Abstract
Background
Whether the volume of coronavirus disease 2019 (COVID-19) hospitalizations is associated with outcomes has important implications for the organization of hospital care both during this pandemic and future novel and rapidly evolving high-volume conditions.
Methods
We identified COVID-19 hospitalizations at US hospitals in the American Heart Association COVID-19 Cardiovascular Disease Registry with ≥10 cases between January and August 2020. We evaluated the association of COVID-19 hospitalization volume and weekly case growth indexed to hospital bed capacity, with hospital risk-standardized in-hospital case-fatality rate (rsCFR).
Results
There were 85 hospitals with 15,329 COVID-19 hospitalizations, with a median hospital case volume was 118 (interquartile range, 57, 252) and median growth rate of 2 cases per 100 beds per week but varied widely (interquartile range: 0.9 to 4.5). There was no significant association between overall hospital COVID-19 case volume and rsCFR (rho, 0.18, P = .09). However, hospitals with more rapid COVID-19 case-growth had higher rsCFR (rho, 0.22, P = 0.047), increasing across case growth quartiles (P trend = .03). Although there were no differences in medical treatments or intensive care unit therapies (mechanical ventilation, vasopressors), the highest case growth quartile had 4-fold higher odds of above median rsCFR, compared with the lowest quartile (odds ratio, 4.00; 1.15 to 13.8, P = .03).
Conclusions
An accelerated case growth trajectory is a marker of hospitals at risk of poor COVID-19 outcomes, identifying sites that may be targets for influx of additional resources or triage strategies. Early identification of such hospital signatures is essential as our health system prepares for future health challenges.
Keywords: COVID-19, Health services research, Outcomes research, Quality of care, SARS-CoV2
Clinical Significance.
-
•
In a study of coronavirus disease 2019 (COVID-19) hospitalizations at 85 US hospitals, despite similar care processes, a rapid increase in COVID-19 cases relative to bed capacity, as opposed to overall hospital case volume, was significantly associated with worse risk-standardized in-hospital case-fatality rate.
-
•
An accelerated case growth trajectory is a marker of hospitals at risk of poor COVID-19 outcomes, identifying sites that may be targets for influx of additional resources or triage strategies.
Alt-text: Unlabelled box
Background
The coronavirus disease-19 (COVID-19) pandemic challenged hospitals to adapt their care procedures for a novel disease process with an unknown trajectory. Simultaneously, hospitals were also overwhelmed by a disease that poses a substantial threat to health care resources, such as intensive care unit (ICU) and ventilator capacity,1 , 2 and puts the health care workforce at personal health risk.3 Therefore, although hospitals caring for COVID-19 patients gained institutional knowledge and experience that may help improve patient outcomes, a surge of patients that overwhelms available resources might negatively impact care. In this respect, care for COVID-19 differs from care for other conditions, in which hospitals with more experience consistently demonstrate better outcomes, especially for new areas of care.4, 5, 6, 7
Thus far, the assessment of COVID-19 outcomes has focused on patient features that portend adverse outcomes.8, 9, 10, 11, 12 However, how hospitals responded to COVID-19 represents an important avenue into evaluating whether our care processes are designed to be resilient to large and dynamic case volumes of acute illnesses and can achieve similar outcomes despite these pressures. The knowledge gained from understanding these associations of volumes and outcomes for COVID-19 can inform care processes such as regionalization of care and sharing of resources across health systems as our health system continues to adapt to the COVID-19 pandemic and considers preparation for future health crises and challenges.
In a nationwide registry of hospitalized COVID-19 patients, we evaluated 2 distinct aspects of the experience of hospitals with COVID-19 case volumes. We assessed the overall case volumes at each participating hospital, which is a commonly used metric for volume-outcome studies, and assessed for its association with survival to discharge after accounting for patient and hospital features. In addition, we constructed a novel case growth measure that accounted for rate of growth of COVID-19 as a function of hospital bed capacity to assess whether rapid acceleration of COVID-19 case volumes relative to hospital capacity was associated with outcomes.
Methods
Data Source
We used patient and hospital data from the American Heart Association (AHA) COVID-19 Cardiovascular Disease (CVD) Registry. The registry was designed to collect high-quality information on patient characteristics and outcomes leveraging the Get With The Guidelines (GWTG) infrastructure in place for collecting data for AHA registries.8 , 13 The registry includes data from patients with completed hospitalizations for COVID-19 at participating hospitals defined by a positive polymerase chain reaction or an antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) and a clinical presentation consistent with COVID-19. The online data entry process ensured completeness of records and consistency in data fields across centers through internal logic checks. The current analysis is based on a data release that includes hospitalizations through August 2020 and captures hospitalizations in the early phase of the pandemic. Because the registry is designed as a quality improvement tool, with no intervention or participant contact, and collection of only a limited data set, informed consent was not obtained. The Duke University institutional review board provided approval.
Study Population and Exposures
Hospitals represented the unit of the analysis, and all hospitals with at least 10 COVID-19 cases were included. All COVID-19 cases reported from these hospitals were included. Two exposures were defined. First, overall COVID-19 case volume was defined as the absolute number of COVID-19 hospitalizations at individual hospitals across the same reporting period. The overall case volume represents the most commonly used unit of analyses in volume-outcome assessments.7 , 14 , 15
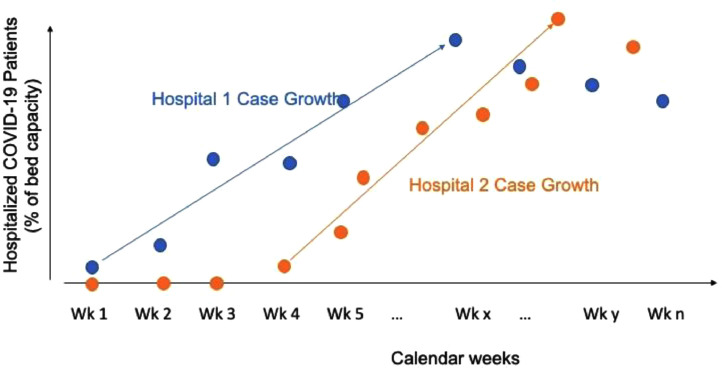
Second, to specifically isolate the potential effect of rapid case growth at hospitals, we constructed a novel measure of case growth rate as a function of a hospital's total bed capacity. This case growth rate was defined as the average weekly change in the proportion of hospitalized COVID-19 patients relative to hospital bed number, beginning with the week when the first case was recorded and ending the week with the peak case load at the hospital across the study period (Supplementary Figure 1, available online). The weekly hospital census of COVID-19 cases was based on hospital admission and discharge dates and included all patients who had a COVID-19 hospitalization spanning a given calendar week.
