Abstract
Background
To characterize their potential use in pre-exposure prophylaxis (PrEP) we compared the pharmacokinetics of raltegravir and lamivudine in genital tissue against ex vivo tissue infection with HIV-1.
Methods
Open-label trial of 36 HIV-negative females and males randomized to 7 days raltegravir 400 mg twice daily and 7 days raltegravir 400 mg+lamivudine 150 mg twice daily (after washout), or vice versa. Blood, saliva, rectal fluid, rectal tissue, vaginal fluid and vaginal tissue were sampled at baseline and on and off PrEP during a total of 12 days, for pharmacokinetics and antiviral activity via ex vivo HIV-1BaL challenge. Ex vivo infectivity was compared with baseline. The trial has been registered in https://clinicaltrials.gov/ with the identifier NCT03205566.
Results
Steady state for both drugs was reached by day 4. Dosing with raltegravir alone provided modest ex vivo HIV protection with higher drug levels in rectal tissue and vaginal tissue than in plasma on and off PrEP. Off PrEP, plasma and vaginal concentrations declined rapidly, while persisting in the rectum. On PrEP, the highest lamivudine concentrations were in the rectum, followed by vaginal tissue then plasma. Lamivudine washout was rapid in plasma, while persisting in the rectum and vagina. Raltegravir/lamivudine increased ex vivo protection on and off PrEP compared with raltegravir alone, reaching maximum protection at day 2 in rectal tissue and at day 8 in vaginal tissue.
Conclusions
Raltegravir 400 mg+lamivudine 150 mg showed high levels of ex vivo HIV protection, associated with high drug concentrations persisting after discontinuation in vaginal and rectal compartments, supporting further investigation of these agents for PrEP.
Introduction
Oral pre-exposure prophylaxis (PrEP) is a rapidly emerging prevention strategy that could help reduce HIV incidence globally.1–4 The use of daily and on-demand oral Truvada (tenofovir disoproxil fumarate/emtricitabine), a combination of two NRTIs, for PrEP has demonstrated high efficacy.1–3 However, its use may be limited by side effects, renal/bone toxicity and emerging drug resistance globally.2,5,6 Furthermore, the use of PrEP agents acting at different stages of the viral replication cycle may allow a more forgiving dosing strategy than a regimen consisting of two NRTIs.
Integrase inhibitors act late in the replication cycle and their role in PrEP is being explored as long-acting injectables.7 Raltegravir is well tolerated, has few drug–drug interactions8 and is available as a generic drug in some countries. Humanized mice models have shown that oral dosing with raltegravir prevents HIV-1 vaginal transmission9 and penetrates well in vaginal and gut tissues.10 In humans, raltegravir rapidly and extensively penetrates the female genital tract and colorectal tissue following oral dosing with higher plateau levels compared with plasma.11–14 However, it is not known whether raltegravir tissue penetration is sufficient to provide protection against vaginal or rectal transmission of HIV-1 or whether combination of raltegravir with another antiretroviral such as lamivudine, an NRTI, is required.
Drug efficacy in preventing mucosal transmission can be assessed by ex vivo challenge of mucosal tissue explants and has been used to evaluate PrEP strategies15–18in vivo in non-human primate studies19–21 and clinical trials.22–27 The ex vivo challenge approach allows evaluation of efficacy, proof of concept and insight into adherence requirements for oral PrEP agents, providing milestone data before embarking on large-scale efficacy studies, which can be used as licensing data should efficacy be shown. Despite the variety of models, consistent results can be obtained among different laboratories through protocol standardization.28
This proof-of-concept study was undertaken to define the pharmacokinetic (PK) and pharmacodynamic (PD) activity of raltegravir using ex vivo HIV-1BaL-treated vaginal and rectal tissue obtained from healthy men and women receiving a 7 day course of raltegravir 400 mg twice daily, administered alone or in combination with lamivudine.
