Abstract
The self-reference effect in memory (SRE), in which stimuli related to self are better remembered than other stimuli, has been studied often in the fMRI literature, but much less with EEG. In two experiments, we investigated how self-referencing modulated event-related potential (ERP) markers of the subsequent memory effect, testing whether the same components that reflect memory success are impacted or whether unique components are modulated by self-referencing. Participants were asked to evaluate whether an adjective accurately described either the self or a given other by making a yes/no key press during EEG recording. Then participants were given a surprise recognition memory test where they judged each adjective as old or new. We observed a main effect of self-relevance on a late positivity at right frontal electrodes. A very similar effect was observed when comparing words subsequently remembered to those that were forgotten. However, no interaction was found between self-relevance and subsequent memory, suggesting the frontal positivity is not exclusive to the SRE, but instead a reflection of deeper encoding that leads to better memory. Thus, this frontal positivity may be a marker of a deeper encoding process that is elicited by self-referencing but not exclusive to the SRE.
Keywords: self-referencing, memory, ERP, self, EEG, subsequent memory
It is commonly said that humans are inherently “selfish” creatures; we are focused on ourselves and our own needs above all else. This idea is supported in memory research by a psychological phenomenon called the self-reference effect: words that are related to the self (self-referential) are remembered better than words related to others (Rogers et al., 1977; Symons & Johnson, 1997). The existence of this effect suggests that either there is a unique mechanism that operates when words are self-relevant that produces this increase in subsequent memory, or that the presence of the self induces a deeper encoding process that is not exclusive to the self. This has led many researchers to investigate the self-reference effect (SRE), highlighting many of its unique features and identifying areas of the brain associated with the SRE. In the cognitive neuroscience literature, functional magnetic resonance imaging (fMRI) has suggested that brain regions including the medial prefrontal cortex may be uniquely related to memory for self-relevant information. The SRE has been less investigated with EEG, and the few EEG studies reported in the literature have primarily focused on retrieval. In an effort to further elucidate the mechanisms underlying the SRE and better link the EEG and fMRI literatures, here we report two event-related potential (ERP) studies of neural correlates of the self-reference effect during memory encoding.
Self-referencing has been shown to benefit both item memory (memory of the specific stimulus) and source memory (memory that adds context to the stimulus) (e.g., Serbun et al., 2011; Leshikar & Duarte, 2012). Old/new judgements are often made to indicate whether items were presented previously. Assessment of specific or source memory requires more detail than being able to discriminate old/new, including memory for information such as specific dates, times, or encoding conditions. Additionally, words more closely related to the self, such as the participant’s name, have higher recall rates than a less relevant stimulus, such as a friend’s name (Burnkrant & Rao Unnava, 1989). The SRE, therefore, is not exclusive to item memory but improves many different aspects of memory via rich encoding of contextual information and responses to the degree of self-relevance of the stimuli.
The self-referencing effect seems especially robust as it develops early in childhood, is preserved in healthy aging, and remains present in early-stage age-related cognitive impairment. The effect has been found to increase source memory as well as recall and recognition memory in children as young as four years old (Cunningham et al., 2014; Sui & Zhu, 2005). In addition, older adults exhibited increased recognition for previously self-referenced items compared to non-self- referenced items, though their memory performance is overall poorer than younger adults (Gutchess, et al., 2007). Older adults with mild cognitive impairment due to Alzheimer’s Disease, who were more susceptible to other memory errors, were at no greater risk for self-related errors than healthy controls (Rosa et al., 2015). The preservation of the SRE with age and mild cognitive impairment makes the effect intriguing for therapeutic application to compensate for memory declines.
A longstanding question is whether the SRE reflects something unique about processing information in relation to the self, or whether it simply reflects the deep level of processing engaged by self-referential conditions. One way to address this question is to investigate whether the SRE is associated with different brain regions than memory for stimuli processed in non-self-relevant conditions, and whether those regions are thought to reflect deep, semantic processing or self-specific processes. Studies examining this question generally use some form of the subsequent memory paradigm, which uses neural differences at encoding to predict whether the stimulus will be later remembered or forgotten (Paller & Wagner, 2002). If a specific subset of activity at encoding is correlated with increases in subsequent memory, then it can be inferred that the process at encoding is related to successful memory formation. The study of subsequent memory formation has helped to delineate which neural regions play a role in creating robust memories, including transforming sensory experiences into internal representations and binding the internal representations into a long-term trace (Paller & Wagner, 2002). Features of stimuli and goals can further modulate the processes and corresponding neural regions that contribute to memory formation (Paller & Wagner, 2002).
A large neuroimaging literature has investigated which unique processes self-referencing brings to bear on memory formation. Primarily using functional MRI, the social neuroscience literature has suggested that self-related processing is particularly associated with the medial prefrontal cortex (mPFC) (Northoff & Bermpohl, 2004; Denny et al., 2012; Wagner et al., 2012; Araujo et al., 2013; Fields et al., 2019). Not surprisingly, a number of fMRI studies conducted to identify regions involved in the SRE have implicated the mPFC as an area important for the memory effect (e.g., Leshikar & Duarte, 2012; Kelley et al., 2002; Morel et al., 2014; Yaoi et al., 2015). The activity of the mPFC during encoding has been shown to predict whether or not a stimulus relating to self will be subsequently remembered in later testing (Macrae et al., 2004). Whereas distinct regions contribute to subsequent memory formation for visual, verbal, and emotional information, medial prefrontal cortex supports the encoding of self-referential information, perhaps reflecting the metacognitive aspects of thinking about the self (Macrae et al., 2004). Other studies have more precisely implicated the ventral medial prefrontal cortex in self-referential thought while relating dorsal medial prefrontal cortex to thoughts about others (Mitchell et al., 2006; Powell et al., 2010). The role of the mPFC in self-referential processing also extends to older adults and is associated with successful encoding of this information (Gutchess, Kensinger, & Schacter, 2007; Gutchess et al., 2015). Therefore, it can be concluded based on the fMRI literature that the mPFC is related to the successful encoding of self-referential memories, and that activity in this region may reflect processes that are relatively unique to encoding self-relevant information, rather than general semantic processes.
There has been less EEG research examining the SRE. A number of ERP studies have examined the effects of self-relevance during stimulus processing without examining the relationship to later memory. The most common finding is a posteriorly distributed late positivity that is sometimes identified as the P300 and sometimes simply as the late positive component (LPC) (reviewed in Knyazev, 2013). For example, a larger posterior positivity is seen when a participant is presented with their own name or face (e.g., Tacikowski & Nowicka, 2010), as well as to other self-relevant words (Gray et al., 2004) and objects (Miyakoshi et al., 2007). This effect may be related to the late positive potential generally seen to emotional stimuli (Hajcak et al., 2012), and is generally taken to reflect increased attention or deeper processing for self-relevant stimuli (Fields & Kuperberg, 2016; Knyazev, 2013). A number of studies have also reported a frontally distributed late positivity to self-relevant conditions (e.g., Fields & Kuperberg, 2012), particularly when participants are asked to judge stimuli for self-relevance or retrieve self-related information from memory (Kotlewska & Nowicka, 2016; Magno & Allan, 2007; Mao et al., 2017; Nowicka et al., 2018; but see Shestyuk & Deldin, 2010). Although a few other ERP studies have examined the effects of self-referential encoding on neural activity at retrieval (Dulas et al., 2011; Tanguay et al., 2018), no ERP study to date has used the subsequent memory paradigm to examine the effects of self-referential processing on memory encoding. This has limited the understanding of the ways in which the self modulates ERP components implicated in memory formation or, akin to the fMRI literature (e.g., Macrae et al., 2004), whether distinct processes that reflect aspects of thinking about the self are recruited to support memory formation.