Supplementary Figure 1.
Schematic representation of the case growth trajectory at 2 hospitals.
Study Covariates and Outcome
To ensure our analyses compared similar patients presenting to similar hospitals, we identified detailed patient and hospital characteristics. For patients, these included demographics and comorbid health conditions (eMethods, available online). Further, in-hospital care features of ICU care and mechanical ventilation or renal replacement therapy were included. We identified several hospital characteristics, including teaching status, census region, number of hospital beds, and average daily patient census in 2019 that were available from the AHA data merged with the AHA COVID-19 CVD registry.
Study Outcome
The outcome of the study was hospital risk-standardized in-hospital mortality. This was computed using hierarchical models that accounted for patient and hospital characteristics. The construction of the model is described in eMethods and Supplementary Table 1, available online.
Statistical Analysis
We classified hospitals into quartiles based on each of the 2 hospital volume exposures: overall hospital COVID-19 volume and hospital COVID-19 case growth. We compared characteristics of hospitals across these quartiles, including their location, teaching status, bed size, and average daily hospital census in the pre-COVID-19 era. Next, we evaluated differences in characteristics of patients across the hospital volume quartiles including demographics, comorbid conditions, and in-hospital care needs and treatments. To evaluate directional difference in characteristics across quartiles, we used the Cochran Armitage test for the categorical variables and Jonckheere-Terpstra trend test for continuous variables. We parametrized quartiles as an ordinal categorical variable from lowest- to highest-volume quartiles for these assessments.
To assess the association with mortality for each of the 2 exposures, we evaluated continuous associations between hospital case volumes and hospital risk-standardized mortality using Spearman correlation. Conceptually, these analyses quantified the differences in hospital rankings for risk-standardized mortality that could be ascribed to overall volumes or their case growth. We repeated an assessment of mean risk-standardized mortality across hospital quartiles of volume and case growth using linear regression with hospital quartiles parameterized as ordinal categories from lowest to highest. We also calculated the odds ratio of above median risk-standardized mortality rates across volume and case growth quartiles with the lowest volume and case growth quartiles as referents, respectively.
Secondary Analysis
To account for temporal evolution of care practices that may have potentially led to the observed improvements in patient outcomes over time16 and may confound volume-outcome assessments, we created a temporally restricted cohort for sensitivity analyses. In these analyses, we limited our assessment of both volume-outcome and case-growth-outcome relationships to hospitals that reached their case peak in the first half of the year, specifically before June 30, 2020.
The level of significance was set at 0.05. All analyses were performed using R 3.9 (R foundation).
Results
Hospital and Patient Characteristics
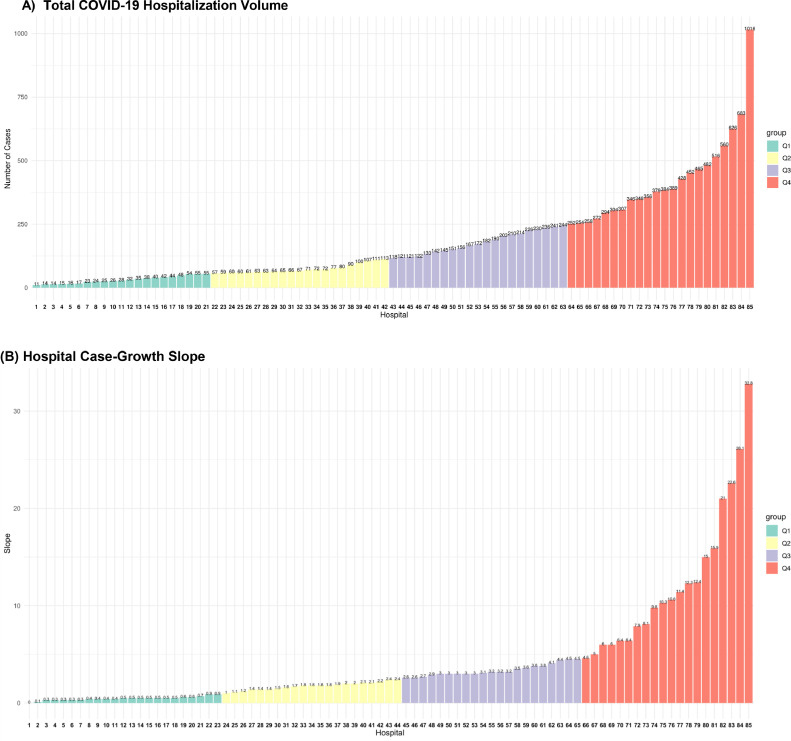
There were 104 hospitals with 15,397 patients in the AHA COVID-19 CVD registry, of which 85 reported at least 10 COVID-19 cases and were included in the study. There were 15,329 COVID-19 hospitalizations recorded at these hospitals during the study period. There was a large variation in volumes across hospitals, with median case volume of 118 (interquartile range [IQR], 57, 252) (Figure 1 ). Hospitals in the highest-case volume quartiles were larger, with larger bed size, and average daily hospital census in the preceding year (Table 1 ). There were no significant differences in teaching status or urban or rural location across hospital quartiles of case volumes.
Figure 1.
COVID-19 (A) hospitalization volumes, and (B) average weekly case growth slope across hospitals in the AHA COVID-19 CVD Registry. The units of the y-axis are number of cases in (A) and case increase per 100 beds per week in (B) and represents the average weekly case growth from the week of first case to that of the peak case volume. The x-axis represents individual hospitals ordered by their case volumes in (A) and their case-growth slope in (B). COVID-19 = coronavirus disease 2019.
Table 1.
