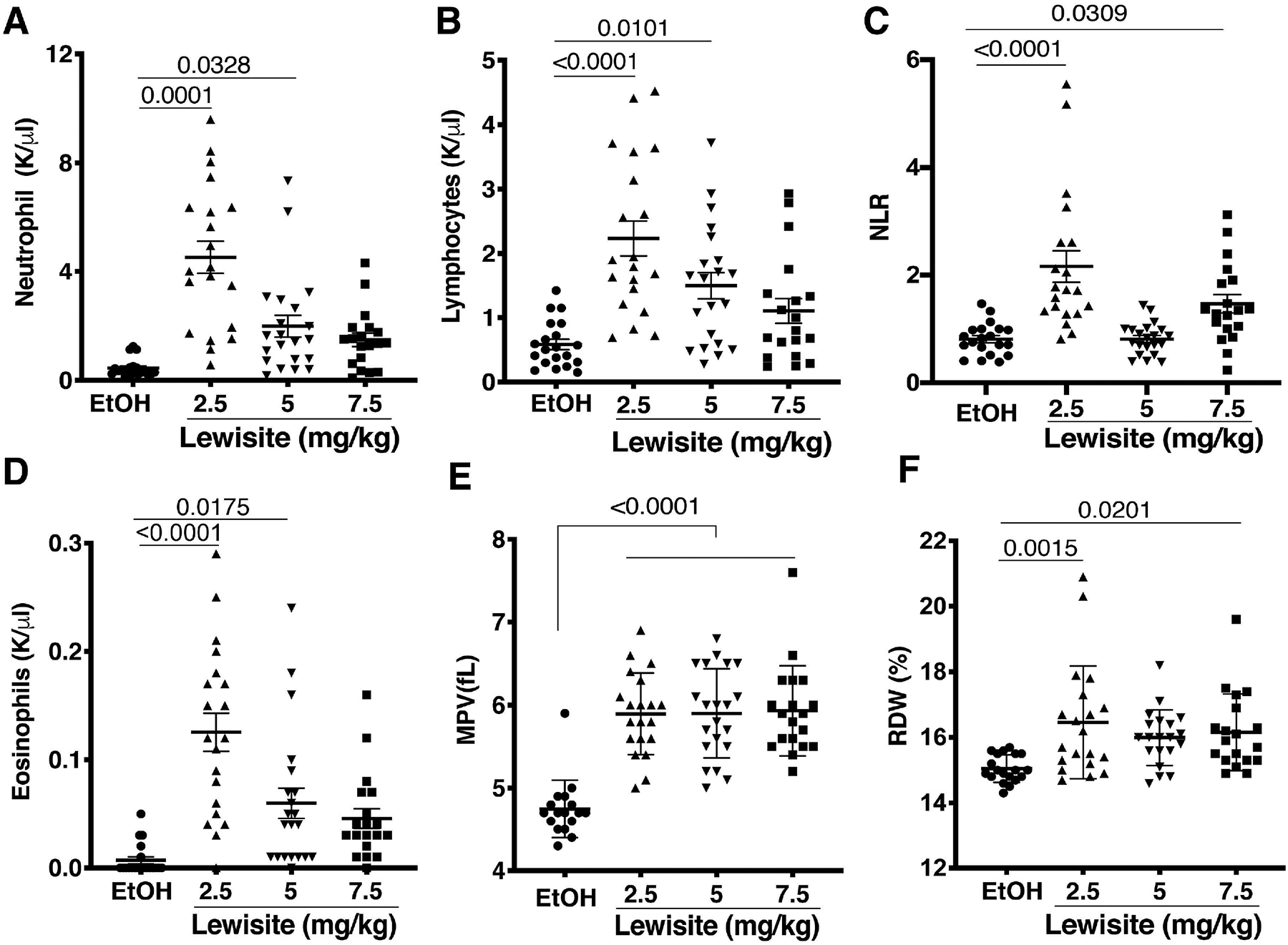
Blood collection and blood count was performed, as described in the ‘Methods‘ section. Counting was accomplished using a 5-part differential blood cell count analyzer (Hemavet, Drew Scientific). (A) Neutrophil count, (B) Lymphocytes, (C) Neutrophil-Lymphocyte ratio (NLR), (D) Eoisinophils, (E) mean platelet volume (MPV) and (F) red blood cell distribution width (RDW) was evaluated in blood of animals exposed to indicated doses of Lewisite, compared to Ethanol (EtOH) control animals. Values are expressed as mean±SEM.