The goal of the present work was to examine whether the ERP subsequent memory effect—that is, the differential neural activity at encoding for items that will later be remembered versus forgotten—is modulated by self-referencing. As such patterns are common in the fMRI literature, but have not been the focus of the ERP literature, these results would better connect these literatures. In addition, as ERP has much better timing and is sensitive to different aspects of neural activity than fMRI, using ERP to investigate the SRE may give further insight into the mechanisms that underlie the SRE.
We recorded EEG while participants encoded trait adjectives in relationship to themselves or a well-known other. Our key question was whether the ERP subsequent memory effect differs in size or nature (e.g., scalp distribution) for self-relevant and non-self-relevant encoding conditions. To the extent that self-referencing leads to unique neural processes that contribute to later memory, we should see differences in the nature of the subsequent memory effect.
Experiment 1
Methods
Participants.
Thirty-two younger adults recruited from Brandeis University participated in the study after providing informed consent. Two participants were excluded due to excessive artifacts in ERP (defined as rejecting more than 25% of trials overall or having fewer than 60 trials in any of the four Valence x Self-Relevance bins), leaving 30 participants included in the analyses (18–32 years old (M=21.90); 22 females, 8 males). Participants had normal or corrected-to normal vision and were all right-handed and native English speakers (defined as either the first language or learned before the age of five). Participants had no history of neurological, affective, or psychiatric disorders. The Institutional Review Board at Brandeis University approved this study.
Stimuli and Procedure.
The stimuli were presented on a computer screen using E-Prime software by Psychology Software Tools. Participants completed an incidental encoding task followed by a surprise memory test later in the study. Participants were given either one of two conditions for each trial. In one condition they judged whether the presented word described themselves. In the other condition, they judged whether the word described Albert Einstein. The visual angle of the words was approximately 6° 46’ wide x 0° 36’ tall. The participants were given a brief training period in which they learned the task and to respond on a computer keyboard with “1” for yes and “2” for no. Afterwards they performed this task while EEG data were being recorded. The participants were instructed to focus their eyes on a jittered black fixation cross that appeared on the screen which was jittered for between 1000 and 1500 milliseconds in the beginning of each trial. After viewing the cross, the participants were shown the condition, either “Self” or “Albert Einstein” for 1000 milliseconds. Albert Einstein was selected for the other condition as he is familiar and most people have a fairly positive view of him (to mirror the self-positivity bias in the self-condition) but do not know him personally. Participants then viewed an adjective for 2000 milliseconds before a question mark appeared and prompted them to make a “yes” or “no” response to whether the adjective described the target person (self or Albert Einstein). After the participant answered or the 2000 milliseconds ended, the next fixation cross appeared. Encoding was separated into three blocks which each contained 100 words for a total of 300 words. The words were equally divided under the self and Albert Einstein conditions (150 each) and not repeated. The words were also divided within conditions to be half positive and half negative words (75 each) and equated for likeability, as per Anderson (1968). There were three different versions of the task such that each set of words was assigned once to each condition: self, Albert Einstein, and those that were only used as “new” in the retrieval task. Those words were then presented in a random order to the participant with the matching condition.
Following the encoding period, there was a twelve-minute period before retrieval in order to control the retention time between participants. During this time, the EEG cap was removed, and participants completed the digit comparison and Shipley vocabulary task to prevent rehearsal between encoding and retrieval. For the digit comparison task (Hedden et al., 2002), participants decided whether two strings of numbers were identical and checked a column to indicate “same” or “different”. Participants were given 45 seconds for each of three sections, consisting of strings of 3, 6, or 9 digits, to complete as many items as possible. The Shipley vocabulary task (Shipley, 1986) consisted of 40 items for which participants attempted to select the correct synonym of a target word, amongst four alternatives on each trial.
Following the twelve-minute period, the participants completed the unexpected retrieval task using the E-prime software on the computer. Participants were presented with an adjective and instructed to indicate whether the word was from the encoding task or a new word. Again, participants responded with a keyboard, using the “1” key press to indicate “old” and the “2” key press to indicate “new”. The task was self-paced such that responding terminated the trial and initiated the next trial. There were no fixed interstimulus intervals but participants were instructed that they could stop responding and take breaks as needed. The task contained 450 words, 300 previously viewed words (150 encoding words from the Albert Einstein condition and 150 self-condition words) and 150 new adjectives. The retrieval task also had three counterbalanced lists corresponding to the encoding versions.
EEG acquisition and processing.
EEG was recorded during the encoding phase of the experiment using a BioSemi Active Two system. Voltage offsets were kept within the recommended range (±40 mV). EEG signals were recorded from 32 Ag/AgCl electrodes in an elastic cap placed according to the international 10–20 system. In addition, electrodes were placed to the lower left corner near the left eye and the upper right corner near the right eye to monitor for blinks and eye movements, along with electrodes on each mastoid to serve as the reference. The signals were amplified, run through an online low pass 5th order sinc response filter with a half-amplitude cutoff of 104 Hz, and continuously sampled at 512 Hz. An online high pass filter was not used.
Data processing and analysis.
Preprocessing and analyses were performed in EEGLAB (https://sccn.ucsd.edu/eeglab/index.php; Delorme & Makeig, 2004) and ERPLAB (https://erpinfo.org/erplab; Lopez-Calderon & Luck, 2014) in MATLAB 2017b. EEG signals were re-referenced to the average of the two mastoid electrodes. Testing with an oscilloscope revealed a consistent delay of 300 ms between the event marker recorded by the EEG system and the presentation of the stimulus, which was corrected in ERPLAB by shifting event markers. Segments of data from breaks in the task (periods of greater than 10 s between event codes) were automatically removed. Then, for each period of continuous EEG data, the DC offset was removed by subtracting the average voltage for the segment and filtered using a 2nd-order Butterworth infinite impulse response high pass filter with a half-amplitude cutoff at 0.1 Hz to remove drift (Kappenman & Luck, 2010).
The next step was independent components analysis (ICA) to correct for ocular artifact. First, the continuous data were visually inspected and any segment containing significant artifact that was not neural, ocular, or muscular was marked and excluded from the data submitted to ICA. Independent components were calculated from the remaining segments using the extended infomax algorithm in EEGLAB (Lee et al., 1999).
Epochs were than extracted 200 milliseconds prior to the event to 1100 milliseconds after and the mean of the pre-stimulus period was subtracted from each (see supplementary figures S1 and S2 for baseline uncorrected data). The previously calculated ICA solution was applied to the now epoched data. Independent components corresponding to ocular activity, specifically blinks and saccades, were identified by visual inspection and removed, which consisted of either 1, 2, or 3 components for each subject. Epochs of raw data with remaining artifacts were identified via artifact detection algorithms in ERPLAB1. Any trial containing a blink or large saccade within the first 200 milliseconds of a trial was rejected even if the artifact was corrected via ICA, as these trials may have a delayed neural response due to eyes being closed or averted during the time of stimulus presentation. The rejection rates ranged from 0.0% to 21.6%, with any participant with a rejection rate higher than 25% or with fewer than 60 trials remaining in each bin (divided by Self-Relevance and Valence) being excluded from the analysis. This number was selected with the goal of approaching 30 trials per bin when further divided into remembered and forgotten bins (information on the number of trials averaged for each bin is included in Figure S3 in the Supplementary Materials). The remaining trials were averaged within the conditions of interest to form ERPs.
The data were initially binned by Valence and Self-Relevance, leading to four bins with a maximum of 75 trials in each (positive self, negative self, positive other, and negative other). Although our predictions focused on comparisons of self and other, lists consisted of positive and negative words. The salience of valence led us to model this factor in analyses. Trials were additionally binned using the behavioral retrieval data by subsequent memory. If the word was correctly remembered (given an “Old” response when the word was previously seen), it was binned as remembered and then separated by Self-Relevance condition. If the word was in the encoding portion but forgotten (given a “N” response incorrectly), it was binned as forgotten and then also separated by Self-Relevance condition (see supplementary Figure S3 for bin counts).
ERP analysis.
