Abstract
A 44-year-old previously healthy woman developed acute myelitis in close temporal relationship with ChAdOx1 nCoV-19 vaccine first-dose administration. The neurological involvement was mainly sensory with neuroimaging showing two mono-metameric lesions involving the posterior and lateral cord at dorsal level. Significant improvement was promptly recorded with high-dose intravenous steroids, with complete recovery within one month.
The strict temporal relationship between vaccination and myelitis, together with the absence of clues pointing to alternative diagnoses, might suggest a conceivable role for anti-SARS-CoV-2 vaccine as immunological trigger, although a causal relationship has yet to be established and our preliminary observation suggests caution.
Keywords: COVID-19, Myelopathy, Vaccine
Graphical abstract
1. Introduction
The medical scientific literature is full of claims and counterclaims on vaccine-related autoimmune diseases. Various and heterogeneous clinical syndromes have been reported as a consequence of different viral vaccinations (e.g., swine flu, influenza, hepatitis B, measles, mumps, rubella, etc.), being neurological pictures the most common (Schattner, 2005). The term post-infectious neurological syndromes (PINSs) stands for a heterogeneous group of neurological disorders affecting the central nervous system (CNS), with or without peripheral nervous system (PNS) involvement, lacking specific biological markers and typically following either infection or vaccination (Brinar and Poser, 2006; Marchioni et al., 2013). Vaccinations may trigger autoimmunity by either a specific mechanism of molecular mimicry (Salemi and D'Amelio, 2010), possibly enhanced by an immunological adjuvant(Ruiz et al., 2017), or a non-specific mechanism of bystander activation (Salemi and D'Amelio, 2010).
Since late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has emerged as the zoonotic viral causative agent of COVID-19, being declared a global pandemic on March 11, 2020, by the World Health Organization (WHO). In 2020, a surprisingly high number of candidate vaccines has been developed, including ChAdOx1 nCoV-19 vaccine (AZD1222), consisting of replication-deficient chimpanzee adenoviral vector ChAdOx1, containing the SARS-CoV-2 structural surface glycoprotein antigen (spike protein; nCoV-19) gene. On January 29, 2021, ChAdOx1 nCoV-19 vaccine received marketing authorization by the European Medical Agency (EMA) due to its safety and efficacy at preventing COVID-19 in people from 18 years of age (Folegatti et al., 2020). As for April 22, 2021, more than 115 million vaccine doses had been administered in the European Union (European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker, 2021 https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab).
Here we report a case of an acute-onset neurological syndrome following ChAdOx1 nCoV-19 vaccine.
2. Case report
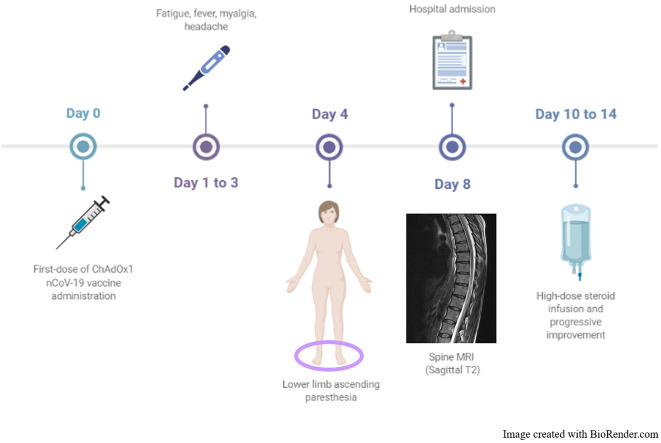
A 44-year-old woman with unremarkable past clinical history received her first dose of ChAdOx1 nCoV-19 vaccine (batch ABV2856) in early March 2021 (day 0). Over the next few days, she reported having minor symptoms (fatigue, myalgia, fever, and headache) and from day 4 she started complaining of bilateral plantar feet ascending paraesthesia evolving over 3 days. She also noticed reduced sensation in her lower back and during micturition. No recent infection nor respiratory symptoms were reported. She was admitted to a local hospital on day 4 from symptom onset. A nasopharyngeal swab tested negative for SARS-CoV-2 reverse-transcriptase-polymerase-chain-reaction assay. Neurological examination showed reduced light touch and pinprick up to ankles and brisk deep tendon reflexes in the lower limbs.