Discussion
Lewisite is a lipophilic compound with a rapid transdermal absorption rate which implies that it is readily available for distribution through systemic circulation4. It is a lethal vesicant and can cause multiple organ failure irrespective of route (ingestion, inhalation or dermal) of exposure4. The mortality caused by vesicating agents, like HD, has been largely ascribed to respiratory failure6. Although the mechanism of Lewisite-induced dermal toxicity has been shown previously2, effect of its cutaneous exposure on the pulmonary system has not been investigated. Therefore, this study was designed to assess the effect of cutaneous exposure to Lewisite on the respiratory system. Here we demonstrate, using Ptch1+/−/SKH-1 mice, a strain previously shown to be susceptible to cutaneous Lewisite exposure2, that a single application of Lewisite caused substantial pulmonary damage, impaired gas exchange and an increase in proinflammatory markers which are characteristics of ALI/ARDS22.
Injury to respiratory system, subsequent to dermal exposure to Lewisite can be caused either via a direct or indirect action of Lewisite or its active metabolite(s). A direct action would involve absorption of Lewisite across the skin and into the circulatory system; its transport to the lungs and eventually the injury at site. In contrast, an indirect injury would be mediated by other molecules. The indirect injury could be similar to one mediated by neutrophils in a model of thermal trauma of skin-induced pulmonary injury23. There the effect was clearly remote/indirect, as lungs were never directly exposed to hot water/thermal trauma and, moreover, blocking of adhesion molecules was observed to attenuate the effect, thus establishing a role of neutrophils. Increased neutrophils following Lewisite exposure, could therefore have a possible indirect effect on the respiratory system. However, there is evidence in the literature to suggest effective absorption of Lewisite from skin and into the circulation with subsequent accumulation in different organs, including lungs, which supports a direct pulmonary injury inducing action of Lewisite. For example, injury caused by cutaneous exposure to Lewisite has been shown to extend beyond skin in pigs, with manifestations in subcutaneous connective and adipose tissue24, 25. Further, sub-cutaneous injection of Lewisite in rabbits has been reported to result in systemic accumulation of Lewisite, with lung, liver and kidney as the organs with highest arsenic concentrations26. The concentrations equilibrate rather rapidly with blood and the average tissue: blood partition coefficients range from 7.41 to 14.5026. These studies indicate a more direct involvement of Lewisite or its metabolites in pulmonary injury. It is possible that cutaneous Lewisite exposure can initiate direct as well as indirect pulmonary injury, which needs further evaluation. Systemic toxicity and effects on the lungs have been described previously with other agents applied on the skin, such as HD and phosgene oxime27, 28. Occupational skin exposures and skin conditions such as atopic dermatitis and systemic sclerosis can sensitize the lungs and lead to manifestation of pulmonary diseases such as asthma and interstitial lung disease29, 30. In our study, we used ethanol as the diluent to ensure efficient absorption of Lewisite. Ethanol, when applied topically, is a skin penetration enhancer and dermal application of ethanol results in measurable concentrations in blood that are still below acute toxic levels31.
The skin is considered as the largest organ and the concept of skin-lung inter organ relationship has been known for long but is being highlighted currently as the knowledge on these organ interactions is progressing30, 32. We observed many characteristic features of ALI/ARDS in Lewisite exposed mice. Hypercapnia, increased PaCO2 and increased total CO2 in arterial blood of Lewisite exposed animals was accompanied by a compensatory increase in HCO3− levels. A decrease in PaCO2 at the highest dose is possibly due to a compensatory hyperventilation. Lewisite exposed animals were also hypoxemic as evident from decreased PaO2 and calculated SO2. These parameters could be very useful in clinical settings for the baseline evaluation of pulmonary gas exchange after admission of a subject and could prove to be of great importance in deciding first line of treatment against acute Lewisite toxicity.