Statistical analysis of ERP data was conducted via the mass univariate approach implemented in the Mass Univariate Toolbox (Groppe et al., 2011) and the Factorial Mass Univariate Toolbox (Fields, 2017). This method involves conducting a statistical test at each electrode and time point and applying specialized multiple comparison corrections (Groppe et al., 2011). This allows for a more data-driven approach to identifying when and where effects occur without significant loss of power (Fields & Kuperberg, 2020). The code, stimuli, and the mass univariate results are available at https://osf.io/svu4c/. We applied a cluster correction: electrodes and time points with effects that exceed the pre-determined threshold (the F-value that would produce p = .01 in an uncorrected parametric analysis) are identified, and the F-values at adjacent time pointes/electrodes are summed to form a cluster statistic. The null distribution of this cluster statistic is estimated by a permutation approach and is used to calculate a p-value for the cluster. 100,000 permutations were performed for each test.
Statistical analysis was conducted on data that were first low pass filtered (2nd-order Butterworth infinite impulse response) with a half-amplitude cut-off at 10 Hz to eliminate high frequency noise that reduces power in mass univariate analysis. We first ran analyses examining the effects of Self-relevance and Valence during encoding without regard to later memory, resulting in a 2 (self, other) x 2 (positive, negative) ANOVA. Based on previously observed effects of self in ERPs (see Introduction) as well as the ERP subsequent memory literature (Wagner et al., 1999; Wilding & Ranganath, 2012), our main analysis focused on the 500–1000 ms time window. Based on previously observed effects of a self-positivity bias on the N400 component (Fields & Kuperberg, 2015), we also conducted an analysis in the traditional N400 time window of 250–500 ms. Because effects have been shown with a range of scalp distributions, all electrodes were included in both analyses.
Because we found no effects of Valence in the main time window of interest and no interactions between Valence and Self-relevance, we simplified analyses and maximized power in our subsequent memory analysis by collapsing across the Valence factor. This led to a 2 (self-relevance: self, other) x 2 (Memory: remembered, forgotten) ANOVA. Based on the results of the initial analyses and the ERP subsequent memory literature, this analysis was conducted at all electrodes from 500 – 1000 ms.
Results
Behavioral Measures:
In presenting the results, we first test behavioral measures to assess whether self-referencing enhances memory performance, compared to other-referencing. Because stimuli consist of adjectives that are positive or negative in valence, we also include valence as a factor. Analyses of reaction time for decisions at the time of encoding are included for completeness. 30 out of 32 participants were included in the final analysis.
Memory Accuracy.
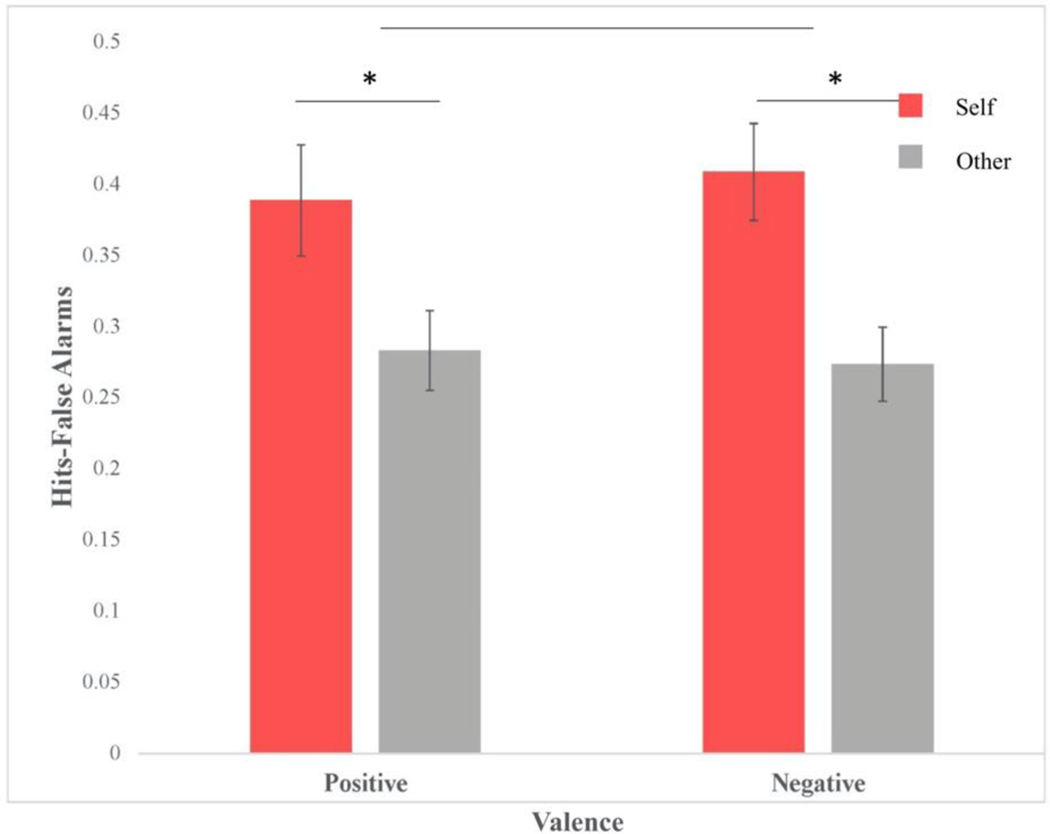
Performance on the retrieval task was assessed by calculating a memory score for each participant in the final sample of 30 for each condition and Valence of the adjective. The memory score consisted of the hit rate for the “old” items minus the false alarm rate for the “new” items. A repeated measures 2 X 2 ANOVA was performed. There was a main effect of Self-Relevance (Figure 1), with higher memory scores for words in the self-condition (M=0.40, SD= 0.19) than for those in the other condition (M=0.28, SD=0.12), F(1, 29) = 23.18, p< 0.001, ηp2= 0.442, as would be expected on the basis of prior work. However, Valence did not have an effect on memory, F(1, 29) = 0.060, p= 0.809, ηp2= 0.002, and there was not a significant interaction between word Valence and Self-Relevance, F(1, 29) = 0.81, p=0.38, ηp2= 0.03.
Figure 1. Self-Referential Processes Enhance Memory.
Memory accuracy, as defined by the rate of false alarms at retrieval subtracted from the rate of correct identifications or hits at retrieval, was compared for Self-Relevance (self or other) and Valence (positive or negative). Memory accuracy was significantly higher for self compared to other regardless of Valence. There was no significant effect of Valence or interaction of Self-Relevance and Valence. This demonstrates that the self-referencing effect of memory was induced for this experiment.
Reaction Time at Encoding.
The reaction time to the adjective at encoding was analyzed based on whether the adjective was presented in the self or other condition and by if it was correctly remembered upon retrieval (see Figure S4). A repeated measures 2 X 2 ANOVA was performed. The ANOVA revealed a significant main effect for memory, F(1, 30) = 5.37, p=0.03, ƞp2 = .15, such that words that were later remembered at retrieval had significantly faster reaction times at the time of encoding (M = 571.87, SD = 215.37) than words that were later forgotten (M =595.14, SD = 216.47). There was no significant main effect of Self-Relevance (p=0.77) and no interaction (p=0.56).
ERP Measures:
Main effects of self-reference and valence are discussed first, divided into earlier (250–500ms) and later (500–1000ms) time windows. Results are then discussed in terms of main effects or interactions involving memory.
N400 time window (250 – 500ms).
Starting around 200 ms, a more positive amplitude was seen to positive words at posterior electrodes. This was reflected in a significant cluster centered around left posterior electrodes and spanning the whole time window (p = 0.004); see supplementary Figures S5 and S7. There was also a more negative amplitude to the self condition at posterior electrodes. A cluster spanning 260 – 455 ms and centered around right parieto-occipital electrodes was significant (p = 0.002) (see Figure 2 and supplementary Figures S5 and S7). Valence and self-relevance did not interact (all clusters p > 0.2).