Whole-spine contrast MRI revealed two single-metamer lesions, in the posterior paramedian cord at D7-D8 level and in the left lateral cord at D10-D11 level, the first one with mild and patchy contrast enhancement (Fig. 1 ). Brain MRI was unremarkable. Cerebrospinal fluid (CSF) examination revealed mildly elevated total protein content (76.7 mg/dl, n.v. 15–45) and 6 lymphomonocytes (n.v. <2/mm3). Lactate was undetectable. CSF viral tests for HSV-1/2, VZV, Enterovirus, EBV and CMV were unremarkable. A complete pro-coagulative screening together with systemic autoimmunity, proved negative. CNS-restricted immunoglobulin G (IgG) oligoclonal bands (OCBs) were absent. MRZ reaction was not performed in the CSF. Antibodies to acquaporin-4 (AQP4) and myelin-oligodendrocyte (MOG) tested negative at once by both live and fixed cell-based assay as well as anti-neuronal surface and onconeural antibodies. Serum IgG neutralizing antibodies targeting SARS-CoV-2 S protein were absent while both IgA antibodies and antibodies to SARS-CoV2-N were not evaluated.
Fig. 1.
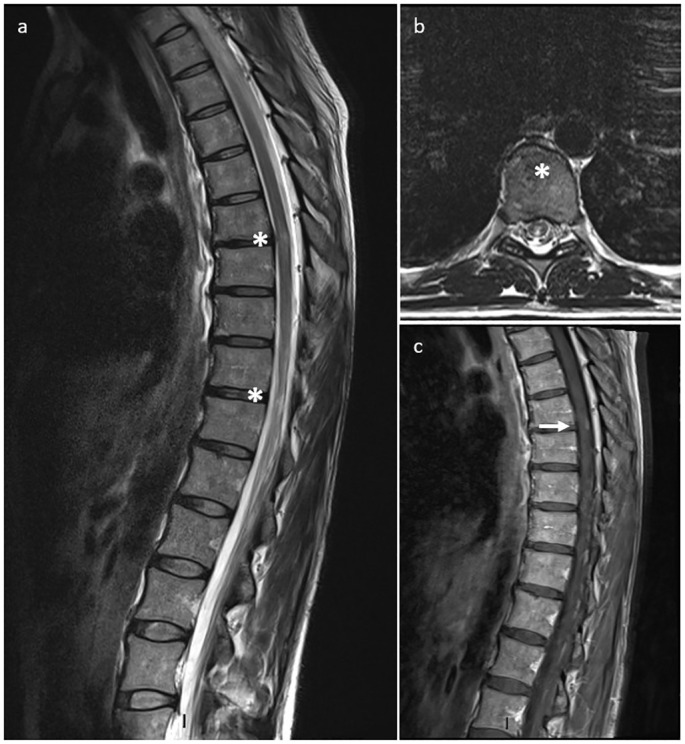
Whole-spine contrast MRI.
T2-w hyperintensity at D7-D8 (posterior cord) on sagittal (a, star) and axial (b, star) plan; T2-w lesion at D10-D11 (lateral cord) on sagittal plan (a, star). Mild and patchy contrast enhancement of D7-D8 lesion on post-contrast T1-w sequence (c, arrow).
Somato-sensory evoked potentials showed bilateral increased latency of both N22-P40 interpeak interval and P40. Both nerve conduction study (including F-wave evaluation) and needle muscle examination were normal. Based both on clinical and radiological features, diagnosis of inflammatory myelopathy was made.