Methods
Study design
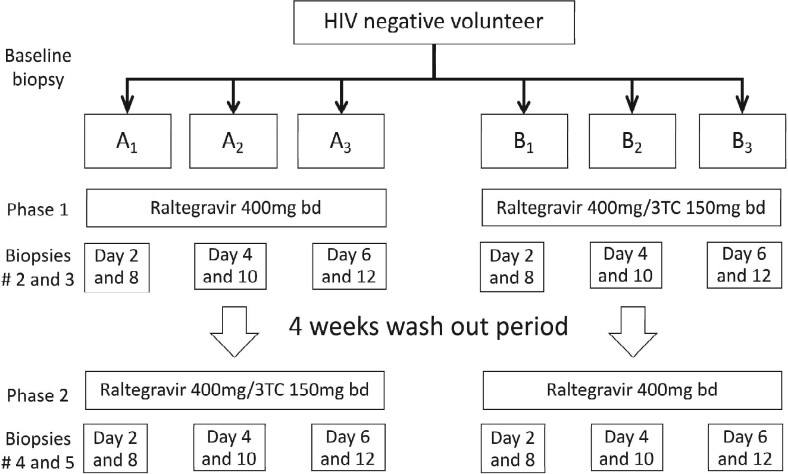
Thirty-six HIV-negative men (n = 18) and women (n = 18), with no sexually transmitted infections, were randomized according to gender in this open-label, PK/PD trial to one of six arms: A1, A2, A3, B1, B2 and B3 (Figure 1). The result being 6 women and 6 men per sampling arm and participants were their own controls. Arm A (A1, A2 and A3) received 7 days of raltegravir 400 mg twice daily, followed by a 1 month washout and then 7 days of raltegravir 400 mg/lamivudine 150 mg twice daily. Arm B (B1, B2 and B3) started with 7 days raltegravir 400 mg/lamivudine 150 mg twice daily, had a 1 month washout and then received 7 days of raltegravir 400 mg twice daily.
Figure 1.
Study design. Two PrEP regimens were investigated and all 36 individuals (18 men and 18 women) received both regimens separated by a 1 month washout. Arm A started with 14 days of raltegravir 400 mg twice daily and arm B started with 14 days of raltegravir 400 mg+lamivudine 150 mg twice daily to remove sequential selection bias. Participants were randomized according to gender to one of six arms with three men and three women per block (A1, A2, A3, B1, B2 and B3). bd, twice daily; 3TC, lamivudine.
Staggered sampling was undertaken at baseline, three timepoints on PrEP and two timepoints in the 5 days after PrEP cessation according to randomization arm. Sampling took place 12 h ± 30 min after PrEP dosing. Collected samples were blood, saliva (by Salivette®), rectal fluid and vaginal fluid (by Weck-Cel sponges; Weck-Cel surgical spear; Medtronic Ophthalmic, Jacksonville, FL, USA), and rectal tissue and vaginal tissue (by Sarratt biopsy forceps obtaining five 3 mm × 3 mm × 1 mm biopsies). Biopsies were frozen for PK analysis or transported immediately in DMEM (median time = 30 min) to the laboratory on ice for ex vivo PD assays. Progesterone levels were measured on sampling visits for women.
Baseline biopsies were obtained from each individual as a control of ex vivo challenge. Lack of productive infection of baseline biopsies resulted in exclusion of data obtained from subsequent biopsies during the trial. Sampling from women avoided menstruation and targeted the luteal phase of the menstrual cycle.
The study was approved by the National Research Ethics Service (ref: 17/LO/0094) and registered on https://clinicaltrials.gov/ct2/show/NCT03205566?term=r-prep&cond=Hiv&rank=1. All subjects provided written informed consent.
PK analysis
Drug concentrations in all matrices were measured by LC-MS/MS.29 Chromatographic separation of raltegravir and lamivudine was achieved using a Synergi polar RP column. Stable isotope-labelled internal standards (raltegravir-d6, 15N213C-lamivudine and 13C-tenofovir diphosphate) were used for all methods. Bioanalytical method validation was carried out in accordance with FDA and EMA guidelines.30
Plasma and tissues
Drug was extracted by protein precipitation [in acetonitrile/water (5:1, v/v)]. Prior to extraction, tissues were homogenized using a MINILYS homogenizer and Precellys–Keramik kit (Bertin Technologies, Bordeaux). Calibration curves ranged between 5–5000 ng/mL (plasma) and 0.35–1000 ng/mL (tissue).
Lamivudine triphosphate (lamivudine-TP) tissue concentrations were determined using weak anion exchange chromatography. Biopsies were homogenized in a solution of methanol and 20 mM EDTA/EGTA (70:30, v/v). A biobasic AX column with a pH gradient mobile phase was used to elute lamivudine-TP and detection was performed on an SCIEX 5500 triple quadrupole mass spectrometer. The assay was validated over the concentration range of 0.075–213 pmol.
Saliva
Drug was extracted using solid phase extraction (SPE) [Oasis HLB (30 mg)] cartridges. The solvent phase was evaporated and reconstituted in acetonitrile/water (1:99, v/v). The calibration curve (0.5–50 ng/mL for both analytes) was prepared using saliva collected from non-medicated healthy volunteers using the Salivette® method.