In addition to physiological changes, ALI is also characterized by cellular infiltration in the lung and increased inflammation. Our multiplex evaluation of cytokines, followed by IPA analysis further confirmed a pro-inflammatory response. Differences in neutrophil levels in the BALF and lung maybe due to differences in transmigration potential of neutrophils from the lung interstitium to the alveolar space33. Our results indicated increased neutrophils and the IPA analysis revealed that such increase could be due to several cytokines whose expression is altered by Lewisite. In addition to granulocyte adhesion, a number of cytokines involved in recruitment of mononuclear leukocytes, a key step in the initiation and progression of inflammation. IPA analysis also suggested differentiation of Th2 cells, which are known to play important role in mediating inflammatory response34. Th2 cells induce recruitment of eosinophils, which could further explain the increased eosinophils in Lewisite exposed animals. Consistent with increased neutrophils, MPO, a marker for neutrophil activation, was also significantly increased in lung tissues. MPO invokes neutrophil movement independent of its enzymatic activity35. Neutrophil activation and transmigration is an important event in the onset and progression of ALI/ARDS36,36–39. In patients with ALI/ARDS, neutrophil numbers in blood and BALF are positively correlated with the severity of injury and outcome36. Neutrophil recruitment is a chemotactic process regulated by various chemokines like CXCL1 and CXCL540, 41. An increase in CXCL1 and CXCL5 along with increase in neutrophils in the lung of Lewisite exposed mice suggests the role of these chemokines in the recruitment process. Of note, GCSF (CSF3), a key factor involved in regulation of neutrophil maturation and differentiation from neutrophil progenitor cells, was also observed to be increased in lung tissues of animals exposed to Lewisite16. This imples that the lung cells not only release the chemokines that would recruit neutrophils to the site of injury but also stimulate neutrophil progenitor cells to undergo maturation after Lewisite exposure. Increased IL-33 as observed in this study potentiates production of Th2-associated cytokines42, thus, further amplifying the inflammatory and immune response.
HMGB1 is a non-histone chromatin binding protein that acts as a damage associated molecular pattern (DAMP) molecule when secreted into the extracellular environment.. HMGB1 has been shown to trigger production of chemo attractants43, 44 and intratracheal injection of HMGB1 was shown to cause inflammation in lungs acutely, underscoring its role as a potent proinflammatory molecule17. HMGB1 can bind to toll like receptors and receptors of advanced glycation end products (RAGE) to promote an inflammatory cascade45. A study reported occurrence of RAGE in lung lining fluid of mice and in sputum of patients after chronic environmental exposure to arsenic46. HMGB1 release has also been implicated in the pathogenesis of ALI/ARDS47,48, 49. Our findings of HMGB1 release in BALF, after cutaneous exposure to Lewisite is therefore consistent with an ARDS-like inflammatory phenotype.
In recent years, few of the blood parameters have received special attention in diagnosis of different lung diseases as independent markers. We observed an increase in lymphocytes and eosinophils in Lewisite exposed mice, as compared to control, which further highlighted inflammatory effects of Lewisite exposure. Neutrophil-lymphocyte ratio (NLR) is considered as a risk factor and prognostic indicator of overall survival in patients with ARDS and various systemic diseases as well as severity of inflammation18, 50. We also observed an increase in NLR of mice exposed to Lewisite. We further observed a significant increase in RDW percentage which has been previously reported to be increased in ARDS patients51. Interestingly, studies carried on burn victims demonstrated that every 1% increase in RDW increased the risk of developing ARDS by 29%21. The animal model for thermal trauma is representative of burn injuries and, as discussed above, provides a mechanism of pulmonary injury secondary to skin trauma and inflammation. Thus, there are possible parallels between cutaneous Lewisite exposure and the thermal dermal burns. In the current study, cutaneous Lewisite exposure in mice caused an increase in RDW percentage by 5–6%, which is suggestive of a significantly increased risk of developing ARDS. The observed increase in RDW percentage signifies that cutaneous Lewisite exposure is injurious to lung and causes ALI/ARDS. Early platelet activation also plays an important role in pathogenesis of lung injury. Although its effect in ALI/ARDS has not been extensively characterized, its contribution to inflammation as well as coagulation has been described52. An early indicator of platelet activation is MPV which has been reported to be proportionally related to disease severity in COPD patients20. Here our injury model presented a significant increase in MPV in Lewisite exposed mice, as compared to control. The cutaneous effects of Lewisite are better characterized and have shown that the injury is progressive with an acute changes in the proinflammatory mediators2.
In conclusion, our data strongly suggests that a single application of Lewisite to the dorsal skin of hairless mice causes lung injury, impaired gas exchange and lung inflammation which are characteristic features of ALI/ARDS. Future studies should focus on therapeutic interventions to mitigate such injuries.
Acknowledgements:
This work is funded by the CounterACT Program grants, National Institutes of Health Office of the Director (NIH OD), the National Institute of Environmental Health Sciences (NIEHS) Grants U54ES030246 (AfA, VA, AnA, MA), U01ES025069 (AfA), U01ES028182 (SA) and U01NS095678 (MA).