The patient was started on high dose 6-methylprednisolone (1 g/day over 5 days) with a subsequent oral tapering starting from 1 mg/kg/day. She showed prompt improvement of her sensory symptoms from day 3 of therapy and was safely discharged at home where complete clinical recovery occurred after approximately one month. Serum IgG neutralizing antibodies targeting SARS-CoV-2 S protein tested three months later were 4320 U/ml.
3. Discussion
Acute myelitis is an acute focal inflammatory disorder of the spinal cord causing bilateral complete or partial motor and/or sensory impairment, with or without sphincter dysfunction, occurring as idiopathic, post-infectious/vaccinic or associated with other autoimmune diseases with a typical relapsing course, including multiple sclerosis (MS), neuromyelitis optica spectrum disorders (NMOSD), myelin-oligodendrocyte glycoprotein-associated disorders (MOGADs) or connective tissue diseases (de Seze et al., 2001). Our patient developed myelitis 4 days after the first dose of ChAdOx1 nCoV-19 vaccine.
The patient presented with distal lower-limb both negative and positive sensory symptoms and spinal-MRI revealed two single-metamer lesions mostly consistent with the clinical picture. No PNS involvement was detected and response to high-dose steroid therapy was almost complete over a course of 30 days. An oral tapering of steroids over approximately 12 weeks was planned according to previous evidence acquired by our group (Berzero et al., 2016).
The case was labelled as PINS in view of the following: - close temporal relationship with SARS-CoV-2 vaccination; − absence of CNS-restricted IgG OCBs; − absence of other CNS inflammatory lesions suggestive of alternative inflammatory disorders, including NMOSD, MOGADs, primary or systemic CNS vasculitis; − absence of serum antibodies to MOG and AQP4, known to be biological markers of MOGADs and NMOSD, respectively; − negativity of the systemic autoimmune and pro-coagulative screening. As expected, IgG antibodies to SARS-CoV-2 S protein, tested within the first week from vaccine first-dose administration, were absent. According to the study registration where antibodies peaked by 28 days (Folegatti et al., 2020), they were found positive at three-month follow-up.
In order to be defined vaccine-induced, any adverse event should meet peculiar requirements established by WHO, including: 1) consistency of evidence i.e., similar or same results generated by studies using different methods in different settings, 2) strength of association i.e., statistical significance to demonstrate that it was not simply a chance occurrence, 3) specificity i.e., vaccine is the only cause of the event that can be shown and 4) temporal relationship i.e., vaccine exposure must precede the occurrence of the event, being only the latter absolutely essential (Wraith et al., 2003). Such stringent criteria were rarely met by the large majority of vaccine-associated events described in the literature. Indeed, so far, a causal relationship has been identified in only the following circumstances with respect to neurological syndromes: a) the occurrence of Guillain Barré syndrome (GBS) after swine influenza (A/New Jersey/76) vaccine in 1976–1977 and, to a lesser extent, after pandemic influenza A (H1N1) 2009 vaccines and b) the occurrence of type 1 narcolepsy after 2009 European Pandemrix-AS03-adjuvanted A (H1N1) pandemic vaccine. In both cases, the most likely mechanism involved was molecular mimicry related to cross-reacting antibodies against peripheral nerve gangliosides or hypocretin II receptor, respectively (Nachamkin et al., 2008; Salmon et al., 2013; Luo et al., 2018).
To date, SARS-CoV-2 infection has been associated with several PINSs, including ADEM, transverse myelitis and inflammatory neuropathy, even though the underlying mechanisms are still unclear. On the other hand, to the best of our knowledge only rare post-vaccination syndromes have been reported to date during this vaccination campaign, including a single case of myelitis occurred 8 days after the first dose of AZD1222 vaccine (April 22, 2021) (Cao and Ren, 2021; Malhotra et al., 2021; Waheed et al., 2021). During the AZD1222 trial transverse myelitis had been described in two patients, 10 days after ChAdOx1 nCoV-19 first dose and 14 days after booster vaccination, respectively. Clinical details were not provided as regards severity of neurological involvement, CSF abnormalities, screening for other diseases, MRI features, treatment strategy, and outcome. However, the first case was later considered unlikely to be related when further investigation revealed preexisting, but previously unrecognized, multiple sclerosis (Voysey et al., 2021). Moreover, a case of GBS has been recently reported in the active arm of ENSEMBLE trial (NCT045505722), 10 days after the administration of the first dose of recombinant, replication-incompetent adenovirus vector vaccine (Ad26.COV2·S, Johnson & Johnson) (Márquez Loza et al., 2021). Interestingly, in a recent study no increased risk of relapse activity was found in a cohort of MS patients treated with BNT162b2 vaccine, over a median follow-up time of 20 and 38 days after the first and second dose, respectively (Achiron et al., 2021).