Vaginal and rectal fluids
Drug was extracted from Weck-Cel sponges with a mixture of acetonitrile/0.1% formic acid. The smples were then passed through SPE [Oasis HLB (30 mg)] cartridges as an additional sample clean-up step. The solvent phase was evaporated and reconstituted in acetonitrile/water (1:99, v/v). The volume of fluid on each sponge was predetermined by subtracting the weight of the ‘dry’ sponge prior to sample collection. The calibration curve (0.15–200 ng/sample) was constructed by spiking plasma calibration standards onto cellulose-based Weck-Cel sponges.
PD analysis
Susceptibility to HIV infection was assessed using an ex vivo challenge model15,31 with a reference clade B R5-tropic isolate, HIV-1BaL,32 provided by the NIH AIDS Research & Reference Reagent Program (http://www.aidsreagent.org/). Vaginal and rectal biopsies were cut in explants, exposed in duplicates for 2 h to HIV-1BaL at a high (104 TCID50/mL) and a low (102 TCID50/mL) titre and then washed with PBS. Different titres of virus were used to mimic in vivo transmission. Rectal explants were transferred onto gelfoam rafts (Pfizer, NY, USA) and vaginal explants onto a fresh culture plate. Explants were cultured for 15 days. Approximately 50% of the culture supernatant was harvested every 2–3 days and cultures re-fed with fresh medium in the absence of drug. Viral replication was measured as p24 concentration (INNOTEST HIV antigen mAb; Fujirebio Europe, Belgium) in culture supernatant at days 3, 7, 11 and 15.
Statistical analysis
Drug concentrations are expressed as ng/mL of plasma or saliva. Tissue homogenate and genital/rectal fluid samples were quantified using an ng/sample calibration curve and converted into ng/mL by adjusting for recorded fluid volumes and tissue weights, assuming an equivalent density for 1 g of tissue and 1 mL of fluid.
Summary statistics of absolute drug concentrations at each visit on days 2, 4 and 6 (on PrEP) and days 8, 10 and 12 (off PrEP) are presented using the arithmetic mean and SEM. Inter-participant variability in plasma concentration following drug administration was assessed by measuring the coefficient of variation (CV = SD/mean × 100). Compartment-to-plasma ratios (parameterCOMP/parameterPlasma) were derived at each visit using detectable drug levels only.
Results
Demographics and safety
Thirty-six subjects (18 males and 18 females) were included in the analysis (Table 1). The luteal phase could not be confirmed for all female participants (Table S1, available as Supplementary data at JAC Online). The study drugs were well tolerated with no serious adverse events reported.
Table 1.
Baseline demographic characteristics; n = 36
| Age, median (IQR) | 32 (20–50) |
| Gender, frequency (%) | |
| male | 18 (50) |
| female | 18 (50) |
| Ethnicity, frequency (%) | |
| white | 23 (64) |
| black African | 11 (30) |
| other | 2 (6) |
| Weight (kg), mean ± SD | 72.74 ± 13.8 |
| BMI (kg/m2), mean ± SD | 24.5 ± 3.60 |
Baseline characteristics are summarized as the mean ± SD (continuous normally distributed variables), median (IQR) (non-normally distributed variables) and frequency (%) (categorical variables).
PK profile of raltegravir with or without lamivudine
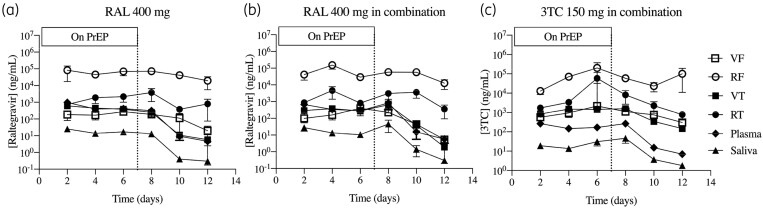
Raltegravir and lamivudine were detectable at day 2 of PrEP and concentrations reached steady state within 4 days of oral dosing in all compartments: plasma, saliva, vaginal tissue, vaginal fluid, rectal tissue and rectal fluid (Figure 2 and Table S2).
Figure 2.
Longitudinal PK analysis. Raltegravir (a and b) and lamivudine (c) levels were measured in vaginal fluid, rectal fluid, vaginal tissue, rectal tissue, plasma and saliva at each sampling point during and after PrEP dosing with raltegravir 400 mg (a) and raltegravir 400 mg+lamivudine 150 mg (b and c). Data are mean ± SEM. The dotted line indicates the timepoint when PrEP dosing stopped. RAL, raltegravir; 3TC, lamivudine; VF, vaginal fluid; RF, rectal fluid; VT, vaginal tissue; RT; rectal tissue.