Figure 2. ERP Results at Encoding Grouped by Self-Relevance and Memory.
ERP waveforms show the response at encoding to words presented with the self or other and grouped by whether they were remembered (correct “yes” response at retrieval) or forgotten (incorrect “no” response at retrieval). ERPs are filtered with a 10Hz half-amplitude low pass filter. The x-axis shows time in milliseconds post stimulus and the y-axis shows voltage in microvolts with positive down and negative up. Frontal electrodes showed a positive deflection beginning around 500 milliseconds for self compared to other as well as remembered compared to forgotten, although this effect was only significant for Self-Relevance.
Late time window (500–1000).
There were no significant effects of Valence or interactions between Valence and Self-Relevance in the 500–1000 ms time window (all ps > 0.08). There was an effect of self-relevance with words in the self condition eliciting a larger positivity at frontal electrodes from around 450 ms to the end of the epoch (see Figure 2). This was reflected in a significant cluster from 525 ms to the end of the time window at a broad range of frontal, central, and parietal electrodes (p = 0.002).
Subsequent memory analysis (500 – 1000 ms).
As shown in Figures 2 and 3, the frontal positivity reported above for the main effect of self vs other (collapsed across remembered and forgotten) was also significant in this analysis (p = 0.015). A very similar frontal positivity emerged for the main effect of memory, larger for words that were subsequently remembered compared to those subsequently forgotten, beginning at around 500 milliseconds (see Figure 2). As seen in Figure 3, a frontally-distributed cluster for the main effect of memory beginning at 861 milliseconds and continuing until the end of the epoch was identified in the mass univariate analysis, but this cluster was not significant (p = 0.053). There was no significant interaction between self-relevance and memory (no clusters found).
Figure 3. Output of the Cluster-Corrected Statistical Analysis for Experiment 1.
The mass univariate approach was used to analyze the effect at all electrodes from 500–1000 ms. The x-axis shows the time in milliseconds while the y-axis shows the electrode (with the left side of scalp on top and the right on the bottom) from front to back of the head. Time point/electrode combinations not included in a significant cluster are in gray. For locations included in a cluster, the color represents F-statistic at that location. Alpha was raised to 0.1 to show the non-significant cluster for Memory.
Discussion
This aim of this study was to assess the neural correlates of the self-reference effect in memory using ERP methods. The manipulation succeeded in inducing the self-reference effect, as seen in the behavioral data for which memory scores were significantly higher for words related to the self than words related to others. Memory scores did not differ significantly for positive compared to negative words, nor did valence interact with self- or other-reference. Of most importance, a significant late positive frontal component emerged that was larger for the self than other. A very similar difference, in terms of scalp distribution and timing also emerged in response to words that were subsequently remembered compared to those that were forgotten, but this effect did not pass the threshold for significance.
Experiment 2
In addition to indicating a role for self-referencing, the results of Experiment 1 suggest that the late frontal positive component may play a role in memory formation as the effect was similar for Self-Other and Remember-Forgotten. However, a limitation of the study is that the memory effect was not significant, possibly due to the old/new task providing a very coarse measure of memory. That is, the items judged as “old” will not only contain trials with varying memory strengthens but also correct guesses. In addition, the presentation of twice as many old as new items could induce biases to respond “old”, potentially inflating the number of correct guesses, which would further weaken the memory signal present in the analyses. In order to get a clearer measure of the ERP response associated with subsequent memory, a second experiment was performed using remember/know judgements in the retrieval phase. In the remember/know paradigm, participants decide whether they are able to re-experience a contextually rich episodic memory (remember) or simply have a sense that they encountered a stimulus or piece of information previously (know), without access to the contextual information necessary to re-experience it. Prior work suggests that self-referencing enhances the experience of recollection (Leshikar, Dulas, & Duarte, 2015), which suggests that self-referencing will increase the number of accurate “remember” responses. ERP methods underscore the distinction between the neural signatures for familiarity and recollection at both encoding and retrieval (e.g., Rugg & Curran, 2007). We adopted this approach to more clearly delineate trials for which a strong memory (remember response) was formed, which reflects a vivid re-experiencing of the event in memory, as opposed to those trials reflecting only familiarity. This design allows true recollection and memory effects to be distinguished from guessing in order to identify the correlates of stronger memory formation. We hypothesize that words relating to self will elicit a frontally-distributed late positive component with a larger amplitude than other-related words, and this component will predict subsequent memory performance in recollection. Additionally, we were interested in whether there would be an interaction of Memory and Self-Relevance for this component, with the subsequent memory effect (remembered-forgotten) being larger for self trials than other trials. Such a pattern would signify that this component is particularly related to self-referencing.
Methods
Participants.
Thirty-five younger adults recruited from Brandeis University participated in the study after providing informed consent. Two participants were excluded due to excessive artifacts in ERP (defined as rejecting more than 25% of trials). One participant was rejected due to receiving a negative hit minus false alarm memory score, signifying that the task was not understood or completed correctly. Two participants were rejected due to using the “R” response less than 10% of the time and four additional participants were rejected due to moderate EEG rejection rates and moderate “R” key press use resulting in too small bin sizes (< 60 trials) for subsequent memory analysis.
Twenty-six participants were included in analyses with a mean age of 19.54 years old (SD = 1.24), ranging from age 18 to 21. Seventeen of the participants were female and nine of the participants were male. Participants were sampled using the same inclusion and exclusion criteria as Experiment 1. The Institutional Review Board at Brandeis University approved this study.
Stimuli and Procedure.
The stimuli were presented using E-Prime software by Psychology Software Tools. The task was the same as in Experiment 1 with a few exceptions. First, Queen Elizabeth was used as the other, replacing Albert Einstein (Leshikar & Duarte, 2012). This was in order to present an alternative other to test the generalizability of the findings in Experiment 1, particularly when a living female target is used as the other. Therefore, participants were shown the following instructions: “Your task is to decide if the word describes either yourself or Queen Elizabeth by indicating yes or no”. In addition, the interstimulus interval between the cue and the adjective was also jittered, randomly varying between 100 and 300 milliseconds, to reduce anticipatory ERPs and to avoid phase-locking of non event-related neural oscillations.
Following the encoding period, there was a twelve-minute period between encoding and retrieval. During this time, the EEG cap was removed, and participants completed the digit comparison task to prevent rehearsal between encoding and retrieval. Following the twelve-minute period, the participants completed the retrieval task using the E-Prime software on the computer. Participants were presented with an adjective and instructed to indicate if the word was from the encoding task or a new word, similar to the task in Experiment 1. However, instead of a yes/no decision, the participants were instructed to make a Remember (“R”) /Know (“K”)/Guess (“G”) /New (“N”) response. They were instructed to press the “7” key for remember and “8” for know. Additionally, participants could press “9” for guess if they cannot discern if the word was in the previous task or not and “0” for new if the word was not from the previous task. Instructions for the remember/know task followed Rajaram (1993). The task was self-paced, and therefore there were no fixation crosses, cues, or breaks built into the task. The task contained 450 words, 300 previously viewed words (150 encoding words from the Queen Elizabeth condition and 150 self-condition words) and 150 new adjectives.
EEG acquisition and processing and ERP analysis.
EEG data collection and analysis were performed in the same manner as Experiment 1 with the following exceptions.
For this study, ICA decomposition was performed after data were epoched rather than on the continuous data. The mean voltage was subtracted from each segment before the data were submitted to ICA (Groppe, 2009); the data were re-baselined to the pre-stimulus period for analysis. This change was due to a general change in the lab approach rather than anything specific to this study.