Characteristics of Hospitals Across COVID-19 Overall Case Volume Quartiles.
| Q1 | Q2 | Q3 | Q4 | P Value | |
|---|---|---|---|---|---|
| Hospitals, N | 21 | 21 | 21 | 22 | |
| Hospital Bed Size | .01 | ||||
| ≥500 | 9 (42.9) | 9 (42.9) | 11 (52.4) | 14 (63.6) | |
| 250-499 | 7 (33.3) | 11 (52.4) | 10 (47.6) | 4 (18.2) | |
| <250 | 5 (23.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NA | 0 (0.0) | 1 (4.8) | 0 (0.0) | 4 (18.2) | |
| Hospital Location | .24 | ||||
| Urban | 19 (90.5) | 20 (95.2) | 21 (100.0) | 18 (81.8) | |
| Rural | 2 (9.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| NA | 0 (0.0) | 1 (4.8) | 0 (0.0) | 4 (18.2) | |
| Teaching Status | .12 | ||||
| Major | 9 (42.9) | 9 (42.9) | 10 (47.6) | 10 (45.5) | |
| Minor | 5 (23.8) | 10 (47.6) | 10 (47.6) | 7 (31.8) | |
| Nonteaching | 7 (33.3) | 1 (4.8) | 1 (4.8) | 1 (4.5) | |
| NA | 0 (0.0) | 1 (4.8) | 0 (0.0) | 4 (18.2) | |
| Annual Hospitalizations, median (IQR)* | 14,840 (5344, 29682) |
18,633 (12174, 25443) |
20,715 (16817, 27910) |
34,368 (24510,41483) |
<.001 |
| Average daily census, median (IQR)* | 207 (69, 463) |
288 (195, 366) |
327 (209, 455) |
645 (367,707) |
<.001 |
| Total hospital beds, median (IQR)* | 281 (152, 594) |
373 (251, 534) |
436 (279, 591) |
820 (502, 942) |
<.001 |
COVID-19 = coronavirus disease 2019; IQR = interquartile range.
P value for Jonckheere-Terpstra trend test
Patients in hospitals in the highest COVID-19 volume quartile were modestly younger (mean, 61.1 years in highest-volume quartile vs 62.0 years in the lowest-volume quartile) but without significant differences in either sex or race or ethnicity distributions or for cardiovascular and noncardiovascular comorbidities (Table 2 , P > .05 for all comparisons). There were similarly no significant differences in proportions of patients receiving ICU care or those requiring mechanical ventilation, hemodialysis, or vasopressors. Lower-volume hospitals had higher use of glucocorticoids, but there was no difference in the proportion of patients receiving remdesivir, interleukin-6 (IL-6) antagonists, or hydroxychloroquine across volume quartiles.
Table 2.
Characteristics of Patients Across Hospital COVID-19 Overall Case Volume Quartiles*
| Q1 | Q2 | Q3 | Q4 | P Value | |
|---|---|---|---|---|---|
| Number of patients | 656 | 1578 | 3724 | 9371 | |
| Age (mean [SD])† | 62.0 (17.2) | 63.0 (17.5) | 61.0 (18.0) | 61.1 (17.6) | .01 |
| Female sex | 261 (39.8) | 704 (44.6) | 1683 (45.2) | 4324 (46.1) | .24 |
| Race | |||||
| Non-Hispanic white | 349 (53.3) | 925 (59.0) | 1524 (40.9) | 2851 (30.4) | <.001 |
| Non-Hispanic black | 133 (20.3) | 351 (22.4) | 1083 (29.1) | 2437 (26.0) | |
| Hispanic | 113 (17.3) | 133 (8.5) | 777 (20.9) | 2690 (28.7) | |
| Others | 60 (9.2) | 158 (10.1) | 340 (9.1) | 1393 (14.9) | |
| Comorbidities | |||||
| Atrial fibrillation/flutter | 79 (12.0) | 211 (13.4) | 350 (9.4) | 767 (8.2) | .33 |
| Cancer | 86 (13.1) | 188 (11.9) | 346 (9.3) | 1403 (15.0) | .38 |
| Cerebrovascular disease (stroke/TIA) | 56 (8.5) | 155 (9.8) | 360 (9.7) | 1418 (15.1) | .43 |
| Currently on dialysis | 15 (2.3) | 49 (3.1) | 124 (3.3) | 346 (3.7) | .46 |
| Diabetes mellitus | 211 (32.2) | 567 (35.9) | 1353 (36.3) | 3269 (34.9) | .31 |
| Dyslipidemia | 199 (30.3) | 659 (41.8) | 1352 (36.3) | 3005 (32.1) | .29 |
| Heart failure | 75 (11.4) | 209 (13.2) | 413 (11.1) | 989 (10.6) | .36 |
| Hypertension | 400 (61.0) | 972 (61.6) | 2206 (59.2) | 5285 (56.4) | .24 |
| Peripheral artery disease | 20 (3.0) | 54 (3.4) | 94 (2.5) | 246 (2.6) | .42 |
| Chronic kidney disease | 84 (12.8) | 247 (15.7) | 455 (12.2) | 1156 (12.3) | .35 |
| History of DVT | 20 (3.0) | 56 (3.5) | 123 (3.3) | 276 (2.9) | .43 |
| History of smoking | 63 (9.6) | 90 (5.7) | 256 (6.9) | 604 (6.4) | .37 |
| Immune disorders | |||||
| HIV | 5 (0.8) | 13 (0.8) | 34 (0.9) | 115 (1.2) | .48 |
| SLE | 7 (1.1) | 6 (0.4) | 21 (0.6) | 54 (0.6) | .45 |
| Rheumatoid | 7 (1.1) | 17 (1.1) | 45 (1.2) | 105 (1.1) | .46 |
| Other immune disorder | 10 (1.5) | 41 (2.6) | 60 (1.6) | 174 (1.9) | .45 |
| History of pulmonary embolism | 16 (2.4) | 40 (2.5) | 72 (1.9) | 173 (1.8) | .43 |
| Pulmonary disease | |||||
| COPD | 60 (9.1) | 176 (11.2) | 277 (7.4) | 702 (7.5) | .36 |
| ILD | 5 (0.8) | 5 (0.3) | 10 (0.3) | 42 (0.4) | .46 |
| Asthma | 60 (9.1) | 150 (9.5) | 271 (7.3) | 892 (9.5) | .38 |
| Other pulmonary | 26 (4.0) | 72 (4.6) | 78 (2.1) | 163 (1.7) | .38 |
| Organ transplant | 11 (1.7) | 24 (1.5) | 42 (1.1) | 152 (1.6) | .45 |
| Congenital heart disease | 2 (0.3) | 3 (0.2) | 9 (0.2) | 19 (0.2) | .48 |
| Prior CABG | 14 (2.1) | 59 (3.7) | 116 (3.1) | 231 (2.5) | .44 |
| Prior MI | 38 (5.8) | 96 (6.1) | 193 (5.2) | 445 (4.7) | .39 |
| Prior PCI | 33 (5.0) | 83 (5.3) | 148 (4.0) | 398 (4.2) | .40 |
| No medical history | 112 (17.1) | 248 (15.7) | 736 (19.8) | 1608 (17.2) | .35 |
| Treatments | |||||
| Glucocorticoid | 238 (36.3) | 471 (29.8) | 1005 (27.0) | 2574 (27.5) | <.001 |
| Interleukin-6 agents | 23 (3.5) | 124 (7.9) | 402 (10.8) | 1124 (12.0) | .20 |
| Remdesivir | 54 (8.2) | 216 (13.7) | 365 (9.8) | 976 (10.4) | .22 |
| Hydroxychloroquine | 297 (45.3) | 458 (29.0) | 1502 (40.3) | 3740 (39.9) | .25 |
| Critical illness | |||||
| ICU use | 267 (40.7) | 678 (43.0) | 1115 (29.9) | 2543 (27.1) | .29 |
| Vasopressor | 77 (11.7) | 176 (11.2) | 456 (12.) | 1058 (11.3) | .45 |
| Mechanical ventilation | 147 (22.4) | 340 (21.5) | 715 (19.2) | 1879 (20.1) | .11 |
| Hemodialysis/CRRT, new | 36 (5.5) | 53 (3.4) | 145 (3.9) | 396 (4.2) | .10 |
CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disorder; CRRT = continuous renal replacement therapy; DVT = deep vein thrombosis; HIV = human immunodeficiency virus; ICU = intensive care unit; ILD = interstitial lung disease; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation; SLE = systemic lupus erythematosus; TIA = transient ischemic attack.