The PK profile of raltegravir was not significantly affected by lamivudine when used in combination. Raltegravir was rapidly absorbed into vaginal and rectal tissues. On day 2 of PrEP, raltegravir plasma concentrations exceeded those in vaginal fluid and vaginal tissue, suggesting a slight lag in raltegravir accumulation in the female genital tract. Higher levels of raltegravir were observed in the rectum than the female genital tract and plasma. Raltegravir tissue-to-plasma ratios on PrEP were, on average, 0.9 for vaginal tissue and 13 for rectal tissue. Off PrEP, the ratios increased to 2.0 (vaginal tissue) and 128 (rectal tissue). Raltegravir levels in rectal fluid were significantly higher than rectal tissue during and after PrEP; however, in the vagina the opposite was observed, with greater concentrations in tissue than in secretions.
Raltegravir concentrations during PrEP in plasma, rectal and vaginal samples were greater than the protein-adjusted IC95 for WT virus (16 ng/mL)0.8 After PrEP cessation, 86% and 58% of rectal tissue samples and 50% and 7% of vaginal tissue samples remained above the IC95 at days 10 and 12, i.e. days 3 and 5 post-ART, respectively, in both arms.
Rectal concentrations of lamivudine (Figure 2c) were higher on average than vaginal and plasma concentrations. Lamivudine tissue-to-plasma ratios were, on average, 8.0 for vaginal tissue and 129 for rectal tissue on PrEP and 17 (vaginal tissue) and 91 (rectal tissue) off PrEP. Furthermore, lamivudine levels in the vaginal and rectal compartments remained high after stopping PrEP, with 100% and 46% of rectal tissue samples and 60% and 43% of vaginal tissue samples above the in vitro IC95 (183.4 ng/mL)33 at days 10 and 12, i.e. days 3 and 5 post-ART, respectively.
Average lamivudine-TP levels in vaginal tissue were 399 pmol/g on PrEP and 305 pmol/g off PrEP (Table S3). Metabolite concentrations persisted in vaginal tissue with 71% of vaginal tissue samples detectable at day 12, i.e. day 5 post-ART. Lamivudine-TP was undetectable in all rectal tissue samples.
Significant positive correlations were observed between raltegravir and lamivudine concentrations in vaginal tissue and rectal tissue with corresponding levels in plasma (Figure S1) and mucosal secretions (Figure S2). In vaginal tissue, there was a significant log relationship between lamivudine and lamivudine-TP concentrations (P = 0.0171) (Figure S3a).
Saliva and plasma concentrations of raltegravir and lamivudine were significantly correlated (r2>0.7; P < 0.0001) (Figure S1). However, levels of raltegravir and lamivudine were lower in saliva, accounting for approximately 4% (raltegravir) and 13% (lamivudine) of total drug concentrations in plasma.
Sampling women rectally and vaginally simultaneously uniquely allowed comparison of compartments following oral dosing. During oral dosing with raltegravir (both alone and in combination with lamivudine), raltegravir levels in female rectal tissue were, on average, 3.6 ± 0.4-fold higher than in vaginal tissue and 98.1 ± 24.8-fold higher in rectal fluid than in vaginal fluid at day 6. Off PrEP, raltegravir levels remained high in the rectum, but began to decline in the vaginal compartment, resulting in 76-fold higher concentrations in rectal tissue than in vaginal tissue and 815-fold higher concentrations in rectal fluid than in vaginal fluid at day 10, i.e. day 3 post-ART (Figure S4). Similarly, lamivudine levels were consistently higher in the rectum than in the vaginal compartment of female subjects both on and off PrEP. Both raltegravir and lamivudine concentrations in vaginal tissue significantly correlated with levels in rectal tissue. For secretions, however, raltegravir vaginal fluid significantly correlated with rectal fluid, but lamivudine did not (Figure S4).
No significant gender differences were observed when comparing the raltegravir and lamivudine plasma, saliva and rectal concentrations (data not shown).