Trials were additionally binned using the behavioral retrieval data by subsequent memory. Items remembered (given an “R” response) were binned as remembered and then separated by Self-Relevance condition (Self or Other). Limiting analysis of remembered items to only “R” responses, excluding “K” responses, allows for the consideration of those items with the strongest memory traces. Memory performance is often near chance for “K” responses, as was the case for the data in this experiment. If the word was in the encoding portion but forgotten (incorrectly given a “G” or “N” response), it was binned as forgotten and then also separated by Self-Relevance condition (see supplementary figure S3 for bin counts).
Time windows and electrodes for analysis were based on results from Experiment 1. The Self-Relevance x Valence ANOVA was conducted in a 500–1000 ms time window at all electrodes included in a significant cluster for the Self-Relevance main effect in Experiment 1 (Fp1, AF3, F7, F3, FC1, C3, CP1, P4, CP6, CP2, C4, FC6, FC2, F4, F8, AF4, Fp2, Fz, Cz). The Self-Relevance x Memory ANOVA (collapsed across Valence) was conducted in a 600–1000 ms time window at all electrodes included in the nearly significantly memory cluster in Experiment 1 (Fp1, AF3, F7, F3, FC1, FC5, T7, C3, CP1, CP5, P3, Pz, PO4, CP2, FC6, FC2, F4, F8, AF4, Fz, Cz). Because these analyses were somewhat more focused, we also conducted an exploratory analysis at all electrodes for the entire epoch to check for unexpected effects.
Results
Behavioral Measures.
In presenting the results, we first test behavioral measures to assess whether self-referencing enhances memory performance, compared to other-referencing. Because stimuli consist of adjectives that are positive or negative in valence, we also include valence as a factor. Analyses of reaction time for decisions at the time of encoding are included for completeness. 26 participants were included in the final analysis.
Memory Accuracy.
Performance on the retrieval task was assessed by calculating a memory score for each participant for each condition, Valence of the adjective, and response type (“R” for remember or “K” for know). The memory score consisted of the “old” terms minus the false alarm rate for the “new” items, in which a “new” item was given the incorrect response of “R” in the remember condition or “K” in the know condition. Repeated measure 2×2 ANOVAs were performed separately for the response types (“R” or “K”), comparing effects of Self-Relevance and Valence due to “R” and “K”. For words given an “R” response, there was a main effect of Self-Relevance (Figure 4), with words in the Self condition (M=0.38, SD=0.16) having higher memory scores than those in the Other condition (M=0.23, SD=0.12), F(1, 25) = 132.58, p< 0.001, ηp2= 0.843. This effect reflects the expected benefit of self-referencing on memory. For words given a “K” response, there was no significant effect of Self-Relevance, with words in the self-condition (M=0.03, SD=0.10) not significantly differing from those in the other condition (M= 0.05, SD=0.08), F(1, 25) = 3.52, p=0.07, ηp2= 0.12. For words given an “R” response, there was no significant interaction of Valence and Self-Relevance, F(1, 25) = 0.03, p=0.87, ηp2= 0.001. For words given a “K” response, there was also no significant interaction of Valence and Self-Relevance, F(1, 25) = 1.76, p=0.20, ηp2= 0.07.
Figure 4. Self-Referential Processes Enhance Recollection, not Familiarity.
Memory accuracy, as defined by the rate of false alarms at retrieval subtracted from the rate of correct identifications or hits at retrieval, was compared for Self-Relevance (Self or Other), Valence (Positive or Negative), and whether the word was given a Remember (“R”) or Know (“K”) response in Experiment 2. Memory accuracy was significantly higher for self compared other when given an “R” response regardless of Valence. When given a “K” response, memory accuracy did not differ by Self-Relevance or Valence. This demonstrates that the self-referencing effect of memory was induced only in the words given an “R” response in this experiment.
Reaction Time at Encoding.
The reaction time to the adjective at encoding was sorted by Self-Relevance, if it was correctly remembered upon retrieval, and if remembered, whether it was given an “R” or “K” response (see supplementary figure S4). A 3×2 ANOVAs for “R” and “K” responses were performed to compare reaction times at encoding based on Self-Relevance condition and Subsequent Memory. There was no main effect of Self-Relevance (p=0.17) or Memory (p=0.20) on reaction time. Additionally, there was no significant interaction of Memory and Self-Relevance for reaction time (p=0.28).
ERP Measures:
Main effects of self-reference and valence are presented first, followed by analyses of main effects or interactions involving memory.
Analyses of Self-Relevance and Valence.
As in Experiment 1, we observed a larger frontal positivity to self than other from around 500 ms to the end of the epoch (see Figure 5). In addition, there was a short-lived Self-Relevance x Valence effect (502 – 572 ms at 7 anterior electrodes, p = 0.04) (see supplementary figures S6 and S7). There was no significant main effect of Valence (ps > 0.2). The exploratory analysis at all electrodes and time points did not reveal any additional effects of any factors.
Figure 5. ERP Results at Encoding Grouped by Self-Relevance and Recollection.
ERP waveforms from Experiment 2 show the response at encoding to words presented with the self or other and grouped by whether they were remembered (given a correct “R” response at retrieval) or forgotten (given an incorrect “N” or “G” response at retrieval). ERPs are filtered with a 10Hz half-amplitude low pass filter. The x-axis shows time in milliseconds post stimulus and the y-axis shows voltage in microvolts with positive down and negative up. There were significant main effects of Self-Relevance and Memory beginning around 500 milliseconds at frontal electrodes, though the interaction did not reach significance.
Subsequent memory analysis.
As shown in Figures 5 and 6, the frontal positivity to the main effect of self vs. other (collapsing across remembered versus forgotten) was again reflected in a significant cluster (604 – 986 ms at frontal central electrodes, p = 0.01). We also observed a main effect of memory (collapsed across self and other) on a similar frontal positivity which was larger to remembered than forgotten and which was reflected in a significant cluster (666 – 1002 ms at frontal and central electrodes, p = 0.002). The critical test of the interaction between self-relevance and memory did not reveal any significant clusters (ps > 0.3).
Figure 6. Output of the Cluster-Corrected Statistical Analysis for Experiment 2.
The x-axis shows the time in milliseconds while the y-axis shows the electrode (with the left side of scalp on top and the right on the bottom) from front to back of the head. Time point/electrode combinations not included in a significant cluster are in gray. For locations included in a cluster, the color represents F-statistic at that location.
The exploratory analysis at all electrodes and time points did not reveal any additional effects of any factors.
Discussion
The second experiment aimed to replicate the Experiment 1 results for the ERP components underlying self versus other-referencing while strengthening the subsequent memory effect by better differentiating the memory signal for strongly encoded items by using the R/K paradigm. This study successfully induced the self-reference effect in memory, with memory scores for self significantly higher than for other, though this pattern only emerged for “R” responses and not for “K” responses. The study also successfully replicated the late frontal positive component for Self-Relevance found in Experiment 1. Additionally, the subsequent memory late frontal positivity component was significant when the “R” responses were used, indicating that this component contributes to successful memory formation. However, no interaction between Self-Relevance and Memory emerged, suggesting that this component may not be exclusive to self-referential memory enhancements.
General Discussion
In two experiments, we investigated how self-referencing affects the processes recruited to support memory formation. The results from both studies revealed a larger frontally-distributed late positive component for words in the self versus other condition. In Experiment 2, this component also predicted subsequent memory performance for items strongly encoded enough to allow for recollection at retrieval, with a larger amplitude for those that are recollected compared to those later forgotten. This finding suggests that the frontal positive component is a marker of successful encoding of the words that leads to better subsequent memory. Additionally, the results of the second experiment converge with literature suggesting differences between familiarity and recollection in that the behavioral memory effect was seen for R but not K (Duarte et al., 2004; Mao et al., 2017). The importance of the distinction between R and K is also indicated across experiments, as the frontal positive component for subsequent memory only emerged as significant when the classes of responses were distinguished in Experiment 2, but not when “Remember” responses were not differentiated on the strength of the memory, as in Experiment 1.