In our case, the definition of the acute myelitis as “vaccine-induced” still remains elusive. The temporal relationship to the vaccine was clear, even though the short temporal interval between vaccination of neurological symptom onset (< 4 days) might suggest non-specific immune activation mechanisms such as bystander activation. The consistency is supported by the occurrence of a similar case during the AZD1222 trial together with another reported case by an Indian study group (Malhotra et al., 2021; Voysey et al., 2021). Possibly, the absence of cases of acute myelitis following other SARS-CoV-2 vaccines exploiting the S protein antigen as an immunogen could depose against molecular mimicry mechanisms. Strength and specificity criteria were not met at once. However, since a causal relation could not be ruled out, we discouraged the patient from booster dose (both homologous and heterologous regimen), also taking into account her low risk-profile and the presence of IgG to SARS-CoV-2 S protein at three-month follow-up.
Currently, we are facing the largest mass vaccination campaign in history, and cases of myelitis or other neurological diseases will inevitably occur, by chance alone, in strict temporal relationship with the vaccination.
Notwithstanding the underlying mechanism, it is important to appreciate that the clinical picture was extremely mild and that the outcome was good after a short steroid course. Therefore, our preliminary observation suggests caution, awaiting further confirmation in larger epidemiological studies and meta-analyses to understand the causality of such rare events following SARS-CoV-2 vaccination.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Acknowledgments
Elisa Vegezzi: clinical assessment, manuscript design and drafting, data collection and interpretation, critical review for intellectual content.
Sabrina Ravaglia: manuscript design, data interpretation, critical review for intellectual content.
Gabriele Buongarzone: manuscript design, critical review for intellectual content.
Paola Bini: clinical assessment, manuscript design, critical review for intellectual content.
Luca Diamanti: clinical assessment, manuscript design, critical review for intellectual content.
Matteo Gastaldi: manuscript design, critical review for intellectual content.
Paolo Prunetti: neurophysiological assessment, critical review for intellectual content.
Elisa Rognone: neuroradiological assessment, critical review for intellectual content.
Enrico Marchioni: clinical assessment, manuscript design, critical review for intellectual content.