Efficacy of raltegravir and the raltegravir/lamivudine combination against rectal and vaginal transmission
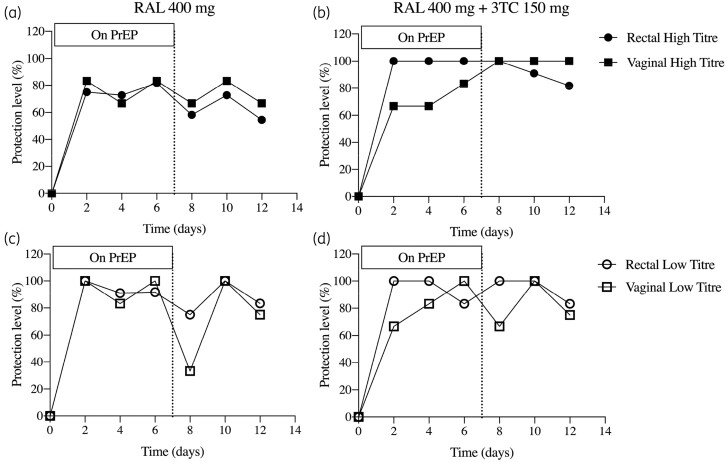
We evaluated the efficacy of oral PrEP in mucosal explants against challenge with HIV-1BaL at two titres: a high titre routinely used to obtain productive infection of explants and a low titre to mimic in vivo transmission. For both titres, reductions in p24 levels compared with those measured at baseline were observed in rectal tissue and vaginal tissue after 2 days of oral dosing with raltegravir alone and progressively decreased after PrEP cessation (Figure S5). Dosing with the raltegravir/lamivudine combination resulted in a greater reduction of p24 levels in both tissues (Figure S5). We then used these reductions in p24 to define ex vivo protection as the percentage of cultures where infectivity after ex vivo challenge was reduced above a certain cut-off compared with baseline samples. We explored a range of cut-offs between 60% and 90% (Figure 3 and Figure S6). For both dosing regimens, with 60%, 70% or 80% cut-offs there was protection of rectal tissue and vaginal tissue from day 2 on drug until 5 days post-drug cessation against high and low titres. Using a 90% cut-off there was rectal, but not vaginal, protection against high and low titre challenge across all timepoints from day 2 on drug to day 5 post-drug cessation.
Figure 3.
Longitudinal analysis of protection level. Ex vivo protection of rectal and vaginal explants was defined as day 15 p24 level >60% lower compared with day 15 p24 of baseline explants following challenge with HIV-1BaL at a high titre (104 TCID50/mL) (a and b) or a low titre (102 TCID50/mL) (c and d). Data are the percentage of samples considered protected under this criterion at each timepoint on and off PrEP with raltegravir 400 mg (a and c) and raltegravir 400 mg+lamivudine 150 mg (b and d). The dotted line indicates the timepoint when PrEP dosing stopped. RAL, raltegravir; 3TC, lamivudine.
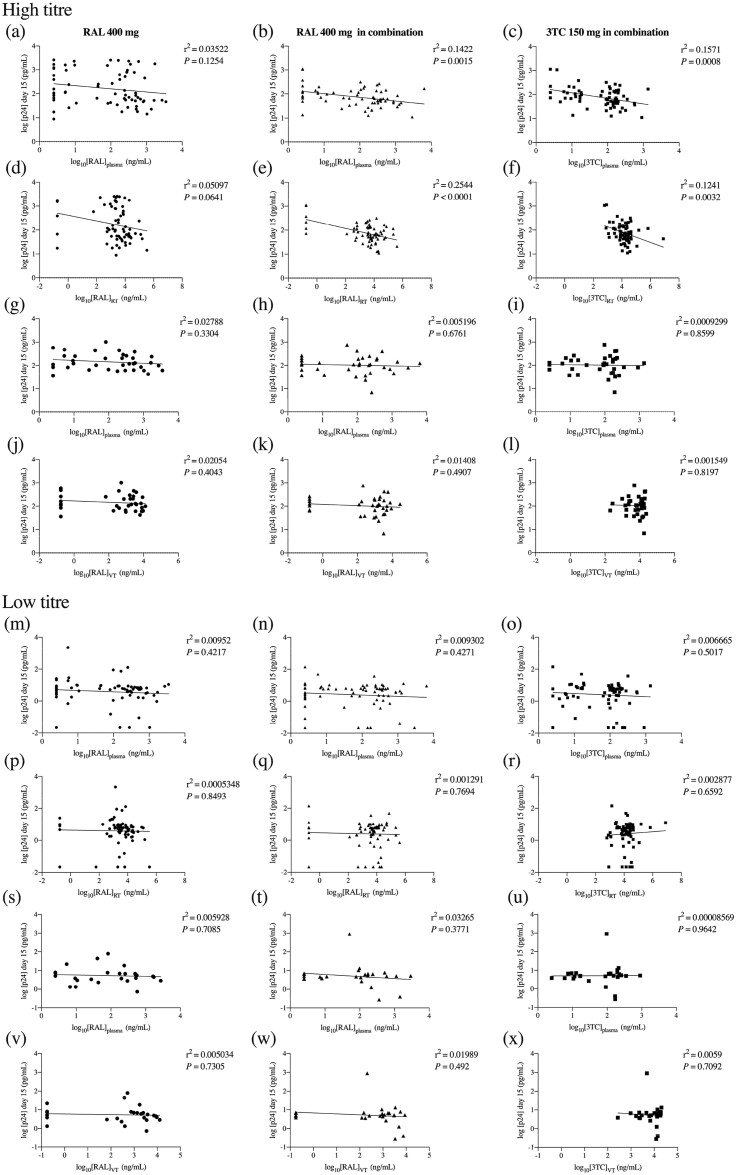
PK/PD correlation
Statistically significant inverse correlations between ex vivo infectivity levels and drug concentrations were only observed in participants dosed with raltegravir 400 mg+lamivudine 150 mg between rectal tissue challenged with high viral titre and raltegravir and lamivudine concentrations in plasma (Figure 4b and c) and rectal tissue (Figure 4e and f). No significant PK/PD correlations were observed with low viral titre challenge infectivity data (Figure 4) or with lamivudine-TP levels in vaginal tissue (Figure S3b and c).