The late positive component in relation to self in comparison to other was supported by both studies, with similar time scales and scalp distribution corresponding to the effect. These results may suggest that this late positive component is elicited in the self-referencing process. Notably, frontal activation is often considered a marker of self-processing in the fMRI literature (Leshikar & Duarte, 2012; Powell et al., 2010; Mitchell et al., 2006; Kelley et al., 2002; Gutchess, Kensinger, & Schacter, 2007). It should be noted, however, that a frontal distribution in EEG does not necessarily mean a frontal neural source; one limitation of this research is that it is unclear whether the component identified in the present study is generated in the mPFC.
Our results are consistent with several previous studies that have shown a frontal positivity when making self-related judgments or retrieving self-related information (Kotlewska & Nowicka, 2016; Magno & Allan, 2007; Mao et al., 2017; Nowicka et al., 2018). Notably, this contrasts with a number of other studies showing posterior effects for self-relevant stimuli such as the participant’s name, face, or objects (e.g., Gray et al., 2004; Tacikowski & Nowicka, 2010; reviewed in Knyazev, 2013). These results suggest that self-relevance has differing effects when manipulated via features of the stimuli versus the task. This may relate to differences in bottom-up capture of attention versus top down modulation of processing, a point we return to below.
In terms of the subsequent memory effect, we saw a similar frontal positivity that was larger for words that were subsequently remembered in both studies. Although this effect was not statistically significant in Experiment 1, Experiment 2 demonstrated that the frontal positive component is a marker of successful memory formation when tested for strongly encoded words. Words that were remembered correctly and associated with recollection during the retrieval task elicited this frontal positive component more than did words that were subsequently forgotten, collapsing across the self and other conditions. Therefore, this component can be considered a marker of a process that supports stronger encoding such that a word is more likely to be retrieved at a later time point with vivid detail.
A diverse set of effects have been associated with subsequent memory in previous ERP research, but the two most common are a posterior positivity, often associated with the P300, and a frontal positivity similar to that reported here (reviewed in Forester et al., 2020; Wagner et al., 1999; Wilding & Ranganath, 2012). The posterior positivity has most often been seen when subsequent memory is most strongly related to aspects of stimuli capturing bottom-up attention. When a task leads participants to engage in elaborative processing, it is instead the frontal positivity that is most strongly associated with later memory. For example, Fabiani, Karis, and Donchin (1990) showed that when participants were instructed to use a simple rote memory strategy, memory was significantly influenced by the distinctiveness of the stimulus and subsequent memory was predicted neurally by a posterior positivity. When participants were instructed to use an elaborative strategy, the distinctiveness of the stimulus had a much smaller effect and memory was predicted by a long-lasting frontal positivity rather than the posterior positivity. This distinction may explain the trends in the ERP literature noted above. When individual stimuli are self-relevant (e.g., the participant’s own name), they are distinctive and lead to deeper encoding as represented by the P300/late positivity. When self-relevance is manipulated via the task rather than the stimuli themselves, deeper encoding takes the form of strategic elaborative processing reflected by the frontal positivity. The present work shows that this deeper encoding can predict later memory.
Based on the fMRI literature, we had predicted there would be an interaction between Self-Relevance and Memory, with the subsequent memory effect being different or larger for the self versus other condition. Although both the effects of self-relevance and subsequent memory appeared to modulate the same component in the present work, there was no significant interaction between memory and self-relevance. Thus, the words that were correctly remembered and elicited the frontal positive component were not exclusive to the self-referencing condition. This pattern of findings is in contrast to the fMRI literature, in which the mPFC uniquely predicts encoding success in the self-referencing condition. This divergence in the results from the two methods suggests that the detected neural signatures indicate different aspects of the memory formation process. Whereas fMRI identifies a medial prefrontal component that contributes to memory formation disproportionately more for self-referential information, the ERP component we identify reflects more general processes that contribute to successful encoding but are not unique to self-referencing. Considered on their own, these ERP findings would not be seen as providing support for the idea that self-referential memory is “special”, and could even be interpreted as evidence against the claim (Gillihan & Farah, 2005; Legrand & Ruby, 2009). Taken together, the findings across methods underscore the multiple processes that contribute to successful memory formation (Macrae et al., 2004; Paller & Wagner, 2002), in line with claims that the self is a multifaceted construct rather than operating as a unitary construct (Gillihan & Farah, 2005; Swann & Bosson, 2010).
Of course, it is possible that such an interaction between self-relevance and memory is present, but that we simply did not have the power to detect it. This could be a limitation of the present research, and is difficult to rule out without very large samples. Indeed, visual examination of the results of Experiment 2 does suggest that the subsequent memory effect on the frontal positivity is larger for the self condition. On the other hand, both studies showed evidence for a main effect of memory without much statistical evidence for an interaction. Although the main effect was just over the threshold for significance in Experiment 1 (p = .053), there were not even a single electrode/time point that surpassed the threshold for cluster inclusion for the interaction. In the second study, the main effect was significant, while the interaction again did not approach significance (p = 0.36). Thus, the results across studies suggest that the main effect of subsequent memory is a stronger pattern in the data than any interaction between the two factors.
The lack of an interaction with significant effects of Memory and Self-Relevance may signify that the component does not demonstrate an encoding process unique to self-referencing. Instead, the frontal positivity we observed may be a more general marker of deeper or more elaborative processing, as observed in a number of previous ERP studies of subsequent memory effects (Fabiani, et al., 1990; Wagner et al., 1999; Wilding & Ranganath, 2012). The self-reference effect is known to improve subsequent memory when words are associated with the self. Therefore, the component would be elicited more for self than other because the self prompts deep processing and better memory than others. However, there would be no interaction because words that are remembered would elicit the component more than those that are forgotten, regardless of whether they were associated with self or other. This explanation of the lack of an interaction suggests that the self-reference effect in memory does not induce a separate encoding process but instead encourages deeper encoding that leads to better subsequent memory.
Although this explanation seems consistent with a depth of processing account of the SRE and accords with the ERP subsequent memory literature, it leaves open the question of why we did not see unique neural contributions to memory for self-relevant stimuli as has been seen in the fMRI literature. ERP and fMRI are complementary techniques that are sensitive to different aspects of brain activity, so results from the two methods will not always mirror each other (Ekstrom, 2010; Murta et al., 2015). One possibility is self-specific activity seen in MRI takes place over a longer time scale and is less reflected in the 1 second time window we examined. Another possibility is that such processes are distributed over time rather than linked to a discrete time-locked component; should this be the case, time-frequency analysis may be well-suited to detect such effects (Knyazev, 2013; Mu & Han, 2010). Alternatively, it may be that bilateral effects generated on the medial surface of the cortex cancel out due to electrical dipoles with opposite orientations. Thus, the activity in the mPFC and other cortical midline structures commonly associated with self-related processing may not generate a strong signal in EEG.
The results of the present studies point to several directions for future research. As this is the first study to examine the effects of self-referencing on the ERP subsequent memory effect, an important first step will be to replicate these results, in particular to confirm the lack of a substantial difference for the subsequent memory effect to self and other conditions. Given the apparent discrepancy between the present results and the fMRI literature, and given the much more significant fMRI literature on the self, it may be valuable to conduct joint ERP-fMRI research to examine how ERP correlates of self-relevance and their relationship to memory are related to the widely examined fMRI correlates of self. Understanding the functional significance of the frontal positivity we observed would be aided by examining a wider range of encoding conditions. In particular, it will be important to test whether effects of self-referencing can be distinguished from other depth of encoding manipulations. For example, future work could compare self-referencing and other-referencing to semantic and structural encoding tasks (cf. Rogers et al., 1977). Above we raised the possibility that self-relevance manipulated via stimuli and tasks elicit different effects. Future research should seek to confirm this pattern by comparing the two approaches in the same dataset. It will also be valuable to examine whether the same holds true for subsequent memory: that is, if self-relevance is manipulated via the stimuli, will subsequent memory be predicted by a posterior positivity rather than a frontal positivity? Finally, given the continuing importance of self-referencing for memory throughout the lifespan, it will be important to examine whether subsequent memory effects with self-referencing are stable or changing as we age (Kensinger & Gutchess, 2015, 2017).