Values represent numbers (percentage), unless otherwise specified.
P value for Jonckheere-Terpstra trend test
Association Between COVID-19 Case Volumes and Risk Standardized In-Hospital Case-Fatality
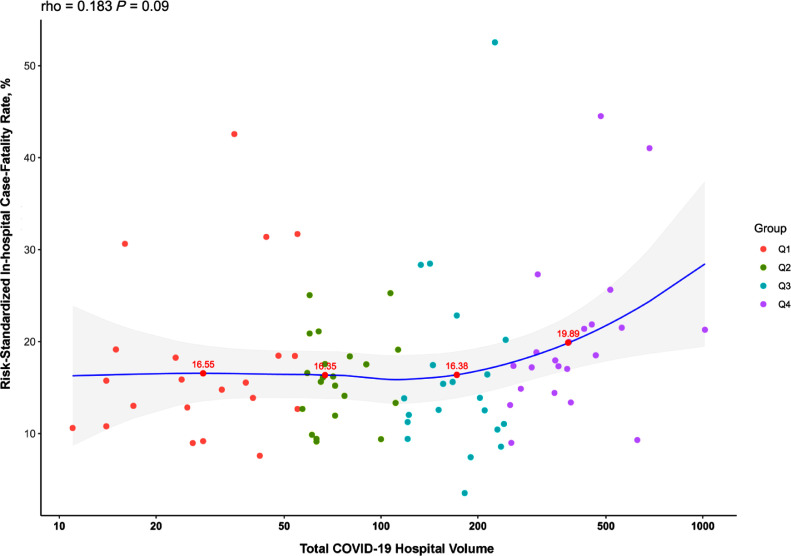
There was a large variation in patient mortality, with a median in-hospital case-fatality rate (CFR) of 14.3% (IQR 8.5%, 18.7%) and risk-standardized case-fatality rate of 15.9% (12.5%, 20.0%) across hospitals. The median unadjusted in-hospital CFR was 14.2% in the lowest-volume quartile (Q1), 12.6% in Q2, 12.8% in Q3, and 16.8% in Q4. There was no significant association between overall COVID-19 case volume and risk-standardized CFR (Spearman correlation, 0.18, P = .09) (Figure 2 ). Mortality was numerically higher but not significantly different across quartiles based on COVID-19 volumes, with median risk-standardized CFR of 15.5% (IQR 12.7%, 18.5%) in the lowest-volume quartile and 18.2% (IQR, 14.9%, 21.5%, P for trend across quartiles, .34). Hospitals in the highest COVID-19 volume quartile had higher odds of having above median risk-standardized CFR compared with lowest-volume quartile hospitals; however, these findings did not reach statistical significance (odds ratio [OR], 3.56; 95% confidence interval [CI] 0.99-12.7, P = 0.05).
Figure 2.
Correlation between COVID-19 hospitalization volume and risk-standardized in-hospital case-fatality rate. Spearman correlation for hospital volume and risk-standardized mortality is 0.18, P = .09. Labeled points represent risk-standardized in-hospital case-fatality rate for the median hospital based on volume in each quartile. COVID-19 = coronavirus disease 2019.
Hospital COVID-19 Case Growth and Risk Standardized Mortality
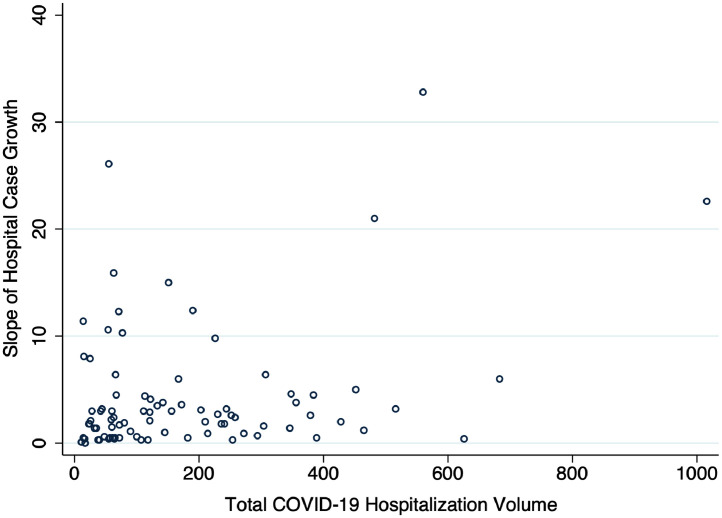
The median COVID-19 hospitalizations case growth was 2 cases per 100 beds per week but varied widely (IQR: 0.9 cases/100 beds/wk to 4.5 cases/100 beds/wk) (Figure 1). There was a modest positive correlation between hospital case growth rate and total hospital COVID-19 volume (Spearman correlation coefficient, 0.22, P = .046, Supplementary Figure 2, available online). There were notable differences in hospital characteristics with large bed size and teaching hospitals less likely to be in the highest-case growth rate quartile, even though they represented the largest group in the highest-volume quartile (Supplementary Table 2, available online). There were, however, no significant differences in patient characteristics across case-growth quartiles (Supplementary Table 3, available online).