References
- Achiron A., Dolev M., Menascu S., Zohar D.-N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 202. Mult. Scler. 2021 May;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzero G., Cortese A., Ravaglia S., Marchioni E. Diagnosis and therapy of acute disseminated encephalomyelitis and its variants. Expert. Rev. Neurother. 2016;16(1):83–101. doi: 10.1586/14737175.2015.1126510. [DOI] [PubMed] [Google Scholar]
- Brinar V.V., Poser C.M. The spectrum of disseminated encephalomyelitis. Clin. Neurol. Neurosurg. 2006;108(3):295–310. doi: 10.1016/j.clineuro.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Cao L., Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol. Belg. 2021 Feb;1:1–3. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control COVID-19 Vaccine Tracker. 2021. https://qap.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., Dold C., Faust S.N., Finn A., Flaxman A.L., Hallis B., Heath P., Jenkin D., Lazarus R., Makinson R., Minassian A.M., Pollock K.M., Ramasamy M., Robinson H., Snape M., Tarrant R., Voysey M., Green C., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Oxford COVID Vaccine Trial Group Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomized controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. 15–21 August. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G., Ambati A., Lin L., Bonvalet M., Partinen M., Ji X., Maecker H.T., Mignot E.J.-M. Autoimmunity to hypocretin and molecular mimicry to flu in type 1 narcolepsy. Proc. Natl. Acad. Sci. U. S. A. 2018 Dec 26;115(52):E12323–E12332. doi: 10.1073/pnas.1818150116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra H.S., Gupta P., Prabhu V., Garg R.K., Dandu H., Agarwal V. COVID-19 vaccination-associated myelitis. QJM. 2021 Mar 31 doi: 10.1093/qjmed/hcab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchioni E., Ravaglia S., Montomoli C., Tavazzi E., Minoli L., Baldanti F., Furione M., Alfonsi E., Bergamaschi R., Romani A., Piccolo L., Zardini E., Bastianello S., Pichiecchio A., Ferrante P., Delbue S., Franciotta D., Bono G., Ceroni M. Postinfectious neurological syndromes: a prospectic cohort study. Neurology. 2013;80(10):882–889. doi: 10.1212/WNL.0b013e3182840b95. [DOI] [PubMed] [Google Scholar]
- Márquez Loza A.M., Holroyd K.B., Johnson S.A., Pilgrim D.M., Amato A.A. Guillain- barré syndrome in the placebo and active arms of a COVID-19 vaccine clinical trial: temporal associations do not imply causality. Neurology. 2021 Apr 6 doi: 10.1212/WNL.0000000000011881. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Shadomy S.V., Mora A.P., Cox N., Fitzgerald C., Ung H., Corcoran A.T., Iskander J.K., Schonberger L.B., Chen R.T. Anti-ganglioside antibody induction by swine (A/NJ/1976/H1N1) and other influenza vaccines: insights into vaccine-associated Guillain-Barre syndrome. J. Infect. Dis. 2008;198:226–233. doi: 10.1086/589624. [DOI] [PubMed] [Google Scholar]
- Ruiz J.T., Luján L., Blank M., Shoenfeld Y. Adjuvants- and vaccines-induced autoimmunity: animal models. Immunol. Res. 2017 Feb;65(1):55–65. doi: 10.1007/s12026-016-8819-5. [DOI] [PubMed] [Google Scholar]
- Salemi S., D’Amelio R. Could autoimmunity be induced by vaccination? Int. Rev. Immunol. 2010 Jun;29(3):247–269. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- Salmon D.A., Vellozzi C., Chen R.T., Halsey N.A. Did the influenza a (H1N1) 2009 monovalent inactivated vaccines increase the risk for Guillain-Barré syndrome? Expert. Rev. Clin. Immunol. 2013 Sep;9(9):795–797. doi: 10.1586/1744666X.2013.824686. [DOI] [PubMed] [Google Scholar]
- Schattner A. Consequence or coincidence? The occurrence, pathogenesis and significance of autoimmune manifestations after viral vaccines. Vaccine. 2005;23:3876–3886. doi: 10.1016/j.vaccine.2005.03.005. [DOI] [PubMed] [Google Scholar]
- de Seze J., Stojkovic T., Breteau G., Lucas C., Michon-Pasturel U., Gauvrit J.Y., Hachulla E., Mounier-Vehier F., Pruvo J.P., Leys D., Destée A., Hatron P.Y., Vermersch P. Acute myelopathies. Clinical, laboratory and outcome profiles in 79 cases. Brain. 2001;124:1509–1521. doi: 10.1093/brain/124.8.1509. [DOI] [PubMed] [Google Scholar]
- Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O’Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J. Oxford COVID Vaccine Trial GroupSafety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Randomized Controlled Trial. Lancet. 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed S., Bayas A., Hindi F., Rizvi Z., Espinosa P.S. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Case Reports Cureus. 2021 Feb 18;13(2) doi: 10.7759/cureus.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith D.C., Goldman M., Lambert P.-H. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003 Nov 15;362(9396):1659–1666. doi: 10.1016/S0140-6736(03)14802-7. [DOI] [PubMed] [Google Scholar]