In summary, the results of these studies implicate a late frontal positive component in both self-referencing and subsequent memory. Words associated with the self condition elicited this component more than words associated with the other condition as did words that were subsequently remembered in retrieval compared to those forgotten. This suggests that this component reflects a deeper cognitive process that is evoked both when information is associated with the self and when information is more strongly encoded into memory. In contrast to prior findings with fMRI, the same component that reflects successful memory formation also responds to referencing the self and these effects were additive. The existence of this component opens up a new avenue for bridging the fMRI and ERP literatures on the self-reference effect, implicating the frontal cortex, but diverges from the fMRI literature in indicating that deeper processing is the locus of self-referencing memory benefits.
Supplementary Material
Acknowledgements
We thank the Brandeis University Provost Undergraduate Research Fund for supporting this work. Additionally, we thank Wanchen Zhao, Amerta Bai, Maayan Sofer, and Nishaat Mukadam for help collecting and analyzing data. Eric Fields was supported by NIH T32 NS007292.
Footnotes
Declaration of Interest
The authors report no conflict of interest.
We used the moving window peak-to-peak function (the difference between the most positive and most negative voltages within a window) and the step function (the difference between the mean voltage from the first half and second half of a window) as implemented in ERPLAB (https://github.com/lucklab/erplab/wiki/Artifact-Detection-in-Epoched-Data). A peak-to-peak function was applied to the difference between a forehead channel (Fp1, or Fp2 if Fp1 showed a lot of non-ocular noise) and the electrode under the left eye in 200 ms windows with a threshold around 75 μV to detect blinks. Step functions were applied in both 400 ms (to detect brief deflections) and 1000 ms (to detect slow drift) time windows at all channels with thresholds around 55 μV. Finally, a peak-to-peak function was applied in 400 ms time windows at all channels with a high voltage threshold (~300 μV) to detect particularly large EMG and other artifacts not picked up by the step function. Precise thresholds were sometimes adjusted for individual subjects’ data based on visual inspection, but applied equally to all trials (and thus all conditions) for each subject (Luck, 2014, p. 191).
Analyses of d’ scores converged with the results from hits minus false alarms. Scores were significantly higher for self (M = 1.20, SD = .54) than other (M = .79, SD = .39), t(29) = 8.05, p < .001.
Analyses of d’ scores converged with the results from hits minus false alarms. Scores were significantly higher for self (M = 1.22, SD = .47) than other (M = .80, SD = .37), t(25) = 11.40, p < .001.
References
- Anderson N. (1968). Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology, 9(3), 272–279. 10.1037/h0025907 [DOI] [PubMed] [Google Scholar]
- Araujo HF, Kaplan J, & Damasio A. (2013). Cortical midline structures and autobiographical-self processes: An activation-likelihood estimation meta-analysis. Frontiers in Human Neuroscience, 7(548), 1–10. 10.3389/fnhum.2013.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnkrant RE & Rao Unnava H. (1989). Self-referencing: A strategy for increasing processing of message content. Personality and Social Psychology Bulletin, 15(4), 628–638. [Google Scholar]
- Cunningham SJ, Brebner JL, Quinn F, & Turk DJ (2014). The self-reference effect on memory in early childhood. Child Development, 85(2), 808–823. 10.1111/cdev.12144 [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience, 24(8), 1742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Winward L, Hayward D, & Knight RT (2004). Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Brain Research, Cognitive Brain Research, 18(3), 255–272. 10.1016/j.cogbrainres.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Dulas MR, Newsome RN, & Duarte A. (2011). The effects of aging on ERP correlates of source memory retrieval for self-referential information. Brain Research, 1377, 84–100. 10.1016/j.brainres.2010.12.087 [DOI] [PubMed] [Google Scholar]
- Ekstrom A. (2010). How and when the fMRI BOLD signal relates to underlying neural activity: The danger in dissociation. Brain Research Reviews, 62(2), 233–244. 10.1016/j.brainresrev.2009.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Karis D, & Donchin E. (1990). Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalography and Clinical Neurophysiology, 75(2), 22–35. 10.1016/0013-4694(90)90149-E [DOI] [PubMed] [Google Scholar]
- Fields EC (2017). Factorial Mass Univariate ERP Toolbox [Computer software]. Available from: https://github.com/ericcfields/FMUT/releases
- Fields E, & Kuperberg G. (2012). It’s all about you: An ERP study of emotion and self-relevance in discourse. NeuroImage, 62(1), 562–574. 10.1016/j.neuroimage.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields E, & Kuperberg G. (2015). Loving yourself more than your neighbor: ERPs reveal online effects of a self-positivity bias. Social Cognitive and Affective Neuroscience, 10(9), 1202–1209. 10.1093/scan/nsv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields E & Kuperberg G. (2016). Dynamic effects of self-relevance and task on the neural processing of emotional words in context. Frontiers in Psychology, 6, 2003. 10.3389/fpsyg.2015.02003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields EC, & Kuperberg GR (2020). Having your cake and eating it too: Flexibility and power with mass univariate statistics for ERP data. Psychophysiology, 57(2), e13468. 10.1111/psyp.13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields EC, Weber K, Stillerman B, Delaney-Busch N, & Kuperberg GR (2019). Functional MRI reveals evidence of a self-positivity bias in the medial prefrontal cortex during the comprehension of social vignettes. Social Cognitive and Affective Neuroscience, 14(6), 613–621. 10.1093/scan/nsz035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester G, Kroneisen M, Erdfelder E, & Kamp S-M (2020). Survival processing modulates the neurocognitive mechanisms of episodic encoding. Cognitive, Affective, & Behavioral Neuroscience, 20(4), 717–729. doi: 10.3758/s13415-020-00798-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillihan SJ, & Farah MJ (2005). Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin, 131, 76–97. [DOI] [PubMed] [Google Scholar]
- Gray HM, Ambady N, Lowenthal WT, & Deldin P. (2004). P300 as an index of attention to self-relevant stimuli. Journal of Experimental Social Psychology, 40, 216–224. 10.1016/S0022-1031(03)00092-1 [DOI] [Google Scholar]
- Groppe DM, Makeig S, & Kutas M. (2009). Identifying reliable independent components via spilt-half comparisons. Neuroimage, 45(4), 1199–1211. 10.1016/j.neuroimage.2008.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe D, Urbach T, & Kutas M. (2011). Mass univariate analysis of event‐related brain potentials/fields II: Simulation studies. Psychophysiology, 48(12), 1726–1737. 10.1111/j.1469-8986.2011.01272.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, & Schacter DL (2007). Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience, 2(2), 117–133. 10.1080/17470910701399029 [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, & Schacter DL (2007). Ageing and the self-reference effect on memory. Memory, 15(8), 822–837. 10.1080/09658210701701394 [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, & Foti D. (2012). ERPs and the study of emotion. In Luck SJ & Kappenman ES (Eds.), The Oxford Handbook of Event-Related Potential Components (pp. 441–474). New York: Oxford University Press. [Google Scholar]
- Hedden T, Park DC, Nisbett R, Ji L-J, Jing Q, & Jiao S. (2002). Cultural variation in verbal versus spatial neuropsychological function across the life span. Neuropsychology, 16, 65–73. [DOI] [PubMed] [Google Scholar]
- Kappenman E, & Luck S. (2010). The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology, 47(5), 888–904. 10.1111/j.1469-8986.2010.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, & Heatherton TF (2002). Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience, 14(5), 785–794. 10.1162/08989290260138672 [DOI] [PubMed] [Google Scholar]