Supplementary Figure 2.
Correlation between total hospital volume and hospital case growth slope. Horizontal axis is the total case volume, and the vertical axis is case increase per 100 beds per week and represents the average weekly case growth from the week of first case to that of the peak case volume. Spearman correlation coefficient, 0.22 (P = .046).
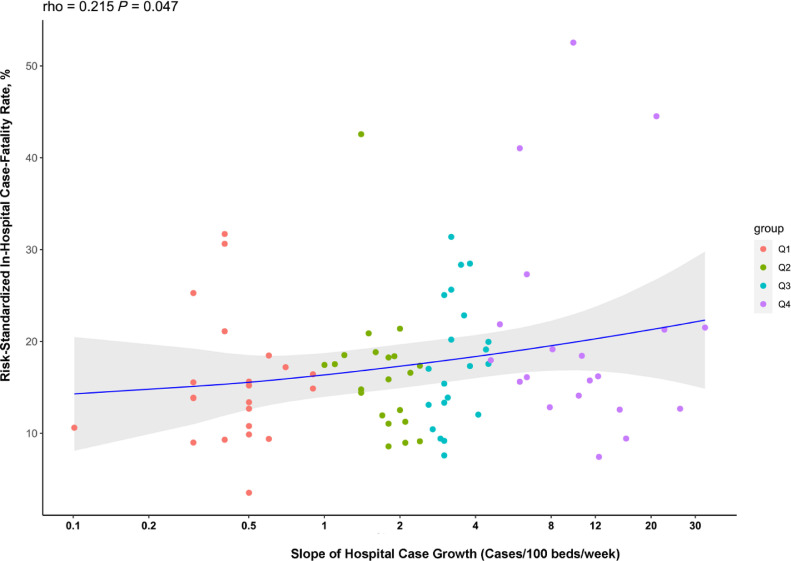
There was a significant positive correlation between case growth and risk-standardized hospital CFR, with hospitals with the most rapid case-growth having a significantly higher risk-standardized CFR (Spearman correlation coefficient: 0.22, P = .047) (Figure 3 ). Median risk-standardized CFR was 13.9 (IQR 10.6%, 17.2%) in the lowest case-growth quartile and 17.8% (IQR, 14.1%, 21.5%, P for trend across quartiles, .03). Hospitals in the highest case-growth quartile had 4-fold higher odds of having above median risk-standardized CFR, compared with hospitals in the lowest case-growth quartile (OR, 4.00; 95% CI, 1.15 to 13.8, P = .03).
Figure 3.
Correlation between COVID-19 case growth slope and risk-standardized in-hospital case-fatality rate. Spearman correlation coefficient 0.22, P = .047. The line represents the loess curve corresponding to the volume slope-outcome relationship. The units of the x-axis are cases increase per 100 beds per week and represents the average weekly case growth from the week of first case to that of the peak case volume. Labeled points represent risk-standardized in-hospital case-fatality rate for the median hospital based on case growth in each quartile. COVID-19 = coronavirus disease 2019.
Secondary Analysis
In analyses that restricted the assessment of volumes and outcomes to early part of the pandemic, a total of 70 hospitals that had a peak case volume in June or earlier were included. Among these hospitals, there was a significant correlation between hospital volumes and hospital risk-standardized CFR (Spearman correlation coefficient, 0.32, P = .008). Among hospitals with an early case peak, those in the highest-volume quartile had 11-fold higher odds of above median risk-standardized CFR (OR 11.43, 95% CI 1.97, 66.36, P = .007). Hospitals in the highest-quartile of case growth who reached their peak volumes in the first half of the year had more than 4-fold higher odds of above median risk-standardized CFR (OR 4.67; 95% CI, 0.96, 22.79, P = .057) similar to the primary analyses but did not reach our predefined threshold of significance.
Discussion
In a large nationwide registry of 85 hospitals with 15,329 confirmed COVID-19 hospitalizations and detailed phenotypic characterization, there was a large variation in both the overall volume of COVID-19 cases as well as the trajectory of case growth. Hospitals had a large variation in patient mortality, even after accounting for differences in patient and hospital characteristics. The overall volume of COVID-19 cases at hospitals was not significantly associated with in-hospital CFR, but in analyses of hospitals with early peaks in COVID-19 volumes, higher COVID-19 volume was associated with higher risk standardized CFR. We also found that the rate at which hospitals experienced an increase in their cases relative to their capacity across the study period was significantly associated with CFR, with faster rise in cases associated with higher risk-standardized CFR.
The challenges faced by hospitals in the COVID-19 pandemic have consistently been interpreted in light of their bed capacity or the vulnerability of their health care providers. However, from a patient outcomes perspective, in addition to volume, the rapidity with which a hospital experiences an increase in their case volume likely has important implications for patients receiving care at those hospitals. Hospital case growth and overall case volumes may potentially create a challenge for staffing, bed capacity, and ICU care needs,17 which are frequently in flux due to the hazard for infection among health care workers. Despite this uncertainty about the mechanism, case growth, which can be assessed prospectively, may play a role in identifying hot spots that may benefit from an urgent influx of additional personnel and resources to care for the surge.
These observations also identify a potential explanation for the observed variation in risk-adjusted outcomes across hospitals that has been suggested in prior studies,12 , 16 especially because this variation in hospital outcomes cannot be explained by differences in measured patient characteristics. Of note, a recent study that addressed variation in outcomes across hospitals included individuals from a single payer and did not have the entire census of hospitalized COVID-19 patients, as was possible in our study using an all-comer registry.16 Although larger hospitals had higher case volumes, there was no correlation of COVID-19 volumes with patient comorbidities or illness severity based on the need for ICU care. Moreover, volume itself is an imperfect surrogate for burden, as has been observed in care for acute cardiovascular conditions and cardiovascular surgery.7 , 14 , 15 , 18 Our hypothesis-driven metric that captures the rate of case growth as a function of a hospital's capacity may offer a more nuanced and accurate assessment of burden on hospitals. Further, our findings may explain the high CFR for certain patient groups in communities with high incidence of COVID-19 infections9 , 12 , 19 because we found that racial or ethnic minorities more frequently presented to hospitals that were more rapidly approaching their capacity. These hospitals may be ideal sites for interventions aimed at reducing mortality from COVID-19 potentially through regionalization of care and sharing of staff and resources to allow continued high-quality supportive care. Further, interventions that decrease hospital length of stay,20, 21, 22 in addition to other outcomes, may have a role in improving the hospital census and the overwhelming of capacity by rapid case growth.