Figure 4.
Correlations of drug concentrations with p24 levels in culture supernatants. Log-transformed p24 levels in day 15 culture supernatants of rectal (a, b, c, d, e, f, m, n, o, p, q and r) and vaginal (g, h, i, j, k, l, s, t, u, v, w and x) explants challenged ex vivo with HIV-1BaL at a high titre (104 TCID50/mL) or a low titre (102 TCID50/mL) were correlated with log-transformed raltegravir and lamivudine concentrations in plasma and mucosal tissue (rectal tissue or vaginal tissue) by Pearson correlation. P < 0.05 was considered statistically significant. RAL, raltegravir; 3TC, lamivudine; RT, rectal tissue; VT, vaginal tissue.
Discussion
We showed that a 7 day course of raltegravir/lamivudine or raltegravir alone results in high drug concentrations at multiple HIV transmission sites by day 2 of dosing. Raltegravir concentrations were consistently higher in the rectum than plasma, saliva and the female genital tract. Higher levels of raltegravir in rectal fluid than in vaginal fluid have also been reported in animal models and in humans.12,34 Raltegravir persisted longest in rectal tissue and rectal fluid in parallel with persistent ex vivo protection and resulting in high rectal tissue-to-plasma ratios towards the end of the sampling interval, as plasma concentrations declined. Vaginal tissue and vaginal fluid raltegravir concentrations remained, on average, above the in vitro IC95 for 3 days after dosing cessation, providing protection levels above 70% with high and low ex vivo challenge titres. Interestingly, drug plasma levels correlated better with those in vaginal tissue than in vaginal fluid and rectal samples. Given that both drugs are metabolized via separate pathways, not surprisingly, the raltegravir PK profile was not affected by the combined dosing with lamivudine. Concentrations of lamivudine and lamivudine-TP in the female genital compartment and in the rectal tract remained above plasma and saliva levels during and after dosing, potentially explaining the continued protection during lamivudine/raltegravir off PrEP. Our study did not include a lamivudine-alone arm, limiting the full assessment of the contribution of raltegravir and lamivudine in the increased activity of the combination compared with both drugs alone. However, our data support that the combination regimen is more ‘forgiving’ than raltegravir alone and perhaps more suitable for daily or even on-demand PrEP. Further studies evaluating the activity of raltegravir/lamivudine at short timepoints post-dosing will be required to fully confirm the potential of this drug combination for on-demand PrEP.
The high raltegravir level in rectal tissue and prolonged presence shows that it accumulated in the tissue and suggests that, following oral dosing, it was absorbed into the circulation. In addition, raltegravir may accumulate within the rectum directly as unabsorbed drug carried in faeces or excreted in a conjugated form by the bile duct and de-conjugates in the large bowel back to raltegravir.
Macaque studies had shown that the luteal phase is associated with increased risk of vaginal HIV transmission;35 however, in our study the luteal phase could not be confirmed for all female participants and no correlation was established with PK/PD results.
All baseline rectal explants were reproducibly infected with high and low challenge titres, except in two participants: for the first, no productive infection was observed; for the second, explants cut for the high challenge titre were too small. All baseline vaginal explants challenged with the high viral titre were infected; however, only 13 of a total of 18 explants were productively infected with the low titre at baseline. Consistent with histological and immunological differences,36 maximum p24 concentrations were 1 log greater in rectal than in vaginal explants.