References
- 1.Goldman M & Dacre JC. 1989. Lewisite: its chemistry, toxicology, and biological effects. Rev Environ Contam Toxicol. 110: 75–115. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Srivastava RK, Weng Z, et al. 2016. Molecular Mechanism Underlying Pathogenesis of Lewisite-Induced Cutaneous Blistering and Inflammation: Chemical Chaperones as Potential Novel Antidotes. Am J Pathol. 186: 2637–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maynard T.C.M.a.R.L. 2007. Organic Arsenicals. John Wiley & Sons, 2007. [Google Scholar]
- 4.Srivastava RK, Traylor AM, Li C, et al. 2018. Cutaneous exposure to lewisite causes acute kidney injury by invoking DNA damage and autophagic response. Am J Physiol Renal Physiol. 314: F1166–F1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pechura CM 1993. History and analysis of mustard agent and lewisite research programs in the United States. 21. [Google Scholar]
- 6.DP P.C.a.R. 1993. “Nonmalignant Respiratory Effects of Mustard Agents and Lewisite”. In Veterans at Risk: The Health Effects of Mustard Gas and Lewisite. DP P.C.a.R, Ed. Washington DC: National Academies Press (US). [PubMed] [Google Scholar]
- 7.Nishimoto Y, Burrows B, Miyanishi M, et al. 1970. Chronic obstructive lung disease in Japanese poison gas workers. Am Rev Respir Dis. 102: 173–179. [DOI] [PubMed] [Google Scholar]
- 8.Tewari-Singh N, Goswami DG, Kant R, et al. 2017. Histopathological and Molecular Changes in the Rabbit Cornea From Arsenical Vesicant Lewisite Exposure. Toxicol Sci. 160: 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henretig FM, Kirk MA & McKay CA Jr. 2019. Hazardous Chemical Emergencies and Poisonings. N Engl J Med. 380: 1638–1655. [DOI] [PubMed] [Google Scholar]
- 10.Johnson ER & Matthay MA. 2010. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 23: 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthay MA, Zemans RL, Zimmerman GA, et al. 2019. Acute respiratory distress syndrome. Nat Rev Dis Primers. 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matute-Bello G, Downey G, Moore BB, et al. 2011. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 44: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmad S & Ahmad A. 2016. Emerging targets for treating sulfur mustard-induced injuries. Ann N Y Acad Sci. 1374: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad A, Ahmad S, Malcolm KC, et al. 2013. Differential regulation of pulmonary vascular cell growth by hypoxia-inducible transcription factor-1alpha and hypoxia-inducible transcription factor-2alpha. Am J Respir Cell Mol Biol. 49: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiland JE, Davis WB, Holter JF, et al. 1986. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 133: 218–225. [DOI] [PubMed] [Google Scholar]
- 16.Roberts AW 2005. G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors. 23: 33–41. [DOI] [PubMed] [Google Scholar]
- 17.Entezari M, Javdan M, Antoine DJ, et al. 2014. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kain V, Van Der Pol W, Mariappan N, et al. 2019. Obesogenic diet in aging mice disrupts gut microbe composition and alters neutrophil:lymphocyte ratio, leading to inflamed milieu in acute heart failure. FASEB J. 33: 6456–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younan D, Richman J, Zaky A, et al. 2019. An Increasing Neutrophil-to-Lymphocyte Ratio Trajectory Predicts Organ Failure in Critically-Ill Male Trauma Patients. An Exploratory Study. Healthcare (Basel). 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed MF, Ali A, Abbas A, et al. 2019. Mean platelet volume as a predictor of pulmonary hypertension in patients with stable COPD. Int J Chron Obstruct Pulmon Dis. 14: 1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao CH, Wan J, Liu H, et al. 2019. Red blood cell distribution width is an independent risk factor in the prediction of acute respiratory distress syndrome after severe burns. Burns. 45: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 22.Ragaller M & Richter T. 2010. Acute lung injury and acute respiratory distress syndrome. J Emerg Trauma Shock. 3: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulligan MS, Till GO, Smith CW, et al. 1994. Role of leukocyte adhesion molecules in lung and dermal vascular injury after thermal trauma of skin. Am J Pathol. 144: 1008–1015. [PMC free article] [PubMed] [Google Scholar]
- 24.Rice P & Brown RF. 1999. The development of Lewisite vapour induced lesions in the domestic, white pig. Int J Exp Pathol. 80: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chilcott RP, Brown RF & Rice P. 2000. Non-invasive quantification of skin injury resulting from exposure to sulphur mustard and Lewisite vapours. Burns. 26: 245–250. [DOI] [PubMed] [Google Scholar]