Our study has certain limitations. First, our observations are limited to hospitals participating in the AHA COVID-19 CVD registry and may not be representative of all US community hospitals. However, the registry has specifically included geographically dispersed hospitals across bed capacity strata. Second, the participation of the hospitals was voluntary, and they have not yet been subject to an audit ensuring all cases were reported. However, the data entry platform was based on a familiar infrastructure that is used by other registries by the AHA in its broader Get With The Guidelines program. Third, we modeled hospital volumes using 2 standards, but there could be other unsupervised models that better capture the trajectory of the curves for each hospital's case volume. However, as we hoped to gain actionable insights from this study, we applied either existing or hypothesis-driven strategies to the choice of our exposures. Nevertheless, a better understanding of the entirety of the case volume trajectory may offer additional insights about the effect of case volumes on patient outcomes. Fourth, the registry included data through August, and the case-growth patterns in the most recent winter peak of COVID-19 cases and its association with outcomes may differ. Fifth, there could be unmeasured variation in patient features, including income and education, that are not accounted for in the models. However, our risk-adjustment models were robust and had a model discrimination of 0.73 at a patient level, suggesting successful capture of patient features that predicted outcomes. We also did not find a significant difference in patient characteristics across hospitals based on their case volumes or their use of drug therapies. Moreover, although there appear to be patterns in sensitivity analyses suggestive of a stronger volume-outcome relationship during the early part of the pandemic, we were limited by statistical power in subsetting the time period into smaller units. Sixth, we did not capture outcomes of patients after hospital discharge, limiting our ability to assess overall case fatality rates for hospitalized patients. Finally, the case volumes and case growth do not explain the majority of variation in outcomes across hospitals, which requires dedicated investigation into differences in other hospital care practices.
Conclusion
In conclusion, an accelerated case-growth trajectory is a marker of hospitals at risk of poor COVID-19 outcomes. These sites may be targets for influx of additional resources or triage strategies. Early identification of such hospital signatures that may portend poor outcomes is essential as our health system prepares for future health challenges.
Acknowledgments1
We thank Laura Stevens and Julie Sizelove of the American Heart Association (AHA) for their assistance in gaining access to the AHA COVID-19-CVD registry and the Precision Medicine Platform.
Footnotes
Funding: RK received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under the grant 1K23HL153775. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest: JAdL reports fees for participating in Data Monitoring Committees from Eli Lilly and Novo Nordisc and consulting income from Jannsen. DJK reports serving on the writing committee of the 2018 American Association for Thoracic Surgery/American College of Cardiology/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons Expert Consensus Systems of Care Document: Operator and Institutional Recommendations and Requirements for Transcatheter Aortic Valve Replacement. WRY reports grants and personal fees from Abbott Vascular, AstraZeneca, Medtronic, and Boston Scientific outside the submitted work. BKN reports being a principal investigator or coinvestigator on research grants from the National Institutes of Health, the Veterans Affairs Health Services Research & Development, the American Heart Association, Apple, Inc, and Toyota; compensation as Editor-in-Chief of Circulation: Cardiovascular Quality & Outcomes, a journal of the American Heart Association; and is a coinventor on US Utility Patent Number US15/356 012 (US20170148158A1) titled “Automated analysis of vasculature in coronary angiograms” that uses software technology with signal processing and machine learning to automate the reading of coronary angiograms, held by the University of Michigan. The patent is licensed to AngioInsight, Inc, in which he holds ownership shares. HMK reports personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, F-Prime, Siegfried & Jensen Law Firm, Arnold & Porter Law Firm, Martin/Baughman Law Firm, and National Center for Cardiovascular Diseases, Beijing; serving as cofounder of HugoHealth, Refactor Health, and Centers for Medicare & Medicaid Services; and grants from Medtronic and the Food and Drug Administration, Medtronic and Johnson & Johnson, and Shenzhen Center for Health Information outside the submitted work. JPC reports receiving support from the American College of Cardiology, CMS, and Medtronic. RK, YL, SRD, AP, WO, SG, CR, KW, ZL, SMB, EJB, KBC report none.
Authorship: All authors had access to the data and a role in writing this manuscript.
The Get With The Guidelines® programs are provided by the American Heart Association. The American Heart Association Precision Medicine Platform (https://precision.heart.org/) was used for data analysis. IQVIA (Parsippany, New Jersey) serves as the data collection and coordination center. AHA's suite of Registries is funded by multiple industry sponsors. AHA's COVID-19 CVD Registry is partially supported by The Gordon and Betty Moore Foundation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjmed.2021.06.034.
eMETHODS
Study Covariates: Patient Characteristics
Patient characteristics included demographics (age, sex, race or ethnicity), comorbid health conditions, including cardiovascular disease (prior myocardial infraction, prior coronary artery bypass grafting, prior percutaneous coronary intervention, cerebrovascular disease, peripheral artery disease, heart failure, atrial fibrillation, atrial flutter), cardiometabolic risk factors (hypertension, diabetes mellitus, dyslipidemia), pulmonary and pulmonary vascular diseases (chronic pulmonary disease, including chronic obstructive pulmonary disease, interstitial lung disease, asthma, and pulmonary embolism), renal disease (chronic kidney disease, chronic hemodialysis), and other disorders (history of deep vein thrombosis, cancer, immune disorders including human immunodeficiency virus infection, systemic lupus erythematosus, rheumatoid arthritis, organ transplant).
Risk-Standardized In-Hospital Case-Fatality Rate
For this, we constructed logistic regression models that included in-hospital case-fatality as the dependent variable, patient characteristics as fixed effects, and hospital site as a random effect. A hospital's risk-standardized case-fatality rate (CFR) represents a ratio of the predicted to expected rate of CFR multiplied by the overall CFR in the population. Predicted rate for the hospital based on its site-specific intercept or the mortality accounting for its own case mix and the expected rate is the overall hospital intercept representing the CFR at an average hospital with the case mix identical to the overall population. The patient-level covariates included detailed demographic characteristics and comorbid conditions selected based on a stepwise regression model applied to all comorbidities and had model c-statistic of 0.73 (Supplementary Table 1 ). Of note, race or ethnicity was not an independent predictor of CFR in our assessment that included comorbid conditions and was not explicitly included in the risk-adjustment model. This is consistent with observation from other studies that also found that race or ethnicity is not an independent predictor of in-hospital CFR.9,12
Supplementary Table 1.