Higher levels of raltegravir in rectal tissue compared with vaginal tissue did not correlate with higher ex vivo protection in rectal explants. However, during oral dosing with raltegravir 400 mg+lamivudine 150 mg, a greater protection level against ex vivo challenge in rectal tissue than in vaginal tissue reflected higher concentrations of raltegravir and lamivudine measured in rectal samples compared with vaginal specimens, resulting in a statistically significant negative correlation between raltegravir and lamivudine concentrations and p24 levels in rectal tissue. Hence, the raltegravir/lamivudine combination maintained protection in a broader range of tissue and for longer compared with raltegravir alone, conferring protection for 5 days after stopping PrEP. The lack of statistically significant PK/PD correlations in vaginal tissue (at high viral titres) could be due to insufficient drug levels in HIV target cells within the tissue to inhibit viral replication or to tissue-specific factors such as cellular activation level and microbiota. In contrast, drug concentrations may exceed the required levels for inhibition in tissues subjected to a low viral challenge titre, thereby preventing the establishment of a concentration–effect relationship.
Currently the ex vivo challenge of mucosal explants remains the only practical way to address PD in humans, aside from Phase III trials, and provides an important tool for risk reduction of late-stage failure when selecting drug strategies for large studies.
The close correlation of drug levels between saliva and plasma indicates that non-invasive saliva sampling could be used to monitor adherence for people on PrEP or HIV treatment taking raltegravir and/or lamivudine.
To the best of our knowledge, this is the first report of the protective activity of raltegravir as an oral PrEP candidate where longitudinal sampling for each participant has been conducted using samples at baseline as their own control and confirms that it is rapidly absorbed with greater accumulation in rectal tissue than in vaginal tissue. Pharmacological boosting of raltegravir with lamivudine resulted in greater and more prolonged protection against ex vivo challenge of rectal and vaginal tissues without a change in drug levels of raltegravir in these compartments. Hence, lamivudine continues to show promise as a PrEP agent. Therefore, the raltegravir 400 mg+lamivudine 150 mg regimen is a potential PrEP candidate for people who are unable to take tenofovir-based PrEP.
Supplementary Material
Acknowledgements
We thank MSD for funding the study, the King’s College London Biomedical Research Centre, the Chelsea and Westminster Hospital for processing blood and the Liverpool Biomedical Research Centre funded by Liverpool Health Partners for infrastructural support.
Funding
This study was funded through an investigator-led grant from Merck Sharp & Dohme (to J.F.). The funder had no role in study design, analysis, writing of the manuscript or the decision to publish.
Transparency declarations
C.H. has received research grants from Gilead. L.D. is supported by PreDiCT-TB and has received a travel bursary from Gilead Sciences. M.B. has received travel and research grants from and has been advisor for Janssen, Roche, Pfizer, ViiV, Bristol-Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Mylan, Cipla and Gilead. S.K. has received research support from ViiV, Gilead and Merck. J.F. has received travel and research grants from Gilead and ViiV. J.L., M.L., S.M., A.A., L.E., S.D.P, D.E., E.C. and R.S.: none to declare.
Author contributions
Conceptualization: C.H., M.B., R.S., S.K. and J.F. Methodology: C.H., J.L., M.L., S.M., A.A., L.E., S.D.P., D.E., E.C., L.D. and S.K. Analysis: C.H., A.A., L.E., S.D.P., D.E., E.C. and L.D. Writing: C.H., L.E., S.K. and J.F. Review and editing: all authors.
Supplementary data
Tables S1 to S3 and Figures S1 to S6 are available as Supplementary data at JAC Online.