- 26.Snider TH, Wientjes MG, Joiner RL, et al. 1990. Arsenic distribution in rabbits after Lewisite administration and treatment with British anti-Lewisite (BAL). Fundam Appl Toxicol. 14: 262–272. [DOI] [PubMed] [Google Scholar]
- 27.Goswami DG, Agarwal R & Tewari-Singh N. 2018. Phosgene oxime: Injury and associated mechanisms compared to vesicating agents sulfur mustard and lewisite. Toxicol Lett. 293: 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tewari-Singh N, Goswami DG, Kant R, et al. 2017. Cutaneous exposure to vesicant phosgene oxime: Acute effects on the skin and systemic toxicity. Toxicol Appl Pharmacol. 317: 25–32. [DOI] [PubMed] [Google Scholar]
- 29.Kimber I, Poole A & Basketter DA. 2018. Skin and respiratory chemical allergy: confluence and divergence in a hybrid adverse outcome pathway. Toxicol Res (Camb). 7: 586–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira C & Torres T. 2019. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 29: 250–258. [DOI] [PubMed] [Google Scholar]
- 31.Lachenmeier DW 2008. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J Occup Med Toxicol. 3: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiu Yu Cherie Leung, P.K.L., Jihang Chen, Kam Ming Ko. 2017. IInter-Organ Relationships among Gut, Lung and Skin beyond the Pathogenesis of Allergies: Relevance to the Zang-Fu Theory in Chinese Medicine. Chinese Medicine. 8: 73–81. [Google Scholar]
- 33.Reutershan J, Basit A, Galkina EV, et al. 2005. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 289: L807–815. [DOI] [PubMed] [Google Scholar]
- 34.Stark JM, Tibbitt CA & Coquet JM. 2019. The Metabolic Requirements of Th2 Cell Differentiation. Front Immunol. 10: 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hickey MJ 2011. MPO and neutrophils: a magnetic attraction. Blood. 117: 1103–1104. [DOI] [PubMed] [Google Scholar]
- 36.Grommes J & Soehnlein O. 2011. Contribution of neutrophils to acute lung injury. Mol Med. 17: 293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons PE, Fowler AA, Hyers TM, et al. 1985. Chemotactic activity in bronchoalveolar lavage fluid from patients with adult respiratory distress syndrome. Am Rev Respir Dis. 132: 490–493. [DOI] [PubMed] [Google Scholar]
- 38.Steinberg KP, Milberg JA, Martin TR, et al. 1994. Evolution of bronchoalveolar cell populations in the adult respiratory distress syndrome. Am J Respir Crit Care Med. 150: 113–122. [DOI] [PubMed] [Google Scholar]
- 39.Abraham E, Carmody A, Shenkar R, et al. 2000. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 279: L1137–1145. [DOI] [PubMed] [Google Scholar]
- 40.Sawant KV, Xu R, Cox R, et al. 2015. Chemokine CXCL1-Mediated Neutrophil Trafficking in the Lung: Role of CXCR2 Activation. J Innate Immun. 7: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koltsova EK & Ley K. 2010. The mysterious ways of the chemokine CXCL5. Immunity. 33: 7–9. [DOI] [PubMed] [Google Scholar]
- 42.Liew FY, Girard JP & Turnquist HR. 2016. Interleukin-33 in health and disease. Nat Rev Immunol. 16: 676–689. [DOI] [PubMed] [Google Scholar]
- 43.Fiuza C, Bustin M, Talwar S, et al. 2003. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 101: 2652–2660. [DOI] [PubMed] [Google Scholar]
- 44.Venereau E, Casalgrandi M, Schiraldi M, et al. 2012. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 209: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu L, Ren L, Chen Y, et al. 2015. Redox status of high-mobility group box 1 performs a dual role in angiogenesis of colorectal carcinoma. J Cell Mol Med. 19: 2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lantz RC, Lynch BJ, Boitano S, et al. 2007. Pulmonary biomarkers based on alterations in protein expression after exposure to arsenic. Environ Health Perspect. 115: 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueno H, Matsuda T, Hashimoto S, et al. 2004. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 170: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 48.Sha Y, Zmijewski J, Xu Z, et al. 2008. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 180: 2531–2537. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa EN, Ishizaka A, Tasaka S, et al. 2006. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med. 174: 400–407. [DOI] [PubMed] [Google Scholar]
- 50.Gunay E, Sarinc Ulasli S, Akar O, et al. 2014. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation. 37: 374–380. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Gong Y, Ying B, et al. 2019. Relation between Red Cell Distribution Width and Mortality in Critically Ill Patients with Acute Respiratory Distress Syndrome. Biomed Res Int. 2019: 1942078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav H & Kor DJ. 2015. Platelets in the pathogenesis of acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 309: L915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]