Patient-Level Covariates Included in the Risk-Adjustment Model (Model C-Statistic 0.73)
| Covariates |
|---|
| Age Sex Cancer Atrial fibrillation or atrial flutter Cerebrovascular disease Current hemodialysis Diabetes mellitus Dyslipidemia Heart failure Hypertension Prior myocardial infarction Systemic lupus erythematosus Rheumatoid arthritis History of pulmonary embolism Chronic obstructive pulmonary disease Interstitial lung disease Asthma Congenital heart disease Smoking history |
Supplementary Table 2.
Characteristics of Hospitals Across COVID-19 Case Growth Quartiles
| Overall | Q1 | Q2 | Q3 | Q4 | P Value | |
|---|---|---|---|---|---|---|
| N | 85 | 23 | 21 | 21 | 20 | |
| Hospital Bed Size | <.001 | |||||
| ≥500 | 43 (50.6) | 14 (60.9) | 16 (76.2) | 11 (52.4) | 2 (10.0) | |
| 250-499 | 32 (37.6) | 7 (30.4) | 5 (23.8) | 6 (28.6) | 14 (70.0) | |
| <250 | 5 (5.9) | 1 (4.3) | 0 (0.0) | 2 (9.5) | 2 (10.0) | |
| NA | 5 (5.9) | 1 (4.3) | 0 (0.0) | 2 (9.5) | 2 (10.0) | |
| Hospital Location | <.001 | |||||
| Rural | 2 (2.4) | 1 (4.3) | 0 (0.0) | 1 (4.8) | 0 (0.0) | |
| Urban | 78 (91.8) | 21 (91.3) | 21 (100.0) | 18 (85.7) | 18 (90.0) | |
| NA | 5 (5.9) | 1 (4.3) | 0 (0.0) | 2 (9.5) | 2 (10.0) | |
| Teaching Status | .004 | |||||
| Major | 38 (44.7) | 14 (60.9) | 12 (57.1) | 9 (42.9) | 3 (15.0) | |
| Minor | 32 (37.6) | 6 (26.1) | 9 (42.9) | 9 (42.9) | 8 (40.0) | |
| Nonteaching | 10 (11.8) | 2 (8.7) | 0 (0.0) | 1 (4.8) | 7 (35.0) | |
| NA | 5 (5.9) | 1 (4.3) | 0 (0.0) | 2 (9.5) | 2 (10.0) | |
| Annual Hospitalizations, median (IQR) | 21,358 (13,790, 30,445) |
20,841 (13,930, 30,400) |
27,207 (19,350, 38,147) |
21,630 (14,736, 31,620) |
12,962 (8796,17,324) |
.002 |
| Total hospital beds, median (IQR) | 457 (262, 637) |
594 (259,655) |
566 (407, 841) |
445 (295, 582) |
274 (160,354) |
.003 |
| Average daily census, median (IQR) | 331 (198, 524) |
437 (212, 526) |
455 (301, 646) |
342 (208, 463) |
178 (106, 240) |
.004 |
COVID-19 = coronavirus disease 2019; IQR = interquartile range.
Supplementary Table 3.
Characteristics of Patients Across Hospital COVID-19 Case Growth Quartiles
| Overall | Q1 | Q2 | Q3 | Q4 | P Values | |
|---|---|---|---|---|---|---|
| Number of patients | 15,329 | 3114 | 3318 | 3875 | 5022 | |
| Age (mean [SD]) | 61.29 (17.71) | 58.69 (18.12) | 60.76 (18.17) | 63.17 (17.38) | 61.81 (17.17) | <.001 |
| Race (%) | ||||||
| Black | 7643 (49.9) | 1599 (51.3) | 1876 (56.5) | 1783 (46.0) | 2385 (47.5) | .267 |
| White | 4101 (26.8) | 958 (30.8) | 995 (30.0) | 1091 (28.2) | 1057 (21.0) | |
| Others | 3569 (23.3) | 557 (17.9) | 446 (13.4) | 989 (25.5) | 1577 (31.4) | |
| Missing | 16 (0.1) | 0 (0.0) | 1 (0.0) | 12 (0.3) | 3 (0.1) | |
| Female sex | 8357 (54.5) | 1598 (51.3) | 1776 (53.5) | 2069 (53.4) | 2914 (58.0) | .28 |
| Atrial fibrillation/flutter | 1407 (9.2) | 299 (9.6) | 349 (10.5) | 403 (10.4) | 356 (7.1) | .36 |
| Cancer | 2023 (13.2) | 385 (12.4) | 387 (11.7) | 438 (11.3) | 813 (16.2) | .393 |
| Cerebrovascular disease (stroke or TIA) | 1989 (13.0) | 307 (9.9) | 367 (11.1) | 348 (9.0) | 967 (19.3) | .437 |
| Currently on dialysis | 534 (3.5) | 94 (3.0) | 131 (3.9) | 137 (3.5) | 172 (3.4) | .431 |
| Diabetes mellitus | 5400 (35.2) | 1191 (38.2) | 1247 (37.6) | 1420 (36.6) | 1542 (30.7) | .264 |
| Dyslipidemia | 5215 (34.0) | 1066 (34.2) | 1257 (7.9) | 1506 (38.9) | 1386 (7.6) | .27 |
| Heart failure | 1686 (11.0) | 396 (12.7) | 445 (13.4) | 456 (11.8) | 389 (7.7) | .328 |
| Hypertension | 8863 (57.8) | 1890 (60.7) | 2028 (61.1) | 2428 (62.7) | 2517 (50.1) | .228 |
| Peripheral artery disease | 414 (2.7) | 104 (3.3) | 85 (2.6) | 136 (3.5) | 89 (1.8) | .412 |
| Prior CABG | 420 (2.7) | 81 (2.6) | 118 (3.6) | 106 (2.7) | 115 (2.3) | .424 |
| Prior MI | 772 (5.0) | 209 (6.7) | 206 (6.2) | 210 (5.4) | 147 (2.9) | .361 |
| Prior PCI | 662 (4.3) | 140 (4.5) | 177 (5.3) | 178 (4.6) | 167 (3.3) | .398 |
| Chronic kidney disease | 1942 (12.7) | 448 (14.4) | 429 (12.9) | 538 (13.9) | 527 (10.5) | .338 |
| DVT | 475 (3.1) | 104 (3.3) | 122 (3.7) | 128 (3.3) | 121 (2.4) | .413 |
| History of smoking | 1013 (6.6) | 235 (7.5) | 312 (9.4) | 206 (5.3) | 260 (5.2) | .359 |
| Immune disorders | 703 (4.6) | 129 (4.1) | 151 (4.6) | 242 (6.2) | 181 (3.6) | .416 |
| HIV | 167 (1.1) | 22 (0.7) | 34 (1.0) | 50 (1.3) | 61 (1.2) | .476 |
| Lupus | 88 (0.6) | 21 (0.7) | 20 (0.6) | 24 (0.6) | 23 (0.5) | .461 |
| Rheumatoid | 174 (1.1) | 33 (1.1) | 49 (1.5) | 58 (1.5) | 34 (0.7) | .447 |
| Other immune disorder | 285 (1.9) | 60 (1.9) | 53 (1.6) | 120 (3.1) | 52 (1.0) | .437 |
| Pulmonary embolism | 301 (2.0) | 80 (2.6) | 77 (2.3) | 74 (1.9) | 70 (1.4) | .417 |
| Pulmonary disease | 2703 (17.6) | 574 (18.4) | 630 (19.0) | 767 (19.8) | 732 (14.6) | .322 |
| COPD | 1215 (7.9) | 232 (7.5) | 333 (10.0) | 331 (8.5) | 319 (6.4) | .375 |
| ILD | 62 (0.4) | 9 (0.3) | 17 (0.5) | 24 (0.6) | 12 (0.2) | .474 |
| Asthma | 1373 (9.0) | 290 (9.3) | 286 (8.6) | 409 (10.6) | 388 (7.7) | .376 |
| Other pulmonary | 339 (2.2) | 118 (3.8) | 68 (2.0) | 91 (2.3) | 62 (1.2) | .393 |
| Organ transplant | 229 (1.5) | 65 (2.1) | 45 (1.4) | 57 (1.5) | 62 (1.2) | .432 |