References
- 1. Baeten JM, Donnell D, Ndase P. et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grant RM, Lama JR, Anderson PL. et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thigpen MC, Kebaabetswe PM, Paxton LA. et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–34. [DOI] [PubMed] [Google Scholar]
- 4. Van Damme L, Corneli A, Ahmed K. et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Molina JM, Capitant C, Spire B. et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373: 2237–46. [DOI] [PubMed] [Google Scholar]
- 6. Mulligan K, Glidden DV, Anderson PL. et al. Effects of emtricitabine/tenofovir on bone mineral density in HIV-negative persons in a randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2015; 61: 572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clement ME, Kofron R, Landovitz RJ.. Long-acting injectable cabotegravir for the prevention of HIV infection. Curr Opin HIV AIDS 2020; 15: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adams JL, Greener BN, Kashuba AD.. Pharmacology of HIV integrase inhibitors. Curr Opin HIV AIDS 2012; 7: 390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neff CP, Ndolo T, Tandon A. et al. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS One 2010; 5: e15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veselinovic M, Yang KH, Sykes C. et al. Mucosal tissue pharmacokinetics of the integrase inhibitor raltegravir in a humanized mouse model: implications for HIV pre-exposure prophylaxis. Virology 2016; 489: 173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Else LJ, Taylor S, Back DJ. et al. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther 2011; 16: 1149–67. [DOI] [PubMed] [Google Scholar]
- 12. Trezza CR, Kashuba AD.. Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: implications for HIV prevention. Clin Pharmacokinet 2014; 53: 611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patterson KB, Prince HA, Stevens T. et al. Differential penetration of raltegravir throughout gastrointestinal tissue: implications for eradication and cure. AIDS 2013; 27: 1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SA, Telwatte S, Hatano H. et al. Antiretroviral therapy concentrations differ in gut vs. lymph node tissues and are associated with HIV viral transcription by a novel RT-ddPCR assay. J Acquir Immune Defic Syndr 2020; 83: 530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrera C, Cranage M, McGowan I. et al. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob Agents Chemother 2009; 53: 1797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrera C, Cranage M, McGowan I. et al. Colorectal microbicide design: triple combinations of reverse transcriptase inhibitors are optimal against HIV-1 in tissue explants. AIDS 2011; 25: 1971–9. [DOI] [PubMed] [Google Scholar]
- 17. Herrera C, Shattock RJ.. Candidate microbicides and their mechanisms of action. Curr Top Microbiol Immunol 2014; 383: 1–25. [DOI] [PubMed] [Google Scholar]
- 18. Shaw GM, Hunter E.. HIV transmission. Cold Spring Harb Perspect Med 2012; 2: a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cranage M, Sharpe S, Herrera C. et al. Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel. PLoS Med 2008; 5: e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dereuddre-Bosquet N, Morellato-Castillo L, Brouwers J. et al. MiniCD4 microbicide prevents HIV infection of human mucosal explants and vaginal transmission of SHIV162P3 in cynomolgus macaques. PLoS Pathog 2012; 8: e1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallace GS, Cheng-Mayer C, Schito ML. et al. Human immunodeficiency virus type 1 nucleocapsid inhibitors impede trans infection in cellular and explant models and protect nonhuman primates from infection. J Virol 2009; 83: 9175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anton PA, Cranston RD, Kashuba A. et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses 2012; 28: 1412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fox J, Tiraboschi JM, Herrera C. et al. Brief Report: pharmacokinetic/pharmacodynamic investigation of single-dose oral maraviroc in the context of HIV-1 pre-exposure prophylaxis. J Acquir Immune Defic Syndr 2016; 73: 252–7. [DOI] [PubMed] [Google Scholar]
- 24. McGowan I, Cranston RD, Duffill K. et al. A phase 1 randomized, open label, rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of three formulations of tenofovir 1% gel (the CHARM-01 study). PLoS One 2015; 10: e0125363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McGowan I, Wilkin T, Landovitz RJ. et al. The pharmacokinetics, pharmacodynamics, and mucosal responses to maraviroc-containing pre-exposure prophylaxis regimens in MSM. AIDS 2019; 33: 237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richardson-Harman N, Hendrix CW, Bumpus NN. et al. Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS One 2014; 9: e111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson-Harman N, Mauck C, McGowan I. et al. Dose-response relationship between tissue concentrations of UC781 and explant infectibility with HIV type 1 in the RMP-01 rectal safety study. AIDS Res Hum Retroviruses 2012; 28: 1422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson-Harman N, Lackman-Smith C, Fletcher PS. et al. Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol 2009; 47: 3530–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Else L, Watson V, Tjia J. et al. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci 2010; 878: 1455–65. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Center for Veterinary Medicine. Bioanalytical Method Validation Guidance for Industry. 2018. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
- 31. Hu Q, Frank I, Williams V. et al. Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med 2004; 199: 1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gartner S, Markovits P, Markovitz DM. et al. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986; 233: 215–9. [DOI] [PubMed] [Google Scholar]
- 33. Drogan D, Rauch P, Hoffmann D. et al. The antiretroviral potency of emtricitabine is approximately 3-fold higher compared to lamivudine in dual human immunodeficiency virus type 1 infection/competition experiments in vitro. Antiviral Res 2010; 86: 312–5. [DOI] [PubMed] [Google Scholar]
- 34. Massud I, Martin A, Dinh C. et al. Pharmacokinetic profile of raltegravir, elvitegravir and dolutegravir in plasma and mucosal secretions in rhesus macaques. J Antimicrob Chemother 2015; 70: 1473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vishwanathan SA, Guenthner PC, Lin CY. et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 2011; 57: 261–4. [DOI] [PubMed] [Google Scholar]
- 36. Poles MA, Elliott J, Taing P. et al. A preponderance of CCR5+ CXCR4+ mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol 2001; 75: 8390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.