- Kensinger EA, & Gutchess AH (2015). Memory for emotional and social information in adulthood and old age. In Addis DR, Barense M, & Duarte A (Eds.), The Wiley Handbook on the Cognitive Neuroscience of Memory (pp. 427–447). West Sussex, UK: John Wiley & Sons. [Google Scholar]
- Kensinger EA, & Gutchess AH (2017). Cognitive aging in a social and affective context: Advances over the past 50 years. Journals of Gerontology: Series B, 72(1), 61–70. 10.1093/geronb/gbw056 [DOI] [PubMed] [Google Scholar]
- Knyazev GG (2013). EEG correlates of self-referential processing. Frontiers in Human Neuroscience, 7(MAY), 264. 10.3389/fnhum.2013.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlewska I, & Nowicka A. (2016). Present-self, past-self and the close-other: Neural correlates of assigning trait adjectives to oneself and others. European Journal of Neuroscience, 44(4), 2064–2071. 10.1111/ejn.13293 [DOI] [PubMed] [Google Scholar]
- Lee T, Girolami M, & Sejnowski TJ (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Computation, 11(2), 417–441. 10.1162/089976699300016719 [DOI] [PubMed] [Google Scholar]
- Legrand D, & Ruby P. (2009). What Is self-specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review, 116(1), 252–282. 10.1037/A0014172 [DOI] [PubMed] [Google Scholar]
- Leshikar ED, & Duarte A. (2012). Medial prefrontal cortex supports source memory accuracy for self-referenced items. Social Neuroscience, 7(2), 126–145. 10.1080/17470919.2011.585242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Dulas MR, & Duarte A. (2015). Self-referencing enhances recollection in both young and older adults. Aging, Neuropsychology, and Cognition, 22(4), 388–412, 10.1080/13825585.2014.957150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck S. (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8(1), 213. 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014). An introduction to the event-related potential technique. MIT press. [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfeld JF, & Kelley WM (2004). Medial prefrontal activity predicts memory for self. Cerebral Cortex, 14(6), 647–654. 10.1093/cercor/bhh025 [DOI] [PubMed] [Google Scholar]
- Magno E & Allan K. (2007). Self-reference during explicit memory retrieval: an event-related potential analysis. Psychological Science, 18(8), 672–677. 10.1111/j.1467-9280.2007.01957.x [DOI] [PubMed] [Google Scholar]
- Mao X, Wang Y, Wu Y, & Guo C. (2017). Self-Referential Information Alleviates Retrieval Inhibition of Directed Forgetting Effects-An ERP Evidence of Source Memory. Frontiers in Behavioral Neuroscience, 11(187), 1–9. 10.3389/fnbeh.2017.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, & Banaji MR (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron, 50(4). 655–663. 10.1016/j.neuron.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Miyakoshi M, Nomura M, & Ohira H. (2007). An ERP study on self-relevant object recognition. Brain and Cognition, 63(2), 182–189. 10.1016/j.bandc.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Morel N, Villain N, Rauchs G, Gaubert M, Piolino P, Landeau B, Mezenge F, Desgranges B, Eustache F, & Chetelat G. (2014). Brain activity and functional coupling changes associated with self-reference effect during both encoding and retrieval. PLoS One, 9(3), e90488. 10.1371/journal.pone.0090488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, & Han S. (2010). Neural oscillations involved in self-referential processing. Neuroimage, 53(2), 757–768. 10.1016/j.neuroimage.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Murta T, Leite M, Carmichael DW, Figueiredo P, & Lemieux L. (2015). Electrophysiological correlates of the BOLD signal for EEG-informed fMRI. Human Brain Mapping, 36(1), 391–414. 10.1002/hbm.22623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, & Bermpohl F. (2004). Cortical midline structures and the self. Trends in Cognitive Sciences, 8(3), 102–7. 10.1016/j.tics.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Nowicka MM, Wojcik MJ, Kotlewska I, Bola M, & Nowicka A. (2018). The impact of self-esteem on the preferential processing of self-related information: Electrophysiological correlates of explicit self vs. other evaluation. PLOS One, 13(7). 10.1371/journal.pone.0200604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, & Wagner AD (2002). Observing the transformation of experience into memory. Trends in Cognitive Science, 6(2), 93–102. 10.1016/S1364-6613(00)01845-3 [DOI] [PubMed] [Google Scholar]
- Powell LJ, Macrae CN, Cloutier J, Metcalfe J, & Mitchell JP (2010). Dissociable neural substrates for agentic versus conceptual representations of self. Journal of Cognitive Neuroscience, 22(10), 2186–2197. 10.1162/jocn.2009.21368. [DOI] [PubMed] [Google Scholar]
- Rajaram S. (1993). Remembering and knowing: Two means of access to the personal past. Memory & Cognition, 21(1), 89–102. 10.3758/BF03211168 [DOI] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, & Kirker WS (1977). Self-reference and the encoding of personal information. Journal of Personality and Social Psychology, 35, 677–688. 10.1037/0022-3514.35.9.677 [DOI] [PubMed] [Google Scholar]
- Rosa N, Deason R, Budson A, Gutchess A, & Brown GG (2015). Self-referencing and false memory in mild cognitive impairment due to Alzheimer’s Disease. Neuropsychology,29(5), 799–805. 10.1037/neu0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, & Curran T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11(6), 251–257. 10.1016/j.tics.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Serbun SJ, Shih JY, & Gutchess AH (2011). Memory for details with self-referencing. Memory, 19(8), 1004–1014. 10.1080/09658211.2011.626429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestyuk AY, & Deldin PJ (2010). Automatic and strategic representation of the self in major depression: Trait and state abnormalities. American Journal of Psychiatry, 167(5), 536–544. 10.1176/appi.ajp.2009.06091444 [DOI] [PubMed] [Google Scholar]
- Shipley (1986). Shipley Institute of Living Scale. Los Angeles: Western Psychological services. [Google Scholar]
- Sui J, & Zhu Y. (2005). Five-year-olds can show the self-reference advantage. International Journal of Behavioral Development, 29(5), 382–387. 10.1080/01650250500172673 [DOI] [Google Scholar]
- Swann WB Jr., & Bosson J. (2010). Self and identity. In Fiske ST, Gilbert DT, & Lindzey G (Eds.), Handbook of Social Psychology (5th ed., pp. 589–628). New York: McGraw Hill. [Google Scholar]
- Symons CS, & Johnson BT (1997). The self-reference effect in memory: a meta-analysis. Psychological Bulletin, 121(3), 371–94. 10.1037/0033-2909.121.3.371 [DOI] [PubMed] [Google Scholar]
- Tacikowski P, & Nowicka A. (2010). Allocation of attention to self-name and self-face: An ERP study. Biological Psychology, 84(2), 318–324. 10.1016/j.biopsycho.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Tanguay AN, Benton L, Romio L, Sievers C, Davidson PSR, & Renoult L. (2018). The ERP correlates of self-knowledge: Are assessments of one’s past, present, and future traits closer to semantic or episodic memory? Neuropsychologia, 110, 65–83. 10.1016/j.neuropsychologia.2017.10.024 [DOI] [PubMed] [Google Scholar]
- Wagner DD, Haxby JV, Heatherton TF (2012). The representation of self and person knowledge in the medial prefrontal cortex. Wiley Interdisciplinary Reviews: Cognitive Science, 3(4), 451–70. 10.1002/wcs.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, & Schacter DL (1999). When encoding yields remembering: Insights from event-related neuroimaging. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 354(1387), 1307–1324. 10.1098/rstb.1999.0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding EL, & Ranganath C. (2012). Electrophysiological correlates of episodic memory processes. In Luck SJ & Kappenman ES (Eds.), The Oxford Handbook of Event-Related Potential Components (pp. 373–396). New York: Oxford University Press. [Google Scholar]
- Yaoi K, Osaka M, & Osaka N. (2015). Neural correlates of the self-reference effect: evidence from evaluation and recognition processes. Frontiers in Human Neuroscience, 9(383), 1–9. 10.3389/fnhum.2015.00383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.