| Congenital heart disease | 33 (0.2) | 11 (0.4) | 4 (0.1) | 11 (0.3) | 7 (0.1) | .471 |
| Pulmonary arterial hypertension | 57 (0.4) | 27 (0.9) | 8 (0.2) | 19 (0.5) | 3 (0.1) | .441 |
| No medical history | 2704 (17.6) | 543 (17.4) | 592 (17.8) | 639 (16.5) | 930 (18.5) | .349 |
CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disorder; COVID-19 = coronavirus disease 2019; DVT = deep vein thrombosis; HIV = human immunodeficiency virus; ILD = interstitial lung disease; MI = myocardial infarction; PCI = percutaneous coronary intervention; SD = standard deviation; TIA = transient ischemic attack.
We included categorical hospital bed number (<250 beds, 250-499 beds, and ≥500 beds) as a hospital-level covariate in the model to allow standardization of outcome assessment for hospitals with different capacity and as a surrogate for their available health services and experience with high acuity conditions. To account for the expected random variation at low-volume centers, all hierarchical models employed an empiric Bayes approach that limits the impact of large random variation of estimates from centers with very few cases.16
References
- 1.Moghadas SM, Shoukat A, Fitzpatrick MC, et al. Projecting hospital utilization during the COVID-19 outbreaks in the United States. Proc Natl Acad Sci U S A. 2020;117(16):9122–9126. doi: 10.1073/pnas.2004064117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emanuel EJ, Persad G, Upshur ., et al. Fair allocation of scarce medical resources in the time of COVID-19. N Engl J Med. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 3.Khera R, Dhingra LS, Jain S, Krumholz HM. An evaluation of the vulnerable physician workforce in the USA during the coronavirus disease-19 pandemic. J Gen Intern Med. 2020;35(10):3114–3116. doi: 10.1007/s11606-020-05854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365(24):2287–2295. doi: 10.1056/NEJMsa1101942. [DOI] [PubMed] [Google Scholar]
- 5.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross JS, Normand SL, Wang Y, et al. Hospital volume and 30-day mortality for three common medical conditions. N Engl J Med. 2010;362(12):1110–1118. doi: 10.1056/NEJMsa0907130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380(26):2541–2550. doi: 10.1056/NEJMsa1901109. [DOI] [PubMed] [Google Scholar]
- 8.Hendren NS, de Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results From the American Heart Association COVID-19 cardiovascular disease registry. Circulation. 2021;143(2):135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 9.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razavian N, Major VJ, Sudarshan M, et al. A validated, real-time prediction model for favorable outcomes in hospitalized COVID-19 patients. NPJ Digit Med. 2020;3:130. doi: 10.1038/s41746-020-00343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association's COVID-19 cardiovascular disease registry. Circulation. 2021;133(24):2332–2342. doi: 10.1161/CIRCULATIONAHA.120.052278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alger HM, Rutan C, Williams JH, 4th, et al. American Heart Association COVID-19 CVD registry powered by get with the guidelines. Circ Cardiovasc Qual Outcomes. 2020;13(8) doi: 10.1161/CIRCOUTCOMES.120.006967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumbhani DJ, Fonarow GC, Heidenreich PA, et al. Association between hospital volume, processes of care, and outcomes in patients admitted with heart failure: insights from Get With The Guidelines-Heart Failure. Circulation. 2018;137(16):1661–1670. doi: 10.1161/CIRCULATIONAHA.117.028077. [DOI] [PubMed] [Google Scholar]
- 15.Kumbhani DJ, Cannon CP, Fonarow GC, et al. Association of hospital primary angioplasty volume in ST-segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302(20):2207–2213. doi: 10.1001/jama.2009.1715. [DOI] [PubMed] [Google Scholar]
- 16.Asch DA, Sheils NE, Islam MN, et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 months of the pandemic. JAMA Intern Med. 2021;181(4):471–478. doi: 10.1001/jamainternmed.2020.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCabe R, Schmit N, Christen P, et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Medicine. 2020;18(1):1–12. doi: 10.1186/s12916-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khera R, Pandey A, Koshy T, et al. Role of hospital volumes in identifying low-performing and high-performing aortic and mitral valve surgical centers in the United States. JAMA Cardiol. 2017;2(12):1322–1331. doi: 10.1001/jamacardio.2017.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wadhera RK, Wadhera P, Gaba P, et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323(21):2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming RM, Fleming MR. FMTVDM quantitative nuclear imaging finds three treatments for SARS-CoV-2. Biomed J Sci Tech Res. 2021;33(4):26041–26083. [Google Scholar]
- 21.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosas IO, Brau N, Waters M, et al. Tocilizumab in Hospitalized Patients With Severe COVID-19 Pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]